R.A. Ryder
Ontario Ministry of Natural Resources
Thunder Bay, Ontario, Canada
and
S.R. Kerr
Marine Ecology Laboratory, Bedford Institute of Oceanography
Dartmouth, Nova Scotia, Canada
ABSTRACT
The introduction of exotic species into native fish communities has been a popular approach toward the management of oligotrophic lakes during the past century. The high level of risk involved, however, all but precludes the usual approach to fish introductions which are as likely to be damaging as successful. Three case histories of introductions, looked at retrospectively, suggest that in some cases, at least, the outcome of an introduction may be predictable. For north-temperate, oligotrophic waters, the greatest likelihood of success lies in the use of species that have co-evolved in glacial refugia but may have become allopatric through the vagaries of redistribution following glacial recession. The risks involved in planting new species may be greatly reduced through a priori consideration of several ecological principles such as niche theory, interactive segregation, dominance-subordinance, and resource partitioning. Each of these hierarchically-associated principles is consistent with the concept of niche, both fundamental and realized, and major niche dimensions of candidate species for introduction should be quantified whenever possible and compared with those species comprising the indigenous fish community. A resulting high level of niche complementarity of the candidate species with the various components of the native community will increase the likelihood of success of an introduction.
RESUME
La faune piscicole des régions du Canada envahies par les glaces au Pléistocène a réintégré son habitat original il y a 8 000–12 000 ans, lorsque les glaciers ont reculé. Elle a survécu à la glaciation dans des refuges situés dans le sud, l'est et le nord-ouest, où il y a en stabilisation des communautés sous l'effet de très longues interactions spécifiques et, par la suite, de la complémentarité des niches. Elle a réintégré son habitat original en passant par les grands lacs du Pléistocène et les cours d'eau qui les relient, et une nouvelle différenciation des niches a eu lieu sous l'effet des interactions collectives et des contraintes écologiques.
L'introduction d'espéces exotiques dans ces réseaux assez fragiles n'a parfois eu aucun effet notable; en revanche, dans d'autres cas, elle a eu des conséquences très importantes, rendant la pêche imprévisible et non rentable, notamment lorsque les dimensions des niches des espèces indigènes se sont trouvées réduites par la concurrence ou le prédatisme des espèces exotiques. Souvent, un seul facteur écologique abiotique a déterminé les résultats de l'interaction.
Les auteurs présentent plusieurs études de cas, avec leurs consêquences pour l'aménagement.
The introduction of an exotic fish species into an indigenous fish community is often viewed as a constructive effort to increase fishery yield by the manager who has experienced repeated frustration in restoring an ailing fishery to a semblance of its pre-stressed condition. Undoubtedly, this approach may have measurable effects on the autochthonous species comprising the natural fish community, but the outcome may be viewed as either beneficial, mitigative or destructive, depending on the anthropocentric values that might be perceived to derive from such introductions. The greatest initial impact, however, will be expressed politically, in the sense that the fishery manager may appear, to both his superiors and the public alike, to have judiciously applied modern management technology in order to enhance a fishery. Essentially, the fishery manager is then perceived to have acted with alacrity and intelligence, and thereby improved his image amongst his clientele public, if not his peers. On the other hand, public or peer retribution for a damaging fish introduction is rarely exacted, as a sufficiently lengthy time span will usually lapse before the effects of an ill-advised planting is conclusively documented. This typical sequence of events precludes the immediate assignment of blame in such actions, the results of which may be amenable to various subjective interpretations.
Because of the somewhat cavalier approach to fish introductions during the past century, when most stocking took place without informed regard for the biological consequences, the practice of management by species manipulations has, perhaps, been overly maligned. In North America, the almost immediate penetration of carp (Cyprinus carpio) into indigenous fish communities, following deliberate introductions, and their subsequent widespread invasion of contiguous waters has been amply documented (e.g., McCrimmon, 1968). Evidence of the effects of the successful establishment of carp on native fish species, particularly in shallow, more fertile bays of oligotrophic lake basins, has been less well documented but is generally felt to be negative (Scott and Crossman, 1973).
Other notable, well publicized, and apparently unfortunate introductions into North American waters emanating from the Old World fauna include those of the grass carp (Ctenopharyngodon idella) and the walking catfish (Clarias batrachus). The former species was first introduced into an open system in Arkansas in 1971 (Guillory and Gasaway, 1978). Special consideration was directed to the ecological role the grass carp might play in the elimination of macrophytes from lakes (Mitzner, 1978) but relatively less scientific rigour was directed toward the potential side effects it might have had on an indigenous fauna, either locally or within the major accessible watershed.
The walking catfish, apparently introduced inadvertently as an escapee from the ornamental aquarium trade in Florida, is usually considered to be an ecological disaster (Courtenay and Robins, 1975). Numerous other examples of potentially disruptive introductions of non-indigenes occur in North America, but some exotic species that have become established in the form of substantial, reproducing populations, are often viewed favourably by certain user groups. Of the latter, two introduced trout species, the brown trout (Salmo trutta), a transplant from European waters, and the rainbow trout (Salmo gairdneri), introduced widely throughout eastern North America following its importation from the west, have often been considered as not only successful emigres (e.g., Courtenay and Robins, 1975) but also as highly desirable ones by the sport fishing faction. In both instances these species probably have displaced the native brook trout (Salvelinus fontinalis) or the Atlantic salmon (Salmo salar) to varying degrees. This displacement has likely been effected through food or reproductive competition in the case of the former species, or because of the proclivity of the two emigres to be better adapted physiologically to partially degraded (on the trophic scale), oligotrophic environments than was the Atlantic salmon. Despite the fact that the rainbow trout was introduced into the Great Lakes region in 1895 (Scott and Crossman, 1973), it is still in the process of adaptive radiation, extending its range as cultural influences on environmental factors swing the balance of survival in its favour, to the detriment of the native brook trout. Cutting of the forests and the attendant increase of mean stream temperatures, appear to be critical factors in this process, which, in many instances, has created marginal or sub-marginal environmental conditions for brook trout, particularly in the lowest reaches of some streams tributary to the Great Lakes (Fig. 1). The rainbow trout/brook trout relationship therefore would seem to be dependent on the ambient range of one or more physical factors such as temperature, calcium carbonate concentration or pH, and the determination of which species might be dominant, in the sense of Svardson (1976) may depend on the values of these variables, or other physical factors at critical periods, such as those of spawning or larval emergence. The ready adaptability and further expansion of the rainbow trout in eastern North America has been viewed by many as an enhancement of the sport fishery resource. Its impact has penetrated the socio-economic system because of the need for specialized gear for the capture of rainbow trout, and the associated requirements for special seasons and creel limits. On the other hand, inveterate brook trout anglers often view the continuing encroachment of the rainbow trout into brook trout territory with dismay, particularly when an obvious decline in the availability of their favoured species ensues. The need for reconciliation of these differences of opinion between two user groups is one that should be anticipated by the fisheries manager before an introduction is effected, in order that appropriate measures may be taken to mitigate the perceived negative effects by one or more client groups. Following an introduction, experience shows that solutions to user group conflicts may become tedious and, often, totally intractable.
To this day, the dilemma persists of judging the relative benefits of exotic fish introductions versus their potentially disruptive influence on native fish communities. We shall approach this problem with the perspective that native fish communities, comprised of both indigenous species and stocks (e.g., Loftus, 1976) in environments relatively unperturbed by man, best optimize the resources of their environment, all factors considered, and consequently produce the highest sustainable yields (Ryder et al., 1981). But in the face of ubiquitous and enduring environmental degradation, a realistic attitude may opt for maximizing yield through introductions of stenoecious fishes (narrow range of environmental tolerance) rather than wait for the implementation of environmental rehabilitation (Regier, 1968), which itself may not be economically practical in the foreseeable future, if ever. In essence, then, our approach condones exotic species introductions in cases of extreme and persistent environmental degradation, or in other exceptional instances, with the rigorous proviso that adequate background studies afford supportive evidence for the likelihood of success, not only of the introduction, but also of the successful integration of the remnants of native fauna that exist in the degraded environment, insofar as practicable.
The decision of when to forego rehabilitation in favour of a fish introduction that may prove ultimately to be irreversible, is not only a philosophically tenuous problem, but is pragmatically intractable as well. For example, a technological solution to a persistent environmental stress may be just around the corner, but its subsequent utilization may be preempted through the introduction of a new species. Consequently, the vital decision between environmental rehabilitation as opposed to species introductions, is one of the most difficult ones that a fisheries manager will ever have to make and should be given careful consideration from ecological and socio-economic points of view, and should be measured against various time-scales in order to determine optimum long-term benefits. In offering these recommendations, we issue the caveat that a substantial to moderate probability of failure can be expected in successfully integrating the emigre with the native fish community. In other words, the introduction of exotics must always, in some measure, be a game of chance (Magnuson, 1976) although the seemingly insurmountable odds against the fisheries manager may be perceptibly reduced through careful, prior application of fundamental ecological principles to any contemplated introduction.
