by
The Fisheries Department
Nairobi, Kenya
INTRODUCTION
Lake Victoria has an area of 68 000 km2 and is shared by the three riparian states: Kenya, Uganda and Tanzania. The Kenya portion of the lake is the smallest and covers an area of about 6 000 km2 with a shoreline of about 760 km. Almost half of the total number of fishermen in Lake Victoria are found in Kenyan waters. For a number of decades fishing has been restricted within the shallow inshore areas and concentrated on the shallow water species, including the tilapiine ciclids and anadromous species. These species have declined with time, to uneconomic levels to the fishermen. Lates niloticus (Nile perch), which is exotic to Lake Victoria, is the main fish species in which the fishing industry is now based in the Kenyan waters of the lake. It has a high relative abundance in deeper waters and this has forced the fishermen to venture into the offshore areas.
Levels and Composition
The dominant species in fish landings up to 1979 were in order of abundance, Haplochromis spp., and Rastrineobola argentea. Other species observed in significant quantities were the tilapiine species, Bagrus docmac, Clarias mossambicus and Lates niloticus. In 1980 and 1981, Rastrineobola had higher landings than Haplochromis (Table 1), and after 1981 the dominant species were Lates followed by Rastrineobola and the tilapiine species, while the other species contributed only modestly to the total landings. Lates seems to have colonized the Kenyan waters completely from 1981 as evidenced from the total landings.
Bottom trawl surveys conducted in the Nyanza Gulf (Muller and Bend, 1981; Okemwa, 1984; Asila and Ogari, in preparation) have suggested a rapid increase of Lates niloticus and Oreochromis niloticus with an almost similar decrease in the catch rates of Haplochromis spp., Bagrus docmac, Clarias mossambicus and Protopterus aethiopicus (Table 2). In 1986, Haplochromis spp. showed signs of revival inside the shallower parts of the Nyanza Gulf where the density of Lates niloticus was lowest (Table 3). Lates niloticus has been observed at all depths in the Gulf, but the highest densities occur in the deeper parts. Wherever catch rates of Lates niloticus were lowest, the density of Haplochromis spp. was highest and vice versa. In 1979, the lowest density of Haplochromis spp. was observed at depth 0–5 m. In 1982, the lowest density of Haplochromis spp. was observed between 10 and 25 m. In 1986, high density of Haplochromis was observed between 3 and 15 m.
A comparison of catch rates in 1986 with other research results reveal that Lates niloticus had increased from under 2 kg.h-1 in 1969 to over 200 kg.h1 in 1986. The expansion of Lates niloticus is also evident from the total landings, e.g., in 1969, 17 t were landed compared with 50 000 t in 1985. Commercial catches reflect the trends shown by the trawl surveys. Total catches of Haplochromis spp. have been declining together with those of Protopterus aethiopicus, Clarias mossambicus, Bagrus docmac, Synodontis spp., Schilbe mystus, Mormyrus kanume, Labeo victorianus and other tilapiine species (except Oreochromis niloticus).
The catches of Oreochromis niloticus and Rastrineobola argentea on the other hand have been rising. Comparing the catch rates of Lates in 1979 and 1981, the density increased four times (46.3 kg.h-1 to 169 kg.h-1), which corresponds to a similar increase in the total catch of Lates from the landings (4 286 t to 22 834 t). Oreochromis niloticus and Haplochromis spp. were observed inside the Gulf only in the 1986 survey.
Food of other Fish Species in Lake Victoria
Studies which have been undertaken in the lake regarding the food of various fish species indicate that all fish species in the lake are carnivores feeding on other fishes, molluscs, insects and crustacea, except for the tilapiine species, certain haplochromines and Labeo victorianus, which feed on detritus and algae.
Graham, 1929; Corbet, 1961; Greenwood, 1966, reported that Clarias mossambicus, Bagrus docmac and Schilbe mystus were mainly piscivorous (preying more on cichlids) augmenting their diet with crustacea and insects. Other less predatory fish species reported by the same authors were Protopterus aethiopicus, Alestes jacksoni and Barbus altianalis, which preyed mainly on molluscs and insects. Mormyrus kannume, Rastrineobola argentea and Synodontis spp. fed mainly on insects and crustacea. Okedi (1970) confirmed that Mormyrus spp. preyed on aquatic insects, mainly chironomids, chaoborus and Povilla adusta. Balirwa (1979) reported that the food of Barbus spp. in Lake Victoria consisted of insects (mainly odonata, Epheroptera, Chironomids and Chaoborids), molluscs and crustacea (mainly copepods).
Fryer and Iles (1972) noted that the main food of haplochromine species consisted of mulluscs, crustacea, insects and fish species. Gee (1969) and Okedi (1970) reported that in the mid and late 1960s the main prey of Lates in Lake Victoria were the haplochromine species. They also observed that to a lesser extent Lates preyed on mormyrids, characids and cyprinids.
The impact of Lates on the fish ecology of the Kenyan waters of Lake Victoria should thus be viewed in terms of predation on other fish species, competition for food with other fish species and distruptions of the food chain by predation on phytoplankton and detritus feeders leading to non-utilization of these food sources.
Impact of Lates on the other Species
The stomach contents of Lates niloticus from the Kenyan wates of Lake Victoria were analysed to determine the food items for the periods 1979, 1982 and 1986. The results indicate that Lates has shifted from one food item as a major prey to another.
In 1979, haplochromine species were the dominant prey among the fish eaten, followed by Rastrineobola argentea, Barbus altianalis, Lates niloticus juveniles and Clarias spp. (Figure 1). Caridina nilotica also featured prominently in the diet. Odonata was the dominant insect prey (Table 4).
In 1982, the most frequent food item was Caridina nilotica followed by Rastrineobola argentea, Lates niloticus juveniles and odonata. Other fish species appearing in the diet included haplochromine species, Oreochromis niloticus, Alestes spp., Xenoclarias and Synodontis spp.
In 1986, the dominant fish species in the diet was Lates niloticus juveniles followed by Rastrineobola argentea, haplochromine species, Oreochromis niloticus and to a much lesser extent Xenoclarias, Synodontis spp., Alestes spp., Clarias mossambicus and Mastacembelus. The most frequent food item was Caridina nilotica. Other food items noted were odonata, Cladecera, Chironomids and Gastropods.
It is evident that Lates has been shifting from one food item to another over the years under consideration. A major contributing factor to the shift might be the decrease in abundance of the target prey as observed from the decreasing catch rates of haplochromine species from 1979 to 1982. It is evident that Lates has been successively taking other species of fish as part of its food. This means that it has had a direct impact on the other fish species, notably Rastrineobola argentea and the haplochromine complex.
The present abundance of Lates in the lake is also possibly influenced by its greater fecundity (in millions of eggs per female) compared to those of the endemic fish species (in thousands of eggs per female) (Ogar, 1984; Okedi, 1970). This would confer a competitive advantage, whilst food abundance is high.
Socio-economic Aspect of Lates niloticus
The social and economic impact of Lates niloticus can be viewed from the influence it has had over the fishing patterns and its acceptability as a substitute for the hitherto preferred endemic species.
The fishing patterns in the Kenyan waters of Lake Victoria have been dictated to a large extent by the size of the boats and the availability of the target species. The success of Lates in the lake has altered the traditional habits. Fishermen have increased the mesh size of their nets and have moved into the deeper waters to meet the market demand created by the shift of interest from endemic species arising from urban demand and the openings of foreign market for the fish.
Original unfavourable attitudes toward Lates were founded on traditional methods of cooking which were poorly adopted to the preparation of the species. However, with improved methods of cooking, interest has shifted from the other species and Lates has found wider acceptance by local consumers. As a consequence the value of the fish is rising.
REFERENCES
Asila, A. and J. Ogari, Growth parameters and mortality rates of Lates niloticus (Nile perch) estimated from length frequency data in the Nyanza Gulf (Lake Victoria).
