1.1 Food and its components
1.1.1 Food
Food is the material which, after ingestion by the animal, is capable of being digested absorbed and utilised. However, not all components of food are digestible.
1.1.2 Components of food
The main components of food are shown in Table VII/1:
Table VII/1
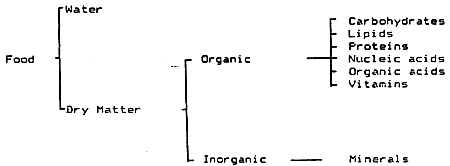
Nutrients are components of food which can be utilised by the animal either as energy sources or for metabolic processes. The energy source components are the proteins, lipids and carbohydrates, while the vitamins and minerals are need to be present in small quantities in the feed for metabolism and life maintenance.
Proteins are complex compounds containing carbon, hydrogen, oxygen, nitrogen, and, generally sulphur. Proteins are found in all living cells. Each species has its own specific proteins. Amino acids are produced when proteins break down through enzyme hydrolysis.
Lipids are a group of substances found in plant and animal tissue. They are insoluble in water, but soluble in organic solvents like ether or chloroform. Table VII/2 below shows the classification of lipids:
Table VII/2
Classification of lipids
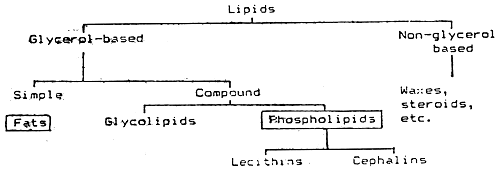
Dietary lipids are sources of essential fatty acids (EFA) and energy. They also act as carriers of fat-soluble vitamins. The term fats includes saturated and unsaturated fats which exist in solid and liquid forms respectiively. Fats contain more energy per unit weight than proteins or carbohydrates.
Nearly all the fatty acids in animal and fish feed as are in the form of triglycerides and phopholipids. Fatty acids have their own classification. The Omega (w)3 and 6 series are important to fish. w3 fatty acids and phopholipids are found at high levels in marine fish, and are therefore important in dietary considerations.
Carbohydrates are chemical components containing carbon, hydrogen and oxygen Most plant material is carbohydrate. Digestible carbohydrate can be utilised by fish as energy source if it is kept in proper balance with the other nutrients. The availability of dietary carbohydrates for energy is low to carnivorous fish. Cooking improves the digestibility of starch and also facilitates feed binding. Carbohydrates like cellulose (fibre) are not digestible to carnivorous fish. Glycogen is the only carbohydrate of animal origin and is present in liver and muscle as a ready energy source.
Vitamins are organic compounds which are required in small amounts for normal growth, life maintenance and reproduction. They are either fat or water soluble. There are 4 important fat-soluble vitamins (A, D, E, K) and 11 water-soluble ones [B1 (thiamine), B2 (riboflavin), B6 (pyridoxine), B12 (cynocobalamin), panthothenic acid, niacin, H (biotin), folic acid, choline, inositol and C (ascorbic acid)].
Minerals have many uses within the animal body. They provide strength and rigidity to skeletal structures, are present in body fluids to maintain balance between the fish and its aquatic environment, integrate nervous and endocrine activities and are components of blood, enzymes, tissues and organs. Minerals are indispensible for metabolism and energy exchange.
Water is not a nutrient, but it makes up about 75% of the adult animal's body weight. Water functions in the body as a solvent in which nutrients are transferred and waste products are excreted into after chemical reactions that involve hydrolysis.
1.2 Finfish nutritional requirements
1.2.1 Dietary requirement for protein/amino acids
Table VII/3 below shows the estimated dietary protein requirement of some fish. The values are generally higher for carnivorous fish.
| Feeding type and fish species | % dietary crude protein | |
| 1 | Herbivorous feeding type | |
| 1.1 Grass carp (Ctenopharyngodon idella) | 34 | |
| 2 | Omnivorous feeding type | |
| 2.1 Common carp (Cyprinus carpio) | 25–38 | |
| 2.2 Tilapia (Tilapia zillii) | 35 | |
| 3 | Carnivorous feeding type | |
| 3.1 Plaice (Pleuronectes platessa) | 50 | |
| 3.2 Gilthead bream (Sparus aurata | 40 | |
| 3.3 Ped sea bream (Chrysophyrs major) | 55 | |
| 3.4 Yellowtail (Seriola quinquer aciata) | 55 | |
| 3.5 Chionook salmon (Oncorhvncus tschawtscha) | 40 | |
| 3.6 European seabass (Dicentrachus labrax) | 45 | |
| 3.7 Seabass (Lates calcarifer) | 45 |
Source: Adapted from Cowey, 1979, Cho et al, 1985, Jhingran and Pullin, 1985, New, 1987.
Generally, younger fish require higher dietary protein for maximal growth. This has been shown to be true for the local seabass (Lates calcarifer), rainbow trout, chinook salmon and channel catfish. Seabass require 52–55% dietary protein from 5g mean weight, and 45% from 50g.
Ten of the 23 amino acids isolated from natural proteins are indispensible to fish i.e. the fish is incapable of synthesising them, and must therefore obtain them from diet. The essential amino acids are arginine, histidine, isoleucine, leutine, lysine, methionine, phenylalanine, threonine, tryptophan and valine. Without these, fish fail to grow. Table VII/4 below shows the quantitative essential amino acid requirements of some fish. As these requirements vary from species to species, the exact requirements for marine carnivorous cultured in this region are still not known.
| Essential amino acid | Chinook salmon | Japanese eel (percentage in feed) | Carp | |
| 1 | Arginine | 2.4 | 1.7 | 1.6 |
| 2 | Histidine | 0.7 | 0.8 | 0.8 |
| 3 | Isoleucine | 0.9 | 1.5 | 0.9 |
| 4 | Leucine | 1.6 | 2.0 | 1.3 |
| 5 | Lysine | 2.0 | 2.0 | 2.2 |
| 6 | Methionine*1 | 1.6 | 1.2 | 1.2 |
| 7 | Phenylalanine*2 | 2.1 | 2.2 | 2.5 |
| 8 | Threonine | 0.9 | 1.5 | 1.5 |
| 9 | Tryptophan | 0.2 | 0.4 | 0.3 |
| 10 | Valine | 1.3 | 1.5 | 1.4 |
| Total | 13.7 | 14.8 | 13.7 | |
*1 In the absence of cystine
*2 In the absence of tyrosine
1.2.2 Dietary requirement for lipid
There is a lack of information on dietary lipid requirement of fish. There is evidence that the essential fatty acid requirement varies from species to species, but that highly unsaturated fatty acids of the w3 series, which are in relatively large concentrations in fish oil, are essential to fish.
