4.1. THE CAUSES OF THE DECLINE IN EMERGENT VEGETATION (exemplified by data on reed)
4.2. CAUSES OF THE DECLINE IN SUBMERSED AND FLOATING-LEAVED MACROPHYTES
4.3. A GENERAL HYPOTHESIS
The nitrogen concentration
For optimum growth of reed (Phragmites australis) the nitrogen (NO3-N) concentration should be lower than 5 mg/l in the water and between 0.3 and 8.0 mg/100g (dry weight) in the sediment (Rodewald-Rudescu, 1974). Reed growing in litter-mats which contain 20-25 mg NO3-N per 100 g (dry weight) showed a reduction in sclerenchyma of the stems (Klotzli, 1971). In the Havel lakes the total dissolved nitrogen concentrations in the water in 1977 ranged between 2 and 5 mg/l. The flexibility of reed shoots increased in this area while the sclerenchyma ring in the reed stems decreased. Thus, the mechanical stability of reed stands is negatively influenced by high nitrogen concentrations (Sukopp et al. 1975; Bornkamm & Raghi-Atri, 1978). Boar & Crook (1984) found a correlation between the NO3-N concentration (more than 2 mg/l) of the water and the degree of deterioration of reed swamps in different Norfolk Broads. The partitioning of biomass between rhizomes and shoots changed with higher NO3-N concentrations. The growth of above ground plant parts increased disproportionally with increasing nitrate concentration.
Filamentous algae
Increasing nutrient concentrations in the water enhances the growth of algae. Filamentous algae, especially Cladophora, form floating mats in which decaying (blue-green) phytoplanktonic algae are caught. These "carpets" become entangled; n the reed swamps at spec; al weather conditions. They fill up the space between bottom sediment and water surface. Wind induced wave action exercises severe stress on these carpets, so the reed will break. The remaining stems are filled with water and suffocate. Decaying stems also will become part of the mat, like all other kind of floating litter (Klotzli, 1971, 1973 & 1982; SchrBder, 1979; Sukopp & Markstein, 1981).
Apart from the mechanical damage, these mats prevent light and oxygen to reach the shoots. The light is intercepted by the carpet, whereas oxygen is kept from penetrating the mat by free exchange of water. The oxygen is largely consumed in bacterial processes within the mat. So anaerobic conditions may occur between the reed stems. Schroder (1979) presented a model in which he showed that these processes lead to the release of phosphorus from the sediment; so the nutrient loading will increase. Moreover, the concentration of ammonia and hydrosulphide ions will increase in the organic substances until hydrogen sulphide gas is released in toxic concentrations (5 mg/l) that kill the rhizomes. In the Grosser Ploner See (Utermohl, 1982). Lake Constance (Schroder, 1979) and Tegelersee (Sukopp & Markstein, 1981) these Cladophora carpets were the main cause of the disappearance of reed.
Floating litter
The floating carpets of Cladophora are not the only cause of mechanical damage. In the Havel lakes without pronounced algal development, the highest decrease in reed stands (up to 27.7% per year)
Occurred at sites with a high volume of floating litter, up to more than cubic metre per metre shore line. The undamaged stands had no or less yhan average (= 0.33 cubic metre per metre; no weight mentioned) floating litter (Sukopp & Markstein, 1981). According to Klotzli (1982) more than 50 kg litter per metre is a serious threat, resulting in "reed death"on the lake front. A number of processes are summarized in his model (Fig. 5).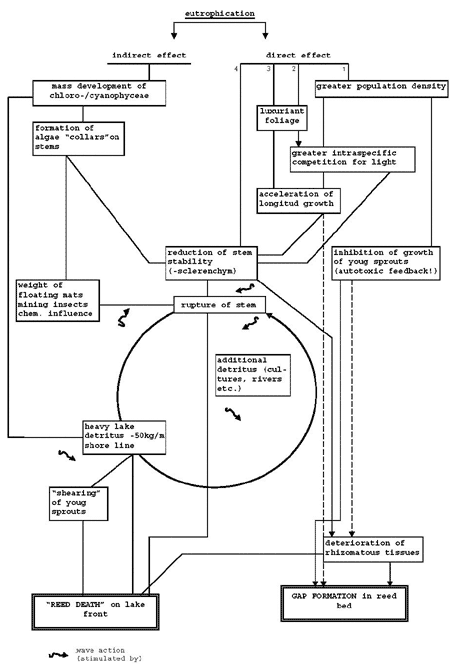
Sedimentation
The terms (soft) mud, silt, bottom substrate and sediment are often interchanged. Because of the complex chemical nature of these categories, the terms are difficult to define. The term sediment is most often used; its dry matter part consists of three basic components:
1) dead organic matter;
2) particulate mineral matter, especially silicate;
3) inorganic components of biogenic origin like calcium carbonate (Wetzel, 1975).
