Shmuel Rothbard
Fish and Aquaculture Research Station
Dor, D.N. Hof-Hacarmel, Israel
ABSTRACT
The zooplanktonic herbivorous rotifer, Brachionus plicatilis, can be used to feed larvae and fry of (marine) aquaculture species.
The rotifers are cultured in a 1.5 m3 tank with a 48 hrs production cycle. They are fed with Chlorella and yeast. The temperature is kept at 30 – 33°C and the pH at 8.0. The Chlorella are cultured in seawater, which is diluted with 60% fresh water prior to the introduction of rotifers. After 10 – 14 hours of rotifer culture yeast supplementation becomes necessary.
RESUME
Le rotifère, Brachionus plicatilis, organisme zooplanctonique herbivore, constitue un aliment approprié à l'alimentation des larves de plusiers espèces aquacoles (marines).
La cycle de production des rotifères s'élève à 48 heurs à une température de 30 – 33 °C et à pH = 8.0 dans des bacs de 1.5 m3. L'alimentation des rotifères est fait à l'aide de Chlorella, produite en eau de mer à laquelle est ajouté 60% d'eau douce avant l'introduction aux rotifères, suivi, après 10 – 14 heures par une supplementation de levure.
The growing importance of marine aquaculture has caused an increasing demand of larvae and fry for stocking mariculture systems. The limiting factor in most larvae and fry cultures is the ability to mass-produce phyto- and zooplankton to be utilized as food for the larvae and fry. One of the most widely used zooplanktonic herbivores is the rotifer Brachionus plicatilis (Müller). Some species of shrimps and fish used in mariculture whose larvae and fry feed on this rotifer are shown in Table I.
One of the advantages of B. plicatilis over other types of zooplankton is the wide range in size, 120–250 μ to which it can be cultured, the other advantage is the tolerance of different salinities Cito, 1960). Hirayama and Watanabe (1975), Hirayama and Nakamura (1976) have shown that this rotifer can be cultured on different diets. In Japan, marine Chlorella spp. and yeast are used in commercial mass-culture of B. plicatilis. Mass culture of B. plicatilis and marine Chlorella as regularly practised in Japan and particularly in SISFFA (1) Centers, are described in this paper.
(1) SETO INLAND SEA FARMING FISHERIES ASSOCIATION.
The volume for Chlorella culture is 4 – 5 times that required for rotifers. The Chlorella is inoculated into tanks filled with sand filtered seawater together with a nutrients medium, which is introduced into the Chlorella growing tanks, once every 4 – 7 days, depending on conditions of temperature and illumination. The medium is contained in aqueous solutions. Different medium compositions used in some SISFFA Centers are described in Table II.
The Chlorella culture tanks are vigorously aerated either through airstones or by submerged pumps. The temperature required varies between 20 – 25 °C. At higher temperatures algal development may be faster, but the addition of fertilizers and nutrients favours rapid development of protozoa which inhibit the growth of Chlorella (Rothbard, 1975).
The system is based on continuous culture. The initial density of Chlorella should be approximately 1.5 × 107 cells/ml. At harvest, after five days of growing the density increase up to 3 – 4 × 107 cells/ml. The Chlorella density can be measured either spectrophotometrically using filter No. 54 or by counting with a hemacytometer.
The basic unit for rotifer mass culture is a rectangular tank with dimensions of 100×100×150 cm (1.5 m3). Two sets of tanks are required, enabling alternate daily cropping in a 48 hours cycle of growing. A 15 inch drain pipe controlled by a value is fitted to the bottom of each tank. The rotifer culture tanks are aerated vigorously. Air is supplied by means of a perforated plastic pipe located parallel to the length of tank bottom. A constant air flow of 24 1/min/m3 keeps the rotifers and the medium in permanent suspension.
To prevent accumulation of faeces and excretions of the rotigers, three filters are immersed in each tank. These are constructed from perforated buckets filled with nylon threads and activated by an airlift. The filters are removed daily and the nylon threads cleaned with water. A schematic presentation of culture tank is shown in Fig. 1.
