Fish production in reservoirs is usually considered secondary to other uses, hence impounded areas may not be favourable habitats for fish. The problems which may arise are associated with unfavourable physico-chemical conditions of water, feeding areas, migration, spawning grounds, excessive growth of aquatic weeds, and change in species composition of the fish. These changes may either encourage or discourage the success of fishery establishment in reservoirs; therefore, modification or manipulation of the habitats is considered necessary to prevent or reduce degradation of the fish populations and/or their food supply.
There has been much discussion on the degree to which reservoir basins should be cleared of timber before they are flooded. Some consider complete removal of timber essential in all reservoirs for reducing deoxygenation of water during the filling period and immediately afterwards, preventing possible damage to the dam or penstock, and for better fishing operations in the newly formed reservoir. Many suggest that only commercial timber should be removed, while others may be left or partly removed. There are many benefits to be obtained from leaving large uncleared areas in the reservoir as recommended, for example, by Hulsey (1959). These include:
A substantial saving in the total cost of a reservoir as compared to complete clearing:
The timbered shallow areas will tend to reduce wave action and protect the dam and shoreline;
The dead timber and litter will temporarily retard erosion when the shoal areas are exposed during drawdown;
The organic material will produce carbon dioxide (from decomposition) which in some cases may help to floculate the colloidal clay turbidity;
The timber areas will give a different type of fish habitat than the open water areas;
The standing timber, litter and debris will considerably increase the surface area for attachment of periphyton and other organisms thereby increasing the productivity of the reservoir.
It has been found that “aufwuchs” algae on drowned trees in African savannah woodland reservoirs provide food for herbivorous fish grazers such as tilapias. The associated fauna, e.g., aquatic insects, molluscs, oligochaetes and microscopic crustaceans is eaten by insectivorous and omnivorous fish. During the process of gradual filling of a new man-made lake, the large volume of hypolimnion may become devoid of oxygen and thus unavailable for the great majority of aquatic fauna. At some time the faunal benthic production may be restricted only to a few top metres of depth around the periphery of the reservoir (Petr, 1972). During such a situation the submerged trees will form a substrate for the benthic fauna, allowing it to exist far offshore in areas with completely deoxygenated bottom waters, thus making it possible for fish to find abundant food supply even outside the narrow and shallow littoral belt.
Il'ina and Gordeyev (1970) reported that the presence of submerged forests near shores in the Soviet reservoirs prevented organic matter from being washed out of the inshore zone. It promotes the mass development of plankton and other food organisms and creates conditions conducive to the feeding of young fish. The abundance of organisms on and in flooded trees varies with location, types of wood, distance of trees from shore, and water turbidity. Petr (1970) reported that burrowing mayfly nymphs (Povilla) in flooded trees of the Volta lake were more dense in the bark of trees than in the wood, and trees with soft wood were attacked more than those with harder wood. McLachlan (1971, 1974) found submerged dead trees in Lake Kariba populated with algae and associated organisms such as chironomid larvae, oligochaete worms, nymphs of trichopterans and ephemeropterans, which comprised a major part of food supply for many species of fish.
The density of fish populations in flooded forests was investigated in many reservoirs by means of fishing pressure and fishing success. Burress (1961) reported that the percentage of successful anglers in Bull Shoals reservoir in the United States was higher in areas of flooded standing timber (94.8 percent) than in open water (90.6 percent). The fishing pressure in 1959 accumulated 12 690 kg/ha in timber areas, as compared to 240 kg/ha in open water. The hook and line harvest in flooded timber areas was 3 430 kg/ha, and in open water was 127 kg/ha. In the Soviet Union, studies of submerged forests were carried out on the Rybinsk reservoir (Poddubnyi, 1963). The highest average fish catch was recorded from the submerged flood-plain forests; it was lower in inshore waters protected by dead trees and lowest in open water.
Since a complete removal of trees and bushes in reservoirs involves large expenditures and capital investment, and since their presence will provide substrate for periphyton and associated organisms and enrich the food resources of the reservoir, a decision on whether to keep or to remove the trees should be a useful analysis of the fish stock, especially according to its feeding preferences, and on the intensity and type of fishing to be expected on the reservoir. Trees need not necessarily be cleared in reservoirs where fishing activities will mainly operate with stationary fishing gears, for example, gillnets, longlines, hooks and traps. It is apparent that catch by using these fishing gears is higher in flooded forest areas than in open water. This has been found in Thai reservoirs and reservoirs in Africa. Selective clearance of trees and bushes up to the draw-down limit is essential for reservoirs where fishing operation is conducted by means of shore seines and dragnets such as operated in reservoirs in India. Removal of trees and other vegetation in areas that will become anaerobic when inundated is necessary for reservoirs in tropical humid forests (Goodland, 1977) for the purpose of reducing the deoxygenated condition and noxious gas development during the decomposition period. A complete clearance together with levelling of the reservoir bottom are necessary only in reservoirs where commercial fishing will operate with trawling (mid-water and bottom) such as operated in reservoirs in the United States and the Soviet Union.
The purpose of a fishway is to provide for the upstream or downstream passage of fish past either a dam or a natural barrier. Therefore, it should be designed suitable for, and passable by, all the native migratory species, and with an attractive location of entrance. To ensure success, the entrance should be located close to the tow of the dam and close to the main current from the spillway or tailrace (Rounsefell and Everhart, 1953). The fish following the current will be led to the fishway entrance. The slope of the fishway, and velocity of flow are also recognized as important factors to influence the upstream migration. Experiments showed that fishways with a slope up to 1 on 8 (a rise of 1 m for every 8 m), and a velocity of 0.6 m/sec were suitable for passing salmonids (Collins and Elling, 1960). Details of different types of fishways and their operation are beyond this study; for further descriptions see Rounsefell and Everhart (1953), Clay (1961) and Eicher (1970).
Fish locks are applied to sites that offer little space for a fishway or where the lift is so high as to preclude the use of a conventional fishway. They have been used much more extensively in Europe, e.g., in The Netherlands (Deelder, 1960) than in North America (Baker, 1967). Many areas have used a fish lift or elevator for passing the fish upstream. An elevator consists of a tank or hopper which fish enter and which is then hoisted mechanically to the stream above the dam where the fish are released. In some instances, methods such as the above have been impractical, because of either mechanical difficulties or the high expense of construction. The fish may be trapped and then transported by tank-trucks to a release point above the dam, or even to other streams (Dill and Kesteven, 1960).
