I. A BIOLOGICAL ANALYSES.
G.J. de GRAAF1,2 , F. GALEMONI3 and B. BANZOUSSI3
UNDP/FAO Rural Fish Farming Development Project, B.P 972 Brazzaville (Republic of Congo)
Present address; (1) NEFISCO, Lijnbaansgracht 14 c, 1015 GN Amsterdam (The Netherlands), (2) Department of Fish Culture and Fisheries, Agriculture University, P.O. Box 338, 6700 AH Wageningen (The Netherlands), (3) Ministry of Rural Development, B.P. 13279, Brazzaville, (Republic of Congo).
ABSTRACT
de Graaf, G.J., Galemoni, F. and Banzoussi, B. Successful recruitment control of Nile tilapia, Oreochromis niloticus, by the African catfish, Clarias gariepinus (Burchell 1822) and the African snakehead, Ophiocephalus obscuris. I. A biological analyses.
Large Clarias gariepinus (6.8 g≤ weight ≤ 130 g) and large Ophiocephalus obscuris (75 g ≤ weight ≤ 206 g) completely controlled the recruitment of Oreochromis niloticus at stocking densities of respectively 8,300 catfish/ha or 725 snakehead/ha. The difference in predation efficiency between the two species was related to their feeding strategies; omnivorous vs piscivorous and the mode of predation; tactile vs visual. The tactile mode of predation of catfish probably causes that a threshold stocking density of 2,000 catfish/ha is needed before recruitment control starts. The elimination of the fingerlings of Nile tilapia increased the specific growth rate of male and female Nile tilapia significantly (P ≤ 0.05) with both predator types but reduced the total yearly net production in case of predation with snakehead (P≤ 0.05). Analyses of the results indicated that food shortage is a limiting factor in the system. This limitation can be removed by increasing the feed supply directly or by increasing the feed supply indirectly through the elimination of the Nile tilapia fingerlings. These results leaded to the conclusion that “stunting” of somatic growth in Nile tilapia is probably mainly related to husbandry techniques used.
Small catfish (weight ≤ 3.65 g) and small snakehead (weight ≤ 2 g) were not able to control the recruitment completely and at harvest 3.7 % and 8.9 % of fingerlings (of total weight) was persisting for respectively catfish and snakehead. The incompleteness of the recruitment control is mainly related to the later onset of piscivorous feeding and the low survival rates of the stocked predators. The large size of the used ponds in combination with an improper feeding technique reduced the survival rate of small catfish to an average of 33.5±6.8% (±S.E.M.). In case of small snakehead the major cause of the low survival rate (24.5±10.2%) was the small size of the stocked snakeheads and predation by the larger stocked Nile tilapia.
A comparison of different methods used in the analyses of recruitment control experiments indicated that a simple predator-prey relation, expressed in numbers per ha, effectively predicts the percentage of fingerlings of Nile tilapia at harvest.
INTRODUCTION
One of the major problems in pond rearing of Oreochromis niloticus (syn. Tilapia nilotica) is its excessive recruitment. At harvest a part of the biomass consists of “low valued” fingerlings. This part may reach percentages varying from 28 – 70 % of the total harvest (McGinty, 1985, Ruwet et al. 1976, Lovshin et al. 1990 and Bardach et al. 1972).
Monosex culture of males has been used in order to overcome this problem. The needed all-male fingerlings are obtained through: manual sexing (Shell, 1968), through hybridization (Lovshin et al, 1990, Pruginin, 1967) or hormone induced sex reversal (Eckstein et al., 1965, Guerrero, 1975, Shelton et al., 1978). Major constraints for the successful use of monosex culture in rural area's are: the relatively large size (35 g) of the fingerlings needed for successful manual sexing, the difficulty to maintain pure strains of parent stock for hybridization and the availability or production of “sex-reversal” feed.
The introduction of a predator fish can control the recruitment and the following species were used successfully in combination with Oreochromis niloticus; Micropterus salmoides (McGinty, 1985), Lates niloticus (Bedawi, 1985; Gamal, 1992, Lazard, 1980), Ophiocephalus striatus (Hopkins et al., 1982), Hemichromis fasciatus (Lazard, 1980), Cichla ocellaris (McGinty, 1983; Verani et al., 1983) and Clarias gariepinus (Janssen, 1985, Lazard 1980). The efficiency of a predator is determined by it capacity to prey upon the larval Nile tilapia and piscivorous species as Ophiocephalus striatus require lower stocking densities in order to eliminate all fingerlings, as compared to the more omnivorous Clarias gariepinus (Hopkins et al., 1982; Janssen, 1985). Results of these more or less “artificial” prey-predator systems have been analyzed mathematically by McGinty (1983), Verani (1983) and Hopkins et al. (1983). The latter authors introduced a multi-linear regression model in order to predict the extend of recruitment control in Tilapia/Predator rearing systems.
The present study investigated the efficiency of Ophiocephalus obscuris and Clarias gariepinus in controlling the recruitment of Oreochromis niloticus in large scale poly-culture in the Republic of Congo (Brazzaville) and covers the biological aspects of the results. In a second paper the economic aspects of the results will be presented.
MATERIALS AND METHODS
Experimental procedures
All experiments were carried out between 1986–1990 at the National Fish Culture Station, Djoumouna, (Brazzaville, lat. 4o15'S; long. 15o15'E), Republic of Congo. Grow-out ponds with an average water depth of 1 m and surface areas between 0.04–1.2 ha were stocked with fingerlings of O. niloticus (15–30 g) in combination with either C. gariepinus (1.5–130 g) or O. obscuris (0.5–206 g). The stocking rate of O. niloticus remained more or less fixed at 20,000–22,000/ha and predator stocking rates varied between 0–20,000/ha for C. gariepinus and 0–4,000/ha for O. obscuris.
The fingerlings of O. niloticus were a hybrid between a stock of un-traceable origin, present in the Congo from the early 50's and a “Red-Benin” stock imported from Ivory Coast in the early 80's. The fingerlings of C. gariepinus were produced through artificial reproduction and nursed in protected ponds as described by de Graaf et al. (1994). Fingerlings of O. obscuris were purchased at the fish market of Brazzaville. Large C. gariepinus and large O. obscuris were obtained from the harvest of preceding poly-culture experiments
The fish were fed six days per week with wheat bran at a daily ratio of 4 % – 11 % of the total biomass of O. niloticus or the combined total biomass of O. niloticus and C. gariepinus in case of poly-culture with C. gariepinus. All ponds were sampled monthly with a cast net and individual weight of males, females and fingerlings of O. niloticus were determined in order to determine the growth rates and in order to adjust the feeding levels for the next month. In the case of poly-culture Tilapia-Clarias it was necessary to assume that the weight of Clarias was equal to the weight of the stocked Nile tilapia as the catfish were rarely caught during the monthly sampling. The biomass of O. obscuris was not taken into consideration for the calculation/adjustment of feeding rates as this species was considered to be piscivorous (Adebesi, 1981).
