Live food used for nursing seabas from newly hatched larvae (after the yolk sac is absorbed) to 30 days old are Tetraselmis, rotifer, marine yeast, brine shrimp and Moina. The culture techniques being used successfully at the Seafarming Development Centre are follows :
Nutrients required for mass production of the diatom are give in Tables 15, 16, 17 and 18. To prevent the diatom settling on the bottom of the culture tank, the culture media should be continuously stirred. To maintain the pH level of the culture medium within 7–8, an excess of CO2 should be provided.
The cultivation of marine green algae is usually done in an outdoor concrete tank with a capacity of 5–50 t with seawater of 1–1.5 m depth. Chlorella cultivation is based on the following procedures :
Pure culture of the algae at density of 10 million cells/ml from 1-gallon bottles are inoculated into a sufficiently aerated 1-ton tank filled up to one third of its capacity with fresh sea water. The chemical fertilizers usually used are ammonium sulphate, calcium phosphate, urea. The concentration of each of these elements (Table 19) is adjusted according to the nature of seawater supplied to the tank, which in turn varies by localities and other factors. The range of optimum temperature for algal growth is 24–25 degree Celsius. It often disappears in water with temperature more than 30 degree Celsius.
As the Chlorella cells increase in number, water is gradually added and appropriate amounts of fertilizers are applied. The amount of Chlorella produced although varying according to several factors, has a range of 2–4 million cells/ml within a 7-day period (Fig. 19). After 5 days a portion of the stock can be harvested. An equal volume of fresh seawater is added to the remaining stock and appropriate amounts of fertilizers are also supplied. A stock of Chlorella can last for a considerable period if there is good water management and contamination is minimized.
Table 13. Treatment of diseases commonly found in seabas fry and fingerlings.
| Size | Disease | Cause | Treatment |
| 3 – 8 days (0.5 cm) | Gas-buble syndrome | Unknown | Formalin 25 – 30 ppm 24 hrs. (Yongprapat, 1988) |
| 10 – 20 days (0.5–1.5 cm) | Black body syndrome | Unknown | Formalin 100 – 200 ppm
15–20 min or Tetracycline 25 ppm 24 hrs. (Yongprapat, 1988). |
| White faeces syndrome | Unkown | ||
| 2.5–8.0 cm | White spot | Cryptocaryon irritans | 1 Formalin 200 ppm, 30–60 mins, depending on the fish's endurance (Chong and Chao, 1986) |
| Ichthyopthirius multifilis | 2. Formalin 100 ppm + acriflavine 10 ppm for 1 har (Chong and Chao, 1986) | ||
| Popeye | Vibriosis | 1. Oxytetracycline 0.5 gm/kg feed for 7 days. | |
| 2. Sulphonamides or potentiated sulphonamides, 0.5 gm active in gradients per kg feed, 7 days. | |||
| 3. Chloramphenicol 0.42 gm/kg feed for 4 days. | |||
| 4. Bath in Nitrofurazone 15 ppm for at least 4 hrs, | |||
| 5. Bath in Sulphonamides 50 ppm active ingredients for at least 4 hrs (Chong & Chao, 1986). | |||
| 4.5–5.0 cm | Kidney | Vibrio sp syndrome | Ampicillin 50 – 100
ppm, 5–7 days (Yongprapat, 1988) |
| 7.5 cm | Colunaris | Flixibacter columnaris | 1. Acriflavine 3 ppm, 3 days or bath NaCl 3–5%, 3 days or Tetracycline 25 mg/kg fish *(Yongprapat, 1988) |
| finrot, tailrot | Aeromonas hyrophilia A. puctata | 2. Nitrofurazone 15 ppm, 4 hrs, or Sulphonamide 50 ppm, 2 hrs, or Neomycin sulphate 50 ppm, 2 hrs, or | |
| Myxobacter | Chloramphenicol 50 pm, 2 hrs, or Acriflavine 100 ppm, 1 min, or 100% freshwater for 1 hr (Chong & Chao, 1986). | ||
| 10.0–17.5 | Lymphocystis | Virus | unknown |
Table 14 Treatment of diseases in young and adult seabass
| Product name | Suggested uses | Dose and treatment |
| 1. Chloramphericol (Chloromycetin) | Bacteria, protozoa, and virus | Food : 50 – 100 mg/kg of fish per day for five day. |
| 2. Furazolidone (nf-180-Furoxone) | Bacteria | Food : 25–75 mg/kg of fish for 14 consecutive days |
| 3. Nitrofurozons | Antimicrobial | Food : 7.5 gm/kg of fish daily for 2 weeks. |
| 4. Oxytetracycline (Terramycine) | Antimicrobial | Food : 1.8 mg/gm of fish approximately 3% of body weight/day for 8 days. |
| 5. Sulfamethazine | Antimicrobial | Food : 100 mg/kg of fish per day for 10 – 15 days. |
| 6. Sulfaguanidine | Antimicrobial | Food : 120 mg sulfaguanidine + 250 mg sulfamerazine/kg of fish per day for 3 days followed by 80 mg sulfaguanidine plus 120 mg sulfamerazine per kg of fish for other 7 days. |
| 7. Sulfamerazine | Antimicrobial | Food : 18 gm/kg of fish per day for 14 days. |
| 8. Sulfasoxazole | Antimicrobial | Food : 200 mg/kg of fish per day for 7–10 days. |
| 9. Copper sulphate | Protozoa | Dissolve in culture medium at concentration of 1 ppm. |
The nutritional value of rotifer is greatly affected by its diet. Food for rotifer, Brachionus plicatilis, are Chlorella, Tetraselmis, yeast, bacteria, and protozoans in which fatty acids have been assimilated. Propagation of rotifer for hatchery can be done using any of three methods : thinning method practiced in large tank, in a canvas cage, and the repeated stocking method in a small tank. Only the first method is described here because it is the one most common used and practiced at the Centre.
