INGEGERD DORMLING
INGEGERD DORMLING is Assistant in the Department of Forest Genetics, Royal College of Forestry, Stockholm.
Preparation of material
Investigations on the anatomy of graft unions have been made with material grafted in the greenhouse using the veneer side and side-slit grafts. The graft unions were cut into pieces 5 millimeters (0.2 inch) long fixed in "Craf", embedded in paraffin, and both gross and longitudinal, sections 15m thick were cut and stained with safranin and fast green.
The anatomical details observed in cross, semi-tangential and radial sections are presented in Figure 29. The structure of Scots pine and Norway spruce stems is similar, but there are some remarkable differences in callus formation and the graft union. The parenchyma inside the cambium (pith, rays, resin ducts) consists in Pinus sylvestris of thin-walled cells, while in Picea abies most of these cells have lignified walls. Multiseriate rays are more numerous in Scots pine than in Norway spruce and are lacking in the 1-year-old shoots of the latter. The suberized part of the periderm in young shoots is larger in Norway spruce than in Scots pine and leaf traces are more numerous in spruce than in pine.
Formation of callus tissue
When a graft is made excretion of resin from out resin ducts immediately occurs at the surface of the wound. There is nothing to show that the resin could constitute a barrier between the graft components; on the contrary it serves as a seal. Contact layers, consisting of directly or indirectly damaged cells are formed on the cut surfaces in places where parenchyma is exposed. The enlargement of cells adjacent to the wound surfaces is the first noticeable reaction from living cells and the epithelial cells of severed, vertical resin ducts in the cortex react particularly quickly. Cell division appears close to the wound surfaces in both rootstock and scion 3 to 4 days after grafting. Frequently the scion shows the first divisions and greater activity during the days immediately following grafting.
The cells surrounding leaf and branch traces and the cells of rays that emerge from leaf gaps are particularly active in the formation of callus. On the whole, the rootstocks produce a larger volume of callus before union than do the scions, but leaf traces adjacent to the cut surfaces of the scions cause locally larger formation of callus. Rays that have been cut far out in the phloem usually form more callus than those which have been cut closer to, or in the cambium. The boundary between phloem and cortex is a very active zone of growth.
The cambial region plays a subordinate role as a callus producer in veneer side grafts. In the rootstocks of side-slit grafts, however, the bark has been loosened from the wood in the cambial region and the cells produce vigorous callus formation in Scots pine. This process is initiated by ray cells, but soon the major portion of the cambial cells participate if they are undamaged by grafting.
Callus formation from the pith and from parenchymatous tissues of the wood occurs only in Scots pine where it can sometimes be rather considerable. The pith of the scion is particularly active when cut.
A comparison of the tendency of various tissues to produce callus in both species is presented in Table C1. Great activity in one graft component appears to stimulate adjacent tissues in the other component to increased activity before direct connection is achieved between the tissues concerned.
TABLE C1. - INTENSITY OF CELL DIVISION IN DIFFERENT TISSUES OF GRAFTS OF SCOTS PINE AND NORWAY SPRUCE
|
Tissue |
Scots pine |
Norway |
|
Periderm |
- |
- |
|
Cortex, ground tissue |
+ + + (1) |
+ + + (1) |
|
Cortex, resin duets |
++++ |
++++ |
|
Phloem, rays |
++++ (1) |
++++ (1) |
|
Phloem, vertical parenchyma |
++ (1) |
++ (1) |
|
Phloem, completely differentiated sieve cells |
- |
- |
|
Cambial region, rays |
+++ (1) |
+++ (1) |
|
Cambial region, other undifferentiated or incompletely differentiated cells |
++ |
++ |
|
Xylem, rays |
+ |
- (+) |
|
Xylem, resin ducts |
+ |
- |
|
Xylem, completely differentiated tracheids |
- |
- |
|
Pith, leaf and branch gaps |
++ |
- |
(1) = Tissues influenced by leaf and branch traces show greater activity than others
++++ = Very high intensity of cell division
+++ = High intensity of cell division
++ = Intensity of cell division varies: in pith, leaf and branch gaps of Scots pine divisions occur at an early stage, other wise not until neighboring tissues have started to divide
+ = Divisions in variable amount can occur but often fan to appear
(+) = When late grafted, ray cells of the current year's xylem may divide
- = No cell divisions
Formation of the graft union
Unions between parenchymatous tissues have been confirmed 8 to 10 days after grafting. Fifteen days after grafting there are parenchyma unions in all the greenhouse grafts that have prospects of developing further. Only cells newly formed after grafting are able to unite. In veneer side grafts, the first unions occur between cells originating from tissues outside the cambial region. In side-slit grafts with radial incision in the bark of the rootstock (Figure 29E), the first unions develop between callus from the rootstock cambial region in the bark flap, and callus from cortex, phloem rays and pith of the scion. In side-slit grafts with tangential incision on the rootstock (Figure 29F) unions also occur at the incision face in a way similar to that of veneer side grafts. Unions of newly formed cells may develop independently of the kind of original tissues.
