G.J. de GRAAF1, F. GALEMONI2 and B. BANZOUSSI2
UNDP/FAO Rural Fish Farming Development Project, Djoumouna (Republic of Congo)
Present address; (1) De Graaf Fisheries Consultancy, Lijnbaansgracht 14c, 1015 GN Amsterdam (The Netherlands),
(2) Ministry of Rural Development, B.P. 13279, Brazzaville, (Republic of Congo).
ABSTRACT
de Graaf, G.J., Galemoni, F. and Banzoussi, B. The artificial reproduction and fingerling production of the African catfish Clarias gariepinus (Burchell 1822) in protected and unprotected ponds.
In order to obtain an appropriate and reliable method for the mass-production of C. gariepinus fingerlings, experiments on artificial reproduction and pond rearing were carried out in the Republic of Congo in 1987–1991. Reproduction could be induced throughout the year using common carp pituitaries (3 mg/kg female). The average relative fecundity of females varied between 1.3 % ± 0.3 (S.E.M) and 14.3 % ± 1.3 (S.E.M). The average hatching percentage of the eggs varied between 28.4 % ± 4.5 (S.E.M) and 59.1 % ± 3.7 (S.E.M) respectively, in the dry and rainy season. The fluctuation of the relative fecundity as well as the fluctuation of the hatching percentage follows the seasonal fluctuation in rainfall and temperature. The use of a net cage made of mosquito netting (1 m3, 0.5 mm mesh size) and the roots of water hyacinth (E. crassipes) as an egg incubator proved to be reliable. In ponds not protected against frogs an average of 5.0 ± 2.9 (S.E.M.) fingerlings/m2/40 days cycle were obtained, whereas in ponds completely surrounded by a wall of aluminum roof plates (0.8 m high) the average production was 32.3 ± 3.3 (S.E.M.) fingerlings/m2/40 days cycle, when they are stocked with approximately 100 larvae/m2. Increasing the larval stocking density or extending the rearing period did not improve the final production. The main causes of low production in unprotected ponds are: competition for food resources due to the presence of phytophagous frog larvae and cannibalism among the fingerlings of Clarias gariepinus.
An analysis showed that the system is labour oriented, technically reliable and economic feasible when the fingerlings can be sold for US$ 0.07 a piece.
INTRODUCTION
Since the 1970's the African catfish, Clarias gariepinus, has been considered to be a fish of great promise for fish farming in Africa. Its growth rate is high, it is very resistant and appreciated in a wide number of African countries. The development of a reliable method for the production of Clarias gariepinus fingerlings is one of the priorities of aquaculture research in Africa (Anonymous, 1987a). Hormone-induced reproduction of the African catfish using deoxycorticosterone acetate (DOCA, 50 mg/kg female), human chorionic gonadotropin (HCG, 2500 I.U./kg female) and common carp pituitaries (3 mg/kg female) has been carried out successfully (Hogendoorn et al., 1975 and 1980a; Micha, 1976; Kelleher et al., 1976; El Bollock, 1976).
The main problem of fingerling production in ponds is the survival rate which is unreliable and varies between 0–60 fingerlings/m2/cycle (Micha, 1973, 1975; Hogendoorn, 1979; Hogendoorn et al., 1976; Kelleher et al., 1976). It has been suggested that the lack of appropriate feed and the presence of predators are likely causes of mortality.
Hogendoorn (1980) and Hogendoorn et al. (1981) successfully developed an intensive production system for fingerlings of the African catfish based on the use of Artemia salina nauplii and commercial trout starter as a feed. The existence of a technically feasible method and a manual in which the techniques are described (Viveen et al., 1985) does not, however guarantee successful implementation, as the impact of local socio-economic and technical conditions are often under-estimated (Anonymous, 1987b). The introduction of intensive rearing methods in the Central African Republic and in Ivory Coast encountered technical and economic problems (Janssen, 1985a, 1985b and 1985c; de Graaf, 1989).
In this study an effort was made to combine technically feasible methods with the socio-economic conditions of the Republic of Congo in order to obtain an appropriate and reliable method for the mass production of Clarias gariepinus fingerlings.
MATERIALS AND METHODS
Throughout all the experiments brood fish with a weight between 200 and 700 were used. They were kept in a pond at an average density of 1.5 fish/m2 and were fed six days a week with wheat bran at a daily ration of 5% of their total biomass and with trash fish irregularly.
