J.M. Kapetsky
Fisheries Department
FAO, Rome
Most experience in sampling fish populations with chemicals for resource assessment has been gained in permanent lakes or in small streams. Methodologies for sampling in floodplains, or in large river channels have only rarely been described, but are urgently needed because of the growing interest in the management of fisheries in such waters. The standing waters of floodplain/river systems are difficult to sample. They are frequently surrounded by dense floating and emergent vegetation, and the floating vegetation is often swept back and forth across the open-water areas by wind action, endangering stationary gears of all types set at the surface. Gillnets, cast nets, traps and longlines, which are the usual commercial gears, are not reliable for estimates of standing stocks because of their selectivity. Many subsistence fishermen use a variety of toxicants derived from local plants for harvesting such waters (see Stauch, 1966). Several workers (e.g., Loubens, 1969, or University of Michigan, 1971) have achieved some success in sampling isolated pools, relatively small lagoons, or coves. Reliable sampling in the open-waters of floodplain lakes, however, depends on the use of an efficient method of block netting. Such a method, using the earlier experience of University of Michigan (1971), was developed during an investigation of the fisheries of the Magdalena River floodplain (Colombia), and involved enclosing a known area and volume of the floodplain lakes and estimating the numbers and weight of fishes in the enclosed area using a toxicant in conjunction with multi-mesh gillnets.
Two types of habitats were recognized within floodplain lakes—bays and open-waters— in which it was hypothesized that fish populations would be quantitatively or qualitatively different. The experimental design, including the sampling frame, selection of lakes for population estimates, and selection of sampling sites within lakes in the two habitat types has been dealt with by Bazigos et al. (1975).
Areas of 2,500 m2 were selected as being the maximum which could be enclosed for population estimates in consideration of the cost of the toxicant and manpower constraints.
At each pre-determined sample site buoys were placed to delimit an area of 2,500 m2 with 50 m on each side. After placing the buoys and taking a number of depth measurements, the area to be enclosed was left undisturbed for 45 minutes to 1 hour, and meanwhile the toxicant was prepared for distribution at a location removed from the sample site. For 16 of the 18 estimates, 5 percent powdered rotenone was put into solution with lowest concentrations initially used in the enclosed area of 1.1 ppm. Concentrations were gradually increased to a maximum of about 4 ppm, as it became obvious that higher concentrations would be required to achieve complete kills. On two occasions Fintrol 15 was used at concentrations of 12.3 and 20.0 ppb.
The block net used to enclose the 2,500 m2 area consisted of two nets of different manufacture about which detailed characteristics are provided in Table 8.1. The two nets were sewn together to make a single net of 205 m × 4.5 m of 2.54 cm stretched mesh size.
The block net was carefully packed into a 3.5 m “Boston Whaler” - type boat powered by a 25 hp outboard motor, and the boat proceeded to the first buoy where its floatline was attached by a snap-on-clip. The boat carrying the net then proceeded as rapidly as possible in reverse toward the second buoy. Meanwhile, a second boat, identical to the first, approached the second buoy arriving there just before the net boat. An individual in the bow of the net boat passed the floatline of the net to another individual in the bow of the second boat, and the latter person attached the floatline of the net to the buoy by a snap-on clip on a short piece of line. By using this procedure at each corner of the square, the net boat was able to continue to lay the net without delay. At the fourth corner the approximately 5 m of net remaining was overlapped with the first 5m of the net to seal the area. This method of setting the block net could be accomplished in as little as 3 minutes, and usually in no more than 5 minutes.
Table 8.1 Characteristics of block nets and of multi-mesh gillnet fleets used to make standing stock estimates
| Characteristics | Block Net No.1 | Block Net No.2 |
|---|---|---|
| Length | 110 m | 95 m |
| Depth | 4.5 m | 4.5 m |
| Floats | Elongated cylinders 4×16 cm at 100 cm intervals | Flattened cylinders 10×8 cm at 50 cm intervals |
| Bottom line | Lead weights at 100 cm intervals | Intergal leadline |
| Stretch mesh | 2.54 cm | 2.54 cm |
| Material | Multi-filament nylon | Multi-filament nylon |
| Multi-mesh gillnet fleets (3) | ||
|---|---|---|
| Length hung | 50 m | |
| Pannel lengths | 8.3 m per pannel | |
| Stretched meshes | 2.54 to 15.24 cm in 2.54 cm increments | |
| Depth | 4 m | |
| Floatline | Intergal foam | |
| Bottom line | Intergal lead | |
| Net material | 2.54 to 12.70 cm meshes; #139 for 15.24 cm mesh | |
The method of setting the blocking net as described above was suspected to result in escapement from the area to be sampled due to fishes being frightened away from the sample area by the disturbance caused by the passage of the motorboat. A simple, inexpensive method was later developed to enclose the sample area within 30 to 50 seconds by releasing the blocking net from the surface simultaneously on all sides. For this method, the blocking net was set floating with the netting and bottomline of the net suspended from the floatline. After an hour or more had passed, the net was approached quietly by paddling and the sides lowered by quick-release ropes incorporated into the floatline. Unfortunately, insufficient population estimates were made thereafter to provide an objective evaluation of this method.