Union between newly formed cork cambia occurs 15 to 20 days after grafting. The old outermost phellogens that enclose the stems do not participate in the formation of the new ones, and a union of new and old phellogens occurs only gradually.
Union of vascular tissues have been confirmed after about three weeks in well-matched grafts. When the matching has been less well done, union may require 5 to 6 weeks. In well-matched grafts it is common that divisions in the cambial region are able to serve in the union of vascular tissues almost at once. Only a small number of short, irregular cells are then formed. When the cambial regions are situated farther apart, they spread through intermediate parenchyma tissues toward each other by induction from one cell to the next.
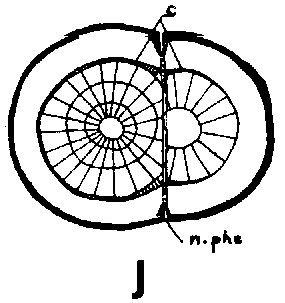
Since the rootstock cambium had entered an active stage already at grafting and since the rootstock has a supply of water and nutrients greater than that of the scion, its cambium will be able to deposit several new rows of tracheids up to the time when union is possible This means that the cambium is forced outward to depart from a scion cambium which has been placed in front, but which is catching up with another cambium that has been placed outside (Figure 29 G, H, I and J)
The first unions of vascular tissues in veneer side grafts are often found between the stock flap and tissues situated on the short cut surface of the scion Leaf traces appear to play an important role in the establishment of vascular connections, especially in Norway spruce where they occur in large numbers The cambial unions in the innermost corner of side slit grafts become complex depending on the mutual position of the tissues of the graft components.
It is not necessary for the space between the wood surfaces of the graft components to be filled with callus tissue. On the contrary, the space often remains empty when the tissues heal rapidly on the outside.
In the summer of the year after grafting the root stocks are cut back with an oblique cut immediate, above the uppermost point of contact with the scion The wound thus caused is callused over from all side in the same manner as in a cut branch.
Veneer side graft
The rootstock flap of veneer side grafts may be fashioned in two ways as shown in Figures 29C and D. Of these two, the downward cut in Figure 29, 4D is to be preferred. In the second case, a slice of wood will adhere all the way along the flap and obstruct a smooth union between the flap and the short cut surface of the scion.
Good fitting of the cambial regions in veneer grafts is essential for rapid union of the graft components. This does not imply, however, that the cambial regions of scion and stock should be placed exactly opposite each other. It is better that the cambium of the scion, is placed a short distance outside that of the rootstock to compensate for the more vigorous growth of the rootstock (Figure G, H, I and J).
It has proved considerably more difficult to arrange a good fit between the graft components in Norway spruce than in Scots pine. Difficulties arising in the grafting of Norway spruce depend on the fact that the rootstocks are often considerably bigger than the scions. A good fit, however, has appeared possible by making a shallow cut in the rootstock, a cut which just reaches the wood, and places the cut surface of the scion opposite that of the rootstock. In Scots pine, however, it is not advisable to make such a superficial incision in the rootstock since this causes a vigorous callus formation over the entire cut surface, which delays the union of the cambial layers or even causes expulsion of the scion. This does not happen in Norway spruce. Of course, the graft components must always be tied firmly to prevent the rootstock from growing over the wood surface before connection with the scion has been established.
A comparison between the 1-year-old shoots of Scots v pine and Norway spruce has shown that the bark of the latter species contains a considerably smaller proportion of living tissues. The Norway spruce shoots are surrounded by a heavy layer of dead cells and the cortex between the ridges formed by the leaf traces is very thin. Since the tissues capable of proliferation e are comparatively limited in extent in scions of Norway spruce, it is very important to arrange a good cambial fit in this species. The investigation has clearly shown e that small young rootstocks should be preferred in the case of veneer side grafts.