ARTIFICIAL REPRODUCTION
Induced ovulation was stimulated in the females with an intra-muscular injection of acetone dried common carp pituitary material suspended in a 0.9 % NaCl solution. The collection of ovulated eggs and their fertilization was carried out using the dry-method described by Hogendoorn et al. (1980) and Woynarowich et al. (1980). Records were kept of the weight of individual females as were on its total weight of eggs produced. Utilizing the natural adhesiveness of the catfish eggs, approximately 300 g of fertilized eggs were attached to the roots of water hyacinth (Eichhornia crassipes) and placed in a cage made of mosquito netting (1 m3, mesh size 0.5 mm), floating in a concrete basin with running water (5–10 litres/minute) obtained by gravity from the river Djoumouna. The water temperature varied between 22 °C (dry season) and 28 °C (rainy season). Ten hours after hatching the water hyacinth was removed from the cage. Three days after hatching when the yolk sac had been absorbed, the larvae were harvested and counted. Hatching rate of the eggs was determined in two ways: one sample of 80 – 100 eggs were counted and attached to the water hyacinth roots (Eichhornia crassipes) and placed in a bucket containing 2 litres of water, while another sample of 80 – 100 eggs was counted and placed in a petri dish containing 15 ml of water. In both samples the total number of hatched larvae was determined 8 – 10 hours after the onset of hatching.
THE POND REARING OF FRY
Three days after hatching, the larvae of Clarias gariepinus were stocked in earthen ponds (100–150 m2, 0.8 m waterdepth) at densities varying between 7 and 200 larvae/m2. Two types of ponds were used: protected ponds, completely surrounded by a wall, 0.8 m high, made of aluminum roof plates, and unprotected ponds. All ponds were filled with water and fertilized with chicken dung (50 kg/100 m2) one week before stocking. From the day of stocking, the fish were fed 6 days a week with wheat bran at a rate of 1 kg/100 m2/day. At harvest the ponds were completely drained and the total number of fingerlings and their average weight was determined. Throughout all experiments frog larvae present in the ponds were captured and their stomach content was analyzed.
ANALYSES OF DATA
The specific growth rate of the fingerlings was calculated with the formula:
Wt = Wo * (1 + a/100)t
Wt = the weight of the fingerlings at harvest
Wo = the weight of the 3 days old stocked larvae.
a = the specific growth rate expressed in % of body weight per day
t = the nursing period, in days
The initial weight of the 3 day old larvae was not determined. It was assumed that the genetic variability is limited, and a weight of 2.3 mg (Hogendoorn, 1980) was used for all calculations.
Statistical analyses of the data were executed by using the computer programme SPSS (Statistical Package Social Science). The mean of individual values are given with the standard error of the mean (S.E.M). The seasonal fluctuation of the relative fecundity and hatching percentage was clearly evident and required no further analyses. The influence of the different incubation techniques on the hatching rates was examined on the basis of paired observation per female, using the Wilcoxon Matched-pairs signed-rank Test (Sokal and Rohlf, 1981).
In order to facilitate analyses, the results obtained from nursing in ponds were grouped. The following classification was used;
A separate-variance Student's t-test or a pooled-variance Student's t-test (depending on the equality of the variances), with a F-probability level 0.05, was used in comparing the fingerling production, survival rate, biomass, weight at harvest and specific growth rate.
RESULTS
ARTIFICIAL REPRODUCTION AND LARVAL PRODUCTION
It proved possible to reproduce Clarias gariepinus artificially throughout the year in the Republic of Congo. Figure 1 shows the relative fecundity ({weight of stripped eggs/weight female}*100) obtained from 155 females, the hatching percentage obtained from 70 females, the average monthly rainfall and the average monthly air temperature registered in Brazzaville. The last two data sets are from Deceuninck (1988). The relative fecundity varies between 1.3 % ± 0.3 (S.E.M) in August and 14.3 % ± 1.3 (S.E.M) in January and follows the seasonal fluctuations in air temperature and rainfall.
Figure 1 indicates that the quality of the eggs obtained changes during the season and the pattern also follows the seasonal fluctuation in rainfall and air temperature. Average hatching percentages of 59.1 % ± 3.7 (S.E.M.) and 28.4 % ± 4.5 (S.E.M.) were obtained during the rainy season (October-May, n=42), and during the dry season, respectively.
No significant differences (P = 0.53, n = 30) were found in using either water hyacinth or a petri dish as an egg incubator. The hatching percentages were 37.1 % ± 4.6 (S.E.M) and 34.1 % ± 4.2 (S.E.M) respectively. (Table I).
