by
W. Ligtvoet and O.C. Mkumbo
Zoologisch Laboratorium
Postbus 9516
2300 RA Leiden
The Netherlands
SUMMARY
The introduction of the Nile perch (Lates niloticus), a large predatorious fish, into Lake Victoria around 1960 has had a large impact on the existing fish community and consequently on the lake fisheries. At the end of the 1970s, the Lates stock expanded enormously and presently forms the dominant demersal fish stock, supporting the most important commercial fishery. Research directed toward monitoring the developments in this new fish stock and its fishery was urgently needed.
From 1986 up to 1989 research on Lates and its fishery has been carried out by the Haplochromis Ecology Survey Team (HEST) and the Tanzanian Fisheries Research Institute (TAFIRI) in the southern waters of Lake Victoria. The main fishery aspects involved comprised a basic description of the developing fishery (gear types, mesh sizes), collection of CUE data, catch distribution and gear selectivity. As to general ecology and population dynamics: habitat, migration, reproduction cycle and maturation, growth and mortalities, and food studies were incorporated in the programme. Presented are the preliminary results concerning most of the subjects mentioned. In the discussion, based on these results, the present state of the Lates stock is assessed. General guidelines for short-term management are suggested.
1. INTRODUCTION
The Nile perch (Lates niloticus) is a large predatorous fish, introduced into Lake Victoria in the early 1960s. The specimens introduced originated mainly from Lake Albert, Uganda, but a small amount was brought from Lake Turkana, Kenya (Gee, 1964).
Although during the first 20 years afters its introduction the abundance of Lates remained insignificant, at the end of the 1970s, beginning of the 1980s an enormous expansion of the Lates stock was observed (e.g., Arunga, 1981; Okemwa, 1981, 1984; Goudswaard and Ligtvoet, 1988). Lates developed into the most important demersal stock, often encompassing more than 90% of trawl catches by weight. It supports a fast growing fishery, which presently constitutes the most important commercial artisanal fishery.
The need for an increased research effort directed towards monitoring the developments in both the Lates stock and its fishery and towards basic ecological and population dynamical studies on this new fish was evident (CIFA, 1985, 1988). In 1986, within the running research programme of the Haplochromis Ecology Survey Team (HEST) and the Tanzania Fisheries Research Institute (TAFIRI), which at that time was directed towards haplochromines, specific research on Lates was incorporated.
The major aim of the research programme was to provide relevant data for fishery management. As to the fishery, the following aspects were incorporated: a basic description of the fishery (gear types, mesh sizes), collection of actual CUE data and catch distribution and selectivity research of main gears. As to ecology and population dynamics, the most relevant parameters for fishery management were studied, involving mainly growth and the reproduction cycle. In addition, to get insight in the role of Lates in the developing ecosystem, food studies were also carried out.
The research programme on Lates came to an end in March 1989. To date, all data are not yet fully processed and evaluated, but with the present paper a preliminary synopsis of the results is presented. Materials and methods are not described; where possible will be referred to previous HEST/TAFIRI publications, but otherwise materials and methods will be described extensively in subsequent papers on particular subjects.
After the presentation of the basic information, the present status of the Lates fishery is discussed and general guidelines for short-term management are formulated.
2. ECOLOGY
2.1 Habitat and Distribution
Lates has a lake-wide distribution, occurring in virtually every habitat. In 1988, in the Ugandan area Lates specimens of a wide range of size classes even have been found in swamps, where after heavy rainfall water levels reached a high level (D.L. Ocenodongo, pers. comm.). Research in Kenya on rivers entering Lake Victoria, revealed Lates specimens of various sizes (largest 15 kg) 9 km upstream (P. Ochumba, pers. comm.). In the lake, the species has been found in the deepest waters (60 m) where trawling surveys have been carried out (Figure 1). The highest catch rates in the Tanzanian waters in 1985 were obtained from waters of 16–35 m deep. Generally, Lates is a bottom dwelling species, but recent observations in the Mwanza area indicate that in deeper waters (20–40 m) it also stays and forages pelagically in water layers of 10–20 m below the surface (pers. obs.). The latter phenomenon is related to the fact that Lates has a relatively high oxygen demand. Due to stratification, especially in the rainy seasons when there are less strong winds, the oxygen concentrations are low (often less than 2 ppm) in the lower-most part of the water column (cf. Wanink et al., 1988). In these periods the species has to move to the upper water layers. The movement to higher water layers probably also is related to the presence of the pelagic cyprinid Rastrineobola, one of its main food items (see below), in that area (Wanink, 1988).
The foregoing pattern concerns the distribution of Lates in the inshore, relatively shallow waters of the lake. The presence of the species in the more central part of the lake is not known, as since the breakdown of R/V IBIS, the largest research vessel on the lake, no suitable, large enough trawler is operating on Lake Victoria for research on those areas. In future trawling and acoustic surveys should reveal the status of Lates in that vast area.
About the distribution of juvenile Lates little specific information for Lake Victoria is available. In Lakes Chad, Turkana and Albert, Lates fry is mainly found in shallow, sheltered bays and young fishes up to a length of approximately 20–30 cm live inshore in the vicinity of submerged vegetation (cf. Hamblyn, 1962; Gee, 1966: Hopson, 1972).
2.2 Migration
From its northern points of introduction at Entebbe, Jinja and Kisumu, Lates gradually spread in a mainly eastward and clockwise direction. At the moment no detailed information is available concerning possible lake-wide movement of the species. Preliminary results of the tagging programme in the Mwanza region, revealed movements of specimens of 50 km in one week, of almost 100 km within two months and of 150 km in six months (Figure 2). Although still incidental, these findings may give an indication of the minimum boundaries of a unit of stock. If, as planned, also tagging programmes in Kenya and Uganda will start, without doubt more knowledge about this aspect will be gained.
On a more local scale, based on trawl surveys, in the Mwanza region an irregular movement of the Lates stock, between shallow and deeper waters seems evident (Figure 3).
2.3 Population Dynamics
2.3.1 Reproduction
Maturation
Lates is sexually dimorphic concerning the anal region: males have two openings (anus and urogential opening) just anterior to the anal fin, while females have the genital opening separated from the urinar opening (Planquette, 1975). Maturity stages for Lates were described by Hopson (1972) and are generally followed by other researchers.
In Figure 4A, the maturity ogives for Lates males and females are given, as found in the Mwanza Gulf, Tanzania. Size of first maturity for males is 60 cm and for females 95–100 cm TL. Okedi (1971) gives sizes of first maturity of 30–33 cm (TL) for males and 33–35 cm for females, which seem very low figures compared with the findings in the Mwanza Gulf. Sizes of first maturity given by Acere (1985) resemble our findings, while Asila and Ogari (1987) found males to mature at 75 cm TL in the Nyanza Gulf (Kenya). In Lake Kyoga where Lates has been introduced as well, both males and females seem to mature at smaller sizes than established for Lake Victoria (cf. Ogutu-Ohwayo, 1988). The maturation pattern as presented in Figure 4A appears to be highly comparable with that found in Lake Chad (Hopson, 1972).
Sex ratio
In Figure 4B the sex ratio per length category is depicted. It reveals that in the smaller size classes, males greatly out number the females with sex ratio M:F ranging from almost 10:1 to 7:1, but that in Lates over 80 cm TL the proportion of males rapidly falls (1:1 at 90 cm; 1:10 at 100 cm TL). Specimens beyond 120 cm, generally are found to be females. In Lake Kyoga a similar pattern has been found (Ogutu-Ohwayo, 1988). To data no satisfying explanation for this highly skewed sex ratio has been found. Hopson (1972) assumes that the numerical superiority of large females is the result of high mortality in the males. Congregation of members of one sex in particular (Hopson, 1982) and sex-inversion from males to females as observed in Lates calcarifer in Australia (Moore, 1979) are possible explanations as well.
Fecundity
For Lake Kyoga the relation between size of females and number of eggs has been established by Ogutu-Ohwayo (1988):
F = 4.436 × 10-6 × L 2.92
F is number of eggs in millions; L is SL in cm
This implies that a 100 cm TL female Lates has a relative fecundity of 140 eggs per gram body weight. Per year, depending on the number of batches of eggs per year, a multiple of ca. 1.8 million eggs are shed.
Breeding cycle
In the Mwanza Gulf, ripe and running males, as well as females, were found in every sampled month of the year. Based on seasonal changes in the proportion of ripe and running (stage 5 and 6) males of the total mature male population (stages 4–6), an annual cycle seems evident with the highest number of males in an advanced stage of maturation in November and December (Figure 5). Acere and Pauly (1988) showed that continuous spawning is caused by maturity fluctuations in the smaller ones. Continuous breeding of Lates has also been observed in Lake Albert (Hamblyn, 1962) and Lake Chad (Hopson, 1972).
Currently in Mwanza, research is undertaken to describe the breeding cycle of Lates more quantitatively in terms of seasonal changes in gonad weight, mesenteric fat contents, liver weight and general condition. Results of the first half year, concurring with Acere and Pauly (1988), indicate that in addition to a spawning peak around November also an increased activity in May/June may occur. The former and latter peak coincide with the start and end of the rainy season respectively. Since the patterns of dry and wet seasons do not run concurrently in the northern and southern part of the lake (Beadle, 1981), the breeding cycle of Lates may not be identical over the whole of the lake.
Breeding behaviour
Little specific information is available about the spawning behaviour. As far as known, no concentrations of ripe and running Lates in particular waters are observed. Spawning seems to occur preferably in shallow sheltered areas, as in Lake Chad the small (0.39–0.50 mm) pelagic eggs, buoyed by a large single oil globule, were recorded most frequently and in the highest numbers in the most sheltered areas (Hopson, 1972).
2.3.2 Growth
General
Lates grows to very large sizes. From Lake Victoria specimens measuring up to 190 cm (Acere, 1985) and 200 cm TL (Okemwa, 1984) have been registered. As shown above, females grow to a much larger size than males. The longevity of the species is not exactly known, but Loubens (1974), regularly recording specimens of approximately 10 years old, assumes that Lates in Lake Chad may age up to 20 years.
