1.1 General criteria for species selection
The fish species selected for netcage farming should meet with the 2 main prerequisites tabulated in Table VI/1:
Table VI/1
Summary of criteria for species selection for netcage farming
1 General
| Wild-caught natural | ||
| 1.1 | Seed (fry/fingerlings) are readily available | |
| hatchery-bred (artificial) | ||
| 1.2 | Conditions for culture of species selected are met. | |
2 For farming in floating netcages, the species selected
2.1 must have a good demand and high market value;
2.2 should be hardy, tolerant to crowded conditions and perform well under such conditions;
2.3 should be able to accept external source of food, especially the carnivorous species which have little or no opportunity to find their food supply under confined conditions. Food from external source may be:
natural eg. trash fish or
artificial eg dry formulation.
1.2 Seed availability
The need to ensure that seed stock is available is important because without a certain and ready supply of seed at stocking time, farming becomes unpredictable.
Seed, which is usually fry or fingerling, can be wild-caught or hatchery-bred. In the former, supply is usually seasonal and unpredictable but are however more robust and hardy as they would already have undergone pre-selection by nature. In the case of hatchery-bred seeds, supply is more predictable, and, depending on whether the parent stocks were wild-caught or farm raised, could be produced on schedule in batch-operation sequence.
Notwithstanding the problems associated with relying on wild-caught seeds for farming, many aquaculture industries have started on this basis and many still are continuing to do so. The large-scale shrimp farming industry in Taiwan for example initially relied on wild-caught shrimp fry for stocking the early ponds. Now the seeds are almost completely hatchery-bred.
In Ecuador, the shrimp farming industry is still heavily dependent on wild-caught seeds for its seed supply, although hatcheries are being established to reduce this dependence.
Similarly in the Philippines the ready availability of milkfish fry from coastal areas has resulted in the establishment of a large-scale milkfish farming industry in that country.
In Singapore, seeds for aquaculture are mainly imported from neighbouring countries. Species selection therefore is based largely on the availability of frys from those countries and the ease and cost of importation. Presently there are only 2 hatcheries, and they specialise in shrimp fry production, in the Republic. It is envisaged that seabass frys can be produced locally in commercial hatcheries that will be established in the near future.
1.3 Site suitability
The availability of suitable conditions for the culture of selected species site is critical as this determines the biological and economical feasibility of farm operation.
In the farming of marine species in netcages, such conditions would include water quality, tidal action and currents. It is therefore important that the species selected for such farming be tolerant of open conditions of coastal waters.
Fine-mesh netcages or netcages with a certain degree of resistance to biofouling could be used for less hardy species or the young frys/fingerlings. The culture of Banana shrimp (Penaeus merguiensis), for example, is possible using fine-mesh netcages of mesh size 2.8 mm initially, and later 8 mm mesh-sized netcages.
Biofouling is also sometimes allowed to accumulate in the netcage during the 3–4 month grow-out culture period for shrimp.
Seabass (Lates calcarifer) frys can also be raised to stocking size in fine plankton netcages of mesh size 250 microns (um) suspended in the sea.
1.4 High-valued species
It is preferable to culture species with high market value so as to off-set the relatively high cost of production of netcage farming.
As the fish can be easily harvested live, the farmer can sell the produce in prime, live condition. In doing this, he obtains a better price for the fish than would be possible if they were sold chilled or frozen as is the usual case in pond culture.
Other than Banana shrimp and seabass mentioned, other high valued species in Singapore include finfish like the groupers, viz the estuarine grouper (Epinephelus tauvina), red grouper (Plectopoma maculatus) and polka-dot grouper (Chromileptis altivelis), golden snapper (Lutjanus johni), mangrove crab (Scylla serrata) and spiny lobster (Panulirus polyphagus).
1.5 Hardy and tolerant species
Species selected should also be hardy and tolerant to confined, crowded conditions and to the rigours of handling during netcage changes.
Stocking in netcages is often more than 10 times that of pond culture eg., 5/m2 compared with 40/m2 for grouper culture in ponds and netcages respectively.
Flan in netcages are also subjected to greater physical contact and stress during feeding as there is often a rush for the food by the main bulk of the population in the netcage. Both estuerine grouper and seabess are found to thrive well under such crowded conditions and do not respond so well to feeding when they are small numbers.
1.6 Ability to accept external source of food
As there are usually no other significant sources of food within the netcage except for small fish which stray in and out, selected fish must be able to accept external source of food especially if species is carnivorous. The selected feed, usually chopped trashfish, would drop through the netcage if it is not eaten by the time it reaches the net bottom.
The loss of feed is greater when dry feed is used. Feeding trays can be suspended in the netcage to catch the pellets as they fall and this is used in netcage culture of shrimp. Netcages can be deepened to allow greater pelleted feed retention time within the netcage, or a slow-sinking dry pelleted feed would also maximise this retention time.
Some fish like the grouper, seabass and golden snapper respond to feed discharged from autofeeders. In fact, the fish will swim around the feeder in anticipation of the feed drop.
Spiny lobsters and Rabbit fish (Siganus canaliculatus) are able to graze on the algas growing on the sides of the netcage and derive part of their food from this source. They can also serve as biofculing controls in netcages. Rabbit fish will also respond to feed given to them.
The success of large-scale fish farming is largely dependent on the continuous and adequate supply of fish fry for stocking. Although fish fry may be collected from natural sources, its supply is usually seasonal and unreliable. A more reliable source is to breed them in hatcheries. This section describes the technique of seabass breeding, with particular emphasis on the operation of a floating hatchery system at a floating fish farm in Singapore.
2.1.1 The floating hatchery system
Natural spawning of seabass
Seabass is an euryhaline and catadromous species. The fry can be cultured to marketable size in marine, brackish or freshwater environments. The matured adults spawn in the mouths of river systems, in lagoons or estuaries during the flood tide. This allows the eggs and the hatchlings to drift upstream to brackishwater areas such as mangrove swamps, where they undergo larval development. Thereafter, they migrate further upstream and spend most of their growing period in the brackishwater or freshwater bodies. By the third to fifth years, the fish migrate downstream to the sea for gonadal development and subsequently to the mouths of rivers for spawning. It is believed that the spent spawners may migrate to the sea or return to fresh or brasckishwaters for further growth.
Characteristics of the floating hatchery system
The major difference of the seabass floating hatchery system described in the section from that of a land-based hatchery is that all breeding operations starting from the development of broodstock to the nursery rearing of fingerlings are done at the floating fish farm. An important consideration for the development of a floating hatchery is its floating nature in the sea, being maintained solely by the numerous bouyant drums attached to the farm structure. Unlike the land-based hatchery where tanks are extensively used, the use of tanks in the floating hatchery is kept to a minimum.
The extensive use of tanks on a floating farm would require large numbers of bouyant drums to support the load. This would not only make it economically unfeasible to operate but would also cause the hatchery to tilt to one side where the tanks are loaded. The structure then loses its stability under strong wave action. As in all breeding operations in the floating hatchery are, as far as possible, conducted in bags or netcages suspended from the floating farm in the sea.
