T. Tattanon and S. Maneewongsa
A. NATURE OF EGGS, JUVENILES AND LARVAE OF THE SEABASS
1. Introduction
In the past before seabass become a cultured fish, no one studied about its eggs and larvae so that there was lack of knowledge on this subject. Only some general background on seabass was known, i.e. that the adult fish live in the sea and migrate to the mouth of rivers that are connected with the sea to spawn during the spawning season. The Songkhla Fisheries Station was established near the mouth of Songkhla Lake and it was observed that during April to September each year, adult seabass were caught at the mouth of Songkhla Lake. By dissecting fish, these were found to be spawners. The finding started the experiments on induced spawning of seabass by stripping eggs and milt conducted in 1971. The trial that year was successful. Since then studies have been done and some knowledge gained on eggs, larvae and juveniles of this species.
2. The Seabass Egg
Immature Eggs in the Ovary
There are no specific studies on the matter made in Thailand at present, but aquaculturists in the country have more or less clear recognition of the immature eggs as found in the ovaries of the fish. Specific studies on this will be needed and should be made in the future.
The Mature Egg
As previously described the mature egg is round with a shell membrane fully distended (no spaces nor distortions) measuring about 0.8 mm in diameter. They tend to stick together and while in groups, the eggs give a golden hue. It has one oil globule inside which measures about 0.2 mm in diameter.
The Milt and Sperm
Some milt will flow freely from a mature male spawner. It should be of good amount, preferably about 10 ml and not very sticky so that it would flow freely if poured from the container. If the milt is examined under the microscope, the sperms can be observed to move very rapidly. There is no detailed study yet of the seabass sperm in Thailand.
The Fertilized Egg and Embryonic Development
When the milt and eggs are mixed by the dry method, fertilization takes place. There appears to be no significant change on the egg from outside observations during the early stages. It was observed that it takes about 35 minutes after the mixing of the eggs and milt that the beginnings of embryonic development take place (Figures 1–2). The approximate time and duration of the various embryonic stages of seabass are enumerated below:
| Embryonic Stage | Period | ||
| Hours | Minutes | ||
| 1) | Fertilized egg | 0 | 0 |
| 2) | One-cell | 0 | 35 |
| 3) | Two-cell | 0 | 38 |
| 4) | Four-cell | 0 | 44 |
| 5) | Eight-cell | 1 | 03 |
| 6) | 32-cell | 2 | 12 |
| 7) | 64-cell | 2 | 43 |
| 8) | 128-cell | 2 | 55 |
| 9) | Pre-bastula | 3 | 11 |
| 10) | Blastula | 5 | 32 |
| 11) | Gastrula | 6 | 30 |
| 12) | Neurula | 8 | 32 |
| 13) | Embryo develops head, optic lobes and tailbuds | 11 | |
| 14) | Heart starts functioning, tail free, body starts to move | 15 | |
| 15) | Hatching | 17 | 30 |
Hatching (Figure 2)
The mechanics of hatching of the seabass embryo has not been studied in detail. However, since the hatching (newly hatched larvae) has by now free tail fin, it can move very freely. The hatchlings tend to confine themselves at levels below the surface (about 0.5 m below) and near parts of the water medium that have aeration or slight water movements.
The period from fertilization to hatching can be affected by temperature. As mentioned earlier, at 27 C the eggs can hatch in about 17 hours while if the temperature were 30–32 C, the eggs can hatch in 12–14 hours. Salinity on the other hand can affect the rate of hatching. This is shown in Table 2.
| Salinity (ppt) | Rate of hatching (percent) |
| 0 | 0.00 |
| 5 | 2.86 |
| 10 | 58.56 |
| 15 | 75.03 |
| 20 | 82.35 |
| 25 | 83.36 |
| 30 | 80.78 |
| 35 | 46.90 |
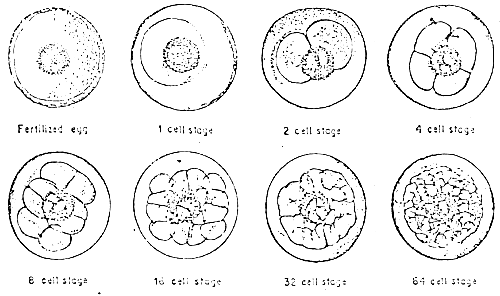
Figure 1. The fertilized egg and embryonic stages of seabass.