The problem of ensuring the safety and free passage of downstream migrants, both young and adult, over, through and around dams is as important as the problem of passing upstream migrants. Probably more ingenuity has been applied to provision of devices for passing fish downstream past obstructions than has been the case in upstream passage (Eicher, 1970). Many factors arise in reservoirs that may seriously affect the downstream migration such as oxygen deficiencies, high water temperature, and slow currents (Trefethen, 1968).
Although many fishways and other devices for guiding and passing fish over, through or around dams have been successful, the installation of the fishways or other means is no guarantee that the run will be preserved. Many fishways built in tropical African impoundments have failed to be used by migratory fish (Jackson, 1966). Need for fishways seems to be less recognized in the tropics than in temperate regions. One reason may be that there are few truly anadromous fish. Several species do, however, ascend the rivers for spawning such as Labeo altivelis, Barbus viviparus, Tilapia sparrmani, in East Africa (Bell-Cross, 1960); Hilsa ilisha in India (Hickling, 1961); Probarbus jullieni in Malaysia and Thailand; Hilsa tori, Pangasius sutchi, Puntius daruphani, and many species of minnows in Thailand. These fishes usually head upstream for spawning. Small fishes like minnows spawn mainly in newly flooded grasses and shrubs along the reservoir shoreline and in floodplains. The spawning season for tropical fishes generally ranges from 1 to 4 months of the rainy season coinciding with the floods. Lateral migrations from river to floodplain are much more common than longitudinal ones. More on this topic can be learned from Welcomme (1979).
Since fish passages are often expensive to build and operate, it should be kept in mind that they may be considered usually only where fish of economic value are known to make regular and necessary migrations to spawn, and when their interruption would seriously affect the fisheries. Serious considerations should also be given in areas where a new artificial obstacle in a stream might lead to a serious decline in or even extinction of a species not necessarily of commercial value. Designing, construction and operation of the fishways requires close cooperation between engineers, fishery biologists and environmentalists.
In temperate latitudes, the availability of spawning grounds often becomes the main limiting factor on fish populations in reservoirs after they have been in existence for a few years. Parts of initial favourable spawning areas are destroyed either by sedimentation or by water level fluctuation. Regulation of river discharge by the dam seriously affects the reproduction of the migratory species such as trout and salmon as a result of blocking their ways to spawning grounds. It has appeared that the construction of Grand Coulee Dam on the Columbia River has shut off the sockeye (Oncorhynchus nerka) migration to spawning areas in the upper Columbia River above the confluence of the Okanagan River (Clay, 1960). This situation leads to a reduction of reproductive success, to a decline in the abundance of the year-classes, and to a decrease in catches.
Improvement in existing spawning grounds for valuable species in reservoirs and on the rivers downstream from the dams is necessary for increasing fishery production. A number of investigators have created artificial spawning grounds and observed the use of them by certain species such as the salmonids. Hourston and Mackinnon (1957) reported their experiment on the use of an artificial spawning channel in Jones Creek, British Columbia, which was built to replace natural spawning ground that was lost to the hydroelectric development of the stream. They claimed this artificial channel was used by the salmonids for spawning in the first few years prior to encounter with sedimentation problems.
Sukhoi Van (1959) set up an experiment of artificial spawning grounds in the Dnieper River (USSR), below the dam of the Kakhovka Hydroelectric Station, by using artificial nests. The nests were built with a substratum of forked roots of various plants. He found as many as 90 percent of the nests were used by Azov roach, Rutilus rutilus heckeli. Nezhivenko (1969) used artificial shrubs to create spawning fields in reservoirs. The shrubs were above the bottom by about 1.5 m, to prevent silting-over and permit being washed off by wave action. Khoroshko and Vlasenko (1970) reported that three experimental stone gravel beds were constructed on the Volga and the Kuban' to provide conditions favourable for natural spawning of sturgeons that approached the dam. The two years of observations on the use of these spawning grounds by sturgeons showed they were less significant in the Volga than in the Kuban River.
In the tropics, particularly in Southeast Asia, lack of suitable spawning grounds for indigenous species rarely occurs, since the majority of fish species in this region are mainly phytophilous with several lithophylous fishes. During the spawning season, which usually coincides with the rainy season, such fishes can easily find spawning grounds in flooded areas and on riverbeds that drain into the reservoir. The brood stock migrating to spawning grounds should be protected by limiting the fishing. Provision of artificial spawning grounds may be needed for some introduced species which cannot find suitable spawning ground in a reservoir and connected water bodies. Modification of the artificial spawning ground should be arranged only when it is considered thoroughly necessary. For those species that cannot spawn naturally in local reservoirs and rivers, artificial breeding should be applied.
In deep reservoirs, such as in the Jatiluhur reservoirs in Indonesia, the introduced Nile tilapia has difficulty in finding suitable spawning ground. For solving the problem, a spawning bed with an area of 3 300 m2 and 1 m depth at low water level was constructed on the shore of the reservoir. The Nile tilapia were stocked and bred in this pond. At high water level this spawning bed was flooded and the fish entered the reservoir (Sarnita, 1977). It was observed in the following year that this spawning bed did not supply enough seed for this impoundment, and it was estimated that the reservoir needs three to four more spawning beds.
The effects of impoundment on downstream water quality depends upon water retention period, impoundment depth, seasonal variation, character of reservoir bottom (whether highly organic or inorganic), the physical and chemical qualities of water entering the reservoir, wind action to provide circulation, and the position and depth of water withdrawal from the reservoir (Sylvester, 1960). A large, long, and deep reservoir with deep-water penstocks appears to have many deleterious effects on discharged water quality. It will discharge water that is low in dissolved oxygen, high in carbon dioxide and exceptionally noxious gases such as hydrogen sulphide, resulting in downstream fish kills. A discharge of water from deep layers may contain higher concentrations of dissolved and suspended materials that may cause a loss of essential nutrients from the reservoir, thus tending to reduce the productive capacity of the impoundment (Wright, 1967).
