SCS/82/SBTC/LEC.1
by
H. R. Rabanal2 and V. Soesanto2
1. INTRODUCTION
1.1 Nature and importance of species
The Lates calcarifer, commonly called the giant seaperch, and in the Southeast Asian region, the seabass, is an economically important food fish in the countries of the tropical and sub-tropical areas of the western Pacific and Indian oceans. It grows to comparatively large size with delicate-flavoured flesh and commands premium price in the market. It is a euryhaline species with catadromous habits within its area of distribution. There is a limited fishery for this species recorded in the different countries, although data available in this regard is very scarce. The possibility of its culture has recently been initiated with high potential for further development.
1.2 Taxonomy
Phylum — Chordata
Subphylum — Vertebrata
Class — Pisces
Subclass — Teleostomi
Order — Percomorphi
Family — Centropomidae
Genus — Lates
Species — Lates calcarifer (Bloch)
The above is a well-accepted taxonomic classification of the giant seaperch. This species has also been placed under the Family Serranidae which includes the groupers, seabasses, coral trouts, coral cods and seaperches. Some authors also place this species under the Family Latidae which includes two genera. Lates and Psammoperca, with a number of species. Presently, however, Centropomidae appears as the predominantly accepted family name of this species.
A close relative, the Lates niloticus is the giant African freshwater fish widely distributed in the rivers Nile, Congo, Volta and Niger and Lake Chad, in Africa.
For the recognized generic name, Lates, there were other names previously listed. These are called synonymy for this genus. These include Perca, Pseudolates, Holocentrus, Coius, Plectropoma, Latris and Plectropomus. Likewise, the specific name has a large number of synonymy given by various authors for collections from different areas within the range of distribution of this species. Bloch (1790) described the type specimen of Lates calcarifer allegedly from Japan and named it Holocentrus calcarifer. A compiled synonymy for the species is found in Appendix A.
The common names attributed to this species varies with language and area. The giant seaperch is the recognized common English name; seabass is the English name commonly used in Southeast Asia. Other English names used are white seabass, silver seaperch, giant perch, Palmer, cock-up and two-finned seabass. In Australia, Papua New Guinea and part of Eastern Indonesia, it is called barramundi, while in most of Indonesia, and in Malaysia and Singapore, it is called kakap; it is pla kapong in Thailand and apahap in the Philippines. A comprehensive listing of common names by countries and areas are listed in Appendix B.
2. DISTRIBUTION
2.1 Geographic distribution
As previously mentioned, the species is widely distributed in the tropical and sub-tropical areas of the western Pacific and Indian oceans. Its range of distribution includes the areas from Australia, Papua New Guinea, the Indonesian archipelago, the Philippines, China, Vietnam, Kampuchea, Thailand, Malaysia, Singapore, Bangladesh, India, Sri Lanka, Pakistan, Iran, Oman and the other Arab countries bordering the Arabian Sea. The first report on this species pointed to Japan as the locality of collection but this is now subject to doubt by some ichthyologists and needs verification. It is interesting to note that the fish is not distributed in the many Pacific island countries beyond its Australian longitudinal boundary of distribution.
2.2 Ecological distribution
The euryhaline and catadromous characteristics of this species result in a very interesting ecological distribution at various stages of its life history. Spawners are known to congregate along the mouths of river systems, lagoons or estuaries where the eggs are laid during the incoming tide. This enables the eggs and the hatchlings to drift into estuarine brackishwaters such as in mangrove swamps where they develop and undergo their larval stages. The fry and fingerlings then migrate actively and have been observed to go as far as 100 km upstream, in freshwater areas if such water bodies are available. In freshwater, they attain the size of 1–5 kg or a length of 30–70 cm. By the third to the fifth year, they would feel the urge to develop their gonads and spawn, thence they migrate downward to the coasts. It is believed that they attain full sexual maturity within a relatively short period of stay in the sea after which they congregate in the mouths of the coastal rivers, and under favourable conditions of salinity, tide, depth and movement of water, phase of the moon, and weather, they would spawn.
3. MORPHOLOGY AND DISTINGUISHING CHARACTERISTICS (Figures 1.2)
The giant seaperch or seabass has an elongate body, compressed, with a deep caudal peduncle. The head is pointed with a very pronounced concave dorsal profile at a level above the eyes, becoming convex in front of the dorsal fin. It has a large mouth which is slightly oblique, with the upper jaw reaching to behind the eyes. The teeth are villiform but with no canines. The lower edge of the preoperculum has one strong spine and a number of serrations. The operculum has a small spine with a serrated flap above the origin of the lateral line. The dorsal fin has seven to nine spines and ten to eleven soft rays. This has a very deep notch that almost divide the spiny from the soft part of the fin. The pectoral fins are short and rounded with several short strong serrations above its base.
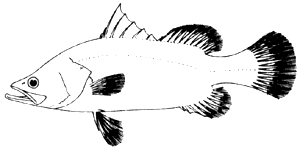
Fig. 1 The giant seaperch or seabass, Lates calcarifer
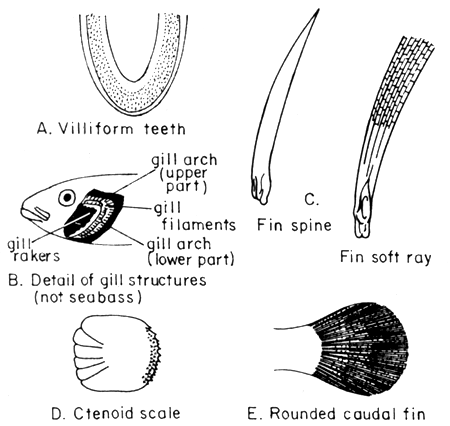
Fig. 2 Morphological features
The dorsal and anal fins have scaly sheaths. The anal fin is rounded with three spines and seven to eight soft rays; the caudal fins are rounded. The scales of Lates calcarifer are large of ctenoid type and are therefore rough to the touch (FAO, 1974).
It could be difficult to distinguish the giant seaperch from closely related species unless one knows its distinguishing characteristics. In the Philippines, for instance, one species of Lates calcarifer and one species of Psammoperca (P. waigiensis) have been identified. These are very closely related and can easily be mistaken for each other. The Lates calcarifer can be distinguished by having the lower boarder of its preoperculum serrated, its maxilla reaching behind the eyes, its nostrils close together and its tongue smooth. The Psammoperca has the lower border of its preoperculum, smooth, its maxilla reaching only below the eye, its nostrils widely separated and tongue with patch of teeth.
The Lates calcarifer has no colour markings, but it is known to have two colour phases. In one phase which occurs among small juveniles of 10 to 15 cm, it is blue brown above and silvery below. In larger fish, it is greenish blue or greyish along the back and silvery along the sides and below (Chan, 1968). The larvae and fry are dark in colour becoming lighter as they assume the juvenile stages. The fins are all greyish in colour in the adult. The eyes of seabass are shiny pinkish-red in colour. At night they produce bright characteristic glow.
4. BIOLOGY AND LIFE HISTORY
4.1 Growth and life in natural waters
Where there are suitable inland freshwater bodies (river systems, lakes, and lagoons with connections with the sea, etc.), the giant seaperch spend most of its growing period in these types of water. It is aggressive and carnivorous subsisting mainly on live food and can grow very fast. They spend two to three or more years of initial growing period in freshwater or brackishwater where they attain weight of 1.5 to 5 kg. The development of the gonads accompanied by the urge to spawn lead them to migrate, towards the coast. It is possible that the spawners migrate to the sea after this spawning and attain further growth or they may return to fresh-brackishwater also to grow further. There seems to be no set migration pattern during this adult stages of this species and it is not known whether the fish return to the same area where they were spawned. This species is known to attain big size. It can reach the maximum length of 200 cm and a weight of over 50 kg.
4.2 Fecundity and spawning
During the spawning run, it is easy to distinguish the male from the female spawners. The males are usually smaller with slender shape and narrower body depth. If ripe, the milt can be observed to issue from the genital opening with slight pressure on the belly. On the other hand, the female spawners can be recognized easily for their big soft round belly and a red-pink papilla extending out at their urogenital aperture. If fully ripe, eggs will be visible through this named opening with slight pressure on the belly.
Observations on the fecundity of spawners very. This is very much due to the size and age of the spawner and the kind of examination made by the observer. In Australia, one report mentioned that a 12 kg fish (107 cm long) contains 7.5 million eggs; a 19 kg fish, 8.5 million eggs; and a 22 kg fish, 17 million eggs (Fisheries Division, Australian Department of Agriculture, 1975). Another observer referring to work done in Thailand and a FAO/UNDP project in Malaysia mentioned that fully matured female fish of 70 to 100 cm total length weighing 7 to 10 kg yield approximately 200 000 to 400 000 eggs on stripping, and a ripe male of 70 to 80 cm can give above 4.5 ml of milt (FAO/UNDP Institutional Support for MAJUIKAN Aquaculture Development Project, 1981). The discrepancy in these figures may be explained by the presence of eggs of different age stages in the mature female enabling it to reproduce two or more times during the year. Therefore, the actual count of eggs contained in the gonads will differ with the number of eggs that can be extruded during each spawning period.