The temperature in culture tanks is kept constant by means of a thermostatically controlled 1Kw heating element immersed in the water. Two systems of rotifer mass-culture are practised in Japan, based on different temperatures in the rotifer tanks. In the first system the rotifers are grown at 30 – 33 °C in a 48 hour cycle, while in the second system they are grown at 26 – 28 °C in a 72 hour cycle. Mean size of the rotifers is about 150 μ or 250 μ in the 48 and 72 hour cycles respectively. Both sizes of rotifers are required to enable feeding the shrimp and fish larvae with zooplankton for longer periods.
The salinity of the Chlorella medium in which the rotifers are grown is about 2.3% (60% sea water). Dense Chlorella cultures are diluted with adequate volumes of fresh water and heated to a suitable temperature before being transferred into rotifer culture tanks. The Chlorella set up and manipulations are presented in Fig. 2.
Before the rotifers are inoculated into the diluted Chlorella medium, the pH is adjusted to 8.0 by addition of HCl. The volume of HCl to be added at different pH ranges is presented in Fig. 3.
The pH and temperature are checked daily.
The rotifers are inoculated into growing tanks by means of fine mesh net (about 0.5 mm) which prevents entry of large pieces of dirt but enables introduction of rotifers.
In 48 hour cycles the initial rotifer density should be 220 – 250 adults/ml. The population with the highest proportion of eggs to adults is chosen for inoculation (see 3.4). This is predicted from the last prestocking count.
The rotifers in each growing tank are counted daily, in the morning and afternoon. These counts are required to monitor the population growth and for estimating the amount of yeast to be fed to the rotifers.
Random samples are collected from each tank into 100 ml. beakers. After mixing contents of the beakers, two samples of 1 ml each are taken from each beaker and placed on watch glasses. Three or four drops of an I-KI solution (consisting of I-5 g and KI-10 g in 200 ml H2O) are dripped into each sample killing the rotifers and enabling the count by means of a binocular microscope.
In each sample the number of adults and eggs is counted separately. The proportional fertility of the population is the ratio of eggs to adults. The population with the highest proportion is used as inoculum for the next growing cycle (see 3.3). The relation between monthly average adult density and proportional fertility in 48 hour cycles is shown in Fig. 4.
The Chlorella provide and adequate food supply for the rotifers only during the first 10 – 14 hours of its growing cycle. When the density of Chlorella decreased to about 2.5 × 106 cells/ml another source of food is required for the rotifers. This supplementary food consists of marine or bread yeasts in slightly diluted sea water (75%) with the addition of EDTA 32:1 g EDTA 32/100 g yeast/1 1 of water. This solution is dripped continuously into the rotifer tanks during the day by means of a drip pump, or manually at frequent intervals. The weight of yeast supplemented depends on the density of rotifers. Yeast feeding regimes during 48 hour and 72 hour cycles are shown in Fig. 5.
Harvesting is carried out by means of 2 inch syphon pipe through a fine mesh sack of 60 – 70 μ with a surface of about 1 m2. This enables concentrating the rotifers into a small volume of water before feeding them to the fry. It takes about 15 minutes to harvest a tank of 1.5 m3. After harvesting each tank is cleaned and prepared for the next stocking.
After harvesting the rotifers can be kept for a short time in a strong aerated portable tank. The work schedule is presented in Fig. 6, for the different manipulations and activities involved in rotifer mass culture, the labor requirement is 2 trained technicians.
Acknowledgements
I would like to thank SISFFA for enabling my participation in the research activities at Tamano Center, and Dr. G. Wohlfarth and Dr. G. Hulata for their help in preparation of the manuscript.
Hirata, H., 1975 An introduction to the rearing method of prawn Penaeus japonicus (Rate), in Japan. Mem. Fac. Fish., Kagoshima Univ., 24: 7 – 12.
Hirayama, K., K. Watanbe and T. Kusano, 1973 Fundamental studies on physiology of rotifer for its massculture. III. Influence of phyto-plankton density on population growth. Bull. Japan. Soc. Sci. Fish., 39: 1123 – 1127.