Length-weight relationship and condition factor
Recent observations on the length-weight relationship are given for Kenya and Tanzania respectively:
W = 0.0000078.TL3.12 (Asila and Ogari, 1987)
W = 0.000006.TL3.17 (Ligtvoet, Chande, and Mosille, 1988)
W in kg; TL in cm. The condition indicated by the Fulton index is:
C = 100.W/L3
Based on the length-weight relationship for Tanzania, an overall condition factor calculated for specimens from 40 to 150 cm TL ranges from C = 1.23 (at 40 cm), via 1.35 (at 75 cm) to 1.52 (at 150 cm). These figures largely resemble the figures as given for other lakes (see summary in Hopson, 1972). Hopson (1972) describes marked seasonal changes in condition of Lates in Lake Chad. To data seasonal fluctuations in the condition factor have been described for the northern part of Lake Victoria (Acere and Pauly, 1988), and soon will be presented for the southern part of the lake (Ligtvoet et al., in prep.)
Growth parameters
Based on length frequency distributions of Lates in trawl catches in the Nyanza Gulf, Asila and Ogari (1987) established the following growth parameters concerning the von Bertalanffy growth curve:
Loo = 205 cm; K = 0.19/year
The longest Lates reported from Lake Victoria measured 200 cm (Okemwa, 1984) which may indicate that the above estimate of Loo is quite adequate.
Based on the length frequency distribution of Lates catches of large beach seine (Figure 6), operating on the southern shore of the Speke Gulf (Tanzania), the growth parameters, using ELEFAN, are estimated at:
Loo = 185 cm; K = 0.17/year
These growth parameters would indicate that Lates in Lake Victoria of 190–200 cm TL may age over 15–20 years.
Direct estimates of the growth parameters may be obtained by tagging experiments. The tagging programme in the Mwanza Gulf has been running for almost two years, but it proves extremely difficult to get reliable TL measures of recaptured specimens. To date, the only reliable information available is on three Lates (Table 1).
Table 1
Growth increment data of tagged Lates in the Mwanza area
| L1 | L2 | Days out | Lm | cm/year |
| 32.0 | 46.8 | 190 | 39.4 | 28.4 |
| 47.4 | 53.0 | 87 | 50.2 | 27.7 |
| 51.2 | 55.9 | 82 | 53.1 | 20.9 |
L1 = TL at time of release
L2 = TL at time of recapture
Lm = mean TL over the period between release and recapture
Based on these three recaptures, with the Gulland-Holt method the following estimates are obtained: Loo = 109.3 cm; K = 0.42. These estimates seem not so realistic, but this is due to the fact that the L data are too close to each other (Figure 7). In such a case, a set value of the asymptotic length (here: 205 cm) may be used to enable an estimation of K (“forced” Gulland and Holt plot; see Pauly, 1980), resulting in an estimate of K = 0.16. When Loo = 185 cm is adopted, the estimate of K results in K = 0.19. Obviously, the K estimates based on the tagging results are of the same order of magnitude as derived from the LF analyses.
As to maturing of the fishes, the above found growth parameters imply that male Lates start to mature at the age of two years, while females start in their third year. For comparison, figures on growth from other lakes are given in Table 2.
Table 2
Von Bertalanffy growth parameters for Lates from various lakes
| Lake | Loo (cm) | K (per year) | t0 (years) | Author | |
| Chad | 93.07 | 0..272 | 0.046 | Hopson (1972) | |
| Chad | M: | 78.10 | 0.281 | -0.510 | Loubens (1974) |
| F: | 95.30 | 0.191 | -0.749 | ||
| Albert | 95.02 | 0.33 | -- | Holden, in Hopson (1972) | |
| Albert | 101.14 | 0.147 | -0.236 | Midgeley (1968) | |
| Turkana | 158.66 | 0.065 | -1.349 | Gee (1965) | |
| Nasser | 101.48 | 0.140 | -0.237 | Latif and Kallaf (1976) | |
Compared with Lates from other areas, Lates from Lake Victoria grows very fast and to large sizes. Lates of 50 cm TL, the size when it recruits to the fishery, grow at least twice as fast as in other lakes (Figure 8).
2.3.3 Mortality
Natural mortality
Natural mortality is difficult to estimate. As a first approximation the empirical formula as given by Pauly (1980) may be used:
log M = 0.1228 - 0.1912.log Loo + 0.7485.logK + 0.2391.logToC
Adopting the estimate of Loo = 185 cm, K = 0.17 and an average water temperature of 23oC (in the lower part of the water column), the natural mortality of Lates in Lake Victoria is calculated at M = 0.27/year. Using the same formula, Asila and Ogari (1987) derive an estimate of M = 0.34. Since this method is a very coarse approximation indeed, an adoption of M = 0.3 as a first estimate seems appropriate.
2.4 Food
Ontogenetic changes
The food of the early stages of juvenile Lates in Lake Victoria has not been described in detail yet, but is currently studied within the HEST/TAFIRI project (Katunzi, in progress). Hopson (1972) gives the following account of diet changes in specimens from 1 to 2.5 cm. The food of the smallest Lates (< 1.5 cm) is essentially planktonic, comprising mainly Cladocera and cyclopoid copepods. The proportion of these planktonic crustacea strongly decreases in fishes between 1.5 and 2.5 cm, to be replaced by small insect larvae.
In Lake Victoria, juvenile Lates up to 4 cm TL have been observed to feed on cyclopoid and calanoid copepods (E.F.B. Katunzi, pers. comm.). In Lates between 1 and 10 cm (SL), small Caridina (3–10 mm) are becoming the most frequent items in the diet, followed by small fish (mainly Rastrineobola, see Figure 9). Insect larvae (Chaoborus and chironomids) were found to constitute a small part of the food (J.H. Wanink, pers. comm.). In specimens between 15 and 40 cm, Caridina and small fish are the dominant food items, with additionally in shallower waters large insect larvae (Odonata, pers. obs.). Between 40 and approximately 70 cm Caridina and small fishes constitute the major part in the diet, and beyond 80 cm primarily fish has been found in the stomachs (cf. Hughes, 1986; Ogari and Dadzie, 1988).
Food composition
From the onset, Lates predominantly preyed upon haplochromines and to a lesser extent upon Mormyridae (Gee, 1964, 1969; Hamblyn, 1966; Okedi, 1971). In 1986, when the haplochromines already had reached a low level of abundance, they still formed the major part of the diet of Lates in the Tanzanian waters (Figure 10). With the decrease of the Haplochromis stock in the lake, the food composition of Lates drastically changed. Currently the benthic shrimp Caridina nilotica, the cyprinid Rastrineobola argentea and young Lates form the main food items (Figure 9) (cf. Ogutu-Ohwayo, 1985; Hughes, 1986; Ogari and Dadzie, 1988). Lates proves to be extremely flexible in its diet: large preys up to 25–30% of TL (cf. Gee, 1969; Ogutu-Ohwayo, 1985) as well as small prey (2 cm Caridina) are readily taken.
The size of the prey which can be taken whole depends on the size of the mouth, volume of the buccal cavity and the volume of the stomach. On average the maximum height of a prey that can be taken by Lates equals about 15% of the predators TL (Figure 11). Of the three main food items, only Lates prey have a size limitation. The maximum height of Lates on average constitutes approximately 30% of TL (Figure 12), which sets the maximum ratio of prey/predator, coinciding with Gee (1969) and Ogutu-Ohwayo (1985) to almost 50%.
Although Oreochromis niloticus often is mentioned as an important prey, especially in shallow waters (Lake Albert: Holden in Hopson, 1972; Lake Chad: Hopson, 1972; Lake Kyoga: Ogutu-Ohwayo, 1985; Lake Victoria: Ogari, 1985), during the studies in the Mwanza Gulf this species only occasionally has been found in the stomachs of large Lates.
Cannibalism
Remarkably is the occurrence of an extensive cannibalism in the Lake Victoria Lates population, which already may act at very young stages: specimens of 15–20 cm TL (and even smaller, viz. 10 cm; J.H. Wanink pers. comm.) have found to eat large amounts of Lates of 2–3 cm (pers. obs.). Also Hopson (1972) noted that cannibalism may be common in very small Lates: in specimens between 4 and 7 cm this accounted for more than half the food intake.
In the Mwanza Gulf, cannibalism seems most prominent in times that the Caridina stock is on low levels (see below), which may indicate that the stock of juvenile Lates serves as a buffer food stock for bigger specimens in times that food shortages arise, due to low abundance of one of the two main other prey stocks. Since juvenile Lates smaller than 4 cm feed mainly on zooplankton and insect larvae (see above), cannibalism enables the species to utilize indirectly also these food sources in the lake which otherwise would not be available for the adult stock.
Seasonal fluctuations
As shown in Figure 10, the diet composition of Lates shows a seasonal fluctuation, possibly reflecting the abundance of the prey stocks, Caridina, Rastrineobola and young Lates.
Diurnal feeding pattern
In Figures 13 and 14, results concerning the diurnal feeding pattern of Lates in the Mwanza Gulf are presented, based on the stomach analysis of specimens in the length range of 40–60 cm TL, which formed the bulk of the sample. The data reveal that there is one main peak in feeding activity between 10.00 and 12.00 a.m. (Figure 13A). During these hours feeding almost exclusively occurs on shrimps since only at that time large quantities of fresh shrimps are found in the stomachs. At night the feeding activity is less intensive (many empty stomachs: Figure 13C) and Lates mainly feed on fish (Rastrineobola and Lates).
During full moon and new moon, the feeding behaviour differs as to the quantities of Rastrineobola and Lates taken (Figures 14A and 14B). There are strong indications that this is due to the vertical distribution of these prey items during these respective nights (cf. Wanink, 1988).
2.5 Interspecific Relations
The interspecific relations concern predator/prey relations with all smaller fishes, like haplochromines, Mormyridae, Alestes, Barbus, Labeo, Synodontis, Schilbe and Rastrineobola, and/or competition with the other large predators in the lake, Clarias and Bagrus.
Almost all of the smaller fish species in Lake Victoria have dropped to very low levels of abundance (e.g., Arunga, 1981; Muller and Benda, 1981; Okemwa, 1981, 1984; Okaronon, Acere, Ocenodongo, 1985; Hughes, 1986; Goudswaard, 1988) with exception of Rastrineobola. Currently, the two most important predator/prey relations in the ecosystem and relevant for the fishery, comprises that of Lates with Rastrineobola and Oreochromis (see also Figure 15). As already mentioned above, Oreochromis mainly is taken by large Lates exceeding 1 m TL. Since the number of specimens of this size is quite low (e.g., Figure 6), the impact of Lates on Oreochromis is expected to be restricted. In spite of the increasing predation pressure from Lates, the Rastrineobola stock seems to increase significantly (Wanink, 1988). As the ecosystem still has not stabilized and the fisheries targetting for these species are still growing, the relationship between Lates and Rastrineobola is still in a stage of development.