4.3 Fishery of the seabass
There is no available data on world production of seabass. There are some incomplete data of the fishery in the Southeast Asian region (SEAFDEC, 1978; and Bureau of Fisheries and Aquatic Resources Philippines, 1980). In Southeast Asia in 1978, it is recorded that there was a total production of 10 192 metric tons valued at US$5 362 000 (SEAFDEC, 1980). This production (mt) came mainly from the following countries: Indonesia, 9 314; Malaysia, 147; and Philippines, 731 or total 10 192 metric tons. There were no available records for Brunei. Taiwan (China), Hong Kong, Kampuchea, Singapore, Thailand and Vietnam.
The methods of capture used include bottom trawls, seines, gill nets, stake traps, hook and line, and lift net. So far, only Indonesia, during that year (1978) reported production through backishwater pond culture of 571 metric tons valued at US$473 000. It is noted from the fragmentary data available that the production from year to year from marine capture fisheries are highly fluctuating and many countries in the region do not reflect the production for this species although undoubtedly, they may have some (e.g., Taiwan (China), Hong Kong, Thailand, Vietnam). An example of radical fluctuation in the amount of marine capture production is from the available data in the Philippines during the period of 1976–1980 (Bureau of Fisheries and Aquatic Resources, 1980).
| Year | Production (mt) |
|---|---|
| 1976 | 212 |
| 1977 | 228 |
| 1978 | 663 |
| 1979 | 294 |
| 1980 | 60 |
The reasons for these fluctuations are difficult to explain, but these show that the fisheries have great uncertainties.
It should also be mentioned that within recent years or after 1978, the culture of the giant seaperch, especially in floating net cages, has developed very rapidly. Production from this source is now available from Hong Kong, Malaysia, Singapore and Thailand although no actual figures are yet available. In other regions, especially in Australia, the fishery for the giant seaperch, called barramundi in that general area, is well-known. It is a popular and highly valued food fish and commands attractive prices in fish markets where quantities are purchased for the hotel and cafe trades (Fisheries Division, Australia Department of Agriculture, 1975). No exact data of production from this area is, however, available.
5. THE ECONOMICS OF SEABASS FISHERY/CULTURE
5.1 Capture fisheries
The profitability of marine capture fishery for seabass is based on the high demand for this species and the comparatively high price it commands in the market. As long as the fishing ground is accessible or provided with convenient transport facilities to the market outlets, the fishery from natural waters could be highly profitable. There are however, many fishing grounds for this species that are quite remote from the marketing areas (e.g., southern coast of Irian Jaya province, Indonesia and the contiguous coast of Papua New Guinea). The high fluctuation in catches such as those observed in the Philippines are unfavourable for maintaining continuing market for the product. For this reason, the innovation of culture fishery for this species has some advantages.
5.2 Culture fisheries
In Australia, culture of this species in brackishwater ponds has been identified as having some potential. It is estimated that a yield of about 2 300 kg per hectare per year may be derived from farm fishponds. This is on the assumption that there will be adequate supply of fry either from natural waters or from hatcheries and a properly designed fish farm in a suitable site is used. The following table shows the estimated running costs of production per hectare per year of a giant seaperch farm.
| Item | Cost ($A) Food at 4.4 cents/kg | Food at 11 cents/kg |
|---|---|---|
| Fry (2 000) | 100 | 125 |
| Labour (2 men) | 160 | 160 |
| Food (8:1 conversion) (18 180 kg) | 800 | 2 000 |
| Miscellaneous expenses | 125 | 125 |
| Total | 1 185 | 2 410 |
| Production cost per kg whole fish | 54 cents | 106 cents |
Note: The market price for gutted fish in 1970–71 in Queensland market ranged from 119–152 cents/kg, average 132 cents/kg.
In analyzing results of cage culture of seabass in a pilot project in Phangnga Bay, Thailand, Rabanal and Maynard (1981), came up with the following viability figures under three different costs of feed. This is shown in the following table.
Table 2.Viability test for pilot cage culture of seabass in Phangnga Bay, Thailand (under three price levels of feed). (After Rabanal and Maynard, 1981)
Amount (Baht)*
| Item | Viability test No. 1 | Viability test No. 2** | Viability test No. 3*** | |||
|---|---|---|---|---|---|---|
| Harvest, 1 500 fish less 10% mortality = 1 350 × 1 kg × Baht 45 | 60 750 | - | 60 750 | - | 60 750 | |
| Less: a) cost of seed | 7 500 | - | 7 500 | - | 7 500 | - |
| b) cost of feed at Baht 1 kg | 12 000 | - | 24 000 | - | 36 000 | - |
| c) cost of raft maintenance & depreciation | 7 000 | 26 500 | 7 000 | 38 500 | 7 000 | 50 500 |
| Margin for 9 months cage/ household | - | 34 250 | - | 22 250 | - | 10 250 |
* Baht 20 = US$1
** Cost of feed is based on Baht 2/kg
*** Cost of feed is based on Baht 3/kg
The above cost/benefit figures for a family pilot cage culture project are based on the following assumptions:
The production unit will be one raft containing six cages, each measuring 3 × 3 × 3 meters (productive volume 135 m3).
Construction will be with styrofoam floatation device supporting wooden pole walkways and nylon mesh cages. Cost of purchased materials for construction is assumed to be Baht 22 000 and life of the raft is assumed to be 3 years.
The cages will be stocked with seabass fingerlings from local nursery, 6 to 8 cm size, 250 pieces per cage or a total of 1 500 per raft. Cost of fry is Baht 5 each, total Baht 7 500 per raft unit.
Feed assumptions will be 8 kg per fish for a 1 kg grow-out size. The feed will be locally produced trash fish with a local landed value of Baht 1, 2 and 3 per kg.
Mortality rate is assumed to be 10 percent.
Growout time is assumed to be 9 months.
Farm gate value is estimated to be Baht 45 per kg.
The economic studies on the pilot project in Thailand were done in 1979 and 1980 and since then there have been rapid development in the cage culture industry for seabass not only in Thailand but also in other countries in the region. Private ventures for hatchery and nursery work have been initiated and are on the increase. Export of fry and fingerlings from Thailand has become an economic proposition and cage culture of the fish for the market is now widespread. In the future, the economic viability of this industry will vary with changes in costs of production and on the prices of marketable fish.
6. LIST OF REFERENCES
BARLOW, C.G., 1981 Breeding and larval rearing of Lates calcarifer (Bloch) (Pisces Centropomidae) in Thailand New South Wales State Fisheries, Australia, 1981: 8p.
BUREAU OF FISHERIES AND AQUATIC RESOURCES, 1980 1980 Fisheries Statistics of the Philippines, BFAR, 1980: 316p.
CHAN, W.L., 1968 Marine Fishes of Hong Kong, Part 1. Hong Kong Government Press, 1968: 17–18, 53–54.
FAO, FAO SPECIES IDENTIFICATION SHEETS FOR FISHERY PURPOSES. 1974 Eastern Indian Ocean (Fishing Area 57) and Western Central Pacific (Fishing Area 71), Vol. 1: Centrp Lat 1.
FAO/UNDP INSTITUTIONAL SUPPORT FOR MAJUIKAN AQUACULTURE DEVELOPMENT PROJECT, 1981 A guide for the induced spawning of seabass, Lates calcarifer in captivity. FAO/UNDP/ 79/018/ISMAO/81/AQUA-LEAFL/2: 13p.
FISHERIES DIVISION, AUSTRALIAN DEPARTMENT OF AGRICULTURE, 1975 The potential of aquaculture in Australia, Aust. Fish. Paper No. 21:41, 100
FOWLER, H.W., 1972 A synopsis of the fishes of China. Repr. Anti-quariaat, Junk, LOCHEM, Netherlands, Vol. 1: 382–5
FOWLER, H.W., and B.A. BEAN, 1930 Contributions to the biology of the Philippine Archipelago and adjacent regions. The fishes of the families Armidae, Chandidae, Duleidae and Serranidae, obtained by the U.S. Bur. Fisheries Steamer Albatross in 1907 to 1910, chiefly in Philippine islands and adjacent seas. Bull. US Nat. Mus. 100 (10) 1930: 174–9
GRANT, E.M., 1965 Guide to the fishes of Queensland. Dept. Harbours Marine, 1965:114
HERRE, A.W., 1953 Checklist of Philippine fishes. Fish Wild Serv., U.S. Dept. Interior, Res. Rep. 20:334–5
HERRE, A.W. and A.F. UMALI, 1948 English and local common names of Philippine fishes. Fish Wild Serv., U.S. Dept. Interior, Circular 14: 128p.
