| FAO Fisheries Synopsis No. 98 | FRm/S98 AST - Prawn |
prepared by
M.J. GEORGE
Central Marine Fisheries Research Institute 1
Mandapam Camp, India
Phylum Arthropoda
Class Crustacea
Subclass Malacostraca
Series Eumalacostraca
Superorder Eucarida
Order Decapoda
Suborder Natantia
Section Penaeidea
Family Penaeidae
Subfamily Penaeinae
Genus Metapenaeus Wood-Mason,
1891
Species Metapenaeus affinis
(H. Milne Edwards, 1837)
Genus Metapenaeus Wood-Mason, in Wood-Mason and Alcock, 1891, Ann.Mag.nat.Hist., (6)8:271. Type species by original designation: Penaeus affinis H. Milne Edwards, 1837. Gender: masculine.
Rostrum dorsally toothed only; carapace without sutures; orbital angle usually sharp; postocular sulcus present; cervical sulcus well defined; hepatic sulcus not well defined behind level of hepatic spine, but pronounced in front with well defined postero-inferior border, usually descending vertically from hepatic spine, then turning towards the pterygostomial angle; antennal and hepatic spines pronounced; pterygostomial angle blunt. Telson with deep dorsomedian sulcus, without fixed subapical spines, and with movable dorsolateral spines which may be microscopic and very numerous. First antennular segment without spine on ventral distomedian border. Antennular flagella usually shorter than carapace. Maxillulary palp with 2 segments, distal small, basal with convex, foliaceous projections on inner and outer edges, and long spine on inner edge. First to 3rd pereiopods with basial spines; 5th pereiopod without exopod; ischium and merus often modified in adult male. Petasma tubular with thickened median lobes; lateral lobes thicker than median, forming distolateral spout-like projections, each with dorsal lobule produced posteriorly into expanded, plate-like projection; median lobes with dorsal lobule produced into thin recurved plate-like or hoodlike structure. Appendix masculina with knoblike distal piece bearing deep posterodistal depression or is sculptured in some way. Thelycum composed of anterior median plate, 2 posterior lateral plates more or less enclosing posterior end of median plate; posterior plates often continuous across sternite. Zygocardiac ossicle with 2 rows of teeth which get progressively smaller. Pleurobranchs on 3rd to 7th thoracic somites, rudimentary arthrobranch on 1st somite, anterior and posterior arthrobranchs on 2nd to 6th, vestigial anterior and fully developed posterior arthrobranchs on 7th somite; mastigobranchs on 1st, 2nd, 4th to 6th somites. Body usually with some dorsal setose depressed areas, remainder of body surface varying from completely glabrous to covered with close irregular setose depressed areas. (Dall, 1957).
Metapenaeus affinis (H. Milne Edwards, 1837)
The type specimens of the species are in the Paris Museum and were examined there in 1938 by Burkenroad (see Burkenroad, 1963), who found them to be identical with the present species. Hall (1958) mistakenly took a specimen of Parapenaeopsis sculptilis in the Paris collection to be the type of Penaeus affinis and concluded that the name affinis could not be used for the present form. This question was put straight by Burkenroad (1963) and the name affinis can be continued to be used in the sense it had always been considered.
Type locality. “Côte de Malabar” (=S.W. coast of Indian peninsula, Kerala, India).
The species is illustrated in Fig. 1.
Body tomentose; rostrum more curved, less uptilted, slightly larger than that of M. monoceros; not less than 9 dorsal teeth; postrostral crest does not extend to posterior part of carapace; anterolateral angles of carapace rounded; antennal spine strong, produced as salient ridge to small hepatic spine; ridge bounding well marked postantennular groove which meets cervical groove. Anterior abdominal terga indistinctly carinated; 5th abdominal somite 2/3 length of 6th; 6th somite little shorter than telson. Telson without marginal spines shorter than endopod of uropod.
Eyes very large, slightly surpassed by antennular scale. Upper antennular flagellum at least 3/4 length of peduncle. 3rd maxilliped barely reaches middle of antennal scale, dactylus of male not modified. Strong spines present on bases of all 3 pairs of chelipeds. Last pair of thoracic legs usually surpassing tip of antennal scale, sometimes by whole length of dactylus; no lobule on posterior edge of ischium in male, notch in the merus bounded by twisted tooth, edge of merus entire beyond tooth.