When environments have been altered or degraded to a point of unacceptable return, an introduction of an exotic species may make possible the development of a viable fishery where none previously existed. In such instances, careful assessment of various ecological factors should be made, insofar as relevant, both in the abiotic and biotic sectors of the ecosystem. A most convenient framework within which to pursue this assessment is the niche concept, which embodies all the environmental limitations and interactions of an organism, together with those affecting other organisms in the community and its abiotic environment. The niche concept we espouse, combines the synecological approach of Hutchinson (1957) with the autecological approach of Fry (1947), as set out by Kerr and Ryder (1977). Suffice it to say here that we consider the ecological niche to be an organismal attribute which is genetically determined and which constrains the role of an organism within the aquatic community and within the abiotic environment, mediated, in turn, by the feedback restrictions imposed on the organism by both the community and its abiotic environment. Accordingly, niche comprises multiple dimensions that describe the feeding, or reproductive or other capabilities of an organism and its ability to survive within the environmental boundaries established by various physical and chemical factors such as temperature, oxygen, light and depth as an important subset of limiting or controlling factors. Each fish species, therefore, might be conceived as enclosed and constrained by a multi-dimensional envelope of survival capability, the contours of the envelope determined by innate genetic characteristics phenotypically expressed as genetic potential or potential niche.
In nature, this genetic potential along each dimensional axis is compressed through the organism's interactions with other organisms as well as with the abiotic environment, to create an operational or realized niche. Within fish communities which have co-evolved, the realized niches of component species have achieved levels of mutual compatability or complementarity, such that a measure of undirected mutual altruism has been attained. We have labelled these aquatic communities as “harmonic” (Ryder and Kerr, 1978; Ryder et al., 1981) because the consequent longterm co-evolution has engendered a perceivable degree of mutually beneficial integration among component species. Harmonic communities lacking severe anthropogenic stresses tend to persist as predictable entities, and exhibit robust levels of resilience under substantial removal rates (e.g., Grossman, 1982).
Astatic communities (Ryder and Kerr, 1978) on the other hand, are consequences of stochastic assemblages of fishes and other organisms, resulting from unplanned introductions or inadvertent invasions of highly opportunistic species, often r-selected, and following environmental degradation (e.g., MacArthur and Wilson, 1967). Astatic fish assemblages are characterized by a boom or bust syndrome, providing highly variable yields and highly variable species composition within composite yields from year to year. The fishing history of Lake Erie over the last 150 years reveals a change from a harmonic community to, more recently, an astatic assemblage of fishes responding opportunistically to various cultural stresses including harvest, eutrophication, toxic wastes and exotic species introductions or invasions (Regier and Hartman, 1973). We suggest that had not the first three stresses been present on a long-term basis, the fortuitous presence of exotic species, from whatever source, would have made much less of an impact on the native harmonic community, whose community niche attributes would have kept them closely in tune with their relatively unstressed environment. This condition, in turn, would contribute to the capacity of the indigenous community to successfully resist the intrusions of new opportunistic species (e.g., Christie et al., 1972).
To propose a “thought experiment”, if all the fish stocks of Lake Erie were catastrophically eliminated, and all other cultural stresses were suddenly removed from the lake, what would be the most appropriate measures a fisheries manager could take to restore a viable and predictable fishery once the environment returned to its previously natural state?
One possible approach might be to select a cross-section of the world's best food or game fish species for introduction into Lake Erie, without regard for any ecological considerations, but with the knowledge that natural environmental rigours would eliminate many species from the mix in short order, usually during the passage of only one winter in the case of tropical species. In time, the effects of interaction among the survivors might run its course, and co-evolution might again be perceived as the sculptor of a new harmonic fish community. Unfortunately, the time scale of evolution hardly coincides with that of a fisheries manager's expectations, and an equilibrium ecological solution to this scenario would not likely ensure before additional perturbations to the system occurred because of realized impatience on the human time-scale.
A more realistic approach to the problem, given Lake Erie in a limnological condition similar to that of 150 to 200 years ago, would be to attempt a reconstruction of the indigenous fish community as it was perceived to be at that time (e.g., Ryder, 1972). For the most part this would involve reintroduction of originally native fish species done in the most appropriate manner, that is, by planting sub-species and individual genetic stocks to fill the community hiatuses that would exist, in numbers and kinds proportional to the energetic processes of each trophic level. While the fishes utilized would be drawn from lakes other than Lake Erie and therefore would not be perfectly nichetuned to that particular lake, on an evolutionary basis they should be sufficiently close to the archtypal genetic strains that one might expect the development of a new harmonic community over several generations, rather than aeons. The greatest chance for success in harmonic community establishment, therefore, would be close emulation of the available, co-evolved natural scheme insofar as possible. In accomplishing this goal, the niche concept is implicit at each taxonomic and hierarchic level of ecological organization, up to that of the integral biotic community.
North-temperate lakes comprise a major portion of the world's natural lakes (Fernando, 1980) and of these, most have formed following the recession of continental glaciers (Flint, 1957). In Canada, because of the immense areas of refractory substrate of the Precambrian Shield, most glacial lakes are relatively low in nutrient content, that is, they are oligotrophic or at the low end of the mesotrophic range. Oligotrophic lakes are apparently more vulnerable to the destabilizing effects of exotic fish introductions than lakes which are eutrophic (Li and Moyle, 1981). Accordingly, we confine the remainder of this discussion to glacially derived lakes of North America, including exemplary case histories, assuming that the principles underlying the debilitating effects of exotic introductions may be clearer, but similar in principle, to introductions in eutrophic lakes and other complex systems. Hence the fundamental concepts revealed by oligotrophic introductions should be applicable to eutrophic and similar systems, but differently scaled. The biological buffering effect of large assemblages of many fish species in complex eutrophic systems may mask the ecological effects of exotic fish introductions that we wish to demonstrate.
Many glacially-derived lakes of North America were repopulated with aquatic fauna from three or more major refugia (McPhail and Lindsey, 1970), while the Laurentian Great Lakes received their fauna principally from two refugia, located within the Mississippi and Atlantic drainages. The native fish fauna of the large to medium-sized inland glacial lakes is moderately depauperate, relative to the Laurentian Great Lakes, and species often number less than thirty (e.g., Keleher, 1972) but more commonly less than twenty in total, depending on post-glacial accessibility (Ryder et al., 1964). Very small, glacially-derived lakes might have ten fish species or less (e.g., Fraser, 1972) and occasionally such lakes are barren of any fish because of the lack of access to Pleistocene refugia or because of episodic events of ecological significance such as periodic winterkill.
Because the numbers of fish species in glacial lakes are low, the numbers of potential interactions amongst them are relatively few, and consequently, alternate pathways for energy transfer are restricted. Egress to glacial lakes by fishes may have occurred on the order of 8 000–12 000 years ago (McPhail and Lindsey, 1970) a short time-scale in evolutionary terms, but it may be assumed that co-evolution of coldwater fish communities occurred not only within glacial refugia, but during several to many interglacial periods as well. Recent (8 000–12 000 BP) natural immigrants to glacial lakes, therefore, may be essentially co-adapted in terms of niche compatability or complementarity, but because of the restricted numbers of alternate pathways for interaction in a moderately depauperate fauna (e.g., Welch, 1967) may be presumed to be in a relatively delicate balance of dynamic equilibrium. Invasion or introduction of exotic species into these relatively uncomplicated, harmonic, species complexes would generate reverberations throughout the community that would decay monotonically over long time periods until new dynamic steady states are reached (Ryder et al., 1981). One current ecological theory suggests that removal of a perturbation from a system does not necessarily guarantee the system's return to the original dynamic equilibrium, if the perturbation took it beyond its response capabilities. In such an instance the system tends toward the equilibrium of a new resident attractor region (Peterman et al., 1979) or new median of central tendency (Ryder and Kerr, 1978). In terms of species introductions into glacial lakes, an essentially salmonid community in a marginally mesotrophic lake could convert readily to a predominantly percid community with the introduction of only one or two of the latter species (Ryder and Kerr, 1978). Events such as this should be predictable by fisheries managers, given the appropriate application of unifying concepts such as niche theory (Kerr and Ryder, 1977), dominance principles (Svardson, 1976) or the concepts of interactive segregation (Nilsson, 1967) and resource partitioning (Schoener, 1974). These hierarchically related concepts, together with their governing principles and modes of utilization, converge in their application, as we shall demonstrate subsequently.
The best documentation showing the effects of the invasion of an exotic species into oligotrophic aquatic systems in North America, is probably that of the sea lamprey (Petromyzon marinus) penetration into the three upper Great Lakes following the construction of the Welland Canal in 1829, which allowed passage around the natural physiographic barrier created by Niagara Falls (Applegate, 1950; Lawrie, 1970). Because of the massive literature on this inadvertently caused invasion, only a brief account will be provided here, specifically related to some of the major ecological considerations.
Prior to the ingress of the sea lamprey into Lakes Huron, Michigan and Superior, the indigenous fish communities of the limnetic waters were essentially of the salmonid (salmonine-coregonine) type (e.g., Koelz, 1979; Lawrie and Rahrer, 1972) which were regulated by two large, piscivorous top predators, the lake trout (Salvelinus namaycush) and the burbot (Lota lota). The former species was an active predator in all zones of the lake where ambient temperatures were favourable, which effectively excluded them only from shallow bays and some epilimnetic waters during summer months. In Lake Superior, numerous phenotypic varieties of lake trout co-existed, which were variously adapted to feed or spawn or otherwise occupy characteristic habitats at particular times. For example, some lake trout were river spawners (Loftus, 1958) while others spawned on shallow or deep lake shoals. Spawning times for lake trout varied from June through November (Eschmeyer, 1957) although most spawning probably occurred in October. Some stocks fed pelagically while others fed on the benthos. The lake trout then, appeared to be exploiting its environment efficiently through a remarkable phenotypic radiation of specialized morphotypes, which themselves developed in response to multiple environmental opportunities.
