(P. Kolář)
Diagnostics of viral diseases in fish can be considered as falling in two major groups:
The basic methods include:
The special methods include:
Sending fish samples to laboratory for virological examination
Virological examination can be made in live fish only. Individual fish are chosen for examination from among those which - still alive though with symptoms of disease - gather close to the water surface or at the inlets or outlets. The water for the transport of the fish must be cooled with natural ice and, if possible, there should be a supply of air or oxygen. If the live fish cannot be sent as described, the dead fish for the laboratory examination should be taken as early as possible and covered by green plants; they may also be wrapped in wet towels or may be put into packages through which air should get inside (hence, not PVC). The fish should always be sent by messengers.
Usually it is necessary also to take water samples according to pertinent instructions. In cases of infectious diseases the sample package usually contains 5–10 fish.
Fish sampling for virological examination
The fish to be used for virological examination should preferably be alive. If the fish are dead there is much less chance to detect the virus agents. Aids and instruments for the sampling must all be sterile. Samples are taken from 5–10 fish. In fry up to a size of 5 cm the whole body makes a sample, though, in fact, it seems better to remove the head and the tail just behind the anus after disinfecting the body surface by means of 70 % alcohol (the disinfection markedly reduces the possibility of bacterial contamination). In the larger fish the body cavity is opened by incisions starting about 1 cm before the anus. The first incision is perpendicular to the lateral line and the other runs along the ventral line of the body cavity. A flap of body wall is thus produced; hold it with pean tweezers or surgical forceps, lift it, and continue the incision along the lateral line and the ventral line up to the gill arches. Turn the whole flap and expose the organs. Disinfect the body surface with 70 % alcohol along the incisions.
Parenchymatous organs are sampled for further examination, including the liver, spleen, fore and rear kidney; pyloric caecaes with the pancreas are also sampled in the case of trout. Also sampled are the eggs, sperm and sometimes the swim bladder.
Isolation of viruses on cell cultures
Isolation of viruses on cell cultures is among the basic methods in studying and diagnosing the viral diseases of fish. Several stable cell lines have been prepared for this purpose. These are:
FMH - cell line from the caudal part of the minnow Pimephales promelas
RTG-2- cell line from the testes of the rainbow trout Oncorhynchus mykiss
PG - cell line from the gonads of the pike Esox lucius
CHSE - cell line from the embryonal cells of the salmon Oncorhynchus tschawytscha
Cell line cultivation
The media.
All cell cultures are cultivated and maintained on media with 10 % of phoetal calf serum (growth) and with 1 % of phoetal calf serum (maintenance).
| Composition: | Growth medium | Maintenance medium |
| MEM Eagle | 90.0 ml | 99.0 ml |
| Phoetal calf serum | 10.0 ml | 1.0 ml |
| Sodium hydrogen carbonate 7.5 % NaHCO3 | 1.0 ml (2.0) | 1.0 ml (2.0) |
| Penicillin-potassium salt + streptomycin 500 I.U. +500 mg | 1.0 ml | 1.0 ml |
| Amphotericin B | 0.1 – 0.2 ml up to 0.5 ml | 0.1 – 0.2 ml up to 0.5 ml |
Every batch of the phoetal calf serum must be tested for cytotoxicity. The store solution of amphotericin is prepared by diluting the content of one phial (50 mg) in 50 ml of distilled water.
The media are replaced if the following criteria are met:
If the cell lines are maintained in thermostats at temperatures specific of each line, it will suffice to replace the media after 1 to 10 days. After about 30 days the lines must be trypsinated again.
Trypsination
The Difco certified trypsin 1:250 (Detroit, Michigan USA) is used for the trypsination. For use it is prepared at a 0.16 % concentration in combination with 0.02 % versene in PBS (pH 7.2–7.4).
| Store solution of trypsin | 10.0 % : |
Trypsin Difco | 10.0 g |
Glucose | 5.0 g |
PBS | up to 100 ml |
All is stirred in a beaker with a magnet for 10 minutes, using an solenoid stirrer. Then the mixture is centrifuged at 2000–3000 r.p.m., the supernatant is filtered and frozen.
| Store solution of versene (Chelaton 3): | |
Sodium chloride | 8.0 g |
Pottasium chloride | 0.4 g |
Sodium hydrophosphate Na2HPO4 | 0.06 g |
Potassium dihydrophosphate KH2PO4 | 0.06 g |
Distilled water | to 1000 ml |
Chelaton III | 0.2 g |
After dissolving, sterilize the solution in autoclave at 1.5 atm for 30 min. For trypsination, always prepare a fresh solution by mixing 25.0 ml of store solution of versene - 0.4 ml of store solution of trypsin.
Trypsination procedure
Maintain the trypsinating solution at 20°C. After sucking off the growth medium or maintenance medium, use the MEM Earle solution with 1 % NaHCO3 and antibiotics (100 I.U. penicillin and 100 μg streptomycin in 100 ml of the cell rinsing medium) to rinse the cell growth in the Roux flask. After rinsing pour the rinsings off and cover the cell monolayer with the trypsinating solution. Leave the cells in direct contact with the trypsin for 30 to 90 seconds (depending on cell quality and type) and pour the solution off. To inspect the process of further “dry” trypsination, watch the Roux flask against light (the growth inside will have a milky colouring) or under inversion microscope (cytoplasmic membranes will become distinct and cells will successively round). Having shaken the cells from the walls of the Roux flask by patting the flask against the hand, resuspend them in 5 ml of growth medium and loosen them by careful pipetting.
Counting the cells.
To count the cells in the suspension, put 1 ml of suspension into a test tube, add one drop of 0.5 % crystal violet and stirr for 2 min. Take one drop of this volume and put it in the Bürger cell. Count only the healthy unstained cells.
Conversion formula: number of cells in 1 large square (arithmetic mean for 10 squares) × 10 000 × amount of stained cell suspension (in the actual case 1).
The Roux flasks for cultivation of the parental line are normally stocked at a rate of 150–250 thousand cells per 1 ml of medium the Leighton or Müller tubes for infection with the tested suspensions are stocked with 300–400 thousand cells per 1 ml. It is necessary that the monolayer should grow over the whole area of the tube in 24–72 hours.
Technique of cultivating cell cultures with the fish tissue suspension being examined
1/ Use a mortar to homogenize the material being examined together with some sea sand. Resuspend the homogenate by means of isotonic phosphate buffer (PBS pH 7.3) or in serum-free culture medium (Earle, Eagle MEM-TM SEVAC VI) at a dilution ratio of 1:10.
| Earle solution in ten-fold concentration: | |
| Sodium chloride NaCl | 68.0 g |
| Potassium chloride KCl | 4.0 g |
| Magnesium sulphate MgSO4.7H2O | 2.04 g |
| Sodium phosphate NaH2PO4.2H2O | 1.5 g |
| Unhydrous calcium, chloride CaCl2 | 2.0 g |
| (may be replaced by CaCl2.6H2O | 3.94 g |
- add the calcium chloride to the solution when all the above mentioned chemicals are completely dissolved, or preferably dissolve it separately in distilled water and add to the solution)
| Glucose | 10.0 g |
| Phenol red. 0.4 % | 15.0 ml |
| Distilled water | to a volume of 1000 ml |
Filter the solution through filter paper. Add 2.0 ml chloroform p.a. to the whole volume and store the solution at 4°C. Sterilize by filtering. Autoclave the diluted working solutions at 1 atm for 20 min.
| Phosphate buffer (PBS), pH 7.2–7.4: | |
| Sodium chloride NaCl | 9.2 g |
| Sodium hydrophosphate Na2HPO4.12H2O | 1.2 g |
| Sodium dihydrophosphate Na2PO4.H2O | 0.115 g |
| Distilled water | to a volume of 1000 ml |
2/ Centrifuge the suspension prepared in this way (3000 r.p.m., 10 min, 20°C).