Immediately after the block net was in place, the rotenone was introduced by mixing it with the propwash of one of the 25 hp outboard motors. In the case of the Fintrol 15, the toxicant was introduced into the propwash directly through a hole punched in the container. Distribution of the toxicant required from 15 to 45 minutes depending on the quantity to be introduced.
Moribund fishes began to appear at the surface within about 15 minutes and were recovered by personnel in the two launches using hand nets. Moribund fishes were in evidence for up to 2 hours after introduction of the toxicant.
Immediately after moribund fishes had ceased to appear at the surface, three fleets of multi-mesh, mono-filament gillnets of 50 m × 4 m (Table 8.1) were set within the enclosed area. These nets were used to determine the effectiveness of the toxicant application and to recover any fishes which had survived the toxicant. The fleets were fished periodically and remained in the enclosed area for an average of 23 hours (Table 8.2).
| Habitat type | Estimate No. | Date | Mean depth of enclosed area (cm) | Total duration (hrs) | Duration of gillnetting (hrs) | Toxicant type* and Concentration |
|---|---|---|---|---|---|---|
| BAY | B1 | 11.6.75 | 112 | 25 | 22.5 | R 1.7 |
| B2 | 13.6.75 | 133 | 33 | 27 | R 1.7 | |
| B3 | 6.7.75 | 377 | 23.5 | 22.5 | R 2.3 | |
| B4 | 7.7.75 | 326 | 22.5 | 21.5 | R 2.5 | |
| B5 | 29.1.76 | 295 | 27.5 | 24.5 | R 4.6 | |
| B6 | 2.2.76 | 334 | 26 | 24.5 | F 20.0 | |
| B7 | 20.2.76 | 211 | 26.5 | 23 | R 4.1 | |
| B8 | 24.2.76 | 147 | 25.5 | 24 | R 4.2 | |
| OPEN-WATERS | 0–1 | 10.6.75 | 105 | 10 | 8.5 | R 1.1 |
| 0–2 | 15.6.75 | 156 | 25.5 | 20 | R 3.1 | |
| 0–3 | 13.8.75 | 377 | 24 | 23 | R 2.3 | |
| 0–4 | 15.8.75 | 355 | 22 | 21 | R 2.3 | |
| 0–5 | 21.8.75 | 225 | 24 | 22 | R 3.2 | |
| 0–6 | 22.8.75 | 239 | 20 | 18.5 | R 3.1 | |
| 0–7 | 27.1.76 | 317 | 25.5 | 24 | R 3.9 | |
| 0–8 | 31.1.76 | 364 | 27.5 | 25.5 | F 12.3 | |
| 0–9 | 18.2.76 | 212 | 21 | 19 | R 4.0 | |
| 0–10 | 22.2.76 | 206 | 27.5 | 25 | R 4.1 |
*R - Rotenone in parts per million; F - Fintrol in parts per billion
During the checks on the gillnets, and at other times, recoveries were made of fishes which had been killed by the toxicant, had sunk and partially decomposed, and had then resurfaced. Some of these fishes resurfaced as early as 4 hours after the application of the toxicant, and most fishes which were to appear had surfaced within about 15 hours.
The duration of the population estimates averaged 25 hours (Table 8.2) although one operation was terminated after 10 hours because few fishes were recovered with the toxicant and because no fishes were caught during 8 hours of gillnetting immediately following the toxicant application.
At the conclusion of each estimation the gillnet fleets were recovered and the block net repacked in the small boat. At this time fishes entangled in the meshes of the blocking net on the enclosed side were removed from the net and later counted and weighed.