Advantages of the floating hatchery system
The operation of a floating hatchery system has several advantages over that of a land-based hatchery. The pumping of seawater at the floating hatchery is independent of the tidal condition and may be performed as and when required. With a small water delivery pump of 1 to 1.5 HP, good quality water can be pumped directly at site at all times.
In contrast, a land-based hatchery requires a long suction hose and a high capacity pump for its seawater intake; the pumping of seawater normally cannot be performed at low tides because the water is either too turbid for hatchery operation or the tidal level is too low for water to be pumped into the hatchery.
To ensure a continuous supply of seawater, the land-based hatchery requires huge reservoir tanks for storing filtered seawater. Such storage tanks are, however not necessary in the floating hatchery system.
The floating hatchery's proximal location to the sea also means that it does not require a regular drainage system.
Site suitability
The success of a floating hatchery depends on the proper selection of a suitable site. It is important that the hatchery should be located in a calm and protected area with little navigational traffic. Strong wave action, especially those caused by passing boats and larger vessels would cause the seawater to splash into the culture bags. This causes contamination the water for the rearing of fish.
The water quality in the area should favour the growth and survival of all the development stages of seabass (i.e. larva, fry, fingerling and adult) and the maturation and spawning of brooders and be free from pollution.
In general, water temperature, salinity and dissolved oxygen should be in the range of 28–31°, 27–31‰ and 5 mg/1 respectively. For larval rearing it is important that the water with high content of heavy metals should be avoided as this will cause high larval mortalities.
2.2 Broodstock maintenance and development
2.2.1 Recruitment of broodstock
Broodstock are obtained by either initially raising young fish in netcages or recruiting adult fish caught from the wild.
In the latter case, gill-nets of 6-10 cm mesh are used. The nets are set in a direction perpendicular to the current. The set gill nets are checked for captured seabass regularly, say once every hour. This is to ensure that the captured fish are not left struggling for long periods, as it will result in excessive injury, especially to the eyes and gills.
After removal of the fish, the captured seabass are stocked immediately in a holding tank (1 m3 capacity or more) on board the fishing vessel. The tank is supplied either with aeration from an air blower or oxygen from a cylinder.
As all fish caught by this method usually suffer to some extent from body damage they are treated directly in the holding tank with antibiotics or an appropriate chemical bath, e.g. 5 ppm acriflavine for 2–3 hours.
The fish should be transferred to a netcage immediately after the fishing operation.
In general, at least 6 months are needed for the fish to recover form stress and injury and to condition to the confined environment in the netcage before they can be used for spawning.
2.2.2 Broodstock fish stocking
All brooders are held in floating netcages. The structure and construction of a floating farm is described in Chapter III.
Fish stocking density and the type of netcage used for broodstock development vary with the size of the fish (Table VI/2).
| Netcage specifications | |||||
| Size of fish (Total length in cm) | Dimensions | Material | Mesh size(mm) | No. per netcage | No./m2 |
| <10 | 2 × 2 × 2 | Nylon ‘hapa’ | 8 | 1000 | 250 |
| 10-15 | 2 × 2 × 2 | Polythylene | 10 | 500 | 125 |
| 15-20 | 5 × 5 × 3 | Polythylene | 12.5 | 1500 | 60 |
| >25 | 5 × 5 × 3 | Polythylene | 25 | - | * <10 kg/m2 |
To ensure water exchange, the holding netcage is replaced monthly with a clean net.
2.2.3 Feeds and feeding
The fish are presently maintained on trash fish. Feeding is done once or twice daily. The size of the food depends on the size of fish.
Fingerlings smaller than 10 cm TL(total length) are fed minced trash fish at the rate of 20% body weight daily while those of 10–15 cm TL are fed on chopped pieces of around 1 cm length at 15% body weight daily. Fish larger than 15 cm TL are fed on larger chopped pieces of around 2.5 cm at 10% body weight daily.
At the size of 1 kg, the fish are ready to accept small trash fish at 5% daily feeding rate.
Mature fish of 3 years or more are fed at 2–3% body weight. This is further reduced to 1–2% during the spawning season.
2.2.4 Gonadal maturation
The success of fish breeding depends primarily on the availability of mature brooders of good condition and numbers for ise in spawning. It is therefore imperative to maintain the broodstock in an environment that is conducive to the well-being of the fish and preferably to gonadal maturation.
Gonadal maturation of fish is basically controlled externally by environmental conditions and internally by the endocrine system. In Singapore, seabass that are stocked in netcages in the East johore Straits have no problems maturing. No special technique is required to induce the fish to maturation.
Under the floating netcage conditions, male and female fish are stocked together in the same netcage of 5m × 5m × 3m (depth) at 20–30 pairs per netcage.
2.3 Spawning
2.3.1 Sex determination and maturity
Sexing of seabass can only be done accurately in mature fish, even though the fish has some dimorphic characters to enable sex determination.
In a group of seabass of the same age, males are generally smaller and with a more slender body and narrower body depth than the females. During the spawning season, the abdomen of the females bulges and the scales around the genital opening are not as thick as those of the males.
As in the case of many other fish, eg golden snapper, seabass male mature at an earlier age than the females, attaining sexual maturity at 2.5 yrs while fish is 3–4 yrs for females.
2.3.2 Determination of the spawning season
When spawning is conducted in a new area where there is no information on the spawning season, it is important to check the gonadal condition of the fish regularly to monitor their level of maturation and to detect the onset of the spawning season. This can be conducted once a month during net cage changing operation.
For seabass held in netcages in Singapore waters, seabass eggs at tertiary yolk stage can be found almost throughout the year. The frequency of occurrence is however higher during the months of May to October. This period may be considered as the peak of the local spawning season for seabass.
2.3.3 Determination of fish maturity
The assessment of maturity of the female fish is made from eggs sampled by catheterization from the mid-portion of the ovary, using a polyethylene cannula (2.5 mm in diameter).
During the sampling process, about 5–7 cm of the cannula is gently inserted into the gonad of the fish through the genital opening, and eggs sucked orally into the cannula as the tube is slowly withdrawn. The eggs are then placed in a test tube containing 1% formalin in 0.6% saline (sodium chloride) solution and stored there for subsequent checking of egg size and appearance. Egg diameters are measured on a glass slide using an ocular micrometer under a microscope. The diameter of elongated egg, if any, is measured by averaging the long and short diameters.
For each sample, about 30 eggs are measured and the mean diameter and percentage standard deviation calculated. This data is used as indicators of mean egg size and egg size variation. Females bearing eggs at the tertiary volk globule stage are considered mature.
The assessment of maturity of males is based on the milting condition. Mature males yield milt when the abdomen is stripped gently in the direction towards the genital opening. Depending on the condition of the testis, the milt is either white and creamy or watery and curdled.
2.3.4 Selection of spawners
Mature of females of 3–7 years [(body weight) 3–12 kg] and males of 3–5 years (B.W. 2.0–7.5 kg) are selected for spawning.
In general, only males and females of the same age are selected. When males and females of different ages are used, the two groups of fish have to be kept together in a same net cage for conditioning well in advance to spawning.
The selection of females is based on the eggs sampled by catheterization. Females bearing uniform (with percentage standard deviation less than 10%), spherical and non-adhesive eggs with a mean diameter 0.45 mm or more are considered ready for spawning.
