by
G.W. Ssentongo and R.L. Welcomme
FAO Fisheries Department,
Rome, Italy
| ABSTRACT |
| The Lake Victoria multiple species fisheries have withstood transient perturbations, chronic shifts in environmental variables, increasing fishing intensity and greater interspecific stress due to fish species introductions since the 1950s. There are a number of individual but overlapping species-oriented fisheries. Fishing has been highly selective of small-sized Haplochromis, mormyrids, tilapiine cichlids, Rastrineobola and other cyprinids in the shallow inshore waters within a depth of 25 m. |
| The actual predominance of the exotic Lates and Oreochromis niloticus eduardinus (= Tilapia nilotica) in the catch is an indication of vital changes in the constancy of taxonomic assemblages. We speculate that as the prey consisting of phytoplanktivores, zooplanktivores and benthos feeders continue declining, Nile perch will suffer from inadequate food supplies and subsequently have reduced growth and recruitment. Hence, the long-term economic viability of the Nile perch fishery remains highly questionable; and the premises for a Haplochromis trawl fishery are even more doubtful. But there is still solace in the historical, artisanal and small-scale fisheries if rational management and development could be accepted. |
1. INTRODUCTION
Lake Victoria straddles the equator (1°00'S, 33°00'E). It lies mainly in Tanzania and Uganda, but it also borders on Kenya. The Lake fills a shallow depression in the centre of a great plateau located between eastern and western Rift Valleys. It has a surface area of about 68 800 km2. The shores are variable but typified by indentations to the east; deep inlets to the south; papyrus as well as ambatch swamps to the west; and a flat, indented and forested coast to the north. The Lake Victoria multiple species fisheries have since the 1950s withstood transient perturbations, chronic shifts in environmental variables, increasing fishing intensity and great interspecific stress due to species transplants.
At the beginning of this century, the level of fishing was determined by the subsistance requirements of the people living around the Lake shore. The introduction of flax gillnets during 1916 stimulated higher catch rates around the entire lake. But uncontrolled entry into the fisheries soon resulted in the decline in catch per unit effort (cpue) of accessible “stocks”, particularly the endemic tilapiine ciclids (O. esculentus and O. variabilis). These circumstances prompted Graham (1929) to recommend formation of a unified lake-wide authority responsible for fishery regulation and collection of fish catch statistics, paving the way to a mesh size limit of 127 mm in 1933. As the fishing effort increased during the late 1940s, the cpue of the 127 mm gillnets dropped more and more and the average size of the marketable fish was significantly reduced. Nevertheless, at first fishing remained profitable and the absence of a big price differential between large and small fish encouraged the use of undersized gillnets to maximize catch in numbers, but this did not stay high for long.
In 1947 the Lake Victoria Fisheries Service (LVFS) was established following Graham's recommendation and in the same year the East African Freshwater Fisheries Research Organization (EAFFRO) was formed in order to carry out biological studies on exploited “stocks” around the Lake.
During the 1950s there were several other developments within the fishing industry which were to assume more significance in the subsequent decades. The synthetic-fibre gillnets, characterized by a high initial cost, but with higher catching efficiency and a longer working life than flax gillnets, were introduced in 1952; and outboard engines were made available from 1953 onwards.
Besides, the complex multiple species fisheries were rendered more fragile by the introduction of exotic Tilapiini (O. niloticus, O. leucostictus, T. zillii and T. rendalli) and the predatory Nile perch (Lates niloticus) during the 1950s and early 1960s. There was neither comprehensive legislation to control the sale and distribution of undersized gillnets nor adequate manpower to police the fishing grounds and consequently by the end of 1956 the prohibition of gillnets of 76–127 mm became unenforceable. Hence, the mesh size limit was repealed in Tanzania and Uganda in that year and finally in Kenya in 1961. Beverton (1959) considered the dynamics of the tilapiine cichlids plus other genera of commercial interest and concluded that direct enforcement was impractical. He suggested certain indirect regulatory methods. Garrod (1957, 1959 and 1960) assessed fishing trends on Lake Victoria.
During the 1960s and 1970s the gillnet fishery was dominated by the tilapiine cichlids although some non-cichlid fish such as Bagrus, Clarias, Protopterus and the anadromous fish species group formed other fisheries around the Lake. Fishing had been highly selective as to species because of several factors: types of fishing gear, mesh size, location of fishing grounds, economic factors and the social preferences of the people utilizing the fishery resources. Hence, some target species were reduced to low population levels. However, a number of exploited species have been resilient to increased fishing and none of the accessible stocks has totally collapsed during the past 80 years of increasing exploitation. But now with the increasing dominance of the Nile perch in the lake fauna, there has been a drastic change in the nature of the inshore fish communities and the resilience of constituent “stocks” is apparently being damaged.
2. CURRENT FISHING REGIMES
The fishery resources of Lake Victoria are mainly exploited by traditional and artisanal fishermen. According to available information, about 54 000 fishermen now actively exploit the Lake using about 11 000 boats (4 000 in Kenya, 4 000 in Tanzania and 3 000 in Uganda). The Nyanza Gulf (Kenya) with an area about 6 000 km2 and a shoreline length of about 760 km has the greatest concentration of fishing units (about 25 000 fishermen and 5 canoes/km of shoreline). The Tanzania sector, with an area of about 34 400 km2 and a shoreline length of about 2 900 km, has less fishing intensity (about 8 000 fishermen and with a density of 1 canoe/km of shoreline) whereas the Ugandan sector with an area of about 28 400 km2 and a shoreline length of about 2 500 km is somewhat lightly fished (about 8 000 fishermen and with a density of 1 canoe/km of shoreline). Thus the intensity of exploitation and relative development of the fishing industry and associated activities has not been equitable around the Lake. The most intense fisheries occur in the Kenyan waters, where about 25 000 fishermen, half of the total number on the Lake, operate along 12 percent of the lake shoreline. Here, agricultural land is somewhat scarce and there are fewer alternative sources of employment. The fishing areas and landing sites are owned by villages or cooperatives and in some instances access to the shallow-water resources (littoral fisheries) is denied to newcomers and outsiders, with the consequence that some fishermen have to migrate to other parts of the Lake.
Most of the traditional fishing craft operate in the inshore waters (bays, gulfs and inlets of less than 25 m depth). Hence, there is considerable pressure on the inshore fish “stocks”. Probably as shown by Bergstrand and Cordone (1971) and by Kudhongania and Cordone (1974), there are good possibilities for deepwater fishing for haplochromine cichlids, but the precise magnitudes and resistance to exploitation of the deepwater resources are unclear.
3. VITAL VARIABLES AFFECTING FISH DISTRIBUTION AND CATCHES
3.1 Rainfall and Water Levels
Welcomme (1969) reports that Lake Victoria levels show seasonal oscillation with a maximum in May–June and a minimum in October to November. Long-term fluctuations of water level also are known to occur. Prior to 1927, Lake Victoria had a 10 or 11 year cycle of water level maxima. From 1927 to about 1961 the pattern of fluctuation changed markedly and the water level rose considerably. In 1964 the water level was 1.4 m above previous records. Welcomme (1969) reports that the rise in water level was accompanied by short-term increases in catch per unit effort for endemic Oreochromis esculentus (formerly Tilapia esculenta, Graham).