FINGERLING PRODUCTION IN PONDS
The overall results obtained in 45 rearing experiments are given in Table II and Table III. The results, after a preliminary grouping of the data, are presented in Table IV. Within the protected ponds, the fingerling production, the survival rate and the final biomass was significantly higher than in unprotected ponds. However, the mean weight of the fingerlings and their specific growth rate was significantly lower in the protected ponds. Increasing the rearing period in protected ponds results in a significant decrease in fingerling production and specific growth rate and significantly increases the final biomass and mean weight at harvest. Increasing the stocking density from ‘low’ to ‘medium’ has its effect of increasing the number of fingerlings at harvest and the growth rate, but decreases the final biomass harvested. A further increase in stocking density, from ‘medium’ to ‘high’ reduces the survival rate significantly.
Table V presents the results, after grouping the data in such a way that the presence of a protecting wall is the only variable within one class. Within the classes; Low & Short, Low & Long and Medium & Short, the results indicated that in protected ponds the fingerling production, survival rate and biomass at harvest were higher and the weight of the fingerlings and their growth rate was lower in comparison to the unprotected ponds. These results proved to be significant in the Medium & Short class only.
The specific growth rate and mean weight of the fingerlings obtained in the unprotected ponds was slightly higher (P= 0.041 and P=0.028) than in the protected ponds. This distinction becomes more visible when these two parameter are related to the duration of rearing (Figure 2). In both cases the specific growth rate decreased and the mean weight of the fingerlings increased over time. The regression describing their relationships were;
| Specific growth rate, unprotected: | Ln(y) = 5.95 - 0.77*Ln(x), R2 = 0.84, P ≤ 0.001 |
| Specific growth rate, protected: | Ln(y) = 6.06 - 0.85*Ln(x), R2 = 0.88, P ≤ 0.001 |
| Mean weight at harvest, unprotected: | Ln(y) = 2.50*Ln(x) - 7.29, R2 = 0.50, P ≤ 0.01 |
| Mean weight at harvest, protected: | Ln(y) = 1.42*Ln(x) - 4.54, R2 = 0.39, P ≤ 0.01 |
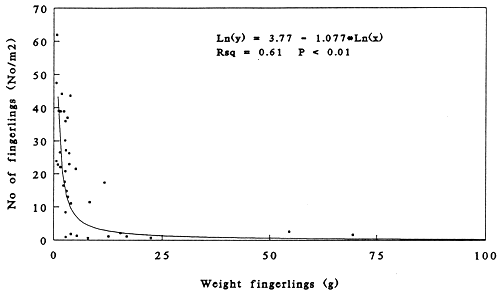
There is a significant correlation (R2= 0.61, P ≤ 0.01) between the average weight of the fingerlings at harvest and the number of fingerlings produced per m2 pond area (Figure 3).
In unprotected ponds 5–8 kg of frog larvae/100 m2 was always present. Three species were found; Rana occipitalis (Gunther 1858), Ptychadena pumilio (Boulenger 1920) and Xenopus tropicalis (Gray 1864). The contents of 126 stomachs showed only material of plant origin.
DISCUSSION
The fact that Clarias gariepinus can be reproduced artificially throughout the year under tropical conditions corresponds with the results obtained by Kelleher et al. (1976), Micha (1976) and Janssen (1985a) in the Central African Republic. The reduction of the relative fecundity of females kept in outdoor ponds during the dry season, when water temperature decreases, was also found by Janssen (1985a) in the Central African Republic and by Me Amoin (unpublished results) in Ivory Coast and by Richter et al. (1987) in Israel. The relative fecundity is less than 5% in the month of July and August, when water temperature falls below 25°C. Similar results were obtained by Richter et al. (1987) in Israel where the relative fecundity was less than 5 % during 6–7 months, when the water temperature was below 25°C. It indicates that maturation processes in Clarias gariepinus, kept in outdoor ponds, are influenced by annual changes in water temperature and that the absolute water temperature is a major factor. The seasonal fluctuation of relative fecundity could be a problem for the year round production of fingerlings. It can however be lessened by keeping the broodstock indoors, under more or less controlled conditions. In this respect, it is interesting to compare the results of some experiments in which the husbandry of the broodstock differed as follows; constant water temperature of 25°C and large fluctuation in light periodicity (Hogendoorn et al., 1980 and Richter et al., 1987), small variation in light periodicity and seasonal fluctuating water temperature of 22–29 °C (Janssen, 1985a). Keeping the brood stock indoors at 25 °C increases the relative fecundity and a high percentage of normal larvae can be obtained but the relative fecundity still follows the variation in photoperiodicity during the 18 month the experiments were carried out (Richter et al., 1987). A complete break down of the natural annual reproductive cycle was obtained by Janssen (1985a) in the Central African Republic after the broodstock was kept over one year indoors at high but still fluctuating temperatures. It indicates that also light periodicity is of importance within the natural reproductive processes of Clarias gariepinus and probably takes care of the “fine-tuning” of the processes, as it is in the long run overruled by high water temperatures. The method of keeping the broodstock indoors was used in Central Africa and Ivory Coast and encountered some serious problems. “Crack head” disease, low growth rates of the broodstock and high mortalities of larvae produced by this broodstock because of an oedemic disease were observed (Janssen, 1985a and de Graaf, 1989). “Crack head” disease has been reported earlier in the intensive rearing of catfish in Thailand and the lack of appropriate feed is the most likely cause (Anonymous, 1981). This is supported by the fact that “Crack head” disease rarely occurs in the indoor hatcheries in the Netherlands where high quality feed is used. During this study the decrease in egg production during the dry season was compensated for by the use of larger numbers of females per reproduction trial.