Changes in volume of flow below the dam may produce several changes in aquatic stocks in different ways. They may increase or decrease feeding areas, available spawning grounds, and the productive capacity of the river below the dam (Dill and Kesteven, 1960). The variation in flow of hydroelectric projects may range from no flow to maximum capacity for the generating facilities with maximum discharge peak occurring daily. A severe problem is produced by a prolonged no-flow period, causing the water to warm beyond the limits desirable for cold water fish, or to expose areas of stream bottom. Reduction in and modification of the flow below a dam may have a highly negative impact especially on the floodplain fisheries (Welcomme, 1979), as well as coastal water productivity. In its extreme, damming a river may lead to complete elimination of floodplains, which in turn will drastically reduce the fish stock. In cases when this may happen, consideration should be given to adjusting the dam operation to minimize the problem. Pirozhnikov et al. (1969) reported that a major technique for increasing the level of reproduction of semi-migratory fishes in the Volga-Caspian region is the supply of adequate water to the delta, which is necessary for the migration of the carp, bream, and roach for spawning, feeding, and growth during the first weeks of their existence, and for the safe migration of the entire population of fingerlings to the principal delta channels and to the northern Caspian Sea.
It has been observed for many years that the temperature of water released from dams depends on the level of the outlets, particularly when reservoirs are thermally stratified. This may be especially of critical importance for fish of temperate latitudes, whose spawning depends on certain water temperature, and where a permanently cool water discharge might make spawning for them impossible. Middleten (1967) reported that the most practical solution to this problem is to design an intake structure or structures suitable for selecting water at the depth of desired temperature. Since outflows through the penstock intakes in a dam produce a “withdrawal layer” (Brooks and Koh, 1969), management of water quality can be achieved either by selecting an appropriate single outlet or through mixed withdrawal according to the requirements.
The dissolved oxygen content in discharged water is often lower than that normally present in the inflow and may closely approach zero at the point of discharge. Churchill (1958) reported that low rates of discharges were reaerated in relatively short distances downstream from the dam, whereas higher discharges required many miles of open-channel flow before oxygen saturation was reached. Studies of the concentration of dissolved oxygen (DO) in water discharged from reservoirs indicated that, during water temperature stratification, dams with deep intakes discharge water of very low DO content, dams with intermediate intakes discharge water of higher DO content, and dams with high-level intakes discharge water with highest DO content (Knight, 1965). Therefore, it is possible to discharge water relatively high in DO content by selecting the level from which water is discharged. This can be done by the same means as temperature control as well as using a submerged weir and artificial aeration. Knight (1965) recommended the submerged weir as a tool for solving the problem. The weir forms an underwater barrier to obstruct passage of deep water into the penstocks so that water is selected for discharge from levels above the crest of the weir. The weir may be impractical for reservoirs with great depth because of the high cost of its construction. A search for other practical methods of treatment has been a subject for continuing study. Wisniewski (1965) introduced the hydroturbine aeration method to resolve the problem. He reported that the use of hydroturbine aeration at power dams has been successful for introducing air into the water in large-scale reoxygenation. The method appears to be low cost under most conditions.
Speece (1970) has proposed four alternative aeration schemes for improving oxygen-deficient impoundment releases. They are:
in-place hypolimnion aeration of the stratified impoundment with commercial oxygen;
injection of commercial oxygen into the penstocks;
down-flow bubble contact aeration of discharge below dam with commercial oxygen, and
U-tube aeration of the discharges.
The experiments for evaluating these methods have been performed on the Appaloosa Dam and the Low Mountain Sheep Dam in the United States. The results are summarized as follows:
Hypolimnion aeration accomplished by injecting small bubbles of commercial oxygen deep in the hypolimnion provides more time for rising bubbles to be completely absorbed. Provision to inject commercial oxygen into the penstocks of a high dam in combination with hypolimnion aeration can provide a “polishing” system for positive DO control in the discharge. The down-flow bubble contact aeration using commercial oxygen simultaneously provides for reasonably efficient oxygen absorption and stripping of dissolved nitrogen. Its use would be dictated by a need to lower dissolved nitrogen levels and produce saturated DO levels in the discharge. The U-tube aeration with air injection appears to be most advantageous for DO addition where a warm water fishery is involved. It can produce a completely saturated water easily and economically, even though it initially contains low DO levels.
In the Ping River below the Bhumipol Dam in northern Thailand local inhabitants living along the river below the dam have complained about poor water quality to fishery officers and engineers who operate the reservoir. This problem still remains to be solved.
Natural and man-made water bodies in the tropics, including Southeast Asia are usually prone to the growth and spread of aquatic plants which causes many problems in the management of water resources. The problem gradually worsens with the increasing number of dams and irrigation development projects, and with increasing enrichment of water bodies with fertilizer run-off and nutrients from human and agricultural wastes. Thus water resources management must give due consideration to the nuisance effects of aquatic vegetation. It is desirable to find ways whereby the aquatic weed problem may be minimized or prevented by instituting measures which would suppress the possibility of excessive growth of aquatic plants.
One is well aware of often undesirable changes caused by blooms of algae and the dense growth of higher aquatic plants, especially in reservoirs. Plants may interfere with, and compete for a variety of uses of impounded water such as irrigation, hydroelectric power generation, navigation and fishing. They also increase water loss by transpiration, reduce the productive capacity of the impoundment, and provide excellent breeding grounds for many disease-carrying and nuisance-causing insects and snails (Little, 1969). Controlling aquatic weeds has become a serious problem in reservoir management. A number of preventing and controlling methods are continuously being developed for more effectiveness and success. In Southeast Asia, the control may be done either by preventive measures - physical, chemical, biological means - or by an integrated method depending on the nature and scope of the existing problem, type and extent of the control desired and comparative costs.
Practical guidelines for appropriate preventive measures for control of aquatic weeds in Southeast Asian countries were prepared at the Southeast Asian Workshop on Aquatic Weeds held in Malang, Indonesia, in 1974 (Soerjani et al., 1976). These include:
Effective legislation for controlling the introduction of exotic plants and for preventing uncontrolled traffic in potential weed species from one country to another, from one island to another, and from one region to another.
Education of people making them aware of the dangers and problems of aquatic weeds, which can be done by either means of education, training, exhibition or mass media.