HORA, S.L. and T.V.R. PILLAY, 1962 Handbook on fish culture in the Indo-Pacific Region. FAO Fish. Biol. Tech. Paper No. 14:37
MANEEWONGSA, S., N. RUANGPANIT, T. PETCHMANEE and T. THADTHANON, 1981 Propagation of seabass, Lates calcarifer (Bloch). Dept. Fisheries, Brackishwater Fish. Div., National Institute Coastal Aquacult. Contrib. No. 1: 24p (In Thai with English abstract)
ROXAS, H.A. and C. MARTIN, 1937 A check list of Philippine fishes. Commonw. Philip., Dept. Agric. Comm. Tech. Bull. 6:314p.
WONGSOMNUK. SWASDI and S. MANEVONK. 1973 Results of experiments on artifician breeding and larval rearing of the seabass Lates calcarifer (Bloch). Songkhla Mar. Fish. Sta. Contribution (5): 20–2
APPENDIX A
RECORDED SYNONYMY OF LATES CALCARIFER
Lates calcarifer (Bloch. 1790)
Synonymy:
Holocentrus calcarifer Bloch, Naturg, Ausl. Fishe. Vol. 4, 1790, p. 100. p. 244 (type locality, Japan)
Walbaum, Artedi Piscium, Vol. 3, 1792, p. 640
Forster, Fauna Indica, 1795. p. 16
Lacepede, Hist. Nat. Poiss, Vol. 4, 1802, pp. 341, 384 (Japan)
Perca calcar Bloch
Schneider, Syst. lchth. 1 1801. p. 83
Holocentrus heptadactylus Lacepede, Hist. Nat. Poiss., Vol. 4, pp. 344 and 389, 1802)
Coius vacti Hamilton Buchanan, Fishes Ganges, pp. 86 and 369. pl. 16. fig. 28. 1822; all mouths of Ganges River
Lates nobilis Cuvier and Valenciennes, Hist. Nat. Poiss., Vol. 2. p. 96. pl. 13. 1828, Pondicherry, India.
Bleeker. Verh. Bat. Gen. XXII, 1849, Percoidem, p. 27
Lates calcarifer Cuvier and Valenciennes, Hist. Nat. Poiss., Vol. 2, p. 100. 1828
Richardson, Ichth. China, Japan, p. 222, 1846; Canton. China
Gunther. Cat. Fishes British Mus., Vol. 1. p. 68, 1859; India: Ganges, China
Day. Fishes Malabar, 1865 1 p. 2: Proc. Zool. Soc., London. 1865. p. 5. (Cochin)
Gunther. Proc. Zool. Soc. London, 1870. p. 824 (Fitzroy River)
Day. Fishes of India. p. 7, pl. 1 fig. 1, 1875–1878. O'Shaughnessy, Zool. Record, 1875 (1878). pisces. p. 9
Castelnau. Proc. Linn. Soc. New South Wales, Vol. 3. 1878. p. 42 (Norman and Fitzroy Rivers)
Klunzinger, Sitz. Bev. Akad. Wiss. Wien, Vol. 80. pt 1. 1879, p. 342 (Cleveland Bay, Queensland)
Meyer. Anal. Soc. Espan. Hist. Nat., Madrid, Vol. 14, 1885 p. 8 (North Celebes)
Day. Fishes of India. Suppl. 1888, p. 779; Fauna Brit. India. Vol. 1. 1889. p. 440. fig. 139
Thurston. Notes Pearl Fisher. Manaar. 1890, p. 91 (Tuticorin and Panban)
Kent. Great Barrier Reef. 1893. ppp. 280. 396 (North of Keppel Bay)
Vinelguerra, Ann. Mus. Civ. Genova 1890, p. 162
Boulenger, Cat Fishes British Mus., ed. 2, Vol. 1, p. 363, 1895; west coast India; Madras Ganger River; Ganger River; Burma; China; Amoy; Malay Peninsula; Baram River; Sarawak, Borneo, Fitzroy River; Queensland
Elera, Cat. Fauna Filip., Vol. 1, 1895, p. 457 (Mindoro and Calapan)
Kent, Naturalist in Australia, 1897, pp. 169 (Fitzroy in Queens- land to Ashburton in West Australia)
Stead, Fishes of Australia, 1906, p. 96 (West Australia and Queensland)
Seale, Philippine J. Sci., Vol. 3, sec. A, p. 517, 1908, Philippines
Seale, Philippine J. Sci., Vol. 5, sec. D, p. 275, 1910, Sandakan, British North Borneo, Philippines
Weber, Siboga Exp., Vol. 57, Fische, 1913, p. 215 (Makassar)
Zugmayaer, Abh. Bayer, Akad. Wiss: Math-Phys. Kl., Vol. 26. pt. 6, 1913, p. 9 (Mekran and Oman)
Sundara Raj. Rec. Ind. Mus. XII prt. VI, 1916, p. 278
Fowler, Copira, No. 58, p. 63, 1918; Philippines
Fowler, Proc. Acad. Nat. Sci. Phila., Vol. 79, p. 275, 1927; Philippines; San Fernando, La Union Province, and Orani and Orion, Bataan Province; Luzon
Herre. Philippine J. Sci., Vol. 34, p. 303, 1927; Lake Naujan Mindoro
Herre, Philippine J. Sci., Vol. 38, p. 494, 1929; bangus ponds about Manila Bay
Weber and de Beaufort, Fishes Indo Australian Archipelago, Vol. 5. p. 396, fig. 96, 1929; 23 East Indian Localities, also the Philippines
Fowler and Bean, Bull. 100, U.S. Nat. Mus., Vol. 10, p. 117, 1930; Aparri market, Cagayan province, Luzon. Macassar, Celebes
Herre. J. Pan Pacific Research Inst., Vol. 8, 1933. p. 4; Sandakan, British North Borneo, p. 8, Dumaguete, Negros Oriental Province
Umali, Edible Fishes Manila, p. 134, fig. 97. 1936; fishponds. Pampanga and Bataan Provinces, Luzon
Villadolid. Philip. J. Sci., Vol. 63, pp. 216 and 217. 1837; Balayan Bay and Pancipit River. Batangas Province, Luzon
Umali, Philip. J. Sci., Vol. 63, p. 234, 1937; San Miguel Bay. Camarines Sur Province, Luzon
Umali, Philip. J. Sci., Vol. 65, p. 182, 1938; Ragay Gulf, Luzon
Domantay, Philip. J. Sci., Vol. 71, p. 98, 1940; Zamboanga Province
Herre, various expeds., 1926–1941; Manila market, bangus ponds and Rio Grande, Pampanga Province; Cagayan River, Cagayan and Isabela Provinces; Malabon, Rizal Province; llocos Sur Province, Luzon; Agusan River, Agusan Province, and Rio Grande, Cotabato Province, Mindanao
Roxas and Martin, A checklist of Philippine fishes, Comm. Phil. Dept. Interior, Res. Rep. 20, 1953; pp. 334–335
Hora and Pillay, Handbook fishculture in the Pacific region; FAO, Fish. Biol. Tech. Paper No. 141, 1962, p. 37
Grant, Guide fishes Queensland, Dept. of Harbours Marine, 1965, p. 114
Munro, Fishes of New Guinea, Dept. Agric. Stock Fisheries, Port Moresby, New Guinea, 1967, p. 256
Chan, Marine Fishes, Hong Kong, part I, Hong Kong Govt. Press, 1968; p. 54
Fowler, Synopsis Fishes China, Vol. I antiquariat Junk, LOCHEM, Netherlands, 1972, pp. 384–385
Fischer and Whitehead, (Eds), FAO. Species identification sheets for fishery purposes, Eastern Indian Ocean, Western Central Pacific, Vol. I, 1974
Wheeler, Fishes of the World, Ferndale, London Edition 1979
Barlow, Breeding and larval rearing Lates calcarifer in Thailand, New South Wales, State Fisheries, Australia, 1981, 8
Lates heptadactylus Cantor J. Asiatic Soc. Bengal, Vol. 18, p. 983, 1850, Penang Sea, Malay Peninsula, Singapore
Lates calcarifer Kent, Great Barrier Reef, 1893, pl. 43, fig. 1
Plectropoma calcarifer Bleeker, Atlas Ichth., Vol. 7, p. 109, 1873–76; Java; Madura; Singapore; Banka; Borneo; Celebes; Vol. 8, pl. 322, fig. 3. 1876–77
Pseudalates cavifrons Alleyne and Macleay, Proc. Linn. Soc., New South Wales, Vol. 1. p. 262, pl. 3, 1877, Torres Straits or coast of New Guinea
Lates darwiniensis Macleay, Proc. Linn. Soc. New South Wales, Vol. 2, p. 345, 1878; Port Darwin, Australia
Plectropomus calcarifer Jordan and Seale, Bull. US. Bur. Fisheries, Vol. 26, p. 19, 1907, Cavite, Cavite Province, Luzon
Plectropoma calcariferum Evermann and Seale, Bull. U.S. Bur. Fisheries, Vol. 26, p. 78, 1907; Zamboanga, Zamboanga Province, Mindanao, 1844
APPENDIX B
COMMON NAMES OF LATES CALCARIFER
I. ENGLISH
II. AUSTRALIA
III. BANGLADESH
IV. CHINA
V. HONG KONG
1. Maang-ChoeVI. INDIA
VII. INDONESIA
VIII. KAMPUCHEA
1. Trey spong
IX. MALAYSIA
X. PAPUA NEW GUINEA
1. Barramundi
XI. PHILIPPINES
XII. SRI LANKA
XIII. THAILAND
XIV. VIETNAM
SCS/82/SBTC/LEC.2
by
B. Sirikul2
1. INTRODUCTION
The seabass, Lates calcarifer (Bloch), is an important cultured food fish in Thailand. The commercial culture of the seabass has developed along the coastal provinces of this country in recent years although the species has for a long time been associated with the other coastal aquaculture systems for shrimps, mullets and milkfish. In earlier years, the seabass could not be propagated artificially so that the fry had to be collected from natural grounds. The culturists had to buy them from the fry collectors. The limited amount of seabass fry did not permit culturists to undertake commercial scale grow-out at that time.