Since the haplochromines formed the major food source for Clarias and Bagrus (Corbet, 1969; Chilvers and Gee, 1974; Ogari and Dadzie, 1988), there has been a strong competition with the apparently superior Lates for this resource. With the decrease of the haplochromine stock, the feeding pattern of both catfishes was affected. The catfishes had to take the prey species that were left, and nowadays the major part of the diet is formed by Rastrineobola (pers. obs.). Being exclusively bottom dwellers, both species probably cannot harvest the generally pelagic living Rastrineobola as efficient as Lates, which is more flexible in its feeding behaviour.
2.6 Food Web
The expansion of the introduced predator, with a hunting capacity not experienced before by the existing Lake Victoria fish community, has had a severe impact on the lake ecosystem. Many species declined. Especially the haplochromine stock, which initially formed the main food for Lates, was severely affected. Presently, the latter stock which played an important role in the original ecosystem has been nearly depleted. Consequently the structure of the food web changed (Ligtvoet, 1989; Ligtvoet et al., in prep.)
In the present food web, Lates is the opportunistic top predator feeding on different trophic levels (mainly primary and secondary, occasionally tertiary consumers). The two main flows of energy, formerly transferred via haplochromines, are currently the detritus food chain via the shrimp Caridina and insect larvae culminating in Lates, and the phytoplankton food chain, via zooplankton and Rastrineobola and juvenile Lates culminating in adult Lates (Figure 15).
3. FISHERY
3.1 Stock Size and Recruitment
Standing Stock
As described above, since the end of the 1970s, the Lates stock in Lake Victoria has increased enormously.
In Figure 16 the mean catch rates of Lates during 1987 obtained with R/V KIBOKO, in various areas of the Tanzanian part of Lake Victoria are given. As can be seen, catch rates strongly fluctuate. In all parts, Lates is the dominant demersal stock, comprising on average 95–100% of the toal catch in weight. Only near Ukerewe, occasionally Clarias was found to dominate the catch (P.C. Goudswaard, pers. comm.). As described above, there seems to be a movement of Lates between deeper and shallower waters during the year (Figure 3). Also the tagging results indicate that there is a long-distance movement of Lates (Figure 2), therefore it seems not unreasonable to lump the catch rates over all depth zones.
Although the swept-area method may result in an underestimation of standing stock, it was applied to get a rough idea of the order of magnitude of the stock size. The mean catch rates over 1987, as presented in Figure 16, are lumped to calculate an overall mean catch rate for that year (151 ± 102 kg/h). Adopting a general 3 knots (5.4 km/h) trawling speed and a 6-m width of the net opening (headrope 18 m), without a correction for the catchability, a conservative standing stock estimate is derived of: 46.6 kg/ha (SD 31.5 kg/ha).
It is not exactly known which proportion of the actual stock is taken by R/V KIBOKO. Two factors are of importance. First, the height of the net opening in the water is around 1.5 m, which without doubt does not cover the depth range over which Lates occurs near the bottom. Sampling with gillnets at various depths revealed that a significant part of the Lates population moves at least over the lowest 8 m in the water column (pers. obs.). Secondly, comparison of the trawl catches in certain areas with catches from other fisheries (longlining, pair trawls and beach seines), indicate that with R/V KIBOKO the proportion of larger Lates is underestimated. This can affect the mean catch rate significantly. Taken the aforegoing into consideration, it seems no overestimation to fix the sampled proportion, as often conducted in other studies (Gulland, 1983), to 50% of the actual stock. This proportion even may be lower for sizes of Lates vulnerable to the professional gillnet fishery of 60 cm and above.
Since trawling normally was restricted to waters up to 40 m deep, the estimate only may be considered representative for waters over that depth range. For the whole of Lake Victoria the total biomass of Lates in waters below 40 m (roughly encompassing approximately half of the area, i.e., 34 500 km2) would amount to 321 540 t (± 68%).
Recruitment
Very little is know about the recruitment pattern of Lates in Lake Victoria. Factors that might endanger a sufficient recruitment are e.g., spectacular overfishing of large (female) Lates and high levels of cannibalism. Cannibalism occurs to a great extent, but the actual impact upon recruitment is unknown.
3.2 Main Fisheries
General
Following the growth of the Lates stock, a Lates fishery developed (Figure 17) and currently this fishery is the most important and most profitable one throughout the countries bordering the lake. Based on interviews, Ligtvoet, Chande, Mosille (1988) found that the percentage of specialized Lates fishermen rose spectacular from almost 15% in 1983 to more than 50% in 1986. In Tanzania, in the Mwanza region four major types of Lates fishing exists, three at artisanal level and one at industrial level. The artisanal fishery comprises a canoe fishery using gillnets, beach seines or longlines; the industrial fishery consists of a fleet of approximately 17 trawlers of various sizes with otter trawl nets. In Mwanza and Musoma two pair-trawl units are operating.
In Tanzania the gillnet fishery is by far the most important one. Presently, on average, an estimated 90% of the total number of canoes used for the Lates fishery is used on behalf of gillnetting and the other 10% encompass the beach seine and longline fisheries, their relative effort varying from site to site.
Gillnet fishery
In Figure 17A the frequency distribution is given of the mesh sizes used by the gillnet fishermen in 1987. The specialized Lates fishermen use meshes of 6–10 inches, and very occasionally 12–16 inches. The relatively small-meshed gillnets (3–3.5 inches), which were formerly used for other fish species, nowadays fetch for an important part Lates. Our surveys in 1988 revealed that in the course of time the range of mesh sizes used for the Lates fishery has narrowed down to mainly 7 and 8 inches. Over 80% of the fishermen were found to use these two mesh sizes. The proportion of 9 inch gillnets has prominently declined.
Due to shortage in genuine netting materials, in Tanzania a wide variety of locally available twines are used for manufacturing gillnets (Ligtvoet, Chande, Mosille, 1988).
In Figures 18, 19, 20 and 21, the length frequency distribution of the gillnet catches in Tanzania are given, compared with those in Uganda and Kenya as recorded during a short survey (Ligtvoet, 1987). Obviously, the overall characteristics of the gillnet catches in the three riparian states greatly resemble each other. The small Lates (30–55 cm) in Figure 18, were caught by fishermen with small mesin Tanzania are given, compared with those in Uganda and Kenya as recorded during a short survey (Ligtvoet, 1987). Obviously, the overall characteristics of the gillnet catches in the three riparian states greatly resemble each other. The small Lates (30–55 cm) in Figure 18, were caught by fishermen with small mesh sized gillnets (mainly 4 inches), aiming at either Tilapias or Bagrus.
Comparing the length distribution of Lates at the landing site Igombe in 1987 (Figure 18) and 1988 (Figure 19) shows that small Lates as recorded at Igombe in 1987 were hardly found anymore in 1988. This supports our general experience that nowadays at the five landing sites covered by the monitoring programme (Ligtvoet, Chande, Mosille, 1988) virtually all fishermen have turned to the Lates fishery. At landing sites in the very shallow waters where other species still can be economically exploited (Oreochromis, Protopterus), this trend is less apparent.
The model lengths in the size distribution of the catch have slightly shifted from 65–75 cm TL early 1987 to 60–70 cm TL over 1988.
Figure 22A, depicting the model length of the Lates catch per canoe at Igombe over 1988, reveals that there is little variation in the lengths of Lates caught over the year. Using the length weight relationship (see above) Ligtvoet, Chande, Mosille (1988) estimated the weight of the catch per boatlanding in Tanzania early 1987 on almost 80 (40–150) kg per night. The mean catch per canoe per night for Lates fishermen as recorded at Igombe over 1988 ranges widely between ca. 25 and 150 kg (Figure 22B). The average catch amounts to ca. 110 kg/canoe/night.
Gillnet selectivity
Research on the selectivity of gillnets to Lates, prosecuted by HEST and the Agricultural University of Wageningen, revealed the following relationship between mesh size and size of fish retained (Figure 23):
1986: TL = 3.5 cm × mesh + 4.94 cm
1987: TL = 2.8 cm × mesh + 16.5 cm
There is a significant difference between the two regressions, mainly due to the catches in 10 cm mesh-sized gillnets; mean length in 1986 was 40 cm versus 47.3 cm in 1987. This difference most likely has to be attributed to a changing size structure of the Lates population, due to growth (cf. Ligtvoet, et al. in prep.).
Lates is easily caught at different body positions, mainly wedged, gilled (or wedged, tangled at the gill position) and wedged at the pre-opercula spine. Tangled specimens normally do not constitute a dominant part of the catch. The overall selectivity curve is asymmetrical, skewed to thee right and has a wide selection range. The parameter K relating mesh size (m) to the fish length caught most efficiently (lo) is estimated at K = 0.37 (Ligtvoet, et al., in prep.).
Beach seine fishery
In Figures 24A and 24B catches of two different types of beach seines, operating on different fishing grounds are given. Figure 23A depicts the catch distribution of a relative small beach seine (net length ca 250 m; ropes ca 200 m) which operates inside the Mwanza Gulf in very shallow, inshore waters. This type of seines formerly was used for fishing on Haplochromis and Tilapia mainly. The other beach seine, representing the types especially designed to fish Lates, is much larger (net length upto 1 200 m; ropes ca 1 000 m) and is operated on shores along the open waters (e.g., Speke Gulf). The catch of the latter seien is orders of magnitudes larger than those of the small seines; in 1987 catches of almost 1 000 kg have been observed, but recently catches of 1 500 kg and more are no exception (pers. obs.). The small beach seine normally does not catch more than 100 kg/haul (Ligtvoet, Chande, Mosille, 1988).
In Kenya, especially along the southern shores of the Nyanza Gulf an extensive mosquito seine fishery is prosecuted, targetting for Rastrineobola. No specific catch records are available concerning the Lates (by) catches, but Asila and Ogari (1987) state that over half of these beach seine catches are composed of juveniles of both Oreochromis and Lates.
In Tanzania it also has been observed that small Lates of TL 2–3 cm may constitute a considerable part of the catch of the mosquito seines aimed at Rastrineobola. It is not known how general this observation may be considered, but this phenomenon surely needs attention as it may influence the recruitment of Lates.
