After hatching, undeveloped embryos and other dirts have to be siphoned out. This can be done by stopping aeration for a few minute to allow the sediments settle on the bottom. The sediments can be siphoned out as required. Hatching rate of seabass larvae also reflects the survival rate of the larvae in subsequent period. It has been observed from culture trials at the Centre as well as from hatcheries in Thailand that the batch with low hatching rate will have also poor survival rate. For economical point of view only the batch with hatching rate above 50 percent will be kept for further development.
The larval rearing can be divided into two phases. The primary rearing phase extends from hatching to a larval size of 4–6 mm TL or 10–14 days after hatching. This rearing phase in mainly in indoor tanks. The secondary rearing phase covers the period up to 6–10 mm TL size or 15–21 days after hatching. This phase is in outdoor tanks.
During the first two weeks in indoor phase, the density of the larvae is about 40–60 larvae/1. Water exchange is about 10–20 % perday. Salinity is maintained at 28–30 ppt. Two systems of larval rearing technique have been developed and tested. Each system has its own advantage and disadvantage depending on management and skill of technician.
17.1.1. Green water system
Algae (both Chlorella and Tetraselmis) and rotifer are given to the larvae after attained two-three days old. Rotifer is given daily at the rate 2–3 pcs/ml on Day 2, 3–5 pcs/ml from Days 3–10, and 5–10 pcs/ml from Days 11–14. The algae does not only serve as a food for the seabass larvae, but also serves as a food for rotifer and helping to improve the water quality. The algae is capable of converting the excretory products toxic to the fish larvae (un-ionized ammonia), which produced by the larvae, and the rotifer, and degrading of other decomposing feeds to less harmful nitrites.
Every day before exchanging the water, the rotifer remaining in the rearing tanks is counted in order to adjust the amount of rotifer given daily. To ensure that the availability of food is adequate for the fry during 24 hrs period, the amount of rotifer remaining in the tank at the following day should not less than 5 pcs/ml. The amount of rotifer consumed by seabass fry relating to the size of fry is shown in Fig. 11. For the fry size about 4 mm TL consumes about 1 500 rotifers a day. The algae and rotifer are given until the fry reached 15 days old. Feeding schedule of seabass larvae and fry from Days 1–40 is given in Fig. 12.
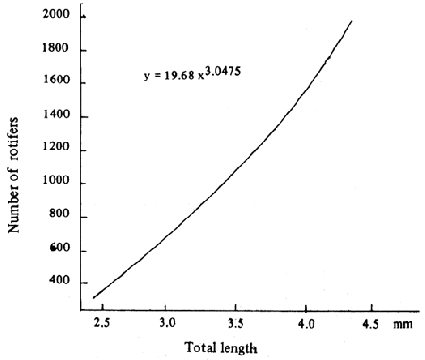
Fig. 11. Number of rotifers consumed by a seabass fry per day corresponding to size.
(Source : Tongrawd and Suteemechanikune, 1983).
Artemia is given when the larvae reached the 8–10 days old or attained the size about 4 mm TL until 20 days old. The density of brine shrimp nauplii is given 1–1.5 pcs/1 first. The density is gradually increased to 4–5 pcs/ml when the fry are 15 days old, and 6–7 pcs/ml for the 20 days old fry. The amount of brine shrimp nauplii daily given should be adjusted according to the number of nauplii and larvae remaining in the rearing tank in the following day. The amount of nauplii eaten by seabass larvae increased linearly with the age of the fish (Fig. 13). For the 15 days old seabass larvae, it consumes as many as 300 nauplii per day.
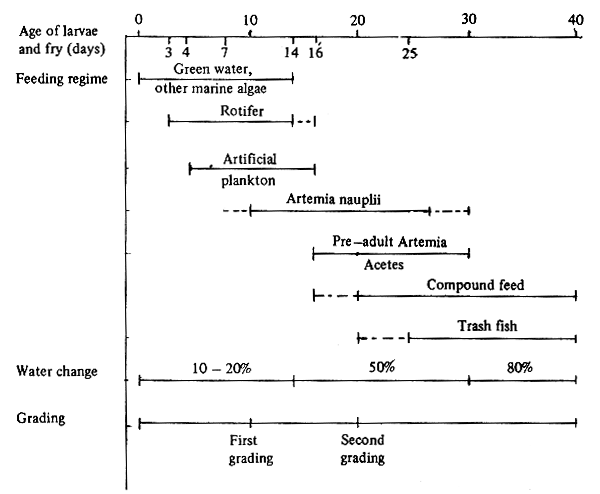
Fig. 12. Feeding schedule and water management for seabass larvae and fry in nursery during the first 40 days period.