In 1973, experiments were conducted on the propagation of the seabass in hatchery ponds at the Songkhla Fisheries Station of the Department of Fisheries. By 1975, the station produced the first successful batch of induced-bred seabass fry.
At present, large number of fish farmers, both in Thailand and overseas and especially countries of the region, are interested in seabass culture. Countries including Australia, Hong Kong, Taiwan (China) and malaysia try to introduce the spawning, larval rearing and grow-out of the seabass due to the high demand for this species in their markets because of its nice taste and high nutritional value.
2. PRODUCTION OF SEEDFISH
2.1 Production from natural sources
Before the seabass could be spawned in captivity, the source of fry for culture depended on supplies from wild stock. Adult seabass migrate from the sea to spawn in the mouth of rivers in April to September. The fry are abundant in mangrove areas, that serve as their nursery grounds. Juveniles were captured in the surrounding areas with fine-meshed nets. The sizes of the juveniles are 10 to 15 cm (4–6 inches) in total length. Sometimes, they enter shrimp farms with tidal sea water let in these farms. The production of juveniles by capture was estimated at less than 500 000 per year. This was a problem for seabass culture in the past.
2.2 Production from hatcheries
Since the introduction of the artificial propagation of the seabass, shortages in the supply of fry have been alleviated in amount of seabass available for culture; most of the requirements of fish farmers were met. The production of fry can reach more than 20 million per year from two private hatcheries and three government fisheries stations. From the National Institute of Coastal Aquaculture alone, 7 073 700 fry were produced in 1981. These were mostly distributed to fish farmers but some were released in natural waters.
3. NURSING SEABASS FRY
There are two stages in the nursing of seabass fry used in Thailand. These are described below.
3.1 Nursery in tank
Nursery tank for larvae and fry should have a drain pipe so that it could be drained easily at any time. The seawater for use must be of good quality. In draining the tank, attention should be focused on the bottom water which is of a poorer quality than that at the surface. Thus, a standard drain pipe in the tank must be installed so that it could be pushed down to drain the bottom water out first. While draining is taking place, fresh seawater should be let in.
The food is one of the most important factors for nursing larvae, and various stages of fry need different kinds of food. The methods of feeding young seabass under culture conditions are described in detail in other lectures and practical work on hatchery techniques.
3.2 Nursery in nylon net cage
After nursing in a tank for 30 to 45 days, the fry can reach 1.5 to 2.0 cm in total length. They can thus be transferred to nylon net cage units each with a surface area of 2 m2 and a depth of 1 m with a mesh size 2 mm and a stocking density of 300 to 500 fry per cage. The fry are fed with finely chopped fish at 8 to 10 percent of body weight daily. The net cages are usually set in running water.
This method is very convenient. There is no need to change water, thus having less problem with diseases. Grading fry has to be done at least once a week during the nursery phase to minimize losses due to cannibalism.
2 Head, Aquaculture Research Section, NICA, Songkhla. Thailand
4. REARING MARKETABLE FISH
The three methods of rearing seabass for the market in Thailand are described below.
4.1 Culture of seabass in brackishwater and freshwater ponds
The stocking rate for pond culture is 3 to 5 juveniles per m2. The average yield average 2 500 to 3 125 kg/ha (400–500 kg/rai1) per year, with the fish at a size of 1.5 kg measuring about 45 cm in total length.
4.2 Culture of seabass in shrimp farms
This method has been used for a long time in mangrove areas. Seabass is cultured in combination with other aquatic animals, such as shrimp and particularly with Tilapia species. The seabass can consume the fry of Tilapia. The stocking of the seabass is at 3 125 to 6 500 juveniles per ha (500–1 000/rai) annually yielding at 1 250 to 1 875 kg/ha (200–300 kg/rai) with the individual fish ranging from 2.2 to 2.5 kg each.
4.3 Culture of seabass in netcages
The size of a netcage usually ranges from about 5 × 5 × 3 m to 10 × 10 × 3 m. The stocking rate is 10 to 15 fish per m2 and the annual yield is 8 to 15 kg/m2 or yield of 200 to 375 kg for the 5 × 5 × 3 m cage and 800 to 1 500 kg for the 10 × 10 × 3 m cage.
4.4 Food and feeding in growout ponds or cages
Artificial diet in seabass culture is not practiced yet, because seabass do not seem to respond well to pellets. So trash fish is suitable for feeding, and the conversion rate ranges from 7 to 10.
5. PRODUCTION
In 1981, the area of seabass culture in Thailand has increased giving an annual yield at nearly 300 000 kg and valued at about 21 million baht2 (US$1 million). Of this weight 150 000 kg were obtained from cage culture in the southern part of Thailand, and the other 150 000 kg from pond and cage culture from the middle and eastern part of the country.
6. PROSPECTS
The expansion of seabass culture is almost certain, because there are vast areas of 5 652 000 ha (or 35 325 000 rai) coastal lands and 160 000 ha (1 million rai) of mangrove swamps that are suitable for this development. In other countries, such as Malaysia, Singapore, the Philippines and Indonesia, there are also extensive shorelines which may be suitable for seabass culture. The future success in the expansion of seabass culture is dependent upon the development of artificial feeds for the species because of the expected shortage of trash fish which form major part of the present diet of the fish.
7. COMMENTS AND CONCLUSIONS
The seabass is suitable finfish for culture, though carnivorous by nature. This is probably the only brackishwater fish which could be considered sufficiently valuable to farm. The wholesale value for marketable-size seabass (from 1 kg) in 1982 ranged from 60 to 80 baht/kg or about US$3 to US$4 or at a mean of 70 baht/kg or US$3.5. The cost of production is estimated at 50 baht/kg.
1 1 rai = 1 600 square meters or 0.16 ha.
SCS/82/SBTC/LEC.16
by
P. Brohmanonda2
1. INTRODUCTION
To raise seabass in any water impoundment, the availability of fish seed is considered to be one of the most important factors for the success or failure of this type of venture. In the previous practice of seabass culture in Thailand (prior to 1970), the fish farmers had to collect seabass fry or fingerlings from the wild and then stocked them in ponds or in cages. The availability of fish seed varied considerably from year to year and the uncertain supply of this commodity had resulted in limited seabass culture in Thailand. At that time, most fish farmers considered the income generated from this practice merely as a secondary source of household income.
With the success made in artificial fertilization and mass production of seabass fry by the Songkhla Fisheries Station in 1973, the supply of fish seed was assured. The rearing of this fish is now widespread in several southern provinces along the east coast of the Gulf of Thailand as well as provinces in the Andaman Sea side. It is anticipated that the rearing of this fish would become a big enterprise in the future. In order to supply adequate fish seed to fish farmers, fish hatchery work has become a very important activity.
2. HISTORY OF DEVELOPMENT OF SEABASS HATCHERY IN THAILAND
In 1973, seabass spawning was successful by artificial means using the wild broodfish. This was done by hand stripping of eggs and milt at the Songkhla Fisheries Station, Brackishwater Fisheries Division of the Department of Fisheries. In 1976, the Department of Fisheries was granted funds for the construction of the first seabass hatchery at the Songkhla Fisheries Station. It can be said that the hatchery at Songkhla Fisheries Station was the first hatchery that succeeded in spawning seabass under controlled condition in the world.