Side-slit graft
The incision in the rootstock in side-slit grafts can be made in two ways - radially and tangentially (Figure 29E and F). When the incision is made radially the entire scion will be placed inside the incision face (Figure 29E), and the cambial edges at this point are far apart. With a tangential incision (Figure 29F) there are possibilities of fitting the cambial layers so as to obtain unions corresponding to those of veneer side grafts. Of the side-slit grafts of Norway spruce used in this investigation only those with tangential incision in the rootstock have succeeded. The major part of the unions occurred at the incision face whereas the bark flap often failed. A cut in the rootstock involving a slice of wood would give a still better contact surface for the scion and less empty space in the corner.
FIGURE 29. - A and B. Gross and tangential sections respectively, from the lower part of a veneer side graft of Scots pine. The broken line marks where the two sections touch each other. A. Part of stock cut away before fixing the sections. - C and D. Radial sections from the lower part of veneer side grafts with stock flap cut upward and downward respectively. E and F. Cross sections from side slit grafts. Radial and tangential incision in bark of rootstock. - G, H, I, and J. Simplified illustrations of the cambial growth in veneer side grafts. Cross sections: a and H Big stock, small scion: G. Common situation just after grafting. Cambial layers matched on one side; the cambium of the scion inside that of the rootstock on the other side. - H. After about three weeks. Growing faster, the cambium of the rootstock has left behind that of the scion on the upper side. On the lower side vigorous development of callus - the graft components are being pushed apart. Cork cambia united on the upper side. - I. Rootstock and scion about equal in size: I. Ideal situation. The cambium of the scion outside that of the rootstock on both sides. - J. After about three weeks. Cambia ready to unite. Cork cambia united.
Figure A
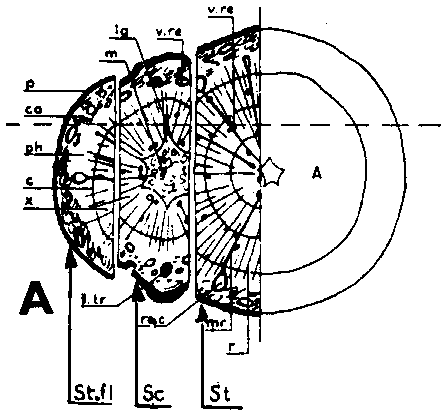
Figure B
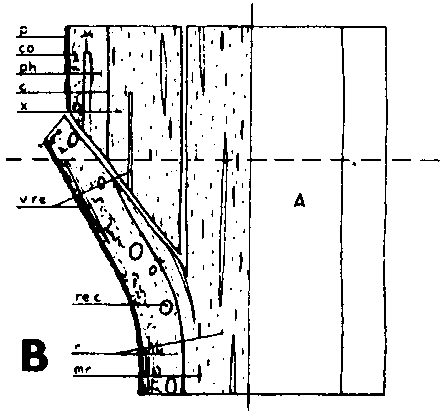
Figure C
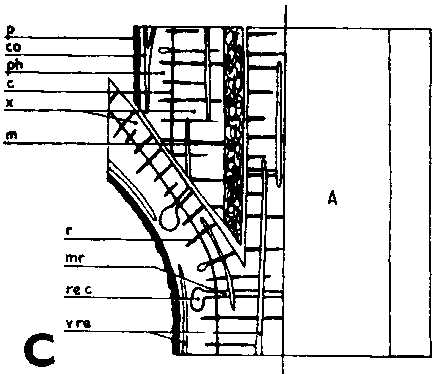
Figure D
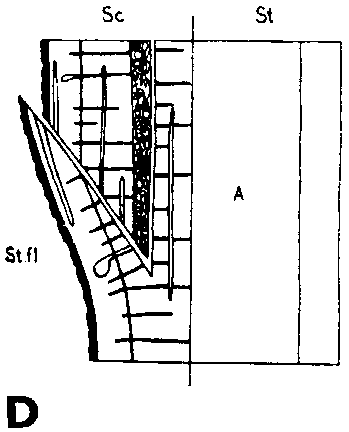
Figure E
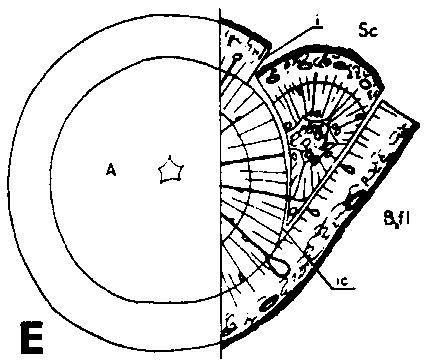
Figure F
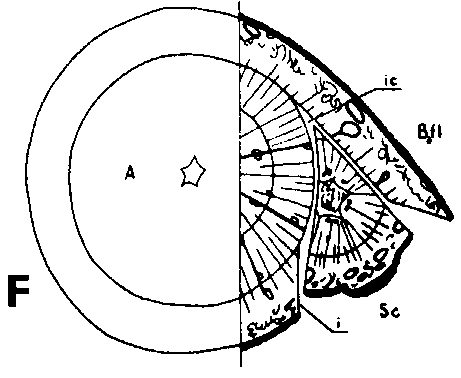
Figure G
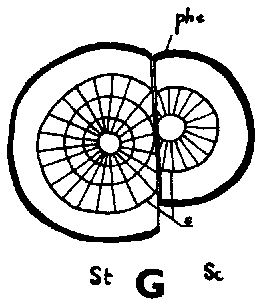
Figure H
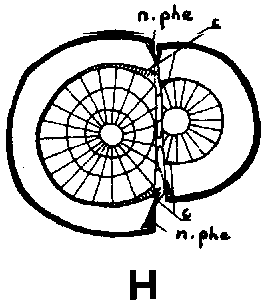
Figure I
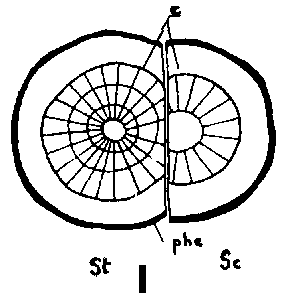
Figure J