High phytoplankton concentrations due to eutrophication will raise the amount of dead organic matter in the water and subsequently the sedimentation rate. Ripl (1984) reported a sedimentation rate of 44 tonnes per ha per year in 1982 in the hypertrophic Dummer lake (1240 ha; Niedersachsen, West-Germany). One tonne (1000 kg) sediment contained 80 kg (8%) dry matter, subdivided in 51 kg biogenic precipitated calcium carbonate, 20 kg organic material and 9 kg silicate. According to Akkermann (1978) the combination of rapid sedimentation with the occurrence of floating\ litter and high nitrate concentrations in the water, was the main cause of "reed death" in the Dummer (Table 4; Section 3.1).
Boorman & Fuller (1981) found a negative correlation between reed stem height and depth of the sediment ("soft mud") layer. The dry matter proportion of the sediment ranged between 12 and 35 %, the organic ("loss on ignition") part ranged between 13 and 48% of the dry matter. In this soft mud anaerobic conditions occurred during summer.
Disturbance and recreational activities
From the landside reed beds are threatened by the invasion of terrestrial plant species like Epilobium hirsutum. Land drainage, resulting in lowering the water table, will favour the introduction of these plants. The reed stands also become vulnerable after disturbance because of human recreation and tracks made by hares (Lepus capensis), roedeer (Capreolus capreolus) and pheasants (Phasianus colchicus) (van der Toorn et al. 1983).
In the 1960s bathing, trampling along the shore line and boating in the Havel area caused considerable damage to the reed beds. Damage by recreational boats has also been reported from Wales (Cragg et al. 1980), while Boorman & Fuller (1981) did not find any relation between decline in reeds and the quantity of pleasure boats in the Norfolk Broads.
Grazing by birds and mammals
Grazing of young reed shoots by mute swans (Cygnus olor), coots (Fulica atra) and muskrats (Ondatra zibethicus) badly influenced the reed beds (Sukopp & Markstein, 1981). In the Norfolk Broads the highest rate of decrease (7%) was found when the population of the exotic rodent coypus (Myocaster coypus) was very high. After the severe winter of 1963 the population declined 14 fold, and the rate of decrease in reed swamp surface slowed down too (Boorman & Fuller, 1981) (Table 4; Section 3.1). Akkermann (1975) studied the muskrats in the Dummer and calculated that a population of ca. 3000 individuals consumed (or damaged otherwise) 0.27 ha Typha spp., 0.15 ha Phragmites australis, 0.86 ha Glyceria sp. and 1.58 ha Scirpus sp. The damaged area of Scirpus and Phragmites did not recover, even when the muskrats significantly decreased in number.
Young reed shoots in soft mud are easier to find by grazing animals (i.e. geese, Anser anser and Branta canadensis) than reed on more solid sediments. The birds tear off the shoots and rhizomes depriving the roots from their oxygen supply. This will eventually cause their death. The excessive amount of sediment, polluted with organic matter, made the reed stems more vulnerable to grazing. This is a consequence of the increased nutrient loading of the water body, thus the damage to reed swamps by waterfowl is in fact an indirect effect of eutrophication (Boorman & Fuller, 1981). Under normal conditions reed stands are able to sustain grazing by wildfowl.
Grazing by cows alongside water bodies is an important cause of reed death in many parts of the Netherlands (Best, 1982), and is also mentioned as a cause in the Havel lakes area (Sukopp & Markstein, 1981). This pertains to areas with intensified agricultural practise with a high density of cattle per ha. Cragg et al. (1980) argue that mild grazing does not harm the aquatic vegetation.
Summary: the emergent vegetation (mainly reed, Phragmites australis), is directly influenced by eutrophication. The increased nitrogen concentration adversely affects the mechanical properties of reed, making them less resistant against damage. Indirectly the reed and other emergent vegetation are threatened by carpet-forming filamentous algae. Under these conditions other activities like human recreation, grazing by cattle, other mammals and waterfowl haye a much stronger negative impact, while under normal conditions reed stands can sustain grazing by wildlife.
Main factor?