Marine fish have more w3 than w6 fatty acids w6/w3 = 0.16) than freshwater fish (w6/w3 = 0.37). Generally however, all fish contain more w3 fatty acids than w6, and have a higher dietary requirement for w3 fatty acids.
1.2.3 Dietary requirement for carbohydrate
Carnivorous fish are generally ill-equipped to deal with carbohydrates which are of plant origin. Herbivorous and omnivorous species can digest carbohydrates more easily. The enzyme amylase which breaks down starch is secreted by all fish, but is less extensive in carnivores than herbivores.
Carbohydrate digestion of the carnivorous rainbow trout decreases as dietary carbohydrate increased beyond 20%.
1.2.4 Dietary requirement for vitamins
Vitamin functions and deficiency signs in fish are well recorded. In almost all cases, anaemia, loss of weight and poor growth are the result of vitamin deficiency.
Table VII/5 shows the dietary vitamin requirements of some fish species, and Table VII/6, a description of vitamin functions and deficiency signs:
| Vitamin | mg/kg feed | Fish species | ||
| 1 | Water-soluble vitamins | |||
| 1.1 | Thiamine (B1) | 1–3 | Channel catfish | |
| 2–3 | Carp | |||
| 2–4 | Turbot | |||
| 2–5 | Eel | |||
| 10–12 | Trout | |||
| 10–15 | Salmon | |||
| 1.2 | Riboflavin (B2) | 5–10 | Atlantic salmon | |
| 7–10 | Carp | |||
| 20–25 | Chinook, Coho salmon | |||
| 20–30 | Trout | |||
| 1.3 | Pyridoxine (B6) | 2–5 | Sea bream | |
| 5–10 | Carp | |||
| 10–15 | Trout | |||
| 10–15 | Atlantic salmon | |||
| 15–20 | Chinook, Coho salmon | |||
| 1.4 | Pantothenic acid (as pantothenate) | 25–50 | Channel catfish | |
| 30–40 | Carp | |||
| 40–50 | Trout and salmon | |||
| 1.5 | Niacin | 30–50 | Carp | |
| 120–150 | Trout | |||
| 150–200 | Chinook, Coho salmon | |||
| 1.6 | Folic acid | 5–10 | Atlantic salmon | |
| 6–10 | Trout; Chinook, Coho | |||
| salmon | ||||
| 1.7 | Cyanocobalamin (B12) | 0.015–0.02 | Chinook, Coho salmon | |
| 1.8 | myo-Inositol | 200–300 | Trout, carp | |
| 300–400 | Chinook, Coho salmon | |||
| 1.9 | Choline | 500–600 | Carp | |
| 600–800 | Chinook, Coho salmon | |||
| 1.10 | Biotin (H) | 1–1.5 | Trout, salmon | |
| 1.5–2 | Brown trout | |||
| 1–15 | Carp | |||
| 1.11 | Ascorbic acid (C) (as ascorbate) | 30–50 | Carp, channel catfish | |
| 50–80 | Coho salmon | |||
| 2 | Fat-soluble vitamins (A & D in IU* | |||
| 2.1 | A | 1000–2000 | Carp | |
| 2000–2500 | Trout | |||
| 2.2 | D | 1600–2000 | Trout | |
| 2.3 | E | 40–50 | Chinook salmon | |
| 80–100 | Carp | |||
| 2.4 | K | 80 | Salmon, trout, catfish | |
Source: Adapted from NAS/NRC 1973, Halver, 1978, Cho et al, 1985
| Vitamin functions and deficiency signs in fish | ||
| Vitamin | Functions | Deficiency signs in fish |
| 1 Water-soluble vitamins | ||
| 1.1 Thiaoine (B1) | Associated with lactic acid formation in the tissues which occurs if thiamine is deficient. | Anaesia, poor appetite, anorexia, opaque eye fatty liver, loss of equilibrica, convulsions darkening in older fish, muscle atrophy, fin paralysis, rolling, whirling movement, vascular degeneration, weakness, growth curtailment. |
| 1.2 Riboflavin (B2) | Respiration and sight. Involved with pyridoxine in the conversion of tryptophan to nicotinic acid. | Anorexia, cloudy lens, lens cataract, darken skin, poor vision, discoloured iris, eye & haenorrhage, poor co-ordination, photo-phobi |
| 1.3 Pyridoxine (B6) | Involved in fat metabolise, especially of essential fatty acids. As a result, carnivorous fish have a specific requirement for B6 in their diet and stores are rapidly exhausted. | Anorexia, convulsions, no reaction to light, anaemia, rapid jerky breathing, muscle spasm weight loss. |
| 1.4 Pantothenic acid | Kidney functioning, production of cholesteral, carbohydrate, fat and protein metabolise. | Anorexia, clubbed gilis, flared opercula, gills sucus, lethargy, jam necrosis. |
| 1.5 Niacin | Respiration, protein metabolisa, food oxidation to energy. | Anaecia, anorexia, gut lesions, stomach |
| 1.6 Folic acid | Blood cell formation, bone narrow conversion, blood glucose regulation, cell membrane function, egg hatchability. | Anaemia, anorexia, darkening, exephthalmia gills, fragile gills, lethargy, pale gills |
| 1.7 Cyanocobelazin (B12) | Involved with folic acid in blood tissue formation, methionine formation, cholesterol metabolism | Anorexia, poor/erratic blood condition. |
| 1.8 myo-Inositol | Involved with choline in lipid metabolise. Critical for maximum growth and liver storage. | Anaemia, blosted stomach, anorexia, skin lesions. |
| 1.9 Choline | Prevents fatty livers, synthesis of phospho-lipids and fat transport. Essential for growth and food conversion. | Anaecia, poor food conversion, poor growth kidney and gut haemorrhage. |
| 1.10 Biotin (H) | Fat metabolisa and various enzyme systems. | Anaemia, anorexia, gut lesions, darkening, blood cell fragmentation, muscular atrophy convulsions. |
| 1.11 Ascorbic acid (C) | Amino acid enzyme systems, collagen formation, blood formation. | Anorexia, impaired collagen, poor backbone formation (lordosis and scoliosis), poor g twisted gill filaments. |
| 2 Fat-soluble vitamins | ||
| 2.1 A | Skin formation, vision, growth of new cells. | Poor growth and vision, thickening of skin tissues, skin lesions, night blindness, haemorrhage of eyes and fins. Enlargement liver. |
| 2.2 D | Calcica and phosphorus balance. | Abnormal bone formation, poor feed convers and growth, lethargy and darkening. High int mobilises phosphorus and calcius from bone causing fragile bones and poor growth. |
| 2.3 E | Antioxicant. Involved in blood capillary permeability. It may affect embyro seabrane peraeability and hatchability of eggs. | Anaemia, ceroid in liver, spleen and kidney clubbed gills, fragile red blood cells, po growth. |
| 2.4 K | Blood clotting, which is important in aquatic environment of fish. | Anaemia, prolonged blood coagulation. |
Sources: Adauted from Halver, 1978, 1979.