The methods of production of fry which have been improved and developed in said Station served as source of technology in this work. Subsequently, the Songkhla Fisheries Station made further progress with the seabass spawning in captivity. This had the advantage of possibly stabilizing the amount of seabass fry for culture. Since this success, the knowledge of seabass spawning was spread to the various fisheries stations under the Brackishwater Fisheries Division. Funds were provided for the construction of seabass hatcheries at Rayong Fisheries Station, Phuket Fisheries Station, Satul Fisheries Station and Prachuab Khirikhan Fisheries Station. At present, the Department of Fisheries can produce 30 million fry per year for distribution to fish farmers and some for export to foreign countries such as Hong Kong, Singapore, Malaysia and Taiwan (China).
2 Director, NICA. Songkhla, Thailand.
3. THE DESIGN AND CONSTRUCTION OF HATCHERY
3.1 Selection of suitable site
Proper site selection is the most important consideration in establishing a hatchery. To achieve this, some of the major factors to consider are enumerated below.
Seawater. To spawn seabass, it requires seawater with high salinity, around 28–32 ppt, for rearing of broodstock as well as for nursing fish fry. Therefore, the most ideal site should be along coastal areas that have high salinity seawater all year round. To transport seawater to a hatchery which is located some distance from the seawater source would not be economically sound due to increased operations costs. And sometimes one may not get the seawater with the sequence needed for the spawning operation. This may lead to failure under such condition.
Freshwater. Clean freshwater is also one of the most important factors in hatchery operation. Sometimes, fresh- water is used to dilute seawater to get the salinity desired to suit the fish fry or to raise the food for them to eat. Besides, fresh- water is used in cleaning instruments and for staff consumption. Therefore, the hatchery should be located in the area where good supply of freshwater is available.
Electricity. Good and adequate electric supply is lso one of the most important factors in hatchery operation. This is because lots of instruments as well as lighting require electricity.
Water pollution. Polluted water always contain large amounts of bacteria or other harmful substances which may result in high mortality or poor state of health of the young seabass. Therefore, the ideal hatchery should be located in an area free of polluted water.
Flood during rainy season. In most estuarine areas, there is excess of freshwater runoff during the rainy season. This water is usually very turbid, of low salinity for about 2–3 months. This water is not suitable for the broodstock and for nursing the fish fry. Besides, low water salinity also affects the rate of egg fertilization and normal development of the embryo. The ideal site should be located in an area that is free of this natural phenomenon.
Proximity to the city. The ideal hatchery should be located near the urban area with good communication network.
Protection against winds and waves. The ideal hatchery should have natural protection against winds and waves. Under this condition, it will be easy to raise the broodstock in cages in coastal areas all year round and to transfer the broodstock without much stress to the spawning tanks during the spawning season.
Proximity to spawning ground. The ideal hatchery should be located near the natural spawning ground of the fish. This will be advantageous for the availability of the parent fish for artificial fertilization.
Nearness to fish farms. The ideal hatchery should be located in the area where there are high concentrations of fish farms. This is to facilitate the sale of fish seeds and transport of fish seeds to farms with low cost and low mortality rate.
3.2 Layout design of parts and structures of seabass hatchery
In the planning of seabass hatchery construction, the design must take into consideration the following:
The facilities and equipment should be arranged at the proper places for convenience in the operation.
The size of facilities and equipment such as broodstock tanks, larval rearing tanks, etc., are of reasonable capacity which depends on the need or quantity of seed production desired.
In order to get seawater along the seashore which is clean, it should be designed so that the place of the water intake should be set in relatively deep water and away from the drainage outlet.
In order to keep out any undesirable quality of seawater for hatchery use, the water supply must be kept free from possible contamination from wastes of chemical laboratory.
For a better understanding of the layout and design of a seabass hatchery, the layout and designs of the existing seabass hatcheries of the Songkhla Fisheries Station, Satul Fisheries Station and National Institute of Coastal Aquaculture are shown (Figs. 1, 2, 3, 4, 5, 11, 12 and 13).
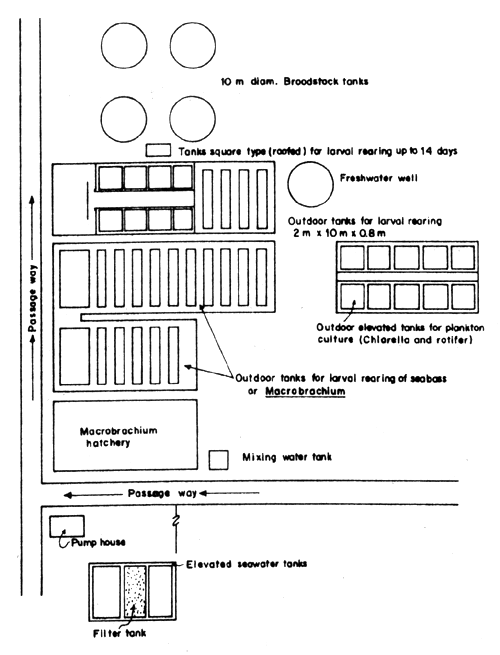
Fig.1 Layout of seabass hatchery of the Songkhla Fisheries Station
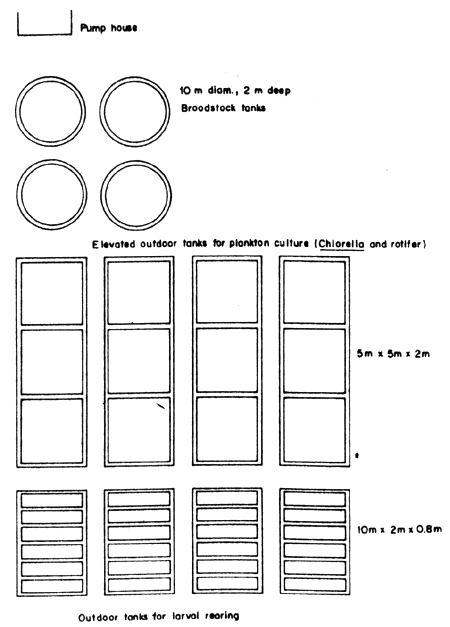
Fig.2 Layout of seabass hatchery of the Satul Fisheries Station
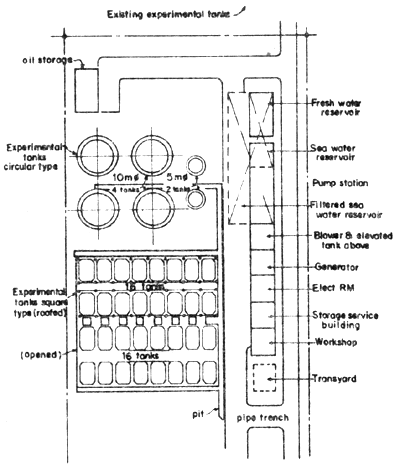
Fig. 3 Layout of seabass hatchery of National Institute of Coastal Aquaculture in Songkhla
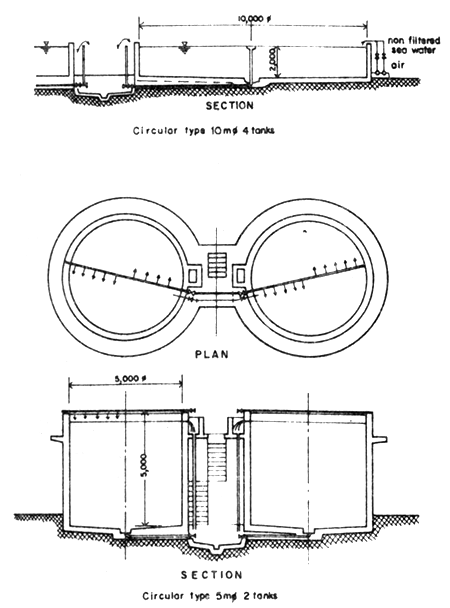
Fig.4 Circular type broodstock tanks
3.3 Equipment and facilities of seabass hatchery
Facilities and systems required in the hatchery operation are grouped into six categories as follows:
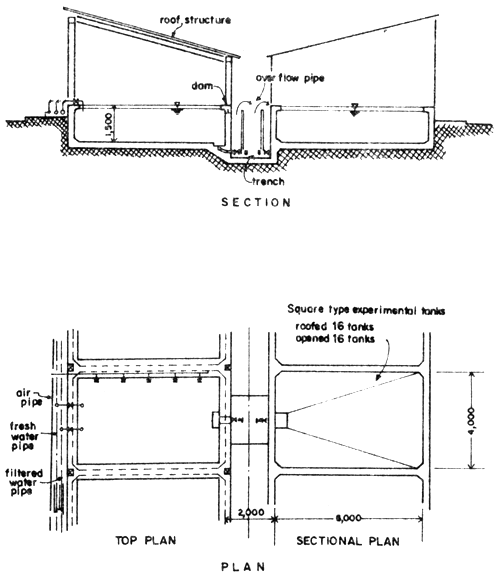
Fig. 5 Square type experimental tanks
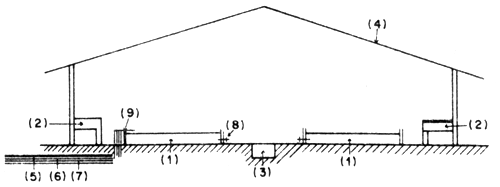
Fig. 11 Details of seabass larval rearing tank
3.3.1 Seawater supply system
Seawater is of prime importance for the rearing of the parent fish and of the young fish and are also used in growing food organisms such as for Chlorella and rotifers. Therefore, good seawater should be available and adequate all the time. The seawater supply system should be passed through the following treatments:
(1) Sedimentation tank. This is a big concrete tank which can retain large amount of seawater. There should be two tanks to be used alternatively. Water will be retained in these tanks for sedimentation.
