IDENTITY
Biological features
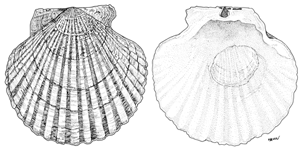
Large (10-22 cm long), almost circular; umbones in centre, between two almost equal ears. Exterior of right valve whitish, with 20 broad, flattened ribs. Exterior of left valve purplish brown with 20 coarse radial ribs. Interior valve whitish, furrowed, with a single adductor muscle scar. Spawning season from March to May.
PROFILE
Historical background
This commercially valuable Pacific Asian low-boreal scallop species supported substantial fisheries until the 1930s; then stocks diminished mainly through over-exploitation. Capture fisheries production appears to have peaked in the mid-1930s when 80 000 tonnes (shell-on) were landed in Japan. At about the same time, the Russian Yesso scallop population along the coast of Primorye was estimated at about 40 million, inhabiting an area of about 16 000 ha. Regional catches declined dramatically thereafter, falling to 6 000 tonnes in Japan in 1968. The development of off-bottom culture, supported by wild seed capture after 1945 led to a sustained upsurge in production, which continued until the year 2000. Since then annual production has stabilised at 1.1-1.2 million tonnes. China and Japan are the major producers, together accounting for over 1.1 million tonnes in 2003.
Main producer countries
This species has also been introduced from Japan to France and Canada
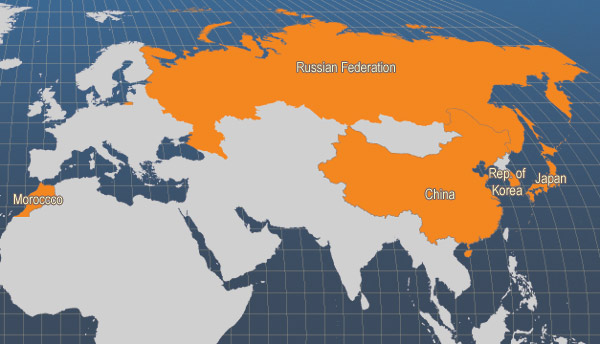

Main producer countries of Patinopecten yessoensis (FAO Fishery statistics, 2006)
Habitat and biology
Yesso scallops occur in sheltered, shallow bays and inlets adjacent to rocky shores, out to a depth of 30-40 m in more open sea areas. They are most abundant within the bathymetric range 4-10 m and occur mainly at salinities of 32-34‰ on sand and firm sandy/mud substrates interspersed with rocks. Optimal growth temperature is 4-8 °C and the tolerance range is from -2 °C to 26 °C. Inshore distribution is limited by ice depth during winter. Unlike many species of scallop, sexes are separate with hermaphrodites rarely observed. Yesso scallops are protandrous hermaphrodites maturing initially as males and changing sex to female as they age. Spawning occurs in spring as water temperature rises and reaches 7-12 °C. Males dominate in younger year classes and females in older year classes. Females of 12-15 cm shell height produce 8-18 million eggs. Spawning begins in March and peaks in April at 7-12 °C in Japan and a month later further north. Larvae, which are planktonic and measure about 110 µm shell length initially, feed on phytoplankton and develop over a period of 30 to 40 days to 250-280 µm, at which size they are fully developed and ready to settle and metamorphose. Duration of the larval stage is temperature-dependent, after which larvae settle on filamentous epifauna and flora, attach by means of byssus threads, and metamorphose. Following growth over a period of 3 to 4 months, when the juveniles (spat) are >10 mm in shell height, they detach and disperse on suitable bottom substrate. Life span is 10-12 years, when scallops will have grown to ~20 cm and weigh 1 kg. The species is amenable to hatchery culture but spat are almost exclusively collected from the wild for commercial culture.
PRODUCTION
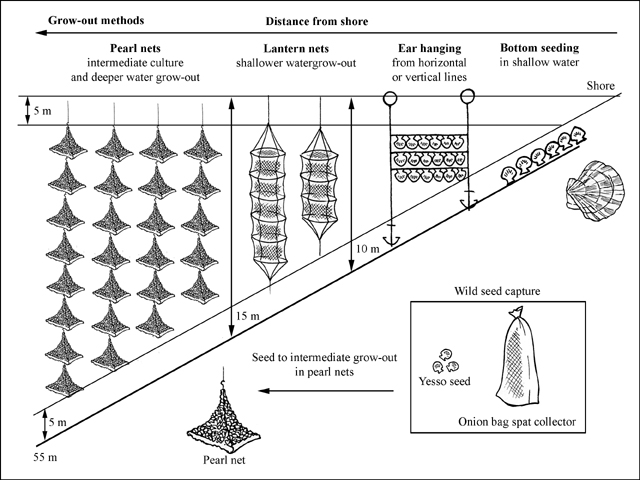
Production cycle

Production cycle of Patinopecten yessoensis
Production systems
World production of the Yesso scallop is almost entirely accounted for by wild seed collection and grow-out of the spat in suspended (hanging) culture or on ground lays. Off-bottom culture methods were developed in Japan and have spread to the other North Pacific Asian countries where culture is practiced. Similar technology based on Japanese experience is utilized worldwide for a wide range of commercially valuable scallop species.