The use of water hyacinth as a support for eggs is comparable with the “kakabans” used for the incubation of common carp (Cyprinus carpio) eggs (Huet, 1972). The yearly average hatching percentage (35%) is low due to the influence of the dry season. Hatching percentage during the rainy season (59.1%) can be compared with results of earlier field studies (Micha, 1976; Hogendoorn, 1979 and Janssen 1985a).
The production in unprotected ponds is highly variable, as observed by others (Hogendoorn, 1979, Hogendoorn et al., 1976; Kelleher et al., 1976 and Micha, 1976). The production in protected ponds is about 8 times higher than in unprotected ponds and proved to be reliable. The fact that increasing the stocking density above 100 larvae/m2 and extending the rearing period to 50 days or more does not improve the final production of fingerlings, indicates the limits of the system. Stocking protected ponds with approximately 100 larvae/m2 and harvesting them after 35–40 days is considered to be optimal.
Three factors are probably influencing fingerling production in ponds;
A final average weight of 2.3 ± 0.3 g and a specific growth rate of 16.9 ± 0.6 %/day obtained in protected ponds with a ‘short’ rearing cycle is comparable with results obtained by Hogendoorn (1980) and Hecht et al. (1987) under controlled hatchery conditions, where the fish were fed to satiation with commercial dry feed and nauplii of Artemia salina. The difference in growth between the protected and not protected ponds indicates that maximum growth is not yet attained in protected ponds and that the maximum carrying capacity of the system has been reached. The limiting factor is most likely the lack of animal protein due to the limited availability of zooplankton.
The production cost of US$ 0.07 (16.4 FCFA) for a fingerling from the studied system (based on the 1989 price index for Republic of Congo) is less than the 29 FCFA, calculated for a hatchery in the Central African Republic, where nauplii of Artemia salina and dry feeds were used (Janssen, 1985a). A comparison of the costs of the two systems is presented in Table VI and it indicates that pond rearing is labour intensive, while hatchery rearing is capital and technology intensive. This, as well as the production price, must be taken into consideration when production units are being planned.
In conclusion, the artificial reproduction and mass rearing of Clarias gariepinus throughout the year is technically possible under tropical conditions by using ‘low cost’ adapted methods and this study does not support the conclusion of Richter et al. (1987) that broodfish should preferably be raised in a hatchery in order to ensure a continuous production of viable eggs.
REFERENCES
Anonymous, 1981. A handbook of diseases of cultured clarias (pla duk) in Thailand. National Inland Fisheries Institute, Freshwater Fisheries Division, Department of Fisheries.
Anonymous, 1987a. Les priorités pour la recherche aquicole en Afrique. Compte rendu d'un atelier à Dakar, Senegal, 1986, le Centre de Recherche pour le Développement International, MR 149f, Ottawa (Canada).
Anonymous, 1987b. Thematic evaluation of Aquaculture. UNDP/FAO/Norwegian Ministry of Development Cooperation.
Deceuninck, V., 1988. National Reviews for Aquaculture development in Africa. No 15, Congo, FAO Fisheries Circular (770.15), in french.
El Bolock, A.R., 1976. Rearing of the nile catfish, Clarias lazera to marketable size in Egyptian experimental ponds. Symp. FAO/CPCA on Aquaculture in Africa. Accra, Ghana. CIFA Techn. Pap. 4(1): 612–620.
de Graaf, G.J., 1989. La reproduction artificielle et l'alevinage de Clarias gariepinus au centre de production d'alevins de Loka en Côte-d'Ivoire, Rapport d'une mission effectuée du 28/10/1989 au 10/11/1989. Projet du développement de la pisciculture rurale, FAO/IVC/87/007. (unpublished).
