Tida Pechmanee*
Introduction
The Songkhla Fisheries Station of the Department of Fisheries produced the first successful batch of artificially bred seabass fry in 1975. Until now, the availability of rotifer (Brachionus plicatilis) and brine shrimp nauplius (Artemia sp.) as larval feed is still the most important factor affecting growth and survival rate of seabass larvae. The larvae are first fed with rotifers until they attain 4 mm in total length when the diet is switched to brine shrimp nauplii until the larvae accept minced fish or formula feed.
BRACHIONUS PLICATILIS
1. Taxonomic Position
| Phylum Trochelminthes | |||||
| Class Rotifera | |||||
| Order Monogononta | |||||
| Family Brachionidae | |||||
| Genus Brachionus Pallas | |||||
2. General Information
The body of most rotifers is divided into three portions: head, trunk and foot. The head carries a corona, which is surrounded by cilia. There are two types of Brachionus plicatilis. One is the S-type which has pointed anterior spines and short lorica (140–220 um). The other is the L-type which has blunt anterior spines and long lorica (230–320 um). S-type grows well in temperature above 20 C, the L-type is less than 20° C. All the mass-cultured rotifers in Thailand are of the S-type. They grow well when the salinity is 15 ppt and temperature is 28° C to 30° C.
* Senior Fishery Biologist, NICA.
3. Food for Rotifer
In nature, rotifers feed on phytoplankton, yeast, bacteria and protozoa. In mass culture, they are usually fed Chlorella, Tetraselmis and yeast. It has been found that rotifers fed on baker's yeast lack n—3 highly unsaturated fatty acids (n—3 HUFA) which are essential fatty acids for marine fishes. Seabass larvae fed with rotifers raised only on yeast (yeast rotifer) show low survival rate. It is therefore necessary to enrich yeast rotifer with squid liver oil or marine fish oil providing the n—3 HUFA at least six hours.
4. Rotifer Culture
Total Harvest System
Large scale hatcheries usually adopt the partial harvest system using large tank. The volume of production tank is 10–100 tons. In small scale hatcheries, the total harvest system is effective using one to 10—ton production tank.
At the National Institute of Coastal Aquaculture (NICA), a 50—percent harvest system is employed for the mass culture of rotifer, using a 26—ton production tank. Rotifers are fed mainly on Chlorella or Tetraselmis. Yeast is used as supplementary food when phytoplankton supply is not sufficient.
The process of culturing B. plicatilis is as follows:
First, 1–2 tons of fresh water is added to 11–10 tons of phytoplankton (Chlorella density, 10 × 10 cells/ml or Tetraselmis, 10 × 10 cells/ml), in order to adjust salinity to 25–30 ppt.
Rotifer seed is introduced at a density of 10–20/ml. After 2 days, this initial food supply is exhausted, and the density of rotifer would have increased to 40–100/ml. At this point, an additional 13 tons of food and water mixture (same as initial batch) is added to the tank, for a total volume of 26 tons.
The next day, the rotifer density would be about 40–100/ml. It is then ready for harvesting by draining the water from the rotifer tank through 63 u mesh bag, leaving half of the original volume to serve as starter for the next batch.
Phytoplankton, or phytoplankton plus baker's yeast (0.2 g per 10 rotifer) or marine yeast (2 × 10 cells/ml) is added to the rotifer tank to the the 26-ton level in order to grow the next batch of rotifer.
The rotifers are harvested daily from the same tank for about 10 days at a constant rate of production.
5. Phytoplankton Culture
Chlorella sp. and Tetraselmis sp. are cultured in 26-ton rectangular tanks. The kind and amount of fertilizer applied to the culture tanks are: 1200 g of (NH4)2SO4, 120 g of calcium superphosphate or agriculture fertilizer formula 16-20-0, and 60 g of urea. It takes about 2–5 days for Tetrasemis and 3 days for Chlorella to grow to harvest level.