Balirwa, J.S., 1979. A contribution to the study of the food of six cyprinid fishes in three areas of the Lake Victoria Basin, East Africa. Hydrobiologia, 66:65–72
Corbet, P.S., 1961. The food of non-cichlid fishes in the Lake Victoria Basin, with remarks on their evolution and adaptation to lazustrine conditions. Proz.Zool.Soc.Lond., 136(1):1–101
Committee for Inland Fisheries of Africa (CIFA), 1982. Report of the First session of the Sub-Committee for the development and management of fisheries of Lake Victoria, Mwanza, Tanzania, 12–14 October 1981. FAO Fish.Rep., (262):73 p. Issued also in French
Fryer, G. and T.D. Iles, 1972. The cichlid fishes of the Great Lakes of Africa. Edinburgh, Oliver and Boyd, 641 p.
Gee, J.M., 1969. A comparison of certain aspects of the biology of Lates niloticus (Linne) in endemic and introduced environments in East Africa. In Man-made lakes: the Accra Symposium, edited by L.E. Obeng. Accra, Ghana Universities Press for Ghana Academy of Sciences, pp. 251–9
Graham, M., 1929. The Victoria Nyanza and its fisheries. A report on the fishery survey of Lake Victoria, 1927–28. London, Crown Agents for the Colonies, 41 p.
Greenwood, P.H., 1966. The fishes of Uganda. Kampala, The Uganda Society, 127 p.
Muller, R.G. and R.S. Benda, 1981. A comparison of bottom trawl stock densities in the inner Kavirondo Gulf of Lake Victoria. J.Fish Biol., 18:399–401
Ogari, J., 1984. The biology of Lates niloticus in the Nyanza Gulf of Lake Victoria (Kenya), with special reference to the food and feeding habits. M.Sc. Thesis, University of Nairobi
Okedi, J., 1970. The food and feeding habits of the small mormyrid fishes of Lake Victoria. Afr.Jr.Trop.Hydrobiol.Fish., 1(1):1–12
Okedi, J., 1971. Further observations on the ecology of the Nile perch (Lates niloticus) in Lake Victoria and Lake Kyoga. Annu.Rep.E.Afr.Freshwat.Fish.Organ., (1970):42–55
Okemwa, E.N., 1984. Potential fishery of Nile perch, Lates niloticus. Linne (Pisces: Centropomidae) in Nyanza Gulf of Lake Victoria, East Africa. Hydrobiologia, 108:121–6
Table 1
Fish landings in the Kenyan waters of Lake Victoria from 1968 to 1985
(in percentages)
| 1968 | 1969 | 1970 | 1971 | 1972 | 1973 | 1974 | 1975 | 1976 | 1977 | 1978 | 1979 | 1980 | 1981 | 1982 | 1983 | 1984 | 1985 | |
| Lates | 0.1 | 0.2 | 0.3 | 0.2 | 0.9 | 0.5 | 0.13 | 0.5 | 1.1 | 4.5 | 14.0 | 16.0 | 59.8 | 54.4 | 67.7 | 57.5 | 56.5 | |
| Haplochromis | 22.8 | 36.8 | 32.7 | 32.0 | 29.0 | 33.2 | 35.0 | 27.9 | 34.0 | 32.4 | 27.8 | 21.6 | 13.5 | 2.4 | 4.2 | 0.8 | 0 | 0 |
| Rastrineobola | 4.5 | 2.9 | 3.2 | 5.1 | 7.8 | 10.5 | 21.8 | 27.4 | 30.3 | 34.7 | 36.5 | 30.5 | 35.1 | 20.0 | 17.1 | 21.3 | 27.1 | 29.2 |
| Tilapiine | 14.8 | 26.6 | 27.5 | 21.1 | 14.8 | 10.1 | 5.6 | 3.9 | 5.4 | 7.4 | 10.9 | 9.0 | 18.6 | 10.2 | 7.3 | 5.5 | 10.4 | 10.7 |
| Clarias | 10.6 | 7.6 | 9.7 | 12.5 | 17.0 | 15.7 | 12.9 | 15.6 | 13.0 | 9.1 | 7.2 | 10.0 | 4.5 | 2.6 | 3.4 | 2.7 | 1.1 | 0.6 |
| Bagrus | 7.0 | 5.5 | 6.7 | 7.1 | 5.4 | 8.6 | 6.4 | 8.4 | 5.5 | 6.0 | 5.9 | 5.8 | 2.4 | 1.1 | 4.2 | 3.1 | 0.1 | 0.1 |
| Protopterus | 17.2 | 9.3 | 11.0 | 12.8 | 12.7 | 13.0 | 8.6 | 1.1 | 5.0 | 4.0 | 2.6 | 1.5 | 1.4 | 0.5 | 0.4 | 0.3 | 0.1 | 0.2 |
| Schilbe | 2.4 | 1.4 | 0.4 | 0.4 | 0.4 | 0.9 | 0.2 | 0.3 | 0.3 | 0.7 | 0.5 | 1.0 | 0.4 | 0.1 | 0.1 | 0 | 0 | 0 |
| Alestes | 2.2 | 0.3 | 0.1 | 0.1 | 0.01 | 0.02 | 0.01 | 0.08 | - | - | - | - | - | 0 | - | 0 | 0 | 0 |
| Barbus | 3.1 | 1.1 | 1.3 | 1.6 | 1.7 | 1.1 | 0.7 | 1.7 | 1.0 | 1.0 | 0.8 | 1.4 | 1.6 | 0.8 | 1.1 | 0.1 | 0.1 | 0.1 |
| Labeo | 3.6 | 2.7 | 1.8 | 1.5 | 2.0 | 0.8 | 0.3 | 0.7 | 0.7 | 0.3 | 0.6 | 1.5 | 1.8 | 0.3 | 1.5 | 0.1 | 0.1 | - |
| Mormyrus | 0.3 | 0.4 | 0.5 | 0.5 | 0.5 | 1.1 | 0.8 | 0.3 | 0.5 | 0.5 | 0.6 | 1.2 | 1.2 | 0.5 | 4.4 | 0.3 | 0.1 | 0 |
| Synodontis | 1.1 | 1.5 | 1.1 | 0.7 | 1.3 | 1.3 | 1.1 | 0.8 | 1.0 | 1.6 | 0.6 | 1.6 | 1.4 | 1.3 | 0.4 | 0.1 | 0.1 | - |
| Small mixed | 0.4 | 3.6 | 5.0 | 5.2 | 7.9 | 3.8 | 1.9 | 3.8 | - | - | - | - | - | - | - | - | 3.3 | 2.6 |
| Total | 16357 | 17442 | 16400 | 14918 | 15989 | 16797 | 17175 | 16581 | 18680 | 19332 | 23856 | 30592 | 26914 | 45667 | 60958 | 77327 | 71854 | 89589 |
Table 2
Catch rate in kg.h-1 of demersal fish species in the Nyanza Gulf
| Species | 1969 | 1979 | 1981 | 1982 | 1986 | Source |
| Lates | 1.12 | 46.3 | 169.0 | 169.0 | 273.0 | |
| Haplochromis | 440.0 | 59.2 | 0 | 0.10 | 8.5 | |
| Mormyrus | 0.34 | 0.01 | X | X | 0.5 | Kudhongonia et al., 1974; |
| Synodontis | 2.31 | 0.16 | 0.20 | 0.01 | 0 | Muller and Benda 1981 |
| Schilbe | 0.54 | 0.01 | X | 0 | 0.03 | |
| Barbus | 0.25 | 0.59 | X | X | 0.03 | Okemwa, 1984 |
| Bagrus | 33.45 | 10.8 | 0.30 | 0.45 | 0.22 | |
| Clarias | 23.23 | 2.5 | 0.10 | 0.48 | 0.14 | |
| Labeo | 0.17 | 0.01 | 0.02 | 0.01 | 0 | |
| O. niloticus | 0.58 | 3.45 | 15.60 | 13.3 | 7.6 |
Source: Fisheries Abstracts 1984, 1985, CIFA Report
Table 3
Catch rates of Lates niloticus and Haplochromis species within the Nyanza Gulf (in kg.h-1)