Table 15. Ingredients of Conway medium.
| Nutrients | Concentration |
| 1. Nutrient enrichment solution for Chlorella, Tetraselmis. | |
FeCl3 6H2O | 2.60 gm |
MnCl2.4H2O | 0.72 gm |
H3BO | 67.20 gm |
EDA (Sodium salt) NaH2 PO4. 2H2O | 90.00 gm 40.00 gm |
NaNO3 | 200.00 gm |
Trace metal solution Distilled water to | 2.00 ml 2.00 l |
| One ml of enrichment solution is added to each liter of seawater. | |
| 2. Trace metal solution | |
ZnCl2 | 2.10 gm |
CoCl2. 6H O | 2.00 gm |
(NH4) 6Mo 7O24. 4H2O | 0.90 gm |
CuSO4. 5H2O | 2.00 gm |
Distilled water to | 100.00 ml |
| 3. Vitamin stock solution | |
Vitamin B 12 | 10.00 mg |
Thiamin HCL Distilled water | 200.00 mg 200.00 ml mg |
10 ml of vitamin stock solution is added to 100 liters of seawater. | |
Feeding rotifer with Chlorella combined with marine yeast may be done as following :
Starting with the culture of Chlorella, Tetraselmis and marine yeast until the density increases to 5, 3, and 1 million cells/ml respectively. On the 4th day a starter of rotifer eith a density of 10–20 cells/ml is inoculated; the rotifer will increase its density to 70–100 cells/ ml after the 10th day, and reach its peak on the 12th day, 100–1 000 cells/ml (Fig.13). To maintain the culture of the rotifer at a considerable level, the Chlorella, Tetraselmis and marine yeast are added after the 11th day. Rotifer can be partially harvested after the 10th day. Harvesting is carried out by draining the culture through a nylon net (60 u) leaving one third of the original volume to serve as a starter for the next batch. Before use, the rotifer should be enriched with cod liver oil (100ml/ton) and raw egg yolk (1 gm/ton) to increase its nutritional value.
The stock of marine yeast can be obtained from local drainage of the hatchery. Nutrients required for the marine yeast culture are 15 gm of brown sugar, 3 gm of ammonium sulphate, 1 gm of potassium phosphate dissolved in one liter of water. One mg of Hydrochloric acid is added to bring pH of the solution down to 4. Within few days, the yeast can be transferred to 10–1 bottle. Adding the same the nutrients but without hydrochloric acid in strongly aerated seawater. The yeast then is further transferred into 100–150 liter tank. After the marine yeast increases its density to 1 million cells/ml, it can be ready for feeding rotifer.
Table 16. Nutrients for 1-liter stock culture of Tetraselmis.
| Nutrients | Concentration |
| Sodium nitrate | 84.0 mg/l |
| Any one of the following : | |
Monobasic sodium phosphate | 10.0 mg/l |
Tribasic sodium phosphate | 27.6 mg/l |
Calcium phosphate | 11.2 mg/l |
| Ferric chloride | 2.9 mg/l |
| EDTA | 10.0 mg/l |
| Thiamin HCl (B1) | 0.2 ug/l |
| Biotin | 1.0 ug/l |
| Vitamin B12 | 1.0 ug/l |
| CuSO4. 5H2O | 0.02 mg/l |
| ZnSO4. 7H2O | 0.04 mg/l |
| NaMoO4. 2H2O | 0.02 mg/l |
| MnCl2. 4H2O | 0.13 mg/l |
| CoCl2. 6H2O | 3.6 mg/l |
Table 17. Nutrients for 3-liter culture of Tetraselmis.