1.2.5 Dietary requirement for minerals
Calcium and phosphorus are usually regarded together because they are combined with each other in the body and an inadequate supply of one limits the nutritive value of both. Most fish are capable of extracting calcium from the environment through the gills.
Calcium, phosphorus, sodium, sulphur, molybdenum, chlorine, magnesium, iron, selenium, iodine, manganese, copper, cobalt and zinc are essential for fish body functions.
Table VII/7 shows the estimated dietary mineral requirements of fish in general, the functions of these minerals, and, where available, the deficiency signs:
| Mineral element | Common level in feeds (g/kg feed) | Functions | Deficiency signs in fish |
| Calcium | 5 | Bone & cartilage formation, blood clotting, muscle contraction | - |
| Phosphorus | 7 | Bone formation, formation of organo-phosphorus compounds | Poor growth and backbone formation |
| Magnesium | 0.5 | Enzyme co-factor in fat, carbohydrate and protein metabolism | - |
| Sodium | 1–3 | Osmoregulation | - |
| Potassium | 1–3 | Osmoregulation & nerve action | - |
| Sulphur | 3–5 | Amino acid & collagen formation, detoxification | - |
| Chlorine | 1–5 | Digestion & acid-base balance | - |
| Iron | 0.05–0.1 | Haemopoeisis | Anaemia |
| Copper | 1–4 | Amino acid and vitamin metabolism | - |
| Manganese | 20–50 | In metabolic enzymes, bone formation and red blood cell regeneration | - |
| Cobalt | 5–10 mg | Part of B12, prevents anaemia | - |
1.2.6 Requirement for dietary water
The need for dietary water varies among fish species. In European marine salmon farms, dry pellets are given mixed with water. However, it did not significantly affect feeding, growth, food conversion, fish body composition or condition. Similar observations have been made with seabass fingerlings.
It has been reported that since marine fish cannot swallow thhe water they require from the environment, moist feeds would replace the water lost osmotically across the gills and body surface, the blood ion concentration of the fish being lower than the ionic concentration of sea water.
Others have reported that teleosts living in a water environment maintain water balance by drinking the water and excreting the accumulated salts. From observations on marine fish like the turbot, salmon and seabass growing well on dry feed, it would appear that at least some are capable of overcoming thsi problem. They could indeed be taking in sea water and excreting large amounts of inorganic ions to maintain electrolyte balance. It is reported that very little urine is produced and that excretion involves mainly the gills.
2.1 Feeds
2.1.1 Function of feeds
The primary function of feed to the fish is the provision of energy for life maintenance, growth and reporduction. The farmer feeds his fish to obtain as high a yield and survival as possible. He also tries to ensure optimal feeding with as little wastage as possible.
Energy metabolism in fish is similar to mammals except that fish do not need to use energy to maintain body temperature and they require less energy to excrete waste nitrogen.
The digestible energy from protein, fat and carbohydrate for locally cultured carnivorous fish species is not known. For the present, rainbow trout values of 4 kcal/g protein, 9 kcal/kg fat and 4 kcal/g carbohydrate are taken.
2.1.2 Choosing feeds
It is important to use a feed which satisfies the nutritional requirements of the fish, as this elicits a positive response from the fish. Most species consume food to satisfy their energy requirements and the energy content of the diet determines the amount of feed consumed.
There are various factors influencing the choice of feed for farmed fish. Of these, the economic factor is usually given precedance. Feeds and feeding can represent about half the operating costs in farming.
2.2 Examples of commonly used feeds
2.2.1 Trash fish
In this region, trash or low quality fish is mainly used as feed for cultured fish. Trash fish is converted into higher quality protein in this manner. However the supply of trash fish is not always consistent. In the developed countries, feed is prepared as complete diets in accordance with known nutritional requirements. This has been essential to the success of intensive aquaculture and is a dominant economic factor in large farms.
In Singapore, trash fish is still the main feed used in seafarming. Trash fish is caught by trawl and landings vary according to the monsoon seasons, being lowest from December to February because of the north-east monsoon.
Generally, the family Mullidae forms the largest proportion (31%) of trash fish used in Singapore for fish farming. Other families that are represented in substantial numbers are Pentopodidae, Platycephalidae and Synodontidae. Trash fish from fishing traps are usually those from the families Engraulidae (69%), Clupeidae (31%), Apogonidae and Gobiidae (<1%). Composition varies with locality and season.
The proximate composition of trash fish is given below:
| Asfed | Dry basis | |
| % | % | |
| Moisture | 76.4 ± 1.7 | - |
| Crude protein (NX6.25) | 18.0 ± 1.1 | 76.3 |
| Crude fat | 0.15 ± 0.1 | 0.6 |
| Ether extractable fat | 0.9 ± 0.3 | 3.8 |
| Ash | 3.7 ± 1.4 | 15.7 |
| - Calcium | (1.0 ± 0.5) | (4.2) |
| - Phosohorus | (0.7 ± 0.3) | (3.0) |
| TVB-N*1 | ||
| (fresh chilled) | 11–15 | |
| (overnight) | 100 | |
| (fresh caught) | 9 |
*1 Total volatile base nitrogen which gives anindication of spoilage.
Trash fish is in moderately good condition when received daily by farmers. There are usually no freezer storage facilities at the farm.
2.2.2 Formulated feed
A formulated feed is basically a compounded mixture of various feedstuffs, microingredients and binder. It is a good alternative to natural food.
Commercially available formulated fish feeds are mainly those for species farmed in temperate countries (eg. salmonids, European sea bass and breams). These may not be entirely suitable for the fish that are farmed in this region. Other commercially available formulated feeds are for species like the tilapia and carps. These feeds originate from countries like Taiwan, Japan, Thailand.
Essentially, formulated feeds are complete or supplementary diets which are used in farming situations where the fish are either totally dependant on an external food source (eg. fish cultured in tanks and raceways), or where they receive part of their daily feed from nature (eg. pond fish) and the rest as supplementary external feed. Total dependance on an external food source is more likely where fish are intensively farmed. All available food from nature becomes insignificant under such conditions.
2.2.3 Practical feed
The term practical feed here refers to those mixes, usually moist, which farmers can make on-farm with simple machinery. The feed could be a combination of natural feed or slaughter-house waste with a supplementary feed binder mix. Practical feeds extend the availability of trash fish and is relatively cheap. It requires simple machinery and can be processed on-farm.
2.3 Feed types
2.3.1 Feed classification
Feeds may be typed by their moisture level (eg. dry or moist feeds), their composition (eg. natural or formulated, high protein, starter, grower or finisher), their form (sinking or expanded/floating, mash, paste or microparticulate), their use (complete or supplementary).