To promote national seminars on aquatic weeds to identify national aquatic weed problems and to create an awareness in the general public as well as among the decision makers about the importance of the problem.
Each country in the region should initiate a periodic monitoring of aquatic bodies and their vegetation by means of appropriate surveys of the distribution and extent of each important species in different seasons of the year, and areas of infestation. It may often be appropriate to establish a weed control team to carry out such monitoring procedures within a specific catchment area of the region.
Control of water quality by means of minimizing eutrophication and erosion control. These may be achieved by applying proper management of agricultural lands and watersheds. The water quality of certain water ways and water reservoirs should be regularly checked, and excess nutrient loads detected and dealt with when necessary.
The physical methods of controlling aquatic plants may involve filtration, screening, weed removal units. The application of these methods depends on type of plants, field condition, the extent of control desired, labour and cost involved. Livermore and Wunderlich (1970) gave several advantages of monitoring the physical control of aquatic vegetation as follows:
It does not introduce foreign substances into the water.
It may actually remove nutrients from the lake cycle and should tend to reduce the rate of lake filling by plant residue deposition.
It can provide immediate relief from nuisance conditions.
During the blooms, algae may be removed by filtration or screening. Raking is the only way at present to remove filamentous algae (Fryer and Makepeace, 1970).
Scythes and sickles for cutting are widely used to clear rooted plants from small areas. A weed saw consisting of a long steel handle can be used to cut fairly sizeable areas. Forking and raking following mechanical cutting or for general shoreline cleanup of weeds and debris is still probably the most widely used collection method (Livermore and Wunderlich, 1970). Cutting by hand is laborious and is suitable in small areas where the use of motorized machinery is impractical. A number of mechanical weed cutters have been developed. Several types of saws, crushers, and choppers have been used to destroy aquatic plants, particularly water hyacinth and alligator weeds. Knife-type cutters, which are dragged through the plant beds and cut by scything action, have been used for cutting both submerged and emergent plants. A continuous chain-type cutter carrying sets of moving blades over stationary shearing knives has been used on some recent models of aquatic weed harvesting machines. Chains and draglines are often pulled along the bottom of water ways behind powerboats as a means of uprooting vegetation. Even though machinery can and will destroy the aquatic plants, it must be clearly recognized that all mechanical devices have their individual limitations. An important aspect in aquatic plant removal is that organic matter and nutrients from the process of the weed decomposition on dry land should not be washed back into the water body from which they have been removed. Therefore, if noxious plants are harvested and then processed by composting, burning or other ways, this should be done well away from the water body from which they originated.
The effect of herbicides on plants varies from one chemical to another. Many herbicides control several to many species within either the submergent or emergent groups; others control plants in both these categories with varying effectiveness. Some chemicals will control higher aquatic plants and algae as well (Zajic, 1971). Mackenthum, Ingram and Porges (1964) state that effective algicides or herbicides used in water must:
Be reasonably safe to use;
Kill the specific nuisance plant or plants;
Be nontoxic to fish, fish-food organisms and terrestrial animals at the plant-killing concentration;
Not prove seriously harmful to the ecology of the general aquatic area;
Be safe for water contact by humans or animals, or provide suitable safeguards during the unsafe period;
Be of reasonable cost.
Some of these factors assume added significance, based primarily on the physical aspects of a particular control operation.
Chemical control has proved the most economical and effective means of rapidly eliminating certain plant species (Montgomery, 1965). Its principal advantages include: ease in application, lasting effect, and covering of a large area in a short time of application (Mackenthum and Ingram, 1967). It may be used in conjunction with mechanical control for areas where mechanical methods only give poor results or are inapplicable, for example, on large water bodies. Sometimes it may be applied in advance to prevent a nuisance problem or to suppress a particular species (Livermore and Wunderlich, 1970). Herbicides are usually sprayed on the foliage of floating and emergent plants. Spray may be applied from the banks, boats, hovercrafts, airplanes or helicopters, depending on the plant and areas covered by them. Manufacturers' instructions should be followed exactly concerning the percentage solution required to provide effective control, and frequently trials are necessary prior to full action. Herbicides for submerged plant and algal control have to be introduced into the water to form a dilute solution. To determine the amount of herbicide to be added to water to obtain the required concentration, the weight of water should first be calculated as follows:
Weight of total volume of water in kilogramme = area in mi2 × average depth in mi × 2 200
The amount of herbicide to be added is then determined by the equation:
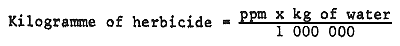
Fryer and Makepeace (1970) suggested that it is often quicker and more convenient to calculate the volume of water in acre-foot (i.e., area in acres × average depth in foot). They stated that since an acre-foot of water weighs a little over 2 700 000 lb, an addition of 2.7 lb herbicide per acre-foot will provide a concentration very close to one part per million.
The use of chemicals for controlling aquatic weed must not only produce the fastest kills, it must also be safe to fish and fish-food organisms. The effects of aquatic herbicides on fish may derive from direct toxicity to fish or indirect effects such as deoxygenation caused by decomposition of killed weeds. Fortunately, it appears that acute toxicity of herbicides to fish is at higher concentrations than those used for weed control. Swan (1967) pointed out that the margin of safety that may exist for a herbicide used in controlling water weeds depends not only on its toxicity, the concentration at which it is effectively phytotoxic, and metabolism in animals, but also on its rate and mode of disappearance from water. He stated that the rate of disappearance of herbicides from treated water is affected by some variables such as the nature of the bottom soil and the amount of agitation after application. A maximum period of 3–4 weeks is recommended as necessary for 2,4-D, and dalapon to decline to about one hundredth of their initial level. Some herbicides such as bipyridylium compounds, paraquat and diquat have the advantage that they are absorbed by weeds and absorbed to soil which leads to their rapid disappearance from water. Sewell (1970) reported that the residues of diquat fell below detectable levels between 4 and 8 days after a cove treatment with 2 gallons diquat per surface acre in Findley Lake, and between 0 and 1 day in Chautauqua Lake. The latter was a typical perimeter treatment of a large lake with the same rate of application. Silvo (1967) found the concentration of paraquat in the water was reduced from 0.1–1.0 ppm to less than 0.001 ppm in 5 days, at the most, after treatment.