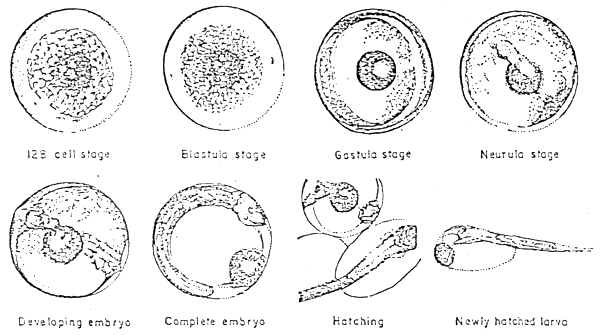
Fig. 2. Advance embryonic stages, hatching and newly hatched larva of seabass
3. The Seabass Larvae
Development of Hatchlings with Egg Yolk
The early hatchling or newly hatched larvae is 1.5 mm long with a big yolk sac (Figure 2). The yolk sac has one big oil globule at its anterior. This enables the larvae to be set in the water with head raised up at 45 to 90 angle of the water surface. The body is slender and pale in colour with a distribution of pigments. The eyes, digestive tract, anus and caudal fins are distinctly seen but the mouth remains closed for a while or a period of about three days.
Under normal conditions, the rate of absorption of the yolk has been observed to be as follows (Table 3):
| Diameter* of yolk in yolk sac (mm) | Time (days) |
| 0.8800 | 0 |
| 0.3525 | 1 |
| 0.2752 | 2 |
| 0.1530 | 3** |
| 0.0050 | 4 |
| 0.00 | 5*** |
* As the yolk is actually elongated, this isa measurement of length from anterior toposterior.
** Mouth opens.
*** Yolk completely absorbed.
Development of Larvae, Fry and Juveniles
The mouth opens when the larvae get to about three days old and the yolk has been almost completely absorbed. This is a sign that the fry can start to feed. Up to seven days the larvae are pale in colour and from the age of seven days to metamorphosis at 18–20 days, they appear dark with distinct vertical stripes on certain parts of the body. After the 18th to 20th days, the larvae again assume pale brownish colour. This time, the vertical stripes can be more clearly distinguished. The stripes are three, one at the caudal peduncle, another at the level between the first or spinous dorsal fin and the second or soft dorsal fin, and a third over the head, all of which are particularly distinct.
In one month, the larvae metamorphose into the fry stage which has the appearance very close to the parent fish. The fry measures 1.5 to 2.0 cm. These further grow and develop into juveniles after the third to the fifth month when they attain 8– 15 cm.
B. FOOD AND FEEDING OF SEABASS LARVAE AND JUVENILES
1. Introduction
Food and feeding are two of the most important factors affecting the survival rate of seabass larvae. The larvae cannot survive if there is inadequate supply of food, which comprises various live organisms, and which varies with the development of the larvae.
2. Food and Sources
Most of the food that seabass larvae feed on is composed of live zooplankton. The larvae first beging to feed on rotifer. Other kinds of food have also been tried with the early larvae but without success. The supply of live zooplankton is expensive and sometimes causes problems because zooplankton culture needs time, facilities and skills. Further, the different kinds of live food required must be prepared in time to satisfy the need of the fast-growing larvae. To maintain a high survival rate, the feeding schedule for the larvae must be closely adhered to.
Live Food Used at Different Age Stage
The live food used for the different developmental stages of the seabass larvae are shown in Table 1.
| Food Type | Days After Hatching | |||||
| 1 | 3 | 8 | 16 | 25 | 30 | |
| Chlorella | ||||||
| Rotifer | ||||||
| Artemia | ||||||
| Moina/Daphnia | ||||||
| Acetes/Trash Fish | ||||||
C. LARVAL REARING OF SEABASS
1. Introduction
Widespread demand for commercial scale culture of seabass is now encouraged by the governments of most Southeast countries including Indonesia, Hongkong, Taiwan (China), Malaysia, Singapore and Thailand. Accordingly, the cost of seabass larvae has become very high and is increasing year after year. In Thailand particularly, it is estimated that at least 50,000,000 larvae are needed to supply the needs of the whole country, The advancement of technology on hatchery operation will be required to insure that the supply of fry for the growout ponds and cages can be met. The seabass seed supply problem is felt not only in Thailand but in the whole region.