The following are commonly used feed classifications:
| Moisture in feed (%) | |||
| 1 | Dry feed | - | <20 |
| 2 | Semi-moist feed (intermediate) | - | 20–50 |
| 3 | Moist feed | - | >50 |
| 1 | Natural | - | unprocessed feed of animal or plant origin eg. trash fish *1. |
| 2 | Formulated | - | feed made up of a variety nutritional ingredients which can be in processed or natural form *2. |
| 2.1 Artificial feed | - | feed made totally from processed ingredients. | |
| 2.2 Practical feed | - | feed comprising an artificial formulated component and a natural component eg: trash fish combination. | |
| 2.3 Purified feed | - | laboratory test feeds for nutritional requirement experiments and not used in actual farming situations. |
| 1 Mash, paste | - | feed in powder form with binder, and to which water has to be added to form a paste, which can be fed directly or in pelleted form. |
| 2 Pellet | - | discrete, dry or semi-moist feed offered in particles to fish. Pellets can be: |
| 2.1 Expanded (floating) | ||
| 2.2 Compacted (sinking) | ||
| 3 Flake | - | flat pieces of bound feed formed in thin layers on a heated drum. |
| 4 Microparticulate | - | small sized dry or liquid feed for larvae |
| 1 Supplementary feed | - | feed formulated to complement food already existing in the farming system (eg. pond fish feed). |
| 2 Complete feed | - | feed whose formulation ensures that the nutritional needs of the fish are satisfied as the fish have no other access to nutrients. |
2.4 Feeding
2.4.1 Definition
Feeding, which is ingestion of food by an animal, is also the input of external nutrient sources into an animal culture system which otherwise will have partial or even no access to food.
The method of feeding employed, its frequency and the rate of feeding all influence production. Improper feeding results in feed wastage, fish malnutrition and poor performance. The acceptance of the feed by the fish is an important consideration, and a nutritionally adequate feed that is unpalatable to the fish cannot function well.
2.4.2 Feed (consumption) intake
Fish feed to maintain their life processes in order to grow and reproduce. Dietary energy, the environment and diet palatibility influence feed.
Feeding rate (usually expressed as % of total fish biomass per day).
The first step to estimating feeding rate is to be able to predict the expected weight gain over a fixed period under the existing conditions of culture eg. at a specific water temperature, stocking density and culture system. This can be done by establishing a growth curve for the fish, say, with trash fish. The life stage of the fish eg. fry, fingerling, grower should also be taken into consideration. The expected feed conversion ratio is also taken into consideration eg. 4.5:1 (feed:weight gain) for trash fish and 1.8:1 for dry pellets.
For a more ideal growth curve, several fish stocks should be covered under similar culture conditions. Feed conversion and even fish survival can be predicted with the standard growth curve (Figure VII/1).
The example below shows how feeding rate is calculated:
Table VII/13
Example 1: To calculate feeding rate for 500 seabass growing from 100g to 300g over expected growth period of 2 months (60 days) and feed conversion ratio of 4.5:1 with trash fish
| Initial fish mean weight | = | 100 g |
| Initial total weight (500 fish) | = | 50 kg |
| Interim fish expected mean weight* | = | 300 g |
| Interim expected total fish weight* assuming 100% survival | = | 150 kg |
| Growth period | = | 60 days |
| Expected feed conversion ratio (as-fed) | = | 4.5:1 |
| Mean weight gain over 60 days | = | 200 g |
| Total expected weight gain after 60 days | = | 200×60 g |
| = | 12 kg | |
| Feed required per fish for 60 days | = | 4.5 × 200 |
| = | 900 g | |
| Feed required per fish per day | = | 900/60 |
| = | 15 g | |
| Feed required per day by 500 fish | = | 7.5 kg |
| Total feed to be given over 60 days | = | 7.5×60 |
| = | 450 kg | |
| Feeding rate at start | = | 7.5/50 |
| = | 15% | |
| Expected feeding rate at end | = | 7.5/150 |
| = | 5% | |
| Expected mean feeding rate | = | 15 + 5/2 |
| = | 10% | |
| * The calculation for actual mean feeding rate is based onthe actual fish interim mean weight (say 350g) and fishnumbers (say 475): | ||
| Actual total interim weight of fish | = | 350×475 g |
| = | 166 kg | |
| Actual feeding rate at end | = | 7.5/166 |
| = | 4.5% | |
| Initial feeding rate | = | 15% |
| Actual mean feeding rate over 60 days | = | 15 + 4.5/2 |
| = | 9.75% | |
When using dry pelleted feed, feeding rate can be calculated on basis of dietary protein equivalent (isonitrogenous basis) to trash fish:
Table VII/14
Example 2: To calculate feeding rate with dry pelleted feed of say 45% crude protein under the same fish conditions of Example 1
| Crude protein of trash fish (as-fed) | = | 18% |
| Crude protein in trash fish given over 60 days | = | 450×0.18 |
| = | 81 kg | |
| Crude protein in dry feed (as-fed) | = | 45% |
| Amount of dry feed containing 81 kg protein | = | 81/0.45 |
| = | 180 kg | |
| Amount of dry feed per day | = | 180/60 |
| = | 3 kg |
3 kg is equivalent to 6% of initial fish total weight (50kg) and 2% of interim total fish weight (150kg) or an average of 4% feeding rate.
Table VII/15 the feeding variables adapted from Weber and Hugenin, 1978.
| Feed form | Live, fresh natural state, moist, dry, sinking/floating meal/paste, pellet/flake, microparticulate/ encapsulated |
| Feeding mechanism | Hand, mechanical (non-automatic), automatic(timed) |
| Feed distribution pattern | Point, arc, linear, area broadcast |
| Feed distribution timing | Intermittent, continuous, diurnal/nocturnal |
| Culture system | Floating cage, submerged cage, raceway, tank, silo, pond, lagoon, bay, pen |
2.5 Weaning
2.5.1 Definition
Weaning is the process by which a change from the usual diet is achieved.
2.5.2 The need to wean fish
The usual diet of farmad carnivorous food fishes in the region eg. seabass (Lates calcarifar), grouper (Epinephelus tauvina) and snappers (Lutjanus johni. L. argentimaculatus) is trash fish. Trash fish is fed fresh cead if caught from nearby fishing traps, chilled, if from inshore landings, or thawed if stored frozen.
Fish that are used to a particular feed can refuse a change of diet unless it is palatable enough, and of a form (eg. softness) and size that is suitable to them. Weaning the fish to the new feed (not necessarily resembling their usual food) can therefore involve several stages.