| Depths (in metres) | |||||
| 3–5 | 5–10 | 10–15 | 15–20 | 20–25 | |
| Lates niloticus | |||||
| 1979 | 79.6 | 92.6 | 49.2 | 28.0 | 30.4 |
| 1982 | 85.4 | 208.0 | 582.0 | 338.0 | 334.0 |
| 1986 | 67.2 | 237.0 | 280.0 | 480.0 | 520.0 |
| Haplochromis | |||||
| 1979 | 30.4 | 69.4 | 131.2 | 180.2 | 191.2 |
| 1982 | 0.18 | 1.00 | 0 | 0 | 0 |
| 1986 | 2.9 | 23.8 | 14.0 | 3.0 | 0.8 |
Table 4
Main food items taken by Lates niloticus 1979, 1982 and 1986 (in percentages)
| 1979 | 1982 | 1986 | |
| Haplochromis | 56.6 | 5.3 | 6.6 |
| Rastrineobola | 4.1 | 12.6 | 9.8 |
| Lates | 0.7 | 12.5 | 19.6 |
| Barbus | 0.8 | 0.06 | 0 |
| Caridina | 4.3 | 64.1 | 22.2 |
| Odonata | 9.3 | 6.9 | 4.8 |
| Chironomids | - | 0.9 | 2.3 |
| Gastropods | - | 3.3 | 0.9 |
| No. fish examined | 2 013 | 1 614 | 1 452 |
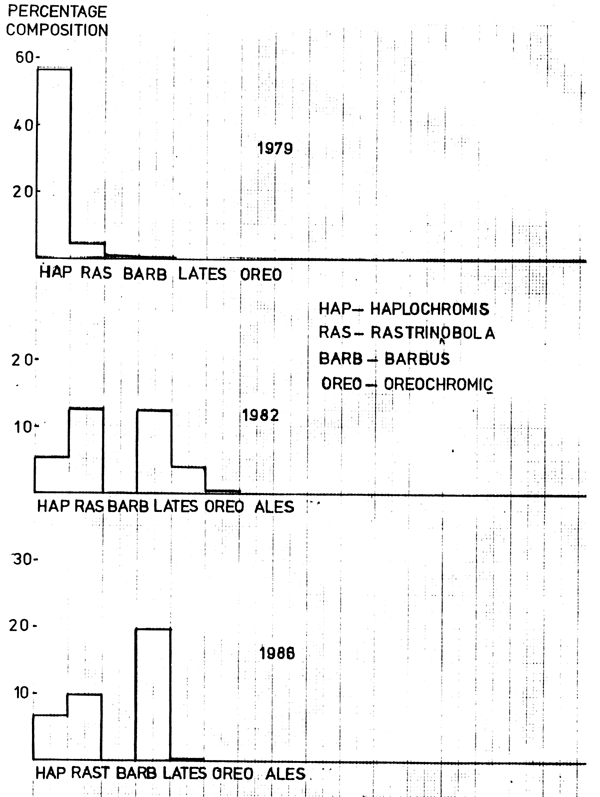
FIG 1 FISH PREY EATEN BY LATES NILOTICUS IN 1979 1982 AND 1986
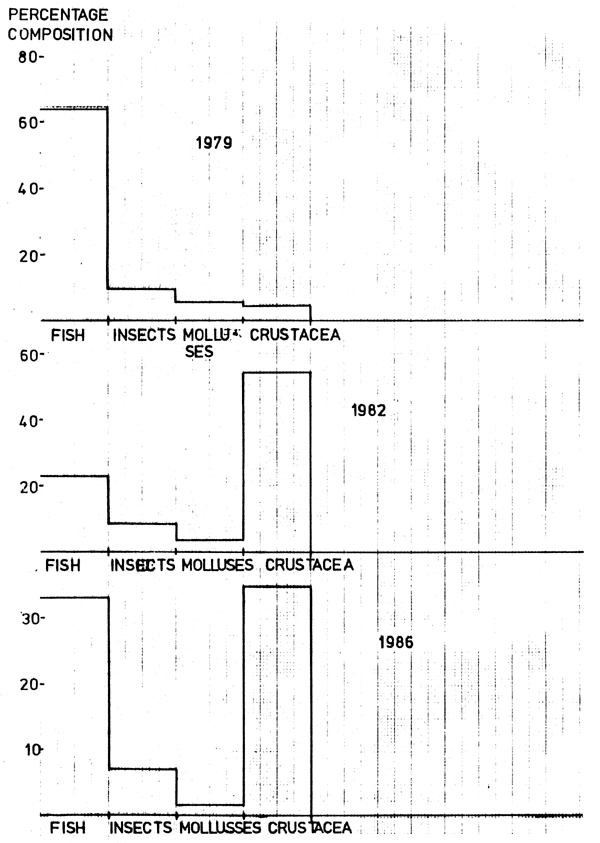
Figure 2 Food items by family groups taken by Lates niloticus in 1979, 1982, 1986
by
Prof. P.O.J. Bwathondi
Tanzania Fisheries Research Institute
Dar-es-Salaam, Tanzania
INTRODUCTION
As early as 1927 it was suggested that large and more palatable piscivores be introduced into the lake to feed on the less valuable bony Haplochromis (Graham, 1929).
FAO/UNDP carried out stock assessments on Lake Victoria fisheries in 1973 and found that Haplochromis constituted about 80 percent of the demersal ichthyomass of the lake. Based on this knowledge, companies were established to exploit the haplochromines by using trawl nets. The haplochromines were to be made into fish meal which would then be used to feed domestic animals and poultry. One such company that was established to make fish meal out of Haplochromis was the Nyanza Fishing and Processing Company (Mwanza).
During the early 1950s and 1960s, Nile perch, Lates spp. was introduced to the lake. The introduction of Nile perch, besides increasing production of fish that could be consumed directly by humans, was also expected to extend the fishing range of the artisanal fishery from nearshore to offshore, open and deeper waters.
The Lake Victoria fishery of about 100 000 t (1985) of the Tanzanian sector constitutes more than 35 percent of the total fish production of Tanzania during the same period. This fishery supports more than 50 000 regular and occasional artisanal fishermen from 302 fishing villages scattered around the lake shore, with a per caput annual income of T Sh 40 000 00. The income accrued from the sale of lake fish is steadily increasing and further gains will most likely be achieved if more gear is made available to the fishermen.
RECENT TRENDS OF LAKE FISHERY
Many indigenous species are faced with a possibility of extinction by the voracious Nile perch. In most parts of the lake, haplochromines, which used to abound in the beach seine catches, have now been replaced by juvenile perch.
Recent surveys carried out in Mwanza indicate that haplochromines and other small fishes are now almost absent from places where previously several kilograms of these fishes could be collected by trawling.
Recent observations indicate that Oreochromis niloticus is increasing. It is suggested that this fish has adjusted to living with the Nile perch in the lake by occupying a different ecological niche.
There has been a dramatic change in the fish species composition of the lake over the past five years (Table 1). Catches of Nile perch increased from 273.8 t (0.4%) in 1981 to 37 608.2 t (38.1%) in 1985. Prior to 1981, the catches of Nile perch were relatively low. Nile perch first established itself in the Mara region then Mwanza and finally the Kagera region with the following percentage compositions in 1984: Mara 55.8%, Mwanza 32.7%, Kagera 51.7%. Catches of tilapiine cichlids were higher in 1981 at 13 260.7 t (18.8%) than in 1984 (8 821.4 t or 9%), but increased again in 1985 (10 650.6 t or 10.8%). This increase is due to greater numbers of Oreochromis niloticus being caught.
Catches of haplochromine fishes have steadily declined during the same period (from 24 593 t or 34.8% in 1981 to 11 572.1 t or 11.7% in 1985). This decline is attributed to heavy predation by Nile perch.
Other species whose catches have declined during the same period are: Labeo, Bagrus, Barbus and Protopterus, whereas Synodontis and Rastrineobola spp. (Dagaa) catches have been increasing. HEST has also observed a dramatic increase in Rastrineobola in the Mwanza area. The greater catches of Rastrineobola are not merely due to an increase in fishing pressure (increase in numbers of fishing gear), but also to an actual increase in the number of fish present. Ssentongo (1985) reported that Rastrineobola produces numerous pelagic eggs and perhaps because of the apparent decrease in number of their competitors (other fish) Rastrineobola are managing to increase in number. Their main predator is the Nile perch.