All experiments were carried out over a period of four years during which the overall performance of the fish culture station improved. During the first two years of the experiments feeding rates (“old rate”) differed, due to budgetary restrictions, from feeding rates (“new rate”) used during the last two years of the experiments. The daily feeding ratio was related to the size of O. niloticus and the “old” and “new” rates are presented in Table 1.
From 46 ponds the sex ratio of O. niloticus at stocking was determined by dissection and subsequent observation of the gonads of 100–200 fingerlings per pond.
From all ponds at harvest the total weight of O. niloticus and the predator was determined and 300–1,000 specimen were sampled at random (sample size depended on pond size), weighed individually and classified as: male, female, fingerling or predator.
TABLE 1
The “old” and “new” feeding rate as used at the fish culture station of Djoumouna during the period 1986-1990
| Average weight fish (g) | Daily ratio (% of total biomass) | |
| Old ratio | New ratio | |
| 0 – 25 | 6 | 11 |
| 25 – 50 | 6 | 10 |
| 50 – 100 | 5 | 9 |
| 100 – 150 | 4 | 7 |
| 150 – 200 | 4 | 6 |
Analyses of data
All experiments were carried out at a “production” station under rehabilitation and not at an established “research” station and consequently several practical problems were encountered during the experiments. Therefore results from ponds which were influenced by calamities originating from “natural” or “human” origin, as broken dikes, contamination with wild fish, especially with Hemichromis fasciatus, and un-authorized harvesting of fish, were excluded from the data set.
The results of all experiments were divided in ten groups, taking into account: the feeding level; “old” vs “new”, the predator type: no predator, C. gariepinus as predator, O. obscuris as predator, and the size of the predator at the moment of stocking; fingerling vs large fish. For semantic reasons the predator-fingerlings are further called “small” predators and the division among the size class of the predator was as follows;
| • | Small predator; | C. gariepinus ≤ 3.65 g |
| O. obscuris ≤ 2 g | ||
| • | Large predator; | 6.8 g ≤ C. gariepinus ≤ 130 g |
| 75 ≤ O. obscuris ≤ 206 g |
The specific growth rate of male and female O. niloticus was calculated with the formula from Huisman (1976).
Daily mortality of recruits/fingerlings (% Md) was calculated with the formula from Pauly (1982);
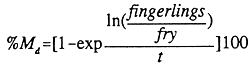
| t | = | The number of days during which the new born larvae of O. niloticus were susceptible to predation. This equals the number of rearing days minus the number of days the female parent stock of O. niloticus needed to reach 30 gramme, which is the average weight at first spawning of the used Nile tilapia stock (de Graaf et al., in press). The number of days between stocking and the moment the females attain a weight of 30 gramme was calculated with the specific growth rates as obtained under the different feeding levels. |
| fingerlings | = | The number of fingerlings at harvest was calculated by dividing the total weight of fingerlings harvested by the average weight as obtained in the sample. |
| fry | = | The number of fry born during the rearing period, which was calculated as follows; the used stock of O. niloticus becomes mature at 30 g and has a fecundity of 7.2 eggs/gramme of female, which gives 5.6 fry/gramme of female or 168 fry per female of 30 gramme using a hatching rate at first spawning of 78.3 % (Siraj et al., 1983). This number is multiplied with the number of stocked females. A sex ratio of 1:1 has been assumed for all ponds where the actual sex ratio was not determined at stocking. |
The percentage of fingerlings at harvest is always calculated on a weight basis.
The number of predators stocked per female of O. niloticus was calculated only for those experiments where the sex ratio of the fingerlings of O. niloticus was determined prior to stocking.
Statistical analyses of the data was executed with the computer programme SPSS and probability levels and correlation coefficients are given in absolutes values as calculated by this programme, unless the parameters were grouped.
The mean of all individual values are presented with the standard error of the mean (S.E.M).
Differences in net yearly production, rearing period and the weight of Nile tilapia at harvest were tested with a separate-variance Student't-test or a pooled-variance Student't-test with a F-probability level of 0.05
The influence of the predator density, the percentage of stocked females of O. niloticus, and the stocking weight of O. niloticus, on the percentage of fingerlings at harvest was evaluated by multiple linear regression analysis according to the model:
yi = b0 + b1x1 + b2x2 + b3x3 + ei
where yi = the percentage of fingerlings at harvest
b0 = the intercept
x1 = the stocked predator density (no/ha)
x2 = the percentage of stocked female Nile tilapia (%)
x3 = the stocking weight of Nile tilapia (g)
b1 to b3 = the regression coefficients
ei = error term
The regression was performed stepwise, disregarding the independent F-values of each variable. The Part correlation coefficient and F-value for change were calculated for each independent variable after it was entered in the model.
Differences in growth rates were analyzed with the Chow-test for structural changes (Greene, 1990) which compares the intercept and slope of the regression lines after logarithmic transformation of the data.
All curves were fitted with the computer programme Slidewrite 5 and significance of correlation was tested with a Spearman rank correlation test (Sokal et al., 1981). Cumulative fits, with a complementary gaussian error function of n, were selected in those cases where it was expected that within the curves an y-asymptote existed (daily mortality and the percentage of predators at harvest for O. obscuris).
RESULTS
Over the entire research period, 92 experiments were carried out, corresponding with an overall production area of 23 ha and in a total production of 79 ton of O. niloticus, 3.4 ton of C. gariepinus, 0.8 ton of O. obscuris. The mean production data of all experiments are presented in Table 2 and 3.
The effect of feeding level on production parameters
Using the higher “new” feeding level (Table 1) in experiments without a predator raised the standing stock at harvest, the net production and the final weight of the male Nile tilapia with 35–45 % (P ≤ 0.05), if compared with the “old” feeding level. The percentage of fingerlings harvested remained stable at 23–25 % of the total harvested biomass and was not influenced (P ≥ 0.991) by a higher feeding level.
The effect of a higher feeding level was also found in the experiments with “large” C. gariepinus and “large” O. obscuris. The higher “new” feeding level increased the net production (P ≤ 0.05) and decreased the rearing period (P ≤ 0.05), while at the same time the weight of the harvested Nile tilapia (males and females) remained unaffected (P ≥ 0.21).