| Nutrients | Concentration (mg/l) |
| Urea 46 | 100.00 |
| K 2 HPO4 | 10.00 |
| Fecl2 | 2.00 |
| Agrimin | 11.00 |
| EDTA | 2.00 |
| Vitamin B1 | 0.005 |
| Vitamin B12 | 0.005 |
Table 18 Nutrients for 200 - liter and 1-ton culture of Tetraselmis
| Nutrients | Concentration (gm/l) |
| KNO3 | 100 |
| Na2HPO4. 12H2O | 50 |
| CaHCO3 | 25 |
| FeCl3 | 5 |
Table 19 Nutrients for 1-ton culture of Chlorella.
| Nutrients | Concentration (gm/t) |
| Ammonium sulphate | 50 – 200 |
| Calsium phosphate | 10 – 50 |
| Urea | 5 – 25 |
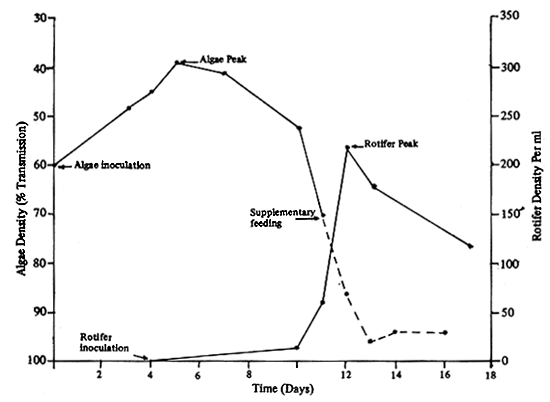
Fig. 19 Rotifer culture showing a blooming peak of rotifer after inoculation.
Table 20. Nutrients for marine yeast culture.
| Nutrients | Concentration |
| Brown sugar | 15 gm/l |
| Ammonium sulphate | 3 gm/l |
| Potassium phosphate | 1 gm/l |
| Hydrochloric acid | 1 mg/l |
Table 21. Content of W3-HUFA of the rotifer fed with different diets.
| Kinds of rotifer | w3-HUFA (%) |
| Rotifer fed on baker's yeast only | 5.9 |
| Rotifer fed on baker's yeast enriched with 8% fish liver oil | 7.0 |
| Rotifer fed on baker's yeast enriched with 15% pollock liver oil | 10.7 |
| Rotifer fed with baker's yeast enriched with 15% cuttle fish liver oil | 13.6 |
| Rotifer cultured with Chlorella | 30.0 |
| Rotifer fed on baker's yeast and secondary fed on Chlorella | |
| 3 hrs prior to administration to the fish larvae | 7.2 |
| Rotifer fed on baker's yeast and secondary cultured on Chlorella 12 hrs | |
| prior to administration to the fish larvae | 20.1 |
(Source : Foscarini, 1988)
Brine shrimp. Artemia salina, is the best live food for seabass larvae aged between 8 and 20 days. At present many brands and varities of brine shrimp cysts are on markets. Each brand contains different nutrition values, survival rates as well as prices.
If the required amount of brine shrimp is 5 nauplii/ml. For feeding the seabass larvae in a 2-t tank, the amount of brine shrimp nauplii require can be calculated as follows :
| Feeding density | 5 | nauplii/ml | |
| Hatching rate (Argentemia grade 1) | 280 000 | nauplii/gm | |
| 2t water | 2 000 000 | ml | |
| Nauplii required (2 000 000×5) | 10 000 000 | nauplii | |
| The brine shrimp cyst required | 36 | gm/2t water | |
| (10 000 000/280 000). |
24.5.1 Decapsulation
In order to improve hatching rate as well as eliminating potential diseases that might be carried with the brine shrimp cysts, the cysts should be decapsulated first before hatching. The decapsulation can be done as follows:
Place required amount of brine shrimp cysts in hatching jar (conical fiberglass container). Add 1 200 ml seawater per 100 gm of a brine shrimp cysts. Aerate for 1 hr. Add 1 000 ml NaOCL or CaO per 100 gm of brine shrimp cysts. Stir thoroughly. Then add 25 gm of bleaching powder per 100 gm of brine shrimps cysts. Continue stirring. During the process of decapsulation, the temperature might gradually increase, if necessary using ice to keep the temperature below 40 degree Celsius. After 5–8 mins, the temperature should become steady. At this stage the cyst should change color from dark brown to white or orange.