Available data indicate dramatic changes in the type of fishing gear used in catching fish in the lake over the past five years (Table 2). The use of small mesh size nets has been declining steadily. The mesh size of gillnets used to catch tilapiine was from 3 to 6 inches (76.2 to 152.4 mm) and that for haplochromines was 1.5 to 2.25 inches (38.1 to 57.2 mm). The decline in haplochromine and small tilapiine fishes is evidenced by the decreasing use of small mesh sizes (up to 5.5 inches or 139.7 mm) between 1981 and 1985.
There is an increase in number of large mesh size nets during the same period, 1981–1985 (see Table 2). Furthermore, the use of beach seines and dagaa seines has also increased. Beach seines catch many fishes of all sizes, including Nile perch, whereas dagaa seines catch mainly Rastrineobola.
Comparative changes in fishery statistics around the Tanzanian sector of the lake are shown in Table 3. It is evident from this data that the Mwanza region produces about 55.3% of the total catch followed by Kagera 28.2% and Mara 16.4%. The large catches of the Mwanza region are partly attributed to the trawl fishery in the region where more than 10 boats operate.
DISTRIBUTION AND NATURE OF NILE PERCH IN THE LAKE
The Nile perch (Lates spp.) is the largest predator in Lake Victoria with specimens weighing up to 200 kg being recorded. Available data indicate that Lates spp. has successfully colonized the Tanzanian waters. The Nile perch spread from their points of introduction in the mid-1950s at Jinja mostly eastward in a clockwise direction. They colonized Kenyan waters before moving to Tanzanian waters where they were first reported from Shirati and Musoma around 1977/78.
Lates also made a westward journey to the north of Sesse Islands and Bunjako Bay (Ssentongo 1985). It is argued that its eastward colonization was facilitated by the numerous shallow bays, inlets and gulfs with more oxygenated waters and also providing more ideal shelter than the deeper inlets on the west coast located between Bunjako Bay and Bukoba.
Recent studies carried out by HEST indicate that Nile perch are also found in deeper waters. For example, Goudswaard and Witte (1985) reported high catches of up to 150 kg of Nile perch per hour in waters of 50–60 m depth. It was formerly believed that Nile perch is an inshore shallow water species, but reports from fish catches in the lake have shown that the Nile perch occupies both shallow and deeper waters (Goudswaard and Witte, 1985; Bwathondi, 1985; Ssentongo, 1985). This has lead to the conclusion that there are possibly three species of the perch in the lake, namely Lates niloticus, L. macrophthalmus and L. longispinis. The entire range of fish species in the lake from both shallow and deep waters are thus prone to heavy predation by the Nile perch. The perch is also reported to feed on pelagic species, such as Rastrineobola.
DISCUSSION
There is a lot of controversy over the usefulness of the introduction of Nile perch in Lake Victoria.
To many fishermen, the perch is considered a real menace to their fishing gear, mainly to the nets. To some fish consumers around the lake, the perch is a stange “beast” which is too difficult to cook. Instead traders, fishmongers and businessmen, consider it is a treasure (sometimes called Mkombozi) and money maker. To some scientists, it is considerd an ecological disaster, while to yet another group, the introduction of the perch is considered an experimental success. To the scientists of the region, it is considered a challenge. It is therefore difficult to describe the effect of Nile perch to the lake fishery in simple terms.
Prior to the introduction of Nile perch, the lake fishery consisted of a variety of fishes. Fishermen and fish consumers enjoyed this variety and had a wide choice, but as time passed and Nile perch spread over the lake, the variety of fish decreased so that people have, in some cases, no choice of fish to eat. They either eat Nile perch or go without.
Despite the decline in the variety of fish, there has been an increase in the volume of catches from the lake, which is mainly due to the increase in Nile perch. Some of the companies that were based on the fishery of smaller fish for fish meal (e.g., Nyanza Fishing and Processing Company) found their “target” fish disappearing and had to change to new targets. Goudswaard and Witte (1985) reported that the 35-foot wooden trawler from Nansio (Ukerewe) which exploited the Nafubo area had to change its fishery from haplochromines to Nile perch.
Scientists have always believed that the Nile perch population will sooner or later stabilize itself. Some contend that the Nile perch will soon overeat its food and starve to death, but from available data it is clear that the Nile perch feeds on a variety of species ranging from insect larvae and shrimps to fish including their own species. This feeding habit should enable it to survive for a longer time without a decrease in population.
One other fishery which has a bright future is that for Oreochromis niloticus. This fish is new forming the second largest demersal species, after Nile perch, in the Mara region. It is believed that the species is now coexisting with Nile perch. Such a phenomenon has been reported in Lake Kyoga (Oguta-Ohwayo, 1985). The coexistance of the two species is believed to be attributed to the fact that Oreochromis take refuge in the aquatic macrophytes. Specimens as heavy as 3–5 kg of Oreochromis are abundant in the Musoma fish markets.
There appears to be a threat to the lake fishery from fish kills. Some research work carried out on fish kills in the lake has confirmed that some of these deaths are due to changes in the oxygen concentration in the water (Bwathondi, Chale and Katunzi, 1984). Some deaths are caused by accidental dumping of pesticides in the lake, but there is still a riddle as to the cause of massive fish kills that are now very common in the lake. Some circles believe that the massive fish kills are caused by pesticides used by neighbouring agricultural areas. In the western side of the lake, the pesticides could come from coffee and banana plantations. In the Mwanza and Mara regions the pesticides could come from cotton farms. The amount of pesticides entering the lake is not known. A recent test survey conducted on Nile perch from Mwanza indicate that the flesh of Nile perch does not contain hydrocarbons, thus dismissing the fears that pesticides are entering the lake. The riddle of the source of fish deaths in the lake still remains unsolved.
REFERENCES
Bwathondi, P.O.J., 1985. The future of the fisheries of the Tanzanian part of Lake Victoria, in view of the predominance of Nile perch, Lates niloticus. FAO Fish.Rep., (335):143–5
Bwathondi, P.O.J, F.M.M. Chale and E.F.B. Katunzi, 1984. Mass mortality of fishes in Kagera side of Lake Victoria 20 April–1 May 1984. Report to the Government
Goudswaard, P.C. and F. Witte, 1985. Observations on Nile perch, Lates niloticus (L.), 1758, in Tanzanian waters of Lake Victoria. FAO Fish.Rep., (335):62–7
Graham, M., 1929. The Victoria Nyanza and its fisheries. A report on the fish survey of Lake Victoria 1927–28 and Appendices. London, Crown Agents for the Colonies, 255 p.