Males are selected when white and creamy milt oozes out from the genital opening upon gentle stripping of the belly. Males yielding watery and curdled milt are not suitable for use in spawning.
2.3.5 Natural spawning
The selected spawners are transferred into a spawning netcage of plankton netting material (0.50mm mesh size) one or two days before the spawning period.
The natural spawning of seabass is closely related to the lunar cycle. In Singapore waters, seabass spawn at 3–7 days after full moon or new moon during each month.
About 10–20 pairs of brooder are stocked in each netcage. The small mesh of the netcage prevents the spawned eggs from being washed away by the tidal current. The brooders are not fed so long as they are in the spawning netcage. This is to prevent deterioration of water quality.
Seabass usually spawn at night at both the new moon and full moon phases. However, the fish will yield more eggs and which are a better quality at full moon than at new moon phase.
The fertilized eggs are collected by seine net the morning after spawning and placed in several 15-1 plastic buckets for separation of good fertilized eggs (ie buoyant eggs) and dead eggs. The dead eggs that sink to the bottom are siphoned away. The good buoyant eggs are washed thoroughly and their numbers estimated before transferring into hatching nets for incubation.
Overall fecundity ranges from 0.8–3.1 million eggs per female. Overall fertilization rate ranges 73–95%, while overall percentage of buoyant eggs 27– 95%.
The spawners are returned to their polyethylene holding netcages after this natural spawning operation.
2.3.6 Incubation and hatching
The hatching nets used for egg incubation are cylindrical (0.7 m dia. X 0.7 m depth, 0.5 m water depth) and of plankton netting materials, (0.50 mm mesh size).
They are suspended in the sea from the floating farm.
All the nets are provided with moderate aeration.
The stocking density is about 0.2 million eggs per net of 0.2m3 capacity.
Fertilized eggs of seabass are transparent, spherical and non-adhesive and appear pale yellowish while in groups. Most of the fertilized eggs are buoyant, and each egg has a single oil globule. The egg diameter ranges from 0.80–0.85 mm with a mean 0.82 mm. the diameter of oil globule ranges from 0.25 to 0.27 mm. The hatching time for seabass is dependent on the incubation temperature. The eggs hatch in about 16–17 hours at 27–28°C.
2.4 Production of fry in the floating hatchery
2.4.1 Seawater and air supply
Filtered seawater and air are required for larval rearing and larval food production. In addition, aeration is also needed for the incubation of fertilized eggs.
Unlike the land-based hatchery which requires high capacity pumps and long intake line, the seawater intake system for floating hatchery is relatively simple. Being in close proximity to the sea, the floating hatchery requires only a small centrifugal water pump or submersible pump of 1 – 1.5 HP for its water supply system. Unfilitered seawater is pumped directly from 3m below the farm to a 500-litre filter tank. To minimize the load of the of heavy sand layers is used to remove plankton, detritus and other undesirable particles from the seawater.
As most of the culture containers are suspended from the raft platform into the sea, the filter tank is elevated to a height of only 30 cm (as compared to more than 1.5 m in land-based hatchery) from the platform for water delivery by gravity to the various culture bags. The filter tank has an outlet of 5cm diameter PVC (polyvinylchloride) which distribute filtered seawater to all the culture bags. A 1-HP air blower supplies aeration to all the brine shrimp (live food, Artemia salina) culture bags for larval rearing and larval food production. Air is distributed by 5 cm diameter, PVC pipes and 6 mm diameter aeration tubes.
As there is no city power supply on the farm, a generator is pumped at the farm.
2.4.2 Larval food production
Larval food production is one of the most important hatchery operation in fish breeding as it is crucial that suitable food organisms are available in sufficient quantities for feeding to the different stages of fish larvae.
In seabass larval rearing, rotifers are cultured in the hatchery for feeding to the early larvae.
Unicellular algae are also cultured and used as food for the production of these rotifers. Algae also conditions the water in larval bags.
Brine shrimp nauplii are hatched from cysts and used for feeding to larger larvae.
2.4.3 Larval rearing
The principle of seabass larval rearing is to ensure an optimal environment and adequate supply of suitable food at different development stages of the fish.
Larvae are raised in indoor larval rearing bags sheltered under a shed until Day 14. (ie 14 days after hatching).
The larval rearing bags are made of canvas material and 1.8 m × 1.8m × 1m (depth). Water depth is about 0.6 m and each bag has a volume of about 2m3. The survival larvae from hatchlings to Day 14 is about 40%.
Buoyant eggs are transferred from the hatching at 20,000 per bag or 60,000 per m3 of water.
Seabass larvae are mainly fed with rotifer and brine shrimp nauplii during the larval stage. The unicellular algae, either Chlorella or Tetraselmis, are added to the larval bag throughout the rearing period. They serve as water conditioner and food for the rotifers. Algal density in the larval bag is maintained at 2.5–5 × 103 for Tetraselmis.
Newly hatched larvae (Day 0) have a uniform size of 1.60 mm TL. Each larva possesses a large yolk sac and an oil globule sited at the anterior-ventral part of the yolk sac. The yolk sac is partially absorbed on Day 1. It is almost completely absorbed on Day 2. By Day 3 only a small oil globule is visible. There is no feeding on Day 0 and 1. The mouth parts of the larvae become apparent in the morning or Day 2, and by the afternoon, the mouth is open and the larva is ready to commence feeding. At this stage, the larva measures 2.5 mm TL and is able to feed on rotifers directly.
The first feeding of rotifers to the larvae is usually on the afternoon or Day 2. Rotifers are fed to the larvae on Day 2 at an minimal low density of 2– 3/ml on Day 2. The density is then maintained at 3–5/ml from Days 3–10, and 5–10/ml from Days 11–14. By Day 11, the larvae have are about 4.5 mm Tl, and are ready to accept brine shrimp nauplii.
Small amounts of the nauplii (less than 0.2 ml) are added to the larval tank from Days 11–12, the density kept at 0.5–1.0/ml from Day 13–14.
2.4.4 Nursery rearing
After the 14-day larval rearing period in larval bags, fish larvae are much stronger and are more adaptable to outdoor conditions.
They are transfered to outdoor netcages for nursery rearing until they reach 2.5–3.5mm TL.
Nursery rearing is conducted in three stages, viz. the primary nursery stage from Day 14 to Day 20, the secondary nursery rearing from Day 20 to Day 35, both being in plankton netcages of 0.5mm and 0.7mm mesh sizes respectively; and the tertiary nursery rearing in polyethylene netcages of 1mm mesh size. All the netcages measure 1.8m × 0.9m × 0.9m (depth) with the upper edge of the net at 0.3m above the water surface. The net cage is set in a square wooden frame with its upper 4 corners tied to the frame, sheltered from strong current movement. The latter is achieved by setting the netcages in an area surrounded by other netcages.
Fry stocking density is 16,000/netcage (10,000/m2) during secondary rearing and 5,000/netcage (3.00/m2) during tertiary rearing.
Unlike the land-based hatchery, the rearing of seabass fry in nursery netcages does not require aeration and water change. To ensure effective water exchange in the netcage, a hand brush with a long handle is used to remove the algae and detritus attached on the sides of the cage once a week. The netcage is changed and washed once every week.