Longlines
Longlining mostly occurs in shallow, inshore waters in the vicinity of Papyrus swamps (mainly targetting for Protopterus and Clarias) or near rocky islands in deeper waters (targetting for Bagrus). In the shallow wters Lates nowadays forms generally more than 50% of the catch. Figure 25 shows the length frequency distribution of Lates caught with hooks of several sizes. In 1987 CPUE was estimated at only 32 kg/canoe/night (Ligtvoet, Chande, Mosille, 1988), but records in 1988 show higher figures (Ligtvoet, et al., in progress).
3.3 Yield
Although the accuracy of the absolute figures produced by the statistical services in the three riparian states may to some extent be disputed (Bernacsek, 1986, cf CIFA 1988), there is no doubt that the Lates catches have increased enormously over the last 10 years. According to these statistics, the total yield of Lates in 1985 amounted to more than 87 000 t for Tanzania and Kenya combined (no figures available for Uganda) (Reynolds and Greboval, 1988). Assuming an equal yield in the Tanzanian and Ugandan waters, the total yield would amount to roughly 120 000 t.
The total effort is not exactly known. Reynold and Greboval (1988) estimate the actual total number of canoes at about 11 000. Again, assuming that the above given CPUE for a gillnet unit in Tanzania, may also hold in Kenya and Uganda, for the gillnet fishery a total yield of 11 000 (canoes) × 0.50 (estimated percentage gillnet canoes targetting for Lates) × 250 (working days) × 80 (kg) = 110 000 t for 1987 can be calculated. Based on the mean catch per night per canoe at Igombe, for 1988 an estimate of 150 000 t is obtained. It should of course be stressed that these estimations are still too rough to base any reliable stock assessment upon, but they are useful in giving an idea of the order of magnitude concerning the yield.
Fishing mortality and exploitation ratio
As a first approximation, total mortality of Lates in the Tanzanian waters can be estimated with the “Beverton and Holt Z equation” based on length data (Beverton and Holt, 1956) and with the “length-converted catch curve” (e.g., Pauly 1983). The Beverton and Holt equation is:
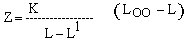
L1 is the smallest length of fishes that are fully represented in the catch samples, and L is the mean length of fish in the catch of length L1 and beyond.
Using the summed catch data over one year (March 1987-February 1988) of the Semba beach seine (cf. Figure 6), a mean length of 58.96 cm can be calculated and with L1 = 39.5 cm, an estimate for Z is derived of Z = 1.10.
With the “length-converted catch curve”, an estimate of total mortality is found of Z = 1.19.
Since Z = F + M, fishing mortality can be estimated at
F = 0.81 (Beverton and Holt data) and 0.90 (catch curve)
To roughly assess the state of the Lates stock concerning the level of the present fishing pressure, based on the above calculated mortality rates an exploitation ratio may be computed from:
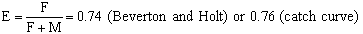
Generally, under the assumption that the sustainable yield is optimized when F equals about M (Gulland, 1971), the optimal exploitation ratio is assumed to equal about 0.5. If these assumptions may hold for the Lates stock in Lake Victoria, the established ratio of around 0.75 may indicate that presently overfishing is occurring. Before adopting this conclusion, however, supporting evidence of, for example, a strong decrease in mean length of Lates in the catches is necessary. The catch records from the gillnet fishery at Igombe and Mkuyuni landing sites show a slight shift of the peak length from 65–75 cm TL early 1987 to 60–70 cm TL in 1988 (Figures 18 and 19), concurring with a shift in mesh sizes from mainly 7, 8, 9 inches to 7 and 8 inches.
The above-used methods for estimating Z, both rest on the assumption of a steady state situation. It should be questioned, whether the Lates stock presently is already in this situation, and actually it seems likely that the whole ecosystem, including the Lates population and the Lates fishery is still in a stage of transition. Nevertheless, as stated by Murphy (1982), even if recognized in error, it is useful to apply these methods, for they provide valuable first estimates and insights into what is taking place.
4. DISCUSSION
Contrary to pessimistic prophecies as expressed in the early days of the Lates increase (cf. Barel, et al., 1985; Fryer, 1972, 1984; Ribbink, 1987; but see Ligtvoet, 1989), after the establishment of the species the Lake Victoria system sustained a high fish production. A profitable, artisanal Lates fishery developed, based on the catch of large sized table fishes (mainly 50–90 cm).
The central question now is whether the present high yields are sustainable. The exceptional fast growth which Lates exhibits in Lake Victoria (Figure 8) indicates favourable environmental conditions for this species. Over the years the composition of prey stocks for Lates has dramatically changed, primarily by overexploiting its initial major food resource, the haplochromine cichlids. Currently, the forage base is formed by Caridina, Rastrineobola and juvenile Lates (Figures 10 and 14). As depicted in Figure 15, the developments of the Lates stock primarily will be determined by developments in these main prey stocks and in the fisheries. Concerning the food chains involving Lates, it is interesting to note that inspite of the increased predation pressure, the Rastrineobola stock increases (Wanink, et al., 1988), and that there are strong indications that the quantity of Caridina grows (F. Witte, J.H. Wanink, pers. comm.).
The present ecosystem is still young and the eventual balance in the lake is uncertain. In view of the developments in the current main prey stocks of Rastrineobola and Caridina, the prospects for a sustained high yield seem not unfavourable. The possible impact, however, of the high level of cannibalism among Lates upon the recruitment and growth of the population cannot yet be assessed.
The fishing intensity is not evenly distributed along the lake and some lightly fished areas do still exist. Since considerable movements of individual Lates specimens have been noted (Figure 2), we may assume that stocks in intensively fished areas to some extent can be replenished by Lates from lightly fished areas. There is, however, a strong expansion of the artisanal fishery and also a wish to increase fishing on an industrial level. In view of the historic developments in Lake Victoria, and with the example of the Nyanza Gulf in mind, without a proper management, in the future overexploitation of the Lates stock is to be expected.
Lates recruits to the main fishery when it is two years old, at a length of 50–60 cm TL (cf. Figures 18 and 19). This is quite young and at a length where males just reach their 50% maturity and females just start their maturation. When the fishing mortality will be too high, recruitment overfishing may occur.
Before Lates was abundant, nearly all stocks in Lake Victoria have been liable to overexploitation (Marten, 1979). Though data from other sources are needed to affirm the conclusion, the exploitation rate as calculated above, might indicate that the Lates stock presently is overfished. To date signs of structural overfishing of Lates are only reported from the probably most intensively fished area of the lake, the Nyanza Gulf, Kenya. From Tanzania and Uganda, however, together comprising more than 90% of the total area, no decrease in the catches has been observed by now (cf. CIFA 1988). When overexploitation will occur, the first signs will come from significantly smaller mean sizes in the catch. Usually fishermen react to this by lowering their mesh sizes. In the Mwanza area, the catch per canoe has not decreased up to November 1988, but a slight decrease in the model length of Lates caught with gillnets is noted (Figures 18 and 19), together with a decline of the proportion of larger (9 inch) gillnets.
If the other fishing types, especially beach seining and trawling, are considered the actual Lc would be lower than that of the gillnet fishery only. Since the yield declines with decreasing Lc, aselective fisheries, also catching large quantities of young Lates, like trawling and beach seining with small mesh sizes, may be considered detrimental for an effective harvesting of Lates.
For short-term management of the Lates fishery, the following guidelines may be suggested: consolidation of the present gillnet fishery with mainly 7 and 8 inch mesh sizes, especially in densely fished parts of the lake; promoting longlining (selective towards mainly larger Lates); prohibiting or control of beach seining (like in Uganda) and trawling (unless with large meshes).
The problems involved with implementing possible management measures will not be discussed here. In general, the shift from a formerly multi-species fishery (based on ca. 12 genera) to a fishery based mainly on three species (Lates, Oreochromis, Rastrineobola) should have simplified the situation for fisheries management. The history of managing the Lake Victoria fisheries does record a well-known example of a detrimental gillnet fishery with increasing use of smaller mesh sizes: the developments in the formerly most important commercial fishery for Oreochromis esculentus, which has been tried to regulate with mesh size regulations, clearly illustrate the difficulties involved with the enforcement of such regulations (e.g., Fryer and Iles, 1972; Lowe-McConnell, 1987).
As already mentioned, the lake presently sustains yields, high as never before and employment and revenues in the fishery and adjacent sectors have raised enormously (Reynolds and Greboval, 1988). In Tanzania for instance, the estimated earnings from the Lates fishery are of the same order of magnitude as those from the major export crops, coffee and cotton.
The countries sharing Lake Victoria have the responsibility to manage the lake's resources. The present situation demands all possible attention and effort to prevent detrimental overexploitation of the fish stocks. It would be a pity to see another example added, of the complete deterioration of a highly profitable fishery.
ACKNOWLEDGEMENTS
The efforts of Dr W.L.T. van Densen in correcting and improving the manuscript are greatly acknowledged.
REFERENCES
Acere, T.O., 1985. Observations on the biology, age, growth and sexuality of Nile perch, Lates niloticus (Linne), and the growth of its fisheries in northern waters of Lake Victoria. In Report of the Third Session of the Sub-Committee for the Development and Management of the Fisheries of Lake Victoria. Jinja, Uganda, 4–5 October 1984. FAO Fish.Rep., (335):42–61
Acere, T.O., 1988. Recent trends in the fisheries of Lake Victoria (Uganda northern part). In Report of the Fourth Session of the Sub-Committee for the Development and Management of the Fisheries of Lake Victoria. Kisumu, Kenya, 6–10 April 1987. FAO Fish.Rep., (388):36–45
Acere, T.O. and D. Pauly, 1988. Artisanal Fisheries Resource Study Project No. 5100.36.47.025 Uganda. Preliminary Report. Manila: 16 p.