Petasma (Fig. 2) symmetrical. In adult, 2 halves form compressed tube ending in pair of 2-lipped spouts like short horns.
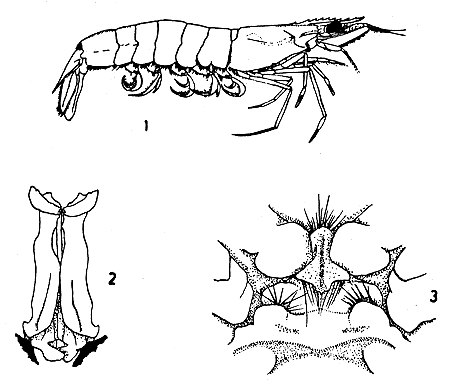
Fig. 1 Metapenaeus affinis.
Fig. 2 Petasma.
Fig. 3 Thelycum.
(from Hall, 1962).
Thelycum (Fig. 3) concave, setose. Lateral lobes fairly flat, transversely out into unequal segments, median plate projects between 2 lobes of sternum between 4th pair of legs.
Body translucent green, uropods tipped with conspicuous green. Maximum length about 180 mm.
For artificial key to the species of Metapenaeus see section 1.1.2 of the synopsis on Metapenaeus monoceros by George (1970), or Racek and Dall (1965).
Metapenaeus affinis (H. Milne Edwards, 1837).
Objective synonymy
Penaeus affinis H. Milne Edwards, 1837, Hist.nat.Crust., 2: 416. (original combination).
Parapenaeus affinis (H. Milne Edwards) Rathbun, 1902, Proc.U.S.Nat.Mus., 26:23–55.
Metapenaeus affinis (H. Milne Edwards) Nobili, 1903, Boll.Mus.Zool.Anat.comp.Torino, 18(447).
Penaeopsis affinis (H. Milne Edwards) De Man, 1911, Siboga Exped., 39a:57.
Subjective synonymy
Penaeus mutatus Lanchester, 1901, Proc. zool.Soc. London, 1901:572.
Metapenaeus necopinans Hall, 1956, Bull. Raffles Mus., 27:82.
Metapenaeus mutatus (Lanchester) Hall, 1962, Fish.Publ., London, 17:76.
In Kerala, on the southwest coast of India, the species is locally known as “Kazhanthan chemeen”. In Bombay, on the northwest coast, it is locally called “Jinga”, and in Bengal, on the east coast, the name “Chingri” is applied to this and related species.
No subspecies or varieties are known for the species.
General distribution of the species is Indian seas through Malaysia and part of Indonesia to Hong Kong and Japan. Under the FAO distribution code (see Holthuis and Rosa, 1965) it occurs in land areas 421, 423, 433, 434 and 437, and water areas ISW and ISEW, in marine and brackish waters.
In Indian waters, the juveniles of the species are found in very small numbers in the backwaters and estuaries, and adults occur in the inshore waters to a depth of about 25 fm (45 m). In Ceylon, it is found on mud banks in the sea at a depth of 8 to 12 fm (14 to 22 m) (De Bruin, 1965). In Malaysian waters the juveniles are found in the Singapore pond fishery in small numbers and the adults in larger numbers in the seas around Malaysia (Hall, 1962).
So far no work has been reported on the eggs and larvae of the species. According to Subrahmanyam (1967) the species may breed in inshore waters of the southwest coast of India.
There does not seem to be such large-scale migration of the postlarval stages into nearby estuaries as in M. dobsoni (Miers) and the species never accounts for more than 20 percent of the catch in the fishery for juvenile prawns in the backwaters of Cochin. In the Singapore pond fishery, according to Hall (1962), juveniles of M. affinis occur sporadically from February to September but never exceed 6.5 percent of the prawn population. The species is present in small numbers in estuaries almost throughout the year and is most common from January to June. In the Chilka Lake, on the east coast of India, Kemp (1915) recorded the species in February and March and concluded that it may be present there at all seasons.
On the inshore fishery grounds of the west coast of India, adults of the species are most common in November and December (George, 1961), and on the offshore grounds they are most abundant from November to January (George, Raman and Nair, 1968a). In Penang, according to Hall (1962), there is some indication that the adults are most abundant in August-September.