Eutrophication, i.e. increasing concentrations of phosphorus (-P) and nitrogen (-N) containing dissolved compounds, is most often mentioned as the main factor of the decline in submersed macrophytes. These nutrients stimulate phytoplankton growth. Jupp & Spence (1977b), Moss (1977), Ozimek & Kowalczewki (1984) and Toivonen (1985) called the increased light attenuation due to water turbidity (phytoplankton blooms, high concentrations of particulate dead organic or mineral matter) an important factor of the decline in submersed macrophytes. Shading by trees, recommended as a management technique to control nuisance growth (Dawson & Kern-Hansen, 1979), is also reported as a cause of the decline in submersed macrophytes in a small lake (Best, 1982). Jupp & Spence (1977a) observed a relationship between P- concentration and the decline in the dominant submersed macrophyte Potamogeton filiformis. Moss & Leah (1982) and Best et al. (1984) stressed that raised P-concentrations are not immediately followed by the decline in aquatic macrophytes. Normally the nutrient loading increases gradually or even remains constant while the decline in aquatic macrophytes is often rapid (Fig. 6).
In general the largest decrease is shown by the submersed aquatic macrophytes while floating-leaved vegetation types with Nuphar lutea (Jeschke & Muther, 1978; Cragg et al. 1980; Moss & Leah. 1982; Ozimek & Kowalczewki. 1984) or Lemna minor (Toivonen. 1985) decreased to a lesser extend, remained constant or even expanded. Whenever the leaves of these plants reach the water surface, they will receive full light, and the growth is no longer hampered by water turbidity.
If the turbidity due to phytoplankton abundance is the main cause of the decline, one ought to find a clear relation between the turbidity (the degree of light attenuation) and the presence of submersed macrophytes. Phillips et al. (1978) did not find such a simple relation. The measured extension of the zone with net photosynthesis did not correlate with the distribution range of submersed macrophytes in different Norfolk Broads. In artificially fertilized experimental tanks the epiphytic and filamentous algae increased more rapidly than the aquatic macrophytes. In the field a higher biomass of epiphytic algae per dry weight of aquatic plant was found on sites with higher concentrations of total P and N in the sediment. The species diversity of the epiphytic community was lower on these enriched sites (Eminson. 1978). Thus, a high nutrient loading of the water in the Norfolk Broads favoured in the first place the amount of biomass (not the number of species) ;n epiphytic algae (Fig. 6).
Epiphytic algae may form several layers which hamper the irradiance of the macrophytic leaves and the exchange of inorganic carbon (HCO3- and CO2). Consequently, the growth of the macrophyte becomes slower or even stops (Sand-Jensen, 1977; Sand-Jensen & Sondergaard, 1981). Under non-eutrophic circumstances the macrophyte may release allelo-pathic substances to suppress the growth of phytoplankton (Wium-Andersen et al., 1982). or to attract selectively epiphytes (Carpenter & Lodge, 1986) and grazing macro-invertebrates (Orth & van Montfrans, 1984; Bronmark, 1985a; Lodge, 1985). Many of these relations are discovered in laboratory conditions; their ecological instance the ability of Chara to suppress phytoplankton growth in fish ponds is well established (Crawford, 1979).
Hence, increased nutrient loading favours few epiphytic species. The epiphytic species diversity decreases, while the biomass increases (Eminson, 1978). The diversity in grazing species like snails decreases. Probably the remaining few epiphytic species are less attractive food for many herbivorous invertebrates (Den Hartog, pers comm.). Maybe the eutrophication disturbs complex but subtle mechanisms (by means of released substances) to suppress the phytoplankton growth and to attract invertebrate grazers, which once existed between the diverse species of epiphytes, macrophytes and grazing invertebrates.

Fig. 6. The relative dominance of the amount of biomass of submersed macrophytes, phytoplankton and epiphytes and the availability of nutrients in an aquatic ecosystem
with gradually increasing nutrient levels (Phase I to III as described by Moss et al, 1985).
A decrease in nutrient loading of the water is probably the panacea to re-establish clear water with abundant aquatic macrophytes and subsequently waters rich in fish. Moss & Leah (1982) warn their readers not to be overoptimistic: after a decrease in the nutrient loading there are several processes maintaining the deteriorated situation.