Hirayama, K. and K. Watanbe, 1973 Fundamental studies on physiology of rotifer for its mass culture. IV. Nutritional effect of yeast on population growth of rotifer. Bull. Japan. Soc. Sci. Fish., 39: 1129 – 1133.
Hirayama, K. and K. Nakamura, 1976 Fundamental studies on the physiology of rotifers in mass culture. V. Dry Chlorella powder as a food for rotifers. Aquaculture, 8: 301 – 307.
Howell, B.R., 1973 Marine fish culture in Britain. VIII. A marine rotifer Brachionus plicatilis (Müller), and the larvae of the mussel Mytilus edulis L. as foods for larval flatfish. J. Cons. Int. Explor. Mer., 35: 1 – 6.
Howell, B.R., 1974 Problems associated with the feeding of certain flatfish larvae. Informes Technicos de Inst. Pesq., 14: 109 – 116.
Hudinaga, M. and Z. Kittaka, 1966 Studies on the food and growth of larval stages of a prawn Penaeus japonicus, with reference to the application to practical mass culture. Inf. Bull. Plank. Japan., 13: 83–94.
Ito, T., 1960 On the culture of mixohaline rotifer Brachionus plicatilis O.F. Müller in the sea water. Rep. Fac. Fish. Mie Pref. Univ., 3: 708 – 740.
Liao, J-chiu, 1969 Artificial propagation of grey mullet, Mugil cephalus L. I.C.R.R. Fish. Secr., 8: 10 – 20.
Ling, S.W., 1969 A brief review on the work done on the induced breeding of Mugil cephalus L. in Taiwan. Jour. Inl. Fish. Soc. India, 1: 1 – 13.
Nash, E.C., C.M. Kuo and S.C. McConnel, 1974 Operational procedures for rearing larvae of the Grey Mullet (Mugil cephalus L.). Aquaculture, 3: 15 – 24.
Rene, F., 1974 Rearing of Gilt-Head Sparus aurata. In: The Early Life History of Fish. (J.H.S. Blaxter, Ed.) Springer Verlag, Berlin. 765 pp.
Rothbard, S., 1975 Control of Euplotes sp by formalin in growth tanks of Chlorella sp. used as growth medium for the rotifer Brachionus plicatilis which serves as food for hatchlings. Bamidgeh, 27: 101 – 109.
Rothbard, S., 1977 Observation on adult forms and experiments in growth of larvae of the freshwater shrimp Macrobrachium nipponense (De Haan). Bamidgeh, 29: 115 – 124.
Table I. Species of shrimps and fish whose fry are cultivated on the rotifer Brachionus plicatilis
| Species | Fry stage | References |
| Penaeus japonicus | zoea, mysis. | Hudinaga and Kittaka (1966) |
| - " - | mysis | Hirata (1975) |
| Macrobrachium nipponense | zoea | Rothbard (1977) |
| Portunus trituberculatus | zoea, megalopa | Rothbard (1977) |
| Mugil cephalus | fry | Ling (1969), Liao (1969) |
| Nash et al (1974) | ||
| Sparus aurata | fry | Rene (1973) |
| Solea solea | fry | Howell (1973, 1974) |
Table II Medium compositions administered to Chlorella culture tanks at different SISFFA Centers
| SISFFA Centers | Chemicals of fertilizers | Weight/m3 | Day intervals at which the medium is administered |
| TAMANO | MG 1(2) | 20 g | every 4 – 5 days |
| (NH4)2 SO4 | 50 g | ||
| Urea | 2.5 g | ||
| Ca (H2PO4) | 7.5 g | ||
| EDTA 32 | 1.5 g | ||
| HAKATAJIMA | Ca (H2PO4) | 15 g | every 7 days |
| Urea | 10 g | ||
| EDTA 32 | 10 g | ||
| (NH4)2 SO4 | 100 g | ||
| KAMIURA | (NH4)2SO4 | 40 g | every 7 days |
| EDTA 32 | 4 g | ||
| Urea | 4 g | ||
| H2PO4 | 4 g | ||
| Acetic Acid | 40 ml |
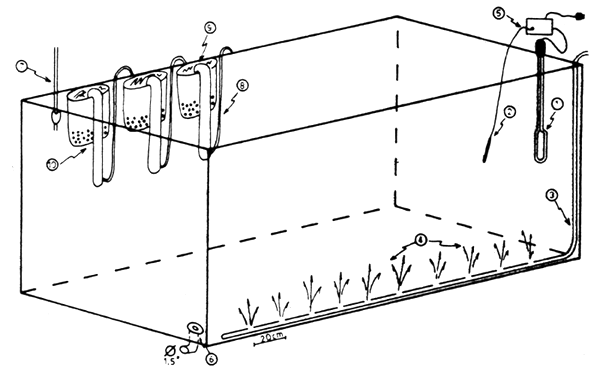
Fig. 1. Schematic view of a 1.5 m3 rotifer culture tank.