M. affinis is heterosexual, as are all penaeid prawns.
Hall (1962) recorded one hermaphrodite in a total of 77 specimens, from a Singapore prawn pond.
Menon (1957) and Subrahmanyam (1967) mentioned that sexual maturity is reached at a length of about 120 mm. Rao (1968) recorded the minimum length at maturity as 88.6 mm, corresponding to the late 0 year class. By ova diameter and other studies he classified the different maturity stages of the female as ‘immature’, ‘early maturing’, ‘late maturing’, ‘mature’ and ‘spent-recovering’. Shaikhmahmud and Tembe (1960) classified specimens from Bombay waters into only 3 stages, namely, immature, maturing and mature.
As in other penaeid prawns, fertilization is external, taking place at the time of spawning. Hudinaga (1941) made some observations on the fertilization, the number of entrance cones, etc.
Fecundity of the species, as estimated by Rao (1968), ranges between 88,000 to 363,000 eggs. According to him there is a linear logarithmic relationship between the number of eggs produced and the size of the prawn. The formula is
Log F = -0.4306+2.7179 Log L
where F is the number of mature eggs and L is the total length in mm.
From a study of the inshore fishery of the southwest coast of India, George (1961) reported the breeding time of the species to be from October to December. Later, from the study of the trawl catches of the species off Cochin, George et al. (1968a) concluded that the active breeding season of the species is from November to February. Rao (1968), using the maturity conditions of the catches off Cochin, also arrived at a similar conclusion, the breeding season being October to March with peak in November to December. January to March is the spawning period in Calicut waters according to Subrahmanyam (1967). In Bombay waters, Shaikhmahmud and Tembe (1960) found most mature and maturing females in October and April to June. Similarly, Hall (1962) reported 2 peak breeding seasons for the species in Malaysian waters, namely, May to June and October to December.
Hudinaga (1942) has found that the species spawns while swimming about or vigorously moving the pleopods when spawning at the bottom. Under other conditions, eggs failed to hatch.
Utilizing the data on the monthly distribution of late maturing and mature females in 10 mm length groups in the catches off Cochin, Rao (1968) concluded that the species spawns more than once during its growth from 91–100 mm to 151–160 mm.
Subrahmanyam (1967) studying the breeding of the species in Calicut, and on the southwest coast of India, observed that the species moves to offshore areas for spawning. Studies at Cochin, on the same coast, indicated that the species may be breeding in the 15 to 25 fm (27 to 45 m) areas. In Malaysian waters Hall (1962) observed the species breeding very close inshore. In Bombay waters, Shaikhmahmud and Tembe (1960) observed that there are indications that the species prefers areas of soft mud, rich plankton and shallow coastal waters for mating and spawning.
The most highly developed ovarian eggs measure 0.352 mm according to Rao (1968). He has given measurements of various stages of maturing eggs. No information is available on the spawned eggs, hatching, etc.
In the embryonic development of the species, Hudinaga (1942) observed the segmentation to be quite similar to that of Penaeus japonicus, which he described in considerable detail.
The larval development of the species is described by Hudinaga (1941). There are 6 moultings in the nauplius stage, 3 in the protozoea, and 3 in the mysis stage, after which the larva passes into the postlarva. The nauplius stages do not feed.
Hudinaga (1941) obtained good results by rearing the zoea of the species on Skeletonema costatum in the laboratory.
Mohamed, Rao and George (1968) described the first postlarva.
Subrahmanyam (1967) recorded that the species may live for 3 years or slightly more. According to George et al. (1968a) from a study of the trawl catches off Cochin only 2 year classes of the species contribute to the fishery.
Like most other penaeids, the species can withstand only a minimum of handling.
The species occurs on the fishing grounds off the coasts of India along with other species, and undoubtedly there will be competitors with them for food.
Penaeids in general are preyed upon by the demersal fishes of the area where they exist. There are records of ‘penaeids’ ‘prawns’, etc., as forming major portion of food of several species of demersal fishes.
There are no reports of parasites or diseases of M. affinis.
The maximum size of the species according to Alcock (1906) and Menon (1957) is 6½ in (165 mm). George et al. (1968a) report a maximum size of 170 mm.