Hecht, T. and Appelbaum, S., 1987. Notes on the growth of Israeli sharptooth catfish (Clarias gariepinus) during the primary nursing phase. Aquaculture, 63: 195–204.
Hecht, T. and Appelbaum, S., 1988. Observations on intraspecific aggression and coeval sibling cannibalism by larval and juvenile Clarias gariepinus (Clariidae: Pisces) under controlled conditions. J. Zool., Lond., 214: 21–44.
Hecht, T., Uys, W. and Britz, P.J., 1988. The culture of sharptooth catfish, Clarias gariepinus in southern africa. South African National Scientific Programmes Report no 153
Hogendoorn, H., 1979. Controlled propagation of the African catfish, Clarias lazera (C&V). I. Reproductive biology and field experiments. Aquaculture, 17 (4): 323–333.
Hogendoorn, H., 1980. Controlled propagation of the African catfish, Clarias lazera (C&V). III. Feeding and growth of fry. Aquaculture, 21: 233–241.
Hogendoorn, H. and Wieme, R., 1976. Preliminary results concerning the culture of Clarias lazera in Cameroon. Symp. FAO/CPCA on Aquaculture in Africa. Accra, Ghana. CIFA Techn. Pap. 4 (1): 621–624.
Hogendoorn, H., and Vismans, M.M., 1980. Controlled propagation of the African catfish, Clarias lazera (C&V). II. Artificial reproduction. Aquaculture, 21: 39–53.
Huet, M., 1972. Textbook of fish culture. Fishing Books ltd, Surrey, England.
Janssen, J., 1985a. Elevage du poisson-chat africain Clarias lazera (C&V) en République Centraficaine. I. Propagation artificielle. FAO projet GCD/CAF/007/NET. Document technique no. 20.
Janssen, J., 1985b. Elevage du poisson-chat africain Clarias lazera (C&V) en République Centraficaine. II. Alevinage en écloserie. FAO projet GCD/CAF/007/NET. Document Technique no. 21.
Janssen, J., 1985c. Elevage du poisson-chat africain Clarias lazera (C&V) en République Centraficaine. III. Alevinage et grossissement en étangs. FAO projet GCD/CAF/007/NET. Document Technique no. 22.
Kelleher, M.K. and Vincke, M., 1976. Preliminary results of studies on the survival of Clarias lazera fryin ponds. Symp. FAO/CPCA on Aquaculture in Africa. Accra, Ghana. CIFA Techn. Pap. 4 (1): 487–496.
Micha, J.C., 1976. Synthèse des essais de reproduction, d'alevinage et de production chez un silure Africain: Clarias lazera Val. Symp. FAO/CPCA on Aquaculture in Africa. Accra, Ghana. CIFA Techn. Pap. 4 (1): 450–473.
Richter, C.J.J., Viveen, W.J.A.R., Eding, E.H., Sukkel, M., Rothuis, A.J., van Hoof, M.F.P.M., van den Berg, F.G.J. and van Oordt, P.G.W.J., 1987. The significance of photoperiodicity, water temperature and an inherent rhythm for the production of viable eggs by the african catfish, Clarias gariepinus kept in subtropical ponds in israel and under Israeli and Dutch hatchery conditions. Aquaculture, 63: 169–185.
Sokal, R.R. and Rohlf, F.J., 1981. Biometry-The principles and practice of statistics in biological research. Freeman & Co., San Francisco, 859 pp.
Verreth, J., Eding, E.H., Rao, G.R.M., Huskens, F. and Segner, H., 1991. feeding practices, growth and nutritional physiology in larvae of the catfish Clarias gariepinus and Clarias batrachus. In: Lavens, P., Sorgeloos, P., Jaspers, E. and Ollevier, F. (Editors), Proceedings International Symposium on Fish and Crustacean Larviculture (in press).
Viveen, W.J.A.R., Richter, C.J.J., Van Oordt, P.G.W.J., Janssen, J.A.L. and Huisman, E.A., 1985. Practical manual for the culture of the African catfish (Clarias gariepinus). The Netherlands Ministry for Development Cooperation, Section for Research and Technology, P.O.Box 20061, 2500 EB The Hague, The Netherlands, 128 pp.
Woynarovich, E. and Horvath, L., The artificial propagation of warm-water fin fishes: A manual for extension. FAO Fish. Techn. Paper 201.