3/ Pour the supernatant into a test tube, add an antibiotic solution (1000 i.u. penicillin, potassium salt and 1000 μg streptomycin). Thoroughly shake the test tube. Check sterility on bacteriological media. After five minutes of exposure, dilute part of the starting material (10 %) ten times, using PBS, to get a final dilution ratio of 1:100 in another test tube. The prepared suspensions can be frozen at minus 15°C to minus 25°C and stored frozen until they are examined.
4/ Put each inoculum diluted 1:10 and 1:100 (volume of 0.1–0.2 ml per 1 test tube) into three test tubes with the grown cell cultures 24 to 48 h old; before doing that, pour off the growth medium with 10 % of phoetal calf serum and rinse the grown test tube cell culture twice with a serum-free medium containing 1 % NaHCO3.
5/ Leave the absorption to go on for 30–60 min at 17 – 22°C.
6/ Having poured the inoculum off, use serum-free medium with NaHCO3 to rinse the test tubes with initial suspension diluted 1:10 and 1:100 and cover the cell cultures in the tubes with 1–2 ml of medium containing 1% phoetal calf serum.
7/ Maintain the infected cultures at a temperature of 15°C for 5–6 days. Inspect the test tubes every day.
8/ If the cytopathic effect (CPE) occurs, identify the virus by immunofluorescence methods (IF), immunoperoxidase test of ELISA.
9/ If no CPE occurs or if the CPE cannot be safely determined, perform two successive passages with both concentrations after prior freezing and thawing of the suspension. Watch for 5–6 days.
10/ If CPE occurs during the watching period, identify the virus by methods chosen from among the following: IF, ELISA, electron microscopy, immunoperoxidase test.
Immunofluorescence methods (IF)
Microscope slides sized to fit into the Leighton test tubes are used for IF diagnosis. The number of cells in the cultures on the slides is the same as in the virus isolations, i.e. 300–400 thousand in 1 ml of medium. Having poured off the growth medium, rinse the slide cell cultures twice in a routine way and infect them with the material being examined, 0.1 ml of the material being applied to each culture. Leave the adsorption to continue for 45 hours and then add 1.5 ml of maintenance medium to the test tubes. Always take the second or third passage for IF examination. Stop the infection in the test tubes when the first visible changes occur on the cell monolayer.
1/ Indirect IF technique for antigen detection
Carefully remove the infected slides from the Leighton tubes and mark them with the code of the sample being examined. Rinse them in IF buffer, dry them thoroughly at laboratory temperature and fix them in cooled acetylene at a refrigerator temperature (+4°C) for 10 minutes. In this stage the preparation can be interrupted and the slides may be maintained at minus 20°C until examination.
In the moist chamber, cover the slides with a specific rabbit hyperimmune serum against the respective virus, the serum having been diluted according to box titration tested in advance. Leave the reaction of fixation of the antigen with the antibody to go on for 30 minutes. The temperature of this reaction depends on the characteristics of the virus under study. Then pour the hyperimmune serum off, rinse the slides and dip them in IF buffer three times, 10 minutes each time. Remove the slides from the buffer, cover them with a layer of SwAR/FITC conjugate (swine antibody against the FITC specific rabbit serum) and leave them so for 30 minutes. Then rinse the slides again and dip them three times in IF buffer, 10 minutes each time. Without drying, apply onto them 1 drop of glycerine (9 parts of IF buffer + 1 part of glycerine). Put a micro cover slip on top of the slide. Watch the sample under IF microscope at a wavelength of 510 nm, using the G 247 filter (Fluoval IF microscope, Carl Zeiss Jena, Germany)
2/ Direct IF technique for antigen detection
The technique differs from the indirect IF in that the FITC is directly fixed to the specific hyperimmune serum against the respective virus.
Generally it is claimed that the direct IF has a lower sensitivity and specificity.
In both the direct and indirect IF the preparations can be examined immediately or can be stored in the refrigerator to examine them the following day.
| Buffer for IF | |
| NaH2PO4.H2O (or KH2PO4) | 0.39 g |
| Na2HPO4.12H2O | 2.69 g |
| NaCl | 8.5 g |
| Distilled water | ad 1000 ml |
Preparing the hyperimmune serum
To have a sufficient amount of the virus with which the hyperimmune serum is prepared, propagate the serum in 6 passages on the respective cell line on which the greatest cytopathic effect is recorded. Freeze and thaw the virus suspension several times, then centrifuge it at 6000 r.p.m. for 10 minutes in a precooled centrifuge. Check the suspernatant by the IF method and administer it to 3 rabbits.
Immunization scheme:
1st treatment: 3 ml of mixture of virus and complete Freund's adjuvant aa, divided for i.m. administration at several sites.
2nd treatment: 2 ml of mixture administered i.m. 3 weeks later.
3rd treatment: 1 ml of virus supernatant administered i.v. three weeks after the 2nd treatment.
Collect blood by intracardial punction into test tubes the following week. Leave the blood to coagulate, separate the blood clot from the walls of the cells, cut the coagulum, and centrifuge the blood at 2000 r.p.m. for 20 minutes. Pipette the serum, distribute it in 2 ml amounts into ampoules and, if necessary, store at minus 25°C for long. The quality of the serum should be tested by box IF titration.
Immunoperoxidase method
Viruses can also be detected in the infected cell cultures by the direct immunoperoxidase method.
It is an advantage of this method that no immunofluorescence microscope is needed: an optical microscope will easily do the job.
The principle of the test, whose practical application depends on the producer, is the reaction of the enzymatic constituent of the conjugate, i.e. peroxidase + the rabbit antibodies against the respective virus, with the substrate. In the positive case, when there is a strong bond between the antigen (virus) and the enzyme-labelled antibody, this bond is visualized by the staining agent, which reacts with the enzyme. The infected cells have clusters of dark brown granules in their cytoplasm. The colour intensity and the number of positive cells are directly proportional to the length of incubation and to the infectivity of the initial organ suspension.
Electron microscopy
Electron microscopy can be used with advantage when there is a need for rapid tentative detection of viruses from isolates on cell cultures and for classification of the viruses in different virus groups.
1. Method of negative staining
- is successful at a virus concentration of about 1.105 TCID in infected cell culture:
Centrifuge 0.5–0.1 ml of cultivation medium from infected cell culture at 1000 r.p.m. for 10 minutes.
Apply a drop of supernatant on the electron-microscope screen, coated in advance with the formvar film.
Suck the drop off with filter paper after several seconds and apply the staining agent (2 % ammonium molybdate) onto the screen. Suck the staining agent off and examine the screen under an EM.
2. Method of ultrathin slices
- not suitable for rapid detection but provides perfect material for documentation:
Pour the cultivation medium off the infected cell culture, fix the culture by a layer of 3 % glutaraldehyde in PBS (pH 7.2) for 1 hour.
Scrape the cells off the glass, centrifuge them and fix them with 1 % OsO4.
Dehydrate the cells in acetone in a decreasing series of 100 %, 90 %, 60 %, 30 % for 30 minutes to 1 hour (depending on the size of the pellet). Encast the cells in Durkupan ACM. Contrast the ultrathin slices by means of a solution of uranyl acetate and citrate.
3. Immune electron microscopy method
- a method of higher sensitivity than the negative staining method.
Inactive the respective hyperimmune rabbit serum at 56°C for 30 minutes before use and dilute it in PBS 1:20, 1:40, 1:80, 1:100, 1:400. Mix each of these dilutions with the same volume of the sample being examined and incubate the mixture at 37°C for 1–1.5 hours. After incubation, apply a drop of the sample onto the electron microscope screen coated in advance with a formvar film. Remove the sample drop by means of filter paper after several seconds and apply a drop of staining agent (2% ammonium molybdate) onto the screen. Suck the staining agent off and examine the sample under EM: evaluate the presence and size of virus clusters (immunocomplexes).