Oguta-Ohwayo, R., 1985. The effects of predation by Nile perch, Lates niloticus (Linne) introduced into Lake Kyoga (Uganda) in relation to the fisheries of Lake Kyoga and Lake Victoria. FAO Fish.Rep., (335):18–41
Ssentongo, G.W., 1985. Recent trends in the fisheries of the Tanzanian sector of Lake Victoria. Rome, FAO, 13 p. (mimeo)
Table 1
Species composition of fish catches in Lake Victoria (Tanzanian waters)
| Species | 1981 | 1982 | 1983 | 1984 | 1985 | |||||
| t | (%) | t | (%) | t | (%) | t | (%) | t | (%) | |
| Tilapiine | 13 260.7 | (18.8) | 10 643.2 | (16.6) | 6 751.8 | (9.3) | 8 821.4 | (9.0) | 10 650.6 | (10.8) |
| Haplochromine | 24 593.0 | (34.8) | 24 022.4 | (37.5) | 21 624.1 | (29.8) | 15 122.1 | (15.5) | 11 572.1 | (11.7) |
| Labeo | 1 637.7 | (2.3) | 1 606.4 | (2.5) | 922.5 | (1.3) | 1 135.1 | (1.2) | 544.4 | (0.6) |
| Bagrus | 12 180.3 | (17.2) | 11 700.4 | (18.4) | 11 600.0 | (15.9) | 8 891.6 | (9.1) | 9 214.5 | (9.3) |
| Barbus | 216.0 | (0.3) | 291.0 | (0.5) | 849.1 | (1.2) | 649.3 | (0.6) | 401.7 | (0.4) |
| Mormyrus | 162.5 | (0.2) | 129.7 | (0.2) | 196.5 | (0.3) | 652.6 | (0.6) | 352.8 | (0.4) |
| Clarias | 2 933.8 | (4.2) | 2 719.4 | (4.2) | 2 528.0 | (3.5) | 5 373.7 | (5.5) | 5 560.2 | (5.6) |
| Schilbe | 2 624.2 | (3.7) | 3 113.4 | (4.9) | 2 317.1 | (3.2) | 4 423.2 | (4.5) | 1 576.5 | (1.6) |
| Protopterus | 7 079.9 | (10.0) | 2 948.1 | (4.6) | 3 704.9 | (5.1) | 3 963.3 | (4.1) | 4 050.3 | (4.1) |
| Synodontis | 1 937.8 | (2.7) | 2 522.5 | (3.9) | 2 478.2 | (3.4) | 5 183.5 | (5.3) | 9 277.7 | (9.4) |
| Alestes | 2.0 | (*) | (*) | 34.2 | 386.9 | (0.4) | 216.9 | (0.2) | ||
| Lates | 273.8 | (0.4) | 2 040.4 | (3.2) | 16 425.2 | (22.6) | 41 613.8 | (42.6) | 37 608.2 | (38.1) |
| Rastrineobola and others | 3 718.3 | (5.3) | 2 258.8 | (3.5) | 3 153.1 | (4.3) | 1 573.6 | 1.6) | 7 878.5 | (8.0) |
| Total | 70 620.0 | (100.0) | 63 996.1 | (100.0) | 72 585.7 | (100.0) | 97 790.1 | (100.0) | 98 971.4 | (100.0) |
Table 2
Summary of fishery statistics from Tanzania territorial waters of Lake Victoria for the period 1981–85
| 1981 | 1982 | 1983 | 1984 | 1985 | ||
| No. of fishermen | 19 787 | 18 263 | 15 194 | 17 827 | 17 556 | |
| No. of canoes | 4 199 | 4 245 | 4 141 | 4 650 | 4 160 | |
| Total catch (t) | 70 619 | 63 996 | 72 586 | 99 686 | 98 971 | |
Value of catch (T Sh '000) | 293 200 | 404 060 | 562 157 | 882 410 | 1 231 349 | |
| No. inboard engines | 8 | 6 | 34 | 24 | 21 | |
| No. outboard engines | 194 | 201 | 115 | 108 | 125 | |
| No. beach seines | - | 1 530 | 1 945 | 640 | 1 029 | |
| No. scoops | - | - | - | 833 | 832 | |
| No. dagaa nets | - | - | - | 350 | - | |
| No. hooks | 324 443 | 407 791 | 563 058 | 485 545 | 373 741 | |
| No. trawl nets | - | - | 17 | 17 | 17 | |
| No. gillnets by mesh size | 189 762 | 121 674 | 164 203 | 92 524 | 65 946 | |
| 28.6 mm | - | 48 | 638 | 543 | ||
| 38.1 mm | 3 229 | 3 035 | 1 840 | 226 | ||
| 44.5 mm | 4 928 | 5 083 | 648 | 2 603 | ||
| 47.6 mm | 7 971 | 13 723 | 6 916 | 2 147 | ||
| 50.8 mm | 12 258 | 18 567 | 8 006 | 7 160 | ||
| 63.5 mm | 26 609 | 45 025 | 19 359 | 7 143 | ||
| 76.2 mm | 11 789 | 10 544 | 8 244 | 5 750 | ||
| 88.9 mm | 6 484 | 4 496 | 4 977 | 4 062 | ||
| 95.3 mm | - | - | 92 | 93 | ||
| 101.1 mm | 13 943 | 15 116 | 7 998 | 6 473 | ||
| 114.3 mm | 23 349 | 27 569 | 14 185 | 8 480 | ||
| 127.0 mm | 8 452 | 15 462 | 2 802 | 4 023 | ||
| 139.7 mm | 1 613 | 1 479 | 8 145 | 2 215 | ||
| 152.4 mm | 807 | 1 142 | 563 | 1 919 | ||
| 165.1 mm | 65 | 6 | 613 | 537 | ||
| 177.8 mm | 177 | 1 483 | 6 003 | 4 900 | ||
| 203.2 mm | - | 206 | 829 | 3 298 | ||
| 228.6 mm | - | 899 | 386 | 1 001 | ||
| 254.0 mm | - | 320 | 280 | 531 | ||
| 279.3 mm | - | - | - | 7 | ||
| 304.8 mm | - | - | - | 88 | ||
Table 3
Catch statistics of Nile perch in the three regions of the Tanzanian sector of Lake Victoria
| Year | Mara region | Mwanza region | Kagera region | ||||||
| Total | Lates | % | Total | Lates | % | Total | Lates | % | |
| 1981 | 8 444.0 | 174.0 | 2.0 | 49 108.0 | 3.0 | * | 18 067.0 | 180.0 | 1.0 |
| 1982 | 11 425.2 | 1 215.0 | 10.6 | 39 394.5 | 680.5 | 1.7 | 13 176.9 | 144.9 | 1.1 |
| 1983 | 13 075.0 | 6 306.5 | 48.2 | 35 229.2 | 6 463.0 | 18.3 | 24 281.5 | 3 655.7 | 15.1 |
| 1984 | 15 845.0 | 8 841.3 | 55.8 | 50 281.0 | 16 452.5 | 32.7 | 31 664.1 | 16 320.0 | 51.5 |
| 1985 | 17 540.6 | 9 691.9 | 55.3 | 54 508.2 | 21 925.6 | 40.2 | 26 922.6 | 6 050.7 | 22.5 |
by
T.O. Acere
Uganda Freshwater Fisheries Research Organization
Jinja, Uganda
INTRODUCTION
This study of the fish stocks in the northern portion of Lake Victoria, Uganda wters, presents data on species composition, relative abundance, catch rates and average weight of individual fish for the period 1979 and 1986. The study area included the shallow (0–30 m) inshore waters of the Napoleon Gulf, Thruston Bay, Buvuma Channel, Lingira Bay, Hannington Bay, Itome Bay and waters around the islands of Lufu and Igwe, all in the Jinja area. In 1981 the study area also covered Bomangi Bay (0–30 m) in the Sesse Islands and Kome Channel (0–47 m) south of Entebbe.
The purpose of the study was to monitor stock changes and species dynamics in order to build up a database.
MATERIALS AND METHODS
Trawling was carried out using R/V IBIS as described by Kudhongania and Cordone (1974) and Okaronon, Acere and Ocenodongo (1985). The trawl nets used were mostly of 19 and 20 mm codend meshes respectively, although in 1981 and 1985 trawl nets with 50 and 60 mm mesh codends were used in 48 and 4 tows respectively. The tows were between 30 minutes and 1 hour 30 minutes duration. Trawling in the vicinity of Jinja area was restricted to daylight hours during which a maximum of 5 hauls were made in a day. In Sesse and Entebbe areas trawling was done both at night and daylight hours.
The artisanal catch data for fresh, sundried and smoked fish was obtained from Masese Fish Landing where the Fisheries Department has permanently stationed staff to record landed fish catches. The artisanal fishery operates gillnets, beach seine nets, longlines (hooks), liftnets for light fishing, basket traps and cast nets. The gillnet fishery incorporates the beating of water by fishermen in canoes to drive fish into the nets set in a U-shape. The daily catch landed at Masese is mostly brought by motorized transport canoes from the various fishing grounds around Jinja.
RESULTS
Species Composition and Relative Abundance
Twenty fish species excluding the haplochromine species were noted in the trawl catches from the Jinja area of the lake (Tables 1, 3 and 4). In the Sesse area (Bomangi Bay) Oreochromis leucostictus, Labeo victorianus, Astatoreochromis and Mastacembelus were not encountered.