ACKNOWLEDGEMENTS
The experimental work reported in this paper was carried out as part of the the UNDP/FAO Rural Fish Farming Development Project in the Republic of Congo. The authors are indebted to Dr. E.A. Huisman and Dr. H. van Zon for their criticism of an earlier draft of this paper. The authors wish to express their gratitude to the International Foundation for Science (IFS), Sweden, for their partial financial assistance (research grant A/1091, B. Banzoussi). The views and conclusions given in this paper were expressed earlier in a final report prepared for the Rural Fish Farming Development Project. They are the responsibility of the authors only and do not imply the expression of any opinion on the part of the United Nations, the Food and Agricultural Organization or the Government of the Republic of Congo. Permission to publish this paper, given by the above organizations, is gratefully acknowledged.
An instruction video on the rearing method described in this paper has been produced by the project “Development of rural fish farming in Congo”, FAO/UNDP/RPC/88/007, and can be obtained through the Audio-Visual Department of the Food and Agriculture Organization of the United Nations, Rome, Italy.
TABLE I
The hatching percentage of eggs (mean ± S.E.M.) from Clarias gariepinus obtained through artificial reproduction
using the roots of water hyacinth (Eichhornia crassipes) or a petri dish as egg incubator.
| Hatching (%) | Number of incubations | |
| Water hyacinth | 37.1 ± 4.6 | 30 |
| Petri dish | 34.1 ± 4.2 | 30 |
Table II
The overall results of the fingerling production of Clarias gariepinus in unprotected ponds.
| No. of larvae/m2 Stocked | No. of fingerlings/m2 harvested | Weight fingerlings (g) | Duration (days) | Survival (%) | Growth rate (%/day) | Class* |
| 8 | 0.02 | 155.0 | 71 | 0.3 | 16.9 | L&L |
| 10 | 2.6 | 54.0 | 115 | 24.8 | 9.1 | L&L |
| 10 | 0.0 | -.- | 60 | 0.0 | -.- | L&L |
| 29 | 8.4 | 2.8 | 36 | 28.7 | 21.8 | L&S |
| 30 | 1.9 | 4.1 | 38 | 6.3 | 21.7 | L&S |
| 32 | 1.2 | 12.8 | 34 | 3.6 | 28.8 | L&S |
| 34 | 0.0 | -.- | 37 | 0.0 | -.- | L&S |
| 39 | 0.0 | -.- | 60 | 0.0 | -.- | L&S |
| 53 | 0.0 | -.- | 45 | 0.0 | -.- | M&S |
| 68 | 0.0 | -.- | 45 | 0.0 | -.- | M&S |
| 68 | 1.3 | 5.5 | 37 | 1.9 | 23.4 | M&S |
| 71 | 0.6 | 8.2 | 37 | 0.9 | 24.7 | M&S |
| 71 | 0.7 | 22.4 | 45 | 1.0 | 22.6 | M&S |
| 75 | 2.1 | 15.5 | 39 | 2.8 | 25.3 | M&S |
| 87 | 0.9 | 2.9 | 37 | 1.1 | 21.2 | M&S |
| 100 | 27.1 | 2.9 | 45 | 27.2 | 17.1 | M&S |
| 100 | 26.5 | 1.4 | 45 | 26.5 | 15.3 | M&S |
| 100 | 0.0 | -.- | 45 | 0.0 | -.- | M&S |
| 100 | 1.2 | 16.9 | 44 | 1.2 | 22.4 | M&S |
| 100 | 0.0 | -.- | 37 | 0.0 | -.- | M&S |
| 200 | 1.7 | 69.5 | 104 | 0.9 | 10.4 | H&L |
* Classification: Density & Duration
TABLE III
The overall results of the fingerling production of Clarias gariepinus in protected ponds
| No. of larvae/m2 Stocked | No. of fingerlings/m2 harvested | Weight fingerlings (g) | Duration (days) | Survival (%) | Growth rate (%/day) | Class* |
| 19 | 11.4 | 8.5 | 107 | 60.3 | 7.9 | L&L |
| 34 | 22.8 | 2.8 | 39 | 60.3 | 19.9 | L&S |
| 44 | 17.4 | 11.9 | 129 | 39.9 | 6.8 | L&L |
| 68 | 22.8 | 0.9 | 39 | 33.7 | 16.6 | M&S |