It has also been noted that fish catches of most species show two peaks a year and that there is good correlation of catch time-series with rainfall and lake levels (Marten and Guluka, 1975). Generally the tilapiine cichlids tend to have the highest catch around October, slightly before the peak of the short rain season when the Lake level is lowest. Catches of Bagrus and Protopterus have peaks coincident with the rains. But the catches of Clarias and Barbus (riverine spawners) appear not to be correlated with the rains.
3.2 Depth of Water
The results of exploratory bottom trawling in Lake Victoria indicated that the depth of water is an important variable affecting spatial distribution and catch rates. The catches of the endemic Oreochromis esculentus and other tilapiine cichlids decline with increasing depth but those of the haplochromine increase with increasing depth, with a maximum occuring at a mean depth of 44.5 m beyond which the catches fall off again (see Table 1). The catch rates of one catfish, Bagrus docmac, follow a similar pattern to those of Haplochromis species. It should be noted that the artisanal fisheries of Lake Victoria are mainly confined to the inshore littoral areas of less than 25 m depth.
3.3 Type of Bottom Deposits
There are spatial and temporal differences in the distribution of trophic groups, particularly among the cichlid species groups. As for the haplochromine species, algal scrapers frequent shallow littoral habitats; detritus-phytoplanktivores and zooplanktivores prefer mud bottoms; whereas the oral shelling molluscivores are associated with sandy habitats (Greenwood, 1974; Witte 1981 and 1983).
The tilapiine cichlids of Lake Victoria occupy contiguous and sometimes overlapping niches. The introduced T. zillii is confined to shallow inshore waters of sheltered bays. Oreochromis leucostictus prefers mud bottoms and swamp fringed shores. The endemic O. variabilis frequents shallow exposed shores with harder bottoms, whereas O. esculentus occurs in shallow bays and inlets with soft silty mud.
In view of these factors, rational exploitation of the fishery resources should be based on proper interpretation of species distributions, ecological habitats and environmental changes.
4. CHANGES IN ECOLOGICAL FEATURES, TOTAL CATCH AND SPECIES COMPOSITION
4.1 Distinctive Ecological Features of “r” and “K” Selection in Lake Victoria
In Lake Victoria, there are multiple species populations of which most constituent species are small and occur in relatively shallow littoral areas. The available “stocks” often comprise assemblages of species with common evolutionary histories and dependences. Hence, the removal of some species by fishing and the introduction of a major predator interfere with the historically established food web systems. MacArthur and Wilson (1967) as well as Pianka (1970) recognize two types of natural selection: (a) “r”-selection (referring to maximal intrinsic rate of natural increase through high reproductive rates), and (b) “K”-selection (denoting greater competitive ability through efficient utilization of environmental resources).
In the case of “r”-selected fish communities, reproduction has the following distinctive features: (a) numerous pelagic eggs, (b) some prolific substrate-spawners, (c) lack of parental care, and (d) protandry; whereas the converse occurs in “K”-selected communities. But in all natural conditions, all species reach a compromise between the “r” and “K” extremes, conforming to what Pianka (1970) refers to as an “r” and “K” continuum and the position of an organism along it.
In the case of “K”-selected fish communities in the littoral and benthic areas of the Great Lakes of Africa, density-dependent effects are dominant. These environments have a diversified assemblage of organisms, e.g., Lake Victoria has more than 170 cichlid species and more than 38 non-cichlid species (Lowe-McConnell, 1975). It is presumed that fish species in Lake Victoria were initially dominated by “r”-selection, but during the course of evolution each niche was filled through selective speciation and species invasions, resulting in a shift from “r”-selection to “K”-selection in the species assemblages.
In the light of the foregoing observations, Lake Victoria ecosystems have been characterized by:
a diverse assemblage of species with more or less equitable distribution;
species schooling more by day, dispersing and feeding more by night;
food web with many trophic levels;
low energy flow per unit biomass (slow turnover);
adaptive feeding types with some specialist species;
more demersal species;
lower and density-dependent natural mortality mostly due to predation.
Nevertheless the indigenous fish species are now increasingly faced with a high cost of maintenance and survival because of the boom of the introduced Nile perch.
4.2 Changes in Catch and Species Composition
There have been significant changes in the fisheries of the Lake since the introduction of synthetic-fibre gillnets and outboard engines in the early 1950s. The total catch of the artisanal fisheries rose steadily from about 59 100 t in 1961 to about 116 000 t in 1968. The total catch dropped to a low level of 80 000 t in 1976, a decline attributed to the disruption of the fishing industry in the Ugandan sector because of civil disturbances and associated economic constraints during the early and mid-1970s. However, the decline in fishing activities and total catch in the Ugandan waters has been recently compensated by increased fishing and high catches of the introduced Nile perch in the Nyanza Gulf (Kenya). At present the total annual catch stands at more than 105 000 t. But considering the delicate nature of exploited predator/prey systems it is difficult to say for how long the catch will stay high. It is likely that the catch will decline to a new “stable” level.
Available catch figures indicate striking differences between fishing trends in Kenyan and Tanzanian sectors of the Lake. Generally, the Tanzanian fisheries have been fairly steady and probably reflect the “stable state”, following on the earlier decline of the endemic tilapiine dominant fisheries. A similar situation would probably have been achieved in Kenyan waters had Nile perch not been introduced. The haplochromini have been a dominant element in the Tanzanian catch, contributing between 36 and 56% of the total annual catch for the period 1976–82 (Figure 1). The total catches of the tilapiine cichlids, Clarias and Bagrus have also remained stable with little fluctuation over the years.
But dramatic changes in catch composition occurred in the Kenyan waters in the late 1970s. In 1976 the dominant species in the Kenyan catch were: Haplochromis, Rastrineobola (Engraulicypris), Tilapiini, Clarias, Bagrus and Protopterus; their combined annual catch was put at about 17 500 t (about 94% of the total). The catch of the same species groups was about 22 300 t in 1982 but this magnitude was just 37% of the total catch for Kenya waters due to a rapid expansion of the Nile perch fishery.
In 1978, the introduced predatory Nile perch first appeared significantly in the catch, contributing about 5% of a total of about 23 900 t. By 1981 the annual catch of Lates had risen to about 22 800 t (60% of the total catch). The increase in catch of Nile perch has been somewhat proportional to the decline in catch of haplochromines (Figure 1). The catch of Rastrineobola has remained steady mainly because it was not directly affected by the switch from the use of small-sized gillnets to large meshed gillnets for capturing Lates. There has been a significant increase in the population of tilapiine cichlids possibly resulting from the change to the use of large meshed gillnets for the Nile perch fishery. The catch of the Tilapiini rose from about 1 000 t (about 6% of the total) in 1976 to about 5 000 t (19% of the total catch) in 1980 (see Figure 1). But the catches of Bagrus, Protopterus and Clarias have steadily declined in the Nyanza Gulf (Kenya).
4.3 The New Fishery for the Introduced Nile Perch
The predatory Nile perch (Lates niloticus) is widespread in Africa occurring naturally in the Nile, Chad, Niger, Volta and Senegal systems and possibly characterized by localized sub-species. Prior to the mid-1950s it was missing from the Victoria Nile above Murchison Falls (Kabalega Falls). It was introduced in Lakes Victoria/Kioga (= Kyoga) basin in the mid-1950s but its presence in Lake Victoria was first noted in 1960. This voracious predator now contributes about 57 000 t a year (about 50%) to the total annual catch of Lake Victoria. Lates niloticus grows to a large size. Individuals over 120 kg have been recorded in Lake Albert (Uganda) (Kinloch, 1956), and in Lake Chad a specimen over 104 kg has been reported (Durand and Louben, 1969). Recently, in Lake Victoria, several individuals weighing over 180 kg have been recorded in the Kenyan, Tanzanian and Ugandan sectors of the Lake.