Clean the cysts in a fine meshed strainer and rinse with freshwater or seawater until the chlorine odor is removed. The cysts can be fed directly to the fish larvae and fry or put in incubation for further nauplii hatching. The cyst can be stored in concentrated brine (salinity of 300 ppt) or in refined salt (30 gm NaCl/100 gm of Artemia cyst) until needed.
Neutralize the chlorine may be required by applying a 0.05 gm of sodium thiosulphate (Na2S203.5h2O) per 100 gm Artemia cysts. Add 100 ml of water and stir for 2–5 mins The decapsulated cysts will float.
24.5.2 Hatching
To obtain the high hatching rate of the brine shrimp nauplii, the following hatching procedures are recommended :
The cyst is hatched in conical hatching tank (Fig. 20) at density of 2 gm/l. For practical reasons, natural seawater is used as hatching medium. However it has been demonstrated that at lower salinities (e.g 5 ppt), the hatching rate increases and the nauplii have a higher energy content.
Water temperature is maintained at 30 degree Celsius and pH is between 8 and 9. Continuous aeration is provided. The dissolved oxygen is maintanined close to saturation level. If necessary Na 2CO3 (1 ml of 0.5 M solution/1 medium) or CaO (65 mg/l) may be added to increase the buffer capacity. The cysts will be hatched out within 20–24hrs.
All cysts are kept in suspension. Accumulation of cysts on the bottom tank creates anaerobic zones which interrupts the cyst metabolism. To assure a maximum hatching rate, the culture is exposed to a continuous illumination of about 1 000 lux. This light intensity is attained when the hatching container is placed at about 20 cm from a florescent light tube of 60 W.
Harvest of brine shrimp nauplii can be done by stopping aeration to allow egg shells afloat. Cover the lid to allow the nauplii to concentrate at the bottom. Drain the nauplii into a strainer and rinse with seawater to remove any remaining empty egg shells. Refill the hatching container with new seawater for further hatching of the remaining cysts.
The brine shrimp cysts used at the Centre is Argetemia brand grade 1. It contained 62% protein, 22.0% 1 carbohydrate, 6.1% ash on dry weight basis. Its fatty acid profile is 5.6%, 20:5 w3-HUFA.
The freshwater Moina can be used to substitute brine shrimp for seabass aged between 15 and 30 days old. It can be produced in large quantities at low cost. The stock is also easily obtained from an aquarium shop or from local drainage. The Moina can be produced in large quantity in outdoor tank. The following procedures are recommended for mass production of Moina in 25-t outdoor concrete tank.
Clean and disinfect the culture tank as necessary. Bring the water in at 25 cm depth. Add 15 l of dried chicken manure and 5 l of rice bran. Stir the tank thoroughly. On the 4th day about 500 ml of Moina stock is added. During this period, all mosquito eggs laid along the surface of water must be removed to minimize the competition in food between mosquito larvae and young Moina. Water level of the tank is gradually increase at the rate of 10 cm per day. The amount of water added can be adjusted according to the growth of the micro-organisms and Moina in the tank. The bloom of the Moina will reach a peak at the 7th day or three days after adding the stock. A partial harvest can be made. The culture will last for three days. After the 10th day the population of the Moina will decline, and the new culture is required.
Since the Moina have a low level of w3-HUFA in its nutrition, the organism should be enriched with enrichment diets to increase its nutritional value before feeding to the seabass fry.

Fig. 20 Artemia hatching tank
Besides increasing the level of w3-HUFA in the Artemia, and rotifer by feeding the organisms with baker's yeast, cod and pollock liver oil as shown in Table 21, the Artemia, and rotifer can also be enriched with enrichment diets which are now commercially available in markets. Among of those are TOPAL, SELCO, SUPER SELCO, SELCO BASE etc. Details and method of enrichment of the selected enrichment diets are given in Appendix 3.
Inert food can also be given as a supplementary feed to reduced the operation costs or to substitute the live food organisms if there are a shortage during the operation.
Micro-encapsulated egg particles size between 150–200 u is given after Day-4 at density of 3–5 pcs/ml, three times a day at 8 00, 10 00 and 12 00 hr. After feeding with the micro-encapsulated egg, 80% of water is changed. Method of preparation of the micro encapsulated egg given in Appendix 2.
This type of inert food is now available on markets under different trade names such as Nippai artificial plankton BP, AS and artificial rotifer. It is convenience and can be used to substitute the rotifer with the similar results. The particle size suitable for seabass Day-3 larvae is 50–150 u. The rate given is 5–10 particles ml water. Feeding interval is 6–8 times/day. After feeding 20% of water is changed.