Scallops spawn in spring at 7-12 °C. The density of settlement depends on the concentration of larvae in the water column; this is closely monitored to predict both the timing and intensity of settlement. Spat collectors are suspended in the water column when monitoring programmes determine that >50 percent of larvae exceed 200 µm in length. Two types of spat collector are used:
Spat are harvested from the collectors about 3 months after settlement when they measure about 10 mm shell height. They are then transferred to intermediate (nursery) culture in pearl nets; this occurs in the autumn. Care is taken to remove predators, which may have settled as larvae within the units, and also organisms that will compete for food and space (e.g. mussels). Each pearl net has a bottom surface area of 0.12 m² and can be stocked with up to 50-60 of 10 mm seed. Pearl nets are strung together to form vertical 'cage' units of variable length, depending on water depth. There may be from 5 to 30 nets per unit, and units are suspended from submerged longlines set 5-10 m below the water surface. After 10 weeks, the seed will have grown to 20-30 mm shell height and occupy about 60 percent of the volume of the net. A survival rate of 90 percent is common during this period. At this time, in October, density is reduced to 15-20 scallops per net. Intermediate culture then continues through winter until the following spring, by which time the scallops have grown to ~50 mm. They are then ready to be transferred to the grow-out stage.
Grow-out to market size is either by sowing year-old seed on bottom lays or in various forms of hanging culture. Scallop culture is mainly a co-operative activity in Asian countries and may be a part of a polyculture system.
Suspension culture
This employs the same basic methods as nursery culture. However, multi-level lantern nets suspended 5 m below water surface are frequently used in water depths from 10-15 m. Strings of pearl nets are preferred in deeper water because they are less prone to swinging in pendulum motion in heavy sea conditions, which can result in mortality among the contained scallops. Stock density is reduced as the scallops grow. One-year old scallops of 20-30 mm are stocked at 15-20 per pearl net and the number is further reduced to 5-7 per net one year later when the scallops are 50-70 mm shell height. Scallops of marketable size (100 mm) are available for harvest in year 2-3 (earlier in more favourable conditions of food supply and temperature). Scallops are often ear-hung in pairs from either horizontal lines in shallow water or vertical lines in deeper conditions when they are approaching 10 cm in size. In this method a hole is drilled in the ear of the shell and a loop of nylon thread is passed through the hole and attached to vertical or horizontal lines in shallow water lease areas.
Bottom culture
When seed quantity is surplus to suspension culture requirements, the excess from nursery culture at 20-30 mm shell height is sown on bottom lays in shallow water at 10-20/m2. However, bottom lays are usually sown with ~50 mm seed in March at densities of 5-6/m2. Bottom sown scallops take a year longer to reach market size than those grown in suspension.
Scallops are harvested at about 100 mm shell height after 2-3 years of culture. Harvesting from bottom culture is by SCUBA diver or by dredging. Harvesting from suspended culture uses craft of various types, often fitted with mechanical winches. The time of harvesting is sensitive to the presence of harmful paralytic shellfish toxins (PSP, DSP, etc.) in waters; this requires careful monitoring.
Scallops lack the ability to hold mantle cavity water and thus will rapidly desiccate and die when out of water. Care needs to be taken to avoid undue exposure to air and sun. Handling methods must therefore ensure that the scallops are removed from the growing units and transported to packing/processing plants swiftly. Processing other than washing and shucking is usually minimal. Whole scallops are transported chilled to local markets and shucked meats are frozen or canned.
Information on production costs is difficult to obtain, not only because the information is proprietary but also because of site specific factors, the diversity of methods used, and the widely varying levels of technology employed. The landed value of scallops is USD 6-7/kg in Japan (2004). There are no feed costs; this is a free resource throughout the culture cycle. Labour is a major recurring cost and scallop culture is very labour intensive. Culture is usually undertaken by Fisheries Cooperatives.
Seed supply
Scallops spawn in spring at 7-12 °C. The density of settlement depends on the concentration of larvae in the water column; this is closely monitored to predict both the timing and intensity of settlement. Spat collectors are suspended in the water column when monitoring programmes determine that >50 percent of larvae exceed 200 µm in length. Two types of spat collector are used:
- Plastic monofilament packed 'onion' bags attached 10 to a rope of 5 m length.