The population estimates, from 9 floodplain lakes and made over a period of 9 months, varied from 21 to 232 kg/ha in the bay habitat, and in the open-water habitat from 0.2 to 251 kg/ha (Table 8.3). Although the range in estimates is very wide, much of the variation among estimates can be accounted for by seasonal changes in fish concentration related to water level changes and trophic movements.
| Habitat Type | Estimate Number | Stocking Stock kg ha-1 |
|---|---|---|
| BAYS | B-1 | 232 |
| B-2 | 73 | |
| B-3 | 24 | |
| B-4 | 21 | |
| B-5 | 83 | |
| B-6 | 26 | |
| B-7 | 86 | |
| B-8 | 156 | |
| OPEN-WATERS | 0–1 | 0.2 |
| 0–2 | 26.26 | |
| 0–3 | 2.5 | |
| 0–4 | 5 | |
| 0–5 | 28 | |
| 0–6 | 8 | |
| 0–7 | 13 | |
| 0–8 | 32 | |
| 0–9 | 167 | |
| 0–10 | 251 |
Two major problems associated with the methodology cause under-estimation of standing stocks of fishes. These are:
Movement of fishes away from the area to be enclosed while the net is being set due to the disturbance caused by the passage of the motorboat.
Incomplete recovery of fishes enclosed by the blocking net.
The first problem, I believe, became negligeable as a source of error in the standing stock estimates after the introduction of the quick-release method of enclosing the sample area. For evaluation of the second problem, the standing stock estimates were categorized according to the following criteria and tabulated (Table 8.4) :
| Habitat Type | Estimate Number | TOXICANT | GILLNETS | BLOCK NET | Total estimate kg ha-1 | |||
|---|---|---|---|---|---|---|---|---|
| kg ha-1 | % | kg ha-1 | % | kg ha-1 | % | |||
| BAY | B-1 | 167 | 72 | 5 | 2 | 60 | 26 | 232 |
| B-2 | 58 | 79 | 5 | 7 | 10 | 14 | 73 | |
| B-3 | 15 | 63 | 0 | 0 | 9 | 37 | 24 | |
| B-4 | 8 | 38 | 11 | 52 | 2 | 10 | 21 | |
| B-5 | 62 | 75 | 14 | 17 | 7 | 8 | 83 | |
| B-6 | 16 | 62 | 6 | 23 | 4 | 15 | 26 | |
| B-7 | 28 | 32 | 36 | 42 | 22 | 26 | 86 | |
| B-8 | 102 | 66 | 41 | 26 | 13 | 8 | 156 | |
| OPEN-WATERS | 0–1 | 0.2 | 100 | 0 | 0 | 0 | 0 | 0.2 |
| 0–2 | 21 | 81 | 1 | 4 | 4 | 15 | 26 | |
| 0–3 | 0.4 | 16 | 0.5 | 20 | 1.6 | 64 | 2.5 | |
| 0–4 | 0 | 0 | 3 | 60 | 2 | 40 | 5 | |
| 0–5 | 15 | 54 | 4 | 14 | 9 | 32 | 28 | |
| 0–6 | 2 | 25 | 2 | 25 | 4 | 50 | 8 | |
| 0–7 | 0 | 0 | 4 | 31 | 9 | 69 | 13 | |
| 0–8 | 2 | 6 | 26 | 81 | 4 | 13 | 32 | |
| 0–9 | 112 | 67 | 38 | 23 | 17 | 10 | 167 | |
| 0–10 | 105 | 42 | 82 | 33 | 64 | 25 | 251 | |
Fishes recovered due to the toxicant — this was the weight of moribund fish collected at the surface in addition to the weight of fish recovered thereafter which had partially decomposed and resurfaced.
Fishes caught in the gillnets — the weight of the survivors of the toxicant treatment which were caught in the gillnets; other survivors may not have been caught.
Fishes entangled in the blocking net — these fishes could have become entangled at any time after the blocking net was set; however, it is assumed here that they represent survivors of the toxicant application.
Consideration of the results of the categorization in the above manner in Table 8.4 indicates that a complete kill was never attained, with one possible exception (0–1). This situation, at least initially, was due to the relatively low concentrations of toxicant employed; however, non-homogeneous distribution of the toxicant in the enclosed area, and loss of the toxicant from the sample area due to currents were undoubtedly other factors which affected the efficiency of the toxicant.