By analysis of the stomach contents of 30 specimens caught during daylight from the Straits of Malacca, Hall (1962) classified the species in a group feeding mainly on vegetable matter. No information is available on feeding time, season, etc.
The items of food, in their order of abundance in the stomachs of the 30 specimens, are vegetable matter (mainly angiosperm tissue), small crustaceans (copepods and ostracods), Polychaeta, echiurid setae, molluscan shell pieces and fish remains. Except for 1 empty stomach, sand grains were always present, some of the grains being large, but the quantity was not great (Hall, 1962). According to Subrah manyam (1967) the species is an omnivorous feeder and larger prawns show a preference to a molluscan diet.
George et al. (1968a), studying the fishery of the species in Cochin trawl catches, recorded a growth of specimens in the 1st year of 20 mm among males and 25 mm among females in about 6 mo.
Differential rates of growth in the sexes, females showing a faster growth rate, has been recorded by George (1961), Subrahmanyam (1967) and George et al. (1968a).
In the Seto Inland Sea of Japan, Yasuda (1956) classified this species in a group in which the young shrimps do not grow for a limited period and thereafter grow very rapidly before the spawning season.
Hall (1962) related weight and carapace length by the formula
W = 0.7079C2.770,
based on 183 observations. The formula according to Subrahmanyam (1967) is
W = .0000495L2.7867,
where L refers to total length.
Juveniles migrate from the sea to backwaters and estuaries, but not to the same extent as some other species like M. dobsoni and Penaeus indicus. In the Cochin backwaters, juveniles of this species are present in very small numbers in most months, but it is probable that most of the postlarvae do not leave the sea.
In the offshore shrimping grounds off Cochin, George, Banerji and Mohamed (1968) recorded some movements. According to them this species and others move to deeper waters during the monsoon period and return after the monsoon.
Subramanyam (1965) studying the migration of the species in the Godavari estuarine system on the east coast of India, found more emigration of the species during the night. According to him, intense emigration was in April, May and early June. Immigration was noticed to be most marked at dawn.
Large concentrations of this species are known to occur in shoals, along with other species, in the mud bank areas off the south-west coast of India and also in the shrimping grounds of the coast.
Observations have been made by Hudinaga (1942) on spawning behaviour in captivity (see section 3.1.6) but there are no other accounts of reproductive habits.
Menon (1957) has described in detail the sex ratio of the species from the inshore catches of the southwest coast of India. The sex ratios according to him are shown in Table I.
TABLE I
Percentage of males in total catch and the same for specimens of over 120 mm
| Year | Total catch | Over 120 mm |
| 1952 | 52.2 | 47.2 |
| 1953 | 50.4 | 47.6 |
| 1954 | 52.0 | 39.0 |
| 1955 | 44.2 | 44.2 |
According to him, the population of immature prawns (up to 120 mm) contains similar numbers of each sex. Among older prawns (above 120 mm), however, there is considerable disparity, females outnumbering males by 11.6 percent. Subrahmanyam (1967) found females dominating in the catches from Calicut waters.
George and Rao (1967) statistically analyzed the data on sex ratios of the species in the offshore trawl fishery off Cochin, and found that, unlike 3 other co-existing species, the two sexes were present in similar numbers throughout the year. Any migration from these grounds must therefore involve similar numbers of each sex. In Bombay waters, Shaikhmahmud and Tembe (1960) recorded more females throughout the year, especially during the months of October, November and December.
George (1961) and George et al. (1968a) have mentioned the age composition of the species in catches of both the inshore and offshore fisheries off Cochin. In both cases the fishery is sustained by the 1st and 2nd year classes. In the trawl fishery, the 2nd year classes generally enter the fishery in the earlier half of the season and the 1st year classes make their appearance in the latter half. The late 0 year class is also represented in the inshore fishery in some months. In the backwater fishery only the 0 year class is represented. 3 year classes are represented in the coastal waters of Calicut (Subrahmanyam 1967). Males and females grow up to 105 mm, 135 mm, 155 mm and 115, 155 mm, 175 mm during the 3 years respectively.
In the inshore fishery of the southwest coast of India (George, 1961), the prominent length groups are 71 to 75 mm and 81 to 85 mm in February to April. In November and December the prominent groups are 121 to 125 mm and 126 to 130 mm. In some years the mode reaches 136 to 140 mm in January or February.
