by
H. J. Schafer
School of Food Technology and Marine Sciences
Technological Institute of Monterrey
Monterrey, Mexico
Abstract
Microscopic examination of the setae of the endopodites of the pleopods gives a clear indication of the molting stages of P. duorarum up to the intermolt stage. On the basis of the observed molts, the duration of postmolt stages A to C represents 19 percent of the total cycle, which lasted for 16 days in animals with an average carapace length of 25.4 mm.
DETERMINATION DE CERTAINS STADES DU CYCLE DE LA MUE DE Penaeus duorarum, PAR L'EXAMEN AU MICROSCOPE DES BROSSES DES ENDOPODITES DES PLEOPODS
Résumé
L'examen au microscope des brosses des endopodites des pléopodes révèle nettement à quel stade du cycle de la mue se trouve la crevette P. duorarum, jusqu'au stade d'intermue. Sur la base des mues observées, la durée des postmues A a C represénte 19 pour cent du cycle total, qui était en l'occurrence de 16 jours pour des animaux mesurant en moyenne 25,4 mm de longuer de carapace.
DETERMINACION DE ALGUNOS ESTADIOS DEL CICLO DE MUDA DEL CAMARON ROSADO, Penaeus duorarum, PRO EXAMEN MICROSCOPICO DE LAS SEDAS DE LOS ENDOPODITOS DE LOS PLEOPODS
Extracto
El examen microscópico de las sedas de los endopoditos de los pleópodos proporciona una clara indicación de los estadios de muda de P. duorarum, hasta la etapa de intermuda. Tomando como base las mudas observadas, la duración de los estadios de postmuda A a C representa el 19 por ciento del ciclo total, el cual duró 16 días en animales con una longitud de carapacho de 25,4 mm.
The molting cycle of Penaeus duorarum Burkenroad has not been described. In a recent review, Passano (1960) states that few aspects of crustacean physiology are as important as molting.
Growth of Crustacea is directly related to the molt cycle, since size increases cannot occur while the animal is encased in its shell.
Important changes in food reserves and metabolism accompany the different stages of the molt cycle. The outgrowth of this study was to be a determination of storage materials utilized by starved shrimp, and it was anticipated that changes in the relative amounts of these products might be obscured by normal variation during the molting cycle. It therefore became obvious that it was essential first to describe the molt cycle of P. duorarum in sufficient detail to identify the specific stage of the cycle in individual animals.
In 1939, Drach devised a scheme to describe the molting cycle of decapod crustacea. This classification has superseded all others. In natantians the cycle is described as follows:
TABLE I
Natantian intermolt stages
(from Passano, 1960, after Drach, 1939)
| Stage | Duration (%) | Characteristics |
| A | 2.5 | |
| A1 | Very soft exoskeleton; matrix fills new spines | |
| A2 | Branchiostegites flexible, bend under slight pressure; spine matrix retracts towards middle of spine | |
| B | 16.5 | |
| B1 | Branchiostegites semi-rigid; exoskeleton of a parchment-like consistency; conical base of spines absent | |
| B2 | Conical base of spines partially formed | |
| C | 21.0 | Exoskeleton fully formed; conical base of spines complete. (The intermolt period) |
| D | 60.0 | |
| Do | Hormonal activation; the epithelium then separates from the old cuticle | |
| D1 | 21.0 | New spine formation begins; initial retraction of nerve fiber from the cavity of the old spine |
| D1" | 14.0 | Secretion of new spines begins |
| D1" | 6.5 | Morphological details of new spines become visible |
| D2 | Formation of pre-exuvial layers of new skeleton | |
| D3 | 17.0 | Resorption of old exoskeleton |
| D4 | 1.0 | Old exoskeleton splits in preparation for ecdysis |
| E | 0.5 | Ecydsis |
Scheer (1960) studied several aspects of the intermolt cycle in natantians using three caridean shrimp, Palaemon xiphias (as Leander), Processa acutirostris, and P. edulis. He described four stages in the intermolt cycle, A, B, C, and D, these stages being further subdivided (Table II).