The second major piscivore of the three upper Great Lakes, the burbot, fed benthically for the most part, and competed with the lake trout for a limited supply of forage fishes (Bailey, 1972) mainly in the form of a species flock of coregonines, Coregonus (Leucichthys sp.), although other prey species were important at times, particularly one of the cottids, Myoxocephalus quadricornus, and the nine-spined stickleback, Pungitius pungitius (Smith, 1968). There is substantial evidence that the lake trout and burbot effectively partitioned their food resources on the basis of temporal and spatial separations, and that the combined effects of these two top predators were the primary force in the maintenance of the steady-state condition within the remainder of the coldwater community (e.g., Smith, 1968).
The effects of the penetration of the sea lamprey into these coldwater communities were difficult to assess initially because of the complications afforded by other exogenous stresses already in place and probably incrementing on an annual basis (Loftus and Regier, 1972). These stresses included exploitation, cultural eutrophication and related effects, and the introduction of, or invasion by, other exotic species such as the rainbow smelt (Osmerus mordax) and the alewife (Alosa pseudoharengus) to mention but two that inhabited the same limnetic areas of the lakes and comprised a large proportion of what had become an astatic assemblage of fishes, particularly relative to the pelagic factions of the coregonine and lake trout complex.
The initial impact associated with the advent of the sea lamprey was the rapid and virtual elimination of the two large predators, the burbot and lake trout, and the subsequent release from predation of various prey species, some of which had apparently been held in predation refugia by these two efficient predators (Ryder et al., 1981). Indeed, it is conceivable that the phenotypic variability expressed in the cisco complex (Koelz, 1929) was created by isolation in predation refugia caused by the highly effective predation of lake trout and burbot. The subsequent release of the small-sized stocks of this genus from efficient and persistent predation was followed by radiation from the predation refugia, which, in turn, may have created conditions favourable for introgression (Smith, 1964). The evidence for introgression between stocks in the cisco complex was expressed in terms of morphological blending, to the point where many of the original systematic characteristics no longer had utility for the identification of the phenotypic stocks originally described by Koelz (1929). In summary, the total coldwater community reverted to an astatic assemblage, a condition of flux and of unpredictability. The loss of the two top predators not only released prey species from predation pressures, but perhaps paved the way for additional exotic prey species to invade or expand (e.g., Christie et al., 1972).
The alewife invaded Lake Superior about 1954 (Miller, 1957). The rainbow smelt, which had already become established prior to the advent of the sea lamprey, continued to expand, presumably at the expense of the native limnetic coregonines, through increased competitive and predation pressures (Anderson and Smith, 1971; Selgeby et al., 1978). By 1954 the lake trout was already well on the way to its precipitous decline in Lake Superior (Lawrie, 1970) a factor which, no doubt, contributed to the opportunistic expansion of the alewife in that lake (Miller, 1957). Both the alewife and the smelt, which benefited from the sea lamprey-induced losses of the lake trout and burbot, in turn, affected other native species through their ability to opportunistically capitalize on abundant food resources. Reverberations within the total fish community persisted (e.g., Smith, 1970: Anderson and Smith, 1971) from the secondary effects of rainbow smelt and alewife invasions, and new steady-state conditions have not yet been attained in Lake Superior, more than thirty-five years following the initial recorded invasion of the sea lamprey.
The case history of the sea lamprey in the Great Lakes has interesting implications within the context of exotic fish introductions because of its clear exemplification of the utility of the niche concept (Kerr and Ryder, 1977). Prior to the advent of the sea lamprey in the upper Great Lakes, there were no terminal predators apart from man, that consistently and effectively preyed upon adult lake trout and burbot. The strategy of the sea lamprey was, in effect, opposite to most predators in which maximum food size may be related to mouth gape or body dimensions (Keast and Webb, 1966). In the case of the sea lamprey, the larger the prey, the greater the likelihood it would survive the attack (Hall and Elliot, 1954) and thereby lessen the need for repetitious food search by the individual lamprey. The niche role of the sea lamprey, therefore, was more akin to that of a parasite than a predator, and its particular niche function was unrealized in the Upper Great Lakes Basin prior to its invasion. It may be hypothesized that given sufficient evolutionary time, a steady-state between the sea lamprey and the lake trout and burbot may be achieved through mean size reduction of the lamprey with a proportional reduction in the mortality rates caused by the lamprey on lake trout and burbot. This relationship has apparently been realized in the finger Lakes of New York where the sea lamprey is believed to be endemic and the primary host (lake trout) maintained itself at high levels (Webster et al., 1959). In that instance, the sea lamprey mean size is substantially less than those found in the Upper Great Lakes.
The management implications of the foregoing considerations are intriguing. If the sea lamprey were economically valuable as it is in Europe, an enterprising fisheries manager may have recommended its introduction into the Upper Great Lakes as an alternative food source, or perhaps even as a valuable gourmet item. Further thought along ecological lines would have revealed that the sea lamprey's niche role was not being realized in the Great Lakes, and that once introduced it would likely have a substantial chance of survival as its potential food resources were abundant, in the form of large standing stocks of lake trout and burbot. Further consideration along ecological lines might have revealed that many of the potential sea lamprey spawning streams had temperature regimes below optimum for sea lamprey reproduction and rearing (e.g., Applegate and Smith, 1950) but that the prevalent cutting of the forests in the Great Lakes watershed promised to provide streams with amenable spawning and nursery conditions (Lawrie and Rahrer, 1972).
In short, the example of the sea lamprey as a successful invader of the Upper Great Lakes could perhaps have been predicted on the basis of analyses of relatively few niche dimensions, that is, preferred food, optimum spawning, and early life history temperature regimes. Similar analyses for other species considered for introduction could be undertaken and while the outcome may not necessarily be as predictable as was the retrospective case with the sea lamprey, reasonable assessments with acceptable measures of confidence could probably be made.
The smallmouth bass (Micropterus dolomieui) has been successfully introduced into many oligotrophic lakes of the Precambrian Shield in southern Canada and the northern United States (Scott and Crossman, 1973). Of particular concern has been its potential effect on the lake trout, another top predator native to these lakes, as well as to the salmonid communities in which the lake trout normally occurs (Ryder and Kerr, 1978).
Although the smallmouth bass on the Precambrian Shield originated from at least one of the same glacial refugia as the lake trout (Mississippi), the total extent of their subsequent northward radiation in Canada following glacial recession, was rather restricted relative to the lake trout. The temperature limitations seem to be the most obvious factor to be considered in this regard (e.g., Hubbs and Bailey, 1938; Rawson, 1945; Coble, 1967; Christie and Regier 1973; Shuter et al., 1981). Generally, temperatures less than 10°C inhibit feeding, reproduction, growth and certain other metabolic processes in the smallmouth bass while the lake trout functions well within these thermal bounds. The temperature limitation, however, has not been nearly as rigorous nor as general as the 18°C summer isotherm limitation proposed by Radforth (1944). Many robust populations of smallmouth bass exist well north of that line in the Hudson Bay watershed of Ontario as a result of introductions and they appear to be extending their range to this day. The northward radiation of the smallmouth bass from its refugium, or refugia, may well have been restricted by physiographic barriers such as watershed divides, rather than thermal ones, of which the latter might have delayed its radiation to a substantially later date relative to another sympatric predator in the Mississippi refugium, the lake trout. Effectively, lake trout reinvasion may have been better assisted by the cold, ephemeral, post-glacial drainage patterns than was the smallmouth bass with its higher temperature optimum. These physiographic barriers then, may have prevented the smallmouth bass from access to both the Hudson Bay watershed and the Algonquin highlands in Ontario, although they demonstrably can thrive there today in both locations following introduction (Scott and Crossman, 1973). Whether these successful smallmouth bass introductions north of their natural range are enduring or not, depends on several facets of climate and demography interacting to determine survival rates (Shuter et al., 1980). Most of these introductions have a history of less than fifty years.
The significance of glacial history, glacial refugia and subsequent radiation patterns of the smallmouth bass, relates to its successful integration into, or at least, coexistence with salmonid communities. Essentially, the smallmouth bass has evolved in at least one glacial refugium in which substantial components of salmonid communities occurred. In a strict sense then, the smallmouth bass should not be considered as an exotic species when reintroduced into salmonid communities, especially in the boreal forest zone of the Precambrian Shield (Ryder and Kerr, 1978).
The smallmouth bass was introduced into the Algonquin Park Region of Ontario in the early 1900s and subsequently spread or was reintroduced into many lakes within the area (Christie, 1957). The native fauna of most of these lakes consisted of salmonid communities, integrated through the predation of lake trout and burbot (Martin and Olver, 1980). There is no indication that the introduction (or reintroduction in evolutionary terms) of the smallmouth bass into the salmonid communities of Algonquin Park had any appreciable effect on any of the community elements with the possible exception of the brook trout (Martin and Fry, 1973).