The disadvantage of the green water system is the rapidly blooming of the phytopplankton in the larval rearing tanks. It may cause high mortality to larvae if the water cannot be changed in time. Rate of sedimentation on the bottom of the tanks is also high when compared with the clear water system. This increases work in cleaning the nursery tank and depresses the larvae during the cleaning process.
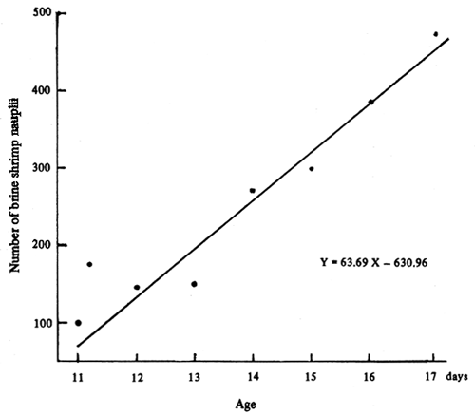
FIG. 13. Number of brine shrimp nauplii consumed per day by a seabass fry of 11–17 days old. (Source : Pechamanee et al, 1984).
17.1.2. Clear water system
On Day-2, after the mouth is fully developed, the rotifer size about 100 μ are given at a density of 2–3/ml. The size of rotifer can be increased to 150 μ at Day-3, and 200 μ at Day-4. After Day-5, the rotifer size bigger than 200 μ are given. The amount of rotifer given is also vary with the size of the larvae. It is maintained at 3–5/ml from Days 3–10, and 5–10/ml from Days 11–14. After Day- 11, the larvae should attain size of about 4.5 mm TL, and are ready to accept brine shrimp nauplii. This technique even though has to grade the rotifer to the size eatable by the seabass larvae, but it requires less work in nursery tank management and gives more consistent result when compared with the green water system.
After 14 days in indoor tanks, the larvae are much stronger and are more adaptable to outdoor condition. The larvae are graded and transferred to outdoor larval rearing tanks until Day-21. At this stage the density of the larvae is reduced to 20–40 larvae/1. Salinity is reduced to 25–26 ppt. Water exchange is around 50% perday. The feed is switched to brine shrimp nauplii. To reduce the production costs, Daphnia or Moina are also used as a supplementary feed, eight times a day.
After Day- 16, Acetes, pre-adult Artemia, or compound feed are given, 8 times a day. Types and relative amounts of food given at various stages shown in Table 7 are only given as a guideline. The actual amount of food required per day should be adjusted by observing the eating and swimming behavior of the fry. If the fry are swimming fast around the tank, it means that more food is required. Alternatively, if the food remains, the amount of food given should be reduced. To minimize the deterioration of rearing water and increase the efficiency of feeding rate, a dropping feeding technique (Fig. 14) is adopted with a good result.
After 21 days old, the fry are graded and transferred to the rearing tanks. Stocking density is around 10–20 fry/1. Salinity is reduced to 20–25 ppt. Water exchange is around 80% per day. Minced fish is given as the main food. At first, the fish might not accept the minced fish but it gradually accepts it, once it is accustomed to it. Amount of fish flesh given is about 10–15 per cent of the body weight. For convenience the calculation is based on the everage weight of the 25 days old fry, 0.15 gm/fry. The amount of feed given should be adjusted according to fish conditions and water quality. Sub-adult and adult brine shrimp are also fed to the fry during Days 21–30. After Day 30, the fry should be transferred into the nursery ponds and/or in floating net cages.