The effect of a predators on production parameters
At both feeding levels the following results were found, if the mean values from polyculture with a predator were compared with the mean values of rearing experiments without a predator.
Poly-culture with “large” C. gariepinus resulted in a significantly higher final weight of males, females and fingerlings of O. niloticus (P ≤ 0.05), in a significant shorter rearing period (P ≤ 0.05), while the standing stock at harvest and the net production did not change significantly (P ≥ 0.35).
In poly-culture with “small” C. gariepinus it was found that the standing stock at harvest (P ≤ 0.05) and the rearing period (P ≤ 0.01) decreased significantly and that the weight of the fingerlings increased significantly (P ≤ 0.01).
The poly-culture with “large” O. obscuris resulted in a shorter rearing period (P ≤ 0.05) or in a higher final weight of the males and females of O. niloticus (P ≤ 0.05), in a lower standing stock at harvest (P ≤ 0.05) and in a lower net yearly production (P ≤ 0.05).
In the case of poly-culture with “small” O. obscuris it was found that the final weight of the males of O. niloticus was lower (P ≤ 0.05).
TABLE 2
Mean production data (±S.E.M.) for the rearing of O. niloticus in combination with small or big predators at “old” feeding level
| PRODUCTION PARAMETERS | NO PREDATOR | “LARGE” C. gariepinus1 6.8 g≤ weight ≤ 130 g | “LARGE” O. obscuris1 75 g ≤ weight ≤ 206 g |
| Stocking weight O. niloticus (g) | 19.9±1.5 | 23.8±4.9 | 23±8.2 |
| % males stocked | 54.8±2.6 | 59.9±7.3 | 50.8±0.8 |
| % females stocked | 45.2±2.6 | 39.3±7.3 | 49.2±0.8 |
| Rearing period (days) | 219±10 | 213±13 | 242±24 |
| Standing stock at harvest (kg/ha) | 3380±297 | 3891±421 | 2735±408 |
| Net production (kg/ha/year) | 5062±535 | 5665±944 | 3555±533* |
| Weight male Tilapia at harvest (g) | 142±9 | 193±31* | 182±23* |
| Weight female Tilapia at harvest (g) | 60±5 | 80±11* | 90±19* |
| Weight Tilapia fingerlings at harvest (g) | 16.5±2.2 | 37.5±11.8 | 19.1±7.5 |
| Weight predator at harvest (g) | -.- | 208±45 | 654±124 |
| Survival rate predator (%) | -.- | 62.1±8.4 | 75.9±13.6 |
| % males at harvest (% of total biomass) | 53.2±3.6 | 56.2±5.1 | 61.1±2.7 |
| % of females at harvest (% of total biomass) | 19.6±3.0 | 16.4±2.6 | 25.7±4.5 |
| % of fingerlings at harvest (% of total biomass) | 25.3±2.4 | 3.5±1.6*** | 4.1±3.0*** |
| % of predator at harvest (% of total biomass) | -.- | 23.9±5.8 | 8.9±2.6 |
1 Compared with no predator,
(* P≤0.05,
** P≤0.01,
*** P≤0.001).
TABLE 3
Mean production data (± S.E.M.) for the rearing of O. niloticus in combination with small or big predators at “new” feeding level.
| PRODUCTION PARAMETERS | NO PREDATOR1 | “SMALL” C. gariepinus2 weight≤3.65 g | “LARGE” C. gariepinus2 6.8 g≤weight≤130 g | “SMALL” O. obscuris2 weight≤2 g | “LARGE” O. obscuris2 75 g≤weight≤206 |
| Stocking weight O. niloticus (g) | 20.3±5.5 | 25.6±2.5 | 22.8±3.7 | 12.4±1.1 | 27.7±2.6 |
| % males stocked | 58.8±8.3 | 62.8±4.8 | 53.8±2.3 | 51.9±3.4 | 68.1±3.9 |
| % females stocked | 41.6±8.3 | 35.8±4.8 | 46.7±2.3 | 46.6±3.4 | 31.8±3.9 |
| Rearing period (days) | 219±8 | 182±9** | 176±16* | 224±11 | 180±10* |
| Standing stock at harvest (kg/ha) | 4814±647* | 3593±357* | 4953±629 | 4282±489 | 3416±232* |
| Net production (kg/ha/year) | 7455±864* | 6584±635 | 8189±847 | 6438±588 | 5962±526 |
| Weight male Tilapia at harvest (g) | 192±7** | 209±17 | 225±8** | 151±12* | 192±6 |
| Weight female Tilapia at harvest (g) | 69±4 | 79±6 | 92±9* | 67±2 | 85±5* |
| Weight Tilapia fingerlings at harvest (g) | 14.6±1.5 | 31.9±3.7** | 34.1±6.6* | 22.7±7.8 | 26.9±7.3 |
| Weight predator at harvest (g) | -.- | 275±54 | 201±19 | 204±21 | 491±73 |
| Survival rate predator (%) | -.- | 33.5±6.8 | 70.5±9.7 | 24.5±10.2 | 60.3±7.1 |
| % males at harvest (% of total biomass) | 65.8±5.4* | 64.1±5.6 | 61.3±6.7 | 55.1±2.3* | 76.1±4.1 |
| % of females at harvest (% of total biomass) | 11.1±2.9* | 11.8±1.8 | 19.4±2.7 | 16.2±4.2 | 11.1±2.8 |
| % of fingerlings at harvest (% of total biomass) | 23.1±3.2 | 4.2±0.9*** | 4.5±1.6*** | 23.3±6.3 | 7.1±1.8** |
| % of predator at harvest (% of total biomass) | -.- | 13.3±2.9 | 10.5±3.7 | 4.0±1.8 | 3.6±0.7 |
1 compared with “old” feeding
level (Table 2),
2 compared with no predator,
(* P≤0.05,
** P≤0.01,
*** P≤0.001).
The effect of predator on the percentage of Nile tilapia fingerlings at harvest
If the data are compared irrespectively of the predator density, the introduction of a predator reduced the average percentage of fingerlings at harvest significantly to a mean level of approximately 5% of the total biomass (P ≤ 0.01). The case of small O. obscuris was an exception as the average percentage of fingerlings did not differ (P ≥ 0.76) from rearing experiments without a predator.
In the experiments with “small” catfish, “large” catfish and “large” snakehead, the variation in percentage of fingerlings of O. niloticus at harvest was mainly related to the stocking density of the predator. Stocking weight and the percentage of stocked females of O. niloticus did not contribute significantly to the correlation coefficient (Table 4). In experiments with “small” snakeheads the percentage of fingerlings of O. niloticus at harvest was not significantly related to the stocking density of the predator but it was significantly related to the number of predator harvested per ha (Table 4).