Fry feeding during the nursery stage varies with the size of the seabass:
Fry smaller than 1 cm TL are fed with brine shrimp nauplii.
Fry of 1 cm TL or more can be weaned to minced trash fish flesh and euphausid shrimp (Acetes sp.)
At 1–1.5 cm TL, the fry are fed with minced trash fish flesh during the day and brine shrimp nauplii in the evening.
When the fry attain 1.5 cm TL, they are trained to accept minced trash fish flesh alone. At this stage, the fish are fed to satiation three times a day, i.e. in the morning, late morning and late afternoon. Minced fish flesh may be fed to the fry manually or by using a feeding cylinder. The feeding cylinder is made of Nylex material covered with 1/8" (3 mm) mesh nylon knotless netting.
The size variation among larvae becomes greater towards the later stage of larval development. Owing to the cannibalistic behaviour of the fish, the bigger and more voracious fry will prey on the smaller ones if they are reared together. The problem becomes more acute after Day 20. At this stage, the fry are stronger and can withstand handling.
To minimise cannibalism, fry are graded into different sizes by graders. Graders are perforated plastic basins of round holes with specific holesize. The specific diameter varies from 2.5–10 mm. Grading is done once a week until the fry turn into juveniles at Days 50–60.
When grading, the fry are netted from nursery nets and transferred into a grader of appropriate holesize. The basket is partially immersed in a 0.5m3 tank. The holes allow only fry with body depths smaller than the specific holesize to pass through. This leaves only the bigger ones in the basket. The bigger fry retained in the grader are then transferred into another grader with larger holes and the exercise repeated. In this way fry can be graded into several size groups.
Fry of different size groups are then raised in separate nets.
2.5 A glossary of terms for Section (2) is found in Table VI/9.
* Commercial name. Use of this name does not imply endorsement.
2.6 Floating netcage systems
2.6.1 Fish farming in floating netcages is an intensive form of aquaculture. The fish under culture are confined in a netcage which is in turn, held within a large body of water e.g. reservoir, lake, river or the sea. The number of fish in a netcage is usually high, and their culture therefore intensive.
2.6.2 The intensive husbandry of fish and management of a floating fish farm include species selection, stocking, feeding, netcage maintenance and occasional treatment of the cultured fish for disease prevention. It is therefore less complicated than intensive fish culture in ponds where considerable effort has to be expended on husbandry and farm management.
2.7 Stocking
2.7.1 Stocking/ restocking of netcages with fish is carried out at various stages of the culture cycle viz in a small-meshed hapa netcage at early nursery stage and polyethylene netcage of larger mesh at late nursery and grow-out stages. These growth stages are arbitrarily defined as those fish sizes that are suitable for the hapa netcage (mesh size 8 mm) polyethelene nursery netcages (mesh size 9.5 – 25 mm) and in polyethylene production netcages (mesh size 25.4 – 50.8 mm). These sizes roughly correspond to the early nursery, late nursery and grow-out life stages of the fish. Thinning carried out at each stage to maximise the use of netcage space, prevent cannibalism and over-crowding.
2.7.2 An example of grouper and seabass stocking management for the floating netcages system is as follows:
Starting out with fingerlings of 7.5 – 10 cm total length (measured from tip of snout to tail), initial stocking in hapa netcages is around 150/m2 (100/m3).
The density is adjusted to 40/m2 (27/m3) when they reach 12.5 – 15.0 cm.
At harvest time, which is the end of the grow-out stage, the fish should have thinned themselves out naturally from 40/m2 to 35–38 /m2 through natural natural mortality (88–95% survival). Fish biomass increases by about 80-fold or more during the culture period because of weight gain. Details are tabulated in Table VI/3 below. Survival improves as the fish grow larger as cannibalism becomes less rampant.
| Stage | Net Dimension ($) | Culture | |||||||||||||
| Net type for | Size of fish | Density | Period | Survival (%) | |||||||||||
| Seabass | Grouper | Seabass | Grouper | Seabass | Grouper | Seabass | Grouper | Seabass | Grouper | ||||||
| (mesh size in nm) | Wt(g) | TL(ca) | Wt(g) | TL(ca) | fish/a2 | fish/a3 | fish/a2 | fish/a2 | days | ||||||
| Nursery | |||||||||||||||
| a hapa | 2×2×2 | 8 | 8 | 2–5 | 5 | 2–5 | 5–6 | 150 | 100 | 150 | 100 | 30 | 30 | 50 | 60 |
| (1 mth) | |||||||||||||||
| b early | 2×2×2 | 8–9.5 | 8–9.5 | 20 | 11–12 | 20 | 10–11 | 70 | 40–50 | 55 | 30–40 | 45 | 60 | 70 | 80 |
| nursery | (1.5 mth) (2 mth) | ||||||||||||||
| c late | 2×2×2 | 9.5 | 25.4 | 100 | 20 | 100 | 20 | 50 | 33 | 45 | 30 | 60 | 90 | 85 | 90 |
| nursery | (2 mth) (3 mth) | ||||||||||||||
| Production | |||||||||||||||
| a grow-out | 2×2×2 | 25.4 | 25.4- | 300 | 30 | 300 | 28 | 40 | 27 | 40 | 27 | 105 | 120 | 85 | 90 |
| 50.8 | (3.5 mth) (4 mth) | ||||||||||||||
| 3×3×3 | 25.4 | 25.4- | 300 | 30 | 300 | 28 | 40 | 16 | 40 | 16 | 105 | 120 | 85 | 90 | |
| 50.8 | (3.5 mth) (4 mth) | ||||||||||||||
| b at | 2×2×2 | - | - | 700 | 38–40 | 700 | 35 | 35 | 23 | 35 | 33 | - | - | - | - |
| harvest | (600–800) | ||||||||||||||
| 5×5×3 | - | - | 700 | 38–40 | 700 | 35 | 35 | 14 | 35 | 14 | - | - | - | - | |
| (600–800) | |||||||||||||||
| Culture period | 240 | 300 | 25 | 38 | |||||||||||
| (8 mth) | (10 mth) | ||||||||||||||
2.8 Feeding (see also Chapter III)
2.8.1 Trash fish, comprising small fishes like goatfish and jewfish, which are low in economic value, is usually fed to cultured fish, which are mainly carnivorous. The size of the feed particle depends on the size of fish in the netcage. For fingerlings, trash fish should be minced or finely chopped; fish in nursery netcages can accept larger pieces of around 1 cm (0.4"); while those in production netcages can accept larger pieces of around 2.5 cm (1").
2.8.2 Feeding is done once or twice daily, usually in the morning and towards evening, at slack tide so that feed is not swept away by the tidal current. The fish usually come up for their food and small quantities of food should be given each time minimise wastage. Trash fish, which is cold after being stored in the freezer, and not sufficiently thawed, may be rejected by the fish, while that which has been constantly taken in and out of the freezer, will be of poor quality because of should be discarded.