Gee (1965) gives information on the habitat preferences of the Nile perch in Lake Victoria: shallow waters of less than 25 m depth. The Nile perch spread from their points of introduction in the mid-1950s at Jinja and Entebbe, mostly eastward and in clockwise direction as far as the Nyanza Gulf. In 1978 the species was recorded in Mara Bay around Musoma, from whence it reached Mwanza and Emin Pasha Gulfs. But Lates also spread a little westward to the north of the Sesse Islands and Bunjako Bay. Preferential colonization of the east coast could be attributed to the numerous shallow bays, inlets and gulfs providing more ideal shelter than the deeper inlets on the west coast. The catch trends of Lates in the Kenya and Tanzania waters are shown in Figure 1.
4.4 The Impact of Lates on Historical Fisheries
The relationship between feeding rate and growth has been investigated by several scientists, e.g., Ivlev (1961) and Winberg (1960) using a “predator oriented” approach. But the effect of feeding rate on the natural mortality of prey species has somewhat been overlooked. Yet natural mortality (M) is closely related to the metabolic level and hence to the growth rate (K) which is usually smaller in longlived populations (Taylor, 1960). Predation is an important agent of natural mortality. Higher metabolic activity in tropical ecosystems is likely to speed up the rate of predation and the natural mortality of prey species (Pauly, 1978). Besides, natural mortality is the key variable in estimating sustained yields (Gulland, 1971; Csirke and Caddy, 1983).
Studies on the feeding habits of Nile perch have been made by Hamblyn (1966), Gee (1969), Okedi (1970), Hopson (1972), Lauzanne (1976) and Moreau (1982). There are physical, chemical and biological differences between ecosystems of Lakes Chad, Mobutu (Albert), Turkana (Rudolf), Kioga and Victoria. Hence, the main food items consumed by Lates in these lakes are somewhat different. Studies on stomach contents of Nile perch from its natural habitat suggest that selection of prey by size is determined by the size of predator; and that Nile perch has a preference for prey whose size is less than a third of its own total length (Hamblyn, 1966). In Lake Albert, Lates of less than 15 cm feed mostly on freshwater prawn, Caridina, plus other invertebrates but Nile perch becomes more piscivorous at later stages. Lake Victoria has a substantial quantity of Caridina whose exact biomass is unknown, and the stomach contents of smaller and young Lates include this prawn species as well as Rastrineobola (Pisces, Cyprinidae). Larger individuals tend to be more piscivorous, preying on haplochromine and tilapiine cichlids, Clarias, Mormyrus and other fish species.
The studies on feeding rates in relation to body size and temperature made by Conover (1978) lead to two main conclusions: (a) an increase in water temperature increases the feeding rate; and (b) small fish require a bigger ration under given conditions than large fish. Caddy (1983) has examined series of data from Conover (1978) for a range of bottom dwelling predatory fish and has fitted a multiple regression to a set of data, viz.
ln (rT,W) = A+B ln w+CT (1)
where A, B and C are constants; where rT,W is feeding rate (percent of body weight per day); w is the mean body weight; and T is the mean water temperature.
On the basis of a set of 59 data points for 13 demersal fish species with weight range 20–633 g, under ambient temperatures 8.6–20°C, Caddy (1983) established the following relationship:
In (rT, W) = 1.841 - 0.286 1n (W) + 0.048T (2)
This relationship, although based on a limited set of data and size range, suggests that over the usual commercial size ranges, a limited range of feeding rates from 1 to 5 percent of body weight per day should cover most of the variation for most marine demersal fish and possibly a number of freshwater species.
The mean weight (W) of Lates caught in large-sized gillnets (mesh > 153 mm) is about 10 kg whereas the mean ambient temperature of the Lake is about 25°C. In these circumstances, it can be shown from equation (2) that the feeding rate (rT,W) expressed as a percentage of body weight per day is about 1.5. We have extrapolated equation (2) for the bigger Lates in Lake Victoria because the feeding rate (expressed as a percentage of body weight per day) does not increase significantly with increase in body weight beyond 1 000 g. Besides, larger predators require smaller rations than small individuals under the same environmental conditions (e.g., temperature) as established by Munro (1967) and Conover (1978).
The present total annual catch (Y) of Nile perch in the Kenyan and Ugandan sectors is about 54 000 t. In the light of current fishing activities, this catch magnitude is considered to be a big fraction of the mean standing biomass (B). There are no exact estimates of mortality rates of Lates in Lake Victoria. But considering the species attains a maximum age (Tmax) of 15 years and maximum length of over 140 cm, the natural mortality (M) should be relatively low. For example, Moreau (1982) estimated M = 0.25. The Nyanza Gulf and the Ugandan sector of the lake extending from Entebbe to Majanji are intensively fished. The actual fishing mortality (F) is greater than M and to our guestimate F = 0.9, so that total mortality (Z) = 1.15.
Gulland (1971) gives an estimator of maximum sustained yield (MSY) of unexploited stocks, viz.
MSY = 0.5 MB∞
where M is natural mortality and B∞ is the virgin biomass.
For fished “stocks” consideration should also be given to the effect of fishing mortality (F) and total mortality (Z). Following the logistic model, it can be shown that in order to obtain the MSY, the biomass of the exploited stock should be half that for the virgin stock, i.e., 0.5 B∞. It is supposed that at MSY, FMSY is roughly equal to M, but it should be noted that this may not be the case for a number of species. Developments of this approximation are also currently used for stocks that are already being exploited to give a rough idea of their potential MSY; thus, MSY = 0.5ZB, where Z = M+F and since Y = FB, this has been rewritten as MSY = .5 (Y+MB) (Cadima, in Troadec, 1977).
If the present total catch in Kenya and Uganda waters is 54 000 t and fishing mortality F = 0.9, it can be estimated that the average standing biomass (B) is 60 000 t. Then we can use the relationship, MSY = 0.5 (Y+MB) and estimate the MSY for the Kenyan and Ugandan sectors of the lake to be 34 500 t. Thus in the heavily fished Nyanza Gulf the present catch of approximately 33 000 t is possibly above MSY.
When Lates has fully established itself in the Tanzanian waters (accounting for half of total lake area), the average biomass for the entire lake would be about 120 000 t and the MSY of Lates for the lake would be about 69 000 t.
The total annual food requirement (X) of the Nile perch can be determined with the estimator given by Caddy (1983), viz.
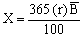
Where r (= 1.5) is the estimated proportion (percentage) of body weight consumed by Nile perch per day, B (120 000 t) is the estimated mean standing biomass of Lates in the Lake. Hence, it can be shown that the estimated population of Lates would consume 657 000 t of food per year.