The brook trout was vulnerable to the invasion of the smallmouth bass for evolutionary, zoogeographic and ecological reasons. It most likely survived Pleistocene glaciation in an eastern refugium (Radforth, 1944) and sympatry in such a refugium with the smallmouth bass is unlikely. Much of the original ranges of the brook trout and smallmouth bass (e.g., Scott and Crossman, 1973) were mutually exclusive and overlapped mainly in the Great Lakes region where fauna originated from both Atlantic and Mississippian refugia (Bailey and Smith, 1981). Even there, the brook trout and smallmouth bass tended not to co-occur and were spatially segregated according to their physiological tendencies to seek different abiotic optima. In lakes, both species are essentially littoral-bound, and the potential antagonistic behaviour may be another possible mechanism for interactive segregation of the species (Nilsson, 1967). In total, too many forces of an evolutionary, zoogeographic and ecological nature are in place to ever allow mutual compatibility in a single habitat between the brook trout and smallmouth bass. The decline of brook trout in lakes in which smallmouth bass were introduced was, therefore, predictable.
The apparent compatibility of smallmouth bass with lake trout was equally predictable on the basis of present knowledge. Sympatric co-evolution has favoured adequate phenotypic or genotypic response to allow each to adequately explore the limitations of its realized niche within the total niche-space of the salmonid community (Ryder et al., 1981). Zoogeographic considerations, such as patterns of glacial recession, have prevented a large overlap in the natural range of these two species in Canada. In certain lakes, both the ecological attributes of the environment, together with speciesspecific and interspecific behaviour, have separated the lake trout from the smallmouth bass in both time and space.
The preferred temperature of the lake trout is 8–10°C (Rawson, 1961) about the same as that at which the smallmouth bass commences feeding on an ascending scale in springtime or reduces feeding activity on a descending temperature cline in the autumn (Coble, 1975). Lake trout, on the other hand, feed actively although at differing rates, in both shallow and deep lake waters from fall overturn, throughout the winter, until the onset of early summer thermal stratification. During most of this period smallmouth bass feed only occasionally if at all, and spend most of their time inactivated in substrate hibernacula (Webster, 1954). During the period of summer stratification, high temperatures prevent lake trout from feeding in the littoral waters of the epilimnion where smallmouth bass actively feed, principally on crayfish, insects and forage fishes (Fedoruk, 1966).
In the boreal forest lakes of Ontario, lake trout spawning normally occurs in October, at temperatures at which the smallmouth bass is inactive, while the latter species spawn in June in shallow littoral areas at temperatures not normally tolerated by lake trout.
Interactive predation between lake trout and smallmouth bass occurs but rarely. Martin and Olver (1980) report only occasional predation of smallmouth bass by lake trout for lakes in which the two species are sympatric. Adult lake trout, by virtue of their size alone, would not be vulnerable to smallmouth bass predation, and young lake trout normally inhabit deep waters (Martin and Olver, 1980) and, therefore, do not co-occur with adult smallmouth bass. In certain isolated instances, young lake trout are found in the stomachs of adult smallmouth bass in the fall of the year (J.A. MacLean, pers. comm.). Adult lake trout predation on young smallmouth bass may be more common in specific circumstances. In four small lakes (200–1 000 ha) of eastern Ontario, adult lake trout predation on young of the year and a few yearling smallmouth bass ranged from 3 to 9 percent frequency of occurrence during a winter creel census (D. Loftus, pers.comm.). It may be conjectured that the small lake sizes, relative environmental homogeneity, large littoral to pelagic zone ratios have forced the lake trout to feed benthically more often than would be normal in a large lake. Smallmouth bass are typically closely associated with substrate materials during the winter months (Webster, 1954).
In summary, available evidence points to the fact that smallmouth bass and lake trout are spatially segregated for much of the year (Carlander, 1975) and any interaction between the two species may occur only through the sequential use of an overlapping food resource. Even here, little evidence can be found to support sequential feeding competition as the primary food requirements of the two predators are substantially different (e.g., Fedoruk, 1966; Martin and Olver, 1980). Any potential interaction between these two species, therefore, must occur indirectly through food web inter-relations or at an even lower hierarchic level through energetic or nutrient “competition”. In the latter instances, if smallmouth bass were introduced into a lake trout lake, the annual production of the latter species may be reduced accordingly, although perhaps not appreciably, through the various interactive and integrative pathways of the energy and nutrient transfer systems which form the basis for a common resource for both species.
From a purely anthropocentric point of view, however, the addition of smallmouth bass to a lake trout lake and its associated salmonid community (brook trout are assumed to be absent), would probably increase both the total yield and variety to the angling fishery without a noticeable decrease in lake trout yield per se. This has already been demonstrated in Lake Opeongo where the smallmouth bass was introduced in 1928 (Martin and Fry, 1973).
Again, a careful evaluation of niche dimensions, resource partitioning, dominance traits and potential or interactive segregation would have provided a fisheries manager with all the scientific information required to have predicted this compatible outcome of the co-existence of lake trout and smallmouth bass.
The cisco (Coregonus artedii) is a co-habitant with the lake trout in many boreal forest lakes of the Precambrian Shield in North America and co-evolved with the latter species in the Mississippi refugium (McPhail and Lindsey, 1970). Because of long-term co-evolution, a natural dynamic equilibrium has been attained by these species through their predator-prey relationship, with adult lake trout preying upon both adult and young cisco. The other major relationship between these species involves “competitive” feeding interactions at the zooplankton level, where young lake trout and all ages of ciscos feed on zooplankton of various size-classes. The lake trout-cisco relationship is a closely coherent coupling in that it is mutually beneficial, although not necessarily mutually obligatory as alternate food pathways may often be available to the lake trout. Hence, the lake trout, through predation, retains ciscos at a predictable steady-state, that is, a level that allows ciscos to operate well within the carrying capacity of its zooplankton food base. Where lake trout predation stress has been removed from cisco stocks, the latter have been observed to expand opportunistically, even beyond the long-term limitations imposed by the environment. Such loss of predation control has been described for the Great Lakes cisco complex following the sea lamprey incursion and the subsequent virtual extinction of the lake trout (Smith, 1968).
Lake trout, in the absence of ciscos in the depauperate faunas of the boreal forest lakes of the Precambrian Shield, and in the absence of alternative forage fishes, must remain with a planktonfeeding habit as they grow. In such instances, the lake trout grow more slowly and do not reach as great an ultimate size as piscivorous lake trout, and mature at a smaller size (Martin, 1966). The other alternative for lake trout in the absence of ciscos is usually to feed on other species of fishes such as the yellow perch (Perca flavescens) or white suckers (Catostomus commersoni) neither of which are as readily available to the lake trout, nor as nutritious as the cisco (Martin, 1970). In some extreme instances, the energy expenditures required to enter a thermally hostile environment in order to capture alternate prey species may not be justified by the caloric augmentation following digestion (e.g, Kerr, 1971).
In a comparative study of lake trout lakes of Algonquin Park, Fry (1939) noted the presence of rapidly growing, large-sized piscivorous lake trout in some lakes and relatively small, slower-growing lake trout in other lakes. Martin (1966) showed conclusively that both the growth rates of lake trout and the upper asymptotic sizes they attained were related to food habits and were essentially dichotomous in nature, depending upon the availability of zooplankton forage or prey species of fish. Most directly, this relationship depends on the particle-size density of the prey relative to predator body size (Martin, 1970) which affects the availability of prey and the ensuing growth efficiency of the predator (Kerr, 1979). Other considerations are equally important to predator efficiency. In instances where ciscos and lake trout were sympatric, it was noted that their migratory and feeding habits were somewhat parallel (Fry, 1939), a condition that augments a continual availability of the prey to the predator and precludes the necessity of the lake trout temporarily invading a hostile thermal environment to capture alternative prey in the epilimnion, such as perch. In addition, Fry (1949) noted a depression in the growth curve of Lake Opeongo lake trout at a size when they had become inefficient in feeding on small particle sizes, but were not yet large enough to prey effectively upon the available forage fishes. The importance of such discontinuities in the prey sizespectrum has since been formalized, and recognized as a useful tool in the management of predator stocks (e.g., Kerr, 1974a, 1979; Ware, 1977).
The obvious management solution contrived to help the lake trout accelerate their growth past this observed growth depression was to introduce a prey species that would provide an optimum size range for adult and sub-adult lake trout to feed upon, and be readily available at all times of the year without the necessity for the predator to enter unfavourable environmental conditions. Accordingly, ciscos were introduced into Lake Opeongo in 1948 (following a failed introduction in 1940) and subsequently became one of the most numerous fish species in the lake (Martin, 1970).
The effects of the cisco introduction into Lake Opeongo have been summarized by Martin (1970) from the data taken from the first three decades of a creel census established in 1936 at the suggestion of the late W.J.K. Harkness (Fry, 1939).
The first effect of cisco introduction has been the elimination of the growth depression in lake trout during the feeding transition from zoo-plankton and other small invertebrates to forage fishes. The fishery perhaps became less attractive to the angler seeking more lake trout rather than fewer, large individuals (Martin, 1970). The lake trout matured one year later, a factor that resulted in more trout being caught before an opportunity to reproduce occurred. Egg production, however, became greater and the eggs were larger and perhaps more likely to survive.
Our assessment of the effects of the cisco introduction into Lake Opeongo in 1948, with the added benefit of another ten years' data, supports the observations of Martin (1970). We have smoothed the available annual data by approximate decades (i.e., 10–12 year units) to eliminate some of the detailed variability in the fishery caused by sampling, socio-economic and climatic changes affecting the fishery, and to accentuate major trends.