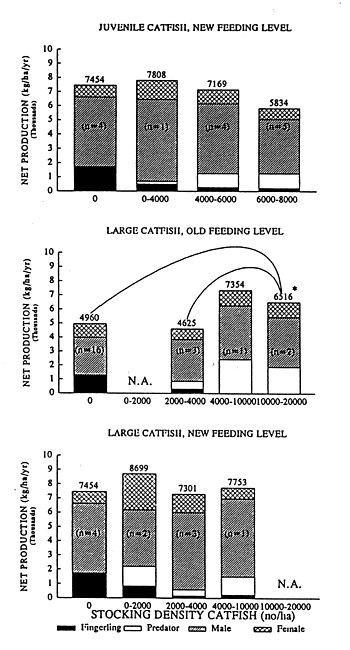
Fig. 1. The relation between the stocking density of C. gariepinus and the net production and the partition of the net production in the different categories of fish for poly-culture with O. niloticus under the “old” or “new” feeding regime. (* P≤0.05 number of experiments between brackets)
TABLE 4
Multiple linear regression of experiments with different types of predators. Three independent variables:
stocking density of predator, percentage of stocked female O. niloticus and the stocking weight of O. niloticus
are related to the dependent variable: the percentage of fingerling at harvest. In the last row of the table the
linear regression between the percentage of fingerlings at harvest and the predator density at harvest is
presented. Part R is the part correlation coefficient, df the degrees of freedom and R is the correlation
coefficient.
| INDEPENDENT VARIABLES | EXPERIMENTAL GROUPS | |||
| “SMALL” C. gariepinus | “LARGE” C. gariepinus | “SMALL” O. obscuris | “LARGE” O. obscuris | |
| Predator density stocked (no/ha) | ||||
| Part R | -0.715*** | -0.517** | -0.108 | -0.748*** |
| F-change | 33.9*** | 10.09** | 0.277 | 39.4*** |
| df | 27 | 28 | 22 | 34 |
| Percentage of ♀ ♀ O. niloticus stocked | ||||
| Part R | 0.041 | -0.023 | 0.088 | -0.083 |
| F-change | 0.116 | 0.021 | 0.151 | 0.217 |
| df | 27 | 28 | 22 | 34 |
| Stocking weight O. niloticus (g) | ||||
| Part R | -0.008 | -0.198 | 0.130 | -0.149 |
| F-change | 0.004 | 1.482 | 0.328 | 0.710 |
| df | 27 | 28 | 22 | 34 |
| Predator density at harvest (no/ha) | ||||
| R | -0.660*** | -0.364** | -0.421* | -0.650*** |
| F | 20.09*** | 15.4** | 4.538* | 24.2 |
| df | 27 | 28 | 22 | 344 |
(* P≤0.05,
** P≤0.01,
*** P≤0.001)
The relation between the stocking density of the predator and the net production and the partition of the net production in the different categories of fish are presented in Figure 1 and 2. “Large” catfish were able to control the recruitment of Nile tilapia effectively at both feeding levels, as less then 2 % of fingerlings were remaining when more then 4,000 catfish were stocked per ha. These high stocking densities of catfish increased the net production significantly in the case of the “old” feeding level but this phenomenon did not occur at “new” feeding levels. “Large” snakeheads effectively controlled recruitment of Nile tilapia at both feeding levels at a stocking density of more then 400 snakeheads/ha. At both feeding levels the net production decreased significantly at these high stocking densities.
“Small” catfish and “small” snakeheads were not able to control the recruitment of Nile tilapia completely, a fingerling percentage of 3.7 % and 8.9 % was persisting for respectively catfish and snakehead.
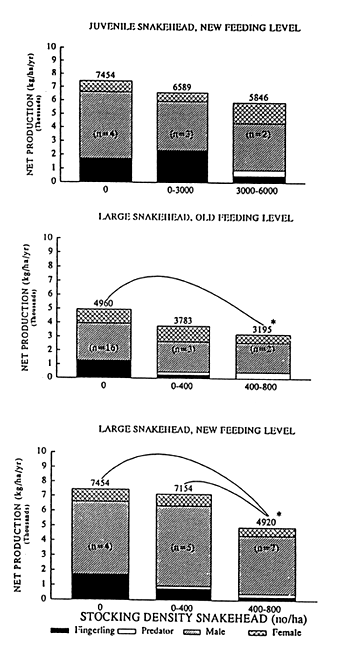
Fig. 2. The relation between the stocking density of O. obscuris and the net production and the partition of the net production in the different categories of fish for poly-culture with O. niloticus under the “old” or “new” feeding regime. (* P≤0.05 number of experiments between brackets)
The daily mortality (% Md) of O. niloticus recruits and the percentage of fingerlings at harvest in relation to the predator density or in relation to the number of predators per stocked female of Nile tilapia of three experimental groups, irrespectively of the used feeding level, is presented in Figure 3. The regression lines were described with the following equations:
Poly-culture with small C. gariepinus
Daily mortality = 1.73 + 1.18*[1 + erf{(no of stocked catfish - 2.36)/154}] (R=0.88, P≤0.01)
% of fingerlings = 3.636 + 21.89*e(-no of catfish stocked/1255) (R=0.84, P≤0.01)
% of fingerlings = 4.09 + 12.08*[1 + erf{(no of catfish per female)- 0.06)/-22.09}] (R = 0.87, P≤0.01)
Poly-culture with large C. gariepinus
Daily mortality = 0.99 + 3.12*[1 + erf{(no of stocked catfish -3899)/4782}] (R=0.88, P≤0.01)
% of fingerlings = 0.095 + 24.65*e (-no of catfish stocked/1357) (R = 0.84, P≤0.01)
% of fingerlings = 20.5*e(-no of catfish per female*5.9) (R = 0.70, P≤0.01)
Poly-culture with large O. obscuris
Daily mortality = 1.24 + 3.00*[1 + erf{(no of stocked snakeheads - 522)/544}] (R = 0.83, P≤0.01)
% of fingerlings = -2.99 + 27.89*e (-no of stocked snakeheads/331) (R = 0.84, P≤0.01)
% of fingerlings = 20.5*e (-no of snakeheads per female*16.20) (R = 0.39, n.s.)