Fish fingerlings, presently purchased by farmers from overseas from total length 2.5 cm, are already accustomed to minced trash fish. Weaning to semimoist or dry feed is likely to be done on-farm in floating netcages.
2.5.3 Weaning procedure
Most weaning studies that have been recorded are those for fish larvae or early fry eg. for flatfishes, salmonids, sea bream, European seabass and eel. The weaning method for these fish are already well established.
The feasibility of weaning seabass fry to dry feed has been demonstrated for as early as 40-day old fish, and studies on the feasibility of weaning them even earlier are on-going.
Several weaning procedures have been tested for the farmed carnivorous fish species in Singapore. They are summarised in the table below:
Table VII/15
Weaning methods
1 Weaning from trash fish to semi-moist formulated feed by:
1.1 direct feeding eg. for seabass fingerlings
1.2 gradual dietary change by feeding with 3:1 feed combination of dry feed binder mash and trash fish, and decreasing the trash fish portion gradually until only the former and water are necessary
2 Weaning from trash fish to dry formulated and pelleted feed by
2.1 direct feeding (not recommended as this can be stressful to fish)
2.2 gradual feeding according to (1.2) and then reducing the moisture content of the formulated feed in stages
2.3 direct feeding on semi-moist formulated feed and then reducing dietary moisture content in stages
2.5.4 Ease of weaning fish fingerlings
The ease of weaning is usually gauged by noting feeding response, the weaning period fish survival. It depends on species, fish size and condition.
Seabass fingerlings (5–150) can accept semi-moist feed within a week of weaning in floating netcages, with good survival. An additional 2 weeks may be required to elicit good feeding response to dry pellets.
Direct weaning to dry feed is not recommended as higher mortalities have been recorded when using this method. Feeding response is never as good as by the gradual weaning method. Growth during weaning is also observed to be slow, and therefore weaning should be as efficient as possible to minimise the period.
2.5.5 Effect of weaning on fish survival
Observations are that this is about 10% in grouper and 5% or less in seabass. i.e. additional to normal mortality.
3.1 Formulated feeds
3.1.1 Need to formulate feeds
Feeds are formulated is to ensure that the nutritional requirements of the animal are supplied. In the absence of information on these requirements, formulation can only be guesswork, often with certain ingredients and their levels used because another successful feed contained them.
A logical step in the development of a complete formulated feed is to use it over extended periods with occasional supplementation with natural food. Although warmwater fish can grow well on incomplete supplemental feeds, they grow better on complete rations.
3.1.2 Feed formula components
Basically they are as follows:
Table VII/16
Feed formula components
Protein source eg. fishmeal, soyabean meal etc.
Fat source eg. fish oil
Micro-ingredients eg. vitamins, minerals
Binder eg. starch
Additives eg. antibiotics, mould inhibitors
Supplements eg. amino acids
The nutrient most needed for weight gain is protein. Protein sources usually represent the main cost component of the feed.
3.2 Principles of feed formulation
3.2.1 Fish nutritional and energy requirements
To successfully formulate feeds, the protein and other nutritional requirements (eg. fat) of the fish should be known. The more expensive ingredients should be substituted with cheaper ones without compromising on nutritional quality.
Knowing the nutritional requirements would also ensure that optimal levels are used in formulations and that fish are satisfied at least cost.
Like other animals, fish eat to meet their energy requirements. Provided food has a satisfactory nutrient balance, fish will, within limits (usually the physical capacity of the digestive tract) be able to compensate for low energy density in food by eating more of it. It will require less of a high energy density diet for unit weight gain. At maximum feed intake, a fish, given such a diet would sustain a higher weight gain.
It is important to provide an optimal balance of energy components because an excess or deficiency of non-protein energy (lipid and carbohydrate) may lower the growth rate. If the diet is deficient in non-protein energy, protein will be used as energy source for basal metabolism and voluntery activity rather than for protein synthesis. If a diet contains an excess of non-protein energy, appetite may be satisfied before enough protein and other nutrients can be ingested for maximum growth.
3.2.2 Working out the feed formula
Balancing crude protein and energy levels
In most animal diets, protein is the most expensive nutrient and is usually the first to be considered;
Once the crude protein of the feed formula is established [see 3.2.1 (a)] it is easier to fit in other energy sources (usually fat) to make up the energy requirement.
Checking on essential amino acids
Total amino acids is best expressed as a percentage of the diet and of the crude protein.
A broad guideline in formulation is to check that feed amino acid pattern matches fish carcass amino acids. Ideally, the essential amino acid requirement of the fish should be studied.
Essential amino acids that appear to be lacking in the formulation could be made up for by using ingredients that have more of it or by supplementation.
Ideally the least cost ingredients should be used, although this is not always possible without compromising in some way, the nutritional quality of the feed. It is therefore important to evaluate the performance of the modified feed.
Best-buy ingredients
A cost-efficient feed is one where feed ingredient prices and their variation throughout the years have been monitored and related to quality and availability.
Feedstuff price should not be only compared on the basis of per metric tonne, but also by per unit of protein, Total essential amino acids or energy.
Since protein is usually the greatest part of the feed cost, the best-buy technique may be employed to identify the least expensive protein source:
Table VII/17
Example 3: To determine the best-buy ingredient in the following two samples of fishmeal:
Fishmeal A costs S$1.20/kg and has a gauranteed minimum crude protein (CP) level of 55%.
Fishmeal B costs S$1.10 and has a gauranteed minimum CP level of 45%.
Fishmeal A evaluation
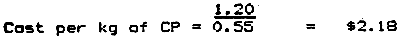
Fishmeal B evaluation
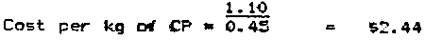
Fishmeal A would be the better buy. However it would be necessary to determine the level of total amino acids to see its relationship to crude protein. Buying Fishmeal A would save $260/metric tonne.
3.2.3 Feed and feed ingredient costs
An approximate division of formulated feed cost is shown below:
| Feed component | % in diet | % cost |
| 1 Protein sources | 70 | 30 – 35 |
| 2 Fat sources | 2 – 3 | 2 |
| 3 Micro-ingredients | 4 – 5 | 30 |
| 4 Binder | 20 | 10 |
| 5 Additives & supplements | 2 | 2 – 5 |
| 6 Processing & bagging | - | 15 |
| 7 Transportation | - | 5 – 10 |
3.3 Feed and feedstuff composition
Feed and feedstuff ingredients may be broken into several components after proximate analysis:
Table VII/19
Proximate composition of feeds and feedstuffs
Crude protein (CP) (N × 6.25) - amino acids
Crude lipid or
Ether extractable fat
Carbohydrat
Crude fibre
Total ash
- calcium
- phospohorus
Nitrogen-free extract
3.4 Feed processing
3.4.1 Feed processing involves the following basic steps:
Table VII/20
Basic steps in feed processing
Grinding/milling (particle size reduction) and sifting/screening (particle size selection)
2 Mixing
- premixing (of microingredients)
- bulk mixing (of bulk or large volume ingredients)
- total mixing (of all ingredients)
Actual processing (pelletisation, extrusion)
Cooling and drying
Crumbling and sizing (where required)
Bagging, storage, transportation
3.4.2 Description of basic feed processing steps
Grinding/milling and sifting of feedstuffs
This is one of the most vital steps in fish feed production as particle size has a direct relationship to feed bindability. Feed bindability affects feed water stability, which is crucial when the feed is used in an aquatic enviromnet. Feedstuff particle sizes of 500 microns bind well.