Of obvious significance is the fact that about 10 000 trout were captured in each of the first three decades (Table 1) but in the fourth decade nearly 19 000 lake trout were taken by the angling fishery. This was undoubtedly a response to a mean fishing effort smoothed by decades, that increased by almost an order of magnitude from the first to the fourth decades. Concurrently, catch-per-unit of effort (CPUE) in both weight and numbers has shown a steady decline from 0.67 trout per hour (0.51 kg/h) in the first decade to only 0.18 trout per hour (0.14 kg/h) in the fourth decade. Mean weight of lake trout (Table 2) increased markedly in the decade following introduction of the cisco but thereafter declined almost to its original value of the 1936 decade. Mean length has shown a similar, but less obvious pattern. Mean age has generally decreased over the four decades (Table 2). Apparently, the observations indicate that the lake trout responded to the cisco introduction first with a growth response and, secondly, with a recruitment response, however, contrary to current ecological theory the conservatism of yield was not demonstrated (Kerr and Martin, 1970) and yield increased by about a factor of two between the first and fourth decades of the fishery (Table 1). This response, however, is correlated with the marked increase in mean annual effort (1 653–11 858 h) and the equally marked decrease in CPUE previously noted. This fishery, therefore, bears many of the signs of a heavily exploited stock as indicated by accelerated fishing effort, increasing catches in the face of decreased CPUEs, decreased mean lengths and weights, and declining mean ages (Tables 1 and 2).
In the light of the dynamic consequences of an increasingly exploitive fishery, which tends to mask the effects of the cisco introduction, we further investigated the changing status of the lake trout stock by preparing estimates of ponderal indices by decade, made from large samples of lake trout both before and following cisco introduction, with the understanding that density-dependent response to exploitation may have about maximized by the third decade (1958–68) and that further incursions into the standing stocks of lake trout would not likely elicit any further demographic response. The ponderal index is a descriptor of the relative wellbeing of a fish and may vary with season, sex, maturity, age and other factors (Carlander, 1969). It is essentially a length-weight relationship where
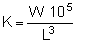
and K is the ponderal index or condition factor. Other analytical procedures may sometimes be more satisfactory for certain purposes, but ponderal indices have the convenient advantage for large and long-term samples of mitigating many of the confounding factors that may contribute to variability when calculated over shorter time spans.
Examination of the ponderal indices by decade (Table 2) shows that the mean index for the second decade was already substantially higher than that of the first decade, despite the fact that the ciscos were not introduced into Lake Opeongo until 1948, the first year of the second decade. Martin (1970) provides evidence that this was, in fact, a transitional decade for the feeding habits of the lake trout. Ponderal indices increased markedly in the third and fourth decades to higher values (Table 2). It is interesting to note that the index in the fourth decade, although similar to that of the third decade, represents appreciably younger trout, with noticeably lower mean weights and lengths. Authors cited by Carlander (1969) noted a general increase in ponderal index with increase in length for lake trout. It would seem, therefore, that the effect of the intensive fishery on Lake Opeongo has been effectively masked by the conservative behaviour of the ponderal index over several age-classes of lake trout. The two indices for the third and fourth decades (ten and twenty years after cisco introduction) were significantly different from that of the decade before introduction as determined by a student's t test (P 0.01).
It must be concluded that this case history exemplifies one of the most successfully planned introductions in the short history of fisheries management in Ontario. Our account has touched only the highlights and does not set out much of the solid background of detailed information that went into the initial management decision and the subsequent evaluations of the results. Essentially, a growth depression in the age-length relationship of the lake trout was observed and attributed to a single factor, namely, a lack of appropriately sized forage fishes accessible to the lake trout at all times of the year, with relatively little energy expenditure required for its utilization by the trout.
A natural safety factor was built into the introduction, that is, the cisco is a co-evolutionary cohabitant of the lake trout in many lakes, and their interrelationships and interactions with other community components of oligotrophic lakes were known. Predictably, it was unlikely that the cisco introduction would harm this cold-water community. Rather, the introduction might have stabilized the indigenous community under the intensive fishing pressure it is increasingly receiving, by providing it with an alternate food resource which would reduce its vulnerability to angling. In a parallel instance, Forney and Eipper (1961) have described angling success in a walleye fishery to be best when natural food supplies are low.
We have provided brief synopses of the histories of invasion or introduction of the sea lamprey, smallmouth bass and cisco, as examples of new species entering natural communities, together with observations of the ensuing consequences. Some lessons may be learned from these examples. As fish communities in oligotrophic lakes can be expected to be more sensitive to change than those in eutrophic systems (Li and Moyle, 1981), the results in our examples have, perhaps, been more decisive and readily discernible than would be likely for similar introductions in eutrophic systems. For that specific reason, study of comparatively simple, oligotrophic systems may sometimes provide useful principles that could be revealed only with great difficulty, if at all, in more complex eutrophic systems in freshwater or marine communities of any kind. At the present stage of our knowledge, and with a restrospective advantage, we can say with some confidence that the eventual major effects of each of these three introductions on the indigenous communities could have been predicted beforehand with reasonable precision by competent managers utilizing available scientific information tempered with simple ecological logic. Lest this statement be misinterpreted as a “carte blanche” for exotic species introductions, we issue the caveat of the “emergent surprise” (Kerr, 1974), which no mathematical theory can be expected to predict. Accordingly, despite our retrospective confidence in predicting the consequences of the foregoing three examples, we caution as a general rule, against introductions. However, as with all general rules, exceptions do occur, and we will attempt to deal with these now.
Natural lakes devoid of any fishes can be advantageously stocked with fish species intended to enhance human cultural values. In these situations an opportunity exists for the fisheries manager to attempt to reconstruct a “natural” fish community by introducing several species in proportions appropriate to their various trophic interactions. The suitable choice of species would involve those indigenous to adjacent lakes with similar habitat characteristics, subject to approximately similar edaphic and climatic regimes.
First, however, an assessment of the habitat status quo should be made, particularly with respect to the reason for the depauperate condition of the lake. If barrenness is attributable to the vagaries of zoogeography, then the likelihood of establishing something reminiscent of a natural community would seem to be good. Otherwise a habitat constraint such as low oxygen concentration or pH level might be suspect and should be investigated. Intermittent and widely-spaced oxygen deficits on the other hand, in lakes with a winterkill or summerkill history, may prove to be ideally suited for management purposes, depending on the length of interval between the episodic events, because of the presumptive ease of managerial control.
Political expediency or public pressure often forces the fisheries manager's hand, and introductions may have to be made in some instances regardless of best personal judgement. On such occasions, the manager has many ecological tools at ready availability and should utilize these to best advantage in order to reduce the relatively high likelihood of failure of the introduction (e.g., Magnuson, 1976) or worse, experiencing a damaging introduction as in the case of the carp in North America.
Many concepts exist in the ecological literature that may be extremely constructive in the process of considering an appropriate species or community for introduction. A hierarchy of four closely related, ecological concepts that are concerned with species interactions within the context of ambient environmental conditions are listed in Table 3. These concepts differ mainly in emphasis, level of generality, or the degree to which they are quantifiable as well as in other, more subtle considerations. Two of these concepts of particular application at the community level of organization, chosen in this instance, from the Swedish literature, are the related ones of interactive segregation (Nilsson, 1967) and dominance-subordinance (Svardson, 1976) together with their further elaboration in marine systems by Skud (1982).
Nilsson (1967) observed that most fish species of north-temperate zones exhibit “interactive segregation”, an expression to be preferred over “competition” which is itself difficult to demonstrate conclusively (Reynoldson and Bellamy, 1971; Fraser, 1978). Interactive segregation depends on the degree of overlap of critical niche dimensions (our terminology) between two allopatric species which might otherwise demonstrate niche complementarity in sympatric situations. That is, the niche dimensions that overlap when the two species are geographically separated become mutually exclusive when the two species co-occur. Each species, therefore, functions in sympatry in a reduced niche space as measured along one or more critical dimensions. In extreme instances, if a formerly allopatric species cannot operate effectively within its newly restricted niche space, continued survival of the species becomes uncertain. Nilsson (1967) has listed some of the possible mechanisms underlying the interactive segregation process, including exploitation, territoriality, agonistic behaviour during feeding, predation and other possible interactions between species.
When considering the introduction of a fish species which is a normal constituent of the fish communities of other nearby and closely similar lakes, careful attention should be directed to the potential of the candidate species for interaction with the native stocks, particularly with respect to the ecological mechanisms indicated by Nilsson (1967). Rigorous perusal of the literature for documentation of observed interactions of the candidate species may lead to the prevention of a potentially catastrophic introduction.
Nilsson (1978) suggests that when a new species of fish is introduced into a lake, several alternative events may potentially occur. The three principal alternatives include rejection, in which the new species cannot compete successfully along one or more of the major niche dimensions; displacement of an indigenous community component by the introduced species, an indication perhaps of successful functioning along major niche dimensions to the point of competitive exclusion of another species (Hardin, 1960) or segregation of the introduced and native species to portions of one or more of their major niche dimensions that are utilized only in the face of major competitive interactions, that is, interactive segregation to avoid undue niche overlap. A fourth consideration that should be included is the potential for total community disruption as it decays into an astatic condition (Ryder and Kerr, 1978).
Ideally, a new introduction into an existing fish community should utilize food and other resources not previously used by the native fish community and it should differ functionally from other community members. For one example, introductions should seek to augment or capitalize upon a particle-size hiatus in the prey resource, as exemplified by the cisco lake trout examples offered earlier. In other words, in looking for a candidate species with a high probability of survival, without creating unacceptable disruption of the native community, the degree of niche complementarity (Werner, 1977) will likely determine the relative level of success. In some respects, the sea lamprey in the Great Lakes fits this definition precisely, except for its apparent inability, as yet to establish a dynamic equilibrium with its principal prey species, the lake trout and burbot.