Arunga, J., 1981. A case study of the Lake Victoria Nile perch, Lates niloticus, fishery. Proc. Workshop Kenya Marine and Fishery Research Institute. Mombasa, Kenya, July 1981: 165–83
Asila, A.A. and J. Ogari, 1987. Growth parameters and mortality rates of Nile perch (Lates niloticus) estimated from length-frequency data in the Nyanza Gulf (Lake Victoria). In Contributions to Tropical Fisheries Biology, edited by S. Venema, J. Moller-Christensen and D. Pauly. Papers by the participants of FAO/DANIDA Follow-up Training Courses. FAO Fish.Rep., (389):272–87
Barel, C.D.N., et al., 1985. Destruction of fisheries in Africa's lakes. Nature, Vol. 315:19–20
Beverton, R.J.H. and S.J. Holt, 1956. A review of methods for estimating mortality rates in fish populations, with special references to sources of bias in catch sampling. Rapp.P.-V.Reun.CIEM, 140:67–83
Chilvers R.M. and J.M. Gee, 1974. The food of Bagrus docmac and its relationship with Haplochromis in Lake Victoria, East Africa. J.Fish Biol., (6):483–505
CIFA, 1985. Report of the Third Session of the Sub-Committee for the Development and Management of the Fisheries of Lake Victoria. Jinja, Uganda, 4–5 October 1984. FAO Fish.Rep., (335):145
CIFA, 1988. Report of the Fourth Session of the Sub-Committee for the Development and Management of the Fisheries of Lake Victoria. Kisumu, Kenya, 6–10 April 1987. FAO Fish.Rep., (338):112
Fryer, G., 1972. Conservation of the Great Lakes of Africa: a lesson and a warning. Biol.Conserv., (4):256–62
Fryer, G., 1984. The conservation and rational exploitation of the biota of African Great Lakes. In Conservation of threatened natural habitats, edited by A.H. Hall. S.Afr.Natl.Sci.Prog.Rep., 92:135–54
Fryer, G. and T.D. Iles, 1972. The cichlid fishes of the great lakes of Africa. Edinburgh: Oliver and Boyd
Gee, J.M., 1964. Nile perch investigations. EAFFRO Annual Report 1964, pp. 13–17
Gee, J.M. 1969. A comparison of certain aspects of the biology of Lates niloticus (Linne) endemic and introduced environment in East Africa. In Man-made lakes, the Accra Symposium, edited by L.E. Obung. Accra, Ghana University Press for Ghana Academy of Sciences, pp. 251–9
Goudswaard, P.C., 1988. A comparison of trawl surveys in 1969/70 and 1984/85 in the Tanzanian part of Lake Victoria. In Report of the Fourth Session of the Sub-Committee for the Development and Management of the Fisheries of Lake Victoria. Kisumu, Kenya, 6–10 April 1987. FAO Fish.Rep., (388):86–100
Goudswaard, P.C. and W. Ligtvoet, 1988. Recent developments in the fishery for haplochromines (Pisces: Cichlidae) and Nile perch, Lates niloticus (L.) (Pisces: Centropomidae) in Lake Victoria. In Report of the Fourth Session of the Sub-Committee for the Development and Management of the Fisheries of Lake Victoria. Kisumu, Kenya, 6–10 April 1987. FAO Fish.Rep., (388):101–12
Gulland, J.A., 1983. Fish stock assessment - a manual of basic methods. FAO/Wiley Series on Food and Agriculture, Vol.1:223 p.
Hamblyn, E.L., 1966. The food and feeding habits of Nile perch Lates niloticus (Linne) Pisces: Centropomidae. Rev.Zool.Bot.Afr., 74(1–2):28
Holden, M.J., 1963. Report on the fisheries of Lake Albert. Uganda Fisheries Department, Entebbe, 112 p.
Hopson, A.J., 1972. A study of the Nile perch (Lates niloticus (L.), Pisces: Centropomidae) in Lake Chad. Overseas Res.Publ.,Lond., (19):93
Hopson, A.J., 1982. The biology of Lates niloticus (L.) in Lake Turkana. In Lake Turkana. A report on the findings of the Lake Turkana Project 1972–75. Vol. 5. Edited by A.J. Hopson. Overseas Development Administration London, pp. 1285–99
Hughes, N.F., 1986. Changes in the feeding biology of the Nile perch, Lates niloticus (L) (Pisces: Centropomidae), in Lake Victoria, East Africa, since its introduction in 1960, and its impact on the native fish community of the Nyanza Gulf. J.Fish.Biol., 19:541–8
Latif, A.F.A. and E.A. Kallaf, 1976. Mortality rates of Lates niloticus in Lake Nasser. Hydrobiol., 50(1):27–32
Ligtvoet, W., 1987. Report of a visit to Kenya and Uganda (4–19 April 1987). Report of the Haplochromis Ecology Survey Team (HEST) and the Tanzanian Fisheries Research Institute (TAFIRI) No. 41. Leiden, The Netherlands, 11 p.
Ligtvoet, W., 1989. The Nile perch in Lake Victoria: a blessing or a disaster? In Crapon de Caprona, edited by M.-D. Fritzsch and B. Fritzsch. Proceedings of the Workshop on the Biology, Ecology and Conservation of Cichlids. Ann.Mus.Roy.Afr.Centr.Sc.Zool., Vol. 257:151–6
Ligtvoet, W., A.I. Chande and O.I.I.W. Mosille, 1988. A preliminary description of the artisanal Nile perch (Lates niloticus) fishery in Southern Lake Victoria. In Report of the Fourth Session of the Sub-Committee for the Development and Management of the Fisheries of Lake Victoria. Kisumu, Kenya, 6–10 April 1987. FAO Fish.Rep., (388):72–85
Loubens, G., 1974. Quelques aspects de la biologie de Lates niloticus du Chad. Cah.ORSTROM Ser.Hydrobiol., 8(1):3–21
Lowe-McConnel, R.H., 1987. Ecological studies in tropical fish communities. Cambridge: Cambridge University Press
Marten, G.G., 1979. Impact of fishing on the inshore fishery of Lake Victoria (East Africa). J.Fish.Res.Board Can., 36(8):891–900
Midgeley, S.H., 1968. A study of Nile perch in Africa and consideration as to its suitability for Australian tropical inland waters. Winston Churchill Mem. Trust. Fellowship Rep., 3:20 p.
Moore, R., 1979. Natural sex inversion in the giant perch (Lates calcarifer). Austr.J.Mar.Freshw.Res., 30:803–13
Muller, R.G. and R.S. Benda, 1981. A comparison of bottom trawl stock densities in the inner Kavirundo Gulf of Lake Victoria. J.Fish.Biol., 18:399–401
Murphy, G.I., 1982. Recruitment in tropical fishes. In Theory and Management in Tropical Fisheries, edited by D. Pauly and G.I. Murphy. ICLARM Conference Proceedings, 9:360
Ogari, J., 1985. Distribution, food and feeding habits of Lates niloticus in Nyanza Gulf of Lake Victoria (Kenya). In CIFA. Report of the Third Session of the Sub-Committee for the Development and Management of the Fisheries of Lake Victoria. Jinja, Uganda, 4–5 October 1984. FAO Fish.Rep., (355):68–80
Ogari, J. and S. Dadzie, 1988. The food of Nile perch, Lates niloticus (L.), after the disappearance of the haplochromine cichlids in the Nyanza Gulf of Lake Victoria (Kenya). J.Fish.Biol., 32:571–7
Ogutu-Ohwayo, R., 1985. The effects of predation by Nile perch, Lates niloticus (Linne) introduced into Lake Kyoga (Uganda) in relation to the fisheries of Lakes Kyoga and Victoria. In CIFA. Report of the Third Session of the Sub-Committee for the Development and Management of the Fisheries of Lake Victoria. Jinja, Uganda, 4–5 October 1984. FAO Fish.Rep., (355):18–41
Ogutu-Ohwayo, R., 1988. Contribution of the introduced fish species especially Lates niloticus (L.) and Oreochromis niloticus (L.) to the fisheries of Lakes Victoria and Kyoga. In CIFA. Report of the Fourth Session of the Sub-Committee for the Development and Management of the Fisheries of Lake Victoria. Kisumu, Kenya, 6–10 April 1987. FAO Fish.Rep., (388):61–70
Ogutu-Ohwayo, R., 1988. Reproductive potential of the Nile perch, Lates niloticus L. and the establishment of the species of Lakes Kyoga and Victoria (East Africa). Hydrobiologia, 162:193–200
Okaronon, J.O., T.O. Acere and D.L. Ocenodongo, 1985. The current state of the fisheries in the northern portion of Lake Victoria (Uganda). In CIFA. Report of the Third Session of the Sub-Committee for the Development and Management of the Fisheries of Lake Victoria. Jinja, Uganda, 4–5 October 1984. FAO Fish.Rep., (355):89–98
Okedi, J., 1970. Further observations on the ecology of the Nile perch (Lates niloticus, Linne) in Lakes Victoria and Kyoga. EAFFRO Annual Report 1970.
Pauly, D., 1980. A new methodology for rapdily acquiring basic information on tropical fish stocks: growth, mortality and stock recruitment relationships. In Stock Assessment for Tropical Small-scale Fisheries, edited by S. Saila and P. Roedel. Proceedings of International Workshop, 19–21 September 1979. University Rhode Island. Intern. Center Mar. Resource Development, pp. 154–72
Pauly, D., 1983. Some simple methods for the assessment of tropical fish stocks. FAO Fish.Tech.Pap., 234:52
Planquette, P., 1975. Dimorphisme sexuel chez Lates niloticus (poisson Centropomidae). Cah.ORSTOM Ser.Hydrobiol., Vol.IX(1):9–12
Reynolds, J.E. and D.F. Greboval, 1988. Socio-economic effects of the evolution of Nile perch fisheries in Lake Victoria: a preliminary assessment. FAO Report RAF/87/099/TECH/02, 167 p.
Ribbink, A.J., 1987. African lakes and their fishes: conservation scenarios and suggestions. Environ.Biol.Fish., 19(1):3–26
Ssentongo, G.W. and R.L. Welcomme, 1985. Past history and current trends in the fisheries of Lake Victoria. In Report of the Third Session of the Sub-Committee for the Development and Management of Lake Victoria. Jinja, Uganda, 4–5 October 1984. FAO Fish.Rep., (335):123–38
Wanink, J.H., 1988. The Pied Kingfisher (Ceryle rudis) and Dagaa (Rastrineobola argentea): estimating the food-intake of a prudent predator. HEST/TAFIRI Report No. 49, Leiden, The Netherlands, 12 p.
Wanink, J.H., et al., 1988. HEST/TAFIRI research in Lake Victoria: some preliminary results and their relevance for fishery management. HEST/TAFIRI Report No. 44, Leiden, The Netherlands, 10 p.