Grinding also improves feed mixing and pelletibility, and increases the bulk of some ingredients. It improves feed accaptability and digestibility.
Grinding is achieved with several types of machines such as the hammermill, dïsc mill, attrition mill and roller mill. They all break up larger-sized particles into finer ones.
Screens or sifters associated with the grinding machine ensure that the suitably-sized particles are retained and collected.
Mixing
Mixing ensures blending, homogeneity and uniformity of ingredients.
In many formulations, some ingredients are present in smaller quantities than the bulk ingredients. Pre-mixing is a step taken to mix such ingredients separately. TYhe premix is then blended with the bulk ingredients during mixing.
Adequate mixing ensures that the finished product contains all the ingredients in proportions similar to the formulation.
Mixing is achieved with horizontal or vertical mixers. The horizontal mixer is more efficient as it eliminates particle segregation through size, shape, bulk density, moisture content or electrostatic charge.
Processing
Steam pelletisation
In steam-pelleted feeds, dry steam is provided by a steam boiler/generator fitted with adequate water separators, steam traps and pressure controls.
Steam quality and specifications depend on the pelleting system, and 2–3 atmospheres, 80–100°C are usual. It is also usual to steamcook the feed mix for about 5–10 minutes before pelleting.
The pellet mill has a rotating die which is vertically mounted. Steam-conditioned feed mix is forced through the die holes which compact the feed. Binding is effected by the gelatinisation of starch in the formulation by steam heat.
Extrusion cooking
Extrusion cooking results in high starch gelatinisation and consequently, good feed water stability. The process can produce both sinking and floating pellets which are more digestible than steam peleted ones. However, as the temperature used in high, denaturing of nutrients is more likely. Extruder cookers are also usually large and expensive, and feed production rate slower. The feed is more expensive and is likely to be economically feasible for high-priced species that require good feed water stability eg. shrimp.
Cooling and drying
Feed coming out from the processing machine is hot (temperature depending on processing temperature eg. 60–70°c for steam pelleted feed and up to 90°c with dry pelleting machines) and damp (15–18% moisture in steam pelleted feeds).
Cooling and drying can be done in vertical or horizontal cooler/dryers. In the former, feed falls by gravity into a chamber from which air is sucked by a fan. Horizontal coolers are better in that feed movement is minimised, and this reduces breaking up. The pellets are conveyed on a moving perforated steel belt over which air is passed.
Crumbling and sizing
Crumbling is the process by which dried and cooled pellets are crushed into smaller particles. Sizing is the fractionisation of the small feed particles or crumbles, into various sizes which can then be used for different fish size groups.
Cooled pellets are ground on corrugated rollers and the product sifted by a sifter into granules or crumbles or various sizes by using screens of appropriate mesh sizes.
During sifting, fines, or the powder-like remnant, may be returned to the pellet mill for repelletisation.
Bagging, transportation and storage
Before bagging, it is important to ensure that the pellets are properly cooled and dry, otherwise trapped moisture in the bags will cause the feed to mould.
Feeds are filled into plastic lined paper bags in predetermined weights (usually 25 kg) and the bags sewn by machine.
Transportation and storage affect feed quality and keepability. Bags containing feed should not be exposed to the sun. Storage should be made in cool, dark, well-ventilated warehouse conditions. Storage problems are insect infestation. mould and fungal attacks which result in stale and musty feed, increased moisture and temperature, rancidity and lipid oxidation.
3.4.3 On-farm feed processing
The method and equipment described in Practical VII/1 for semi-moist feed are adaptable for smaller-scaled fish farms such as can presently be found in Singapore's coastal waters.
4.1 Evaluation of feed ingredients
4.1.1 Nutrition is based on the intake, digestion and metabolic utilisation of feed. Finfish comprise mainly of muscle, which is protein tissue, while bones reflect the need for minerals. Fat dominates as the energy source, while vitamins and trace elements are necessary for maintenance of vital functions.
4.1.2 Chemical analysis is usually the starting point in determining the nutritive value of feeds, while digestibility determines feed utilisation.
4.1.3 Raw materials (feedstuffs) that have potential use as ingredients in fish diets may be evaluated as follows:
Table VII/21
Feedstuff evaluation
Analyse ingredient for proximate composition;
Determine digestibility of ingredient to the fish species concerned;
Formulate a test feed containing 2–3 levels of this ingredient;
Bioassay the test feed with the fish species concerned by measuring feed intake, fish growth and feed conversion;
Calculate nutrient and energy retention
4.1.4 Feed and feedstuff evaluation
True digestibility takes into account nutrient intake and absorbed nutrients:
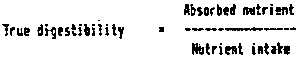
where
| Absorbed nutrient | = | Nutrient | - | [Faecal | - | Metabolic nutrient] |
| intake | [nutrient | excreted with faeces] |
Apparent digestibility is the measurement where no correction is made for metabolic faecal losses:

In digestibility determinations, faeces markers or indicator substances are incorporated in the feed. Such indicators are inert, in that they do not themselves affect digestibility. Chromic oxide is the commonly used faeces marker. Apparent digestibility is expressed as a percentage and calculated as follows:

Such studies involve feeding the fish with feed containing the test ingredient and collecting faeces over a period of time. Faeces and feed are determined for marker concentration, and the nutrient whose digestibility is to be measured eg. protein or fat is calculated.
4.2 Assessment of feed performance
4.2.1 Feed efficiency and conversion
Feed efficiency is the weight gain per unit feed intake:
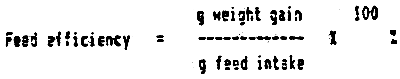
Feed conversion ratio (FCR) expresses the feed consumed to unit weight gain:
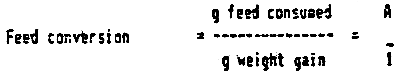
FCR = A:1
4.2.2 Protein efficiency and retention
Protein efficiency ratio (PER) expresses the weight gain to protein consumed:
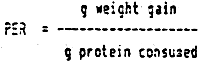
Protein retention is percentage protein deposited in the fish to its protein intake:
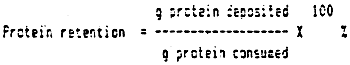
Fat and energy retention are calculated by the same method.