A second concept of use to the fisheries manager in the context of a fish species introduction, is the level of interspecific population dominance in fish communities, or more simply, the dominance-subordinance principle (Svardson, 1976). In freshwater fish communities relatively few species dominate numerically, and the standing stocks of fishes in a lake are heirarchically organized. Reduction or elimination of a dominant species will tend to elicit drastic changes in the trophic hierarchy. In general, the terminal predators, unless heavily exploited, are governed by abiotic variables generated by climatic and edaphic conditions or lake morphometry, while the lower ranked species are primarily regulated through one or more interactive pathways determined by the dominant species. Svardson (1976) has elaborated on this pattern by the careful empirical observation of dominant and subordinate sympatric species pairs and has shown that species with pelagic capabilities are dominant over those species that live, generally, in the littoral zones of lakes. The survival advantage conferred on pelagic species apparently reflects their ability to be efficient plankton-feeders.
In a recent elaboration of Svardson's (1976) dominance-subordinance principle, Skud (1982) demonstrated that changes in relative abundance of dominant and subordinate marine fishes, caused by changes in the physical environment, improved the survival of the dominant species while reducing the survival of subordinate species. The subordinate species, therefore, were controlled by the dominant species although environmental changes could reverse the dominance relations between species.
In a recent review of replacement of depleted marine stocks by other species, Daan (1980) was unable to demonstrate, with any degree of confidence, direct replacement of one species by another in exploited stocks. Only for the closely coupled sardine-anchovy fisheries of California, was approximate compensatory replacement of one species by another a reasonable explanation for changes in stocks. However, Daan used only species pairs in his analysis of complex marine communities. In such instances, compensatory mechanisms may not be particularly obvious without a careful examination of all community components. Even where a total yield conservatism is demonstrated in a fishery of many species, individual species yields may fluctuate widely (e.g., Sutcliffe et al., 1977). Perhaps most marine systems are too complex with too many alternative energetic pathways available for such changes to be easily detected by dominance-subordinace analysis alone, reinforcing the notion that such mechanisms are best studies first in relatively simple freshwater systems before extension to the marine environment.
Application of the dominance principle is especially relevant to culturally disturbed lakes, those subjected to acidification or eutrophication to cite just two examples. Changed pH or nutrient levels may be sufficient to confer advantage on a species other than the one currently dominant (e.g., Fig. 1) and corresponding reverberations may occur throughout the fish community. In environments that have been severely degraded beyond any realistic prospect for rehabilitation, non-indigenous species more tolerant of the agents of degradation may be the only option for a successful introduction. Again, careful evaluation of the dominance-subordinance relationships of many species pairs may provide additional clues of the likelihood of niche complementarity of an introduced species.
Another important consideration for the fisheries manager is the partitioning of resources by fishes in ecological communities in general and, more specifically, how different fish species divide the available resources of the environment (Schoener, 1974). In some respects this concept is complementary to the concept of interactive segregation (Nilsson, 1967), particularly with respect to food resources, but in another sense it adds a different perspective to the competition concept. Of particular interest in what follows, is the effect of habitat partitioning by related species.
In a study of three centrarchid species in a small Michigan lake, Werner et al., (1977) observed that two species occupied the same habitat in the littoral zone where they utilized broad but disjunct ranges of food size. The two species were segregated in terms of food-size rather than habitat, with the larger species being functionally better adapted to capture and ingest large food particles than the smaller species, which selected correspondingly the small food particles. A third centrarchid species that was functionally intermediate between the first two species did not overlap with them on a spatial basis. By feeding in isolation in extremely shallow waters, the third species effectively avoided direct food competition with the two functionally efficient occupants of deeper waters. The third species thus demonstrated a niche complementarity that allowed it to co-exist with the other two centrarchids which, in turn, were effectively segregated along the food-size dimension of nichespace.
Whether or not a candidate species for introduction will be spatially segregated from other components in a natural community is an important consideration. Supporting evidence required empirical observation from a broad range of habitat types. The lake trout, for example, generally occupies approximately similar habitat to the cisco on a time-space basis (Fry, 1939), such that a steady-state predator-prey relationship is typical. Such a steady-state relationship was observed by the late D.S. Rawson (in Cuerrier, 1954) in Lake Minnewanka, Alberta, prior to the construction of a major impoundment in 1941. However, by 1952 Cuerrier observed that the lake trout no longer preyed upon ciscos and attributed this fact to a spatial separation of the species, induced by a marked increase in water levels following impoundment. Accordingly, spatial separation of species may be caused by environmental heterogeneity, including changes in depth, temperature, light or other abiotic factors, as well as by interactive segregation per se. Earlier, we demonstrated that effective time-space separation of the walleye (Stizostedion vitreum) and sauger (S. canadense), when sympatric, is attributable to both the particle-size of the food ingested and the respective capabilities of these predators to feed most efficiently at differing light intensities (Kerr and Ryder, 1977). In this instance, at least two niche dimensions come into play in the time-space separation of these two closely similar ecological homologues.
Besides the concepts of interactive segregation, dominance-subordinance and resource partitioning, many other approaches to assessing the consequences for fish introduction exist in the literature, such as the application of graph theory (Saila and Parrish, 1972) or loop analysis (Li and Moyle, 1981). Regardless of the approach taken, quantification of both fundamental and realized niche properties as defined by Ryder et al. (1981) are critical to the evaluation process for a species introduction. Realized niche boundaries may be determined empirically from direct observation of natural communities comprising various species. Unfortunately, as many candidate species for introduction evolved in communities other than those in which they are destined to be planted, this type of evidence can be difficult to interpret. Because community interactions, together with a multitude of abiotic variables, shape the realized niche, only broad generalizations will likely be obtained in this manner for truly exotic species. For example, a fish species functioning as a piscivore in one community will likely retain this characteristic if introduced in a community in which it did not evolve, assuming that appropriate forage fish species are available. Similarly, a filter-feeder will likely retain the planktivore habit, again assuming that the right kinds and sizes of food particles are readily available. Even these assumptions may be tentative, however, as shown by the occurrence of numerous stocks of plankton-feeding lake trout in highland lakes lacking suitable forage species. In most fish communities in which lake trout occur, however, they function typically as piscivores (Martin, 1966). Despite the existence of these and similar emergent surprises, attempts to evaluate the realized niches of candidate species will increase the safety factor of introductions, by emphasizing incompatibilities that may not otherwise be obvious. For situations where quantification is impossible, or tenuous at best, some indication of interactive processes may be determined qualitatively through the establishment of a multi-dimensional interaction matrix between the candidate species and the indigenous community. Each of the major interactive niche dimensions of the candidate species should be examined in this fashion (e.g., food, reproduction, behaviour, etc.), with respect to environmental and prey resource factors of primary ecological importance (type, size, time, space, density, nutrients, energy). The latter set of factors may be further subdivided as necessary to any level of resolution for which relevant information exists such as day-night, pelagic-littoral, eutrophic-oligotrophic, predation type or other appropriate division. Each niche dimension, in turn, should be considered in relation to the broader concepts of interactive segregation, dominance-subordinance and resource partitioning.
Careful evaluation of the fundamental (potential) niche (Ryder et al., 1981) is also desirable in order to determine the likelihood for adaptation of a candidate species into a particular environment. Usually, evaluation of major limiting or controlling environmental factors such as temperature, oxygen, light or pH (e.g., Fry, 1947; Scherer, 1971; 1976) will provide suitable information to determine the potential of a species to utilize the resources of its environment, or to survive at times of the year when some of these dimensions may be suppressed. Multi-variate or matrix analysis may be used where appropriate, depending on the quality and resolution of available data.
Fish community manipulation, through the introduction of new species, is generally risky and therefore the degree of success likely to be experienced is relatively unpredictable. Conservatively, the probability for damaging an indigenous community is at least as great as that of enhancing it. These odds against the fishery manager can be reduced by taking a stand toward the protection of, or rehabilitation of, indigenous co-evolved fish communities.
Where introductions are unavoidable, because of public or political pressure, efforts may be most effectively directed toward barren lakes, lakes degraded to the point of irreversibility, or lakes with an obvious functional community component missing. In each instance native community stocks should be utilized for introduction where possible, although in irreversibly degraded systems of the boreal forest zone, it is unlikely that a useful candidate species will exist. In such cases it may be necessary to search for a likely species elsewhere, where fish communities or individual species have perhaps developed some evolutionary tolerance to culturally degraded systems.
An alternative option which should always be considered as a means for reducing the risk of introductions, is to choose stenoecious species or sterile hybrids as candidates for planting, thereby increasing the possibility that catastrophic errors can be rectified.
In summary, intelligent approaches to community manipulation involve careful evaluation of appropriate niche dimensions of both the candidate species for introduction and the indigenous community. A naive approach to complex systems by a fisheries manager will likely produce disappointing results at best, or worse, result in a degraded fishery of long-term instability and unpredictability.
We are extremely grateful to Nils Nilsson of Sweden, who evaluated, placed in context and summarized this contribution at the EIFAC Symposium on Stock Enhancement in the Management of Freshwater Fisheries, held in Budapest, Hungary, in 1982.