6. Marine Yeast Culture
Marine yeast seed is collected from the fish culture water. The nutrient solution for growing marine yeast is 15 g sugar, 3 g of (NH4)2 SO4, and 1 g of KH4 PO2 dissolved in one litre of water. One ml of HCl concentrate is added to the the solution to produce a pH level of 4. The yeast is reared for 2–3 days, then transferred to grow in a 10-litre vessel for 1–2 days. Add the same nutrient mix but without the HCl. The yeast is then transferred to a 100 to 500-litre tank and the sugar supply reduced to 8 g/l. One day later, it can be used for feeding rotifer.
7. Rotifer Enrichmnent
Yeast rotifers should be enriched before using. To enrich, 100 ml of cod liver oil, 1 g of raw egg yolk and 1 litre of water are homogenated for 2–3 minutes by mixer then added to a 1-ton tank of rotifer with about 500–1000/ml for at least 6 hours. The rotifers are cleaned before using.
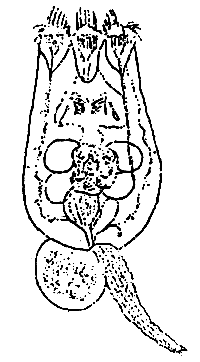
Fig. 1. Rotifer
(Brachionus spp.)
ARTEMIA SP.
1. Taxonomic Position
| Phylum Arthropoda | |||||
| Class Crustacea | |||||
| Order Anostraca | |||||
| Family Artemiidae | |||||
| Genus Artemia Leach | |||||
2. General Information
Salt lakes and brine ponds with Artemia populations are found all over the world. At certain moments of the year, cysts of Artemia floating at the lake surface are washed ashore by wind and waves. The cysts are harvested, processed, packed and sold. Artemia nauplii can be produced easily from these cysts. The first larval stage of Artemia measures about 500 microns in length and has 3 pairs of appendages. An important criterion in selecting a suitable brand of Artemia cyst is the amount of nauplii that can be derived from a unit weight of cyst. This can be determined by the hatching efficiency, that is, the number of hatched nauplii obtained from 1 g of cyst.
3. Hatching
Soak the cysts for 1 hour in a 20 ppm hypochlorite solution in tap water with good aeration. After soaking, wash the cysts with tap water.
Add not more than 5 g of cyst for 1 litre of sea water. The hatching containers should be cylindrical tanks with conical bottom, aerated from the bottom. The hatching containers should be illuminated at least during the first hour of hatching. NaHCO is added to adjust the pH to 8–9 whenever necessary. Artemia cysts usually start to hatch after incubation at 25–30° C.
4. Harvesting
Harvesting of Artemia nauplii is done after 5 to 10 minutes interruption of the aeration. Empty cyst shells float to the surface, while the nauplii concentrate in the lower part of the tank and the unhatched cysts accumulate underneath the nauplii. Since most nauplii are positively phototactic, their concentration can be hastened and increased by shading the upper part of the hatching container with a black plastic sheet so that light reaches the lower part of the container only. Remove the unhatched cysts for the second hatching, after which the nauplii can be collected. A second collection of nauplii may be done 5 to 10 minutes after the first.
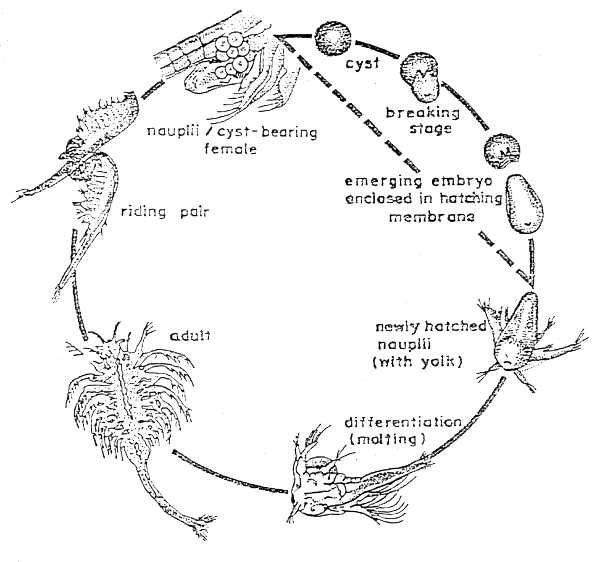
Figure 2. Life cycle of Artemia.