There has been a sharp decline in the catch rates of the haplochromine species from 523 kg/hour (91.1%) in 1981 to 17.8 kg/hour (6.9%) in 1985, whereas in 1969–71, Kudhongania and Cordone (1974) report, the pooled mean catch for the 0–30 m depth zones was 674 kg/hour. Lates niloticus, on the other hand, increased from 5.3 kg/hour (0.9%) in 1981 to 234.7 kg/hour (90.4%) in 1985. Oreochromis niloticus declined from 12.3 kg/hour (2.1%) in 1981 to 1.9 kg/hour (0.8%) in 1984 and suddenly rose to 5.1 kg/hour (1.9%) in 1985 and to 7.1 kg/hour (8.1%) in early 1986. Table 1 shows the mean catch rates for the various species, the areas having been pooled together.
Mean Sizes
The average weight of individual fish of several species trawled declined while those of others increased (Table 4). The mean size of Nile perch declined from 5.3 kg in 1982 to 1.08 kg in 1985 and 0.66 kg in early 1986. Clarias declined from 5.0 kg to 4.0 kg in 1982 and 1985 respectively. The mean weight of Protopterus increased from 5.3 kg in 1981 to 14.0 kg in 1985. The mean weights of Oreochromis esculentus, O. variabilis and O. leucostictus remained more or less constant. However, Bagrus, Synodontis and O. niloticus scored general increases in mean weights.
The mean weights of individuals of fish species in the artisanal catch landed at Masese are shown in Table 4. The average weight of Clarias mossambicus declined from 5.38 kg to 2.58 kg in 1981 through 1985. The mean weight of Lates also declined from 8.63 kg to 1.57 kg in 1982 through 1985. Oreochromis niloticus, however, steadily increased in mean size from a low average weight of 0.98 kg to 1.42 kg in 1982 through 1985. Similarly, Bagrus docmac increased, although slightly, from 1.7 kg in 1982 to 1.81 kg in 1985. The rest of the commercial fish species maintained more or less constant average weights, as shown in Table 4.
Predation by Lates
During the period under review Nile perch extended its range constituting its major food items from the Haplochromis species, Caridina, Rastrineobola, Xenoclarias, gastropods and bivalves (Acere, 1985) to also include juveniles of its own and those of Oreochromis niloticus. The predominance of the food item is proportional to its abundance in the habitat. Caridina formed a very significant food item in the stomach contents of all sizes of Nile perch caught by trawling.
DISCUSSION AND CONCLUSION
Gnathonamus longibarbis and Alestes spp. continued to be missing from trawl catches since the last report by Okaronon, Acere and Ocenodongo (1985). The absence of records on some species in the artisanal catch landed at Masese may be due to:
retention for consumption by the fishermen of some species of little commercial import, such as Synodontis, Labeo and Schilbe which happen to be delicacies.
The multi-species fisheries, composed of 12 species recorded at Masese, has persisted. However, in the Nyanza Gulf, Mainga (1985) reported that the artisanal catches were dominated (96%) by three species only, viz., Lates niloticus (71.2%), Rastineobola argentea (19.6%), O. niloticus (5.2%) and the rest was made up of the haplochromine species (1.4%), Clarias mossambicus (1.1%) and other fish species (1.0%). The Nyanza Gulf fish stocks have been subjected to uninterrupted and continuously increasing fishing effort.
The apparent recovery of some species in the Uganda waters of Lake Victoria may be due in part to frequent on and off imposition of restrictions limiting fishing activities to day time hours only and, in some very drastic instances, total ban on all fishing operations. These restrictions which have been common in Uganda since the early 1970s have given fish stocks breathing space for recovery from reduced numbers resultant from uncontrolled fishing pressure and unregulated exploitation. It is therefore an inescapable fact that uncontrolled access to the fishery and ever increasing effort lead to biological overfishing. Biological overfishing continues to be demonstrated in the Nyanza Gulf thereby affecting most of the species. This practice has been responsible for the decline of some species and for the virtual disappearance of others such as Labeo victorianus and Oreochromis esculentus. This conclusion is supported by well documented evidence reported by Graham (1929), Beverton (1959), Garrod (1960, 1961, 1961a), Cadwalladr (1965), Jackson (1971), Benda (1979, 1981), and Marten (1979). The historical evidence has been either overlooked or simply dismissed by some eminent scientists spearheaded by Barrel et al. (1985), when they completely attributed the decline of the native fish species of Lake Victoria to predation by the Nile perch. It may be recalled that the prized food fish reported by Barrel et al. (1985) is the exotic Oreochromis niloticus and not the endemic O. esculentus; the latter virtually disappeared long before Lates had established itself and its population had exploded in the 1980s.
The evolution of the Rastrineobola fishery to a dominant role in the Uganda waters of Lake Victoria is a notable development, especially as it contributed an estimate of 1 641.11 t (58.31%). The two leading exotics, Lates niloticus and Oreochromis niloticus provided in equal parts about 34% of the total recorded catch of 2 814.53 t landed at Masese in 1985. There is therefore need for serious investigations on the dynamics of Rastrineobola.
There has been a decrease in pooled mean catch rates for all the species from 573.8 kg/hour in 1981 to 364.3, 358.0, 264.4 and 259.6 kg/hour in 1982 through 1985. This may be attributed to the decline of the haplochromine species from 543.3 kg/hour in 1981 to 223.4, 269.5, 110.6 and 62.8 kg/hour in 1982 through 1985. The decline of the haplochromine species has not been matched by the increase of Lates and O. niloticus in terms of total ichthyomass. The decline of the haplochromines in the study area is partly due to predation by the Nile perch (Acere, 1985; Goudswaard and Witte, 1985; Ogari, 1985; Ogutu-Ohwayo, 1985), and is also partly due to overfishing as trawling effort was concentrated along the same transects. The haplochromine species, being strongly habitat restricted, are incapable of withstanding overexploitation by man and predation by Nile perch concurrently. A similar phenomenon was also observed in the Tanzania waters of Lake Victoria by Witte and Goudswaard (1985).
There were differences in the response of various species of stocks to a particular fishing gear. From the trawl catch data the fish species may be grouped into six classes according to the trends of their relative abundance:
increasing (Lates niloticus).
The artisanal fishery, however, may be considered to be exploiting four different groups of fish stocks on the basis of species catch landed at Masese:
increasing (Oreochromis niloticus, O. variabilis and Rastrineobola).
The decline in mean size of Lates is due, to a large extent, to the increase in the population size of juveniles in the areas under study (pers. observation). This has resulted in the dampening effect on the weight contributed by the few large individuals of the adult Nile perch caught both in the trawl and artisanal fisheries. The decline in mean sizes of various fish species caught in the artisanal fishery may be attributed to the use of beach seine nets and cast nets near the shores. The shores are heavily populated by predominantly younger individuals of the different fish species. There is also the rampant use of small meshed gillnets which are combined with beating of the water to scare fish and other fishing malpractices which hardly give fish any chance of escape.
Nile perch, by turning to cannibalism, is directly curbing its population size. In this way it is likely to attain a state of equilibrium with other species in a manner similar to that obtained in its natural habitats of Lake Chad, Lake Turkana, Lake Albert, River Nile and some rivers in West Africa. In its endemic state Lates is found to coexist with many other fish species without damaging the multi-species fisheries characterizing those habitats. Some of the water bodies, such as Lake Albert and Lake Tanganyika, where Lates is endemic, are among the richest assemblage of ichthyofauna in the world.
ACKNOWLEDGEMENTS
The preparation and presentation of this paper was supported by the International Development Research Centre (IDRC), which is funding the Nile Perch Study Project in Uganda. I am grateful to my colleagues in the UFFRO Lake Victoria Research Team for the collaborate research. I am particularly indebted to Miss Namulemo, Messrs. D.L. Ocenodongo, J, Kamanyi and J.O. Okaronon, with whom I have constantly had some discussions on our research findings. Mention must be made to the staff of the Fisheries Department, Jinja Region, who recorded the artisanal catch data for the fish landed at Masese.