At the Southeast Asian Workshop on Aquatic Weeds in 1974, it was agreed that chemical control of aquatic weeds in Southeast Asia should be applied only when the following conditions are fulfilled:
Correct identification of the weed problem and other plants that are likely to be affected;
Knowledge of the nature of the chemicals, their persistence in the environment, and potential toxicological and handling problems;
Availability of the recommended herbicide, equipment and trained personnel;
Information regarding the uses or disposal of water in which the weed problem exists, such as its intended use for agricultural, industrial, recreational and other direct human purposes;
Determination of the relative urgency of the weed problem in terms of economic losses, or potential losses if the problem is left unsolved, and the benefit likely to be gained by the proposed treatment;
Availability of operational funds for purchases of chemicals, supplies, application equipment and payment of wages.
Algal control treatment can be marginal or complete, the type applied to a given body of water must be determined by the size, shape, and relative fertility of the water, and the estimated cost of the project (Mackenthum and Ingram, 1967). Marginal treatment refers to a method designed to obtain temporary relief in a restricted area where more extensive activity is not feasible or financially possible. In this procedure a strip, lying parallel with the shore, and all protected bays are sprayed. No other part of the area is treated even though much algae may be present. On the other hand, complete treatment is applied over the entire surface area of a water body. It ensures that a major portion of the total algal population is eliminated, so that it requires a longer period to recover. The interval between necessary treatments will be directly correlated with climatological conditions and the available nutrients released from dead algal cells. One to three complete treatments per season may be sufficient to give reasonable control. Zajic (1971) suggested that algae and rooted submerged plants should be treated while the plants are developing and before they reach nuisance levels. During this period the chemical will provide more effective control of the plants and there will be less problem of oxygen depletion as a result of decomposition of a large algal mass.
Funk and Gaufin (1971) reported that the effectiveness of individual algicides in Deer Creek Reservoir appears to be the result of several variable conditions. For example, excessive alkalinity interferes significantly with the solubility of most algicides. Field investigations showed that most effective algicides were rendered useless in water with a total alkalinity of over 300 ppm. Varying temperatures stimulate seasonal blooms and algal succession; and increasing temperatures may enhance the capabilities of some algicides. Extremely resistant algal species do not appear to respond to strong algicides even under favourable conditions. Therefore, the dosage required for control depends upon the chemistry of the water and the toxicity of algicides as well as the susceptibility of particular organisms.
Some algicides are listed in Table 2.
These two kinds of aquatic plants are considered jointly, because of similarity in control methods. Many of these plants have waxy coating on their leaves which resists penetration of chemicals unless oil carriers or sticker-spreaders are used. Surber (1961) stated that one of the most effective, economical, and safest herbicides for control of these aquatic plants is 2,4-dichlorophenoxy-acetic acid (2,4-D). It is a growth regulator type of weed killer which is absorbed by affected plants and translocated to all of their parts. Plants affected by this chemical may grow themselves to death, or simply wilt and die from the toxic effects. This herbicide has been used in liquid form, either in kerosene, household detergent or in other organic chemicals, such as in formagens, for the control of these aquatic plants. Other herbicides are also widely used; the results of their effectiveness are summarized in Table 3.
Table 2
Some suggested herbicides and their effectiveness in controlling algae
| Algicides | Rate to Kill | Name of Algae | References |
| Algaecidex | 1.0 ppmwa (CuSo4) | phytoplankton | Hiltibran, 1970 |
| Atrazine | 0.2 ppmw | filamentous | Hiltibran, 1970 |
| Copper sulphate | 0.3–0.5 ppm (soft water) 1.0 ppm (hard water) | blue-green and green | Surber, 1961 |
| Cutrine | 1.0 ppmw (CuSo4) | filamentous, phytoplankton | Hiltibran, 1970 |
| Dichlobenil | 4.5 kg/ha | Chara vulgaris | Hiltibran, 1970 |
| 1.0 ppm | Vaucheria | van Busschbach and Elings, 1967 | |
| Diquat | 1.0 ppm | Cladophora | Jennings, 1967 |
| Diuron | 1.1 kg/ha | filamentous | Hiltibran, 1970 |
| 0.1 ppm | filamentous | Bungenberg de Jong, 1967 | |
| Pennsalt TD-47 | diatoms | Funk and Gaufin, 1965 | |
| Pennsalt TD-188 | green, diatoms | Funk and Gaufin, 1965 | |
| Pennsalt TD-191 | green, diatoms | Funk and Gaufin, 1965 | |
| Paraquat | 0.5–1.0 ppm | phytoplankton | Silvo, 1967 |
| Simazine | 0.2–0.4 ppmw | filamentous, phytoplankton | Hiltibran, 1970 |
a ppmw = part per million by weight
Table 3
Some suggested herbicides and dosages recommended
to control floating and emergent aquatic plants
| Herbicides | Rate to Kill | Name of Plants | References |
| 2,4-D, acetamid (20% act.ingr.) | 11.2 kg/ha | needlerush | Surber, 1961 |
| 2,4-D, amine | 0.5% | pickerel weed, burreed, water hyacinth, arrow- head | Surber, 1961 |
| 2,4-D, ester | 0.5% | cattail, pickerel weed, lotus, alligator-weed, softstem, bulrush, needlerush, parrot-feather, water lettuce, water shield, white water lily, spatterdock, burreed, arrowhead | Surber, 1961 |
| 2,4-D, granulated | 112.3 kg/ha | water shield | Surber, 1961 |
| 2,4-D, iso-ester | 5.6 kg/ha | cattail | Surber, 1961 |
| 2,4,5-T | 1 000 ppmw | water hyacinth, Pistia, Spirodela, Ipomoea | Misra and Das, 1969 |
| Amitrole | 11.2 kg/ha | cattail | Surber, 1961 |
| Amitrole formulation | 2.3 kg/ha | water hyacinth | Gallagher, 1962 |
| Dalapon | 11.2–22.4 kg/ha | cattail, cutgrass, manna grass | Surber, 1961 |
| Dichlobenil | 1.0 ppm | Phragmites communis, Acorus calamus | van Busschbach and Eling, 1967 |
| Diquat | 0.5 ppmw | Hydrilla verticillata | Mackenzie and Hall, 1967 |
| 1.0 ppm | Callitriche stagnalis | Surber, 1961 | |
| Endothall | 0.24 kg/1 | duckweed | Bennett, 1970 |