2.8.3 Fish fingerlings can be given up to 10% of body weight daily e. g., a 50 g fingerling should receive 5 g of trash fish daily. Larger fish, such as those in nursery netcages can be given 8% each of their body weight daily e.g., a 100 g fish should receive 8 g of trash fish daily, while fish in production nets can be given 3–5% each of its body weight daily e.g., a 300 g fish should receive 15 g of trash fish daily at 5% of its body weight, and a 500 g fish 15 g of trash fish daily at 3%. It is estimated that the Feed Conversion Ratio (FCR) for the grow-out period is 4.5:1, that is, every unit gain in weight of the fish requires 4.5 times this weight in trash fish.
2.9 Growth
2.9.1 The 3 types of fish mentioned, namely grouper, seabass and snapper, are fast growers gaining 80–100 g/fish/month and can attain market size of 600–800 g in about 6–8 months from 80–100 g initial mean weight (12.5–15 cm Total Length) when fed on trash fish. Seabass grow better in less saline conditions, of 10–25 parts per thousand.
2.9.2 A farmer can estimate the mean weight of his fish by bulk weighing say 20–30 fish and dividing the total weight by the actual numbers. However, to obtain an accurate assessment of growth in fish, it is necessary to first determine the minimum sample number required for measurement from a pilot sample. The tolerance limit is calculated as shown in Table VI/4.
Table VI/4
Calculation of tolerance limit in pilot sample
| Mean TL (Total length) | = | a cm |
| Standard deviation (s) | = | ± b cm |
| Number of fish (n) | = | c (usually 25) |
 | ||
If the tolerance limit is unacceptable and a lower limit is required, say ± 0.1 cm, ± 0.25 cm or ± 0.5 cm, the minimum sample number at each tolerance limit can be calculated as shown in Table VI/5.
Table VI/5
Calculation of minimum sample number at specific tolerance limits
Substitute the tolerance limits of 0.1, 0.25, 0.5 cm into the formula below and calculate n
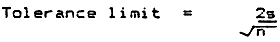 | ||
| Where Tolerance limit | = | 0.1, 0.25 or 0.5 cm |
| s | = | Standard deviation of the TL of the pilot sample |
| n | = | minimum sample of fish to be measured (to be calculated) |
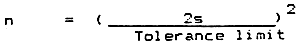
Example
| Tolerance limit | = | 0.5 cm |
| s(pilot sample of 25 fish) | = | ± 3 cm |
| Minimum number of fish to be measured for ± 0.5 cm TL accuracy in mean value | = | 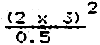 |
| = | 72 |
2.9.3 The minimum sample number, once derived when the tolerance limit is acceptable can be drawn out at random from the netcage.
2.9.4 The use of quinaldine is found to be effective in the anaesthesia of fish. The stock solution is prepared by dissolving 1 part of quinaldine to 9 parts of acetone by weight. From the stock solution, 20 ml is mixed with 50 litres of seawater, stirred well and aerated. 2–3 fish may be anaesthesized at one time. Fish condition must be observed during anaesthesia and fish condition must be observed during anaesthesia and fish removed immediately when immobilised. After measurement, the fish should be treated in a 10ppm acriflavine bath for 1 hour before transferring back to the netcage.
2.9.5 From the total and standard length and weight measurements, the arithmetic mean (x) and standard deviation (s) of each set of data can be computed as shown in Table VI/6.
Table VI/6
Calculation of arithmetic mean (x) and standard deviation (s)
1. Arithmetic mean
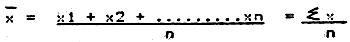
where 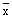 | = | arithmetic mean |
| x | = | individual sample reading |
| n | = | minimum sample size (number of fish) |
2. Standard deviation*
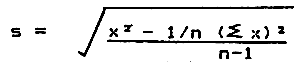
| where √ | = | square root |
| x | = | individual sample reading |
| n | = | size of sample |
| Σ | = | summation |
2.9.6 Determination of the growth curve with a series of measurements in fish is carried out by plotting weight (vertical axis) against days (horizontal axis) on graph paper. The correlation coefficient (r) of 4 possible curves can be determined using a microcomputer programme. The value of the coefficients nearest to 1.0 represents the best fit to one of the 4 curves. The formulae for the 4 types of curves are listed in Table VI/7.
Table VI/7
Types of growth curves
| 1. Linear (straight line) | Y = A + Bx |
| 2. Exponential curve | Y = AeBx |
| 3. Logarithmic curve | Y = A + Blnx |
| 4. Power curve | Y = AxB |
| where A, B are constants provided by the microcomputer and | |
| Y = days | |
| x = weight in g | |
The best fit curve is plotted by substituting four Y (weight) values of between 50–700 g to calculate x (days) for each Y. The curve would give an indication of the growth performance of fish when subsequent measurements are made.
2.10 Disease control (see Chapter VII)
2.10.1 Regular netcage-changing would ensure that there is good water exchange in the cage for maintaining optimal environmental conditions to the fish cultured. Proper farm management e.g. feeding sufficient quantities of food, ensuring that the food is adequately frozen and not kept for long periods, stocking the netcages at the recommended levels, would also help to minimise disease outbreak. Handling stress e.g. during netcage changing, should likewise be kept to a minimum.
2.10.2 Major losses are most often suffered in the first few weeks following stocking. In Singapore most of the fry and fingerlings are imported from abroad. The stress during transport and adjustment to a new environment can be severe. Improved survival of imported fry and fingerling can be obtained through sanitization.
2.10.3 Protozoan parasites appear to account for the majority of local marine fish diseases. The most commonly encountered protozoan parasite is Cryptocaryon irritans. The affected fish show loss of scales and skin particularly from the head region. These clinical signs are also produced by other protozoan parasites. Identification of the parasites is made by microscopic examination of skin smears and gill lamellae. Standard treatment for all such ectoparasites is a formalin bath of 200 ppm for half to one hour depending on the endurance (condition) of the fish.
2.10.4 Vibriosis is another common disease occurring mainly as a sequel to protozoan infestation or mechanical damage sustained after importation or handling. This bacterial disease, caused by Vibrio parahaemolvticus and V. alginolyticus is usually characterized by skin inflammation and haemorrhage and ulceration of the musculature. The treatment of vibriosis in the early stages of infection is relatively straightforward. Antibiotics can be mixed with chopped trashfish and fed to sick fish. A variety of antibiotic treatments has been found effective:
Oxytetracycline 0.5 g per kg feed for 7 days.
Sulphonamides or potentiated sulphonamides, 0.5 g active ingredients per kg feed, for 7 days.
Chloramphenicol 0.2 g per kg feed for 4 days.
Bath treatment can also be attempted if fish are off-feed:
Nitrofurazone, 15 ppm for at least 4 hours.
Sulphonamides, 50 ppm active ingredients. for at least 4 hours.
2.11 Plankton blooms
Periodic plankton blooms may occur in the farm water. The bloom is a result of a sudden rapid increase in the population of certain plankton species due to favourable conditions in the water. Such conditions may arise due to changes in water conditions caused by land run-offs and river discharge. The bloom is usually restricted to the first 1–1.5 m of the water layer.
2.12 Biofouling (See also Chapter IV)
Fouling organisms of the netcage can be controlled biologically to some extent by using grazer fish species within the culture fish. Alternatively, copper-nickel alloy (which does not attract fouling organisms) could be used but these tend to make the netcages rigid. Frequency of rotation of the netcages to allow regular cleaning may be reduced by application of anti-fouling paint. The use of such paint has been tested under local conditions and found to be effective for up to 3 months.