TABLE II
Characteristics of intermolt cycle stages in natantians (from Scheer, 1960)
| Stage | Characteristics |
| A (early postmolt) | Integument soft, parchment-like, internal cones of setae of uropods absent |
| B (late postmolt) | Integument firm but yielding to pressure, internal cones of setae incomplete |
| Ca (early intermolt) | Integument firm to hard, internal cones of setae nearing completion |
| Cb (late intermolt) | Integument hard, internal cones of setae complete, slight separation of epidermis from cuticle between setae |
| Do (beginning premolt) | New setae present at base of old, epidermis clearly separated from cuticle, bases of new setae at the outer surface of the epidermis |
| D1 (early premolt) | New setae in course of development |
| D1 | New setae extending partly into old, their bases beginning to invaginate below the surface of the epidermis |
| D1" | New setae extending well into old, the bases invaginated into the epidermis for approximately ¼ the length of the setae |
| D1" | Bases of new setae invaginated for approximately ½ the length of the setae |
| D2 (middle premolt) | Epidermis with a clearly developed cuticular layer |
| D3 (late premolt) | Epidermis with a chitinous pigmented cuticular layer |
| D4 (final premolt) | The old carapace separates readily from the new cuticle on slight pressure |
Scheer's work is essentially a modification of Drach's criteria, since the changes introduced were suggested by Drach personally (Scheer, 1960). This work is used as the foundation to determine the stages of the molting cycle in P. duorarum.
Live shrimp were obtained from a local bait fisherman. The animals were captured in the southern part of Biscayne Bay. A preliminary study conducted by Costello (1963), has shown that the shrimp catches in this area comprise two species of penaeid shrimp, P. duorarum and P. brasiliensis Latreille. The relative numbers of these species change during the seasons of the year.
Since a small percentage of the sample could be expected to be represented by P. brasiliensis, it was important to determine the species of each shrimp in the sample. The criteria described by Voss (1955) were used. Individuals were also compared with unpublished drawings by Boschi. Species identification was uncertain in the smaller males, therefore only the females in the samples were used.
At the beginning of the experiments the shrimp were transferred to 10 gallon (45 l) aquaria. Each aquarium was equipped with a sub-sand filter and was subdivided by six fiberglass-plastic partitions. Each compartment contained one shrimp. The shrimp were examined daily to identify those which had molted overnight. Individual records were kept of the molts.
The endopodites of pleopods were removed, mounted in sea water on microscope slides and the setae and spines were photographed under the low power (100X) of a compound microscope. The photographs were later used to determine the stage of the molting cycle, according to the criteria employed by Scheer (1960) and observations of molts in the aquaria.
A comparison of the molting histories of individuals and the microscopic observation of their appendages revealed that changes in the molting stage could be detected by observing the internal structure of the setae on the pleopods (Figs. 1, 2, 3 and 4). During the early part of what is here called the postmolt period, each seta is filled with a translucent cellular matrix. Near the end of the postmolt stage, this material remains only at the basal end of the seta, close to a cone-shaped structure that begins to form at this time. The intermolt period begins when the translucent material has disappeared from the seta and the cone has become complete. The setae of pleopods removed from animals the day before the night of the molt showed no structural evidence of premolt changes.
The observations on the molts of the experimental animals are summarized in Table III. Forty four percent of the animals molted during the first seven days of captivity, and this, coupled with observations by Ewald (personal communication) on P. duorarum and Zein-Eldin (1961) on P. aztecus, suggests that the molt cycle might have a duration of 16 days. The postmolt period was about three days, or 19 percent of the suggested 16-day cycle.
TABLE III
Percentage of total population molting
| Number of Days | Number of Animals | Number Molted | Percent Molted |
| 0 | 30 | 0 | 0 |
| 1 | 30 | 4 | 13 |
| 2 | 23 | 2 | 9 |
| 3 | 17 | 1 | 6 |
| 4 | 17 | 1 | 6 |
| 5 | 10 | 0 | 0 |
| 6 | 19 | 0 | 0 |
| 7 | 9 | 1 | 11 |
4.1 Molting cycle
In his recent review, Passano (1960) states, “Few aspects of crustacean physiology are as important as molting”. In natantians this process is regulated by the central nervous system through specialized neurosecretory hormones. Welsh (1961) points out that the action of neurons cannot be explained solely in electrical terms, but that their most characteristic feature is the ability to synthetize and release chemical agents which act in a hormone-like manner. Some of these agents, such as acetylcholine, act at close range at synapses and neuromuscular junctions; others, such as the molting hormones of Crustacea, enter the blood and produce general systemic effects.
The sinus gland system in crustaceans consists of neurosecretory cells in various ganglia which send their axons to a common release center in each eyestalk, called the sinus gland. The nerve endings are arranged in such a way that released materials traverse a thin membrane before they contact the haemolymph. Of particular interest here is the group of secretory nerve-cell bodies that comprise the papilla X-organ in natantians. The neurosecretory molt-inhibiting hormone, is synthesized in the cell body and migrates via the axoplasm to the axon terminals where it is stored in the form of discrete granules. The hormone is then released through the sinus gland into the blood stream. Welsh favors the theory of cycles of production and nonproduction of the molt-inhibiting hormone. The nonproduction cycle is triggered by external and internal factors. The external factors are light, temperature, and seclusion. Among the internal factors regulating the release of molting hormones are the accumulation of organic reserves and other demands on nutrients. When the X-organ hormone concentration in the blood is reduced, the Y-organ is activated. The Y-organ, a neurohemal organ, liberates a molting hormone, ecdysone.