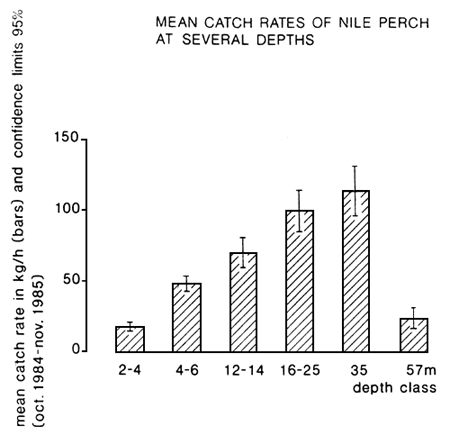
Fig. 1. Depth distribution of Nile perch. Te halen uit: Goudswaard & Ligtvoet 1987, Hest report no. 35. Uit fig. 6 en 7 de Nijlbaars grafieken kombineren.
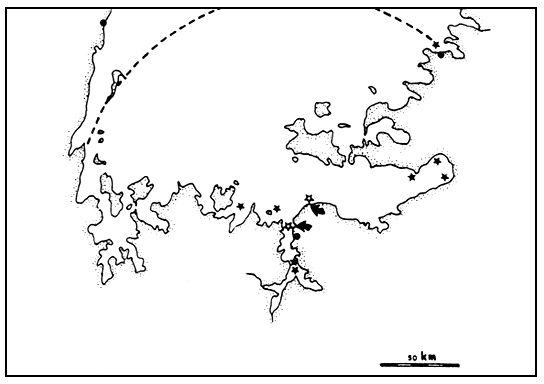
Fig. 2. Recaptures of Lates specimens in southern Lake Victoria. Tagging stations are indicated by the solid arrows; open stars represent areas with numerous recaptures, black stars denote individual recoveries. The broken line may reflect the minimum area or the unit of stock, as based on the maximum distance observed.
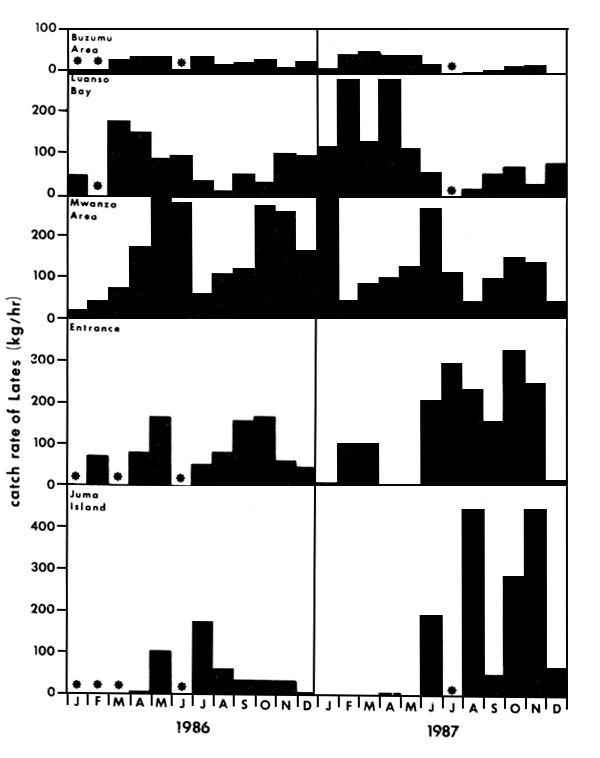
Fig. 3. Monthly catch rates of R.V. Kiboko at five stations near Mwanza: Buzumu (depth 2–4 m), Luanso (4–6 m), Mwanza (12–14 m), Entrance (16–25 m) and Juma Island (35 m). Asteriks indicate months in which no trawl shots were made. (Form Goudswaard, in prep.)
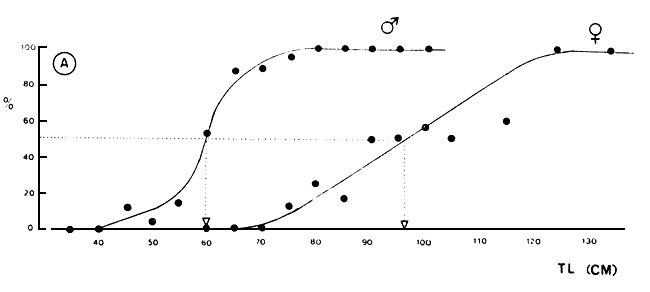
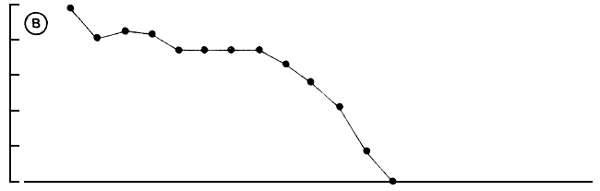
Fig. 4. Maturity ogives of male and female Lates niloticus (A) and the sex-ratio expressed as the percentage of males in a particular length class) over 5 cm length classes (B). The lines in A, are fitted by eye.
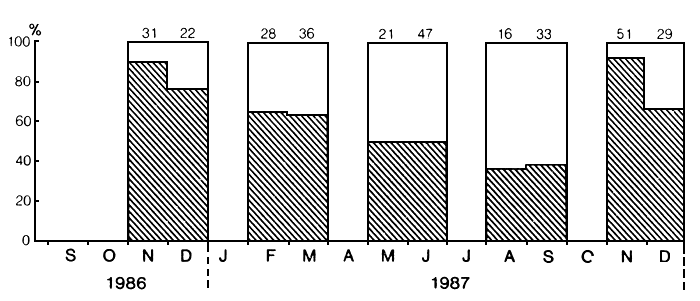
Fig. 5. Seasonal changes in maturity of male Lates niloticus, stage 4, 5 and 6. Shaded is the percentage of ripe and running males (stage 5 and 6) in the samples. Sample sizes are indicated at the top.
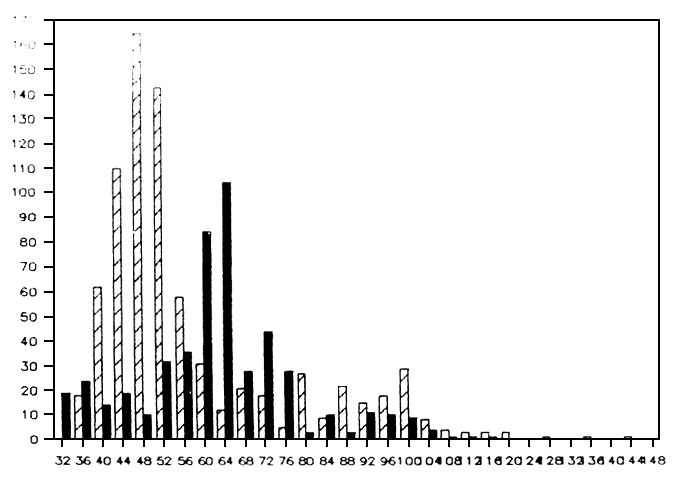
Fig. 6. Length frequency distributions of Lates catches in March 1987 and January 1988 (black), obtained with a large beach seine operating on the southern shore of the Speke Gulf. Over the 10 months, a clear shift of the main peak can be noted from (midpoint of 4 cm class) 48 cm in March to 64 cm in January.
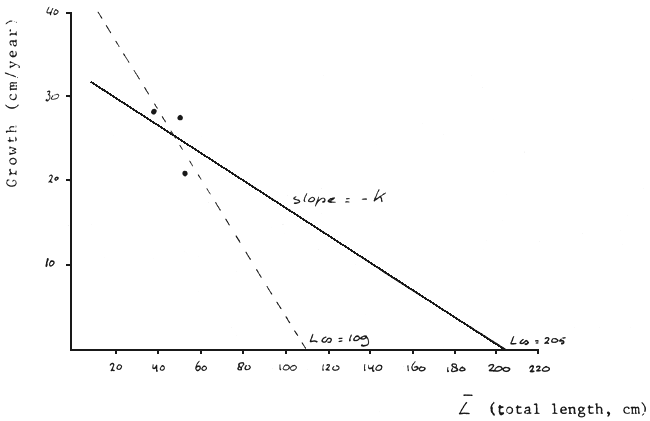
Fig. 7. “Gulland and Holt Plot” (broken line) and “forced Gulland and Holt Plot” (bold line) based on three recaptured Lates specimens. For explanation see text.
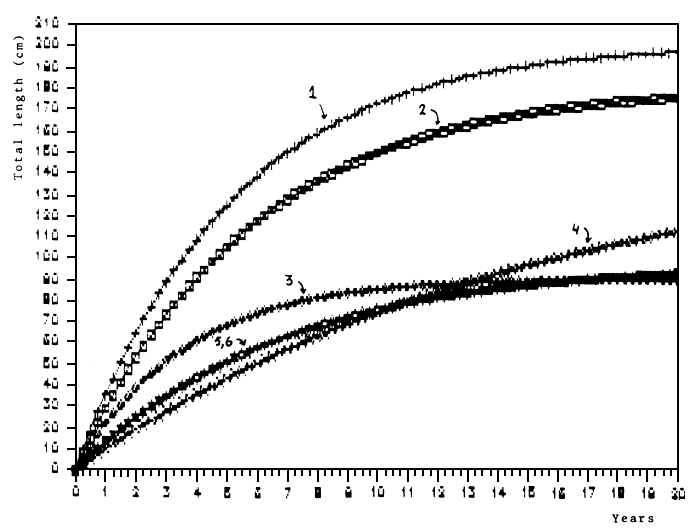
Fig. 8. Von Bertalanffy growth curves for Lates from various areas as given in table 2. 1: Lake Victoria - Kenya; 2: Lake Victoria - Tanzania; 3: Lake Chad (Hopson, 1972); 4: Lake Turkana (Gee, 1965); 5: Lake Albert (Midgeley, 1968); 6: Lake Nasser (Latif & Khallaf, 1976).