Veneer side grafting is clearly the superior of the two methods tested and the one recommended for Norway spruce. Side-slit grafting may be applied to Scots pine when big rootstocks are grafted with small scions. The incisions in the rootstock should be tangential.
Heteroplastic grafting
Heteroplastic or interspecific grafting is used in fruit tree breeding to produce small trees with early and rich flowering. The same effect is aimed at when grafting forest trees for seed orchards.
Some experiments have been made in Sweden, but with unsatisfactory results. Pinus mugo has been used as a rootstock for Pinus sylvestris. These species form good graft unions if the former is of a vigorously growing race but the grafted tree then has characters similar to those of intraspecific grafts. If the rootstock is of a slow-growing race the scion will grow faster than the rootstock. A swelling is formed above the graft union which weakens and is easily broken by the wind. Too rapid growth of the rootstock may cause expulsion of the scion. Experience has shown that seedlings of the same species and of a provenance as near that of the scion as possible are the best rootstocks o Scots pine and Norway spruce. From some mother trees it is very difficult to obtain grafts, and incompatibility between graft components of the same species must sometimes be expected.
In countries with several species available the possibilities of successful interspecific graft combinations are considerably greater. Studies with forest trees have been made by Mergen (1954 a), Pitcher (1960) and Ahlgren (1962).
Incompatibility between rootstock and scion has been subject to many investigations (Proebsting, 1926, 1928: Bradford and Sitton, 1929; McClintock, 1948; Herrero, 1951; Thiel, 1954; Stigter, 1956; and Pitcher, 1960). It is a common occurrence that the scions persist one or more years and then die. In some grafts of Populus reported by Schönbach (1960) the scions lived up to 7 years. In these grafts the xylem unions are almost complete but the phloem junctions are unsatisfactory. Cork layers are involved in the graft region. The continuity of the cambium seems to be broken at the end of the growing season. A new union may be established in the spring but often fails to appear. Some graft combinations have proved to be compatible if leaves are left in the rootstocks (Stigter, 1956; Pitcher, 1960).
The mutual influence between rootstock and scion is often discussed but its nature is not fully understood (Brix, 1952; Thiel, 1954; Rogers and Beakbane, 1957). It is quite clear, however, that hereditary characters are never influenced in this way.