Moreau (1982) considers the food items of Lates, of length 30–70 cm, in Lake Victoria. The haplochromine cichlids are predominant accounting for 84% of the stomach contents. The “tilapia” and Clarias contribute 8% and 2.8% respectively, whereas Mormyrus and Rastrineobola (Engraulicypris), each provides 0.4% of the diet. Other food organisms provide about 4.2% of the food requirements. In these circumstances, the present stock of Lates niloticus (of 30–70 cm) would require about 551 800 t of Haplochromini, 52 560 t of Tilapiini, 18 400 t of Clarias, 2 600 t of Mormyrus and 2 600 t of Rastrineobola. The other fish species (e.g., Protopterus, Bagrus, Synodontis, etc.) would possibly provide about 28 960 t of food for Lates. It should be noted that Lates of length less than 30 cm feed mostly on invertebrates, particularly the prawn (Caridina).
Kudhongania and Cordone (1974) give estimates of standing mean biomass (B) for various species in Lake Victoria and these estimates are shown in Figure 2. But no biomass estimates were made for Tilapiini and Protopterus in the lake sector within 5 m depth. Considering the available catch and effort data, the MSY of the Tilapiini in Lake Victoria is about 23 000 t. Considering M = 0.2, FMSY = 0.6, the mean standing biomass (B) of the “tilapias” would be about 71 900 t.
It is evident from Figure 2 that the introduced Nile perch has a tremendous impact on the fisheries of the indigenous species. But there are also pelagic resources on which Nile perch, particularly the small-sized ones, feed. Okedi (1982) gives a biomass of Rastrineobola as 273 kg/ha/year for the Tanzania waters. Assuming eguitable distribution of this species in various habitats of the Tanzanian sector, the standing biomass of Rastrineobola in Tanzania is possibly 93 700 t. Extrapolating for the entire Lake, the standing biomass of Rastrineobola would be about 187 400 t. It is noted that in the situation of declining prey species, Lates could temporarily persist by increasing its rate of cannibalism (Moreau, 1982).
There is a difference in the types of food item consumed by Nile perch in Lakes Kioga and Victoria (Moreau, 1982). In Lake Kioga where prey species are declining, 58% of the Lates' diet is supplied by the tilapiine cichlids; Rastrineobola accounts for 23% of the food requirements; whereas 8% of food energy is provided through cannibalism. There is enough evidence in the Nyanza Gulf where the haplochromine cichlids have almost completely disappeared, to show that with the decline of Tilapiini, Haplochromini and other fish stocks Lates has switched to feeding more on Rastrineobola and the invertebrates, particularly Caridina whose biomass and dynamics in the Lake are completely unknown.
4.5 The Developing Pelagic Fishery for Rastrineobola
There is a new fishery for Rastrineobola argenteus, a small-sized species with maximum length (L∞) 5–8 cm which inhabits open inshore and offshore waters and feeds mainly on zooplankton. It has been observed that within the Nyanza Gulf Rastrineobola also feeds on invertebrates (e.g., Ephemeroptera) and diatoms. The species shoals; it is strongly attracted by light and its fishery is partly dependent on lunar cycles (Okedi, 1981). For the monthly cycle, fishing begins during the last quarter of the lunar phase, continues through the new moon phase up to the first quarter of the lunar phase. The duration of this fishing cycle is 14–18 days (Okedi, 1981). The ideal annual fishing season or seasons correspond to the dry season when the use of kerosene pressure lamps is practical. The Rastrineobola fishery, which initiated with beach seines in Kenyan and Tanzanian waters, has expanded to include ringnets and scoopnets, thus becoming a genuine pelagic fishery. Information on the biology of Rastrineobola is still scanty. The nature of the pelagic community is in need of more investigation not only to determine its biomass and yield potential but also to describe its interaction with other fish groups feeding on zooplankton, for instance, a suspected pelagic Haplochromis species group. Additionally, it is necessary to define the effect of predation and fishing on the available stocks (see Figure 2).
4.6 Traditional Fisheries in the Littoral Areas
During the period 1940–60, Labeo victorianus, Barbus altianalis and some Mormyrus species were abundant in the shallow inshore waters of the Lake and its affluent rivers. But these anadromous fish species became increasingly vulnerable to small mesh-sized gillnets and basket traps set in rivers and across river mouths. Cadwalladr (1965 and 1969) describes the fishing trends of Labeo victorianus as follows: during the period 1935–50, the mean catch per net (in numbers) of Labeo was 10. But after the introduction of synthetic-fibre gillnets in 1952, the cpue dropped progressively falling to a mere 0.5 by 1963. Since the mid-1970s, the fishermen have switched to using larger mesh gillnets for the Nile perch fishery. These gillnets are inefficient for catching smaller fish. Consequently, populations of Barbus altianalis, Labeo victorianus and Mormyrus species are recovering slowly; for their total catch rose from 360 t in 1976 to 1 600 t in 1982 in the Nyanza Gulf. But Schilbe mystus and Alestes jacksonii are still very rare in the catch.
The tilapiine cichlids appear to be recovering around the Lake even in the heavily fished Nyanza Gulf. The catch in the Kenya sector of the Lake rose from 1 000 t in 1976 to 4 500 t in 1982 whereas in the Tanzanian waters the catch increased from 3 600 t in 1976 to 9 900 t in 1982. The catch of tilapiine cichlids in Ugandan waters is over 4 000 t out of which the introduced Oreochromis niloticus eduardianus (formerly T. nilotica) contributes 50%. The slight recovery of the “tilapias” is most probably due to a switch from use of small-meshed gillnets to the use of large-sized gillnets for capturing the bigger Nile perch.
The present catch of Protopterus, Clarias and Bagrus in the Lake is about 19 000 t. But during recent years the catch of the three genera has declined particularly in the Nyanza Gulf. It appears that there is inter-specific competition between these fish groups and the predatory Lates.
The haplochromine cichlids inhabiting the shallow waters include insectivores, detritivores, phytoplanktivores, zooplanktivores, molluscivores and piscivores. This fish species group has been fished over many years using gillnets of 25–63 mm. The Haplochromini have declined in the Kenya waters but still persist in Tanzanian waters where the Nile perch population is still at a low level. The status of this cichlid species group in the Uganda waters is unknown. But it is certain that haplo-chromini in shallow waters are now confronted with increasing stress beyond their historical experience. Studies in the Mwanza area indicate that most Haplochromini are habitat restricted (Hoogerhoud, Witte and Barel, 1983; van Oijen, 1982; Witte, 1981 and 1983). The detritivores, phytoplanktivores and zooplanktivores are dominant in shallow waters, comprising more than 40% of haplochromine cichlids in the littoral areas (Witte, pers. comm.). This fish group is therefore a good “target” prey for the Nile perch which is somewhat intolerant of moderately deoxygenated waters beyond a depth of 20 m.
4.7 New Fisheries in Shallow and Deeper Waters
The new pelagic fishery for Rastrineobola poses certain conflicts. The use of beach seines is destructive, catching also immature haplo-chromine and tilapiine cichlids, young Lates and at times young anadromous fish species. Fishing with scoop nets and ring nets might be less hazardous. Available data on fishing activities and catches of this cyprinid fish species indicate underexploitation. However, lack of biological and ecological information on the species still constrains rational exploitation and management. The fishery for the deepwater Haplochromini, based on the findings of the UNDP/FAO Lake Victoria Research Project RAF/65/045, which identified about 563 500 t of standing stock of haplochromines, has yet to be developed. Indeed, current findings on the low resistance of haplochromine cichlids to exploitation (Witte, 1981 and 1983) indicate that extreme caution is needed in planning and implementing this type of fishery.