| Diuron | 0.6–1.1 kg/ha | Chara, water primrose | Blackburn and Weldon, 1970 |
| 2.0 ppmw | Hydrilla verticillata | ||
| Silvex | 9 kg/ha | Alternanthera philoxeroides | Surber, 1961 |
| 3.4 kg/ha | Softrush |
Table 4
Some suggested herbicides and dosages recommended to control submerged squatic plants
| Herbicides | Rate to kill | Name of plants | References |
| 2,4-D, ester (20% granular) | 22.5–45 kg/ha | Myriophyllum spicatum | Smith and Hall, 1967 |
| Copper sulphate | 112.3 kg/ha | Elodea densa | Ware, 1966 |
| Diquat | 0.5–1.0 ppm | most submerged weeds | Newman, 1967 |
| 1.0 ppm | Elodea canadensis, Ceratophyllum demersum, Potamogeton spp., Hydrocharis morsus-ranae, E. densa | Jennings, 1967 | |
| 8.5 l/ha | water milfoil | Sewell, 1970 | |
| Diuron | 0.6–1.1 kg/ha | black willow, coontail, naiads | Dalrymple, 1971 |
| 2.3 kg/ha | all above, plus lotus, pond weeds | Dalrymple, 1971 | |
| 3.4 kg/ha | all above, plus curly- leaf pondweed, rushes, water smartweed | Dalrymple, 1971 | |
| 4.6 kg/ha | all above | Dalrymple, 1971 | |
| Endothal (granular) | 0.5 ppm | Potamogeton diversifolius | Surber, 1961 |
| 1.0 ppm | P. pusilus | Surber, 1961 | |
| 5.0 ppm | P. americanus, P. foliosus | Surber, 1961 | |
| Hydrothol | 3.0 ppm | Najas guadalupensis | Frizzell, 1962 |
| Hydrothol 191 (granular) | 2.5 ppmw | Elodea spp. | Ware, 1966 |
| Paraquat | 0.5–1.0 ppm | most submerged weeds Elodea canadensis | Newman, 1967 Silvo, 1967 |
| Silvex | 0.5 ppm | white water lily | Younger, 1958 |
| 1.0 ppm | yellow water lily | Younger, 1958 | |
| 2.0 ppm | water weed, mud plantain, water milfoil | Younger, 1958 | |
| Simazine | 11.2 kg/ha | P. diversifolius | Surber, 1961 |
| Sodium arsenite | 2.5–4.0 ppm | most submerged weeds | Surber, 1961 |
The chemical control of submerged plants has been investigated as much as the others. Many chemical substances have been tested for this purpose. Surber (1961) reported that sodium arsenite is a cheap and very effective chemical for controlling nearly all species of submerged aquatic plants. Smith and Hall (1967) found that applying butoxyethanol ester of 2,4-D in a 20 percent granular form was the most effective control of Eurasian watermilfoil (Myriophyllum spicatum L.). Jennings (1967) reported that a concentration of 1.0 ppm of diquat was extremely effective against certain underwater weeds such as Elodea canadensis, Potamogeton spp., etc. Newman (1967) stated that the application of paraquat or diquat to water at rates of from 0.5 to 1.0 ppm will kill most submerged weeds. The treatment had no direct toxic effect on fish or on invertebrates in water. The applied herbicide was removed from the water rapidly, and was ultimately destroyed by microbiological breakdown, or was inactivated by absorption in the bottom-mud. The results of application of some common herbicides on submerged plants are summarized in Table 4.
Biological control of aquatic weeds involves the use of natural enemies to suppress pest species. This method is considered as the most feasible method in controlling aquatic weeds in areas where the cost of chemical or mechanical control is prohibitive, or impossible, such as areas too large to be efficiently treated by the above methods. Biological control usually results in relatively low costs; however it requires ready supply sources. The ease of application often requires no special equipment and minimal training of unskilled personnel (Butler, Ferguson and Berrios-Duran, 1968). The success of this method of controlling has been accomplished mainly by the use of insects, snails, fishes, and manatees as the controlling agents. It should be realized, however, that thorough tests under strict quarantine conditions should precede any introductions of exotic biological control animals into new environments, to establish or reconfirm firmly their specificity of action.
Insects are one of the most effective biological control agents. They may kill their established host plants either directly by destruction of photosynthetic tissue, or indirectly by causing depletion of the food reserves. Blackburn, Sutton and Taylor (1971) listed the essential characteristics of an insect as a biological controlling agent:
The ability to kill aquatic vegetation or prevent its reproduction in some direct or indirect way.
An ability to disperse and locate its host plant.
An adaptability to weed host and to environmental conditions in which it occurs.
A reproduction capacity sufficient to overtake an increase of its host plant.
Host specificity to prevent damage to desirable plants.
The most outstanding example of an insect controlling aquatic vegetation is the flea beetle, Agasicles sp., on alligator weed, Alternanthera philoxeroides (Mart.) Griseb. This insect was introduced to the United States from Argentina in 1964 (Hawkes, 1965); since then the flea beetle has become established throughout most of the region infested by alligatorweed, particularly in the southeast states. This insect feeds only on alligator weed. Blackburn, Sutton and Taylor (1971) found the beetle population declined rapidly after the alligator weed leaves had been eaten, and the flea beetles then were forced to eat the stems, and/or move to other areas. Sailer (1972) reported that the stemboring moth, Vogtia malloi Pastrana is also an effective controlling agent on alligator weed. Laboratory studies showed that the damage caused by the moth-larval feeding was more injurious to alligator weed than that of the flea beetle. Furthermore, he found that the adult of the weevil Neochetina sp., feeds on water hyacinth leaves, and their larvae tunnel in the stem and root crown of this plant. It seems possible to use them as a controlling agent on water hyacinth.
Floating fern, Salvinia molesta, has been successfully controlled by wingless aquatic grasshopper, Paulinia acuminata. There are very strong indications that Paulinia was responsible for a decline in area of Salvinia on Lake Kariba in Africa from about 400 km2 to 50 km2 (Mitchell and Rose, 1979). On the Linyanti River in northern Botswana, although the insect is successfully established, it has not as yet made a significant impact on the amount of Salvinia in the system. It has been suggested that this may be a reflection of the climate of the Linyanti area where mean daily temperatures drop as low as 17.5°C, close to the developmental zero for Paulinia of approximately 15°C (Thomas, 1974, Edwards and Thomas, 1977).