D42 feeds
1 Objective
To prepare formulated seabass/grouper semi-moist grow-out feed.
2 Equipment and materials
2.1 Equipment for feed processing
Electrical slow grinder with 3mm–7.5mm dies and cutting blade attachment;
Vertical heavy duty mixer with 20 and 40-litre stainless steel bowls, aluminium hook and beater attachments;
Mechanical platform or top-pan scales of 2 kg × 100g units, 10 kg × 500-g units.
2.2 Other apparatus
Plastic basins, scrapers, buckets and plastic bags
2.3 Materials
Feed ingredients according to formulation;
Vitamin and mineral premix supplements;
Alpha-starch binder or wheat flour and hot water.
3 Method of feed preparation
3.1 Feed formulation
| Ingredients | % | 10kg | ||
| a | Bulk ingredients | |||
| i | Fishmeal (min. CP*1 50%) | 40 | 4 | |
| ii | Soyabean meal, solvent extracted (CP 45%) | 20 | 2 | |
| iii | Meal and bone meal (CP45%) | 19 | 1.9 | |
| b | Micro-ingredients | |||
| i | Vitamin premix | 2 | 0.2 | |
| ii | Choline chloride 50% | 2 | 0.2 | |
| iii | Mineral premix | 1 | 0.1 | |
| c | Binder | |||
| i | Alpha-tapioca starch or | 12 | 1.2 | |
| ii | Wheat flour | (20) | ||
| d. | Oil | |||
| i | Fish oil | 4 | 0.4 | |
| TOTAL | 100 | 10 | ||
3.2 Feed mix preparation
Weigh out micro-ingredients (vitamin and mineral premixes and choline chloride) and binder separately in plastic bags.
Mix these in the smaller mixing bowl for 10–15 minutes, using the beater attachment and making sure that ingredients sticking to the side of the bowl are scraped into the centre of the bowl.
Weigh the bulk ingredients into a basin and transfer into large mixing bowl.
Mix the bulk ingredients well for 20 minutes, using the beater attachment.
After 20 minutes, add in micro-ingredient-binder mix a little at a time into the bulk ingredient mix. Continue to mix thoroughly using the beater attachment. Mix for a further 10 minutes to ensure homogeneity.
Weigh and add the oil slowly to the feed mix.
Add enough water to form a slightly moist loose crumble. Record the actual amount of water used and calculate percentage added to feed in (6). If using wheat flour which will gelatinise only with hot water, use near boiling water.
3.3 Feed pelletisation
It is necessary to compact the feed mix to improve water stability.
Set up the slow grinder by fixing a die of appropriate holesize (3mm for early grow-out fish of 50–100g mean weight, 5mm for 300g fish and 7.5mm for market fish of 600g) and cutting blede. The speed of the grinder has adjusted so that about 8mm pellets will be cut off.
Pass the prepared feed mix gradually through the grinder without forcing too much at a time.
Re-pelleting is necessary if a normal speed grinder is used to obtain better compaction. A slow grinder is best.
4. Results
| a | Weight of dry feed mix in bowl | = | kg |
| b | Weight of freshwater added to mix | = | kg |
| c | Percentage of water added | = | a/b × 100 |
| = | % |
1. Objective
To assess the water stability of feeds.
2. Equipment and materials
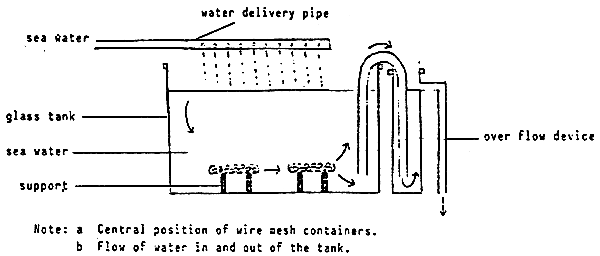
2.1 Equipment
200-litre tank with water delivery and outflow (eg. is shown below) and supports on tank base to hold net baskets;
Electrical top-pan weighing balance ± 0.001g;
Moisture oven;
Dessicator.
2.2 Other apparatus
Wire baskets, 10cm × 10cm × 5cm; stop watch; spatula; forceps, aluminium drying dishes with covers.
2.3 Materials
Commercial prawn dry pelleted feeds, semi-moist pellets prepared from Practical VII/1.
Figure VII/1
Simple set-up to assess the relative water stability of feeds
3 Method of feed water stability assessment
3.1 Determination of dry matter in feed
Pre-heat moisture oven to 130°C;
Weigh about 2 × 10g of the feed samples and place into two previously weighed aluminium drying dishes. Evenly spread the feed in each dish to ensure even drying. Weigh each dish with feed. Label each dish. Place dishes in moisture oven with dish covers slightly displaced;
Dry the feed, checking on the weight of dish and feed every 4 hours until constant weight for 2 consecutive weighings. Note the final weight of dish and feed.
3.2 Determination of feed water stability
Start seawater through flow in tank system and adjust flow rate to 8 litres/min;
Weigh another two feed samples (around 10g). Spread each evenly on a wire basket. Place baskets on supports in tank;
Leave for 10 minutes (for fish feed) or 30 minutes (shring feeds) under through flow conditions;
Remove wire basket. Slant to drain. Pick up discrete places of feed left and place in previously weighed aluminium dish for drying describes in (3.1).
4 Results
| Feed description | Dry matter in feed before innersion | Dry matter of feed after immersion | |||||||||
| Feed type*1 and replicates | Pellet diaceter | length | Initial weight | Final weight | Dry matter | Moisture in feed | Moisture in feed | Initial weight | Final weight | Dry matter | Water stability*3 |
| (cm) | (mm) | (g) | (g) | (g) | (g) | (%) | (g) | (g) | (g) | (%) | |
| A*2 | B*2 | B-A | A(B-A) | A(B-A)×100 | C*2 | D*2 | D-C | (D-C)×100 | |||
| A | (B-A) | ||||||||||
*1 eg. comercial dry pelleted feed for shrimp and semi-acist pelleted test feed for seabass.
*2 (Weight of dish - feed) - (Weight of dish alone)
*3 The water stability of feed is expressed as the percentage of dry matter retained as a descrete after thefeed has been immersed in water for a standard period (eg. 10, 30, 60 minutes).
41/frpvii.1
1 Objective
To demonstrate the methodology for peroxide value (POV) in fish material.
2 Principle
2.1 Unsaturated fish oils are particularly susceptible to oxidation. They develop peroxides if stored too long. Peroxides eventually break down into products that cause rancid flavour in the material. The peroxide concentration is indicative of oxidation during the early stages of lipid deterioration. It is therefore a measure of the extent of lipid oxidation in the material.