5. STATUS OF PRESENT DAY FISHERIES
The present fishing patterns in Lake Victoria can be divided into a number of individual but overlapping species-oriented fisheries. Experimental bottom trawling between mid-1960s and mid-1970s revealed a substantial quantity of Haplochromini which could form a basis for a trawl fishery. But in 1980 drastic changes in catches and stocks particularly in the Nyanza Gulf became apparent. These changes are attributed to several factors: (a) introduction of exotic tilapiine cichlids since the 1950s; (b) introduction of the voracious Nile perch; and (c) an intensive artisanal fishery in the inshore water of less than 25 m depth around the Lake.
The multiple-gear fisheries of Lake Victoria have been highly selective of small-sized Haplochromis species, mormyrids, tilapiine cichlids, Rastrineobola and other cyprinids in the shallow inshore waters. These species with a high fishing mortality easily loose their resilience and so their biomass declines rapidly. This phenomenon is already evident in the Nyanza Gulf (Kenya) and also in Mori and Mara Bays (Tanzania).
If the foregoing arguments are accepted, then it follows that Nile perch in the long-run will suffer from reduced food supplies and subsequently reduced growth and recruitment. Lates appears to be a “high level” predator which elects or chooses to consume the most abundant prey (Haplochromis, Rastrineobola and Caridina). This feeding strategy tends to reduce chance variability in prey populations. Therefore, an intensive selective fishery for Nile perch will result in temporary high yields. But this fishery will possibly cause long-lasting instability in species assemblages.
6. CONCLUSIONS
The structurally complex ecosystem of Lake Victoria has been one of a long history of coevolution of its constituent indigenous fish groups since the Pleistocene and this has endowed the ecosystem with long-term stability. The current predominance of the exotic Lates and Oreochromis niloticus eduardianus in the catch is an indication of vital changes in the previous constancy of the fish fauna. Obviously the close links between genetic and ecological attributes of Lake Victoria fish communities have been disrupted (see Figures 1 and 2). Increased “stress” from predation and the various intensive fisheries are rendering the once stable ecosystem more and more fragile. It appears doubtful that the population of Lates has attained some form of stability just in a matter of 20 years. Therefore, we speculate that as the prey consisting of phytoplanktivores, zooplanktivores and benthos feeders continue to decline and lose ecological stability, the Nile perch fishery will inevitable decline also, although, an increase in the rate of cannibalism could delay or postpone indefinitely the day of reckoning. Even then, it might take a very long time of ecological manipulation and evolutional adaptation before the Nile perch population and the lake ecosystem attain a new steady state. Hence, the long term economic viability of the Nile perch fishery remains highly questionable; and the premises for a haplochromine trawl fishery are even more doubtful. But there is great solace in rational management and development of the historical, artisanal and small-scale fisheries which have resisted eight decades of increasing exploitation. It might be disastrous for the riparian States to overcapitalize the fishing industry now. Adoption of fishery technology appropriate to the changing stocks could possibly bring more long-lasting economic benefits to the fishing industry.
7. REFERENCES
Bergstrand, E. and A.J. Cordone, 1971. Exploratory bottom trawling in Lake Victoria. Afr.J.Trop.Hydrobiol.Fish., 1(1):13–23
Beverton, R.J.H., 1959. Report on the state of the Lake Victoria fisheries. Lowestoft, England, Ministry of Agriculture Fisheries and Food, Fisheries Laboratory (mimeo)
Caddy, J.F., 1983. Trophic interactions and stock assessment - some ideas and approaches. Paper presented at the International Symposium on the most important upwelling areas off Western Africa (Cape Blanco and Benguela). 21–25 November, 1983. Instituto de Investigaciones Pesqueras de Barcelona (mimeo)
Cadwalladr, D.A., 1965. Notes of the breeding biology and ecology of Labeo victorianus Blgr. (Cyprinidae) of Lake Victoria. Rev.Zool.Bot.Afr., 72:109–34
Cadwalladr, D.A., 1969. A discussion of possible management methods to revive the Labeo victorianus fishery of Lake Victoria, with special reference to the Nzoia River, Kenya. Occas.Pap.Fish.Dep. Uganda, (2):1–4
Conover, R.J., 1978. Transformation of organic matter. In Dynamics of marine ecology; New York, John Wiley and Sons, Vol. 4:221–456
Csirke, J. and J.F. Caddy, 1983. Production modelling using mortality estimates. Can.J.Fish.Aquat.Sci., 40(1):43–51
Durand, J.R. et G. Loubens, Courbes longueur-poids de 46 poissons du bassin tchadien. Fort-Lamy, 1969 ORSTOM, 70 p. (mimeo)
Garrod, D.J., 1957. A review of Lake Victoria fishery service records, 1951–56. Pamph.E.Afr.Freshwat.Fish.Res.Org., (1)
Garrod, D.J., 1959. The growth of Tilapia esculenta Graham in Lake Victoria. Hydrobiologia, 12(4):268–98
Garrod, D.J., 1960. The fisheries of Lake Victoria, 1954–59. E.Afr. Agric.For.J., 26:42–8
Gee, J.M., 1965. Nile perch investigation. Annu.Rep.E.Afr.Freshwat. Fish.Res.Org., (1964):13–7
Gee, J.M., 1969. A comparison of certain aspects of the biology of Lates niloticus (Linne) in some East African lakes. Rev.Zool. Bot.Afr., 80:244–62
Graham, M., 1929. The Victoria Nyanza and its fisheries 1927–28. London, Crown Agents for the Colonies
Greenwood, P.H., 1974. The cichlid fishes of Lake Victoria, East Africa: the geology and evolution of a species flock. Bull.Brit.Mus. (Nat.Hist.) (Zool.)., Suppl. 6:134 p.
Gulland, J.A., 1971. The fish resources of the ocean. West Byfleet, Surrey, Fishing News (Books) Ltd. for FAO, 225p. Rev.ed. of FAO.Fish.Tech.Pap., (97):425 p. (1970)
Hamblyn, E.L., 1966. The food and feeding habits of Nile perch, Lates niloticus (Linné) (Pisces: Centropomidae). Rev.Zool.Bot.Afr., 74(1–2):1–28
Hoogerhoud, R.J.C., F. Witte and C.D.N. Barel. The morpho-ecological differentiation of two closely resembling Haplochromis species (Pisces: Cichlidae) from Lake Victoria. Neth.J.Zool., in press
Hopson, A.J., 1972. A study of the Nile perch (Lates niloticus (L.), Pisces: Centropomidae) in Lake Chad. Overseas Res.Publ., Lond., (19):93 p.
Ivlev, V., 1961. Experimental ecology of the feeding of fishes. (Translated from the Russian). New Haven, Yale University Press, 302 p.
Kinloch, B.G., 1959. Fishing in Uganda. Uganda Wildl.Sport, 1:13–21
Kudhongania, A.W. and A.J. Cordone, 1974. Bathospatial distribution pattern and biomass estimate of the major demersal fishes in Lake Victoria. Afr.J.Trop.Hydrobiol.Fish., 3(1):15–31
Kudhongania, A.W., A.J. Cordone and A.J. Wetherall, 1973. Summary of the “IBIS” bottom trawl survey results for Lake Victoria in general. Annu.Rep.E.Afr.Freshwat.Fish.Res.Org., (1971)
Lauzanne, L., 1976. Régimes alimentaires et relations trophiques des poissons du lac Tchad. Cah.ORSTOM (Sér.Hydrobiol.), 10(4):267–310
Lowe-McConell, R.H., 1975. Fish communities in tropical freshwaters: their distribution, ecology and evolution. London, Longman, 337 p.