Blue-green algae may remain if large-bodied cladoceran zooplankters stay away because of intensive predation by small (young-of-the-year) fish (see Section 2.5.4.). The slow-growing, colony-forming, blue- green algae like Aphanothece are more difficult to handle by the small remaining zooplanktonic species. In the absence of large-bodied cladocerans this alga may predominate even at lower than preferred nutrient concentrations because it has a low death rate, a low sinking rate and it is able to use the nutrients more efficiently by internal recycling (Moss & Leah, 1982). Excretion of allelopathic substances by blue-green algae that suppress the growth of aquatic macrophytes is proved in the laboratory (van Vierssen & Prins, 1985) and may help to preserve the situation with predominance of phytoplankton.
Reduction of zooplankton eating fish in an eutrophicated lake may reduce the symptoms of eutrophication (Hrbacek et al. 1961; Andersson et al. 1978; Leah et al. 1980; Reinertsen & Olsen, 1985; Braband et al., 1986). In that case the phytoplankton will be grazed more effectively by the larger (mainly cladoceran) zooplankters and the water will become clear. However, if the fish population has considerably decreased, invertebrates (e.g. mysids) may occupy part of the niche of the young fish and prey on the cladocerans. The introduction of Mysis relicta will significantly decrease zooplankton densities (Langeland, 1981; among others) and therefore alter the trophic state of the water body.
Moss et al. (1986) reported the reactivation of PO4-P from the sediment after reduction of the nutrient loading of the water in a small lake in the Norfolk Broads. At first the water became clear and submersed water plants successfully recolonized the lake within four years. Six years after the reduction, activated phosphorus from the sediment caused a comeback of the phytoplankton and the growth of aquatic plants became negligible.
In the Norfolk Broads large flocks of birds bring guano (nutrients) in some lakes (Section 3.2) (Moss & Leah, 1982).
Especially in soft (non-alkaline) waters acid precipitation causes dramatic changes in the species composition and biomass of aquatic macrophytes and fish populations (Hendrey, 1982; Roelofs, 1983; Roelofs et al., 1984). These phenomena have been excluded from the scope of this review because most attention has been paid to productive, hard, nutrient-rich waters. However, the increased nitrogenous deposition in some areas also may influence these waters and will help to maintain undesirably high blue-green algae concentrations.
i. Wave action The submersed macrophytes rooted in the sediment become more and more restricted to the shallowest parts of the water, where they can receive enough light for growth. They also become more vulnerable to wave action at these shallow parts. From shallow, wind- exposed sites the nutrients will be flushed out. Direct mechanical destruction and hampered growth because of nutrient-poor sediments were the causes of the deterioration of the whole Potamogeton vegetation in Loch Leven (Jupp & Spence, 1977b).
ii. Grazing by birds. mammals and fish Mute swans ( Cygnus olor )and coots (Fulica atra) in the Havel lakes (Sukopp & Markstein, 1981) and the Mazurian and Northbrandenburgian lakes (Ozimek & Kowalczewki, 1984; Jeschke & Muther, 1978), whooper swans (Cygnus cygnus), geese and ducks in Loch Leven and the Norfolk Broads (Jupp & Spence, 1977b; Boorman & Fuller, 1981) intensively grazed on submerged and floating-leaved macrophytes.
These animals also contributed to the decrease in l. aquatic macrophytes. However, Kiorboe (1980) proved that the effect of grazing by waterfowl on the abundance of submersed macrophytes in spring and summer is small, although the birds consume quantities up to 60% of the annual plant biomass production. Because grazing by waterfowl takes place in autumn and winter, when the aquatic plant population already produced its survivals organs (diaspores), the macrophyte populations are not significantly damaged.
Because the decrease of submersed aquatic vegetation is multicausal, the grazing by waterfowl may become harmful when these birds have neither decreased at all, nor lessened their population in the proportion to the amount of aquatic macrophytes. Their grazing pressure in relation to the remaining amounts of aquatic plants is stronger.
Van der Velde et al. (1982) reported grazing and damage to Nymphoides peltata stands by coots (Fulica atra), but also by snails, insects, muskrats (Ondatra zibethicus) and cattle (mainly Bos taurus). Animals were responsible for the disappearance of 22% of the total leaf area produced during the growing season, being the combined effect of consumption and microbial decay after damage.