REFERENCES
Acere, T.O., 1985. Some observations on the biology, age, growth, maturity and sexuality of Nile perch, Lates niloticus (Linne), and the growth of its fishery in the northern waters of Lake Victoria. FAO Fish.Rep., (335):42–61
Barrell, C.D.N., et al., 1985. Destruction of fisheries in Africa's lakes. Nature, (315):19–20
Benda, R.S., 1979. Analysis of catch data from 1968 to 1976 from 9 fish landings in the Kenya waters of Lake Victoria. J.Fish Biol., (15):385–7
Benda, R.S., 1981. A comparison of bottom trawl catch rates in the Kenya waters of Lake Victoria. J.Fish Biol., (18):609–913
Beverton, R.J.H., 1959. Report on the state of the Lake Victoria Fisheries. Fish Laboratory, Lowestoft. (Mimeo)
Cadwalladr, D.A., 1965. The decline in the Labeo victorianus BLGR. (Pisces:Cyprinidae) fishery of Lake Victoria and an associated deterioration in some indigenous fishing methods in the Nzoia River, Kenya. E.Afr.Agric.For.J., 30(3):249–56
Garrod, D.J., 1960. The fisheries of Lake Victoria 1954–59. E.Afr.Agric. For.J., 26(1):42–8
Garrod, D.J., 1961. The rational exploitation of Tilapia esculenta stock of the North Buvuma Island area, Lake Victoria. E.Afr.Agric. For.J., 27(2):69–76
Garrod, D.J., 1961a. The history of the fishing industry of Lake Victoria, East Africa, in relation to the expansion of the marketing facilities. E.Afr.Agric.For.J., 27(2):95–9
Goudswaard, P. and F. Witte, 1985. Observations on Nile perch, Lates niloticus (Linne) 1958, in the Tanzania waters of Lake Victoria. FAO Fish.Rep., (335):62–7
Graham, M., 1929. The Victoria Nyanza and its fisheries. A report on the fishing survey of Lake Victoria 1927–28, and Appendixes. London, Crown Agents for the Colonies, 255 p.
Jackson, P.B.N., 1971. The African Great Lakes fisheries: past, present and future. Afr.J.Trop.Hydrobiol.Fish., 1(1):35–49
Kudhongania, A.W. and A.J. Cordone, 1974. Batho-spatial distribution patterns and biomass estimates of the demersal fishes in Lake Victoria. Afr.J.Trop.Hydrobiol.Fish., (3):15–32
Mainga, O.M., 1985. Catch effort assessment survey work in the Kenya waters of Lake Victoria from 1981 to 1982. FAO Fish.Rep., (335):110–6
Marten, G.G., 1979. The impact of fishing on the inshore fishery of Lake Victoria (East Africa). J.Fish.Res.Board Can., 36(8):891–900
Ogari, J., 1985. Distribution, food and feeding habits of Lates niloticus in the Nyanza Gulf of Lake Victoria (Kenya). FAO Fish.Rep., (335):68–80
Ogutu-Ohwayo, R., 1985. The effects of predation by Nile perch, Lates niloticus (Linne) introduced into Lake Kyoga (Uganda) in relation to the fisheries of Lake Kyoga and Lake Victoria. FAO Fish.Rep., (335):18–41
Okaronon, J.O., T.O. Acere and D.L. Ocenodongo, 1985. The current state of the fisheries in the northern portion of Lake Victoria. FAO Fish.Rep., (335):89–98
Witte, F. and P.C. Goudswaard, 1985. Prospects for the haplochromine fishery in Southern Lake Victoria. FAO Fish.Rep., (335):81–8
Table 1
Mean catch rates, kg/hour and percentage for various fish species trawled in Northern Lake Victoria (Uganda) between 1981 and February 1986
| Species | 1981 | 1982 | 1983 | 1984 | 1985 | 1986 | ||||||
| kg | % | kg | % | kg | % | kg | % | kg | % | kg | % | |
| Haplochromis sp. | 391.7 | 88.0 | 295.7 | 79.8 | 264.6 | 76.3 | 113.9 | 46.7 | 17.8 | 6.8 | - | - |
| Lates niloticus | 3.5 | 0.8 | 45.3 | 12.2 | 54.3 | 15.7 | 140.2 | 57.4 | 234.7 | 90.4 | 80.1 | 91.6 |
| Oreochromis niloticus | 12.2 | 2.8 | 7.3 | 2.0 | 5.2 | 1.4 | 1.8 | 0.8 | 5.0 | 2.0 | 7.1 | 8.1 |
| O. esculentus | 0.5 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | - | - | - | - | - | - |
| O. variabilis | 5.6 | 1.2 | 2.5 | 0.7 | 0.9 | 0.3 | - | - | 0.0 | 0.0 | 0.0 | 0.0 |
| O. leucostictus | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | - | - | - | - |
| Tilapia zillii | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | - | - |
| Bagrus docmac | 9.0 | 2.0 | 9.3 | 2.5 | 11.3 | 3.2 | 4.3 | 1.8 | 1.5 | 0.6 | 0.3 | 0.3 |
| Clarias mos sambicus | 14.4 | 3.2 | 8.0 | 2.2 | 4.4 | 1.3 | 1.8 | 0.8 | 0.1 | 0.0 | - | - |
| Protopterus aethiopicus | 4.9 | 1.1 | 0.1 | 0.0 | 2.7 | 0.8 | 1.5 | 0.6 | 0.2 | 0.1 | - | - |
| Barbus altianalis | 0.0 | 0.0 | 1.5 | 0.4 | 2.1 | 0.6 | 0.2 | 0.1 | 0.0 | 0.0 | - | - |
| Mormyrus kanume | 0.1 | 0.0 | 0.0 | 0.0 | 0.6 | 0.2 | 0.3 | 0.1 | 0.0 | 0.0 | - | - |
| Synodontis (2 species) | 2.9 | 0.6 | 0.5 | 0.1 | 0.4 | 0.1 | 0.2 | 0.1 | 0.1 | 0.0 | - | - |
| Rastrineobola argentea | a | a | a | a | a | a | a | a | a | a | a | a |
| Labeo victorianus | - | - | 0.0 | 0.0 | - | - | 0.0 | 0.0 | - | - | - | - |
| Schilbe mystus | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | - | - |
| Xenoclarias | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | - | - |
| Astatoreochromis | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | - | - | - | - |
| Mastacembelus frenatus | - | - | - | - | - | - | - | - | - | - | - | - |
| Total catch/hour | 444.9 | 370.4 | 346.6 | 244.0 | 259.6 | 87.5 | ||||||
| Total time in hours | 266.2 | 230.1 | 278.7 | 110.6 | 62.7 | 15.0 | ||||||
| Total hauls | 199.0 | 202.0 | 270.0 | 110.0 | 67.0 | 15.0 | ||||||
a This species present although not separated from the haplochromines
Table 2
Artisanal fish production in tons and percentage in Northern Lake Victoria landed at Jinja (Masese) between 1979 and 1985. Data for 1979 and 1980 are estimates by the Fisheries Department Uganda
| Species | 1979 | 1980 | 1981 | 1982 | 1983 | 1984 | 1985 | |||||||
| t | % | t | % | t | % | t | % | t | % | t | % | t | % | |
| Haplochromis sp. | 2.1 | 0.6 | 8.2 | 2.8 | 8442.9 | 92.7 | 932.8 | 43.2 | 45.4 | 2.6 | 2.4 | 0.2 | - | - |