A summary of the overall results presented in Table 8.4 takes the following form:
| Habitat | Rotenone | Gillnets | Block nets |
|---|---|---|---|
| Open-waters | 48% | 30% | 22% |
| Bays | 65% | 17% | 18% |
This summary indicates that the toxicant was much more efficient in bays than it was in open-waters. From the standpoint of the methodology employed, bays and open-waters differ in that the bays are usually more sheltered from winds, and consequently, wind-generated currents are less likely to occur in them. Thus, the toxicant concentration was maintained effectively higher in bays than in open-waters because the toxicant was dispersed less rapidly from the enclosed area.
In deeper waters the method of distribution of the toxicant, by outboard propwash, was probably not effective. This problem was overcome by the University of Michigan (1971) by using a gasoline-powered pump with a venturi device for distribution of the toxicant.
Again, considering Table 8.4 and the summary table above, it is evident that the standing stock estimates, especially in the open-water areas, depended to a large extent on the efficiency of the gillnets to capture the survivors of the toxicant treatment. Therefore, the standing stock was under-estimated by the extent to which the toxicant survivors were able to avoid capture by the gillnets. In hindsight, the approximate efficiency of the gillnets in capturing the survivors of the toxicant treatment could have been estimated over a number of trials to provide a correction factor for the population estimations. A second toxicant treatment could have been made at a high concentration after the gillnets had been recovered from the sample area. The efficiency of the gillnets could then have been approximated by the weight of the catch of the gillnets in relation to the weight of fishes which had escaped capture by the gillnets recovered from the second toxicant application.
Incomplete recovery of fishes which are killed by the toxicant which then die and sink
to the bottom can be a source of under-estimation in standing stock estimates if the time
accorded to each operation is not sufficient for fishes to partially decompose and resurface.
In the Magdalena situation, with relatively high prevailing water temperatures on the bottom
(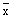 = 29.8°C; range 26.8° to 31.2°C), this was probably not a serious problem. In a study
conducted both in the laboratory and in the field, Parker (1970) showed that at water
temperatures of 27°C from 0.5 to 2 days were required for all dead fishes to resurface.
In situations were waters are clear, the weight or number of unrecovered fishes on the
bottom can be estimated by a diver making transects along the bottom; however, in the
floodplain lakes of the Magdalena system the waters were too turbid for this method of
estimation.
= 29.8°C; range 26.8° to 31.2°C), this was probably not a serious problem. In a study
conducted both in the laboratory and in the field, Parker (1970) showed that at water
temperatures of 27°C from 0.5 to 2 days were required for all dead fishes to resurface.
In situations were waters are clear, the weight or number of unrecovered fishes on the
bottom can be estimated by a diver making transects along the bottom; however, in the
floodplain lakes of the Magdalena system the waters were too turbid for this method of
estimation.
In conclusion, the methodology described above undoubtedly resulted in under-estimation of standing stocks of fishes in the floodplain lakes sampled, more so in open-waters than in bays. More accurate results could have been attained by increasing toxicant concentrations; however, financial constraints and the necessity of avoiding the killing of large numbers of fishes outside the enclosed areas because of adverse public relations effects from fishermen precluded this solution.
Anderson, R.S., 1970 Effects of rotenone on zooplankton communities and a study of their recovery patterns in two mountain lakes in Alberta. J.Fish.Res.Board Can., 27 (8):1335-56
Antonioni, M.E. and P.C. Baumann, 1975 Antimycin as a management and sampling tool. EIFAC Tech.Pap., (23) Suppl.1, Vol. 1:266-86
Bazigos, G.P., J.M. Kapetsky and J. Granados, 1975 Integrated sampling designs for the complex inland fishery of the Magdalena River Basin, Colombia. FAO Working Paper. Rome, FAO, FI:DP/COL/72/552/4:58 p.
Balon, E.K. and A. Coche, 1974 Lake Kariba: a man-made tropical ecosystem in central Africa. Monogr.Biol., 24:767 p.