Grazing by carp (Cyprinus carpio) and other cyprinids, often stocked and managed to improve fisheries, can contribute to the decline of Chara weedbeds (Jeschke & Muther, 1978; ten Winkel & Meulemans, 1984).
iii. Effects of pleasure-boats Turbidity is also caused by pleasure- boat traffic. However, Hilton & Phillips (1982) did not find a long-term build-up turbidity in the River Ant as a consequence of frequent pleasure-boat traffic. Like Moss (1977), they state that turbidity is mainly caused by phytoplankton. Yousef et al. (1980) found evidence for increased phosphorus content due to water mixing by recreational motorboats in Florida. In all cases the direct mechanical damage to macrophytes by motorboats is evident. Strong correlations have been found between the intensity of boat traffic and the decrease in submersed and floating-leaved macrophytes, especially Potamogeton natans and Nuphar lutea (Cragg et al. 1980; Jeschke & Muther, 1978; Murphy & Eaton, 1983). Increased pleasure-boat traffic is often accompanied by the construction of jetties and ports which have a considerable effect on the reed vegetation and may have caused the rapid decline in Hydrocharis morsus-ranae dominated (aquatic) vegetation in the Northbrandenburgian lakes (Jeschke & Muther, 1978).
All processes leading to the decline in submersed aquatic macrophytes can be summarized in a model (Fig. 6 & 7). This model is mainly based on the ideas of Phillips et al. (1978) and Moss et al. (1985), with modifications added by van Vierssen et al. (1985a).

Increased inputs of nutrients will cause initially an increase in aquatic macrophytes (Phase II in Fig. 6). Later on the epiphytic algae start to increase. If the macrophytes are successful in the production of diaspores, they will build up sufficient biomass in the next season. Thus a dynamic balance between macrophytes and epiphytes exists in the mildly eutrophicated situation. The phytoplankton growth may be inhibited by the excretion of suppressants. In mildly eutrophic situations the macrophytes can also compete successfully with phytoplankton for nutrients (Goulder, 1969, van Vierssen et al. 1985a) and light (Fig. 7).
When nutrient levels increase the total biomass of epiphytes increases, hence there is more food available to the grazer population, which will increase too. Subsequently there is enough food for fish, substrate (=aquatic macrophytes) to deposit their eggs and sufficient shelter for fish larvae and fry (Phase II in Fig. 6). These eutrophicated water bodies are more productive because the extra input of nutrients will cause an energy flow to the adult (commercially important or game) fish through the aquatic macrophytes, epiphytes or phytoplankton, via herbivorous invertebrates and small fish. This positive relationship between trophic state and fish productivity is well-established (Grosch, 1980, Hoyer et al., 1985).
As a consequence of increased abundance of the few epiphytic species, the macrophytes become gradually hampered in their growth if the consumption of the extra growth of epiphytes becomes insufficient. As long as they produce enough diaspores (survival organs), the aquatic macrophytes will appear the next season. When the production of diaspores diminishes, fewer plants will start to grow the following year. The few aquatic macrophytes (which are then strongly overgrown with epiphytic algae) no longer produce suppressants to inhibit phytoplanktonic growth. More phytoplankton will be produced at the end of Phase II consequently the light does not reach the young plants. A catastrophic decrease of submersed aquatic macrophytes will occur, while the phytoplankton predominates (Fig. 6 & 7). Some macrophytes, especially species which will rapidly grow to the water surface, like P. pectinatus endure this situation for a relatively long time. However, sometimes within one season the whole aquatic vegetation disappears (Phase III in Fig. 6). et al. (1985a)
Summary:
(i) At moderate nutrient levels the submersed aquatic macrophytes can compete successfully for inorganic carbon and light with phytoplankton and epiphytes. The water is clear.
(ii) The grazing of epiphytes by macro fauna, the grazing of phytoplankton by zooplankton and the excretion of inhibiting or attracting compounds by aquatic macrophytes are of importance for the survival of the aquatic vegetation.
(iii) Because of (ii) the predation of fish on zooplankton and macro-fauna, and subsequently also the predation of piscivorous fish on small fish, indirectly affects the growth of the aquatic vegetation.
(iv) At higher nutrient levels the epiphytes increase. Incomplete consumption of the extra growth causes hampered growth of the submersed macrophytes and insufficient diaspore production. The survival of the macrophyte in the next season will become uncertain.
(v) At about the same concentration level of nutrients. the macrophytes disappear. while the phytoplankton start to predominate. The light inhibition caused by the phytoplankton and probably the excretion of inhibiting compounds by blue-green algae. prevent the resettlement of submersed plants.
(vi) The decline in aquatic macrophytes is a multicausal process. not only caused by an increased concentration of NO3-N or PO4-P. Therefore growth of macrophytes will be possible at different levels of nutrient concentrations.