Table 7 Types and relative amounts of food given at different stages of seabass larvae and fry.
| Age (days) | Chlorella etraselmis (cells/ml) ×10000 | Rotifer (pcs/ml) | Artemia (pcs/ml) | Artificial plankton (pcs/ml) | Acetes pre-dult Artemia (pcs/ml) | Minced fish compound feed (% body wt) |
| 3 – 7 | 5 – 10 | 5 – 7 | 2 – 3 | |||
| 8 – 15 | 5 – 10 | 6 – 10 | 1 – 2 | 3 – 5 | ||
| 16 – 20 | 4 – 5 | 0 | 1 | 30 – 35 | ||
| 21 – 30 | 6 – 7 | 0 | 2 | 25 – 30 | ||
| 31 – 40 | 3 | 15 – 20 | ||||
| >41 | 15 |
In the nursery pond, a density of 25–50 fry/m2 are recommended. To keep the fry healthy and fast growth, the daily water exchange rate should be about 30 per cent. The food given in this period can be either trash fish or compound feed or both.
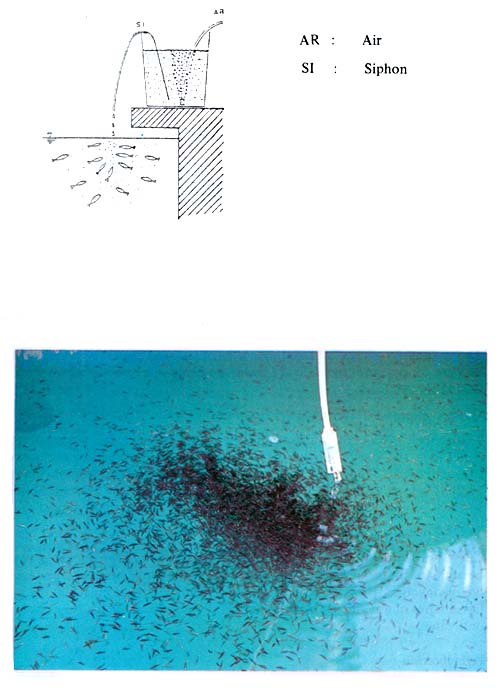
Fig. 14. Dropping feeding technique
Nursing of seabass fry in netcage has been successful since conducive environmental conditions. The size of cages suitable for the operation are vary from 2.0×2. 0×1.0 m to 5.0×2. 0×1.0 m. The mesh size of the net used for nursery netcages is 1.0 mm. Seabass fry size betwen 1.0 and 2.5 cm TL are stocked at the rate of 80–100 fry per m2 . After 45 days in nursery ponds or netcages, the fry attain weight of about 10 gm (body weight) which are ready for the grow out phase.
Rearing the fry in confinement is subjected to adverse effects of over-crowding and ecological problems inherent in the culture system. As environmental parameters fluctuate and other factors extend its adaptive response, the fry attempts to maintain or reestablish its normal physiological balance. If this process is within the adaptive range the chance of survival of the fry is high. The behavior and size distribution of the larvae and fry in the tank reflect the management and culture techniques that have been employed for the particular batch.
Swimming behavior
If the fry actively swim with head slightly downwards, and aggregate at the level near the bottom of the tank or at the certain level in the water column due to light activating, it suggested that the fry are in good condition and healthy. Healthy fry also prefer staying at a certain distance from the aeration spot, and move actively.
Feeding behavior
The healthy fry shows fast swimming behavior around the tank seeking for food and accepts food vigorously. The fry swim slow and relax on the water surface after having enough food.
Size distribution
If the stock is properly managed, the fry are uniform in size. Under confinement conditions, the uneven growth of the larvae and fry promotes competition among the individuals for food, space and other essentials of survival. The resulting additive effects of stresses on the smaller and weaker fry, are witnessed by the dark to black color, making them more susceptible to being preyed upon and contracting disease.
Uneven growth can cause a significant nursery mortality. The uneven growth could be due to the cannibalistic behavior of the species, dietary and environmental factors involved. However each batch of fry, there are the normal and the subnormal fry in terms of growth and other biological characteristics, which cannot be detected until later.
Pigmentation
The healthy fry have bright pigment and lively look. Head, body and tail are well developed.
The survival rates of seabass larvae and fry are determined by temperature, salinity, light intensity, stocking density, feed and feeding, water quality, grading and cannibalism.