Boccardy, J.A. and E.L. Cooper, 1963 The use of rotenone and electrofishing in surveying small streams. Trans.Am.Fish.Soc., 92(3):307-10
Bonetto, A.A., C. Pignalberi and E. Cordiviola, 1965 Contribucion al Conocimiento de la populaciona de peces de las lagunas islenas en el Paraná medio. Ann.Congr.Latinoam. Zool., 2:131-44
Carlander, K.D. and W.M. Lewis, 1948 Some precautions in estimating fish populations. Prog. Fish-Cult., 10:135-7
Davies, W.D. et al., 1975 Validity of rotenone sampling in reservoirs. EIFAC Tech.Pap., (23), Suppl.1, vol.1:236-48
Engstrom-Heg, R. 1974 Use of powdered activated carbon to eliminate rotenone toxicity in streams. N.Y. Fish Game J., 21(2) :153-62
Hall, G.E., 1975 Sampling reservoir fish populations with rotenone. EIFAC Tech.Pap., (23), Suppl.1, vol. 1:249-59
Hayne, D.W. et al., 1968 An evaluation of cove sampling of fish populations in Douglas Reservoir, Tennessee. In Reservoir Fisheries Resources Symposium, presented by Reservoir Committee, Southern Division, American Fisheries Society, Athens, Ga., April 5–7 1967. Athens, Ga., University of Georgia, pp. 244-97
Hocutt, C.A. et al., 1973 Rotenone methods in a large river system. Arch.Hydrobiol., 72(2) : 245-52
HolĎik, J., 1972 Abundance, ichthyomass and production of fish populations in three types of water-bodies in Czechoslovakia (man-made lakes, trout lake, arm of the Danube River). In Productivity problems of freshwaters. Proceedings of the IBP/Unesco Symposium on Productivity Problems of Fresh Waters, held in Kazimierz Dolny, Poland, 6 May 1970, edited by Z. Kayak & A. Hillbricht-llkowska. Warszaw, Polish Scientific Publishers, pp. 843-55
Jensen, K.W., 1975 Population estimates of perce (Perca fluviatilisL.) by marking-recapture and by rotenone poisoning. FAO Fisheries EIFAC Tech.Pap., (23) Suppl.1, Vol.2: 600-10
Lennon, R.E. et al., 1970 Reclamation of ponds, lakes and streams with fish toxicants. A review. FAO Fish.Tech.Pap., (100):99 p.
Little, J.D., 1966 Reclamation of Pine Creek, Tennessee. Proc.South.Assoc.Game Fish.Comm., 19: 302-15
Loubens, G., 1969 Etude de certains peuplements ichthyologiques par des pêches au poison (1 re note). Cah.ORSTOM(Hydrobiol.), 3(2):45–73
Loubens, 1970 Etude de certains peuplements ichthyologiques par des pêches au poison (2e note). Cah.ORSTOM(Hydrobiol.), 2(1):45–61
Marking, L.L., 1975 Effects of pH a toxicity of Antimycin to fish. J.Fish.Res.Board Can., 32 (6) :769-73
Meadows, B.S., 1973 Toxicity of rotenone to some species of coarse fish and invertebrates. J. Fish Biol., 5:155-63
Meyer, F.P. et al., 1976 Registration status of fishery chemicals, February 1976. Prog. Fish-Cult., 38(1):3–7
Morrison, B.R.S. and G. Struthers, 1975 The effects of rotenone on the invertebrate fauna of three Scottish freshwater lochs. J.Inst.Fish. Manage., 6:81–91
Olmsted, L.L. and D.G. Cloutman, 1974 Repopulation after a fish kill in Mud Creek, Washington County, Arkansas, following pesticide pollution. Trans.Am.Fish Soc., 103:79–87
Parker, R. Jr., 1970 Surfacing of dead fish following application of rotenone. Trans.Am.Fish. Soc., 99(4):805-7
Rupp, R.S. and S.E. De Roche, 1965 Standing crops of fishes in three small lakes compared with C14 estimates of net primary productivity. Trans.Am. Fish.Soc., 94:9–25
Stauch, A., 1966 Le bassin Camerounais de la Bénoué et sa pêche. Mém.ORSTROM, 15:152 p.
Sumari, O., 1975 Estimation of the standing crop of fish in small lakes by rotenone treatment. EIFAC Tech.Pap., (23)Suppl.1, vol.1:260-5
University of Michigan, 1971 The fisheries of the Kafue River Flats, Zambia, in relation to the Kafue Gorge Dam. Report prepared for FAO/UN acting as executing agency for UNDP. Ann Arbor, Michigan, University of Michigan, FI:SF/ZAM 11:161 p. Technical Report 1