Research on the propagation of seabass L. calcalifer commenced at Songkhla in 1969 under the direction of Mr. Sawasdi Wongsomnuk, who has later joined by Mr. Sujin Maneewongsa. The objective of the project was to develop methods of mass propagatuon of fry and to study propagation methods applicable to the physical and economic environment in Thailand.
The first successful mass production technique for seabass fry was achieved in 1973.
2. Care of Fertilized Eggs and Hatching
Fertilized eggs are dipped into 5 ppm of acriflavine solution for one minute then washed out 2 to 3 times before placing in hatching-rearing tanks, with size of 3.5 × 4.75 × 1.20 m. Each tank is filled with filtered seawater at the salinity of 28–30 ppt and aerated from the bottom.
Approximately, 800,000 to 1,000,000 eggs are stocked in each tank. The eggs hatch out in 17 hours at average water temperature of 27 C or at 12 hours at water temperature of 30 C. Various salinities of water give different hatching rates; the best salinity for hatching appears to be between 20–30 ppt (Table 1).
| Salinity (ppt) | Hatching Rate (%) |
| 0 | 0.00 |
| 5 | 2.90 |
| 10 | 58.5 |
| 15 | 75.0 |
| 20 | 82.4 |
| 25 | 83.4 |
| 30 | 80.8 |
| 35 | 49.9 |
3. Care of Newly Hatched Larvae Before Feeding
After hatching, aeration is stopped for a few minutes in the tank so that the sediments of undeveloped embryos and other dirts can be siphoned out. During this period, running water system is used in the rearing tank for 2 to 3 days before feeding.
4. Start of Feeding and Care of Growing Larvae
Feeding of Various Age Stage
From 3 days old larvae, the yolk is almost completely absorbed, the mouth opens and the larvae start their feeding habit. By this time, the rotifer (Brachionus) is required to feed the larvae. Enough rotifer is added or approximately 5–10 rotifers per ml are stocked into the larval tank. Larvae are fed with rotifer up to 10–14 days, later on they are fed with Artemia salina until 20 days. Daphnia or Moina is the last living organism that is fed on by 20–30 days old larvae. After this period, they are trained to change their predatory habit by feeding trash fish which are chopped or minced into bite—sized pieces.
Nursery Tank Management
The rearing tank should be cleaned up every time before using. The rate of water replacement in the rearing tanks depend on feeding period of each age stage. In the period of rotifer feeding to prevent the loss of rotifer through the outlet, approximately 10–20 percent of the water in the rearing tank is drained out only for the replacement of rotifer supply each day. During Artemia feeding period, approximately 50 percent of water is changed while almost complete change is made during trash fish feeding period.
The sediment of dead organisms, larvae or leftover food are siphoned out everyday. The management of seabass nursery is shown in Figure 1.
Grading Techniques
Due to cannibalistic nature of the fish, size selection or grading or sorting us of prime importance, The first sorting should start at the second week since during this period, the bigger fish can eat the smaller ones. The easiest way of sorting is to use screen with various mesh size so that the various sizes of fish can be separated easily. Stocking the same size of fish will reduce the rate of cannibalism, thus the survival rate will be increased and the growth rate of the fish could also be faster and more uniform.
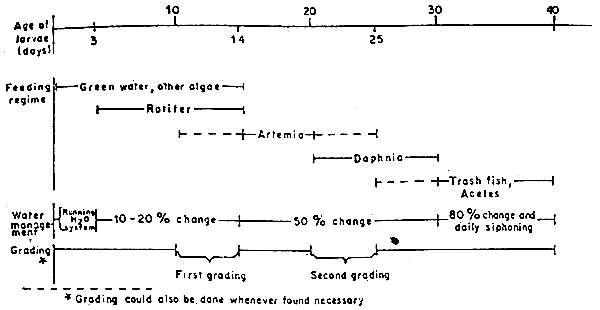
Fig. 1. Chart showing management method for seabass nursery tank within the first 40-day period
Growth and Care of Larvae as They Develop to Fry and Juveniles
Up to 40–45 days, the larvae develops to juvenile stage. They are moved from the rearing tanks for culture in netcages. The netcages are of 1 × 2 × 1 m in dimensions are are usually set in open waters. Stock of 2,000–3,000 fry are raised to the fingerling size in these cages.