Temperature affects the growth and survival of the seabass fry. Within the temperature ranges between 26–32 degree Celsius survival rate of the fry increased as the temperature increased (Table 8, Fig. 15). From rearing trials, poor survival rate was observed if the temperature fluctuated according to the ambient temperature (between 26–32 degree Celsius) during the 24-hr cycle. Good survival rate was obtained, when the temperature was kept consistent either at 28 degree Celsius or above. On contrary slow growth and poor survival rate were observed when the larvae or fry were kept at 26 degree Celsius or below.
Table 8. Survival rate of seabass fry from Days 21–34 rearing in 20 ppt at different temperatures.
| No. | Initial number of fry | Control | 26°C | 28°C | 30°C | 32°C |
| 1 | 200 | 36 | 47 | 68 | 114 | 185 |
| 2 | 200 | - | 54 | 57 | 146 | 182 |
| 3 | 200 | - | 52 | 71 | 135 | 145 |
| Total | 600 | 36 | 153 | 196 | 395 | 512 |
| Average | 200 | 36 | 51 | 65 | 132 | 171 |
Salinity affects survival rate of seabass larvae and fry. The results of the hatchery trials suggested that for good survival rate, the salinity of the larval tanks should be maintained at 28–30 ppt during the first tow weeks. After 2 week, the salinity can be reduced to 25–28 ppt, and maintained at 25 ppt after the 3rd week (Table 9).
Table 9. Survival rate of seabass larvae and fry from days 1–30 at different salinities.
| Age (days) | Salinity (ppt) | Survival rate (%) |
| 1 – 7 | 28 – 30 | 90 |
| 8 – 14 | 28 – 30 | 80 |
| 15 – 23 | 25 – 28 | 75 |
| 24 – 30 | 25 | 85 |
Aeration should be provided in as many spots as possible to allow even distribution of air in the tank. Keep the flow rate at minimal in order to facilitate even distribution of the fry in the culture tank.
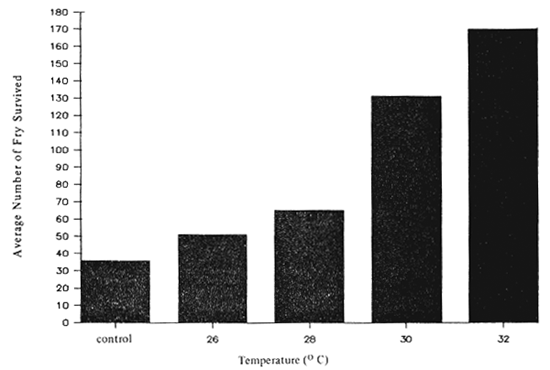
Fig. 15. Survival rate of seabass larvae kept in 20 ppt at different temperature.
Light intensity enhances the blooms of micro-organisms in the culture tanks. If the blooms occur too rapid and the water cannot be changed in time, the high mortality of the larvae might incur. Covering the tank is not only keeping the light intensity minimal and evenly distributed in the tank during the day time, but also makes the water temperature less fluctuation during the 24 hrs period.
Stocking density of the larvae and fry in the nursery tank also varies according to the age of fry (Table 10). During the first two weeks a stocking density should be about 60– 80 larvae/1. It should be reduced to 20–40 larvae/1 in 2nd week, and 10–20/1 in the 3rd week. After 21 days, the density of 1–10/1 is preferable. The stocking density used in hatchery trials at the Nationel Seafarming Development Centre, Hanura, Teluk Betung, Lampung, with a good survival rate are given in Table 10.
Table 10. Stocking density and survival rate of seabass larvae and fry at different ages.
| Age (days) | Total lengt (mm) | Stocking density (per m3) | Survival rate % |
| 1 – 14 | 1.5 – 5 | 60 000 – 80 000 | 70 – 80 |
| 15 – 20 | 5 – 8 | 20 000 – 40 000 | 60 – 80 |
| 21 – 28 | 8 – 10 | 10 000 – 20 000 | 70 – 80 |
| 29 – 35 | 10 – 13 | 5 000 – 10 000 | 80 – 90 |
| 36 – 42 | 13 – 30 | 1 000 – 5 000 | 80 – 90 |
High stocking density is another common cause of high nursery mortality especially in the absence of stock management measures. Chan (1982) observed that in the absence of stock management initial stocking density of fry size ranging between 2.0 and 2.2 cm TL at 375 fry/m3 would be reduced of 200 fry or by 47 percent after 25 days of culture. The remaining stock is much more even in size, comprising roughly 2% of 6.7 cm TL. 88% of 4.5 cm TL and 10% of 2.5–3.0 cm TL. Of this remaining stock, the lowest size group similarly exhibits a contrasting color pattern signifying a state of stress while the highest size group is also silvery except when disturbed.