MacArthur, R.H. and E.O. Wilson, 1967. The theory of island biogeography. Princeton, N.J., Princeton University Press, 215 p.
Marten, G.G. and L. Guluka, 1975. Fluctuations in fish catches and prices and their correlations with climatic factors. Annu.Rep.E.Afr. Freshwat.Fish.Res.Org., (1974):69–75
Moreau, J., 1982. Exposé synoptique des données biologiques sur la perche du Nil Lates niloticus (Linnaeus, 1762). FAO Synop.Pêches, (132):44 p.
Munro, J.L., 1967. The food of a community of East African fish. J.Zool., Lond., 151(1966):389–415
Oijen, M.J.P. van, 1982. Ecological differentiation among the piscivorous haplochromine cichlids of Lake Victoria (East Africa). Neth.J. Zool., 32:336–63
Okedi, J., 1970. Further observations on the ecology of the Nile perch in Lake Victoria and Lake Kioga. E.Afr.Agric.For.J., 30:42–5
Okedi, J., 1981. The Engraulicypris “Dagaa” fishery of Lake Victoria, 49 p. (mimeo)
Okedi, J., 1982. Standing crop and biomass estimates of the Lake Victoria “Dagaa” (Engraulicypris argenteus Pellegrin), 6 p. (mimeo)
Pauly, D., 1978. A discussion of the potential use in population dynamics of the interrelationships between natural mortality, growth parameters and mean environmental temperature in 122 fish stocks. ICES C.M. 1978/G:21 p. (mimeo)
Pianka, E., 1970. On r- and K- selection. Am.Nat., 104:592–7
Taylor, C.C., 1960. Temperature, growth and mortality - the Pacific cockle. J.Cons.CIEM, 26:117–24
Troadec, J.P., 1977. Semi-quantitative methods of assessment. FAO Fish. Circ., (701):131–42. Issued also in French
Welcomme, R.L., 1969. The effect of rapidly changing water level in Lake Victoria upon the commercial catches of Tilapia (Pisces: Cichlidae). In the Accra Symposium, edited by L. Obeng. Accra, Ghana Universities Press for Ghana Academy of Science, pp. 242–50
Winberg, G.G., 1960. Rate of metabolism and food requirements of fishes, Transl.Ser.Fish.Res.Board Can., (194):1–202
Witte, F., 1981. Initial results of the ecological survey of the haplochromine cichlid fishes from the Mwanza Gulf of Lake Victoria (Tanzania): breeding patterns, trophic and species distributions. Neth.J.Zool., 31:175–202
Witte, F., 1983. Ecological differentiation in Lake Victoria haplo-chromines: a contribution to the comparison of cichlid species flocks. Paper presented at the Species Flock Symposium, Sixty-third Annual Meeting of the American Society of Ichthyologists and Herpetologists (mimeo)
Table 1: An index of spatial distribution of species and catch rates (kg/hr) at various depths based on the results of exploratory bottom trawling in Lake Victoria in the late 1960s (Kundhongania, Cordone and Wetherall, 1973)
| Fish species | Main depth in metres | |||||||
| 6.5 | 14.5 | 24.5 | 34.5 | 44.5 | 54.5 | 64.5 | 74.5 | |
| Haplochromini | 213.3 | 388.8 | 350.7 | 363.2 | 393.2 | 410.7 | 156.5 | 23.1 |
Oreochromis esculentus | 47.2 | 25.4 | 4.6 | 0.5 | 0.1 | 0.0 | 0.0 | 0.0 |
| Other Tilapiini | 13.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Bagrus docmac | 17.6 | 42.6 | 39.1 | 36.5 | 35.4 | 36.2 | 21.1 | 0.2 |
Clarias mossambicus | 25.4 | 36.0 | 31.1 | 20.8 | 15.3 | 16.8 | 15.5 | 7.6 |
Xenoclarias eupogon | 0.0 | 0.2 | 0.2 | 0.3 | 0.5 | 0.8 | 0.5 | 0.4 |
Protopterus aethiopicus | 31.2 | 23.8 | 7.1 | 5.5 | 1.4 | 0.4 | 0.0 | 0.0 |
| Lates niloticus | 21.1 | 0.7 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Synodontis victoriae | 0.4 | 1.7 | 6.6 | 9.0 | 10.8 | 23.8 | 22.0 | 16.1 |
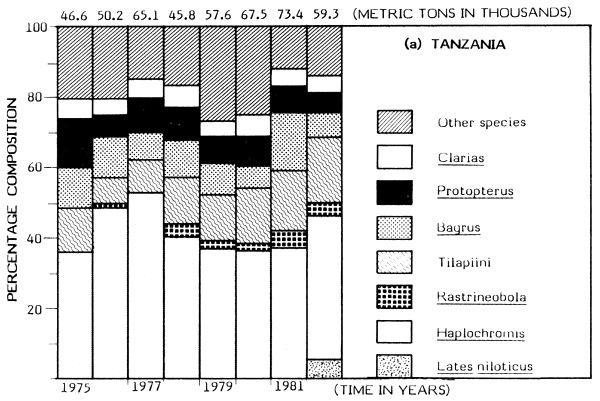
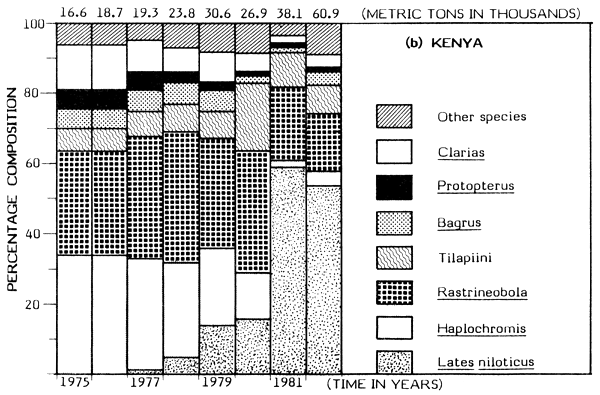
Figure 1 Change in catch and species composition in the Kenya and Tanzania waters of Lake Victoria

Figure 2 A simplified food web including possible biomass transfers in Lake Victoria (East Africa)
by
A.W. Kudhongania and T.K. Twongo
UFFRO, Jinja, Uganda
The significance of Lake Victoria, size apart, can be gauged from its tremendous potential for two renewable resourses: fisheries, for nutritional and economic benefits and water, for domestic, industrial and agricultural applications. The Lake falls under three national jurisdictions, Kenya, Uganda and Tanzania, supports a multispecies fishery and is influenced by urban, industrial and agricultural developments along its shore.
The multispecies stocks of Lake Victoria are separated geographically, by their behaviour, by their extreme trophic diversity, by bathymetric segregation and the different levels of scientific knowledge about each. Due to constant fish movements, including obligate potamodromys and diel vertical migrations within specific taxa, spatial segregations among the stocks become indistinct.