References
AHLGREN, C. E. 1962. Some factors influencing survival, growth, and flowering of intraspecific and interspecific pine grafts. J. For., 60: 785-789. (H) 1
BRADFORD, F. C. & SITTON, B. G. 1929. Defective graft union in the apple and the pear. Mich. Agric. Exp. Sta. Tech. Bull. 99. 106 p. (A, H).1
BRAUN, H. J. 1958. Die normalen Verwachsungavorgänge nach Pfropfung von Laubbäumen. I. Des Verfahren des seitlichen Einspitzens. Z. Botan., 46: 309-338. (A) 1
BRAUN, H. J. 1959. Die normalen Verwachsungavorgänge nach Pfropfung von Laubbäumen. II. Die Verfahren des seitlichen Anplattens und der Kopulation. Z. Botan., 47: 145-166. (A) 1
BRAUN, H. J. 1960a. Die normalen Verwachsungevorgänge nach Pfropfung von Laubbäumen. III. Das seitliche T-Schnitt-Verfahren, die Geissfuss- und Keilpfropfung, sowie das Ablaktieren. Z. Botan., 48: 58-65. (A) 1
BRAUN, H. J. 1960b. Neuere Erkenntnisse über die Vorgänge beim Pfropfen von Bäumen. Mitt. Deut. Dendrol. Ges., 61: 32-44. (A) 1
BRIX, KÄTHE. 1952. Untersuchungen über den Einfluss der Pfropfung auf Reis und Unterlage und die Möglichkeit einer Übertragung eventueller Veränderungen auf die Nachkommen. Z. Pflanzenzücht., 31: 261-288. (H) 1
DORMLING, I. 1964. Anatomical and histological examinations of the union of scion and stock in grafts of Scots pine (Pinus silvestris L.) and Norway spruce (Picea abies [L] Karst.). (To be published in Sludia Forestalia Soecica) (A) 1
EVANS, G., WATSON, D. P. & DAVIDSON, H. 1961. Initial evolution of grafting some species of the Rosaceae. Proc. Am. Soc. Hort. Sci., 78: 580-585. (A, H) 1
HERRERO, J. 1951. Studies of compatible and incompatible graft combinations. J. Hort. Sci., 26: 212-237. (H) 1
JULIANO, J. B. 1941. Callus development in graft union. Philippine J. Sci., 75: 245-251. (A)1
LAUNAY, J. 1961. Phénomènes d'histogenèse produits lors du greffage du pin maritime. Rec. Trav. Lab. Vég. Fac. Sc., Bordeaux 2. (A) 1
MCCLINTOCK, J. A. 1948. A study of uncongeniality between peaches as scions and the Marianna plum as a stock. J. Agric. Res., 77: 253-260. (H) 1
MERGEN, F. 1954a. Anatomical study of slash pine graft unions. Quart. J. Florida Acad. Sci., 17: 237-245. (A) 1
MERGEN, F. 1954b. Heteroplastic micrografting of Slash pine. Southeast. Forest Expt. Sta., Sta. Paper 47, 17 p. (H) 1
PITCHER, J. A. 1960. Heteroplastic grafting in the genera Acer, Fraxinus, Picea and Abies. Proc. 7th Northeast. For. Tree Impr. Conf., p. 52-57. (H) 1
PROEBSTING, E. L. 1926. Structural weakness in interspecific grafts of Pyrus. Bot. Gaz., 82: 336. (A, H) 1
PROEBSTING, E. L. 1928. Further observations on structural defects of the graft union. Bot. Gaz., 84: 82-92. (A, H) 1
ROGERS, W. S. & BEAKBANE, A. B. 1957. Stock and scion relations. Ann. Rev. Plant Physiology., 8: 217-236. (H)1
SASS, J. E. 1932. Formation of callus knots on apple grafts as related to the histology of the graft union. Bot. Gaz., 94: 364-380. (A, H) 1
SCHÖNBACH, H. 1960. Beiträge zur Pappelforschung nr V. Beobachtungen an heteroplastischen Pfropfungen innerhalb der Gattung Populus. Wiss. Abh. Landw.-wiss, Berlin, 47: 27-41. (H)1
SHARPLES, A. & GUNNERY, H. 1933. Callus formation in Hibiscus Rosa-sinensis L. and Hevea brasiliensis Müll. Arg. Ann. Bot., 47: 827-840. (A) 1
STIGTER, H. C. M. DE. 1956. Studies on the nature of the incompatibility in a cucurbitaceous graft. Mededel. Landbouwhogeschool, Wageningen, 56 (8). (H) 1
THIEL, K. 1954. Untersuchungen zur Frage der Unverträglichkeit bei Birnendelsorten auf Quitte A. (Cydonia EMA). Gartenbauwiss., 1: 127-159. (H) 1
1 (A) = anatomy; (H) = heteroplastic grafting.