ELISA
This method is used
This is only a description of the principle. Its practical application depends on the producers of the kits.
| Indirect ELISA for antibody detection | Double antibody sandwich ELISA technique to detect antigens (viruses) | ||
 | 1/Antigen is adsorbed onto bottom of the pit |  | 1/Antibodies are adsorbed onto bottom of the pit |
 | 2/Fixing the specific antibodies onto adsorbed antigen |  | 2/Fixing the antigen onto adsorbed antibodies |
 | 3/Fixing the enzyme-labelled antiglobulin | 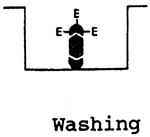 | 3/Fixing the specific enzyme-labelled antibodies against |
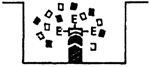 | 4/Adding substrate | 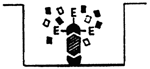 | 4/Adding substrate |
The positive reaction will manifest itself in the colour of the pit: colour intensity is directly proportional to the amount of the antibodies or antigen present there.
Important viral diseases of freshwater fishes
There are about 50 fish diseases that are regarded as being of virus origin. Seventy-five percent of these diseases affect freshwater fishes, twenty-five percent the marine fishes.
Fish viruses fall within the following families:
| ds-DNA-pelleted | - | Herpesviridae |
| ds-DNA-naked | - | Iridoviridae |
| - | Adenoviridae | |
| es-RNA-naked | - | Caliciviridae |
| es-RNA-pelleted | - | Rhabdoviridae |
| - | Orthomyxoviridae | |
| ds-RNA-naked | - | Birnaviridae |
| - | Rheoviridae |
The following viral diseases cause significant economic losses in intensive fish culture:
Channel catfish virus (CCV)
Virus of the family Herpesviridae, causing acute disease in the channel catfish (Ictalurus punctatus). The young channel catfish are killed by the disease. The virus is endemically spread in the U.S.A.
Diagnosis:
Infectious pancreatic necrosis (IPN)
The IPN virus (Birnaviridae) is responsible for a highly contagious disease affecting the fry and the young fish (not older than 20 weeks). The losses may amount up to 100 %. The condition usually has an acute course with a high mortality. In the older fish the course of the disease is usually inapparent. The disease occurs in Europe, North America and Asia.
Diagnosis:
laboratory: direct: virus isolation and identification in
acute stage of infection,
indirect: detection of antibodies.
Stable cell lines RTG-2 and PG, electron microscopy, immunofluorescence, immunoperoxidase test and ELISA are the best for the isolation and identification of the virus.
The IPN virus exerts a marked cytopathic effect on the cell lines. Antibodies can be detected by ELISA.
Spring viraemia of carp (SVC)
This disease affects carp. Its clinical manifestation is most marked in spring at water temperatures below 15°C. The mortality may be up to 90 % under experimental conditions. Poor state of nourishment of the fish and decreased immunity worsen the course of the disease. The disease occurs in Europe.
Diagnosis:
laboratory: direct: virus isolation and identification
indirect: detection of antibodies.
The causative agent of the disease, Rhabdovirus carpio, belongs to the group of Vesiculoviruses. It readily propagates on the FHM and CHSE stable cell lines. It exerts a marked cytopathic effect.
The virus can be detected by immunofluorescence, electron microscopy or by the immunoperoxidase test. Antibodies can be detected by ELISA.
Viral haemorrhagic septicaemia (VHS)
An acute disease of salmonids. It is endemically spread in Europe. Its causative agent is a virus of the family Rhabdoviridae, subgroup Lyssaviruses. The mortality is up to 80 %.
Diagnosis:
Infectious haematopoetic necrosis (IHN)
This is an acute virus disease whose causative agent belongs to the family Rhabdoviridae. The disease affects young populations of salmonids with an up to 100 percent mortality. IHN occurs in North America and Japan.
Diagnosis:
Recommended literature
Ahne W., Wolf K. (1980): Viruskrankheiten der Fische. In: Reichenbach-Klinke H.H. Krankheiten and Schädigungen der Fische. Gustav Fischer Verlag, Stuttgart, New York, 56–106.
Hornych M., Pospíšil Z., Tománek J. (1986): Diagnostics of IPN in trout by immunofluorescence. Med. Vet., Praha, 31, 55–64 (In Czech).
Pospíšil Z., et al. (1986): Isolation and identifications of IPN virus in trout. Med. Vet., Praha, 31, 103–112 (In Czech).
Pospíšil Z., et al. (1987): Epizootology of IPN in salmonids. Veterinářstvi, 37, 489–490 (In Czech).
Pospíšil Z., Tománek J. et al. (1991): Diagnosis and prevention of infectious pancreatic necrosis in salmonids. Research Institute of Fish Culture and Hydrobiology, Vodňany, pp. 15.
Rodák L., et al. (1986): Diagnostics of spring viraemia of carp by ELISA method. Med.Vet., Praha, 31, 505–512 (In Czech).
Rodák L., et al. (1988): Enzyme linked immunosorbent assay detection infection pancreatic necrosis virus. Fish. Dis., 11, 225–235.
Tománek J., et al. (1988): Causes of swimming bladder inflammation in carp. Veterinářství, 38, 76–77, (In Czech).
(P. Vladík)
Most of the bacteria associated with fish diseases are naturally saprophytic organisms, widely distributed in the aquatic environment. Comparatively few species are classified as true obligate pathogens. Both groups of organisms may be present on the external body surface or in the tissues of apparently healthy fish and their pathogenic role will only manifest itself as a consequence of stress.
Fish presented for bacteriological examination fall into two categories (diseased fish, healthy fish), although the dividing line is not always obvious. If clinically diseased fish are subjected to bacterial examination, the effort is focused either on the detection of the causative agent in external lesions such as dermal changes, changes in the gills and so on, or on the examination of the internal organs, especially kidneys, if there is suspicion of a septicaemic disease. If the presence of a specific germ (e.g. vectorship) is to be eliminated or the bacteriological examination is performed for another reason, such bacteriological examination is mainly focused on the kidneys and/or other parenchymatous tissues.
Methods of examination
1/ Wet preparations
These are useful for the rapid presumptive diagnosis of myxobacterial infections, including columnaris, cold water or bacterial gill diseases. Myxobacteria (Flexibacter spp.) can be seen as long slender rods exhibiting a characteristic flexile motility.
2/ Stained smears
Gram, Giemsa and Ziehl-Neelsen stains are commonly used. Though the detection of bacteria in tissues may be conducive to presumptive diagnosis, confirmation by culture is required in the majority of cases, only in the case of diagnosis of bacterial kidney disease (BKD) in salmonid fish are stained smears used for diagnosis. The presence of small, strongly Gram-positive bacilli in kidney smears is considered diagnostic for infection with Renibacterium salmoninarum.
3/ Isolation of bacteria
There are essentially two major approaches: the use of selective media or the use of non-selective media. Our laboratory prefers the non-selective media because such a method of bacteriological examination provides a more complete picture of the bacterial status of the fish. The majority of the bacterial pathogens require no special media for isolation, Tryptone Soya Agar (TSA) and the Blood Agar (BA) being regarded as sufficient; in some cases we can also use other media, e.g. Endo Agar. The exception are Myxobacteria, which require comparatively low levels of both nutrients and agar. Cytophaga Agar (ANACKER, ORDAL 1959), composed of (in g per litre of distiled water) tryptone 0.5, sodium acetate 0.2, beef extract 0.2 and agar 0.9, provides a suitable medium for the isolation of freshwater strains. Renibacterium salmoninarum is an extremely fastidious organism. For its isolation there exist a number of modifications of the ORDAL, EARP (1956) medium. Commonly used is the KDM-2 medium after EVELYN (1977), which contains (in g per litre of distilled water) peptone 10, yeast extract 0.5, cysteine HCl 1.0, agar 15.0; it is supplemented with foetal calf serum (10 % v/w). Mycobacteria spp. can be isolated on any of the glycerol or egg-based media commonly used for this group of organisms (e.g. the Lowenstein-Jensen medium, Dorset medium etc.). Incubation temperatures of 18–23°C are generally used for isolation and visible growth may be expected to appear within 48 hrs in most cultures. R. salmoninarum may require 6 weeks and Mycobacteria 3 weeks.