During the first 21 days period, the larval fish are fed with live food. Quality and quantity of feed given to the larvae and fry during this period are significantly affect on tis growth and survival rate. It has been clearly shown in rearing trials that seabass fry fed with rotifer and Artemia containing high level of the highly unsaturated fattay acid (w3-HUFA) gave higher survival rate than those fed with the low w3-HUFA diet (Chantarasri et al., 1989). The result suggested that larvae and fry of Lates calcalifer are similar to seabass, Dicentrarchus, (Franicevic et al., 1986) and seabream, Sparus auratus) (Lisac, et al., 1986) that require diet containing high level of 20:5w3 and 22:6w3. The quantitative requirement is approximately 1.80% in dry weight of the diet for good growth, high efficiency and free from chronic essential fatty acid (EFA) deficiency symptom.
The quantity of feed given must kept under control. Water from rearing tanks should be taken seven or eight times a day to determine the concentration of the feed to assure the sufficient amount and continous supply of food to the fish larvae.
After Day-21, minced fish is given. It takes about 2–3 days to wean the seabass fry to the minced fish. After getting use to the feed, it accepts the minced fish nicely.
Water quality in nursery tanks have to be maintained as good as in the nature or at least as good as suggested in Table 2. The nursery tank should be cleaned as often as necessary. The rate of water replacement in nursery tanks depends on feed and feeding at different ages. During the period of rotifer feeding 10–20% of water is changed every day. It is increased to 50% during the Artemia feeding period, and almost complete change during minced fish feeding period. Water exchange is siphoned through strainer box (Fig. 16). Dirts and wastes settled on the tank bottom are siphoned out.
| SB : Strainer box. SI : Siphon, PP : 1 inch PVC pipe, FH : Flexible hose, | 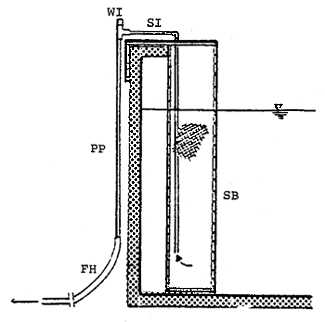 |
Fig. 16 Strainer box to facilitate water exchange.

Fig. 17. Grading of seabass fry.
First grading starts after the fry attained 12 days old using a net mesh size of 1.5 mm (Fig. 17) A small fish pass through the net but retained the larger ones. The second grading is needed after the fry attained 15 days old or whenever the difference in size is distinguished to separated the fast-growing fry from slogrowing ones. In order to reduce unnecessary injury and stress to the fish fry, and to speed up the operation, the second grading is done by using a series of fish graders with different pore sizes. The pore size of graders to retain the different sizes of fish is given in Table 11. Fish are placed in the fish grader and floated in the newly prepared larval tank.
Table 11. Pore size of grader and retained size of fish.
| Pore size (mm) | Minimum retained size (mm) | |
| 2 | 3 – 4 | |
| 6 | 10 | |
| 10 | 25 | |
| 15 | 50 | |
| 20 | 75 |
The smaller size fish pass through the pore to the new tank. The remainings are transferred into another tank. This procedure is able to sort the fish into different size groups as required and faster. Each grading may cause at least 5 percent mortality to the stock due to stress or injury. The rate will alleviate, if technicians lack of experience in handling the fry during the grading process. After grading, the larvae and fry are treated with 5 ppm acriflavine solution for disinfection purposes.
Seabass is highly cannibalistic especially in early stages when the individuals tend to congregate at high density in a certain place. This offers an opportunity for the stronger fry to take their prey. Upon its first success to prey on its kind, a seabass fry will exhibit increasing vigour and urge to feed mainly through its cannibalistic behavior.