- Conical plates of perforated plastic that are strung together in batches, usually of 25, by rope to form a string of 2.5 m length, which is then covered by fine mesh.
Nursery
Spat are harvested from the collectors about 3 months after settlement when they measure about 10 mm shell height. They are then transferred to intermediate (nursery) culture in pearl nets; this occurs in the autumn. Care is taken to remove predators, which may have settled as larvae within the units, and also organisms that will compete for food and space (e.g. mussels). Each pearl net has a bottom surface area of 0.12 m² and can be stocked with up to 50-60 of 10 mm seed. Pearl nets are strung together to form vertical 'cage' units of variable length, depending on water depth. There may be from 5 to 30 nets per unit, and units are suspended from submerged longlines set 5-10 m below the water surface. After 10 weeks, the seed will have grown to 20-30 mm shell height and occupy about 60 percent of the volume of the net. A survival rate of 90 percent is common during this period. At this time, in October, density is reduced to 15-20 scallops per net. Intermediate culture then continues through winter until the following spring, by which time the scallops have grown to ~50 mm. They are then ready to be transferred to the grow-out stage.
Ongrowing techniques
Grow-out to market size is either by sowing year-old seed on bottom lays or in various forms of hanging culture. Scallop culture is mainly a co-operative activity in Asian countries and may be a part of a polyculture system.
Suspension culture
This employs the same basic methods as nursery culture. However, multi-level lantern nets suspended 5 m below water surface are frequently used in water depths from 10-15 m. Strings of pearl nets are preferred in deeper water because they are less prone to swinging in pendulum motion in heavy sea conditions, which can result in mortality among the contained scallops. Stock density is reduced as the scallops grow. One-year old scallops of 20-30 mm are stocked at 15-20 per pearl net and the number is further reduced to 5-7 per net one year later when the scallops are 50-70 mm shell height. Scallops of marketable size (100 mm) are available for harvest in year 2-3 (earlier in more favourable conditions of food supply and temperature). Scallops are often ear-hung in pairs from either horizontal lines in shallow water or vertical lines in deeper conditions when they are approaching 10 cm in size. In this method a hole is drilled in the ear of the shell and a loop of nylon thread is passed through the hole and attached to vertical or horizontal lines in shallow water lease areas.
Bottom culture
When seed quantity is surplus to suspension culture requirements, the excess from nursery culture at 20-30 mm shell height is sown on bottom lays in shallow water at 10-20/m2. However, bottom lays are usually sown with ~50 mm seed in March at densities of 5-6/m2. Bottom sown scallops take a year longer to reach market size than those grown in suspension.
Harvesting techniques
Scallops are harvested at about 100 mm shell height after 2-3 years of culture. Harvesting from bottom culture is by SCUBA diver or by dredging. Harvesting from suspended culture uses craft of various types, often fitted with mechanical winches. The time of harvesting is sensitive to the presence of harmful paralytic shellfish toxins (PSP, DSP, etc.) in waters; this requires careful monitoring.
Handling and processing
Scallops lack the ability to hold mantle cavity water and thus will rapidly desiccate and die when out of water. Care needs to be taken to avoid undue exposure to air and sun. Handling methods must therefore ensure that the scallops are removed from the growing units and transported to packing/processing plants swiftly. Processing other than washing and shucking is usually minimal. Whole scallops are transported chilled to local markets and shucked meats are frozen or canned.
Production costs
Information on production costs is difficult to obtain, not only because the information is proprietary but also because of site specific factors, the diversity of methods used, and the widely varying levels of technology employed. The landed value of scallops is USD 6-7/kg in Japan (2004). There are no feed costs; this is a free resource throughout the culture cycle. Labour is a major recurring cost and scallop culture is very labour intensive. Culture is usually undertaken by Fisheries Cooperatives.
Diseases and control measures
No specific diseases of the Yesso scallop are reported in the various databases such as AAPQIS (http://www.aapqis.org). Neither have any that have been implicated in unusual mortalities been reported in the literature. Like most bivalves, the shells are often bored into by the polychaetes, Polydora sp. and Dodecaceria concharum, the parasitic sponge, Cliona sp., and the Myxosporidian, Myxosporidia. The sporozoan parasite, Perkinsus sp. is endemic in most populations.