| 68 | 35.9 | 2.8 | 44 | 53.0 | 17.4 | M&S |
| 68 | 11.1 | 4.1 | 38 | 16.3 | 21.8 | M&S |
| 68 | 47.4 | 0.6 | 36 | 69.5 | 16.5 | M&S |
| 69 | 17.6 | 2.6 | 36 | 25.6 | 21.5 | M&S |
| 69 | 30.1 | 2.7 | 36 | 43.7 | 21.7 | M&S |
| 69 | 26.2 | 3.7 | 38 | 38.0 | 21.4 | M&S |
| 69 | 16.4 | 2.3 | 44 | 23.8 | 17.0 | M&S |
| 70 | 43.7 | 3.9 | 37 | 62.8 | 22.2 | M&S |
| 72 | 14.8 | 3.1 | 39 | 20.5 | 20.2 | M&S |
| 75 | 23.8 | 0.6 | 43 | 33.3 | 13.8 | M&S |
| 75 | 37.0 | 3.2 | 37 | 49.2 | 21.6 | M&S |
| 81 | 61.9 | 0.7 | 40 | 75.9 | 15.3 | M&S |
| 100 | 44.2 | 1.9 | 46 | 44.2 | 15.7 | M&S |
| 100 | 38.9 | 1.6 | 40 | 38.9 | 17.7 | M&S |
| 100 | 38.9 | 2.4 | 45 | 38.9 | 16.7 | M&S |
| 100 | 39.1 | 1.1 | 35 | 39.1 | 19.2 | M&S |
| 100 | 21.5 | 5.2 | 59 | 21.5 | 13.9 | M&L |
| 116 | 13.0 | 3.4 | 72 | 11.2 | 10.6 | H&L |
| 138 | 22.0 | 1.5 | 36 | 15.9 | 19.7 | H&S |
| 146 | 22.9 | 3.7 | 46 | 15.7 | 17.4 | H&S |
* Classification: Density & Duration
Table IV
Fingerling production, survival rate, mean weight at harvest, total biomass harvested and growth rate of
C. gariepinus obtained from protected or unprotected nursery ponds, stocked at low, medium or high larval
densities with a short or long rearing period. Values are means ± S.E.M. Data are compared among one
class difference only.
| Class | Fingerlings (no/m2) | Survival (%) | Weight at harvest (g) | Biomass (kg/ha) | Growth rate (%/day) | N |
| protected | 28.3±2.7*** | 38.7±3.7*** | 3.1±0.5 | 735±95** | 17.7±0.9 | 24 |
| not protected | 3.6±1.7 | 6.0±2.7 | 26.74±11.2* | 359±117 | 20.1±1.5* | 2 |
| protected & short | 30.7±2.9** | 39.9±3.9 | 2.3±0.3 | 652±85 | 18.7±0.5*** | 20 |
| protected & long | 15.8±2.3 | 33.2±10.7 | 7.2±1.8* | 1149±339* | 9.8±1.6 | 4 |
| protected & low | 16.5±2.7 | 53.5±6.7 | 7.7±2.6 | 1203±448* | 11.6±4.1 | 3 |
| protected & medium | 31.7±3.7* | 40.4±3.9 | 2.4±0.3 | 689±96 | 18.4±0.7** | 18 |
| protected & medium | 31.7±3.7 | 40.4±3.9*** | 2.4±0.3 | 689±96 | 18.4±0.7 | 18 |
| protected & high | 19.3±3.2 | 14.3±1.5 | 2.8±0.6 | 540±157 | 15.9±2.7 | 3 |
* P ≤ 0.05
** P ≤ 0.01
*** P ≤ 0.001
Shelton, W.L., Hopkins, K.D. and Jensen, G.L., 1978. Use of hormones to produce monosex tilapia for aquaculture. In: R.O. Smitherman, W.L. Shelton and J.H. Grover (Eds.), Culture of Exotic Fishes Symposium Proceedings. Fish Culture Section, American Fisheries Society, Auburn, AL, pp 10–33.
Sokal, R.R. and Rohlf, F.J., 1981. Biometry, The principles and practice of statistics in biological research, second edition, Freeman and Co., New York, 859 pp.
Teugels, G.G., 1984. The nomenclature of African Clarias species used in aquaculture. Aquaculture, 38: 373–374.
Verani, J.R., Marins, M.A., da Silva, A.B. and Sobrinho, A.C., 1983. Population control in intensive fish culture associating Oreochromis (Sarotherodon) niloticus with the natural predator Cichla ocellaris, a quantitative analysis. In: L. Fishelson and Z. Yaron (Editors) Proc. Int. Symp. On Tilapia aquaculture, 1983, Nazareth, Israel, pp 580–587.
Winberg, G.G., 1956. rate of metabolism and food requirements of fishers. Beloruss State Univ., Minsk. (Fish. Res. Board Can., Transl. Ser. no 194 (1960).