Myxobacterial infections
Although the term Myxobacteria is commonly used to describe the group of gliding bacteria associated with a number of fish diseases, all the pathogens belong to the genus Flexibacter. The organisms can be recognized by the characteristic yellow, spreading colonies and gliding motility on low nutrient media (ANACKER, ORDAL 1959). Further identification is seldom required because it would need a special bacteriological techniques. Flexibacter columnaris is pathogenic only at water temperatures above 15°C. Flexibacter psychrophila causes disease at temperatures below 12°C.
Aeromonas infections
Aeromonas infections of freshwater fish may, in the essence, be divided into those caused by the motile species (septicaemia) and those caused by the non-motile species (furunculosis, carp erythrodermatitis).
Present characteristics of the genus
Cells straigth, rod shaped with rounded ends to coccoid. Gram-negative, generally motile (Table 1), only one species is nonmotile (Table 2). Facultative anaerobes. Metabolism of glucose is both respiratory and fermentative.
Table 1: Differentiation between motile aeromonads
| A.hydrophila | A.caviae | A.sobria | |
| Esculin hydrolysis | + | + | - |
| Growth in KCN broth | + | + | - |
| L-histidine, L-arginine | + | + | - |
| Fermentation of: | |||
| Salicin | + | + | - |
| Sucrose | + | + | + |
| Manitol | + | + | + |
| Acetoin from glucose | + | - | d |
| Gas from glucose | + | - | + |
| H2S from cysteine | + | - | + |
Oxidase positive. Catalase positive. Resistant to vibriostatic agent 0/129. Optimum temperature range 22–28°C.
Table 2: Differentiation between non-motile subspecies of A. salmonicida
| subssp. salmonicida | subssp. achromogenes | subssp. masoucid | |
| Brown pigment | + | - | - |
| Indol production | - | + | + |
| Esculin hydrolysis | + | - | + |
| Growth in KCN broth | - | - | - |
| L-histidine, L-agrinine | - | - | - |
| Utilization of: | |||
| L-arabinosa | + | - | + |
| Fermentation of: | |||
| Salicin | d | d | d |
| Sucrose | - | + | + |
| Manitol | + | - | + |
| Acetoin from glucose | - | - | + |
| Gas from glucose | + | - | + |
| H2S from cysteine | - | - | + |
Pseudomonas infections
Pseudomonas fluorescens and Pseudomonas putida are considered as casual pathogens responsible for bacterial septicaemia of fish, which as a rule cannot be distinguished from aeromonas-induced septicaemia occurring in fish stocks exposed to different stresses.
Present characteristic of the genus (Table 3)
Straight or slightly curved rods. Aerobic, with a strictly respiratory type of metabolism with oxygen as the terminal electron acceptor; in some cases nitrate can be used as an alternative electron acceptor. Oxidase and catalase positive. The fish pathogens belong to the group of rRNA group I.
Table 3: General characteristics of species 1–5 of Pseudomonas (sec.I)
| Ps. aeruginosa | Ps. fluorescens | Ps. putida | |
| Pyocyanin production | + | - | - |
| Pyoverdin production | +/- | +/- | + |
| Oxidase | + | + | + |
| Levan from glucose | - | + | - |
| Gelatin liquefaction | + | + | - |
| Starch hydrolysis | - | - | - |
| Lecithinase | - | + | - |
| Growth at 4 °C | - | + | + |
| Growth at 41 °C | + | - | - |
| Denitrification | + | + | - |
| Arginine dihydrolase | + | + | + |
Edwardsiella tarda and Edwardsiella ictaluri infections
Edwardsiella tarda causes gas-filled malodorous lesions in the muscle tissue of channel catfish (MEYER, BULLOCK 1973). Edwardsiella ictaluri causes intestinal disease in the catfish (HAWKE 1979).
Table 4: Differentiation within the genus Edwardsiella
| E. tarda | E. hoshinae | E. ictaluri | ||
| most strains | biogroup 1 | |||
| Indole production | + | + | - | - |
| H2S production (TSI) | + | - | - | - |
| Motility | + | + | + | - |
| Malonate utilization | - | - | + | - |
| Fermentation of: | ||||
| D-manitol | - | + | + | - |
| Sucrose | - | + | + | - |
| Trehalose | - | - | + | - |
| L-arabinose | - | + | - | - |
| Tetrathionate | ||||
| Reduction | + | - | - | - |
Present characteristics the genus (Table 4)
Motile peritrichously flagellated rods, not encapsulated. Gram negative, facultative anaerobes. Oxidase negative. Catalase positive. Nitrates are not reduced. Fermentation of glucose with gas. H2S is produced abudantly from TSI agar, but poorly producing biotypes may occur. Indole is produced. Manitol is not fermented by most strains.
Enteric redmouth disease (Yersinia ruckeri infection)
Enteric redmouth is an economic ally important disease of salmonid fish especially in North America.
Present characteristics of the genus (Table 5)
Straigth rods to coccobacilli. Endospores are not formed. Gram negative. Non-motile at 37°C, but motile with peritrichous flagella when grown below 30°C, except for Y. pestis which is always non-motile. Facultatively anaerobic, having both respiratory and fermentative type of metabolism. Oxidase negative, catalase positive. Nitrate is reduced to nitrite. Glucose and other carbohydrates are fermented with acid production but little or no gas. Phenotype characteristics are often temperature-dependent and a greater number of characteristics are usually expressed by cultures incubated at 25–29°C rather than at 35–37°C.
Table 5: General characteristics of the species of Yersinia
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Indol production | +/- | + | + | - | - | - | - |
| Methyl red | + | + | + | + | + | + | + |
| Voges-Proskauer | - | - | - | - | - | - | - |
| Simmons citrate | - | - | - | - | - | - | - |
| Urea hydrolysis | + | + | + | + | + | + | - |
| Lysin decarboxylase | - | - | - | - | - | - | +/- |
| Arginin dihydrolase | - | - | - | - | - | - | - |
| Ornitine decarboxylase | + | + | + | + | - | - | + |
| D-glucose/acid/ | + | + | + | + | + | + | + |
| Fermentation of: | |||||||
| Sucrose | + | + | + | - | - | - | - |
| Lactose | - | - | - | - | - | - | - |
| Manitol | + | + | + | + | + | + | + |
1 - Y. enterocolitica,
2 - Y. frederiksenii,
3 - Y. intermedia,
4 - Y. kristensenii,
5 - Y. pestis,
6 - Y. pseudotuberculosis,
7 - Y. ruckeri.
Vibrio infections
Vibrio anguillarum is a pathogen of marine fish and eels and a major cause of disease in fish culture. Several other species not properly described are also pathogenic for fish.
Present characteristics of the genus
Gram negative straigth or curved rods. Motile. Do not form endospores. Chemoorganothrops and facultative anaerobes capable of respiratory and fermentative metabolism. Most are oxidase positive. All utilize D-glucose as a sole principal source of carbon and energy. Most species require 2–3 % NaCl or seawater base for optimum growth.
Characteristics of Vibrio parahaemolyticus:
Gram negative curved rods. Endospores not formed. Swarming on solid complex media negative. Non-pigmented. Arginin dihydrolase negative. Oxidase positive. Reduction of nitrate positive. Not producing gas from glucose. Voges-Proskauer (acetoin production) negative. Growing at 40°C. Sucrose not fermented. D-gluconate positive.
Bacterial kidney disease (R. salmoninarum infection)
Renibacterium salmoninarum is an obligate pathogen of the subfamily Salmoninae (salmon, trout and char) of the family Salmonidae, which produces a slowly developing chronic infection characterized by enlarged gray-white necrotic abscesses primarily in (but not restricted to) the kidney.
The bacterium belongs to the group of regular, non-sporing Gram negative rods (Section 14, Bergey s Manual 1986).
Present characteristics of the genus
Short Gram-positive rods. Non-capsulated. Non-motile. Endospores not formed. Aerobic. No acid production from sugars. Catalase positive. Oxidase negative. Grows very slowly at 5 and 22°C. No growth at 37°C. Cysteine required for growth.