L.M. Dickie, K.H. Loftus and J.A. MacLean provided insightful reviews of a preliminary draft and D. Loftus, J.A. MacLean and N.V. Martin supplied data from Ontario lake trout lakes.
Anderson, E.D. and L.L. Smith, Jr., 1971 Factors affecting abundance of lake herring (Coregonus artedii LeSueur) in western Lake Superior. Trans.Am.Fish.Soc., 100:691–707
Applegate, V.C., 1950 Natural history of the sea lamprey (Petromyzon marinus) in Michigan. Spec.Sci.Rep.U.S.Fish Wildl.Serv., (Fish.), (55):237 p.
Applegate, V.C. and B.R. Smith, 1950 Sea lamprey spawning runs in the Great Lakes in 1950. Spec.Sci.Rep.U.S.Fish Wildl.Serv., (Fish.), (61):49 p.
Bailey, M.M., 1972 Age, growth, reproduction, and food of the burbot, Lota lota (Linnaeus), in southwestern Lake Superior. Trans.Am.Fish.Soc., 101:667–74
Bailey, R.M. and G.R. Smith, 1981 Origin and geography of the fish fauna of the Laurentian Great Lakes basin. Can.J.Fish.Aquat.Sci., 38(12):1539–61
Carlander, K.D., 1969 Handbook of freshwater fishery biology. Ames, Iowa, Iowa State University Press, vol. 1:752 p.
Carlander, K.D., 1975 Community relations of bass - large natural lakes. In Black bass biology and management, edited by R.H. Stroud and H. Clepper. Washington, D.C., Sport Fishing Institute pp. 125–30
Christie, W.J., 1957 The bass fishery of Lake Opeongo. M-A. Thesis, Department of Zoology, University of Toronto, Toronto, Ontario, 77 p.
Christie, W.J. and H.A. Regier, 1973 Temperature as a major factor influencing reproductive success of fish - two examples. Rapp.P.-V.Reun.CIEM, 164:208–18
Christie, W.J., J.M. Fraser and S.J. Nepszy, 1972 Effects of species introductions on salmonid communities in oligotrophic lakes. J.Fish.Res.Board Can., 29(12):969–73
Coble, D.W., 1967 Relationship of temperature to total annual growth in adult smallmouth bass. J.Fish.Res.Board Can., 24(1):87–99
Coble, D.W., 1975 Smallmouth bass. In Black bass biology and management, edited by R.H. Stroud and H. Clepper. Washington, D.C., Sport Fish. Institute, pp. 21–33
Courtenay, W.R., Jr. and C.R. Robins, 1975 Exotic organisms: an unsolved, complex problem. BioScience, 25:306–13
Cuerrier, J.P., 1954 The history of Lake Minnewanka with reference to the reaction of lake trout to artificial changes in environment. Can.Fish-Cult., 15:1–9
Daan, N., 1980 A review of replacement of depleted stocks by other species and the mechanisms underlying such replacement. Rapp.P.-V.Reun.CIEM, 177:405–21
Eschmeyer, P.H., 1957 Note on the subpopulations of lake trout in the Great Lakes. Spec.Sci.Rep.U.S.Fish Wild.Serv., (Fish.), (208):129 p.
Fedoruk, A.N., 1966 Feeding relationship of walleye and smallmouth bass. J.Fish.Res.Board Can., 23(6):941–3
Fernando, C.H., 1980 Tropical man-made lakes, African fish and cheap protein. ICLARM Newsl., 3(1):15–7
Flint, R.F., 1957 Glacial and Pleistocene geology. New York, John wiley and Sons, 553 p.
Forney, J.L. and A.W. Eipper, 1961 Oneida Lake walleyes. N.Y.Conserv., Feb–Mar. issue:18–20
Fraser, J.M., 1972 Recovery of planted brook trout, splake and rainbow trout from selected Ontario lakes. J.Fish.Res.Board Can., 29(2):129–42
Fraser, J.M., 1978 The effect of competition with yellow perch on the survival and growth of planted brook trout, splake and rainbow trout in a small Ontario lake. Trans.Am.Fish.Soc., 107:505–17
Fry, F.E.J., 1939 A comparative study of lake trout fisheries in Algonquin Park, Ontario. Univ.Toronto Stud., 42(58):69 p.
Fry, F.E.J., 1947 Effects of the environment on animal activity. Univ.Toronto Stud.(Biol.Ser.), 55(68):62 p.
Fry, F.E.J., 1949 Statistics of a lake trout fishery. Biometrics, 5(1):27–67
Grossman, G.D., 1982 Dynamics and organization of a rocky intertidel fish assemblage: the persistence and resilience of taxocene structure. Am.Nat., 119:611–37
Guillory, V. and R.D. Gasaway, 1978 Zoogeography of the grass carp in the United States. Trans.Am.Fish.Soc., 107:105–12
Hall, A.E., Jr. and O.R. Elliott, 1954 Relationship of length of fish to incidence of sea lamprey scars on white suckers, Catostomus commersoni, in Lake Huron. Copeia, 1954(1):73–4
Hardin, G., 1960 The competitive exclusion principle. Science, Wash., 131:1292–7
Hubbs, C.L. and R.M. Bailey, 1938 The small-mouthed bass. Bull.Cranbrook Inst.Sci., (10):1–89
Hutchinson, G.E., 1957 Concluding remarks. Symp.Quant.Biol., 22:415–27
Keast, A. and D. Webb, 1966 Mouth and body form relative to feeding ecology in the fish fauna of a small lake, Lake Opinicon, Ontario. J.Fish.Res.Board Can., 23(12):1845–74
Keleher, J.J., 1972 Great Slave Lake: effects of exploitation on the salmonid community. J.Fish.Res.Board Can., 29(6):741–53
Kerr, S.R., 1971 Prediction of fish growth efficiency in nature. J.Fish.Res.Board Can., 28(6):809–14
Kerr, S.R., 1974 Structural analysis of aquatic communities. Proc.Int.Congr.Ecol., 1:69–74
Kerr, S.R., 1974a Theory of size distribution in ecological communities. J.Fish.Res.Board Can., 31(12):1859–62
Kerr, S.R., 1979 Prey availability, metaphoetesis, and the size structures of lake trout stocks. Invest.Pesq.Barc., 43(1):187–98
Kerr, S.R. and N.V. Martin, 1970 Trophic-dynamics of lake trout production systems. In Marine food chains, edited by J.H. Steele. Edinburgh, Oliver and Boyd, pp. 365–76
Kerr, S.R. and R.A. Ryder, 1977 Niche theory and percid community structure. J.Fish.Res.Board Can., 34(10):1952–58
Koelz, W., 1929 Coregonid fishes of the Great Lakes. Bull.U.S.Bur.Fish., 43(2):643 p.
Lawrie, A.H., 1970 The sea lamprey in the Great Lakes. Trans.Am.Fish.Soc., 99:766–75
Lawrie, A.H., and J.F. Rahrer, 1972 Lake Superior: effects of exploitation and introductions on the salmonid community. J.Fish.Res.Board Can., 29(6):765–76
Li, H.W. and P.B. Moyle, 1981 Ecological analysis of species introductions into aquatic systems. Trans.Am.Fish.Soc., 110:772–82
Loftus, K.H., 1958 Studies on river-spawning populations of lake trout in eastern Lake Superior. Trans.Am.Fish.Soc., 87:259–77
Loftus, K.H., 1976 Science for Canada's fisheries rehabilitation needs. J.Fish.Res.Board Can., 33(8):1822–57
Loftus, K.H. and H.A. Regier, 1972 Introduction to the proceedings of the 1971 symposium on salmonid communities in oligotrophic lakes. J.Fish.Res.Board Can., 29(6)613–6
MacArthur, R.H. and E.O. Wilson, 1967 The theory of island bio-geography. Princeton, N.J., Princeton University Press, 203 p.
Magnuson, J.J., 1976 Managing with exotics - a game of chance. Trans.Am.Fish.Soc., 105:1–9
Martin, N.V., 1966 The significance of food habits in the biology, exploitation and management of Algonquin Park, Ontario, lake trout. Trans.Am.Fish.Soc., 95:415–22
Martin, N.V., 1970 Long-term effects of diet on the biology of the lake trout and the fishery in Lake Opeongo, Ontario. J.Fish.Res.Board Can., 27(1):125–46
Martin, N.V. and F.E.J. Fry, 1973 Lake Opeongo - the ecology of the fish community and of man's effects on it. Tech.Rep.Great Lakes Fish.Comm., (24):34 p.
Martin, N.V. and C.H. Olver, 1980 The lake charr, Salvelinus namaycush. In Charrs: salmonid fishes of the genus Salvelinus. Perspectives in vertebrate science, edited by E.K. Balon. The Hague, Netherlands, Dr. W. Junk, Publishers, vol. 1:205–77
McCrimmon, H.R., 1968 Carp in Canada. Bull.Fish.Res.Board Can., (165):93 p.