Table V
Fingerling production, survival rate, mean weight at harvest, total biomass harvested and growth rate of
C. gariepinus obtained from protected or unprotected nursery ponds, stocked at low, medium or high larval
densities with a short or long rearing period. Values are means ± S.E.M. Within each class the protected
and unprotected ponds were compared
| Classnote | Fingerlings (no/m2) | Survival (%) | Weight at harvest (g) | Biomass (kg/ha) | Growth rate (%/day) | N |
| Low & Short & Not protected | 2.8±1.8 | 9.6±6.4 | 6.5±3.1 | 153±46 | 22.3±2.2 | 4 |
| Low & Short & Protected | 20.7 | 60.2 | 2.7 | 571 | 18.3 | 1 |
| Low & Long & Not Protected | 0.6±0.6 | 6.3±6.2 | 104.8±50.2 | 712±681 | 12.3±3.7 | 4 |
| Low & Long & Protected | 14.3±3.0** | 50.1±10.2** | 10.2±1.7 | 1519±550 | 6.9±0.5 | 2 |
| Medium & Short & Not protected | 5.0±2.9 | 5.2±2.9 | 9.4±2.7* | 247±88 | 19.9±1.2** | 12 |
| Medium & Short & Protected | 32.3±3.3*** | 41.5±4.0*** | 2.3±0.3 | 664±98** | 16.9±0.6 | 17 |
| Medium & Long & Not protected | n.a | n.a | n.a | n.a | n.a | 0 |
| Medium & Long & Protected | 21.5 | 21.5 | 5.2 | 1118 | 12.9 | 1 |
| High & Short & Not protected | n.a | n.a | n.a | n.a | n.a | 0 |
| High & Short & Protected | 22.4±0.4 | 15.8±0.1 | 2.6±1.1 | 589±259 | 16.9±1.3 | 2 |
| High & Long & Not protected | 1.6 | 0.8 | 3.4 | 1160 | 9.8 | 1 |
| High & Long & Protected | 13.0 | 11.2 | 69.5 | 442 | 9.8 | 1 |
note Classification: Density
& Duration & Protection
* P ≤ 0.05
** P ≤ 0.01
*** P ≤ 0.001
TABLE VI.
Division of costs as percentage of the total production costs of two Clarias gariepinus fingerling
productions systems in Africa: Pond rearing (this study) and hatchery rearing using nauplii of Artemia salina
and composed feeds (Janssen, 1985b).
| Costs of pond rearing (% of total costs) | Costs of hatchery rearing (% of total costs) | |
| Man-power | 68.0 | 16.2 |
| Depreciation of investment | 6.1 | 56.2 |
| Operating costs | 25.9 | 27.6 |
Figure 1. The relative fecundity (% of total body weight), hatching percentage (% of total eggs) of Clarias gariepinus, average monthly rainfall* (cm) and average air-temperature* (°C) measured in Brazzaville, bars indicate S.E.M.
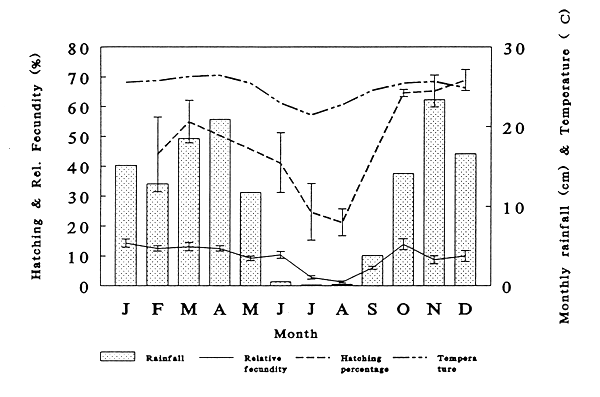
Figure 2. The mean weight at harvest (g) and specific growth rate (% of body weight/day) of Clarias gariepinus after the start of exogenous feeding in protected and unprotected ponds.
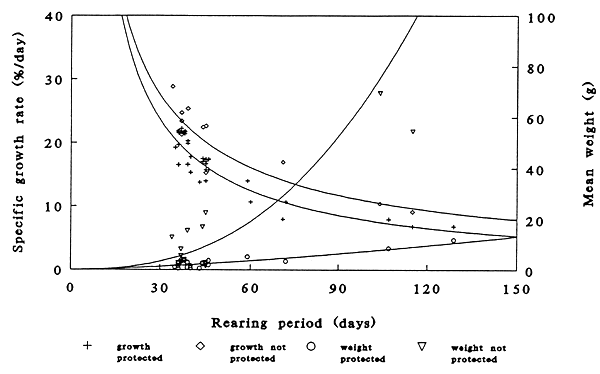
Figure 3. Relation between weight at harvest (g) and the number of Clarias gariepinus fingerlings harvested from protected and unprotected ponds (no/m2/cycle).