In the trawl fishery (George et al., 1968a) from October to January larger sizes, with lengths between 121 and 140 mm, are represented in larger numbers than in the inshore fishery. Towards the middle of the season, i.e. after February, smaller sizes between 111 and 115 mm come into the fishery. In the Bombay fishery (Shaikhmahmud and Tembe, 1960) the species ranges in size from 45 to 156 mm in length.
In the inshore fishery (George, 1961), recruitment of bigger specimens into the fishery commences in October. After December to January the smaller specimens get recruited into the fishery. In the offshore trawl fishery off Cochin also more or less the same pattern is observed (George et al., 1968a). Recruitment of postlarvae into the backwaters is on a small scale compared to other species.
In the Bombay area, the ‘dol’ net or bag net is the gear used for catching prawns. The details of the ‘dol’ nets and its operation have been described by Setna (1949).
On the north Kanara coast, the prawns are caught by shore seines (‘yendi bale’), the details of which are given by Pradhan (1956).
On the southwest coast of India, various types of boat seines (locally called, ‘thangu vala’, ‘vatta vala’, ‘koru vala’), shore seines (‘kamba vala’, ‘nona vala’), drag nets (‘vadi vala’) and cast nets are employed.
Powered boats use shrimp trawls of various sizes.
The inshore fisheries are mainly operated from dugout canoes and plank-built boats with out-rigger. Powered fishing vessels are generally medium sized 7 to 11 m pablo boats, having 10 to 30 bhp engines.
The species is fished in Indian waters, particularly on the west coast, and round the coasts of Ceylon and Malaysia.
Juveniles are fished in small numbers in the backwaters and estuaries, including paddy fields, of the west coast of India in shallow waters ranging from 1 to 5 m.
Young adults and adults are caught from depths up to about 30 fm (54 m) from the sea. In the trawl fishery off Cochin, George et al. (1968a) reported a concentration of the species in the 10 to 11 fm (18 to 20 m) depth region.
In Bombay (Shaikhmahmud and Tembe, 1960), the depth from which these prawns are caught ranges from 4 to 7 fm (7 to 13 m).
The Singapore pond, from which Hall (1962) recorded this species, had an average depth of 4 ft (1.2 m) at high tide.
In Ceylon, M. affinis is found in 8 to 12 fm (14 to 22 m) (De Bruin, 1965).
The fishery for M. affinis tends to be seasonal in all areas where it occurs, but the periods of peak abundance vary between the different fisheries.
See section 5.3.3
In the Singapore pond fishery this species is represented sporadically by juveniles in April, May and June (Hall, 1962).
In the backwater fishery of Cochin the species is most abundant from January to June. The percentage contribution of the species, given by Menon and Raman (1961), is shown in Table II.
George (1961) gave the percentage contribution of the species to the inshore fishery of the southwest coast of India for 1956 through 1960. Its highest percentage is reached in the post-monsoon months, November and December, and it is fished from October to May.
TABLE II
Showing percentage values (numerical and weight) of M. affinis in the monthly catches in the stake net catches at Cochin from February 1957 to January 1958
| Months | Feb. | Mar. | Apr. | May | June | July | Aug. | Sept. | Oct. | Nov. | Dec. | Jan. |
| By weight | 9.5 | 10.3 | 9.9 | 5.9 | 12.7 | 0.5 | - | - | 2.5 | 1.4 | 5.9 | 13.4 |
| By number | 11.4 | 15.3 | 15.3 | 11.4 | 16.9 | 2.1 | 2.1 | 0.4 | 1.6 | 5.2 | 6.9 | 15.8 |
The peak season for the species in the trawl fishery off Cochin (George et al., 1968a) is from December to February. Detailed distribution of the species from 1958 through 1963 is given by them. In Bombay waters (Shaikhmahmud and Tembe, 1960), although the species is available throughout the year it is more plentiful from January to March. In 1952 to 1954 the species formed 12.3 percent of the Bombay prawn catch. In Calicut inshore waters the fishing season is January to August (Subrahmanyam, 1967).
The inshore fishery on the Malabar coast intensifies after the annual formation of mud banks, on which the prawns concentrate. The fishery in the backwaters of Kerala is probably influenced by rainfall and lunar periodicity (Menon and Raman, 1961). Subrahmanyam (1967) found a positive relationship between bottom salinity and abundance of the species.