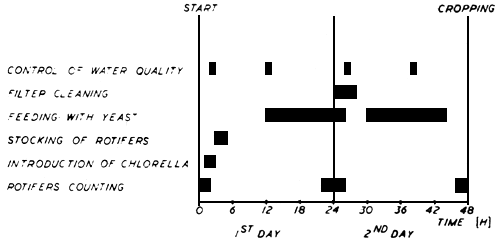
Fig. 6. The activities and manipulations involved in 48 hours cycle of rotifers growth.
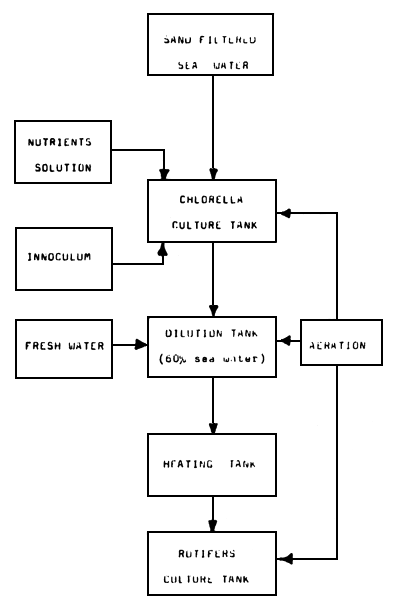
Fig. 2. Flowchart showing activities and manipulations concerned with Chlorella culture.
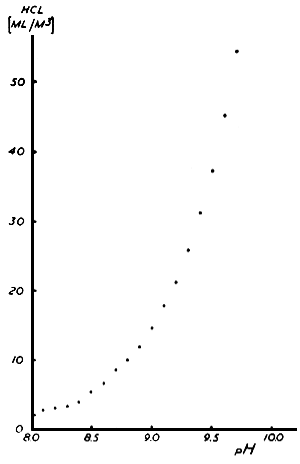
Fig. 3. The amount of HCl to be added into rotifer culture tanks for the control of pH level.
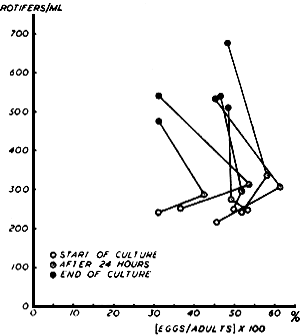
Fig. 4. The relation between monthly average adult densities and proportional fertility in 48 hour cycles (Each line represents monthly average of 150 culture tanks).
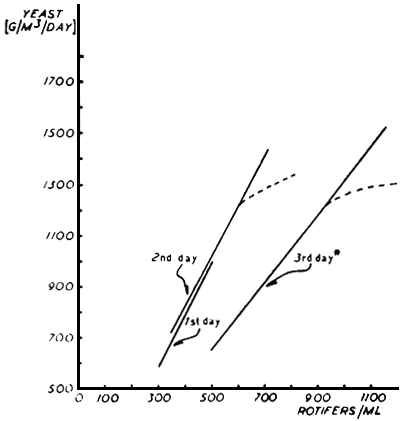
Fig. 5. The amount of yeast used for feeding the rotifers in relation to density of rotifers during 48 and 72 hour cycles (Broken line describes the addition of yeast in practice).