With the release of the molting hormone, proecdysis is initiated. This initial stage is denominated Do. It is not characterized by morphological changes. When stage D1 is reached, the formation of the new exoskeleton begins. This process is evidenced by the formation of new spines within the old ones. As proecdysis continues, resorption of organic and inorganic materials increases, and exoskeleton formation continues. The skeletal alterations coincide with changes in behavior of the animal which soon ceases to feed; this is stage D2. The tissues suffering major changes during this period of starvation are the integument, the hepatopancreas, and the blood.
Ecdysis, or stage E, is divided into a passive and an active phase. Natantians exuviate late at night. At the beginning of this stage, the blood osmotic pressure rises. When the old gastric lining breaks down, water is swallowed, increasing the hydrostatic pressure in the digestive tract. These two factors combined accelerate the movement of water to the haemolymph, increasing its volume. The animal swells and the carapace ruptures. The active phase begins after the carapace has split, and the animal begins to withdraw from the exuvium. Once the animal has moved out of the old shell, the appendages become turgid.
Water uptake continues during the early part of the postecdysal stages (A1). Calcification of the exocuticle begins and the new endocuticle begins to form. Tissue growth, measured as increase in dry weight, begins during stage B2 or C1, continuing during C2 and C3. Protein and DNA show the highest turnover rates during this time (Passano 1960).
4.2 Changes in the integument
At the stage C4 (end of the intermolt period), the integument consists of five layers (Fig. 5). The epidermis (A), consists of an epithelium composed of closely packed simple subcuboidal cells and associated gland cells, connective tissue, and sensory nerve endings. This layer is the only living part of the integument and secretes the other layer. There is a thin, membranous layer (B), proteinaceous and noncalcified, overlying the epidermis. During proecdysis the membranous layer splits, giving rise to the mucilaginous layer which helps the animal during exuviation. The main portion of the exoskeleton is composed of the endocuticle (C). This layer accounts for 80% of the integument thickness, and is secreted and mineralized after ecdysis during stages A2 through C3. The layer is rich in chitin, which comprises 70% of the organic matter, and is low in protein. It is mineralized with calcium salts. The pigmented layer (D), is composed of 40–45% chitin, the rest being protein. This layer is secreted during proecdysal stages D2 and D3. The layer is mineralized all at once by salts, shortly after ecdysis. The layer is often strongly pigmented, and together with the chromatophores gives the crustacean its coloration. The very thin epicuticle (E) consists of lipid-impregnated protein and contains no chitin. It is secreted by the epidermis during stage D1 and may be for the protection of the new exoskeleton from the molting fluid during exuviation.
No marked histological changes are evident during Do, but during D1 the epithelium separates from the membranous layer and chitin formation begins. At D2, full secretory activity is reached. The muscle attachment to the old cuticle breaks down and the muscles become attached to the new skeleton. The pigmented layer is secreted and resorption of mineral and organic matter from the old skeleton begins. Resorption of the organic matter is 23% in Panulirus, 79% in Carcinus (Passano, 1960), and 70% in P. duorarum (Schafer, 1967).
In P. duorarum, no structural changes in the setae of the pleopods could be detected up to the afternoon prior to the night when they molted. This may indicate that the proecdysis portion of the molting cycle is very short in this form. Studies on other natantians, however, suggest that this part of the cycle comprises 59% (D1 to D4) (Passano, 1960) to 53% (Do to D4) (Scheer, 1960) of the total cycle. The calculated total for the cycle is 16 days. This agrees well with the length of the cycle observed by Ewald (personal communication) in P. duorarum; he reports 12 to 20 days for animals with carapace length of 18 to 22 mm, 14 to 30 days for animals with carapace length of 23 to 27 mm, and 27 to 38 days for animals with carapace length of 28 to 32 mm. Zein-Eldin (1961) observed a molting cycle length of 16 and 15 days for male P. aztecus with initial carapace length of 21.4 and 22.5 mm respectively.
Observations in this study, of the structural changes in the setae of the pleopods, indicate that the postmolt period, from A1 to the onset of C1 has a duration of 3 days, or 19% of the total cycle. These observations agree well with the 19% given by Passano (1960) and the 15 to 17% given by Scheer (1960). The early part of this stage is characterized by the presence of a translucent matrix filling the setae (Fig. 1). As the stage advances, the matrix begins to disappear at the distal end and continues to do so until it is evident only at the basal end (Fig. 2). A cone-shaped structure begins to form during late postmolt, stage B2. By the end of this period, the cone-shape is almost complete and the matrix almost disappeared (Fig. 3). The beginning of the intermolt period C1 is marked by the completion of the cones and the total absence of the matrix from the setae (Fig. 4).