Chan (1982) observed that while the bigger individual readily takes the smaller fry, it is also not uncommon for one to take another of the same size. The cannibalistic behavior is most pronounced at dawn and dusk when light intensity is low and also when flow rate of culture medium is minimal. The cannibalistic behavior of the species is also pronounced when the fry congregate in high densities, fighting for food during feeding time. The cannibalistic rate increases with increasing stocking density, clarity of water, and the number of feeding sessions per day. It also increases with decreasing percentage satiation per feeding, the number of feeding sessions per day, and the intensity of light.
After 30 days, the fish fry attaining a size of about 12 mm TL (Table 12, Fig. 18), the fry can be either transferred into nursery ponds, netcages or sold to farmers for furhter growth.
Harvesting of the fry can be done either using a small seine net with 2 mm mesh size after the water of the rearing tank is lower to 10–15 cm depth for large scale operation or collecting at the outlet for small scale operation.
Under normal conditions, average hatching rate of fertilized eggs is around 80% Survival rate of larvae at day-15 and fry at Day-30 are expected at 70–80%, and 30–50% respectively.
Table 12. Normal growth of seabass fry in 30 days.
| Age (days) | Total length (mm) | |
| Fertilized egg (dia) | 0.87 | |
| 0 | 1.60 ± 0.04 | |
| 1 | 2.20 ± 0.08 | |
| 2 | 2.52 ± 0.06 | |
| 3 | 2.61 ± 0.08 | |
| 4 | 2.78 ± 0.15 | |
| 5 | 3.08 ± 0.09 | |
| 6 | 3.10 ± 0.13 | |
| 7 | 3.44 ± 0.09 | |
| 8 | 3.58 ± 0.13 | |
| 9 | 3.49 ± 0.26 | |
| 10 | 3.81 ± 0.27 | |
| 11 | 3.87 ± 0.24 | |
| 12 | 4.41 ± 0.29 | |
| 13 | 4.58 ± 0.17 | |
| 14 | 4.75 ± 0.32 | |
| 15 | 5.41 ± 0.50 | |
| 16 | 6.56 ± 0.56 | |
| 21 | 8.91 ± 1.19 | |
| 30 | 12.05 |
(Source : Konsutarak and Watanabe, 1984)
Disease infection is another major cause of nursery mortality. The term “disease” here generally refers to viruses, bacteria, fungi, protozoans and other harmful pathogens.
Disease control in the hatchery will not be efficient, if any of the three factors; namely diagnosis, preventive measure of treatment, is omitted. The recommendations to control diseases in the seabass hatchery are listed below:
Correct diagnosis, as well as an understanding of the life cycle and ecology of the pathogen, is essential in order to identify a proper control programme. The diseases infected seabass fry show symptoms of loss of appetite, loss of scales, change in body color from gray to black and occurrence of white spot on the body (for white spot syndrome).
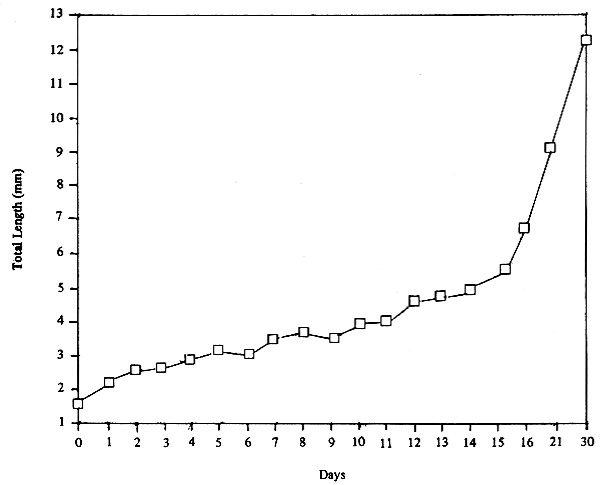
Fig. 18. Growth of seabass fry during the first 30 days.
To control the disease, the following preventive measures should be applied :
Chemical control should be considered as a “last resort” in disease control. Before treatment, it is better to have base line information on water quality such as pH and temperature of the culture media. When treating the fish, few fish should be treated to see how they react before treating the entire group. Only those drugs and chemicals which have been cleared by an authorized agency should be used on food fish. Treatments for specific groups of pathogens are suggested in Table 13 and Table 14.