Natural daily mortality of the Nile tilapia recruits was 1.73 ± 0.10 %/day (± S.E.M). Key parameters as calculated with the regression lines are presented in Table 5. The maximum daily mortality, due to predation by “large” C. gariepinus and “large” O. obscuris, was 7.2 %/day and was reached at stocking densities of 12,670 catfish/ha and 1,435 snakeheads/ha. Maximum daily mortality, due to predation by “small” C. gariepinus was 4.1 %/day at a stocking densities of 4,400 catfish/ha.
Large catfish and large snakehead were able to control the recruitment of Nile tilapia effectively, less then 0.15 % of fingerlings were remaining at a stocking density of 8,290 catfish/ha and 725 snakeheads/ha. “Small” catfish were not able to control completely the recruitment of Nile tilapia, a fingerling percentage of 3.7 % was persisting stocking density of 7,330 catfish/ha.
One large catfish can control the recruitment of 1.2 female Nile tilapia's and for large snakehead this value is 3.3.
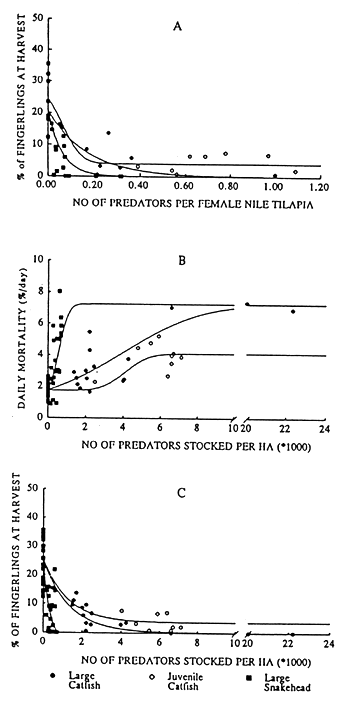
Fig 3. The percentage of fingerlings at harvest in relation to the number of predators per stocked female of Nile tilapia (A), the daily mortality (% Md) of O. niloticus recruits and the percentage of fingerlings at harvest in relation to the predator density (B,C) of small and large C. gariepinus and O. obscuris irrespectively of the used feeding level.
TABLE 5
Key parameters from polyculture of O. niloticus with a predator.
| PARAMETERS | PREDATOR TYPE | ||
| “SMALL” C. gariepinus | “LARGE” C. gariepinus | “LARGE” O. obscuris | |
| Predator stocking density in no/ha and obtained minimal percentage of fingerlings of O. niloticus (between brackets). | 7,330 (3.7%) | 8,290 (0.15%) | 725 (0.15%) |
| Predator stocking density in no/ha and obtained maximal daily mortality (%Md) of O. niloticus recruits (between brackets). | 4,400 (4.1%) | 1,2670 (7.2%) | 1,435 (7.2%) |
| Ratio of predator per female O. niloticus and obtained minimal percentage of fingerlings of O. niloticus (between brackets). | 0.41 (4.1%) | 0.83 (0.15%) | 0.30 (0.15%) |
Growth rates of Nile tilapia
The specific growth rate (SGR) of O. niloticus in experiments with a “new” feeding level, was not significantly different if data from all experiments with a predator were compared with data from all experiments without a predator. The predator density at stocking and the resulting quantity of fingerlings at harvest, however, obscures the results. Figure 4 presents the significance levels of a stepwise execution of the Chow-test. In each step the percentage of fingerlings at harvest was increased with 2%. Then the growth rate of O. niloticus from all experiment where no predators were used was compared with the growth rate of all experiments where a predator was stocked and where the percentage of fingerlings at harvest was lower or equal then the given step-level. The results of the test indicates that male and female O. niloticus were growing faster in experiments where a predator reduced the percentage of fingerlings at harvest to a level of 8% in the case of male-growth and to a level of 15% in the case of female-growth. The relations between body weight and specific growth rate of O. niloticus from experiments under a “new” feeding regime, with and without a predator, are given in Figure 5 and the growth rates can be described by the following regression lines;
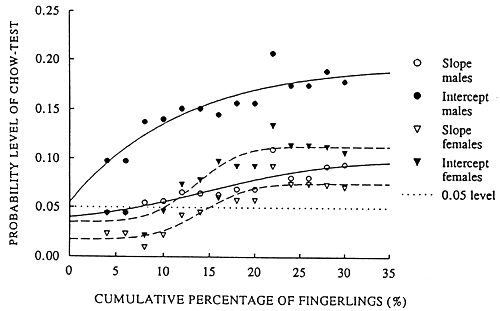
Fig. 4. The probability levels of a stepwise execution of the Chow-test for structur al changes. The percentage of fingerlings at harvest was stepwise increased with 2%, followed by a comparison of the growth rate of O. niloticus from all experiment where no predators were used with the growth rate of all experiments where a predator was stocked and where the percentage of fingerlings at harvest was lower or equal then the given step-level.
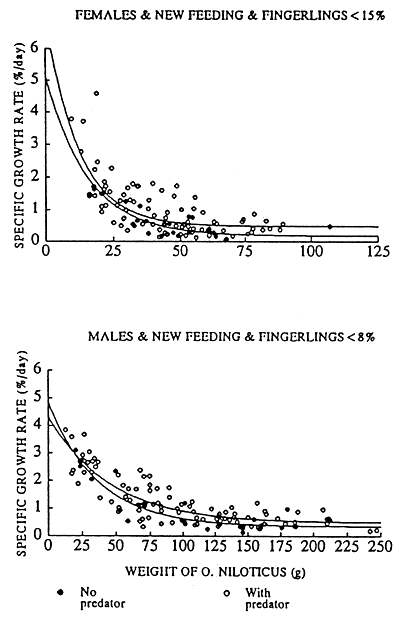
Fig. 5. The relation between the weight of male and female O. niloticus and their specific growth rate for experiments, under the new feeding regime and where a predator reduced the percentage of fingerlings at harvest to respectively 15% for female-growth and 8% for male-growth.
Male O. niloticus, No predator
SGR = 0.35 +4.48* e(-weight/35.8) (R = 0.92, P≤0.01)
Male O. niloticus, With predator, Percentage of fingerlings≤8%
SGR = 0.48 +3.82* e(-weight/45.1) (R = 0.86, P≤0.01)
Female O. niloticus, No predator
SGR = 0.17 +4.92* e(-weight/15.3) (R = 0.86, P≤0.01)
Female O. niloticus, With predator, Percentage of fingerlings≤15%
SGR = 0.48 +6.50* e(-weight/12.1) (R = 0.64, P≤0.01)
Survival rates
The overall survival of Nile tilapia was 96 ± 5% and 82 ± 8% (± S.E.M.) for respectively males and females. The survival rate of stocked C. gariepinus and O. obscuris varies from 24.5 ± 10.2 to 75.9 ± 13.9 (± S.E.M., Table 1 & 2) and was not affected by differences in feeding level (P≥0.279). The results clearly indicate that “small” predators have a lower survival rate (P≤0.008).