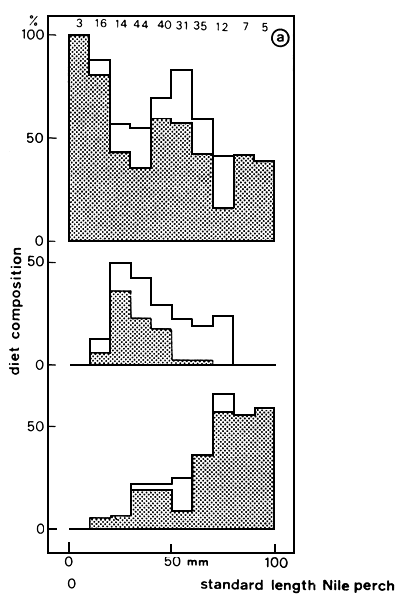
Fig. 9. Diet composition of juvenile Lates. Figures denote the occurrence of the shrimp Caridina (upper panel), insect larvae (middle) and fish (lower) per 10 mm size class of perch. Sample sizes are indicated at the top (From Wanink, first Quarterly Report 1987 of the Haplochromis Ecology Survey Team).
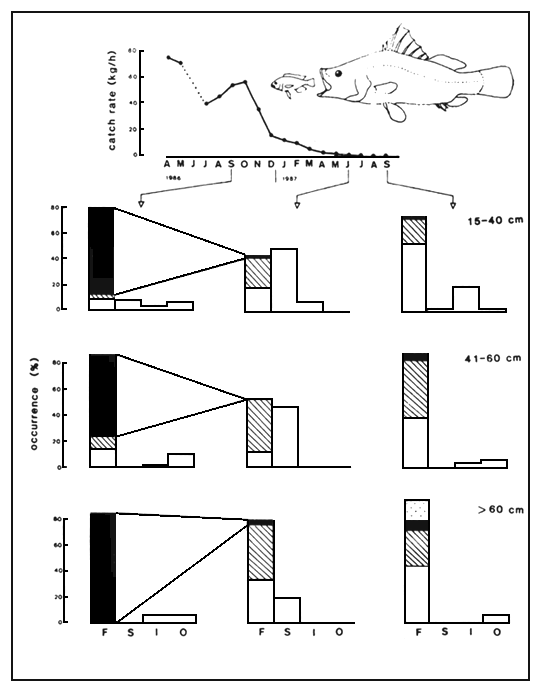
Fig. 10. Comparison of food composition of Lates of three length classes, caught in the Mwanza Gulf in waters of 2–15 m depth in September 1986, and June and September 1987. Figures denote the relative occurrence (percentage) of the various food items in the stomachs. Unidentified fish remains have been allocated proportionally to the recognizable fish prey species. The top figure (drawn after Goudswaard & Katunzi, in prep.) illustrates the decrease of the haplochromine stock over this period of time.
Notation: F = fishes (black: haplochromines; shaded: Rastrineobola; white: juv. Lates; stippled: Synodontis); S = shrimps Caridina nilotica; I = insect larvae (mostly Odonata); O = other items (mostly snails).
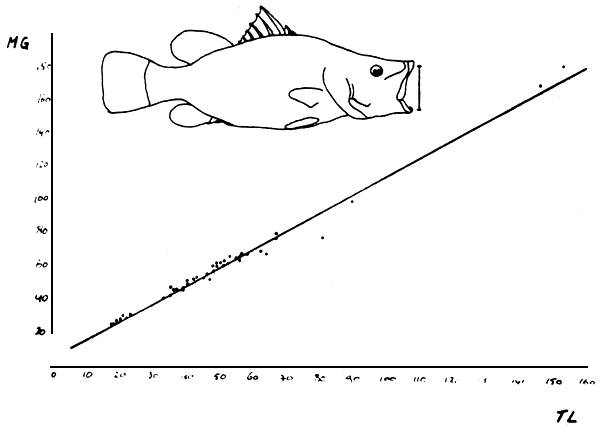
Fig. 11. The relationship between total length and the maximum width of mouth gape for Lates niloticus: MG = 1.12 × TL + 4.3. (MG, TL in cm).
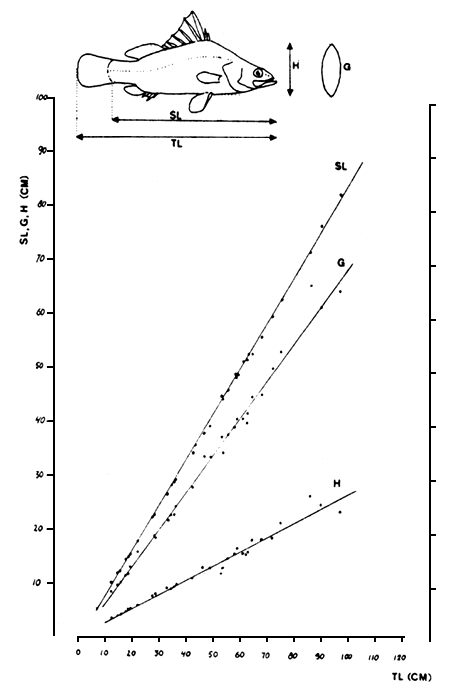
Fig. 12. The relationships between total length (TL) and standard length (SL), maximum body heigth (H) and maximum girth (G) for Lates niloticus.
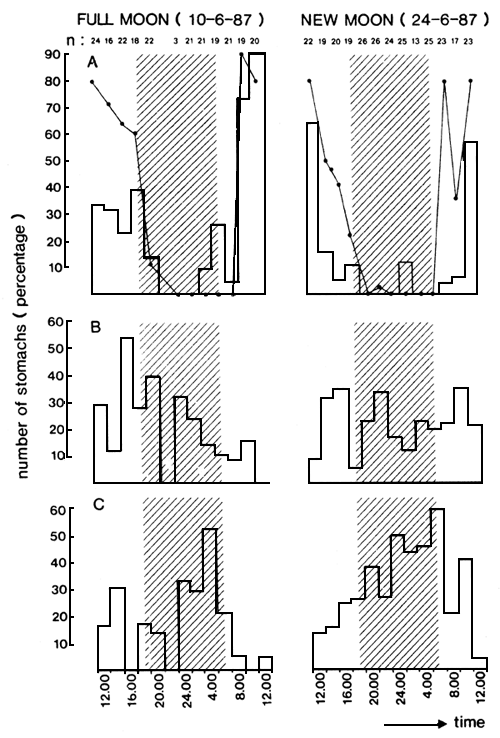
Fig. 13. Number of full (A), half full (B) and empty (C) stomachs of Lates specimens (TL 40–60 cm) during two 24 hour sessions. Blank: daytime; grey: nighttime. In fig. 13A the occurrance of fresh shrimps in the stomachs is indicated (bold line).
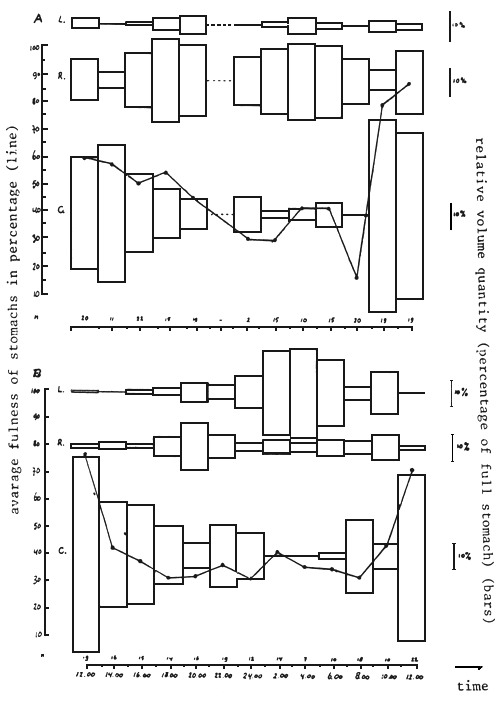
Fig. 14. Relative volume quantities (as percentage of full stomachs) of the three main prey types of Lates through two 24 hour sessions. A: full moon, 10/06/87; B: new moon, 24/06/87. Indicated with the bold lines is the ‘mean fullnes’ of the not-empty stomachs. Sample sizes are given at the bottom.
C = Caridina; L = Lates; R = Rastrineobola.
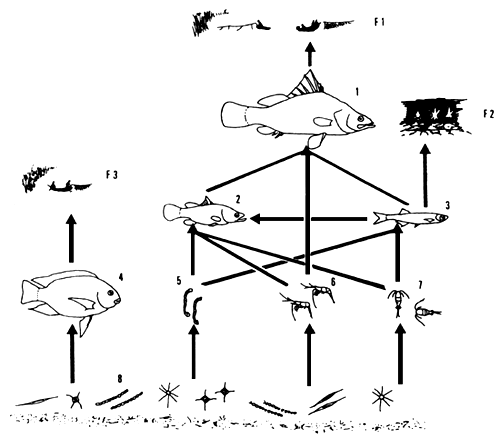
Fig. 15. Simplified food web of Lake Victoria depicting the relationships between the three commercially most important fish stocks, their main food sources and their fisheries. Figures are not drawn to scale. 1: adult Lates niloticus; 2: juvenile Lates; 3: Rastrineobola argentea; 4: Oreochromis niloticus; 5: insect larvae; 6: Caridina nilotica; 7: zooplankton; 8: phytoplankton and detritus.
Main fisheries: Fl: gillnet, longline, beach seine fishery for Lates; F2: nightly light fishery with mosquito seines for Rastrineobola; F3: gillnet fishery for Oreochromis.
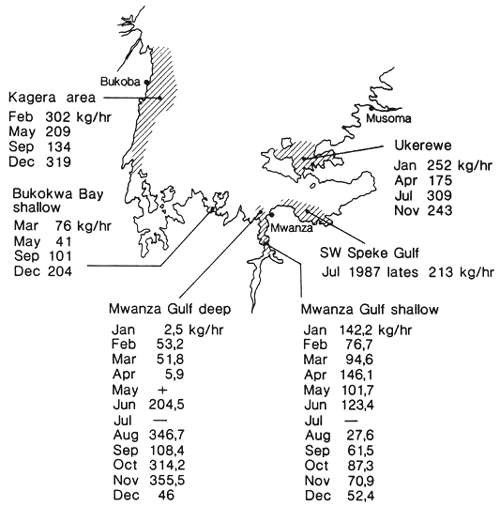
Fig. 16. Mean catch rates of Lates niloticus in 1987 during the monitoring program of R.V. Kiboko at different areas in southern Lake Victoria. (From Goudswaard, Progress Report 1987 of the Haplochromis Ecology Survey Team).