Streptococcal fish infections
A less common bacterial disease of fish. HOSHINA et al. (1958) isolated Streptococcus faecalis from dying juvenile rainbow trout. Streptococcal sepsis, accompanied by exopthalmus (the so-called pop-eye) in rainbow trout was described by BARHAM et al. (1979). Streptococcal infections in the channel catfish were described by PLUMB et al. (1974).
Characteristics of the genus
Cells spherical or ovoid, occurring in pairs or chains when grown in liquid media. Endospores are not formed. Gram positive. Most are facultatively anaerobic. Catalase negative. Metabolism is fermentative. Growth on solid media is enhanced by addition of blood, serum or glucose.
Serological identificatin is important for determination of haemolytic streptococci. This is a method of classification suitable for use in animals and humans. On the other hand, many so-called viridizing and non-haemolytic streptococci lack the existing group antigens, so they are very difficult to classify.
Streptococcus faecalis belongs to Lancefield serological group D, whose latest denomination is Enterococci (Table 6).
Table 6: Differentiation between enterococci
| S. faecalis | S. faecium | S. avium | S. gallinarum | |
| Hydrolysis of esculine | + | + | + | + |
| Ammonia from arginine | + | + | - | d |
| Reduction of potassium tellurit | + | d | d | - |
| Fermentation of: | ||||
| L-arabinose | - | d | + | + |
| Melecitose | + | - | + | d |
| Melibiose | - | + | d | + |
| Sorbitol | + | d | + | + |
| Sorbose | - | + | + | - |
Mycobacterial infections of fish
Mycobacterial infections (mycobacterioses) have so far been observed in more than 150 species of marine and freswater fish (NIGRELLI, VOGEL 1963). Glycerol or egg based media, commonly used for these group of organism, serve for their isolation. Mycobacterium fortuitum and Mycobacterium marinum are isolated most commonly. Identification of Mycobacteria is best carried out by a specialist laboratory.
Characteristics of the genus
Slightly curved or straight rods, mycelium-like growth may occur but on slight disturbance it usually becomes fragmented into rods or coccoid elements. Acid-alcohol fast. Non-motile. Growth slow. Easily visible colonies produced in 5 days to 3 weeks. Incubation temperature for fish pathogens 25°C. Identification requires special bacteriological techniques.
Recommended literature
Anacker R.L., Ordal E.J. (1959): Studies on the myxobacterium Chondrococcus columnaris. I Serological typing. J. Bacteriol., 78, 25–32.
Barham W.T., Schoonbee H., Smit G.L. (1979): The occurence of Aeromonas and Streptococcus in rainbow trout, Salmo gairdneri Richardson. J. Fish Biol., 15, 457–460.
Evelyn T.P.T. (1977): An improved growth medium for the Kidney Disease bacterium and some notes on using the medium. Bulletin de l'Office International des Epizooties. 87, 511–513.
Hawke J.P. (1979): A bacterium associated with disease of pond cultured channel catfish, Ictalurus punctatus. J. Fish. Res. Bd. Can., 36, 1508–1512.
Hoshina T., Sano T., Morimoto Y.A. (1958): A streptococcus pathogenic to fish. J. Tokyo Univ. Fish, 44, pp. 57.
Krieg N.R., Holt J.G. (ed.) (1984): Bergey's Manual of Systematic Bacteriology. Vol. 1.
Meyer F.P., Bullock G.L. (1973): Edwardsiella tarda, a new pathogen of channel catfish (Ictalurus punctatus). Appl. Microbiol., 25, 155–156.
Nigrelli R.F., Vogel H. (1963): Spontaneous tuberculosis in fishes and other cold-blooded vertebrates with special reference to Mycobacterium fortuitum Cruz from fish and human lesions. Zoologica, New York, 48, 130–143.
Ordal E.J., Earp B.J. (1956): Cultivation and transmission of etiological agent of kidney disease in salmonid fishes. Proc. Soc. Exp. Biol. Med. 92, 85–88.
Plumb J.A. et al. (1974): Streptococcus sp. from marine fishes along the Alabama and northwest Florida coast of the Gulf of Mexico. Trans. Amer. Fish. Soc., 103, 358–361.
Sneath P.H.A, Mair N.S., Sharpe M.E., Holt J.G. (ed.) (1986): Bergey's Manual of Systematic Bacteriology. Vol. 2, pp. 1599..
(J. Řehulka)
Moulds, which cause mycoses, are microscopic organisms producing filamentous coatings on various substrates. Basically, moulds are fungi, which are heterotropic organisms nutritionally dependent on organic substrate. They are classified as a separate phyllum, Mycota, of thalloid plants (Thallophyta).
It is still widely believed that mould infestation of fishes is largely a secondary phenomenon. However, there is evidence that fungi may affect healthy fish in certain circumstances. From the viewpoint of the fish pathologist, there are mycoses that hinder the function of organs and kill the fish on mass and also mycoses that successively deprive the fish body of its natural strength, bringing it up to a cachectic state.
I. Determining moulds and filamentous fungi parasitizing freshwater and marine fishes: laboratory methods
1. Methods of isolation
Mycological examination is an integral part of the complex of examinations of health of the fish and is done more or less simultaneously with the bacteriological examination. Semiparasitic and parasitic fungi are obtained fresh from the host by taking an inoculum from the suspect tissue onto agar medium, using a scorched and cooled snare or dissecting needle. If mycelium is present in the tissue, the sample can be surface-disinfected to prevent secondary contamination by airborne spores. The disinfection is done by dipping in 1% formaldehyde for 1 to 5 minutes. Then the sample is transferred to 70% alcohol and finally to sterile water in which it is throughly rinsed. When isolating an actual species it is also possible to hold the sample under a fast stream of water to wash the secondary infection off.
2. Methods of cultivation
a) Cultivation
The purpose of cultivation is to obtain pure cultures for studying the fungus thalli, their variability and conditions of growth, and for investigating the whole ontogenesis. The cultivation is usually done on synthetic agar media. By the cultivation, an individual is left to produce its progeny which is then regarded as a cultured strain. Differences between strains of the same species are physiological (e.g. production of enzymes or toxins) a may also be morphological (e.g. different colours, the appearance of the fungal growth etc.). On the culture medium the strain produces monospore or multispore colonies which fuse to form a growth. A colony consists of surface and substrate mycelium and these forms may differ on different substrates.
b) Culture media
Besides the common bacterial culture media such as the blood agar or Ordal's agar, parasitic fish fungi can also be cultivated on media of natural substrates. Of these, we recommend the sweet wort agar (1000 ml of wort diluted to half the concentration, 20 g of agar), and of the synthetic media were recommend Sabouraud's agar (40 g glucose, 10 g peptone, 1000 ml distilled water, 15 g agar), Sabouraud's preserving agar for collection cultures (30 g peptone, 1000 ml distilled water, 20 g agar) or the Czapek agar (3 g NaNO3, 1 g KH2PO4, 0.5 g KCl, 0.5 g MgSO4.7H2O, 0.01 g FeSO4.7H2O, 30 g saccharose, 15 g agar, 1000 ml distilled water). These media are suitable for morphological study of the mycelium and the sporulation organs. Low-nutrient media are suitable for maintaining the strains in collection cultures.
c) Inoculation
Perfect cleanness must be maintained in the rooms where the inoculation is performed. The table on which the work is done should be wiped with a cloth soaked in a disinfectant (ajatin); 4% formaldehyde or 3% chloramin with detergent should be used when the work is finished (preferably before leaving the lab). When the Petri dishes are to be opened, the lids should be lifted with care. Small containers (test tubes) are opened when necessary just for a short while, the necks of the containers should be heated before and after transferring the inoculum, and the cotton wool stoppers should be singed on surface. Straight or bent hollow dissecting needles are used for reinoculation of moulds and filamentous fungi. The spores must be transferred together with the mycelium to avoid sterility of the strain. The inoculated test tubes and Petri dishes are put in thermostat at a room temperature.
d) Preserving the cultures
To continuously maintain the fungus cultures, reinoculate them twice or three times annually. The fast growing species need reinoculation every other month, others once in 4–6 months. Low temperatures (4–10°C) slow down growth and allow the cultures to survive up to a year without reinoculation. Abundantly sporulating fungi are reinoculated by transferring the spores with a snare. In the case of fungi with poor sporulation, culture cutouts with agar are transferred. Bent inoculating needles made of a thick wire are suitable for this purpose.