Information on insects that feed on submerged plants has been obtained. The larvae of the moth, Paraponyx stratiotata (L.), clearly prefer to feed on Myriophyllum spicatum, and also feed to varying degrees on other submerged aquatic plants (Sailer, 1972). He also reported that two weevils of the genus Bagous, feed on Hydrilla verticillata (Casp.) However no details of the success in controlling submerged plants by using these insects has been reported so far.
The investigation of a large tropical freshwater snail, Marisa cornuarietis L., for the biological control of aquatic weeds was begun in 1961 at Fort Lauderdale, Florida (Blackburn and Weldon, 1965). Seaman and Porterfield (1964) reported that this snail attacked a variety of submerged aquatic plants with complete control of Ceratophyllum demersum L., Najas guadalupensis (Spreng) Magnus, Potamogeton illinoiensis Morong, and partial control of water lettuce, Pistia stratiotes, and alligatorweed, Alternanthera philoxeroides. Water hyacinth, Eichhornia crassipes, was not completely eaten; however, its growth and flowering were greatly retarded by root pruning action of the snails. Marisa preferred submerged weeds to floating or emersed weeds, but the floating Salvinia rotundifolia Wild. was eaten nearly as readily as submerged weeds. The problem of using Marisa in controlling aquatic vegetation is that they will eat young rice plants when other food is not available. This feeding behaviour has prevented the release of this snail in rice growing regions of the world (Butler, Ferguson and Berrios-Duran, 1968). Experiments in New Zealand showed that this snail can convey cercaria larvae of the liver fluke parasite and for effective weed control it requires very high population densities of the snail. Therefore, the introduction of Marisa as a biological control agent was not accepted in this country (Chapman et al., 1974). Another species of freshwater snail that shows promise as a biological control of submerged vegetation is Pomacea australis d'Orbigny. This snail is native to northern Brazil. The preliminary experiments showed that the snail fed on many aquatic weeds, even more vigorously than does Marisa (Blackburn, Sutton and Taylor, 1971).
Many herbivorous fishes have been used as biological control agents for aquatic vegetation. These fishes may limit the growth of aquatic weeds either by ingesting the plant tissue or by stirring the hydrosoil to increase turbidity which inhibits light penetration and thus reduces photosynthetic activity (Blackburn, Sutton and Taylor, 1971). They listed the fishes that appear to have promising potential in controlling aquatic weeds: tilapias Tilapia rendali (Boulenger); Tilapia1 mossambica Peters; Tilapia1 nilotica L; Tilapia zillii Gervais; grass-carp, Ctenopharyngodon idella Val.; Silver dollar, Metynnis roosevelti Eig.; Silver dollar, Mylossoma argenteum E. Ahl.; Common carp, Cyprinus carpio L.; and Israeli carp, Cyprinus carpio L. (Israeli strain). Other species which have been tested and appear to have value include; Silver carp, Hypophthalmichthys molitrix (Val.) (Prowse, 1969); Carp, Puntius sp., and Osteochilus hasselti Cuv. and Val. (Hora and Pillay, 1962); Carassius carassius L. (Swingle, 1957); Osphronemus sp., Alestes macrophthalmus Gunther, and Distichodus sp. (Hickling, 1961); channel catfish, Ictalurus punctatus Rafinesque; goldfish, Carassius auratus L. (Avault, 1965); and Mugil sp. (Grizzell and Neely, 1962).
1 Sarotherodon mossambicus, Sarotherodon niloticus
Tilapia rendalli feeds mainly on plankton, filamentous algae, and higher aquatic plants. Maar (1960) reported that this tilapia effectively controlled both submerged and floating plants in reservoirs in Zimbabwe. Investigation showed that their stocking rates in ponds of approximately 3 700 to 4 950 per ha could control Pithophora sp.; Spirogyra sp.; Eleocharis acicularis; Elodea densa; Hydrochloa sp.; Utricularia biflora, and Rhizoclonium sp., in three months (Avault, 1965). Pierce and Yawn (1965) reported that T. rendali and S. niloticus are excellent control of filamentous algae and submerged vegetation where these fishes can overwinter and are stocked alone or allowed to become well established prior to the stocking of bass. Experiment in Thailand showed the daily feeding rate of S. niloticus with an average of 27.58 g in weight (11.45 cm), on duckweed (Lemna) was 79.50 percent of body weight (Tanthong, 1973). The introduction of both species of tilapias in Puerto Rico resulted in control of Spirogyra, Chara, Najas, Nitella, and some surface plant covers (Butler, Ferguson and Berrios-Duran, 1968). The stocking of Tilapia aurea in reservoirs in Israel resulted in a significant decrease in the amount of filamentous blue-green algae and submerged vegetation (Eren, Yashouv and Langer, 1972). In Malacca, Prowse (1969) reported that Tilapia zillii eat most aquatic vegetation except the most woody water weeds; however, bigger fish apparently are quite able to control the coarser sedges, possibly by uprooting them when the fish build their nests. He also stated that this species feeds on some floating plants such as Lemna and Spirodella. In Africa, Sarotherodon macrochir was introduced into Lake Kariba as an alga-controlling agent (Jackson, 1960).
Experiments in Thailand showed that aquatic plants are important food for the Thai silver carp, Puntius gonionotus. This fish feeds mainly on aquatic plants and its feeding rate increases gradually as the fish grows (Pawhorm, 1970). He found aquatic plants constituting 64.7 percent of total food (in weight) consumption for fish of less than 75 mm in total length; the feeding rate would increase to 71.4 percent and 83.3 percent for fish ranging between 7.5–12.5 cm and over 12.5 cm respectively.