Compound feed with crude protein higher than 40 percent can be used as a feed for seabass fry after Day-20 to replace minced fish and Artemia. The compound feed available in markets are salmon starter, trout starter, and shrimp starter. At the Seafarming Development Centre, the shrimp starter of President feed was used to feed the seabass fry after Day-20 to reduce the operation costs such as the costs of Artemia, fish flesh, and to reduce the labor in preparation of feed and changing the water. The fry accept the feed nicely after 1–2 days weaning period. The compound feed is given 3 times a day at satiation. The excess feed is siphoned out after feeding. After feeding about 50% of the water is changed. The result showed that the fry fed with compound feed has a better growth and survival rate when compared with those fed with minced fish.
The seabass fry can be transported in airtight carriers with oxygen. The basic needs are plastic polyethylene size 40×60 cm, with 0.11 mm thick, insulating boxes, rubber bands and pure oxygen. The main causes for high mortality in transportation of live fish are :
A few days before transport, fish are kept in clean water in separate tanks. The fish should not be fed for several days, depending on size. The last feed for larvae should be 12 to 24 hrs before transhipment while for fish up to 3 gm body weight it is 48 hrs. Larger fish should not be fed for three days.
Weak or diseased fish have to be removed. The fry are graded according to size. Before packing, the fry is conditioned for 24 hr in plastic baskets floating in a concrete tank (Fig. 21). This procedure is necessary to allow the fish to empty their stomach, and as a part of sanitary control to prevent transporting any disease that might be carried with the fry. The fry is treated with 10 ppm acriflavine solution for 30 mins or 0.5 ppm of copper sulphate solution for 5–10 mins.
The plastic bags should be filled with three parts oxygen to one part seawater. After filling with water and fish, the plastic bags are inflated with oxygen. The oxygen level is measured by collapsing the bag to water level then placing hand down 10 inches from top and filling with oxygen until there is a feel of resistance. The top of the bag is then bent and tied with six or eight rubber bands (Fig.22)
A crushed ice and sawdust can also be used to control the water temperature in plastic bags during the transporting period. For maintaining the water temperature between 19–23 degrees Celsius the ratio of crushed ice and sawdust (by weight) is 1:2 for 4–5 hrs transporting time and 1:1 for 12–16 hrs transporting period.

Fig. 21. Conditioning of seabass fry before packing
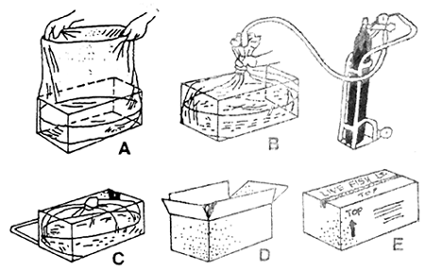
Fig. 22. Sequence of packing fish in plastic bag.
Dry ice can be also used as an effective refrigerant to maintain the temperature in the plastic bag. However, the ratio of dry ice and sawdust to maintain the water temperature between 19–23 degree celsius at the required transporting time is subjected for further studies
Density of fry per bag depends on age and size of fry, duration of transport, temperature control system, nature of container, and climate. If the temperature in the plastic bag is maintained between 19 and 23 degrees Celsius, about 500 fry size of 2–3 cm can be packed per bag with good survival rate after the 16 hr transporting period (Table 22).
Table 22. Survival rate of seabass fry packed in 40×60 cm plastic bags at different age, size and density.
| Age (days) | Size(TL) (cm) | fry/bag | water temperature(°C) | Duration (hrs) | Survival (%) |
| 7 – 15 | 0.2–0.3 | 10 000 | 19 – 23 | 16 | 90 |
| 20 – 22 | 0.5 | 5 000 | " | 16 | 90 |
| 30 | 1.0–1.5 | 1 000 | " | 16 | 90 |
| 60 | 2.0–3.0 | 5 000 | " | 16 | 90 |
(Source : Tatanon and Maneewongsa, 1982b).
Before arrival, tanks containing clean seawater should be prepared. The water should be aerated and of appropriate temperature, If possible, a separate tank should be made available for each bag of fish.
The main reason for mortalities after arrival in hasty transfer from transport water into new water. By the time of arrival, fish have become acclimatized to conditions in the bag namely high concentrations of carbon dioxide and ammonia, and pH between 5–6. These concentrations may be reduced gradually by a simple method. First open the bags and leave them in the boxes or in baskets. Then pour new water from the tanks into the bags, until the water volume is three or four times the initial quantity. This procedure should last about half an hour. The transport water must not be aerated, as this would drive out carbon dioxide, increase the pH and turn harmless ionized ammonia into poisonous unionized ammonia.
A simple device to allow the gradual changing of water is shown in Fig.23. After the fish acclimatized to the new water, the fish may be transferred into the tanks. The tanks should be covered to avoid stressing the fish and preventing them from jumping out the tank, especially at the corners. The fish should be fed the day after arrival.