| Lates niloticus | 30.6 | 9.3 | 13.8 | 4.8 | 81.3 | 0.9 | 781.1 | 36.2 | 1153.8 | 66.7 | 647.7 | 25.8 | 476.9 | 16.9 |
| Oreochromis niloticus | 76.8 | 24.4 | 114.6 | 39.5 | 286.9 | 3.2 | 207.9 | 9.6 | 253.0 | 14.6 | 289.9 | 23.6 | 478.0 | 17.0 |
| O. esculentus | 3.5 | 1.1 | 4.8 | 1.7 | 1.6 | 0.0 | 1.0 | 0.0 | 1.1 | 0.1 | - | - | - | - |
| O. variabilis | 61.1 | 18.7 | 70.6 | 24.4 | 128.0 | 1.4 | 44.0 | 2.0 | 30.5 | 1.8 | 42.5 | 3.5 | 59.4 | 2.1 |
| O. leucostictus | 3.9 | 1.2 | 5.2 | 1.8 | 6.1 | 0.1 | 2.6 | 0.1 | 2.9 | 0.2 | 3.0 | 0.2 | 3.4 | 0.1 |
| Tilapia zillii | 5.5 | 1.7 | 5.0 | 1.7 | 6.1 | 0.1 | 2.6 | 0.1 | 23.5 | 1.4 | 112.9 | 9.2 | 119.7 | 4.3 |
| Bagrus docmac | 14.2 | 4.3 | 13.8 | 4.8 | 25.1 | 0.3 | 16.8 | 0.8 | 12.6 | 0.7 | 5.2 | 0.4 | 5.4 | 0.2 |
| Clarias mossambicus | 17.8 | 5.4 | 20.2 | 7.0 | 16.9 | 0.2 | 36.7 | 1.7 | 38.3 | 2.2 | 7.9 | 0.6 | 5.8 | 0.2 |
| Protopterus aethiopicus | 13.3 | 4.1 | 33.7 | 11.6 | 56.4 | 0.6 | 50.6 | 2.3 | 95.7 | 5.5 | 27.7 | 2.3 | 18.3 | 0.7 |
| Barbus altianalis | 4.2 | 1.3 | 1.8 | 0.6 | 2.9 | 0.0 | 28.4 | 1.3 | 22.8 | 1.3 | 2.5 | 0.2 | 2.4 | 0.1 |
| Mormyrus kanume | 1.2 | 0.4 | 2.6 | 0.9 | 4.6 | 0.1 | 6.7 | 0.3 | 12.0 | 0.7 | 5.2 | 0.4 | 4.4 | 0.2 |
| Rastrineobola argentea | 93.4 | 28.7 | - | - | 46.2 | 0.5 | 47.2 | 2.2 | 37.3 | 2.2 | 80.0 | 6.5 | 1641.1 | 58.3 |
| Total | 327.6 | 289.8 | 9104.9 | 2158.3 | 1728.8 | 1226.6 | 2814.6 | |||||||
Table 3
Trends in species stock changes in biomass observed in trawl (T) and artisanal (A) catches in the northern part of Lake Victoria from 1979 to 1986
| Species | (a) | (b) | (c) | (d) | (e) | (f) | ||||||
| T | A | T | A | T | A | T | A | T | A | T | A | |
| Haplochromis sp. | X | X | ||||||||||
| Lates niloticus | X | X | X | |||||||||
| Oreochromis niloticus | X | X | ||||||||||
| O. esculentus | X | X | ||||||||||
| O. variabilis | X | X | ||||||||||
| O. leucostictus | X | X | ||||||||||
| Tilapia zillii | X | X | ||||||||||
| Bagrus docmac | X | X | ||||||||||
| Clarias mossambicus | X | X | ||||||||||
| Protopterus aethiopicus | X | X | ||||||||||
| Barbus altianalis | X | X | ||||||||||
| Mormyrus kanume | X | X | ||||||||||
| Synodontis (2 species) | X | |||||||||||
| Rastrineobola argentea | X | |||||||||||
| Labeo victorianus | X | |||||||||||
| Schilbe mystus | X | |||||||||||
| Xenoclarias | X | |||||||||||
| Astatoreochromis | X | |||||||||||
| Mastacembelus frenatus | X | |||||||||||
(a) Decreasing
(b) Decrease followed by stability
(c) Decrease then increasing
(d) Increase then decline
(e) Increase followed by stability
(f) Increasing
Table 4
Average weights (kg) of various fish species caught in trawling operation (T) and artisanal fisheries (A) in the northern portion of Lake Victoria (Uganda) from 1981 to early 1986
| Species | 1981 | 1982 | 1983 | 1984 | 1985 | 1986 | ||||||
| T | A | T | A | T | A | T | A | T | A | T | A | |
| Haplochromis sp. | 0.009 | 0.026 | 0.005 | 0.022 | 0.005 | 0.028 | 0.005 | - | 0.006 | - | - | - |
| Lates niloticus | 5.609 | 4.815 | 5.213 | 8.638 | 4.84 | 5.786 | 2.374 | 4.595 | 1.08 | 1.567 | 0.658 | - |
| Oreochromis niloticus | 0.767 | 1.207 | 0.667 | 0.978 | 0.852 | 1.02 | 0.915 | 1.436 | 1.418 | 1.423 | 1.5 | - |
| O. esculentus | 0.267 | 0.349 | 0.343 | 0.343 | 0.441 | 0.347 | - | - | - | - | - | - |
| O. variabilis | 0.318 | 0.383 | 0.299 | 0.332 | 0.267 | 0.291 | - | 0.263 | 1.18 | 0.352 | 0.18 | - |
| O. leucostictus | 0.225 | 0.328 | 0.077 | 0.337 | 0.1 | 0.604 | - | 1.432 | - | 1.254 | - | - |
| Tilapia zillii | 0.206 | 0.334 | 0.225 | 0.376 | 0.145 | 0.293 | 0.34 | 0.279 | - | 0.298 | - | - |
| Bagrus docmac | 0.592 | 1.069 | 0.902 | 0.696 | 0.973 | 0.726 | 1.001 | 1.376 | 1.016 | 1.807 | 1.0 | - |
| Clarias mossambicus | 3.927 | 5.353 | 4.667 | 5.859 | 4.848 | 4.742 | 4.06 | 3.227 | 4.0 | 2.582 | - | - |
| Protopterus aethiopicus | 4.792 | 9.14 | 5.394 | 7.756 | 8.273 | 8.613 | 2.836 | 7.238 | 14.0 | 6.624 | - | - |
| Barbus altianalis | 4.792 | 2.395 | 0.511 | 3.159 | 0.384 | 3.582 | 0.227 | 1.378 | 2.24 | 0.933 | - | - |
| Mormyrus kanume | 0.259 | 0.262 | 0.24 | 0.284 | 0.688 | 0.301 | 1.179 | 0.397 | 0.55 | 0.5 | - | - |
| Synodontis spp. | 0.146 | 0.278 | 0.18 | 0.085 | 0.137 | |||||||
| Rastrineobola argentea | 0.003 | 0.003 | 0.002 | |||||||||
| Labeo victorianus | 0.105 | 0.05 | 0.08 | 0.12 | ||||||||
| Xenoclarias | 0.022 | 0.025 | 0.51 | |||||||||
| Astatoreochromis sp. | 0.105 | 0.077 | 0.258 | |||||||||
| Mastacembelus frenatus | ||||||||||||
| ITEM | State of Lake Victoria Fisheries |
| 1. | Transmit to the Secretariat a precis of current regulations (Paragraph 9(i) of the report). |
| 2. | Seek assistance in standardization of product development and quality control of products (paragraph 9(ii) of the report). |
| 3. | Undertake research on fish kills (paragraph 18 of the report). |
| 4. | Adopt the Length Frequency Analysis Method in stock assessment and seek funds for training courses on the promotion of this method (paragraph 22 of the report). |
| ITEM | Fishery Statistics of Lake Victoria |
| 1. | Produce a field guide on fish identification and provide training for enumerators (paragraph 40(i) of the report). |
| 2. | Use the newly designated documentation centre at Mombase (paragraph 41 of the report). |
| 3. | Establish a Regional Fisheries Statistic Programme (paragraph 43 of the report). |
| ITEM | Introduction of exotic species |
| 1. | Convene a workshop on introductions and formulate a suitable mechanism for control and regulation (paragraph 47 of the report). |
| ITEM | Cooperation in Management of Lake Fisheries |
| 1. | Establish a Commission for joint management of the lake fisheries (paragraph 49 of the report). |