TABLE 6
The correlation coefficient between the survival rate of “small” predators and the stocking weight of the
predator, the stocking weight of O. niloticus, the predator density at stocking and the surface area of the ponds.
| Predator type | Stocking weight predator (g) | Stocking weight O. niloticus (g) | Predator density (no/ha) | Pond area (ha) |
| “small” O. Obscuris | 0.968** | -0.953** | 0.727 | 0.592 |
| “small” C. gariepinus | -0.619 | -0.404 | 0.522 | -0.654* |
The correlation coefficients between the survival rate of “small” predators and some rearing parameters are presented in Table 6. The survival rate of “small” O. obscuris was positively related to the stocking weight O. obscuris (P≤0.01) and negatively related to the stocking weight of O. niloticus (P≤0.05). The survival rate of “small” C. gariepinus was negatively related to the surface area of the ponds (P≤0.05).
DISCUSSION AND CONCLUSIONS
Recruitment control
“Large” catfish and “large” snakehead were able to control the recruitment of Nile tilapia effectively, less then 0.15 % of fingerlings were remaining at a stocking density of 8,290 catfish/ha (ratio predator:parent-prey=1:2.7) and 725 snakehead/ha (ratio predator:parent-prey = 1:30). Similar predation efficiencies in the successful control of the recruitment of O. niloticus were reported for other piscivorous species; Lates niloticus, ratio 1:37 (Lazard, 1980), Ophiocephalus striatus, ratio 1:32, (Hopkins et al., 1982), Hemichromis fasciatus, ratio 1:17 (Lazard, 1980).
The difference in efficiency between the two species is probably caused by their feeding habits and mode of predation. C. gariepinus is an omnivore (de Kimpe et al., 1974, Munro, 1967) and feeds on the supplied wheat bran which could ease its motivation to search for Nile tilapia fry. O. obscuris is strictly piscivorous (Adebisi, 1980).
Hecht et al (1988) found that C. gariepinus with barbels were 22.6% more efficient at catching prey than those without. This indicates that tactile behaviour is of importance in prey catching processes. It can be expected that a tactile predator covers a smaller area per unit time than a visual predators, consequently a higher density of tactile predators is needed in order to acquire an equal number of contacts between prey and predator.
Successful recruitment control in a mixed culture of O. niloticus at a predator:parent-prey ratio of 1:5 or a stocking density of 2,500 predators per ha was reported for C. gariepinus by Lazard (1980). Middendorp (in press) however found that C. gariepinus was not able to control the recruitment of O. niloticus in a monosex culture with a 0.9% sexing error, a predator:parent-prey ratio of 1:0.1 (females only), or a stocking density of 400–1,500 predators per ha, while in a mixed culture the recruitment was controlled effectively at a predator:parent-prey ratio of 1:8 or a density of 2,000 predators per ha. This could indicate that a minimum threshold stocking density of approximately 2,000 catfish per ha is needed in order to obtain successful recruitment control of Nile tilapia. Fagbenro (1987) found that C. lazera (syn C. gariepinus) controls the recruitment of Tilapia guineensis, a substrate breeder, at a stocking density of 500–1,000 catfish per ha, which indicates that the existence of a threshold is probably related to the interaction of the tactile mode of predation and a mobile or non-mobile prey.
However it could also be speculated if the revision of the taxonomy of the african catfish and the regrouping under C. gariepinus by Teugels (1984) did overlook behavioral differences. This because natural reproduction of C. gariepinus occurs in the experiments of Middendorp (1994b), which has not been reported for C. gariepinus in ponds before.
“Small” catfish and “small” snakeheads were not able to control the recruitment of Nile tilapia completely, a fingerling percentage of 3.7 % and 8.9 % was persisting for respectively catfish and snakehead at a stocking density of 7,330 catfish/ha and 3,140 snakeheads/ha. Similar results for C. gariepinus were found by Janssen (1985) and for Channa striata by Hopkins et al. (1982) if only their data set A1–A12 is considered. The difference in efficiency of predation between “small” or “large” fish is probably related to the survival rate and the onset of piscivory. The low survival rate in our experiments with C. gariepinus caused that the real existing predator density drops below the required level of 8,000 per ha. Janssen (1985) found that at high stocking densities “small” C. gariepinus (20,000 per ha) still fails to control the recruitment of O. niloticus completely which indicates that also other factors are of importance.
The large size of the Nile tilapia fingerlings harvested from ponds with a “small” predator indicates that the first born Nile tilapia larvae escape predation. The question is whether “small” C. gariepinus are not able to predate on the Nile tilapia larvae or whether they have another food preference shifting later to a more piscivorous feeding habit. The size of the born Nile tilapia larvae/fry is not a limiting factor as Hecht et al (1988) found that C. gariepinus in a similar size range as use in this study can predate on fish with a length of 30 mm. Munro (1967) reports that the stomach of small C. gariepinus (0.02–4 gramme), obtained from natural waters, contained mainly chironomid larvae, Cladocera, Copepoda and Ostracoda. This, combined with the results that C. gariepinus larger than 7 gramme, completely controls Nile tilapia recruitment, could indicate that “small” C. gariepinus have a specific food preference for zooplankton and probably shifts to a more piscivorous behaviour once they reach a weight of 7–8 gramme.
Yield and growth
Recruitment control through a piscivorous predator as O. obscuris reduces the net yearly production while in experiments with the omnivorous C. gariepinus the net yearly production increases in case of the “old” feeding level or remains stable in case of the “new” feeding level. A reduction of the yield has been observed before in similar experiments for other piscivorous species as; Lates niloticus (Ofori, 1988); Megalops cyprinoides (Fortes, 1980); Micropterus salmoides (McGinty, 1985) and Cichla ocellaris (McGinty, 1983). Lazard (1980) reports that yields with C. gariepinus as a predator are higher then the yields of experiments with Lates niloticus or Hemichromis fasciatus. This phenomenon may be explained by the fact that with piscivorous species the fingerling biomass is converted into lower a predator biomass and this loss is obviously not completely compensated by the observed higher growth rates of male and female Nile tilapia. C. gariepinus feeds on the Nile tilapia fry and on the supplied wheat bran and consequently the lost fingerling biomass is replaced by an equal or higher biomass of C. gariepinus.
Ofori (1988) found that the loss in biomass is economically compensated by the higher price level of the larger Nile tilapia. The situation in the Republic of Congo is somewhat different and the economic consequences of the different predator systems are presented in a separate article (de Graaf et al., in press).