Diseases
If hygienic conditions are maintained, the larvae are generally resistant to diseases. However, since the larvae are stocked in the tank for a long period, sometimes they show their abnormal swimming character, stop feeding, and turn black. These are signs of disease or poor health so that if these occur, they should be treated with 1:2,000 parts formalin solution for 10–15 minutes for 2–3 days continuously.
Survival Rate
The system of culture outlined above gives about 85 percent hatching rate and a survival rate of 1–7 days old larvae of 30 percent. For 8–15 days old larvae the survival is 80 percent, after which they can be maintained indefinitely with negligible mortality (Table 2).
| Age (days) | No. of larvae* per liter | Survival Rate (%) |
| 1–7 | 30–40 | 37.2 |
| 8–15 | 15–20 | 80.9 |
| 16–23 | 5–10 | 70.0 |
| 24–30 | 2–5 | 85.3 |
* Normal stocking density used in nursery tanks.
D. GROWTH OF SEABASS LARVAE AND JUVENILES
1. Introduction
There is no information or previous studies made on the growth of seabass larvae and juveniles from the wild. It is commonly known that the seabass fry when collected from natural areas are big enough so that they can be suitable for stocking growout ponds and cages. Only approximate estimates of their possible age can be made.
As we are now able to spawn the fish and grow the larvae and juveniles under controlled conditions, we have better knowledge on their growth. We are also successful in nursing the seabass larvae and juveniles in controlled conditions are relatively high survival rates.
2. Growth of Larvae and Juveniles
Growth in Relation to Food
In the nursery of seabass larvae, the most important factor to consider is to have the right kind and amount of food prepared for the larvae. Various stages of larvae need different kinds of food. The food to be given to the first stage of the larvae when they start to feed is rotifer, Brachionus plicatilis. This kind of food is very suitable for the young larvae making them grow well and giving high survival rates. Therefore, adequate amount of rotifer is very necessary for nursing seabass larvae. The density of rotifer should be 5–10 rotifers per ml in the nursery tank. Feeding larvae with rotifer is started when the seabass larvae are 3 days old until their 14th day. Artemia is fed to seabass when they get to the 8th day until the 20th day. Daphnia or Moina is fed to the larvae on the 17th day until the 30th day. Acetes and minced fish are fed to the fry after the 21st day. These feeding stages are shown in Table 1..
| Age (Days) | Percent of Food Given | |||||
| Chlorella | Rotifer | Antemia | Daphnia/Moina | Acetes | Minced Fish | |
| 3–7 | 10 | 90 | ||||
| 8–15 | 10 | 75 | 15 | |||
| 16–20 | 50 | 50 | ||||
| 21–30 | 80 | 10 | 10 | |||
| 31–40 | 50 | 25 | 25 | |||
| 41 | 100 | |||||
Experiments were conducted on nursing of seabass larvae with 3 kinds of zooplankton, namely: rotifer, Artemia and Moina. The larvae were reared during their 3–15 days of age. The results are shown in Table 2.
| Type of Food | Number of larvae | Number survived | Survival rate(%) |
| Rotifer | 2,000 | 808 | 40.40 |
| Rotifer + Artemia | 2,000 | 951 | 47.55 |
| Rotifer + Moina | 2,000 | 381 | 19.05 |
The results showed that rotifer and combination of rotifer and Artemia were the more suitable food of seabass larvae at this age stage.
Growth in Relation to Space
The tanks used as nursery for the seabass larvae measure 3.50 m × 4.75 × 1.20 m deep. This is filled with good fresh seawater 1.10 m deep and aerated at 4 points of the tank bottom. The density of stocking of this size of nursery facility ranges from 600,000 to 1,000,000 larvae/tank. Larval densities that are usually used in nursing tanks per ton are shown in Table 3.
| Age (Days) | Density of Larvae per ton | Survival Rate (%) |
| 1–7 | 60,000–100,000 | 37.23 |
| 8–15 | 15,000–20,000 | 80.91 |
| 16–23 | 5,000–10,000 | 70.05 |
| 24–30 | 2,000–5,000 | 85.33 |
Growth in Relation to Water Quality
Good fresh seawater has to be filtered by the phytoplankton net to prevent predators from entering the nursery tank. The salinity of water should be maintained at 10–30 ppt.