3) Methods of preparation
Moulds and filamentous fungi should first be evaluated for their general habit by examination under binocular lens or, just slightly enlarged, under a microscope, preferably with phase contrast. Preparations that serve for detailed study may be native, semipermanent or permanent.
a) Native preparations are examined in a drop of water. They are stained with either an aqueous solution of neutral red (dilution 0.01 %) or methylene blue (0.01 %). These dyes stain the plasm. To stain the cell wall, use aqueous solution of cresyl blue or a solution of Congo red in ammonia (3 g Congo red per 100 ml H2O plus an addition of 2 ml ammonia).
b) Chloral hydrate, lactophenol or lactofuchsin are used for better clarification of the object. Lactophenol is the best: methylene blue is added to it to enhance its colour, and part of mycelium with the reproduction organs is transferred to. it by means of a dissecting needle. The preparation treated in this way is covered with a micro cover slip and is carefully heated over moderate flame to let the air bubble out; during this it is carefully compressed with the dissecting needle. Lactophenol composition: pure crystalline phenol 20 g, lactic acid 20 g, glycerol 40 g, distilled water 20 g. The preparation is stored in a brown flask.
c) Glycerol gelatine after Kaiser is used for the permanent preparations: distilled water 42 ml, glycerol 30 ml, gelatine 7 g, phenol 1 g. Acetone lacquer or synthetic lacquer can be used for the framing. DuNoyer's cement is also suitable (heat 20 g anhydrous lanolin over a sand bath for 30 min, then continue the heating while mixing the lanolin with 80 g rosin which is added in lumps). A thick wire bent into a triangle can be used to apply the cement: the wire, heated to a high temperature, is laid onto the surface of the cement which melts and can be easily applied onto the edges of the cover slip.
II. The special part
Oomycetosis
Affections caused by fungi of the Oomycetes class are among the most widely known and most widespread mycoses of fishes. The characteristic trait by which the Oomycetes are distinguished from other classes of fungi is the presence of motile spores (zoospores) with two flagella (feather and smooth), which develop in the zoosporangia. In a large part of Oomycetes this is the main mode of nonsexual reproduction; the other modes are through chlamydospores and gemmae. Union of the nuclei of immobile gametes produces a thick-walled spore (oospore). Oomycetes also differ from the majority of fungi by having cellulose in their walls. Besides that, most of the Oomycetes have a diploid nucleus when they are in a vegetative stage. All Oomycetes produce filaments called hyphae which, unlike in the higher fungi, have a limited number of septa. A grouping of hyphae makes a mycelium.
The Oomycetes class breaks into four orders: Legenidiales, Perenosporales, Leptomitales and Saprolegniales. Of these, the genus Pythium of the order Perenosporales, the genus Leptomitus of the order Leptomitales and eight genera of the order Saprolegniales (Achlya, Aphhanomyces, Calyptralegnia, Dictyuchus, Leptolegnia, Pythiopsis, Saprolegnia, Thraustotheca) are considered to be parasites of fishes. The most widespread species parasitizing fish include representatives of the genera Achlya and Saprolegnia, which will be treated in detail below.
The species of the genus Saprolegnia (Fig. 1) have two kinds of zoospores. The primary zoospore, having the shape of a grain with flagella on the end, encysts short after leaving the zoosporangium. Then the cyst may die and produce a mycelium or form a secondary kidney-shaped zoospore with lateral flagella: the feathery one will point forward and the smooth one will point back. The secondary zoospore lives longer and also forms a cyst. The cyst may proliferate owing to mycelium formation, or may produce a secondary zoospore.
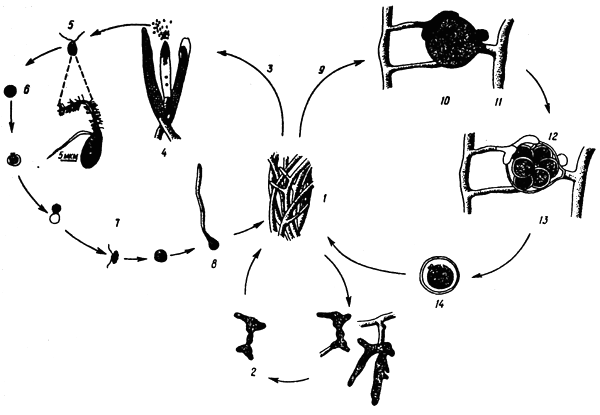
Fig. 1: Developmental cycle in Saprolegnia: 1 - mycelium, 2 - gemmae, 3 - zoosporogenesis, 4 - zoosporangia, 5 - primary zoospore, 6 - encysted spora, 7 - secondary zoospore, 8 - proliferating zoospore, 9 - oosporogenesis, 10 - meiosis, 11 - antheridium, 12 - antheridial cell, 13 - oogonium, 14 - oospore
There are no freely flowing primary zoospores in the fungi of the genus Achlya; secondary oocysts emerge from the cyst and appear at the sporangium opening. In species of the genus Aphanomyces the cysts are produced in the same way as in Achlya but unlike the latter, the former have much thinner hyphae and the zoospores in their zoosporangium are arranged in a single line.
Fish infestation by Saprolegnia, saprolegniasis, occurs in all types of waters all over the world and is one of the most widespread diseases of inland fishes both in ponds and in water courses. The disease breaks out where pathogenous strains are present: absence of saprolegniasis is ascribed to the absence of pathogenous strains. The spores most easily and most frequently penetrate into the fish body when surface of the skin or gills is damaged (mechanically or by a parasitary or bacterial infection) and the fish is weak, unable to produce substances that would protect it. In salmonids the susceptibility to saprolegniasis is induced by exposure to stress: stress raises the corticosteroid level in the blood plasma, thus suppressing the inflammatory reaction and boosting protein catabolism, regulated by the corticosteroids. In the final stage, this results in protein deficit, which in turn is conducive to atrophy of the skeletal muscles and suppression of collagen synthesis. Lack of collagen leads to poor regeneration of lesions on the skin. The Pacific salmon has a high level of corticosteroids in the blood plasma when it migrates and reaches sexual maturity.
Laboratory diagnosis of the order Saprolegniales is performed after routine isolation on common agar media (blood agar, Ordal's agar). A bacterium-free pure fungus culture is obtained by the method of repeated tapping, preferably onto an agar medium with pea extract or onto sweet wort agar. The species are best identified in hemp water after incubating the culture at 18–20°C. Another procedure that can be used is to take a piece of mycelium from the diseased fish or eggs and put it in sterile Petri dishes with sterile distilled water where sterilized halved hemp seeds are added. The fungi start growing two to three days later. Washing and transfer in sterile water are repeated many times to successively obtain a more or less bacterium-free macro culture. The fungus species are then diagnosed according to the morphology of the hyphae and the reproductive organs.
The saprolegnia are fixed by means of a mixture of 90 % alcohol (94 parts) and 40 % formaldehyde (6 parts).
Depending on the degree of infestation, the affected fish stop taking food, lose their escaping reflex and stay close to the shore. They carry white-grey woll-like fungus growths of different sizes, prominent on their body surface. New growths may be difficult to distinguish; the older ones are usually grey-green. The transparent mycelium with a large amount of sporangia can be seen under the microscope. The genus can be identified according to the morphology of the sporangia. When the fungus is removed the primary injury appears underneath. Its margins are dark red and in an advanced stage of the disease the area under the fungal growth is necrotic. The form and location of the fungus may be specific, e.g. in the Atlantic salmon affected by UDN (head); in some fishes it may depend on the sex or may occur with Staff's disease, which sometimes affects the olfactory pits. A reliable histological determination of the fungus hyphae is by staining after Grocott and by the PAS method. Ten percent neutral formol would suffice to fix the tissue for the histological examination. The inflammatory response is surprisingly weak in saprolegniosis: muscle is infiltrated by the lymphocytes and histiocytes and there are proliferated monocytes in the blood; the inflammatory cellulization may be completely absent.