3 Application
3.1 Peroxide value determination is usually made for oils (liquid fat) but may be extended to fish materials which also contain unsaturated fatty acids.
4 Sequence
Peroxide value determination involves first, the extraction of lipids (fats, oils) from the material, and then the determination of POV.
5 Lioid extraction
5.1 Materials
Purified and distilled chloroform
Wash chlcroform (CHCl3) 2–3 times with distilled water (DW) and add anhydrous calcium chloride (CaCl2), Stand overnight, then transfer to distillation flask, Distil and collect fraction which distills over at 60,50C. Add purified and distilled methyl alcohol (1% by volume) as stabiliser. Keep in the dark. Should be used within one month.
Purified and distilled methyl alcohol (methanol)
Add granular potassium hydroxide (KOH) to methyl alcohol (CH3OH) to remove acids, aldehydes and moisture. Distill and collect fraction which distills over at 64.5°C. Keep in the dark. Should be used within one month.
2:1 chloroform - methanol mixture
Mix reagents (a) and (b) in the proportion of 2:1.
1% BHA-BHT antioxidant solution
Dissolve 1g of butylated hydroxyanisole (BHA) and 1g of butylated hydroxytoluene (BHT) in 100-ml of (c).
5.2 Apparatus and equipment
Homogeniser
Buchner filtration apparatus
Separating funnel
Rotary evaporator, water bath and other accessories
Volumetric flasks, pipettes
Centrifuge
5.3 Procedure
Weigh the finely divided (chopped, if in solid mass) material, depending on the lipid content*1, into a homogeniser cup.
Add the chloroform-methenol mixture at a volume which is about 3.5 times the weight of the sample, and 2–3 drops of anti-oxidant solution.
Homogenise for 1 minute and filter with Whatman No. 1 filter paper, using a Buchner funnel.
Transfer the residue into the cup and repeat homogenisation twice (there is no further need to add anti-oxidant solution).
Transfer the combined filtrate into a separating funnel.
Pour distilled water (at about the same volume as the extract) into the separating funnel.
Shake very gently 2–3 times and stand overnight (if the mixture does not separate well, centrifuge at 2000 rpm for 10 min).
Drain off the chloroform phase through Whatman No. 1 filter paper, into an evaporating flask.
Wash the filter paper 2–3 times with the chloroform-methanol mxiture.
Concentrate the extract with a rotary evaporator, under reduced pressure at 40°C (water bath temperature).
Dissolve the concentrated extract with the chloroform-methanol mixture and transfer to a 25 ml or 50 ml volumetric flask, using a pipette.
Make up to the flask mark with the chloroform-methanol mixture.
Flush with nitrogen gas, and store at -20°C until required.
*1 Sample size of 20–50g for fish carcass
6 Peroxide value (POV) determination
6.1 Materials
N/100 sodium thiosulphate (Na2B2O3) solution
Dissolve 25g Na2B2O3.5H2O in freshly boiled distilled water and make up to 1000 ml, Stand for 2–3 days. Add 10 ml of isoamylalcohol as stabiliser. When required, dilute 10 times with freshly boiled distilled water. Keep in a dark brown bottle. Standardise the solution according to the method below:
Standardisation of Na2S2O3 solution
Take 20 ml of N/100 K2Cr2O7 solution in a 500 ml flask with stopper.
Add 10 ml of 10% KI solution and 5 ml of 25% H2SO4.
Immediately stopper the flask and stand for 5 minutes in the dark.
Add 100 ml of distilled water and shake.
Titrate with N/100 Na2S2O3 solution until the yellow colour disappears.
Add 1 ml of 1.5% starch solution as indicator, and continue the titration until the dark blue colour disappears.
2:3 chloroform-acetic acid mixture
Mix chloroform (CHCI3) and acetic acid (CH3COOH) 2:3 by volume.
Saturated potassium iodide solution
Dissolve 100g potassium iodide (KI) in 70 ml of freshly boiled distilled water. Keep solution with precipitated crystals in a dark brown bottle.
1.5% starch solution
Weigh 1.5g of soluble starch in a beaker. Add 100 ml of distilled water. Heat and boil for 30 seconds.
N/100 potassium dishromate standard solution
Weigh 4.9035g of potassium dichromate (K2Cr2O7) which has been dried at 100–110°C for 3–4 hours. Dissolve in distilled water and make up to 1000 ml. When required, dilute 10 times with distilled water.
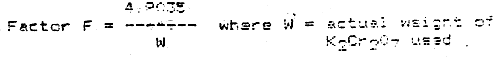
10% (weight by volume) potassium iodide solution
25% sulphuric acid solution
Mix 25g (13.5 ml) of concentrated sulphuric acid (H2SO4) and 75 ml of distilled water.
6.1 Apparatus and equipment
250 ml flask with stopper
Burette and beakers
Rotary evaporator and accessories
Water bath
6.3 Procedure
Take a volume (A ml) of the extract which contains about 0.3g of fat into a 250 ml flask with stopper.
Remove the solvent with the rotary evaporator under reduced pressure and at 40°C (of the water bath).
Add 10 ml of the chloroform-acetic acid mixture (1.1, b) and dissolve the fats by shaking.
Add 1 ml of saturated KI solution.
Immediately stopper and stand in the dark for 5 minutes.
Add 20 ml of distilled water and shake.
Titrate the liberated iodide with N/100 Na2S2O3 solution until the dark blue colour disappears. Use 1 ml of 1.5% starch solution as indicator.
Carry out a blank test in the same manner using a sample which is without fats.
7 Calculation for PV
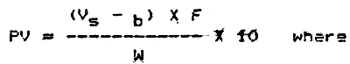
Vs = titration volume of sample (ml)
Vb = titration volume of blank (ml)
F = factor of n/100 Na2S2O3 solution
W = weight of fat in volume of extract used, (in g)
8 Interpretation of results
Oils with PV well below 10 meq/kg are considered fresh. A rancid taste begins to be noticeable when the POV is between 20–40 meq/kg.
D42
naca-7
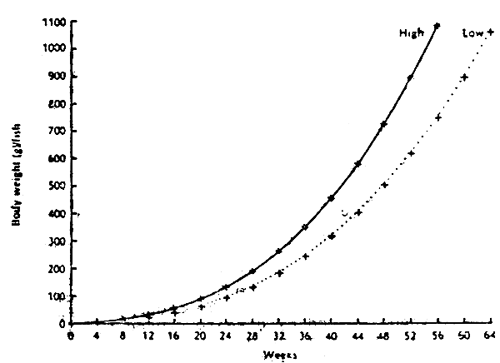
Figure VII/1
Standard growth curves of seabass
(Lates calcarifer)