George (1961), studying the prawn fishery of three centres of the Kerala coast for the years 1956 through 1960, used the total effort and intensity of fishing and established a relationship between catch-per-man-hour and recruit sizes. M. affinis, however, makes only a minor contribution to this fishery.
In the trawl catches of Cochin, George et al. (1968a) studied the effort and intensity of fishing through the years 1958 to 1963 and concluded that there is no overfishing in the area. In Table III the catch and effort for one season is shown.
None of the fishery as it exists at present is very selective as regards size on species.
Tham Ah Kow (1954) gave the total prawn catch (including this species) from a 20 acre prawn pond in Singapore, and Hall (1962) gave the weight of prawns (again including M. affinis) passing through Singapore markets in 1952 to 1954.
George (1961) and George et al. (1968a) gave the total catches of prawns, including this species, for certain Indian areas. Menon and Raman (1961) gave total catches of prawns at two centres in the backwaters of Cochin.
Subramanyam (1965) gave the total catches of this and other species for the new moon and full moon periods in the Godavari estuarine system, on the east coast of India. He also gave high tide and low tide catches of the prawns.
In southwest India, the paddy field fishery is legally restricted to the period from the middle of November to the middle of April. This regulation is imposed in the interests of rice cultivation, and not to protect the prawn stocks.
In the paddy fields of Kerala, prawns may be held in the fields for a few days before trapping, but this is mainly to wait for a convenient tide. A few decades ago, young prawns were allowed to grow in the paddy fields for 2 or 3 mo before trapping, but this practice is no longer followed.
TABLE III
Total catch and catch per hour of Metapenaeus affinis in the trawl catches for the season 1959–60
| Year | Month | Percentage by weight | Total catch of prawns (kg) | Total effort in hrs | Catch of M. affinis | Catch per hour for all prawns (kg) | Catch per hour for M. affinis (kg) |
| 1959 | November | 20.8 | 2306 | 301.17 | 479 | 8.0 | 1.6 |
| December | 28.9 | 10027 | 430.00 | 2898 | 23.0 | 6.7 | |
| 1960 | January | 49.6 | 22725 | 491.25 | 11272 | 46.0 | 22.9 |
| February | 28.3 | 50113 | 692.68 | 14182 | 72.0 | 20.5 | |
| March | 55.8 | 14119 | 608.75 | 7878 | 23.0 | 12.9 | |
| April | 20.8 | 18944 | 660.42 | 3940 | 29.0 | 6.0 | |
| May | 29.2 | 20712 | 387.33 | 6048 | 54.0 | 15.6 |
Alcock, A., 1906 The prawns of the Peneus group. In Catalogue of the Indian decapod Crustacea in the collection of the Indian Museum. Calcutta, Part 3, Macrura, Fasc. 1, 55 p.
Burkenroad, M.D., 1963 Comments on the petition concerning penaeid names (Crustacea, Decapoda) (2.N(S.)962). Bull.zool.Nomencl., 20(3):169–74
Dall, W., 1957 A revision of the Australian species of Penaeinae (Crustacea, Decapoda: Penaeidae). Aust.J.mar.freshwat.Res., 8(2):136–231
De Bruin, G.H.P., 1965 Penaeid prawns of Ceylon (Crustacea, Decapoda, Penaeidae). Zoöl. Meded., Leiden, 41(4):73–104
de Man, J.G., 1911 The Decapoda of the Siboga expedition. Part 1. Family Penaeidae. Siboga Exped., 39a:131 p.