Outdoor tank for seabass larval culture
Fig. 12 Details of seabass nursery tank
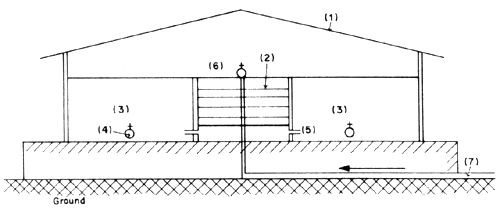
Fig. 13 Details of elevated seawater tank with filter
(2) Seawater tank. This elevated tank is located next to the sedimentation tank which is usually used to retain water from the sedimentation tank. This serves as storage of water ready for immediate use.
(3) Seawater filter. Water filter in general is in the form of filter tank that is filled with crude sand; fine sand and sometimes charcoal, shells and corals. The size of the filter tank varies according to the quantity of water needed. If there is high demand for seawater, pressured filter pump will be used in order to give higher quantities of clean seawater and also at a taster rate. Water is then retained in the seawater tank.
3.3.2 Seawater intake system
To pump in seawater to the hatchery system, five different methods are used. These are as follows:
Pumped in directly from the sea through a pipe that is laid along a jetty toward the sea where the water level is lower than the lowest tide level (Fig.6).
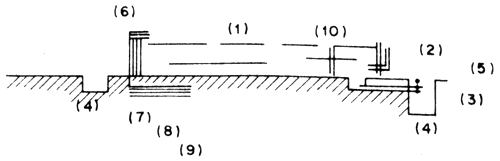
Fig. 6 Sea water intake
The same as (1), but the intake opening of the pipe is submerged in filter tank constructed below the seafloor beyond the lowest tide level. This method is usually used for hatcheries with little water requirement (Fig. 7).
Fig. 7 Details of one type of seawater intake
Another type has the pipe on the seabed up to some distance from the shore which is usually beyond the lowest tide level, then the pipe opening is raised above the seabed by some contraption such as a float (Fig. 8).
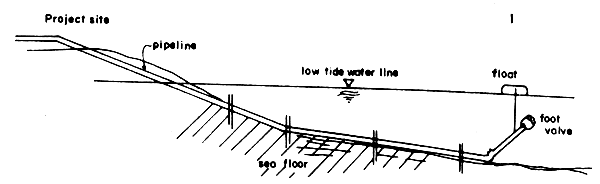
Fig. 8 Details of another of seawater intake
A fourth type consists of a deep well on the beach. Here the sandy beach is used as a natural filter and seawater is pumped directly into the storage tank (Fig. 9).
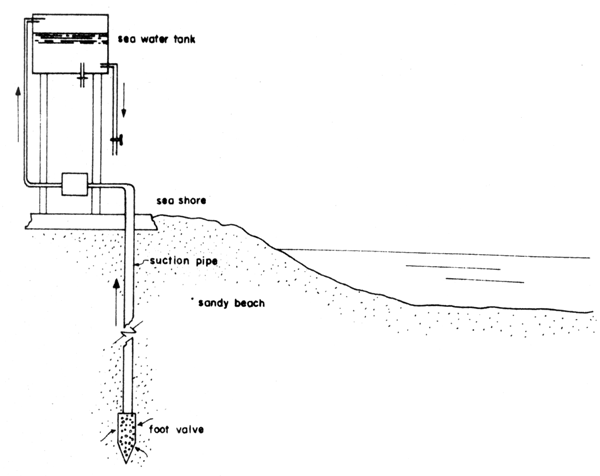
Fig.9 Details of a deep well type seawater intake
The same type as in (4) where a deep well is dug on the shore and seawater is then pumped in as shown in Fig. 10.
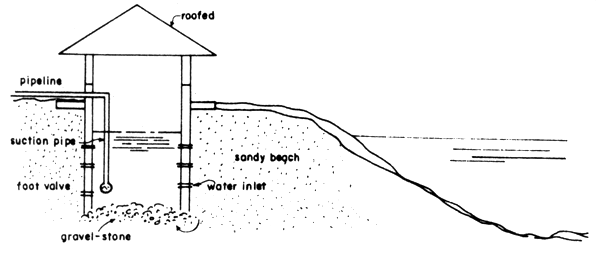
Fig. 10 Details of shallow well type seawater intake
3.3.3 Freshwater supply system
Freshwater is of prime importance in hatchery operations. Water is used to clean the instruments, tanks, etc. Besides, it is also used to dilute the saline water to suit the requirements of the fish, and also for use in culturing water flea (Moina. Daphnia) to substitute for Artemia to lower cost of production. Freshwater system may be composed of the following:
Sedimentation tank. Freshwater from the water supply authority is usually treated with some chlorine. It should therefore be kept in the open air to allow the chlorine to evaporate. And if water is obtained from natural sources, it should also be allowed to stand for sometime so that its sediments can settle prior to its use in the hatchery.
Water storage tank. This tank is connected with the sedimentation tank to retain clean freshwater from the sedimentation tank. The size of this depends upon the quantity of water needed.
3.3.4 Air supply system
Air supply is necessary for all stages of hatchery operation such as in the broodstock tank, nursery tank and receptacles for culturing of Chlorella, rotifer, Artemia, etc. Therefore, in each hatchery there should be at least three sets of air supply system, one for current use and the others as standby sets.
3.3.5 Drainage system
In the hatchery, there is a need to change some water almost everyday. Therefore, the drainage system must be laid out to enable the removal of waste water without any disruption to the hatchery operations.
3.3.6 Facilities of seabass seed production
The process of seed production is composed of articial spawning by stripping or natural spawning in environmentally controlled tanks. This is followed by nursing of the fish larvae and fry. The above processes require the following facilities:
Broodstock tank. The tank is a round concrete tank. It is rather big with good water system as well as air system (Fig. 4). After spawning the fertilized eggs or newly hatched fish will be moved to be nursed in the larval rearing tank.
Larval rearing tanks. The larval rearing tanks are rectangular. These may be well-protected under the shade, but these may also be outdoor tanks. These tanks should be in greater number due to the fact that when the larvae grow bigger, they must be spread to other tanks to make them less dense in order to obtain higher survival rate. When the young fish reach the age of 20 days, they can be kept in the outdoor tanks.
Tanks for culture of food organisms. Seabass larvae live on microorganisms so that live food culture is needed. These tanks have the same size and shape as the nursery tanks, but they are outdoor tanks for culturing Chlorella, rotifers, etc.
3.3.7 Equipment and facilities
Equipment and facilities needed in seabass hatchery are as follows:
Generator. This is used for electric supply in order to run the various necessary equipment, such as marine pump, airblower, water analyzers, and as standby in case of public power failure.
Air blower used for the air supply system. The size varies from 1–4 inches in diameter of hose delivery; 1–10 hp of motor. At least two air blowers will be provided for back-up and alternate use.
Marine pump. This is used for seawater pumping to the sedimentation tanks and for reserve water in the elevated seawater tanks. This pump may have pipes of 4–8 inches (10–20 cm) in diameter, with 10–15 hp of motor.
Freshwater pump. This pump is also needed for the freshwater system. The ones using 3–6 inches (7.5–15 cm) pipe diameter, 7–10 hp of motor may be used.
Water analyzer. Water quality monitoring is always needed such as for dissolved oxygen, carbon dioxide, nitrite, pH, etc. The water quality analyzer is needed for these purposes.
Others. Some other equipment such as electric meat chopper, submersible pumps, various sizes of pan-lite tanks, or fiberglass tanks, etc., can be handy if available for the hatchery work.
SCS/82/SBTC/LEC.7
by
S. Maneewongsa2 and T. Tattanon2
1. INTRODUCTION
Before controlled spawning of seabass was discovered, we tried to produce fertilized eggs by using spawners from natural waters. We had to study and investigate about spawning seasons, spawning grounds, and spawning time of seabass in the natural grounds. If we can get all of these information clearly, then success in seabass spawning can be assured. This will be of prime importance in promoting the seabass culture industry.