Differential diagnosis is necessary in the early stage of the disease when the symptoms may be the same as those of pox.
Branchiomycosis
Branchiomycosis is a much feared fungal disease of fishes almost all over the world, especially on carp farms. The disease occurs most frequently in the warm climatic regions.
Branchiomycosis occurs in two types:
The two species can be distinguished from each other by their morphological traits and by peculiarities of their development. These can be characterized as follows:
Branchiomyces sanguinis (Fig. 2) is generally located in the blood vessels of the gill arch and gill filaments. The diameter of the hyphae is 8–20 μm, the thickness of the hypha wall is 0.2 μm and the diameter of the spore is 5–9 μm.
Branchiomyces demigrans produces hyphae which are able to penetrate into the gill filaments and spread on their surface. The diameter of the hyphae usually is 13–14 μm and may be up to 22–28 μm at the end of the hypha. The thickness of the wall is 0.5–0.7 μm and the diameter of the spore is 12–17 μm.
B. demigrans differs from B. sanguinis by having thicker-walled hyphae and by being able to proliferate from the blood wessels to the adjacent tissue of the gills.
The spectrum of hosts harbouring these fungi has recently been extended and includes also the salmonids.
Laboratory diagnosis is based on the examination of the gill filaments by the compression method. Fungal hyphae are visible in the blood vessels of the gills at a 150-fold magnification under the microscope. Spores can also be identified when the diseases are in its acute stage: tear the affected tissue into pieces on the microscope slide and examine it in a drop of 50 % glycerol solution in water or alcohol. The branchiomycetes are also well discernible in preparations from dead fish bodies decomposed by rot. For isolation and cultivation, scrape part of the affected tissue on a watchglass with water, pour it into a centrifuging test tube with water and centrifuge for 3 minutes. Pour the supernatant out of the test tube, pour new distilled water in and centrifuge again. Wash the material again until the liquid is clear. Then use a pipette to transfer the sediment onto the microscope slide and, under the microscope, separate the hyphae of the mycelium from the rest of the tissue. Transfer the mycelium again into the test tube with water and centrifuge it. Put the washed hyphae in a 2 % formaldehyde solution, wash in water again, sow them on the Sabouraud's medium, 0.5 % semiliquid agar or blood agar and cultivate them at 20–22°C.
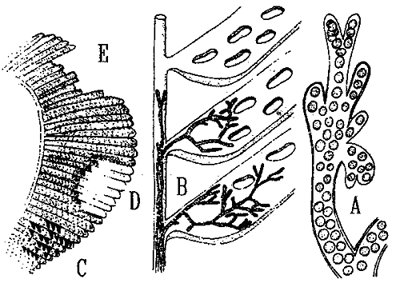
Fig. 2: Branchiomycosis of fish: A - branching hyphae of the fungus Branchiomyces sanguinis, B - fungus hyphae spreading in the branchial blood circulation, C - congested and poor-supply areas arise on the gill filaments, D - parts of the gill filaments necrotize, E - necrotic the gill filaments fall
The rise and course of the disease depend on factors that underline them; water temperature is one of the factors that play the most important part. The disease breaks out most frequently when the water temperature is above 20°C.
The infection comes via the gills where the spores are intercepted. The digestive tract is another infectious route. The spores produce hyphae which proliferate in the branchial blood vessels. The disease may take a peracute course which is very rapid and leads to the blocking of a number of blood vessels: the fish die of suffocation. In the case of an acute course of the disease, necrotic processes take place in the gills, the blocking of the blood vessels is not so extensive but when the water is poor in oxygen the fish may die on mass. The chronic stage of the disease is characterized by the shedding of the necrotic parts of the gill filaments and by a regeneration process accompanied by hyperplasia of the stratified cuboidal epithelium.
In the early stages of the disease, dark red strips can be seen on the gill filaments when the fish are examined; these strips signal the blocking of the blood vessels by the hyphae of the fungi. Later these strips are dirty grey. In an advanced stage of the disease the gill tissue disintegrates and the necrotic parts are shed; this is accompanied by secondary development of the saprolegnia. The fish killed by the disease are usually found dead in the morning. The fish that withstood the disease have gaps in their gill filaments.
Differential diagnosis should be performed to eliminate sanguinicolosis, branchionecrosis and intoxications caused by environmental factors.
Ichthyophonosis
This genus comprises two species: Ichthyophonus hoferi and I. gasterophilum. From the viewpoint of pathogenicity, I. hoferi (Fig. 3) is more important as the causative agent of ichthyophonosis (ichthyosporidiosis). The disease has until now been diagnosed in more than 80 fishes among which sea fishes prevail. Most of the affected freshwater fishes belong to the salmonids.
Microscopic appearence of the organism is dependent on its stage of development. The stages include (1) spore at “resting” stage, (2) germinating spore, (3) hyphal stage.
It is believed that there are two forms of Ichthyophonus, both belonging to one genus. One of them is known as the “salmon” form, occuring in freshwater and cold-preferring sea fishes: this form is characterized by its ability to produce long tubulose germ hyphae. The other is called the “aquarium fish” form, typical of the tropical freshwater fishes. This form is completely devoid of hyphae.
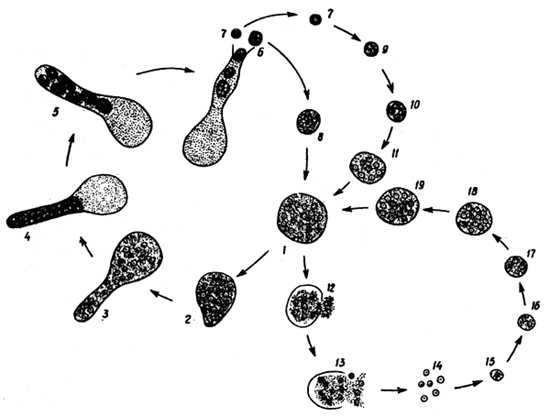
Fig. 3: Developmental cycle of Ichthyophonus hoferi: 1–5 - development of “daughter” spores, 7–11 - development of resting spore from the “daughter” spore, 12–19 - development of resting spore by fragmentation
For mycological diagnosis in the lab, the affected organs should be cultivated on gelatine or in fish broth.
After cultivation the fungus germinates in 1 to 7 days. Fine filaments free of septa with conical ends are sent forth: the conical ends, filled with protoplasm, become round and are throttled off to produce globose or cystose formations. In old cultures, large resting-stage formations (usually unable to germinate in artificial media) appear on the ends of the filaments. In 60 days the fungus culture produces in the medium a growth 5–12 mm in size. The fungus grows at temperatures between 3 and 20°C, ten degrees being the optimum.
The disease is introduced in the fish culture environment by the latently sick fish: they may shed the fungus from the attacked skin or the infection may spread from their urinary tract if their kidneys are invaded. Decaying bodies of the fish killed by the disease may also be a copious source of infection. The infection is contracted via the digestive tract. The clinical signs depend on the intensity of invasion of the different organs. Rainbow trout shows inappetence and inco-ordination of motion in swimming in the early stages; later the flanks darken and finally the dark colour spreads over the whole body. Sometimes the fins disintegrate and may fall off: the affected fins are frayed and white-rimed. If the liver, kidney and spleen are invaded, they enlarge and so does the whole belly of the fish; the eyes often bulge and erode.
The symptoms that accompany severe infection of the kidneys and liver include exophthalmus, distended scales and accumulation of exudate in the body cavity. If the skin is invaded, ulcers develop. No organ is safe from the disease; generally it can be said that tissues abundantly supplied with blood are most vulnerable (heart, spleen).
The organisms elicit a severe focal granulomatous response which replaces much of the organ it occupies. Masses of epithelioid cells surround the developing and germinating spores. The whole granuloma is bounded by a thin connective tissue capsule. Foreign body giant cells may rarely be present.
