We have already referred to interaction of pH and CO2. As evident from the Henderson-Hasselbach equation, it is the change in concentration of H+ ions (coupled with HCO3- and unionized H2CO3) which decides the level of pH of water/blood. pH, as well known, is the negative logarithm of hydrogen ion concentration within the pH range of O to 14. Fish grow between 6.5 and 9, and would prefer slightly alkaline water, close to neutral pH (Fig. 13). Very low (acid) and very high (alkaline) pHs are recorded in natural waters. A point in question close to us is the acid waters of the mangrove swamp; which goes very acid and acid waters develop pH down to two, occasions; alkaline pH is more a problem under drier conditions, when dissolved solids, and hardness of waters (see below) increase.
Both these conditions can be rectified by proper management for example by liming low pH soil/waters and adding organic matter (manures) to alkaline soils. These will be discussed separately in ‘liming and fertilization’ under “Pond Culture”. The acid pH of mangrove swamps can also be corrected by other management methods such as by repeated drying and flushing with tide water (see “acid-sulfate soils” in this section).
With reference to Site Selection, the important aspect is to determine the relative costs of such recovery, if at all possible, and then decide if the site is to be selected or rejected under the specific set of conditions.
Effect of pH on biological systems are significant. In the case of fish acid pH causes more or less the same effect on blood-oxygen capacity as the increase in pCO2 (Bohr effect). We already referred to lethal and sublethal effects of pH on fish (Fig. 13).
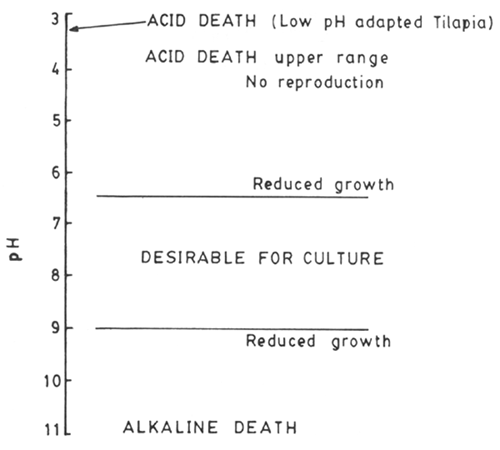
Fig. 13. pH limit of biological activities in fish. (After Boyd, 1979)
pH is often measured using an electrode which is automatically compensated for temperature change. Details of standardization and use are indicated in the practicial handout for water analysis. pH papers can only be used for rough estimations in the field.
A common problem encountered is the calculation of average pH value explained succinctly in EIFAC (1986). It is wrong to directly average pH values obtained; the correct method is to convert all the pH values to H+ ion concentrations and then average pH. To cite the example given in EIFAC (1986):
| pH | = | (H+) |
| 7.5 | = | 3.2 × 10-8 |
| 8.5 | = | 0.32 × 10-8 |
| 9.0 | = | 0.1 × 10-8 |
| 9.1 | = | 0.79 × 10-8 |
| 10.2 | = | 0.0063 × 10-8 |
| 3.7053 × 10-8/5 = 0.74 × 10-8 | ||
| (H+) | = | 7.4 × 10-9 |
| pH | = | -(log 7.4 + log 10-9) = 0.87 + (-9) |
| pH | = | 8.13 |
It is shown that the error that could be caused by using a direct mean of pH values (8.86) and the value (8.13) by reconverting average of H+ concentrations, can be serious and could be rightly judged by the differences in effect of the pH on bio-functions (lethal and sublethal) and also from the calculated values of unionised ammonia which could be estimated. For the example we have, assuming a total of 1 mg/L of total ammonia (NH3 + NH4+) at 22°C the respective unionised ammonia fractions calculated for both the mean pH values indicated would be 6% and 30% of total ammonia or 0.06 mg and 0.3 mg NH3/L i.e. a difference by a factor of 5. To take an example of lethal pH, the low pH tolerant tilapia (T. guineensis) can survive for long period at pH above 3.5, but at pH 3.2, is survival is only for few hours (Wokoma, 1986).
Most information available concerns shrimps. A low pH is especially dangerous in waters with low content of inorganic carbon. In Penaeus monodon in high concentration of inorganic carbon there was no mortality at a pH of 6.4, but growth was reduced 60%. At the same pH in inorganic carbon less than 10 mg/l both growth and survival of P. aztecus and P. merguiensis were reduced, and at a pH of 5.0 heavy mortalities occurred (Wickins, 1976).
Low water pH is caused in these ponds by acid soils - acid and potential acid sulfate soils are discussed separately. Usually the top soil is non-acid and subsoil is acidic (acid sulfate). The correction of acid sulfate soil is very laborious and costly and so it is suggested that dykes are made keeping the top soil as pond bottom. If excavated the acid sulfate subsoil exposed it would be profitable to put back the non-acid top soil. Liming also can reduce acidity. Arrangements may also be made to prevent rainwater run off from the dykes (cutting a channel through top of dyke) entering the pond. High pH also is toxic, as discussed separately.
During day time when photosynthesis is high, say around noon, CO2 removal from water is also high. Since the CO2 (acid) removal causes the bicarbonate portion in the water to be proportionately high and this causes the pH to rise. Therefore there will usually be a pH peaking during the day when the O2 evolution is high with increase in light intensity as shown by Boyd (1979) (Fig. 12). Similar results were obtained by Ali (1986) in his study of fish ponds at Aluu.