12.13 Yield
Market-size groupers are around 600–800 g (1 kati), while those of seabass and snappers usually range from 600–1000 g. Based on 600 g per fish at harvest, a 5m × 5m × 3m(depth) netcage would yield 600 kg of fish after 6–7 months culture. A raft unit comprising 32 interlocking units each of 5m × 5m × 3m(depth) would yield 19.2 tonnes per harvest or 38.4 tonnes per annum with 2 harvests per year. Since such a structure is sited within 5 000 m2 water space, actual yield is 76.8 tonnes/ha/annum. The high yield compares favourably with polyculture of fresh water carps in ponds which yields only 2 tonnes per hectare per annum.
1. Objectives
1.1 To learn important criteria used in the selection of male and female spawners.
1.2 To familarize with techniques used for checking the gonadal maturity of male and female spawners.
1.3 To observe the setting of spawning net.
2. Procedure
2.1 Selection of spawners
Select healthy male and female fish with no physical deformities injuries or signs of disease infection for examination of gonadal maturity.
2.1.1 Selection of female fish
Select female fish preferably with bulging abdomen, protruding or pinkish genital papilla.
Collect egg samples by catheterization:-
Gently insert 5–7 cm of the cannula through the genital pore.
Obtain egg sample by gently sucking the cannula as it is being withdrawn slowly.
Place eggs in a test tube and add formalin mixture (1% formalin in 0.6% saline).
Examine egg visually and under microscope.
Select fish with uniform eggs of >0.45 mm in dia.
Measure weight of the selected female using a spring balance.
2.1.2 Selection of male
Check milting condition of male fish by applying pressure along the abdomen towards the genital opening.
Observe the condition of the milt.
Select males yielding white and creamy milt.
Measure weight of selected males using a spring balance.
2.3 Preparation
Untie two corners of the polyethylene holding net (Polyethylene net) that hold the selected brooders for spawning. Drive the brooders to one side of the cage by lifting up the net from the opposite side in stages.
Set a clean Polyethylene (PE) net in the same cage.
Set a spawning net inside the clean PE net.
Drive the brooders from the old PE net into the spawning net.
3. Exercise
List the dimorphic characters of male and female fish.
Do you use the same catheterization technique in your country? If you use a different technique, compare the present technique with that used in your country.
| 1 | Artemia nauplii | : | Artemia larvae of the earliest development stage. |
| 2 | Cannibalism | : | Practice of eating one another of the same kind. |
| 3 | Catadromous species | : | Fish species which migrate from freshwater to seawater, usually for the purposes of gonadal maturation and spawning. |
| 4 | Catheterization | : | The process of sampling of eggs from the ovary of a live female fish. |
| 5 | Cysts | : | Resting eggs of the Artemia. Under suitable conditions, the resting eggs can be hatched into nauplii. |
| 6 | Dimorphic characters | : | Two different forms of character which are peculiar to males and females respectively. |
| 7 | Endocrine system | : | The system of endocrine glands and their secretions of hormonal substances. Endocrine glands are organs of internal secretion. |
| 8 | Enrichment | : | The addition of nutrients to increase the fertility of water with the aim of inducing bloom of phytoplankton. |
| 9 | Euryhaline species | : | Species which is adaptable and resistant to a wide range of salinity. |
| 10 | Fecundity | : | The ability to reproduce. In fish, it refers to the number of eggs in ovary or that is produced during a spawning. In the text, it refers to the number of eggs produced by a female during a spawning. |
| 12 | Inocula | : | Cells that are introduced into a medium for starting a new culture. |
| 13 | Inoculation | : | The process of adding inocula into a medium for starting a new culture. |
| 14 | Tertiary yolk globule stage | : | A final phase of the yolk globule stage in oogenesis (egg formation). At this phase, the yolk globules accumulate throughout the ooplasm. Fusion of yolk globules and oil globules is apparent. |
3.1 The farm constructed by the Department comprises 32 interlocking units and a hut. The cost of material for such a structure is approximately $60,000.
3.2 With proper maintenance the farm would have a minimum 5-year life. (See Figures VI/1 and VI/2). In addition to the farm, the farmer would also require a workboat for transportation, water pumps for washing the nets and a freezer to keep the trashfish. Some fibreglass tanks would also be needed to quarantine or treat the fish. The following series of tables (Table VI/8) are important economic projections for the operation of each farm.
| Years of operation | ||||||
| Notes | ||||||
| 1 | 2 | 3 | 4 | 5 | ||
| 1. Sales | ||||||
| 1.1 a.tons/no. of harvests in parenthesis | A | 4.9(4) | 28.18 (23) | 28.18 (23) | 28.18 (23) | 28.18 (23) |
| 1.2 market price @ $13.50/kg | 66.2 | 360.4 | 380.4 | 380.4 | 380.4 | |
| 2. Cost of sales | ||||||
| @ $5.70/kg | B | 27.9 | 160.6 | 160.6 | 160.6 | 160.6 |