Fortunately, this has been successful in Thailand with test trials started in 1971. Success in producing consequential number of seabass fry (200 000) was first achieved in 1973. This triggered and encouraged the establishment of hatchery facilities.
2. CONDITIONS OF THE SPAWNING GROUND OF SEABASS ALONG SONGKHLA LAKE
The spawning ground of the seabass along Songkhla lake is at the mouth of the lake. Spawning begins from April and lasts till October or before the rainy season. Salinity during the spawning season is high at about 28 to 32 ppt and water temperature ranges from 28° to 34°C. The spawning period depends on the phase of the moon, that is it usually occurs from full moon to six days after full moon during low tide at 1800 to 2100 hours or in the evening hours.
3. GEARS USED FOR COLLECTION OF SPAWNERS
Along the mouth of Songkhla lake, gears used to catch mature seabass are enumerated below:
3.1 Bottom gillnets
3.1.1 Movable bottom gillnet
3.1.2 Fix bottom gillnet
3.2 Hooks and line
3.2.1 Single hook and line
3.2.2 Long line
3.3 Cast nets, traps
4. SELECTION OF RIPE SPAWNER
The wild spawner that are obtained from the natural spawning grounds should be selected. They should be of suitable size for stripping eggs and milt; too big or too small spawners should be avoided as the survival rate of larvae and fry from such spawners seems to be comparatively lower.
4.1 Distinction of male and female seabass adult fish
Shape of male spawner is more slender and generally it has less weight than the female spawner even with the same total length. Abdomen of the male is not bulging out like the female spawner and the depth of the body in male is less than female. The scales around the anal area of male become thicker than the female. The female spawner has large quantities of eggs causing the abdomen to bulge out. With slight pressure by hand, the eggs will flow out. In the case of the male, if pressed on the abdomen, the milt will come out from the urogenital opening.
In general, male spawners used for stripping method-range from 2 to 10 kg in body weight. The milt obtained from the male spawner should not be sticky and strongly concentrated. If one male spawner yields milt of less than 2 ml in volume, this may not be very satisfactory. The male spawners that can give milt between 5 to 15 ml by stripping would be more suitable.
Female spawners at the size of more than 3 kg in weight can yield eggs of 1.0 to 1.5 million by stripping per time.
In the case of broodstock raised in nursing nets and later in grow-out cages, before they are brought into the tanks for natural spawning, careful selection is made for both the female and male spawners. The characteristics to look into for good female and male spawners are the following:
2 Fisheries Biologists, NICA. Songkhla, Thailand.
SCS/82/SBTC/LEC.8
by
S. Maneewongsa and T. Tattanon
1. INTRODUCTION
Induced spawning of seabass by hand stripping of eggs and milt from wild spawners that are collected from the natural spawning grounds is one of the best methods for collecting adequate amount of eggs for mass production of seabass fry. This method is suitable for the hatcheries that are near the spawning grounds and have no spawner in grow-out culture for broodstock.
2. SPAWNING TECHNIQUE
The process of stripping the spawner from natural spawning grounds needs a fair amount of skill to avoid injuring the eggs as well as the fish.
2.1 Stripping ripe female spawners
This is usually done by two men working as a team. One man grasps the ripe female over a container while the other gently extrudes the eggs by applying gentle pressure on the abdomen from the anterior towards the posterior with the thumb and forefinger; the eggs will flow into the container. The characteristics or stage of eggs that are ready to be fertilized are enumerated below.
Eggs round in shape and with distended surface (no wrinkles).
Yolk distributed in whole area of inner egg shell, no perivitelline space.
Eggs are separated and do not stick together in groups.
Eggs are transparent but when in groups, they appear to have light yellow or golden colour.
Eggs float in the water at 28–32 ppt salinity.
Diameter of the egg averages 0.8 mm.
Oil globule is present in the centre with diameter of 0.2 mm.
No yolk vesicle.
2.2 Stripping male spawner for milt
For stripping the male spawner for milt, the same technique as with the female spawner is used. Good sperms must not be thick and sticky with plasma. When it is poured from a container, it should flow easily and on examination under the microscope, the sperms can be seen to be moving rapidly.
Sometimes the mature male spawner is encountered before you can find suitable running ripe female. In this case, the milt can be placed in glass vials or test tubes, stoppered and kept in the ice box. By this method, the sperms remain viable up to five days.
2.3 Method of fertilization
As soon as the female has been stripped, ripe male has to be stripped to the container with the eggs. The eggs and sperms are then gently mixed with chicken feather until well mixed. This is the dry method of egg fertilization.
After that, add some water and mix again. Then the eggs have to be washed out again repeatedly with clean saline water.
3. CARE OF FERTILIZED EGG, EMBRYO AND EARLY LARVAE
The fertilized eggs are put in vinyl plastic bags with some seawater of proper salinity (28–32 ppt) and transferred to the hatchery. Before putting the eggs into the hatching nursery tanks, the unfertilized eggs have to be taken out and the fertilized ones washed again. If necessary, the eggs are disinfected with acriflavine before being placed in the tanks.
If the hatchery is far from the spawning ground, aeration should be supplied to the bags of eggs and placed in low temperature conditions. This will prevent development of egg which will appear to be damaged. At lower temperatures (about 27°C) hatching will occur in about 17 hours. At higher temperature (30°), the eggs hatch earlier or in about 12 hours. After hatching, care of the young larvae starts.
SCS/82/SBTC/LEC.9
by
T. Tattanon and S. Maneewongsa
1. INTRODUCTION
In 1971, artificial spawning of seabass was first discovered. Broodfish were caught from natural open water during the spawning season and in 1973, 200 000 fingerlings were produced and distributed to the fish farmers. However, commercial culture as practiced at that time was of low intensity. This was due to the unpredictable natural supply of broodfish.
Natural spawning of seabass in controlled environment was first successful in 1975. By this method, much greater number of seedfish supply can be produced for commercial seabass culture. This operation has been conducted since that time.
2. SOURCE, COLLECTION AND SELECTION OF SPAWNERS
Parent fish are raised from the juvenile stage in cages. These can come from fry originally collected from natural waters or from larvae and fry spawned in the hatchery. There is not much selection at this stage except for the regular routine grading of the growing fry. When this stock attain the juvenile stage, the healthy ones that grow very fast are usually separated to be reared as future broodstock. Second selection is done after the first year and on the second year for the healthy and fast growing individuals. All these stages are still being reared in netcages in the sea.
After the second year, some of the male individuals can be identified as males when they would extrude milt with pressure on the belly. However, the females and other males cannot still be separated at this stage.
If during the elimination process for slow-growing individuals not enough stock is available, the deficiency is usually filled by selective purchase from private fishcage owners. Care must be taken that fish of some age group are put together. The reared broodfish would be ready to start spawning at the end of their third year when they attain about 3.5 kg in their body weight. The best male milt is furnished by male of from 2–4 years and female fish are not used before their third or fourth year. Approximately one month before the spawning season, the parent fish are moved from cages into the spawning tanks.
Round tanks, 10 m in diameter and 2 m in depth are used as spawning tanks at NICA. Approximately 24 spawners are kept in each tank with relative proportion of male and female of 1:1.
3. CARE AND MATURATION OF SPAWNERS IN THE HATCHERY
Water supply in the spawning tanks is seawater with average salinity of 30 ppt. Every other day 80–100 percent of the total volume of water is drained out for new clean seawater.
Fish, sardine or anchovy, with intestines and head removed are used for feeding the spawners by chopping these food into bite-sized pieces. Spawners are fed one time a day in the morning using food equivalent to 1 percent of their body weight. The excess food which falls down to the bottom of the tank should be removed by siphoning.
Generally, during spawning season, the female will appear with their big abdominal portion and swim awkwardly. Approximately 1–2 weeks before spawning, the female fish separate from the school and their feeding activity decreases. The male fish still keeps on eating normally, schools and swims actively.
4. METHOD OF SPAWNING IN THE HATCHERY
Natural spawning in controlled tanks takes place at the same time as natural spawning in open waters. It starts from the beginning of April to the end of September. Spawning activity always occurs between 1900–2300 hours on the first to the eighth day of a full moon. As the female approaches full maturity there is an increase in spawning play activity. The ripe male and female swim together often turning laterally when swimming and then spawn. The fertilized eggs then float at the surface while the unfertilized eggs sink down to the bottom. The floating fertilized eggs are scooped out of the tank and placed in hatching/nursery tanks. The unfertilized eggs and other dirt at the bottom of the tank are cleaned out by siphoning.
5. PROCEDURE FOLLOWED AFTER SPAWNING
The spawners are free in their tank habitat while they are spawning. After the fertilized eggs are scooped out, the spawning tank should be cleaned and a volume of new seawater is supplied in preparation for repeated spawning activity in the future. It is also possible that different fish of the confined spawners in each tank spawn in different days within a spawning period.