In differential diagnosis, ichthyophonosis must be distinguished from bacterial diseases accompanied by a chronic proliferative granulomatous response - especially from the mycobacteriosis of aquarium fishes. Imprints of the affeced tissue or histological slices are stained after Ziehl-Neelsen: if acid resistant rods are identified, the ailment is mycobacteriosis (tuberculosis). In a certain stage the disease may look like myxosporidiosis, bacterial disintegration of the fins or deficit of vitamin C and tryptophan. In cases of dermal defects in salmonids, bacteriological or histological examination should be performed to exclude haemophilosis and furunculosis.
Moniliales
Moniliales, also known under their former name, Hyphomycetes - philamentous fungi, belong to the group of imperfect fungi (Fungi imperfecti).
Most of the Moniliales are outside the range of potential parasites of fishes, though in recent years researchers have studied them with increased attention from the point of view of their possible primary pathogenicity. Some species, especially Exophiala salmonis, Exophiala pisciphila and Ochroconis tschawytschae, are particularly suspected in this respect. These fungi can be generally characterized by their forming either sterile mycelium or mycelium with conidiophores on which conidia (spores developing in nonsexual reproduction) are produced. They are classified by morphology, colour and distribution of the spores and hyphae and morphology, colour and distribution of the spores and hyphae and also by the mode of conidium production by conidiogenous cells.
The genus Exophiala
Fungi of this genus produce conidia on the end of the conidiogenous cells called anellide. The important fish pathogens of this genus include E. salmonis and E. pisciphila.
E. salmonis (Fig. 4) produces grey-brown colonies 5–8 mm in size on the Czapek-Dox agar at 25°C in 14 days. It does not grow at 37°C. The anellideconidia are yellow cinnamon in colour and cylindrical in shape, with a rounded distal end and a flat outgrowth on the proximal end. The number of septa is 0 to 2. The septated conidia are 3x11 μm in size and the size of the nonseptated conidia is 3x5 μm. The sporulation is good on grain and maize agar and/ or completely absent on the Sabouraud agar.
E. pisciphila (Fig. 5) produces dark grey to dark olive colonies, 25–28 mm in size, under the same cultivation conditions on potato agar with dextrose and on the grain agar. Like E. salmonis, it fails to grow at 37°C. The anellideconidia are yellow cinamon in colour and round in shape with a rounded distal end and cut outgrowth. The septated conidia are 2×5 μm in size. Sporulation is good on potato agar with dextrose and grain agar and bad on the Sabouraud's agar.
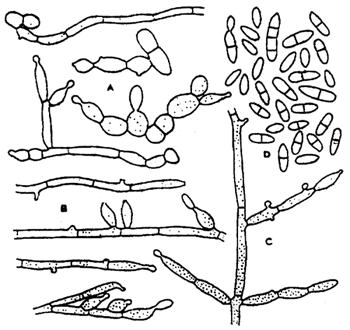
Fig. 4: Exophiala salmonis: A-C - conidial apparatus, D - conidia
E. salmonis and E. pisciphila can be isolated with success on the above-mentioned media and also on the Ordal's and blood agars. The best medium for their cultivation is sweet wort agar.
The epizootics caused by E. salmonis, recorded in Salvelinus namaycush, showed symptoms such as ataxia, whirling, exophthalmus and led to death.
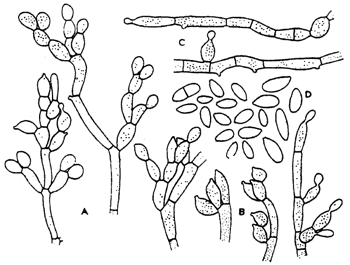
Fig 5.: Exophiala pisciphila: A-C - conidial apparatus, D - conidia, various strains
On histological examination, there was chronic granulomatous response with foreign body giant cells. The pathogenic action of E. pisciphila was demonstrated in a number of hosts, especially in aquarium fishes. The pathogen invades all internal organs, particularly the swim bladder. The sick fish refuse food, keep aloof, stagger, and the final symptoms before death include ascites, distended scales and exophthalmus.
Differential diagnosis should exclude mycobacteriosis.
The genus Ochroconis
Species of this genus produce ellipsoid and cylindrical conidia with wide rounded ends.
Ochroconis tschawytschae was isolated from two fishes and also from the kidneys of Oncorhynchus kisutch yearlings. Olive-coloured colonies 30 to 40 mm accross will grow on potato agar with dextrose at 25°C in three weeks. As distinct from other species of the genus Ochroconis tschawytschae produces small needle-like colonies with three septa ovoid in shape.
The genus Fusarium
Fusarium tricinctum (Fig. 6): Aerial mycelium sparse to floccose, white becoming carmine red to purple on the surface of the agar. Microconidia ovate to pyriform with a minute apiculum; some later become 1 septate. Microconidia are formed initially from simple lateral conidiophores bearing one or two simple cylindrical to subulate phialides. Macroconidia are falcate or more strongly curved and with a well marked foot cell. Sporodochia are pale to orange and formed of a plectenchymatous stroma covered with a palisade of short profusely branched conidiophores each branch of which terminates in 2–3 phialides. Chlamydospores are intercalary, singly or in chains, or occasionally terminal on short lateral branches.
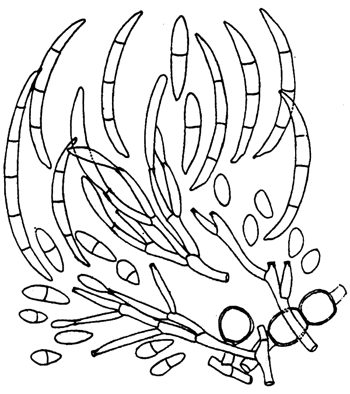
Fig. 6: Fusarium tricinctum: Conidia and conidiophores
This species has recently been isolated from the swim bladder of an intensively farmed rainbow trout. The fish invaded by this fungus swim with difficulty because their swim bladders are filled with a clear or slightly haemorrhagic exudate.
Coelomycetosis
This class is characterized by their conidia's being produced in several types of fruiting bodies, chief among which are picnidia, acervuli and stromata.
Phoma herbarum produces hyaline unseptated conidia 4.5 x 2.5 μm in size, oval to cylindrical in shape, in single or compound picnidia. Cultivation on artificial media must be performed in order to identify the species. All standard media will do, including their variations.
Ailment caused by this fungus has so far been recorded in the fishes of the genus Oncorhynchus and occurs mainly in specimens up to an age of 100 days. The infection starts in the swim bladder, which is ascribed to the fact that the disease is contracted by the fry during the first filling of the bladder: spores may get in together with the air. In cases of spontaneous infection the fish have an enlarged anus with haemorrhages and their belly is laterally constricted. Petechial haemorrhages can also be seen on the caudal peduncle and in the dorsal and ventral part of the body. The invaded fish usually swim on their side with their caudal fin bent down. The yearlings' swim bladder is filled with a liquid and older specimens have a liquid also in their stomach. In the early stage of the infection, mycelium of P. herbarum can be found inside the swim bladder. The mycelium may later completely fill the swim bladder and proliferate into the internal organs.
In conclusion it should be noted that the aetiological spectrum of the diseases of the swim bladder of salmonids includes another six fungus species which have been isolated recently. These are Acremonium koliense, Tolypocladium inflatum, Alternaria consortiale, Fusarium avenaceum, Volutella salmonis, Pyrenochaeta accicola
Recommended literature
Amlacher E. (1986) : Taaschenbuch der Fischkrankheiten. VEB Gustav Fischer Verlag Jena.
Neish G.A., Hughes G.C. (1990) : Fungal diseases of fishes. T.F.H. Publications, Inc., Ltd.
Wolke R.E. (1975) : Pathology of bacterial and fungal diseases affecting fish. In: The Pathology of Fishes (ed by W. E. Ribelin and G. Mikagi), pp, 33–116. The University of Wisconsin Press, Madison, Wisconsin.