Experiments were conducted using various salinities of water for nursing larvae of 1–30 days of age. The results reveal that the salinity of 20 ppt gave the highest survival rate as shown in Table 4.
| Salinity (ppt) | Survival Rate (%) |
| 0 | 0.0 |
| 5 | 24.0 |
| 10 | 28.0 |
| 15 | 28.0 |
| 20 | 68.0 |
| 25 | 22.0 |
| 30 | 18.0 |
| 35 | 10.0 |
The effects of other water quality factors (temperature, pH, DO, etc.) have not been fully studied.
3. Growth Rate of Seabass Larvae
By nursing seabass with enough food of the kind described above within 30 days, the larvae should normally attain a length of 1.205 cm. The normal growth within the first 30 days is shown in Table 5.
| Age (Days) | Total Length (mm) | Remarks |
| Fertilized egg | 0.870 | Diameter of fertilized egg. |
| 0 | 1.5175 | |
| 1 | 2.1850 | Beginning to hatch out.* |
| 7 | 3.5916 | |
| 14 | 4.3650 | |
| 20 | 8.10 | |
| 30 | 12.05 |
* Hatching is at 12th to 17th hour depending on temperature.
E. DISTRIBUTION AND TRANSPORT OF SEABASS FRY
1. Collection and Conditioning of Fry Before Transport
Fry are collected from the rearing tanks and placed in smaller receptacles.
Fry are treated with 5 ppm of acriflavine solution or 0.5 ppm of copper sulfate solution for 5–10 minutes.
There should be no feeding within 1–2 hours before packing.
2. Packing and Amounts that can be Transported
Plastic bags of 40 × 60 cm of proper gauge are filled with 6–7 litres of fresh seawater and saturated with oxygen; 10–12 litres of oxygen gas are used for packing. The amount of transportable fry depends on size of fry, water temperature in plastic bags and duration of travel and handling from source of fry to its destination.
| Age Stage (Days) | Size (TL) (cm) | No. of fry per bag | Water temp. | Duration (hours) | Survival rate (%) |
| 7–15 | 0.2–0.3 | 10,000 | 19–23 C | 16 | 90 |
| 20–22 | 0.5 | 5,000 | -do- | 16 | 90 |
| 1 month | 1.0–1.5 | 1,000 | -do- | 16 | 90 |
| 2 months | 2.0–3.0 | 500 | -do- | 16 | 90 |
3. Transport
In transporting by truck, a mixture of crushed ice and sawdust is needed to control the water temperature in the plastic bags during transport. The mixture is spread uniformly on the floor of the truck before the plastic bags are laid upon it. The proportion of crushed ice and sawdust is 1:1 for long—period transport (12–16 hours) and 1:2 for short periods (4–5 hours). Transportation should be carried out at night time. By this method, it is possible to control the water temperature between 19–23° C.
Figure 1 shows the observed fluctuation in temperature of the water in the plastic bags during transport. It was also observed that the dissolved oxygen starting initially at 5.3 to 5.0 ppm will drop to 2.3–2.6 ppm at destination.
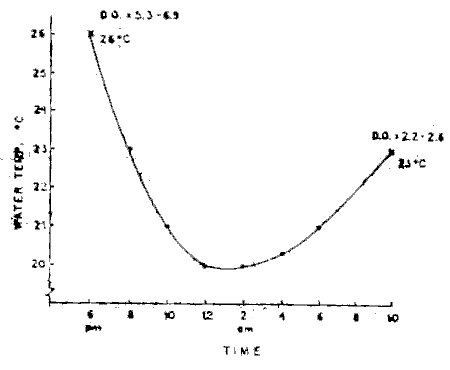
Figure 1. Fluctuation of water temperature under transportation.
However, another and more convenient way for land transport of live fish is by using refrigerated truck or air—conditioned bus where temperature of 20–22 ° C can be controlled during transport.
Air transport is of course a very convenient and fast but expensive way. In this case, the plastic bags are required to be placed in a receptacle. The bags can be covered with some crushed ice before loading. This method takes about 3–4 hours duration while it takes 13–16 hours to transport by truck over the same distance. The price of air transportation can vary from one country to another.
4. Distribution
Usually 7–14 day—old larvae are distributed to the fish farmers who have their own nursery to continue nursing the larvae. After the larvae attain fry sizes of 1.5 to 2 cm they are distributed to the local fishermen to be reared in grow-out cages or ponds.