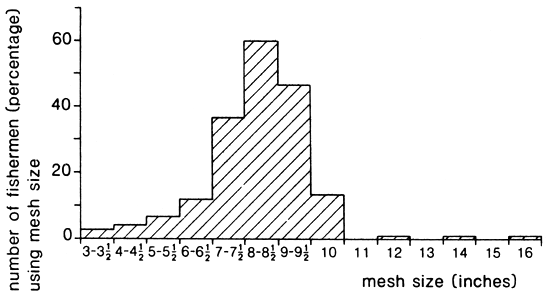
Fig. 17. Composition of the net fleet, with regard to the mesh sizes, of fishermen in the Mwanza area who are targetting for Lates. (Ligtvoet, Chande, Mosille, 1988)
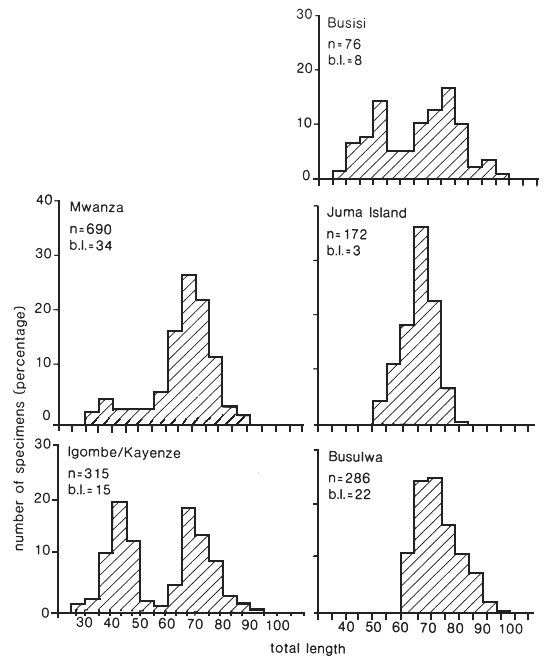
Fig. 18. Length frequency distributions of the gillnet catches of Lates recorded at five landing sites in the Mwanza area, Tanzania. b.l. = number of boat landings. (Ligtvoet, Chande, Mosille, 1988)
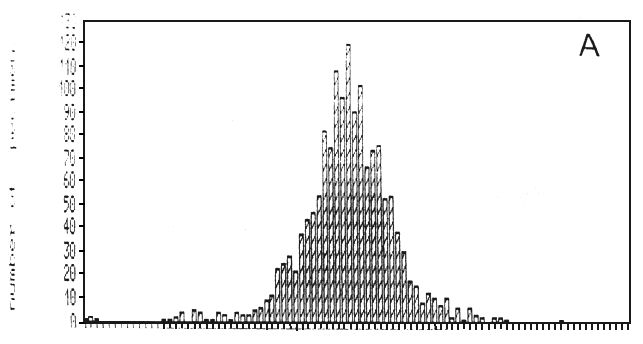
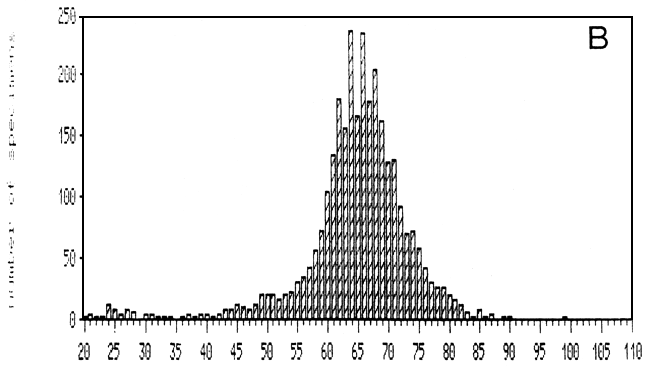
Fig. 19. Length-frequency distributions of the artisanal gillnet catches over 1988, as recorded at two landing beaches in the Mwanza area: A = Mkuyuni, situated inside the Mwanza Gulf; B = Igombe, situated along the Speke Gulf (Ligtvoet, Chande, Mosille, 1988)
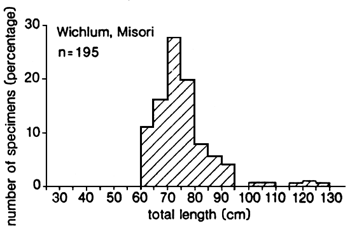
Fig. 20. Length frequency distributions of the gillnet catches of Lates recorded at three landing sites along the northern shore of the Nyanza Gulf, Kenya. (From Ligtvoet 1987).
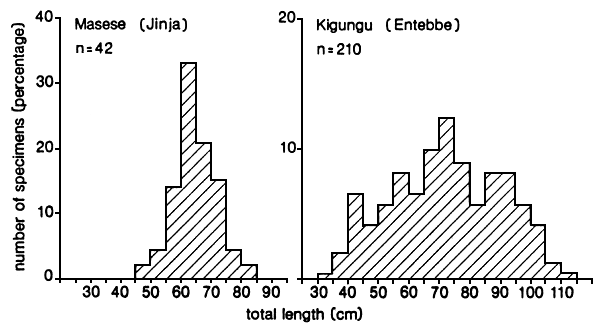
Fig. 21. Length frequency distributions of the gillnet catches of Lates recorded at two landing sites near Jinja and Entebbe, Uganda. (From Ligtvoet 1987).
Artisanal gillnet catches at Igombe
(Speke gulf) in 1988
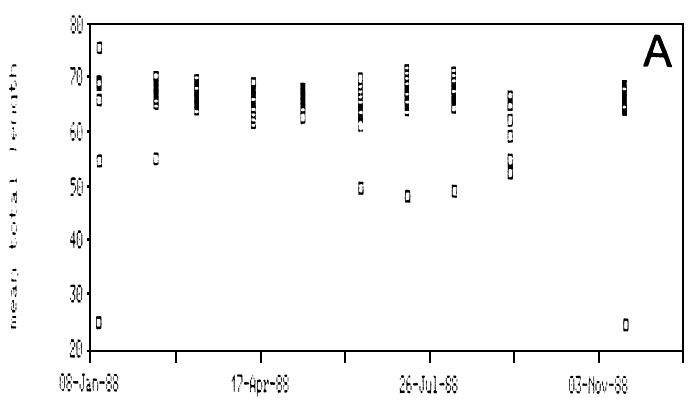
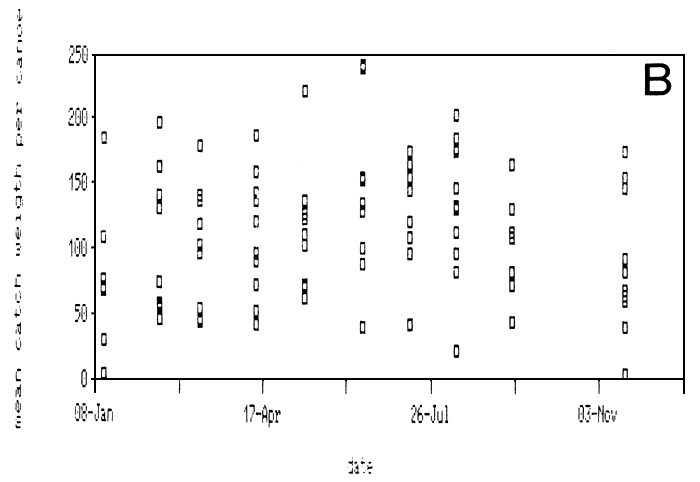
Fig. 22. The modal length (A) and average catch (B) per canoe over 1988 as recorded at the landing beach Igombe (Speke Gulf). One square represents one canoe.
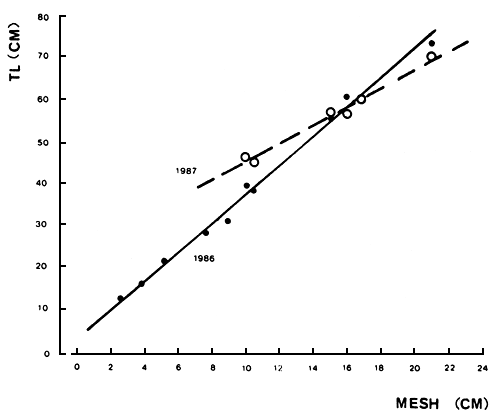
Fig. 23. Relationship between gill-net mesh size (stretched mesh in cm) and the mean total length (cm) of Lates specimens retained in 1986 and 1987. (From Ligtvoet et al. in prep.).
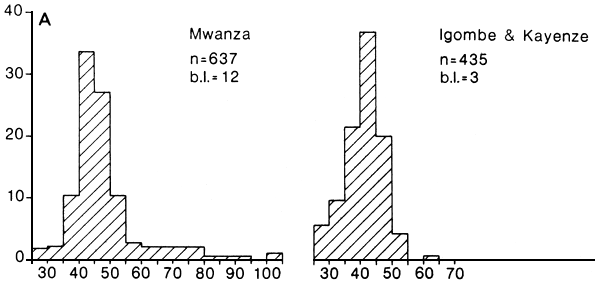
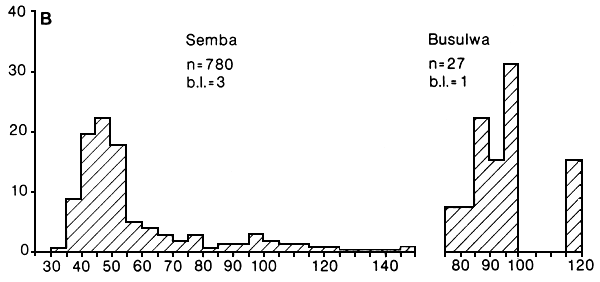
Fig. 24. Length frequency distributions of medium sized (A) and large (B) beach seine catches of Lates recorded at four landing sites in the Mwanza area, Tanzania. b.l. = number of boatlandings. (Ligtvoet, Chande, Mosille, 1988)
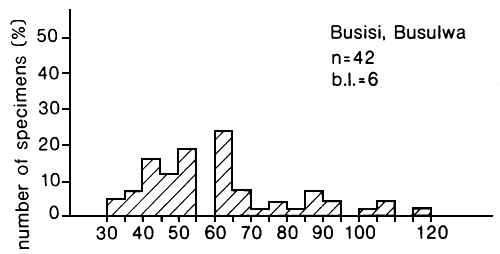
Fig. 25. Length frequency distribution of the long line catches of Lates recorded at two landing sites in the Mwanza area, Tanzania. (Ligtvoet, Chande, Mosille, 1988)