All fish should be prophylactically treated against ectoparasites (0.6 mg Malachite Green per 10 liters of water) the day after arrival. Heavy bacterial and fungal infections can be treated with Tetracycline (2 gm per 100 l) and Chloramphenicol (1 gm per 100 l).
One of the most crucial problems of spawning seabass is that the eggs and sperm are not available at the same time. Sometimes viable eggs are available but sperm is not, or vice versa. The problem could be reduced if the milt are collected first and preserved at low temperature. Seabass sperm can be kept for five days in the refrigerator at a temperature of 4–8 degree Celsius. The sperm is more active if suspended in 10% of dimethyl suphoxide before chilled storage (Withler and Lim, 1982).
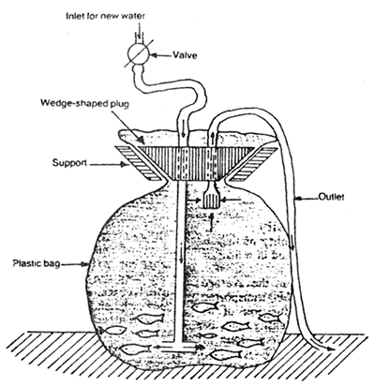
Fig. 23. A device for gradual changing of water in a transport bag (After Frose, 1986).
The long term preservation technique can be done by storing the fish sperm in liquid nitrogen at a temperature of-196 degree Celsius. To prevent cell breakdown due to extremely low temperature, a drop of dimethyl sulphoxide can be mixed with the sperms. By this method the sperm can be kept for about 2 years; and approximately 98 percent survival of sperm is obtained (Bhinyoing, 1980). The following method of freezing fish spermatozoa is modified from Harvey (1983) which have been tested successfully in Tilapia, goldfish, seabass, and grouper.
Add 1 ml methanol to 9 ml 0.9% Na Cl in tap water. Add 1.5 gm dry powdered milk and mix well. Make up fresh daily; keep powdered milk frozen until use.
Add 1 part milt to 5 parts diluent in a plastic vial. Mix well and bury in dry ice for 10 mins., then transfer to liquid nitrogen. Freeze no more than 0.5 ml (total volume) in one container. Milt frozen in liquid nitrogen will keep indefinitely.
Remove from nitrogen and agitate in a 40 degree Celsius water for 30 second or until ice just melts. Dilute 10:1 with seawater and add immediately to eggs.
Seabass hatchery operation is a new kind of business. All techniques described in the manual can be considered as pioneering and need to be applied with caution. Although there have been rapid advances in developing appropriate seabass hatchery techniques since 1970, the techniques are still far from perfect.
As with any art from, mastering the art of producing fish fry requires inherent ability, training and hard work. The required inherent ability, although hard to define, is indeed real. Some are better at rearing fish larvae and fry than others. The skill of those who are able to make decisions on day-to-day operation is probably the most critical factor in success or failure of the hatchery. The art of producing fish fry cannot be learned in any depth by reading, by discussion or by observing, but learning to handle fish fry under good supervision of experts would broaden the skill. Training could consist, in whole or in part, of gaining experience by trial and error. The hard work must be intelligently directly towards improving skill and know-know by whatever means. As in any business, success or failure will depend largely on the manager's ability to manage well.
Broadhead, G.C. 1953 Investigations of black mullet, Mugil cephalus L In North-west Florida. State Board of Conservation Technical Series No. 7, Marine Laboratory, University of Miami, Fla., 34p.
Bhinyoying, S. 1980 Sperm preservation technique, hormone bank, and fish seed production in Thailand. Asean Meeting of Technical Experts on Aquaculture, 3–4 Jan 1980 National Inland Fisheries Institute Bangkok, Thailand, Asean 80/Aquaculture 1/XI, 5 p.
Chan, W.L. 1982 Management of the nursery of seabass fry. In : Report of training course on seabass spawning and larval rearing. SCS/GEN/82/39. South China Sea Fisheries Development and Coordinating Programme, Manila, Philippines p.34–37.
Chantarasri, 1989 Hanung Santosa, Hardoto and Sumbodo Kresno Yuwono. Induced spawning and larval rearing of seabass, Lates calcarifer in captivity. INS/ 81/008/Technical Paper No.8, 13pp.
Dunstan, D.J. 1959 The barramundi in Queensland waters. Technical Paper Division of Fisheries and Oceanography CSIRO Australia, No.5, 22p.