STATISTICS
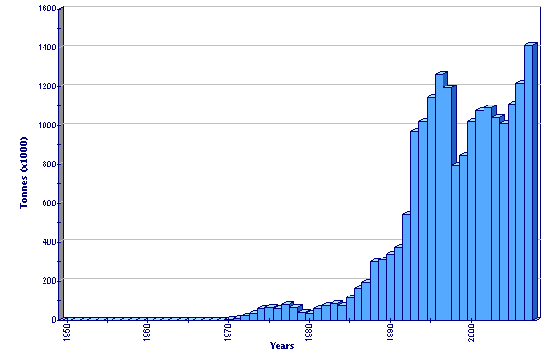
Production statistics
Global aquaculture production of Patinopecten yessoensis
(FAO Fishery statistics)
(FAO Fishery statistics)

Market and trade
Yesso scallop production is mainly absorbed by local markets in the countries in which it is cultured. The short shelf life of live scallops dictates that chilled live product is only available close to cultivation sites. Otherwise the market is for frozen meats. Quantities amounting to a few thousand tonnes are exported, mainly as frozen meats. The USA and France are the principal importers of such products.
STATUS AND TRENDS
Production in Japan showed a steady increase from 1970 until it reached 200 000 tonnes in 1992, a level has been exceeded thereafter with annual fluctuations. Peak production was in 2002 at almost 272 000 tonnes. Scope for future increases is limited by the availability of suitable lease areas and by concern for sustainability, where the carrying capacity of the areas utilized is an issue. Market saturation may also be a factor in the flattening trend of production in the past 10 years.
Chinese production showed dramatic increase from around 147 000 tonnes in 1990 to 916 000 tonnes in 1995 and to over 1 million tonne by 1997. Production from 1998-2003 has shown wide variability (from 629 000-960 000 tonnes), which may be related to the availability of seed.
Potential exists for development in the Republic of Korea and the Russian Federation.
Chinese production showed dramatic increase from around 147 000 tonnes in 1990 to 916 000 tonnes in 1995 and to over 1 million tonne by 1997. Production from 1998-2003 has shown wide variability (from 629 000-960 000 tonnes), which may be related to the availability of seed.
Potential exists for development in the Republic of Korea and the Russian Federation.
MAIN ISSUES
Availability of reliable seed supply in sufficient quantity from nature is always a concern when industry is dependent on this source. While hatchery culture can to some extent supplement seed supply from the wild, production on the required scale to sustain industry requirements from hatcheries is more technically challenging than it is with oysters or clams. Of similar concern is the potential for environmental imbalance, which already exists in some areas of major production. Semi-intensive methods of production occupy very large areas where suitable environmental conditions exist and compete for the available food supply with other filter feeding animals and also for oxygen. Scallop farming can remove excess nutrients from a watershed and thus help prevent the development of eutrophication. However, this can cause ecological imbalance in itself, as has been observed in areas such as Jioazhou and Sungo Bays in Northern China, where intensive mollusc culture has so depleted essential nutrient levels that primary production has been adversely affected.
Responsible aquaculture practices
A number of important issues have been identified above which are being addressed in the development of more responsible and sustainable practices in the production of this species. These are very much in line with the FAO Code of Conduct for Responsible Fisheries and include limiting lease areas within bays and estuaries to keep within the carrying capacity of the waters, together with other aspects of environmental and fish health awareness and mechanisms to minimize impacts.
REFERENCES
Bibliography
| Anonymous. 2001. Scallops. Korea-U.S. Arrangement for Scientific and Technical Cooperation in Integrated Coastal and Ocean Resources Management. |
| Bourne, N., Hodgson, C.A. & Whyte, J.N.C. 1989. A manual for scallop culture in British Columbia.Canadian Technical Report of Fisheries and Aquatic Sciences No. 1694. Fisheries and Oceans Canada, Pacific Biological Station, Nanaimo, BC., Canada. 215 pp. |
| FAO. 1995. Code of Conduct for Responsible Fisheries. FAO, Rome, Italy. 41 pp. |
| FAO. 1997. Aquaculture development. FAO Technical Guidelines for Responsible Fisheries No. 5. FAO, Rome, Italy. 40 pp. |
| Gardner Pinfold & IEC International. 2001. Economic potential of sea ranching and enhancement of selected shellfish species in Canada: (Section IV Scallop Culture in Japan). Office of the Commissioner for Aquaculture Development, Department of Fisheries and Oceans, Ottawa, Canada. 89 pp. |
| Kan-no, H. & Hayashi, T. 1971. The present status of shellfish culture in Japan. Proceedings of the First U.S.-Japan Meeting on Aquaculture at Tokyo, Japan, October 18-l9, 1971 . William N. Shaw, editor. Seattle WA: U.S. Dept. of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, 1974. NOAA technical report NMFS CIRC ; 388. |
| NACA/FAO. 2004. Emerging trends and experiences in Asia-Pacific Aquaculture 2003. Prepared by the Network of Aquaculture Centres in Asia-Pacific and Food and Agriculture Organization of the United Nations for the 15th NACA Governing Council Meeting, 21-25th April 2004, Colombo, Sri Lanka 146 pp. |