George, M.J., 1961 Studies on the prawn fishery of Cochin and Alleppey coast. Indian J. Fish., 8(1):75–95
George, M.J., 1970 Synopsis of biological data on Metapenaeus monoceros (Fabricius). FAO Fish.Rep., (57)Vol.4:1539–57
George, M.J. and P.V. Rao, 1967 Distribution of sex ratios of penaeid prawns in the trawl fishery of Cochin. Symp.Ser.mar.biol.Ass.India, 2(2):698–700
George, M.J., S.K. Banerji and K.H. Mohamed, 1968 Size distribution and movement of the commercial prawns of the southwest coast of India. FAO Fish.Rep., (57) Vol.2:265–84
George, M.J., K. Raman and P.K. Nair, 1968a Observations on the offshore prawn fishery of Cochin. Indian J.Fish., (1963), 10A(2):460–99
Hall, D.N.F., 1956 The Malayan Penaeidae (Crustacea, Decapoda). Part 1. Introductory notes on the species of the genera Solenocera, Penaeus and Metapenaeus. Bull. Raffles Mus., 27:68–90
Hall, D.N.F., 1958 Distinctions between Metapenaeus monoceros (Fabr.) and Metapenaeus ensis (de Haan) (Crustacea, Decapoda). Ann.Mag.nat.Hist., 1(8):537–44
Hall, D.N.F., 1962 Observations on the taxonomy and biology of some Indo-West Pacific Penaeidae (Crustacea, Decapoda). Fish.Publs colon.Off., 17:229 p.
Holthuis, L.B. and H. Rosa, Jr., 1965 List of species of shrimps and prawns of economic value. FAO Fish.tech.Pap., (52):21 p.
Hudinaga, M., 1941 On the nauplius stages of Penaeopsis monoceros and Penaeopsis affinis. Proc.scient.Fishery Ass., Tokyo, 8(3–4)
Hudinaga, M., 1942 Reproduction, development and rearing of Penaeus japonicus Bate. Jap.J. Zool., 10(2):305–93
Kemp, S., 1915 Fauna of the Chilka Lake. Crustacea Decapoda. Mem.Indian Mus., 5(3):201–325
Lanchester, W.F., 1901 On the Crustacea collected during the Skeat Expendition to the Malay Peninsula, together with a note on the genus Actaeopsis. Part 1. Brachyura, Stomatopoda, and Macrura. Proc.zool.Soc.London, 1901:534–74
Menon, M.K., 1957 Contributions to the biology of penaeid prawns of the southwest coast of India. 1. Sex ratio and movements. Indian J.Fish., 4(1):62–74
Menon, M.K. and K. Raman, 1961 Observations on the prawn fishery of the Cochin backwaters with special reference to the stake net catches. Indian J.Fish., 8(1):1–23
Milne Edwards, H., 1837 Histoire naturelle des crustacés, comprenant l'anatomie, le physiologie et le classification de ces animaux. Paris, Vol.2, 532 p.
Mohamed, K.H., P. V. Rao and M. J. George, 1968 Postlarvae of penaeid prawns of southwest coast of India with a key to their identification. FAO Fish.Rep., (57) Vol.2:487–503
Pradhan, L.B., 1956 Mackerel fishery of Karwar. Indian J.Fish., 3(1):141–85
Racek, A.A. and W. Dall, 1965 Littoral Penaeinae (Crustacea Decapoda) from northern Australia, New Guinea and adjacent waters. Verh.K.ned.Akad.Wet., 56(3):1–119
Rao, P.V., 1968 Maturation and spawning of the penaeid prawns of the southwest coast of India. FAO Fish.Rep., (57)Vol.2:285–302
Setna, S.B., 1949 Bombay fishermen's ingenuity. J.Bombay nat.Hist.Soc., 36:887–97, 40(3):444–9
Shaikhmahmud, F.S. and V.B. Tembe, 1960 Study of Bombay prawns: the seasonal fluctuation and variation in abundance of the commercially important species of Bombay prawns with a brief note on their size, state of maturity and sex ratio. Indian J.Fish., 7(1):69–81
Subrahmanyam, C.B., 1967 Notes on the bionomics of the penaeid prawn Metapenaeus affinis (Milne Edwards) of the Malabar coast. Indian J.Fish., (1963), 10A(1):11–22
Subramanyam, M., 1965 Lunar, diurnal and tidal periodicity in relation to the prawn abundance and migration in the Godavari estuarine systems. Fishery Technol., Ernakulam, 2(1):26–33
Tham Ah Kow, 1955 The shrimp industry of Singapore. Proc.Indo-Pacif.Fish.Coun., 5(2–3):145–55
Wood-Mason, J. and A. Alcock, 1891 Natural history notes from H.M. Indian marine survey steamer INVESTIGATOR. Ann.Mag.nat.Hist., 8(6):268–86
Yasuda, J., 1956 Shrimps of the Seto Inland Sea of Japan. Proc.Indo-Pacif.Fish.Coun., 6(3):378–86