| 3. Gross cargin | ||||||
| [(1) – (2)] | 38.3 | 219.8 | 219.8 | 219.8 | 219.8 | |
| 4. Operating expenses | C | |||||
| eg., manpower, fuel, maintenance, misc., depreciation | 86.4 | 86.4 | 86.4 | 86.4 | 86.4 | |
| 5. Interest on loan | D | |||||
| @ 8% per annum | 12.0 | 12.0 | 4.0 | - | - | |
| 6. Profit/(Loss) | (60.1) | 121.4 | 129.4 | 133.4 | 133.4 | |
| 7. Less Tax @ 33% | E | - | 20.4 | 42.7 | 44.0 | 44.0 |
| 8. Profit after tax | (60.1) | 101.0 | 86.7 | 89.4 | 89.4 | |
| 9. Retained Profit (Loss) | c/f | (60.1) | 40.9 | 127.6 | 217.0 | 306.4 |
B. Cash Flow Projection for a 32 - 5a×5a raft farming seabass (S $'000)
| Years of Operation | ||||||
| Notes | ||||||
| 1 | 2 | 3 | 4 | 5 | ||
| 1. Cash Inflow | ||||||
| Capital ) | F | 150.0 | ||||
| Loan ) | 150.0 | |||||
| Sales | Sea P/L | 66.2 | 380.4 | 380.4 | 380.4 | 380.4 |
| Projection | ||||||
| Total Cash Inflow | 366.2 | 380.4 | 380.4 | 380.4 | 380.4 | |
| 2. Cash Outflow | ||||||
| Fixed assets | F(1) | 151.0 | - | - | 11.7 | - |
| Expenses | ||||||
| Direct cost of sales | F(2)/ (8) | 136.0 | 158.0 | 153.8 | 158.0 | 159.4 |
| Operating cost | F(3) & (C) | 62.0 | 62.0 | 62.0 | 62.0 | 62.0 |
| Interest | ||||||
| @ 8% per annua | D | 12.0 | 12.0 | 4.0 | - | - |
| Tax @ 33% | E | - | 20.4 | 42.7 | 44.0 | 44.0 |
| Total Cash Outflow | 361.0 | 252.4 | 262.5 | 275.7 | 265.4 | |
| 3. Cash surplus/(Deficit) | 5.2 | 128.0 | 117.9 | 104.7 | 115.0 | |
| 4. Loan repayment | D | - | 100.0 | 50.0 | - | - |
| 5. Net cash flow | 5.2 | 28.0 | 67.9 | 104.7 | 115.0 | |
| 6. Accumulated cash flow | 5.2 | 33.2 | 101.1 | 205.8 | 320.8 | |
Notes for Profit and Loss Projection
A. 1. Yield per harvest
| Year | No. of harvests | Qty (st) | Remarks | |
| 1 | 4 | 4.9 | • • • • | 1st 2 1/2 months for raft construction; 1st cycle commences 1 1/2 months after raft construction commences. 1st cycle completes in 8 months, with 1 more month for completion of harvest, net maintenance, etc., before same nets are re-used for next stocking. Completion of harvesting of 2 nets at end of every cycle every month, commencing from the 10 1/2 month of operation (inclusive of the raft construction period) [see diagram below]. |
| 2 | 23 | 28.18 | ||
| 3 | 23 | 28.18 | ||
| 4 | 23 | 28.18 | ||
| 5 | 23 | 28.18 | ||
| Year 1 | |||||||||||||||||||||||||
| Months | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
Constr. -2 1/2 oths-- Raft construction
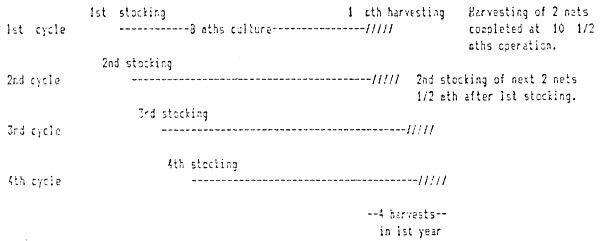
Figure VI/I
Diagramatic representation of 1st 4 cycles at start-up of a 32-5a×5a seabass netcage farm
B. Cost of sales
| 1. | Cost of fingerling | : | 53.43 | |
| Basis of derivation | : | • | 1 kg marketsize fish has 1.4* fish @ 700g each. | |
| • | if survival from stocking (2g ea) to marketsize (700g) is 25%, no. of fingerlings needed to produce 1 kg marketsize fish | |||
| = 1.4 × 4 = 5.6 | ||||
| • | @ 50.60/fingerling, 5.6* fingerlings cost $3.43. | |||
| *(theoretical calculations) | ||||
| 2. | Cost of feed | : | $2.25 | |
| Basis of derivation | : | • | Initial weight of fingerlings @ 2g ea = 2 × 5.6 | |
| = 11.2 g | ||||
| • | Weight gain per kg fish harvested = 1 kg | |||
| (since initial weight is negligible) | ||||
| • | If Feed Conversion Ratio (FCR) on trashfish = 4.5 : 1, and cost of trashfish @ 50.50/kg, cost of trashfish for entire culture/kg cultured fish harvested = 4.5 × 0.5 | |||
| = $2.25 | ||||
| 3. | Total cost of sales | : | $3.43 + 2.25 | |
| = $5.68 | ||||
| Say $5.70/kg soid | ||||
| 4. | Cost of sales/year | : | Year 1 = $5.70/kg × 4,900 kg = $ 27,930 | |
| Years 2–5 = $5.70/kg × 28,180 kg = $160,626 | ||||
C. Operating expenses
| Sub-total | Total | ||||
| ($'000) | (S'000) | ||||
| 1. Manpower cost | |||||
| 1.1 | Supervisor | 10.4 | |||
| @ $800/mth × 13 mths | |||||
| (incld 1 mth bonus) | |||||
| 1.2 | Workers (3) | ||||
| @ $600/mth × 13 mths | 23.4 | 33.4 | |||
| 2. Fuel | |||||
| 2.1 | for boats | ||||
| @ $1000/mth × 12 mths | 12.0 | ||||
| 2.2 | for generators & misc. | ||||
| @ $200/mth × 12 mths | 2.4 | 14.4 | |||
| 3. Maintenance | 5.0 | 5.0 | |||
| 4. Food (for staff) | |||||
| @ $600/mth × 12 mths | 7.2 | 7.2 | |||
| 5. Others eg., lease ($500/annum), administrative expenses, etc. | 2.0 | 2.0 | |||
| 6. Depreciation cost | |||||
| 6.1 | Depreciation of raft | ||||
| @ $78,500* for 10 yr-life | |||||
| × 1 unit | 7.85 | 7.85 | |||
| 6.2 | Depreciation of netcages | ||||
| 6.2.1 | hapas | ||||
| @ $418 ea* for 3 yr-life | |||||
| × 28 units | 3.90 | ||||
| 6.2.2 | nursery netcages | ||||
| @ $620 ea* for 5 yr-life | |||||
| × 24 units | 2.98 | ||||
| 6.2.3 | production/grow-out netcages | ||||
| @ $614 ea* for 5yr-life | |||||
| × 36 units | 4.42 | 11.3 | |||
| 6.3 | Depreciation of equipment/ items | ||||
| 6.3.1 | workboats (2 units) | ||||
| @ $2,000 for 5 yr-life | 0.8 | ||||
| 6.3.2 | outboard engines (2 units) | ||||
| @ $2,000 for 3 yr-life | 1.3 | ||||
| 6.3.3 | generator | ||||
| @ $3,000 for 5 yr-life | 0.6 | ||||
| 6.3.4 | aerator | ||||
| @ $3,000 for 5 yr-life | 0.6 | ||||
| 6.3.5 | pumps | ||||
| (2 units) | |||||
| @ $600 for 5 yr-life | 0.24 | ||||