Biological communities are structured along trophic hierarchies which influence every aspect of ecosystem function. Freshwater community structure is a result of biotic interactions such as predation or competition and the abiotic environment (de Bernardi, 1981) which act through reciprocal feed-back mechanisms. Consequently the Lake Victoria community should be considered to be in a period of transition which is being directed, among other things, by four current circumstances.
The influence of different management and development strategies as separately determined by the three riparian states. This influence was increased with the collapse of the East African Community.
Modifications imposed by the changing fishing regimes, including changes in gillnet mesh sizes, uncontrolled use of beach seines, illegal use of the tycoon in certain areas and trawling. These changes arise from the differing needs for effective exploitation of specific categories of the stocks.
The bio-dynamic effects of the continuing disappearance of conventional species (Labeo victorianus, Oreochromis esculentus, etc.) and the introduced Oreochromis niloticus and Lates niloticus. These originate both from a previous lack of effective management policy and the appearance of exotic species into the ecosystem.
Allochthonous impacts derived from developments along the lake shore including sewage and industrial effluents, petroleum oils and their by-products, forest and vegetation clearing and agro-chemicals. This impact is difficult to control because of inadequate knowledge of the qualitative and quantitative requirements for the maintenance of a healthy fish stock.
The cumulative effect of these and similar considerations has not been investigated so that the current status of the exploited fish stocks is not easy to define.
Any fishery is renewable but is also fragile. Fishery resources are liable to irreparable damage through malpractices even though these be confined to limited periods only. If little is known about the resources, little can be done to improve them. At the same time, recolonization by mismanaged exploited populations should be expected to be fortuitous. Yet, while surveying for mechanisms to improve the nutritional and economic potential benefits for current needs, there is the additional need to perpetuate the “renewable” resource for incoming generations, given:
that nutritional deficiencies contribute to deaths, kwashiorkor, marasmus, nutritional blindness, etc.
that fish is a comparatively cheap and good source of protein, vitamins and minerals;
the future dimensions of population levels and food requirements.
the employment opportunites in the fishing industry.
It is our duty to dedicate all our efforts and foresight to the rational management and development of the Lake Victoria fisheries and at the same time ensure the conservation of exploited species. To this end, management should be conceived as an essential and continuing tool based upon the magnitude of the resource, its distribution, recruitment and interrelationship within the community structure, etc. A stockrecruitment relationship is the basis of present management of most stocks of fish (Parrish, 1973). This requires comprehensive data on catch, fishing effort and relevant biological parameters. There are several practical models of fish stocks recruitment, such as the Beverton-Holt (Beverton and Holt, 1957), the Schaefer model (Schaefer, 1968), Ricker (1954) and stochastic-dynamic programming for mixed stocks (Hilborn, 1976), but the choice of application should depend upon the characteristics of a given fishery.
Management measures are either qualitative or quantitative in their effects on the fish stocks, catches and profits. Effective management requires adequate institutional support, which include professional research ability, appropriate laws, timeliness in implementing research and management decisions, and the necessary means of surveillance and enforcement, focused on the entire ecosystem. Consequently, a unified approach on the exploitation, development and management policies on the shared resources of Lake Victoria appears necessary, timely and justifiable for several reasons:
There are no jurisdictional barriers to bio-dynamic events including pollution within the aquatic medium. Modifications occurring in one ragion of the lake eventually spread into other areas.
Changes in the dynamics of the fishery often proceed in association. This ultimately requires a study of the entire system including the reciprocal interactions between spatially segregated biotic and abiotic components of the ecosystem.
Fragmented management, by riparian states, may be detrimental to the whole Lake since differing policies might conflict, reverse, restrain or nullify the objective of such management.
Cooperation in fisheries management provides shared professional skills at reduced costs, minimizes the triplication of research facilities and effort, and encourages technical assistance and training from donor agencies.
The volume of scientific information and intricacies of monitoring needed for efficient management regimes exceed individual national capacities and lower the priority given to fisheries research and management.
Just as there is the justification for regional collective planning, management and development on the Lake Victoria fishery, there is even greater urgency to upgrade collaboration between the scientific and the decision-making machineries for the following reasons:
Fishery research and fishery exploitation (administrative) are interlinked processes in time aimed at quantifying the benefits to be obtained from the fishery. Any discord between the executing agencies weakens their shared strategy. As the measures for successful management should be formulated and implemented with the involvement of the administrators, scientists and fishermen, the fishermen would be mobilized more directly by the administrators.
Targets for fishery development should be formulated on the basis of scientific assessment and adequate examination of ecological implications of the available fishery resources and not vice versa.
Current knowledge of the biology, dynamics and ecological inter-relationships of the stocks comprising the Lake Victoria fisheries is at best fragmentary and inadequate as a basis for the formulation of comprehensive management strategies for the fishery or any of its component stocks. The principal gaps in our knowledge are as follows:
1. Structure and function of the food chains:
Knowledge of the structure and function of the food chain is essential. This requires accurate taxonomy based on the morphology, biology and ecology of the component species, and it also requires an understanding of their role as members of the various biological communites and food chains. Data on basic limnological and biological processes including nutrient dynamics, primary and secondary production, and the basic producer/consumer food chains is highly limited. This knowledge would be useful in assessing fishable potentials, reaction to exploitation, etc.
2. Target stocks
The dynamics of an exploited multispecies fishery do not merely affect the target stocks. There is insufficient knowledge of the interrelationships among the various trophic levels and the resilience of specified “target stocks” to given levels of exploitation within the entire eco-system. As such it may be premature to insist on the exploitation of “target stocks” as a management policy rather than the general objective of improving and perpetuating the viability of the fishery resource as a whole.
3. Exploitation of the haplochromine cichlid stocks
One of the most disturbing phenomena on Lake Victoria is the persistent trend toward the severe depletion of certain fish stocks from the Lake for example, Labeo or Oreochromis esculentus. At the same time, the increasing predation on the preponderant Haplochromini by Lates and the independent findings by HEST (Mwanza) and UFFRO, Jinja, that haplochromine stocks do not endure heavy exploitation, strongly reflect how feeble the base of the current Lake Victoria fishery is. Accordingly the options for the exploitation and utilization of haplochromines need to be re-assessed. A case in point is the exploitation of these species for fishmeal production which is trophically uneconomic and demographically segregative.
4. The impact of introduced species
The introduction of Oreochromis niloticus and Lates niloticus into Lake Victoria has brought dramatic changes in predation, trophic structures, spatial distribution and other bio-dynamic parameters. Such changes may lead to the disastrous situation similar to that of Cichla ocellaris introduced in Gatum lake in the Panama Canal Zone (Zaret and Paine, 1973). It is thus imperative to investigate the ecological implications of these exotic fish species as part of the Lake Victoria fishery.
5. Species diversity and fish density
Bathospatial distribution patterns could result from various factors including speciation, response to biotic and abiotic factors, strategy to minimize predation and competition, and for breeding. To relate these factors to the distribution patterns improves knowledge of species requirements, response to exploitation and acts as a guide for management schemes.
6. Littoral zone, fringing swamps, affluent river systems
This is known to be a highly productive zone which comprises terrestrial and aquatic components of production. Its high nutrient load makes it rich in plankton and invertebrates, and a favoured feeding, breeding and nursery ground. The fringing swamps act as biological filters for silt and reservoirs of nutrients. The zone is, therefore, a major functional constituent of the lake system where very little scientific investigation has been carried out.