The presence of approximately 25 % (on weight basis) of fingerlings in a mixed culture interferes within the production process through feed competition between the originally stocked specimen and their offspring. This interference is rather strong because the metabolism of small fish is higher than the metabolism of large fish (Winberg, 1956) and consequently 1 kg of fingerlings consume more feed as 1 kg of adult fish. This food shortage can be eliminated by increasing directly the feeding level (“old” vs “new” feeding rate), or by increasing the feeding level indirectly by removing the fingerlings through the introduction of a predator. Within this respect it is interesting to look at the so called “stunting” process of Tilapia, which has been often mentioned as a limiting factor in Tilapia rearing (Guerrero, 1980, Hepher et al., 1980, McGinty, 1983, Mires, 1980, Ruwet et al., 1976). This process of “stunting” has been discussed extensively by Noakes et al., (1982) and by Fryer et al., (1972) and they concluded that the phenomenon is not one of “stunting” of somatic growth, but one of an earlier breeding of the fish. This study confirms this theory as the Nile tilapia were “stunted” if their size of first reproduction (30 gramme) is considered. However, from an aquaculture point of view no limitations were encountered as long as sufficient feed was available for the stocked specimens. Therefore it could be concluded that “stunting of the growth” in Nile tilapia is more a matter of husbandry techniques and the carrying capacity, then a matter related to the species itself.
Comparison of prey-predator relations
Comparison of different experiments on the recruitment control of Nile tilapia is hampered by a rather large variation is used rearing techniques; the stocking density of Nile tilapia fluctuates between 3,000–20,000 per ha (Hopkins et al., 1982, Janssen, 1985, Lazard, 1988, McGinty, 1985, Verani et al., 1983); monosex male culture is used (Anonyme, 1980, Lovshin et al, 1990, Middendorp, in press (b)) or Nile tilapia is reared in combination with other non-predator species (Hopkins et al., 1982, Ofori, 1988). Differences in stocking densities of Nile tilapia can be described with predator-parent prey ratio's or by multi-linear regression and both methods are equally valid if the significance of correlation is considered (Hopkins et al., 1982). The variation in stocking density of Nile tilapia in this study was limited and multi-linear regression analyses did not provide significant equations.
From a practical point of view predicting the recruitment control in terms of the percentage of fingerlings at harvest is accurate enough, since for a fish farmer 1–5 % of fingerlings is an acceptable level. The application of daily mortality (%Md), a methodology used in marine predator-prey systems (Pauly, 1982), over-estimates the number of predators needed. This overestimation is mainly caused by the asymptotic character of the fitted curve equation and this phenomenon is also illustrated by the curve-fit equation of the percentage of fingerlings obtained by “small” catfish.
The obtained predator-parent-prey relations are however not applicable for monosex or mixed species culture and here a relation between the number of predators and the number of stocked female Nile tilapia could be applied. For C. gariepinus the predicted values are close to the measured values but the existence of the earlier discussed threshold could interfere with the final results. For O. obscuris the measured ratio of 0.3 and the predicted stocking density of 3,300 per ha is too high and is caused by the non significance of the curve fit equation.
Survival rates
The survival rate of “small” C. gariepinus is rather low and negatively related as the survival rate reduces with increasing pond size. This phenomenon was also found by Hogendoorn et al (1983) where a survival rate of 63 % was obtained in ponds of 50–100 m2. The real cause is probably the used feeding technique used. Both small and large ponds were fed once a day and had only one feeding place. Wheat bran floats on the water surface and in small ponds it covers quickly the whole pond area. In large ponds (0.25 ha or more), all the wheat bran is consumed before it has a chance to cover the whole pond, consequently the fish far away from the feeding place are deprived of their supplementary feed.
The survival rate of “small” O. obscuris is positively related to their own stocking weight and negatively related to the weight of the stocked Nile tilapia, indicating that Nile tilapia predates upon the 15-times smaller O. obscuris. From an ecological point of view this negative relation between the survival rate of O. obscuris and the weight of the stocked Nile tilapia is in contradiction with the “match-mismatch” concept (Adams et al., 1987, van Densen, 1993) whereby the inverse is expected as larger Nile tilapia are closer to their first spawning size and will produce suitable preys at an earlier moment consequently the survival rate of O. obscuris should increase.
ACKNOWLEDGEMENTS
The experimental work reported in this paper was carried out as part of the programme of the UNDP/FAO Fish Farming Development Project in the Republic of Congo.
The authors are indebted to Prof. Dr. E.A. Huisman (Department of Fish Culture and Fisheries, Agriculture University, Wageningen, The Netherlands) and Dr. H. van Zon (Euroconsult, Arnhem, The Netherlands) for their criticism of an earlier draft of this paper and to Mr. R. Stam for his advice on statistical analyses.
The views and conclusions given in this paper were expressed earlier in an final report prepared for the Fish Farming Development Project. They are the responsibility of the authors only and do not imply the expression of any opinion on the part of the United Nations, the Food and Agricultural Organization or the Government of the Republic of Congo. Permission to publish this paper, given by the above organizations, is gratefully acknowledged.
REFERENCES
Adams, S.M., and DeAngelis, D.L., 1987. Indirect effects of early bass-shad interactions on predator population structure and food web dynamics. In: W.C. Kerfoot and A. Sih (Eds.) Predation in aquatic ecosystems. The University Press of New England. pp 103–117.
Adebesi, A.A., 1981. Analyses of the stomach contents of the piscivorous fishes at the upper Ogun river in Nigeria. Hydrobiologica, 79: 167–177.
Anonyme, 1980. Les recherches sur les peches continentales et la pisciculture, Rapport Annuel 1980, Centre Technique Forestier Tropical, Nogent-sur-Marne, France, pp 9–11.
Bardach, J.E., Ryther, J.H. and McLarney, W.O., 1972. Aquaculture, the farming and husbandry of fresh water and marine organisms. Wiley-interscience Inc. New York, 868 pp.
Bedawi, R.M., 1985. Recruitment control and production of market size Oreochromis niloticus with the predator Lates niloticus L. in the Sudan. J. Fish Biol., 26: 459–464.
van Densen, W.L.T., 1993. Predator enhancement in the freshwater fish community. In: I.G. Cowx (Eds.) Rehabilitation of inland fisheries. Blackwell.
Eckstein, B. and Spira, M., 1965. Effect of sex hormone on gonadal differentiation in a cichlid, Tilapia aurea. Biol Bull. (Woods Hole, Mass.) 129: 482–489.