1962 The barramundi in New Guinea waters. Papua New Guinea Agriculture Journal 15: 23–31.
FAO. FAO 1974 Species identification sheets for fishery purposes. Eastern Indian Ocean (Fishing area 57) and Western Central Pacific (Fishing area 71) Vol 1
Foscarini, 1988 Roberto. Intensive farming procedure for red sea bream (Pagrus major) in Japan. Aquaculture 72:191–246.
Franicevic, 1986 V.D. Lisac, J. Buble, Ph. Leger, and P. sorgeloos. International study on Artemia XLII. The effect on the nutrition qauality of Artemia on the growth and survival of seabass (Dicentrarchus labrax L.) larvae in a commercial hatchery. In : Proceedings of the Conference on Production in marine hatcheries. Rovinj-Zadar (Yufoslavia) 10–28 Feb., 1986, 10pp.
Frose, Rainer. 1986 How to transport live fish in plastic bags. Infofish Marketing Digest 4: 35–36.
Greenwood, P.H. 1976 A review of the family Centropomidae (Pisces Perciformes). Bulletin of the British Museum of Natural History (Zoology) 29 : 1–81.
Harvey, B. 1983 Cryopreservation of Sarotherodon mossambicus spermatozoa. Aquaculture matozoa. Aquaculture 32:313–320.
Konsutarak, P. And T. Watanabe. 1984 Notes on the development of larval and juvenile stages of seabass, Lates calcarifer. Report of Thailand and Japan Joint Costal Aquaculture Research Project No. 1 : 36–45.
Lisac, D.,V. Franicevic, Z. Vejmlka, J. Buble, Ph. Leger and P. Sorgeloos. 1986 International study on Artemia. XLIII. The effect of live food fatty acid content on growth and survival of sea bream (Sparus aurata) larvae. Paper presented at the conference Ichtyphotology in Aquaculture October 21–24, 1986, Inter-University Center, Dubrovnik, 10 pp.
Maneewongsa, S. and T.Tattanon. 1982 Nature of eggs, larvae and of the seabass. In : Report of training course on seabass spawning and larval rearing. SCS/GEN/82/39. South China Sea Fisheries Development and Coordinating Programme, Manila, Philippines p. 22–24.
Pechmanee, T., 1984 P. Ugkayanon and S. Maneewong. Growth comparison of 11–18 days old seabass larvae, Lates calcarifer, fed with birne shrimp nauplii, Artemia salina, and with rotifer, Brachionus plicatilis. Report of Thailand and Japan Joint Coastal Aquaculture Research Project. No 1 : 134–139.
Reynolds, L.F. The population dynamics of barramundi Lates Calcarifer (Pisces : Centropomidae) in Papua New Guinea. MSc Thesis, University of Papua New Guinea, Port Moresby.
Tattanon, T. and S. Maneewongsa. 1982a Larval rearing seabass. In : Report of training course on seabass spawning and larval rearing. SCS/GEN/82/39, South China Sea Fisheries Development and Coordinating Programme, Manila, Philippines p. 29–30.
Tattanon, T. and S. Maneewongsa. 1982b Distribution and transport of seabass. In : Report of training course on seabass spawning and larval rearing. SCS/GEN/82/39. South China Sea Fisheries Development and Coordinating Programme, Manila, Philippines p. 33.
Tongrawd, S. and N. Suteemeechaikune. 1983 Feeding rate of seabass larvae fed on larvae rotifer. Contribution No.8 Satul Brackishwater Fisheries Station (in Thai)
Withler, F.C and L.C. Lim 1982 Preliminary observations of chilled and deep frozen storage of grouper (Epinephelus tauvina) sperm. Aquaculture 27 389–92.
Phylum Chordata
Subphylum Vertebrata
Class Pisces
Subclass Teleostomi
Order Perciformes
Family Centropomidae
Genus Lates
Species Lates calcarifer ( Bloch)
Taxonomic description
Body elongate, compressed, with a deep caudal peduncle. Head pointed, with concave dorsal profile become convex in front of dorsal fin. Mouth large slightly oblique, Upper jaw reaching to behind eye; teeth veliform, no canines present. Lower edge of preoperculum with a strong spine; operculum with a small spine and with a serrated flap above origin of later line. Dorsal fin with 7–9 spines and 10–11 soft rays; a very deep notch almost dividing spiny from soft part of fin; pectoral fin short and rounded, several short, strong serration above its base; dorsal and anal fins both have scaly sheaths; anal fin rounded. Scales large, ctenoid (rough to touch).
Color : two phases, either olive brown above with silver sides and belly (usually juveniles) or green/blue above and silver below. No spots or bars present on fins or body.
(Source : FAO, 1974)
As mentioned in the text, many different inert food are used in rearing seabass larvae and fry. Some inert food can be prepared and used local available materials. This appendix describes method of preparation of two types of the inert food that currently use at the Centre. These tow feeds can be prepared as follows.