7. Pollution
Pollution in Lake Victoria may arise from industrial effluents, vegetation clearance which may lead to increased silting, agro-chemical, petroleum oils, and results of urban development along the lake shore. The direct and indirect influence of pollutants into the lake system have neither been fully identified nor quantified to assess their potential effects on the lake ecosystem.
8. Fisheries statistical data
There is a strong need for a comprehensive fishery statistical data system based on standard procedures for collection and analysis which should be kept at a Regional Fisheries Statistics bank for readiness of interpretation, retrieval, dissemination and re-appraisal.
REFERENCES
Beverton, R.J.H. and S.J. Holt, 1957. On the dynamics of exploited fish populations. Fish.Invest.Minist.Agric.Fish.Food G.B.(2 Sea Fish.) (19):533 p.
de Bernardi, R., 1981. Biotic interactions in freshwaters and effects on communities. Boll.Zool., 48:353–71
Hilborn, R., 1976. Optimal exploitation of multiple stocks by a common fishery: a new methodology. J.Fish.Res.Board Can., 33(1):1–5
Parrish, B.C. (ed.), 1973. Fish stock and recruitment. Rapp.P.V. Réun., CIEM, 164:372 p.
Ricker, W.E., 1954. Stock and recruitment. J.Fish.Res.Board Can., 11(5):559–623
Shaefer, M.B., 1968. Methods of estimating effects of fishing on fish populations. Trans.Am.Fish.Soc., 97:231–41
Zaret, T.M. and R.T. Paine, 1973. Species introduction in a tropical lake. Science, Wash., 182:449–55
by
P.O.J. Bwathondi
Tanzania Fisheries Research Institute
Dar-es-Salaam, Tanzania
INTRODUCTION
Lake Victoria is the biggest lake in Africa and has one of the most important freshwater fisheries on the continent. The three riparian countries, Kenya, Tanzania and Uganda attach great importance to the fishery of the lake which has in the past been based upon several types (species) of fishes, which is a common feature of a tropical fishery.
People around the Lake have in the past enjoyed consuming quite a variety of fishes, including cichlids, lung-fish, etc. Due to food preferences prevailing in the area, fishermen can be grouped by fish consuming habits. For example, one finds lung-fish consuming groups like the Luos (Kenya) and haplochromine consuming groups like the Wakerewe in Tanzania. Such habit groups have occurred in the area for many years now which makes it difficult for such people to change overnight their fish eating habits in response to any change in the fishery.
STATUS OF NILE PERCH IN THE LAKE
The Nile perch, Lates niloticus was first introduced to Lake Kyoga in the 1950s from Lake Albert. Previously Kaba Legga Falls on the River Nile, between the two lakes, prevented the Nile perch and other elements of typical nilotic fish population from naturally reaching Lake Kyoga. This fish was later introduced into Lake Victoria in the 1960s (1960–63) in the Northern waters around Jinja and Entebbe in Uganda, so as to feed on haplo-chromine fishes which were then regarded as trash fish. A second lot of fish (Nile perch) was introduced from Lake Turkana (Greenwood, 1976). The Nile perch which is believed to consist of two groups, the shallow water and deep water species (L. niloticus, L. longispinis and L. macrophthalmus) has spread very rapidly in the Lake. The spread was eastward toward Kenya then southward (from Kenya) to Tanzania and westward to the Bukoba area so that at present the percentage catch composition of this fish is highest in Kenyan and Ugandan waters.
A survey carried out in the Lake in 1983/84 has shown that more than 60% of the catch has been reported as Nile perch in Shirati. This consisted of both juvenile fish and fairly large ones (1–50 cm). In Musoma, the percentage was lower than that of Shirati but higher than that of Mwanza. This indicates the southward migration intensity of this fish.
Lates is now the largest predator in Lake Victoria where specimens of well over 100 kg body weight are fairly common in the trawl and beach seine catches. (A Lates of over 140 kg was caught at Lake Kyoga in Uganda and another specimen of over 200 kg body weight was caught in Musoma waters in Tanzania).
Previous studies of Lates species in the lake and elsewhere showed that this fish feeds on a number of species of fish but prefers haplo-chromine cichlids. Juveniles feed on aquatic insects, benethic invertebrates and fish fry.
The ecosystem of Lake Victoria had most likely reached some level of stability between the indigenous predators and prey before the introduction of Lates to the Lake. Bagrus docmac was the largest and the most important predator in the Lake before the introduction of the Nile perch. Bagrus, though it used to be the largest predator in the lake, was comparatively smaller than Lates (maximum size of Bagrus being about 20 kg body weight). Bagrus is a deep water species and is therefore ecologically separated from the shallow water type of Lates. The presence of these two ecologically separated predators in the Lake makes them fairly effective in preying on available fish species.
Recently outcries from fishermen have been voiced in both private and public on the destruction of this fish in the lake. The most popular fishes like Tilapia and Haplochromis have either been displaced completely or have been reduced to a very low percentage. Traditional fishermen have failed to accept the Nile perch as food which means that the Nile perch caught are usually transported to Dar-es-Salaam and inland towns. Thus the fishermen on the Lake are deprived of fish protein.
ACTION TO BE TAKEN
Suggestions from politicians and fishermen to TAFIRI range from total elimination of the Nile perch to the introduction of a new species which would be palatable as well as competitive to the Nile perch. But for TAFIRI, a different stand should be taken:
Elimination: Although it is premature to dismiss the idea of totally eliminating the fish from the Lake, its rapid spread in the area within a decade indicates that the lake is a favourable habitat for the species. Lates is establishing itself in the lake so fast that any attempt to eliminate it would be futile, or would eliminate other species as well.
Overfishing: TAFIRI believes that the large fishes could be actively over-fished to reduce the predation intensity. This would require large mesh-sized nets. These gillnets should allow smaller fish (including other small fish species) to escape capture.
The study of the Nile perch: Although some knowledge of the ecology, biology and behaviour of the Nile perch is available, much needs to be explored. For example, it appears that there are two main groups, the shallow water and the deeper water groups, with possibly three species. It is not known which species are most destructive and which ones could be accommodated. It is known that the species is a prolific spawner. Unpublished reports give the maximum number of eggs laid by this fish to be over three million.
The fat content: Large specimens have plenty of fat in the body cavity and in the underskin. The local population have used this fat in many ways including crudely processing it to produce cooking fat, or using it as a medicine, whereas some people use it in soap manufacture. However, it is not known how safe the fat is, and in view of the fact that the lake zone is an agricultural area with a lot of pesticides which are eventually washed into the lake.
Marketing: Whereras the inhabitants around the lake dislike the fish, people in large towns have developed a good taste for it. It is therefore important to carry out market survey studies and find out how best it can be utilized.
Other fish species in the lake: The fish group that is receiving much attention in the lake is the Haplochromis being studied by HEST in collaboration with TAFIRI (in Mwanza). There are other studies being carried out on Protopterus, the lung-fish and Oreochromis species. This work involves breeding Oreochromis to a length of about 3–4 cm and restocking them into Lake Victoria. The impact of this work is fairly small because they are not able to restock more than 20 000 specimens per annum which in Lake Victoria is an almost negligible number. It is important, therefore, to study the effect of Nile perch on the other exploited fish stocks and attempt to estimate its exact abundance.