El Gamal, A.A., 1992. Predation by Nile perch Lates niloticus (L.) on Oreochromis niloticus (L.), Cyprinus carpio (L.), Mugil sp. and its role in controlling tilapia recruitment in Egypt. J. Fish Biol. 40: 351–358.
Fagbenro, O.A., 1987. Recruitment control and production of Tilapia guineensis (Dumeril) with the predator, Clarias lazera (Valenciennes). Nig. J. Basic and Applied Sci., 2: 135–140.
Fortes, R.D., 1980. Tarpon as predator to control Java tilapia young in brackish water ponds. Fish Res. J. Philipp. 5(2): 22–35.
Fryer, G. and Iles, T.D., 1972. The ciclid fishes of the great lakes of Africa: their biology and evolution. T.F.H. Publ., Neptune City, New Jersey.
McGinty, A.S., 1983. Population dynamics of peacock bass, Cichla ocellaris and Tilapia nilotica in fertilised ponds. In: L. Fishelson and Z. Yaron (Editors) Proc. Int. Symp. on Tilapia aquaculture, 1983, Nazareth, Israel, pp 86–94.
McGinty, A.S., 1985. Effects of predation by largemouth bass in fish production pods stocked with Tilapia nilotica. Aquaculture, 46: 269–274.
Greene, W.H., 1990. Econometric Analysis. Macmillan, New York, pp 211–214.
de Graaf, G.J., Galemoni, F. and Banzoussi, B., 1994. The artificial reproduction and fingerling production of the African catfish Clarias gariepinus (Burchell 1822) in protected and unprotected ponds. Aquaculture and fisheries management.
de Graaf, G.J. and Galemoni, F., in press. The reproductive biology of pond reared Nile tilapia (Oreochromis niloticus).
Guerrero, R.D., 1975. Use of androgens for the production of all-male Tilapia aurea (Steindachner). Trans. Am. Fish. Soc. 104(2): 342–348.
Guerrero, R.D., 1980. Control of tilapia reproduction. In R.S.V. Pullin and R.H. Lowe-McConnell (Eds), The biology and culture of tilapia, ICLARM Conference Proceedings 7, Manila, Philippines, pp 309–317.
Guerrero, R.D. and Garcia, A.M., 1983. Studies on the fry production of Sarotherodon niloticus in a lake based hatchery. In: L. Fishelson and Z. Yaron (Editors) Proc. Int. Symp. on Tilapia aquaculture, 1983, Nazareth, Israel, pp 388–393.
Hecht, T. and Appelbaum, S., 1988. Observations on intraspecific aggression and coeval sibling cannibalism by larval and juvenile Clarias gariepinus (Clariidae: Pisces) under controlled conditions. J. Zool., Lond. 214: 21–44.
Hepher, B. and Pruginin, Y., 1980. Tilapia culture in Ponds under controlled conditions. In R.S.V. Pullin and R.H. Lowe-McConnell (Eds), The biology and culture of tilapia, ICLARM Conference Proceedings 7, Manila, Philippines, pp 185–205.
Hogendoorn, H. and Koops, W.J., 1983. Growth and production of the African catfish, Clarias lazera (C&V). I. The effects of stocking density, pond size and mixed culture with tilapia (Sarotherodon niloticus L.) under extensive field conditions. Aquaculture, 4: 227–248.
Hopkins, D.K., Pauly, D., Cruz, E.M. and van Weerd, J.M., 1982. An alternative to predator-prey ratio's in predicting recruitment. Meeresforschung, Reports on Marine research. Bd 29, H.3.S.: 125–135, Verlag Paul Parey, Hamburg.
Huisman, E.A., 1976. Food conversion efficiencies at maintenance and production levels for carp, Cyprinus carpio L., and rainbow trout, Salmo gairdneri Richardson. Aquaculture, 9: 259–273.
Janssen, J., 1985. Elevage du poisson-chat africain Clarias lazera (C&V, 1840) en République Centraficain; Alevinage et grossissement en etangs. Document technique no 22, Projet FAO/GCP/CAF/007/NET.
de Kimpe, P. and Micha, J.C., 1974. First guidelines for the culture of Clarias lazera in Central Africa, Aquaculture. 4: 227–248.
Lazard, J., 1980. Le developpement de la pisciculture intensive en Cote-d'ivoire. Exemple de la ferme piscicoles de Natio-Kobadara (Korhogo). Bois et Forets de Tropiques, 190: 45–66.
Lovshin, L.L., Da Silva, A.B., Carneiro-Sobrinho, A. and Melo, F.R., 1990. Effects of Oreochromis niloticus females on the growth and yield of male hybrids (O. niloticus female × O. hornorum male) cultured in earthen ponds. Aquaculture. 88: 55–60.
Middendorp, A.J., in press (a). Pond farming of Nile tilapia (Oreochromis niloticus) in Northern Cameroon. I. Feeding combinations of cotton seed cake and brewery waste in fingerling culture, hand-sexed male monosex-culture and mixed culture with police-fish (Clarias gariepinus), Aquaculture and Fisheries Management.
Middendorp, A.J., in press (b). Pond farming of Nile tilapia (Oreochromis niloticus) in Northern Cameroon. III. Controlling a sexing error of 1 % in hand-sexed male tilapia monosex-culture by african catfish (Clarias gariepinus), Aquaculture and Fisheries Management.
Mires, D., 1980. A study of the problems of the mass production of hybrid tilapia fry. In R.S.V. Pullin and R.H. Lowe-McConnell (Eds), The biology and culture of tilapia, ICLARM Conference Proceedings 7, Manila, Philippines, pp 309–317.
Munro, J.L., 1967. The food of a community of East African freshwater fishes. J. Zool., Lond. 151: 389–415
Noakes, D.L.G. and Balon, E.K., 1980. Life history of Tilapias: An evolutionary perspective. In R.S.V. Pullin and R.H. Lowe-McConnell (Eds), The biology and culture of tilapia, ICLARM Conference Proceedings 7, Manila, Philippines, pp 61–83.
Pauly, D., 1982. A method to estimate the stock recruitment of shrimps. Trans. Am. Fish. Soc., 111: 13–26.
Pruginin, Y., 1967. Report to the Government of Uganda on the experimental fish culture project in Uganda, 1965–66. FAO/UNDP (Technical Assistance). Reports on Fisheries TA Report 2446. 19 pp. FAO, Rome.
Ruwet, J.C., Voss, J., Hanon, L. and Micha, J.C., 1976. Biologie et élévage des Tilapias. In: Symp.sur l'aquaculture en afrique. CIFA Technical paper no 4: 331–359.