| 6.3.6 | grinders (2 units) | ||||
| @ $800 for 5 yr-life | 0.32 | ||||
| 6.3.7 | fish cutter (2 units) | ||||
| @ $1,000 for 5 yr-life | 0.4 | ||||
| 6.3.8 | fish tanks (5 units) | ||||
| @ $1,000 for 5 yr-life | 1.0 | 5.26 | |||
| Total (1) to (6) | 86.41 | ||||
* [Cost details given in Addendum to (C)]
| D. Interest on Loan | ||
| Years 1 & 2: | Assuming Share Capital | = $150,000 |
| Loan | = $150,000 | |
| Total Capital Structure | = $300,000 |
Addendum to (C)
Cost of raft & netcages
I. Cost of raft
| One 5m × 5m raft | 23 - 5m ×5m raft | ||
| (S$) | (S$) | ||
| 1. | Cost of materials | 1,300 | 60,000 |
| (%) | |||
| Timber | 24 | 15 | |
| Metal | 14 | 17 | |
| Floatation system | 32 | 37 | |
| Anchor system | 29 | 21 | |
| Others | 1 | 10 | |
| 2. | Labour cost | 230 | 18,500 |
| 3. | Man-days | 2.5 | 70 |
| 4. | Total cost [(1) + (2)] | 1,530 | 78,500 |
II. Cost of netcages (5m × 5m × 3m depth)
| Hapa | Nursery | Production | ||
| (S$) | ||||
| 1. | Cost of materials | 190 | 392 | 386 |
| (%) | ||||
| Netting | 90 | 96 | 93 | |
| Rope | 9 | <4 | 6 | |
| Twine | 1 | <1 | 1 | |
| 2. | Labour cost | 228 | 228 | 228 |
| 3. | Man-days | 3 | 3 | 3 |
| 4. | Total cost [ (1) + (2) ] | 418 | 620 | 614 |
| D. | Interest on loan @ 8% per annum | = | $ 12,000 |
| (8% × $150,000) | |||
| Yr 3 | Assuming loan repayment at end of Yr 2 | = | $100,000 |
| Balance of loan | = | $ 50,000 | |
| Interest on loan @ 8% per annum | = | $ 4,000 | |
| (8% × $50,000) | |||
| Yr 4 | Assuming loan repayment at end of Yr 3 | = | $ 50,000 |
| Balance of loan | = | nil |
E. Less Tax @ 33%
| Yr 1 | : | deficit - no tax incurred | ||
| Yr 2 | : | 1. Profit | = | (Profit for Yr 2) less (deficit incurred in Yr 1) |
| = | $121,400–60,100 | |||
| = | $ 61,300 | |||
| 2. @ 33% | = | $ 20,433 | ||
| Yr 3 | : | 1. Profit | = | $129,400 |
| 2. @ 33% | ||||
| = $ 42,702 | ||||
| Yr 4 & 5 | : | 1. Profit | = | $133,400 |
| 2. @ 33% | = | $ 44,022 | ||
F. Capital & Loan
| $'000 | ||||
| 1. | Fixed Assets Expenses | |||
| 1.1 | raft | 78.5 | ||
| 1.2 | netcages | |||
| 1.2.1 | hapas (7 sets × 2/set × 2 = 28 nos) | |||
| @ $418 ea × 28 nos | 11.7 | |||
| 1.2.2 | nurseries | |||
| (6 sets × 2/set × 2 = 24 nos) | ||||
| @ $620 ea × 24 nos | 14.9 | |||
| 1.2.3 | production/grow-out | |||
| (9 sets × 2/set × 2 = 36 nos) | ||||
| @ $614 ea × 36 nos | 22.1 | |||
| 1.3 | equipment/items | |||
| 1.3.1 | workboat (2) | |||
| @ $2,000 ea × 2 | 4.0 | |||
| 1.3.2 | outboard engine (2) | |||
| @ $2,000 ea × 2 | 4.0 | |||
| 1.3.3 | generator | 3.0 | ||
| 1.3.4 | aerator | 3.0 | ||
| 1.3.5 | pumps (2) | |||
| @ $600 ea × 2 | 1.2 | |||
| 1.3.6 | grinders (2) | |||
| @ $800 ea × 2 | 1.6 | |||
| 1.3.7 | fish cutters (2) | |||
| @ $1,000 ea × 2 | 2.0 | |||
| 1.3.8 | fish tanks (5) | |||
| @ $1,000 ea × 2 | 5.0 | |||
| Total Fixed Assets Expenses | 151.0 | |||
2. Direct Cost (Yr 1)
| 2.1 | Fingerlings | ||
| 2.1.1 | No. of fingerlings per stocking | = 7,000 | |
| (@ 3,500/netcage × 2 netcages) | |||
| 2.1.2 | No. of stockings in Year 1 | = 21 | |
| (@ 1 stocking every 6 mths starting from 1 1/2 mths after commencement of raft construction - see Notes A, Diagram 1). | |||
| 2.1.3 | Total no. of fingerlings reqd. | = 147,000 pcs | |
| (21 × 7,000) | |||
| 2.1.4 | Cost of fingerlings | = $88,200 | |
| (@ $0.60 ea) | |||
| 2.2 | Feed | ||
| 2.2.1 | No. of complete cultures | = 14 | |
| (> 4 mths) | |||
| 2.2.2 | No. of incomplete cultures | = 7 i.e. 35 | |
| (< 4 mths) | complete | ||
| cultures. | |||
| 2.2.3 | Total no. of cultures | = 17.5 | |
| 2.2.4 | Cost of feed/culture | = $2,730 | |
| (ie 2 netcages) | |||
| 2.2.5 | Total cost of feed | = $47,775 | |
| 2.3 | Total Direct Cost [ (2.1) + (2.2) ] | = $135,975 | |
| ie $136,000 | |||
| 3. | Operating cost (less depreciation) | = $ 62,000 |
| - see Notes C | ||
| 4. | Total Operating Expenses for 1st year | = $ 198,000 |
| [ (2) + (3) ] | ||
| 5. | Less 3 stockings after cash inflow from 1st harvest at 10.5 mths | = $ 12,600 |
| 6. | Working Capital [ (4) - (5) ] | = $185,000 |
| 7. | Total Capital Structure | = $320,975 |
| [ (1) + (6) ] | Say $300,000 |
8. Direct Cost (Yr 2-5)
| Yr 2 | Yr 3 | Yr 4 | Yr 5 | ||
| 8.1 Fingerlings | |||||
| 8.1.1 No. of stockings | = | 23 | 22 | 23 | 23 |
| 8.1.2 Total no. of fingerlings reqd. | = | 161,000 | 154,000 | 161,000 | 161,000 |
| 8.1.3 Cost of fingerlings | = | $96,600 | 92,400 | 96,600 | 96,600 |
| (@ $0.60 ea) | |||||
| 8.2 Feed | |||||
| 8.2.1 Complete cultures | = | 15 | 15 | 15 | 15 |
| (> 4 mths) | |||||
| 8.2.2 Incomplete cultures | = | 15 | 15 | 15 | 16 |
| (< 4 mths) | (7.5 complete cycles) | ||||
| 8.2.3 Total no. of cultures | = | 22.5 | 22.5 | 22.5 | 23 |
| 8.2.4 Total cost of feed | = | $61,425 | 61,425 | 61,425 | 62,790 |
| (@ $2,730/culture) | |||||
| 8.3 Total Direct Cost | = | $158,025 | 153,825 | 158,025 | 159,390 |
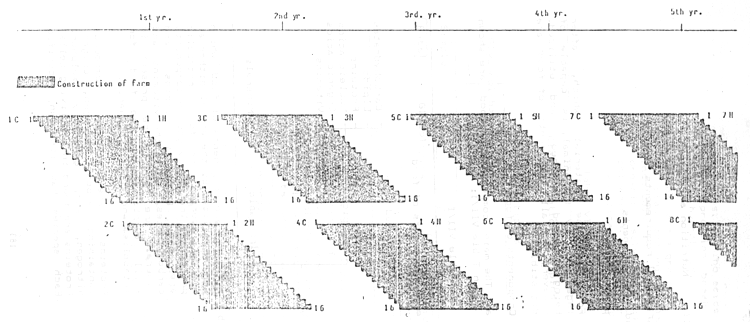
Denotation
C = Culture cycle
H = Harvest
Numerals of 1 culture cycle represent 2 netcages in use.
Figure VI/2
Diagramnatic representation of entire 5 years of operation of a 32-5m×5m seabass netcage farm