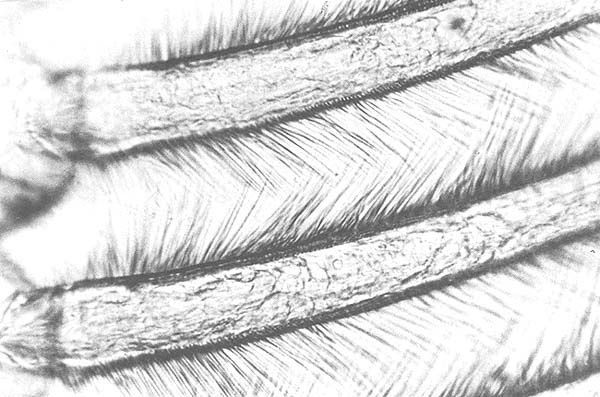
Fig. 1. Setae of endopodite of pleopod, a few hours after molting.
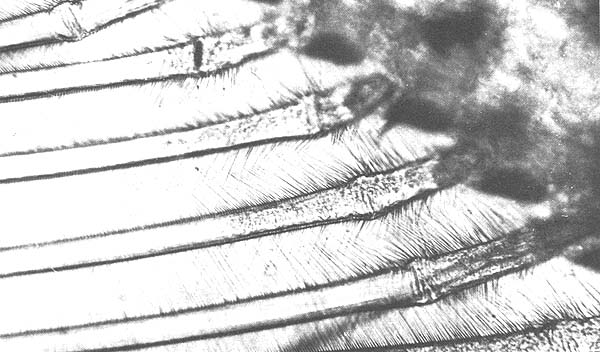
Fig. 2. Setae of endopodite of pleopod, 2 days after molting.

Fig. 3. Setae of endopodite of pleopod, 3 days after molting.

Fig. 4. Setae of endopodite of pleopod, intermolt period.
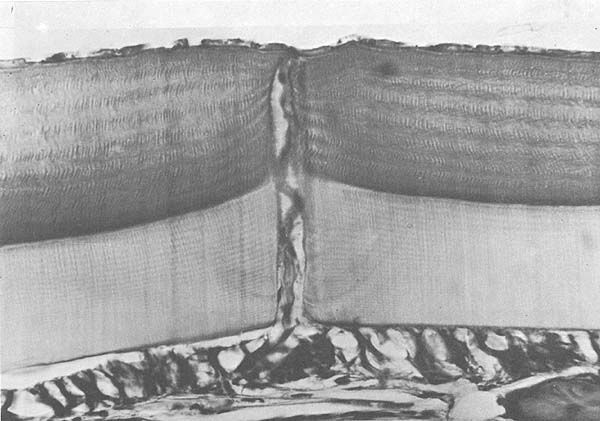
Fig. 5. Integument of P. duorarum. A, epidermis; B, membranous layer; C, endocuticle; D, pigmented layer; E, epicuticle.
The molting cycle of P. duorarum under the condition encountered appears to last about 16 days. The structural changes within the setae of the endopodites of the pleopods are easily discernible by microscopic examination under 100X magnification during the postmolt period. This stage of the cycle apparently lasts 3 days, or 19 percent of the total cycle.
Costello, T. J., 1963 Pink shrimp life history. Annual Report, Fishery Research Galveston Biological Laboratory. Circ. Fish.Wildl.Serv.,Wash., (161):35–7
Drach, P., 1939 Mue et cycle d'intermue chez les crustacés décapodes. Annls Inst. oceanogr., Manaco, 19:106–377
Passano, L. M., 1960 Molting and its control, In The physiology of Crustacea, edited by T. H. Waterman, New York, Academic Press, vol. 1, pp. 473–536
Schafer, H. J., 1967 Chitin resorption by Penaeus duorarum. Contr. Fdn mar. Sci., Mexico, 1:1–8
Scheer, B.T., 1960 Aspects of intermoult cycle in natantians. Comp. Biochem. Physiol., 1:3–18
Voss, G. L., 1955 A key to the commercial and potentially commercial shrimp of the family Penaeidae of the western North Atlantic and the Gulf of Mexico. Tech. Ser. Fla St. Bd Conserv., (14) : 1–23
Welsh, J. H., 1961 Neurohumors and neurosecretion. In The physiology of Crustacea, edited by T. H. Waterman, New York, Academic Press, vol. 2, pp. 281–312
Zein-Eldin, Z. P., 1961 Shrimp physiology. Annual Report, Fishery Research Galveston Biological Laboratory. Circ. Fish. Wildl. Serv., Wash., (129):44–8