1. Micro-encapsulated egg particles
Crack an egg into heat resistent container. Soak 20 gm of soya been in water for 24 hrs. Wash it two-three times and blend it with a blender. Filter the soya milk through strainer of 150–200 u. Add 10 gm of soybean milk and 10 gm of fish meal per egg. Add a drop of cod liver oil or vitamins as required. Homogenize egg and soyabean and milk with a mechanical blender. Steam it for about 15 mins. A fine opalescent suspension is obtained. Particles of the required size for food can be obtained by passing through sieve of appropriate size. Wash thoroughly until the wash water is clear. Feed directly to larvae. Unused food can be stored in a tight container in refrigerator for 2–3 days.
Micro-encapsulated egg particles with 44% crude protein
| Ingredient | Quantity |
| Egg | 1 pc |
| Soy bean | 20 gm |
| Fish meal | 10 gm |
| Non-fat dry milk powder | 10 gm |
| Cod liver oil | 1 drop |
| Vitamin C | 1 tablet |
| Antibiotic | 1 capsule |
2. Minced fish
Skipjack tuna, caranx, bonito, and mackerel are the fish of choice for preparing this feed.
Fillet the fish, discarding head, bones, and viscera. Grind and liquidize the flesh in a blender. Force the flesh through the stainless steel sieves. The mesh sizes should be chosen to produce particles of size relevant to the age of the seabass fry. The fish flesh may be used directly or formed into bals of known weight for storage. It may be kept in the refrigerator for 2–3 days.
ENRICHMENT OF Artemia WITH SELCO OR SUPER SELCO
ENRICHMENT OF ROTIFER WITH SUPER SELCO
| < | Less than. |
| > | Greater than |
| μ | Micron (sometimes written as um). |
| hr | Hour. |
| 1 | Litre. |
| mm | Millimeter. |
| cm | Centimeter. |
| m | Meter. |
| m2 | Square meter. |
| m3 | Cubic meter |
| ml | Milliliter. |
| min | Minute. |
| mg | Milligramme |
| gm | Gramme |
| kg | Kilogramme |
| ppm | Parts per million. It is equivalent to 1ml/m3 1 gm/t, lug/g, 1mg/l |
| ppt | Parts per thousan (also expressed as o/oo). |
| DO2 | Dissolved oxygen. |
| TL | Total length. |
| HUFA | Highly unsaturated fatty acid. |
| HCG | Human chorionic gonadotropin. |
| IU | International unit |
| RU | Rabbit unit and Rat unit. |
| PG | Pituitary gland. |
| LENGTH: | 1 um = 0.001 mm 1 um = 0.0394 in = 0.001 m 1 cm = 0.394 in = 10 mm = 0.01 m 1 m = 3.28 ft = 1.094 yd 1 in = 25.38 mm = 2.54 cm 1 ft = 0.305 m = 12 in 1 yd = 0.915 m = 3 ft |
| WEIGHT: | 1 gm = 0.0353 oz 1 kg = 1 000 g = 2.205 lb 50 kg = 110.25 lb 1 000 kg = 1 t 1 t = 0.9842 UK t = 1.102 US t 1 oz = 28.349 g 1 lb = 16 oz = 453.59 g 1 cwta = 112 lb = 50.80 kg 1 US cwt= 100 lb = 45.36 kg 1 ta = 20 cwta = 2 240 lb 1 US t = 20 cwt = 2 000 lb 1 UK t = 1.016 t = 1.12 US t |
| VOLUME: | 1 liter = 1 000 ml = 0.220 gallona = 0.264 US gallon 1 m3 = 35.315 ft3 = 1.308 yd3 1 m3 = 1 000 liters = 219.97 gallonsa = 264.16 US gallon 1 ft3 = 0.02832 m3 = 6.229 gallonsa = 28.316 liters 1 gallona = 4.546 liters = 1.2009 US gallon 1 US gallon = 3.785 liters = 0.833 gallona 1 MGDa = 694.44 GPMa = 3.157 m3 / minute = 3 157 liters/minute |
| AREA: | 1 m2 = 10.764 ft2 = 1.196 yd2 1 ha = 10 000 m2 = 2.471 acres 1 km2 = 100 ha = 0.386 mi2 1 ft2 = 0.0929 m2 1 yd2 = 0.836 m2 1 acre = 0.405 ha 1 mi2 = 640 acres = 2.59 km2 |
| TEMPERATURE: | 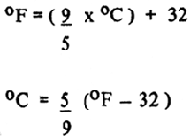 |
| PRESSURE: | 1 psi = 70.307 gm/cm2 |
a “British” or “Imperial” units