The grass carp is one of the most promising herbivorous fish for controlling aquatic plants. This fish feeds primarily on submerged plants as well as small floating plants, but with different degrees of preference (Cross, 1969). When preferred plants are not available, they turn to feed on overhanging terrestial plants and bank grasses. Active feeding begins when water temperature rises above 10°C with an optimum feeding temperature of near 26°C. Small fish, less than 1.2 kg, may eat aquatic plants several times their body weight daily. Large fish, under favourable conditions, consume at a rate of around their body weight daily (Anon., 1976). However, feeding may be interrupted by abrupt changes in temperature and by the ripples produced by wind, making it difficult to assess food selectivity (Alabaster and Stott, 1967). They also claimed that feeding becomes less selective as its intensity increases with increase in temperature up to 25°C and also as the total supply of food and variety of species is reduced. Weed control is rapidly achieved if more than 75 fish per ha are stocked (Anon., 1976).
In Thailand the grass carp shows promise as an aquatic plant-controlling agent, but also as a valuable food fish. Experiments were conducted by feeding grass carp of sizes ranging between 223.5–241.5 g (or 26.0–31.0 cm long) with Najas, Azolla, Hydrilla and water hyacinth. The average water temperature during the experiments was between 16.2–25.0°C. The fish fed heavily on these aquatic plants. Average daily feeding rates on Najas, Azolla, Hydrilla, and water hyacinth were 2.18; 1.78; 1.52 and 0.44 times their body weight respectively (Chatmalai, Bhukaswan and Pongpangan, 1976).
Avault (1965) reported that the Israeli carp was an effective controlling agent for Pithophora, Rhizoclonium and Eleocharis acicularis at a stocking rate of 60 to 120 fish per ha. He also found goldfish gave good control of Pithophora when stocked at a rate of 1 690 fish per ha. Blackburn, Sutton and Taylor (1971) mentioned that the silver dollar fish, the small, herbivorous fish of South America, Metynnis roosevelti and Mylossoma argenteum can control a variety of submerged aquatic weeds such as horned pondweed, Zannichellia palustris L., American pondweed, Potamogeton nodosus Poir., and sago pondweed, Potamogeton pectinatus L. The silver dollar fish prefer new growth plants to older plants as do many other plant-feeding fish.
The manatees, or sea cows, Trichechus spp., are widely distributed in tropical and sub-tropical countries facing the Atlantic Ocean. These animals are capable of living both in freshwater and marine environments and are often observed in estuaries. They are large, warm-blooded, air-breathing herbivores that have been suggested as aquatic weed control agents for Central and South America, the Caribbean, and West and Central Africa (Anon., 1976).
Manatees eat a wide variety of aquatic plants but seem to prefer submerged to floating, and floating to emergent plants. Their feeding habits are very systematic, eating first the luscious, submerged plants and then the less attractive and more fibrous floating and emergent plants (Allsopp, 1969). In Guyana and Florida, manatees have been known to consume many species of aquatic plants, for example, Cabomba, Elodea, Hydrilla, Najas, Myriophyllum, Potamogeton, Chara, Eichhornia, Salvinia, Azolla, Pistia, Lemna, Typha, Montrichadia, Nymphaea, and many others (Anon., 1973). There are many advantages of manatees as an aquatic weed controlling agent:
Manatees are docile, unobtrusive, and harmless to man.
Manatees are voracious feeders, consuming as much as a quarter of their body weight per day. An adult manatee, exceeding a half ton in weight and over 3 m in length, consumes more than 90 kg of wet vegetation daily.
Manatees are remarkably unselective in their choice of food. Although they prefer succulent aquatic plant, if denied this they will consume almost any water plants.
Manatees adapt readily to confinement, provided they are assured an adequate food supply.
Manatees can survive in water that is fresh or saline, acid or alkaline, turbid or clear.
Despite their size and weight, manatees are relatively easy to transport. They tend to remain passive and immobile out of water, but care has to be taken to prevent them from rolling on their back.
Manatees are a potential source of protein.
The use of manatees as a means of water weed control is far more effective and lasting than the usual chemical control (Allsopp, 1969). It had been found that two manatees 2 m in length were capable of clearing a canal 7 m wide and 1 500 m long with a massive growth of submerged plants in 17 weeks. Sguros, Monkus and Phillips (1965) reported that one male and 4 female manatees ranging in weight from 160 to 900 kg and being up to 3.8 m in length could eat the submerged weeds in half a mile of canal with almost normal water flow in about one week. Blackburn, Sutton and Taylor (1971) claimed that in an experiment using the same weight of manatees they could clear the same canal infested with water hyacinth in 8 weeks. After the canal was cleaned of submerged and floating weeds, the manatees began to eat the bank vegetation that extended into the canal water. They also reported that the canal was free of vegetation for 6 to 8 months after the manatees were removed.
The manatees are harmless to fish and other aquatic animals. Their capability appears as a feasible means of controlling aquatic weeds particularly in areas where other means are impractical such as in canals of British Guyana1.
An integrated control of aquatic weeds is defined as the application of two or more different types of control measure to maintain aquatic vegetation at an acceptably low level. The approach may incorporate chemical, physical and biological control methods, together with preventive measures, but must be based on sound ecological principles. Soerjani et al. (1976) emphasized that the combination of different control measures will rarely result in simple additive effects. There are likely to be potentially valuable, synergistic interactions between methods to be exploited, and likewise there are the possibilities of antagonistic, detrimental interactions which must be avoided.
At present, the most useful measure for controlling some aquatic weeds in reservoirs of Southeast Asia and the Indian sub-continent appears to be biological control with herbivorous fishes. Several herbivorous fishes have been used to fullfil this purpose in this region for years. Herbivorous fishes not only give good promise in controlling aquatic weeds but also provide an excellent source of animal food for human consumption. However, other methods need to be applied as alternative measures whenever herbivorous fishes cannot fullfil the requirements of controlling floating and emergent vegetation. If only a small area is infested, mechanical removal using human labour is recommended. This method has dual benefits. It is not only capable of controlling aquatic weeds effectively, but it also provides income in terms of wages to poor rural inhabitants. If the infestation covers vast areas and needs to be eradicated rapidly, it may need the use of chemicals. However, this measure should be given the last priority because it is dangerous to human lives, live-stock, and aquatic ecosystem, as well as requiring foreign exchange for purchase of chemicals and equipment. Use of aquatic weeds as animal feed for cattle, pigs, ducks, for making household items, e.g., mats, baskets, containers, and as a source of fertilizer should be encouraged. Such utilization of aquatic weeds has been widely applied in Southeast Asian countries.