McPhail, J.D. and C.C. Lindsey, 1970 Freshwater fishes of northwestern Canada and Alaska. Bull.Fish.Res.Board Can., (173):1–381
Miller, R.R., 1957 Origin and dispersal of the alewife, Alosa pseudoharengus, and the gizzard shad, Dorosoma cepedianum, in the Great Lakes. Trans.Am.Fish.Soc., 86:97–111
Mitzner, L., 1978 Evaluation of biological control of nuisance aquatic vegetation by grass carp. Trans.Am.Fish.Soc., 197:135–45
Nilsson, N.-A., 1967 Interactive segregation between fish species. In The biological basis of freshwater fish production, edited by S.B. Gerking. Oxford, Blackwell Scientific Publications, pp. 295–313
Nilsson, N.-A., 1978 The role of size-biased predation in competition and interactive segregation in fish. In Ecology of freshwater fish production, edited by S.B. Gerking. Oxford, Blackwell Scientific Publications, pp. 303–25
Peterman, R.M., W.C. Clark and C.S. Holling, 1979 The dynamics of resilience: shifting stability domains in fish and insect systems. In Population dynamics, edited by R.M. Anderson et al. Twentieth Symposium of the British Ecological Society. London, Blackwell, opp. 321–41
Radforth, I., 1944 Some considerations on the distribution of fishes in Ontario. Contrib.R.Ont.Mus.Zool.Palaentol., (25):1–116
Rawson, D.S., 1945 The experimental introduction of smallmouth black bass into lakes of the Prince Albert National Park, Saskatchewan. Trans.Am.Fish.Soc., 73:19–31
Rawson, D.S., 1961 The lake trout of Lac la Ronge, Saskatchewan. J.Fish.Res.Board Can., 18(3):423–62
Regier, H.A., 1968 The potential misuse of exotic fish as introductions. Res.Rep.Ont.Dep.Lands For., (82):92–111
Regier, H.A., and W.L. Hartman, 1973 Lake Erie's fish community: 150 years of cultural stresses. Science, Wash., 180:1248–55
Reynoldson, T.B. and L.S. Bellamy, 1971 The establishment of interspecific competition in field populations with an example of competition in action between Polycelis nigra (Mull.) and P. tenuis (Ijima) (Turbellaria, Tricladida). In Dynamics of populations. Proceedings of the Advanced Study Institute on Dynamics of Numbers in Populations, Oosterbeck, The Netherlands, 7–18 September, 1970, edited by P.J. den Boer and G.R. Gradwell. Wageningen, Centre for Agricultural Publishing and Documentation, pp. 282–97
Ryder, R.A., 1972 The limnology and fishes of oligotrophic glacial lakes in North America (about 1800 A.D.). J.Fish.Res.Board Can., 29(6):617–28
Ryder, R.A., W.B. Scott and E.J. Crossman, 1964 Fishes of northern Ontario, north of the Albany River. Contrib.R.Ont.Mus., (60):30 p.
Ryder, R.A. and S.R. Kerr, 1978 The adult walleye in the percid community - a niche definition based on feeding behavior and food specificity. Spec.Publ.Am.Fish.Soc., (11):437 p.
Ryder, R.A. et al., 1981 Community consequences of fish stock diversity. Can.J.Fish.Aquat.Sci., 38(12):1856–66
Saila, S.B. and J.D. Parrish, 1972 Exploitation effects upon interspecific relationships in marine ecosystems. Fish.Bull.NOAA/NMFS, 70:383–93
Scherer, E., 1971 Effects of oxygen depletion and of carbon dioxide buildup on the photic behaviour of the walleye (Stizostedion vitreum vitreum). J.Fish.Res.Board Can., 28(9):1303–7
Scherer, E., 1976 Overhead light intensity and vertical positioning of the walleye, Stizostedion vitreum vitreum. J.Fish.Res.Board Can., 33(2):289–92
Schoener, T.W., 1974 Resource partitioning in ecological communities. Science, Wash., 185:27–39
Scott, W.B. and E.J. Crossman, 1973 Freshwater fishes of Canada. Bull.Fish.Res.Board Can., (182):966 p.
Selgeby, J.H., W.R. MacCallum and D.V. Swedberg, 1978 Predation by rainbow smelt (Osmerus mordax) on lake herring (Coregonus artedii) in western Lake Superior. J.Fish.Res.Board Can., 35(11):1457–63
Shuter, B.J. et al., 1980 Stochastic simulation of temperature effects on first-year survival of smallmouth bass. Trans.Am.Fish.Soc., 109:1–34
Skud, B.E., 1982 Dominance in fishes: the relation between environment and abundance. Science, Wash., 216:144–9
Smith, S.H., 1964 Status of the deepwater cisco population of Lake Michigan. Trans.Am.Fish.Soc., 93(2):155–63
Smith, S.H., 1968 Species succession and fishery exploitation in the Great Lakes. J.Fish.Res.Board Can., 25(4):667–93
Smith, S.H., 1970 Species interactions of the alewife in the Great Lakes. Trans.Am.Fish.Soc., 99:754–65
Sutcliffe, W.H., Jr., K. Drinkwater and B.S. Muir, 1977 Correlations of fish catch and environmental factors in the Gulf of Maine. J.Fish.Res.Board Can., 34(1):19–30
Svardson, G., 1976 Interspecific population dominance in fish communities of Scandinavian lakes. Rep.Inst.Freshwat.Res., Drottningholm, (55):144–71
Ware, D.M., 1977 Spawning time and egg size of Atlantic mackerel, Scomber scombrus, in relation to the plankton. J.Fish.Res.Board Can., 34(12)2308–15
Webster, D.A., 1954 Smallmouth bass, Micropterus dolimieui, in Cayuga Lake. Part 1. Life history and environment. Mem.Cornell Univ.Agric.Exp.Stn., (327):1–39
Webster, D.A., W.G. Bentley and J.P. Galligan, 1959 Management of the lake trout fishery of Cayuga Lake, New York, with special reference to the role of hatchery fish. Mem.Cornell.Univ.Agric.Exp.Stn., (357):1–83
Welch, H., 1967 Energy flow through the major macroscopic components of an aquatic ecosystem. Ph.D. Dissertation, University of Georgia, Athens,
Werner, E.E., 1977 Species packing and niche complementarity in three sunfishes. Am.Nat., 111:553–78
Werner, E.E. et al., 1977 Habitat partitioning for a freshwater community. J.Fish.Res.Board Can., 34(3):360–70
Table 1 Mean values for numbers, yield, effort and catch per unit of effort of lake trout entering the sport fishery at Lake Opeongo, Algonquin Park, for the four decades (approximate) between 1936 and 1979
| Years1 | Total no. lake trout | No. .yr-1 | Yield .yr-1(kg) | Effort .yr-1(hr) | C.U.E. .yr-1(no.hr-1) | C.U.E. .yr-1(kg.hr-1) |
| 1936–47 | 9 227 | 839 | 1 047 | 1 653 | 0.51 | 0.67 |
| 1948–57 | 10 298 | 1 030 | 1 449 | 4 103 | 0.28 | 0.40 |
| 1958–68 | 10 044 | 913 | 1 645 | 6 857 | 0.13 | 0.24 |
| 1969–79 | 18 604 | 1 691 | 2 114 | 11 858 | 0.14 | 0.18 |
1 Data for each variable was not available for every year
Table 2 Age, fork length, weight and ponderal indices (K = W105/L3) for lake trout from the angling fishery of Lake Opeongo, Algonquin Park, for the years 1936 to 1979 by approximate decade. The indices for the last two decades (1958–79) were significantly different from that prior to the cisco introduction according to a Students t-distribution (p 0.01)
| Years1 | Mean age | Mean fork length (cm) | Mean weight (kg) | Ponderal index value (K) | Comments | |
| Mean | Range | |||||
| 1936–47 | 8.0 | 47.9 | 1 271 | 1.14 | 0.99–1.27 | Ciscos not present |
| 1948–57 | 8.1 | 47.8 | 1 446 | 1.32 | 1.22–1.50 | After cisco introduction (1948) |
| 1958–68 | 7.8 | 48.8 | 1 906 | 1.58 | 1.42–1.76 | " |
| 1969–79 | 7.5 | 43.5 | 1 273 | 1.55 | 1.44–1.64 | " |
1 Data for each variable was not available for every year
Table 3 A hierarchy of four ecological concepts relating to the interrelationships of two or more species within the background of ambient environmental conditions. Despite the difference in nuance or emphasis among the four concepts, they all describe a conceptually similar phenomenon.
| Ecological Concept | Objective Mode | Level of Generality | Level of Quantification | Authors |
| Realized niche | Most passive | Most general | Low | Fry (1947) Hutchison (1957) |
| Interactive segregation | Nilsson (1967) | |||
| Dominance-subordinance | Svardson (1976) | |||
| Resource partitioning | Most active | Most specific | Relatively high | Schoener (1974) Werner et al. (1977) |
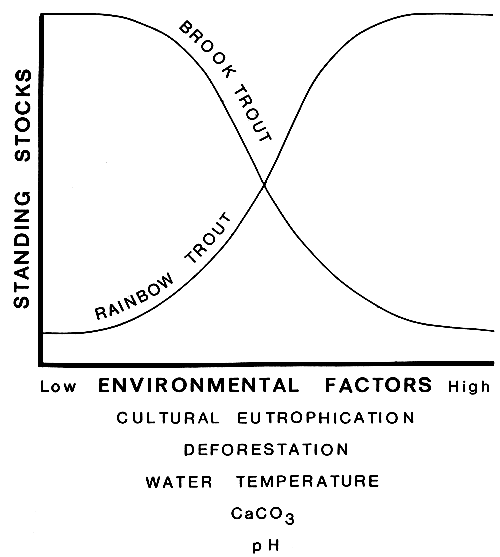
Fig. 1 Changes in relative proportions of rainbow trout and brook trout in response to changing environmental factors
2 Bedford Institute of Oceanography Contribution