As mentioned earlier, the fertilized eggs are placed in a separate tank for embryonic development and hatching. To safeguard proper development of the eggs, the salinity should be maintained at levels of 25–32 ppt. Salinities above or below this range can result in weak larvae. The water temperature of the big tanks is usually stable and does not fluctuate much, generally at an average of 28°C. Radical temperature fluctuations which may occur in smaller tanks can harm the developing eggs and early larvae.
SCS/82/SBTC/LEC.20
by
S. Maneewongsa
1. METHOD FOR SEABASS SPAWNING
At present, spawning of seabass is very popular and has been done among the fisheries stations located along the coast in Thailand. Two methods for induced spawning of seabass have been done in hatcheries, they are:
1.1 Natural spawning in concrete tanks
1.1.1 Spawning without hormone injection
Two hatcheries, Songkhla and Satul are successful in spawning of seabass in concrete tanks without hormone injection. This is probably because these fisheries stations are located near the spawning grounds and the spawners are kept in the netcage before putting them in the concrete tanks during the spawning season.
1.1.2 Spawning with hormone injection
Many seabass hatcheries such as Rayong, Cholburi, Phuket and Prachuab Kirikhan have to use hormone injection for seabass spawning in concrete tanks. Even stock previously obtained from Songkhla reared in Rayong, for unknown reasons, failed to spawn naturally, but with hormone injection, it is able to spawn. These hatcheries mentioned appear to have different environment for the rearing of the spawners. Although on the spawning trials, the female spawners have ripe eggs, they failed to spawn in the concrete tanks. Thereafter, use of hormone injection of one to two doses to the seabass spawner were done and the spawners easily spawn in concrete tanks.
1.2 Spawning by stripping
For this method, the spawners have to be collected from the spawning grounds. For the male spawner, there is usually no need to use hormone injection. In the case of the female spawner, the eggs have to be checked before stripping. If the eggs are good or in running stage, there will be no need of using hormone injection. But if the eggs are not in running stage hormone injection have to be done for the female spawner. The dose of hormone is rather high compared with female spawner reared in concrete tanks.
2. NATURAL SPAWNING IN CONCRETE TANKS
2.1 Some factors that may affect spawning
Food. Good quality and suitable amount for spawners should be given. Only about one percent of body weight using trash fish is feed to spawners. Overfeeding can result in failure to spawn.
Water quality. Good fresh running water supply with adequate dissolved oxygen should be used or water with not less than 6 ppm Do, and pH ranges of 7.5 to 8.5
Salinity. Salinity in the concrete tanks should be 28–32 ppt and maintained at this range.
Clean tanks. Spawning concrete tanks should be clean.
Stress. Disturbances to spawners during spawning season in concrete tanks are not conducive for the spawning of the seabass spawners.
Water management. Usually changing of water or running water system have to be done in the spawning tanks.
Uniform size of spawners. The size of male and female spawners should not be very different.
Age. Age of spawners should also be suitable so that spawners can spawn readily.
Sex ratio. Sex ratio in the spawning tank is usually 1:1 but the male spawners are often very much less in weight and their number should sometimes be increased.
2.2 Types and doses in using hormone for injection
Mainly 3 types of hormones are used for injection to induce spawning:
But the most popular hormone that is used is the HCG hormone. The dosage in using hormone for injection of seabass spawner in concrete tanks ranges 50–100 IU/kg of body weight. For Puberogen 200 IU/kg is used.
3. SPAWNING BY STRIPPING
The hormone that is used for injection is HCG, the same as used in concrete tanks. But the dosage is higher; ranging between 50–1 000 IU/kg of body weight. The interval of injection is about 12 hours. Three areas of the seabass that are usually used for hormone injection are: (a) behind pectoral fin, (b) on the lateral line, and (c) between the first and second dorsal fin. The best area usually used for scaled fish such as the seabass is behind pectoral fins.
4. SOME RESULTS AFTER INJECTION
In the experiments on induced spawning of seabass by hormone injection carried out in 1974, the results are shown below.
The foregoing experiments show some of the experiences on induced spawning of seabass at NICA, through hormone injection. It should be noted that after the spawning by stripping running ripe spawners, and natural spawning in tanks were achieved at this Institute, these latter methods were found much more convenient with the facilities in hand.
| Specimen No. | Body Weight (kg) | Time | HCG (IU) | Pitituary (doses) | Remarks |
|---|---|---|---|---|---|
| 1 | 5.2 | 10.00 | 2500 | - | |
| 22.00 | 2500 | - | |||
| 10.00 | 2500 | - | |||
| 11.00 | - | - | Dead, some parts showed ripe eggs. Sperm added and 10% fertilized. | ||
| 2 | 5.0 | 08.30 | 2800 | - | |
| 20.30 | 2800 | - | |||
| 08.30 | 3000 | - | |||
| 20.30 | - | - | Killed, found some ripe eggs. | ||
| 3 | 5.0 | 09.45 | 3000 + | 2 doses | |
| 21.45 | 3500 + | 4 doses | |||
| 00.00 (midnight) | stripped | - | All running ripe eggs. 70% fertilized eggs. Hatched out 80%. |
SCS/82/SBTC/LEC.10
by
S. Maneewongsa and T. Tattanon
1. INTRODUCTION
In the past before seabass became a culture fish, no one studied about its eggs and larvae so that there was lack of knowledge on this subject. Only some general background on seabass are known, i.e., that the adult fish live in the sea and migrate to the mouth of rivers that are connected with the sea and for spawning during the spawning season. The Songkhla Fisheries Station was established near the mouth of Songkhla Lake and it was observed that during April to September each year, the adult seabass were caught at the mouth of Songkhla Lake. By dissecting fish, these were found to be spawners. This finding started the experiments on induced spawning of seabass by stripping eggs and milt conducted in 1971. The trial that year was successful. Since then studies were made and some knowledge were gained on eggs, larvae and juveniles of this species.
2. THE SEABASS EGG
2.1 Immature eggs in the ovary
There are no specific studies on this matter made in Thailand at present, but aquaculturists in the country have more or less a clear recognition of the immature eggs as found in the ovaries of the fish. Specific studies on this will be needed and should be made in the future.
2.2 The mature egg
As previously described, the mature egg is round with its shell membrane fully distended (no spaces nor distortions), measuring about 0.8 mm in diameter. They tend to stick together and while in groups, the eggs give a golden hue. It has one oil globule inside which measures about 0.2 mm in diameter.
2.3 The milt and sperm
Some milt will flow freely from a mature male spawner. It should be of good amount preferrably about 10 ml in amount and not very sticky so that it would flow freely if poured from container. If the milt is examined under the microscope, the sperms can be observed to move very rapidly. There is no detailed study yet of the seabass sperm in Thailand.
2.4 The fertilized egg and embryonic development (Figures 1, 2)
When the milt and eggs are mixed by the dry method, fertilization takes place. There appears to be no significant changes on the egg from outside observations during the early stages. It was observed that it takes about 35 minutes after the mixing of the eggs and milt that the beginnings of embryonic development take place. The approximate time and duration of the various embryonic stages of seabass are enumerated below (Table 1).
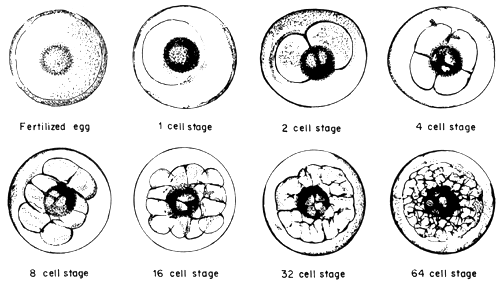
Fig.1 The fertilized egg and embryonic stages of seabass
| Embryonic stage | PERIOD | ||
|---|---|---|---|
| Hours | Minutes | ||
| (a) | Fertilized egg | 0 | 0 |
| (b) | One-cell | 0 | 35 |
| (c) | Two-cell | 0 | 38 |
| (d) | Four-cell | 0 | 44 |
| (e) | Eight-cell | 1 | 03 |
| (f) | 32-cell | 2 | 12 |
| (g) | 64-cell | 2 | 43 |
| (h) | 128-cell | 2 | 55 |
| (i) | Pre-blastula | 3 | 11 |
| (j) | Blastula | 5 | 32 |
| (k) | Gastrula | 6 | 30 |
| (l) | Neurula | 8 | 32 |
| (m) | Embryo develops head, optic lobes and tailbuds | 11 | 20 |
| (n) | Heart starts functioning, tail free, body starts to move | 15 | 50 |
| (o) | Hatching | 17 | 30 |
2.5 Hatching (Figure 2)
The mechanics of hatching of the seabass embryo has not been studied in detail. However, since tha hatching (newly-hatched larva) has by now free tail fin, it can move very freely. The hatchlings tend to confine at levels below the surface (about 0.5 m below) and near parts of the water medium that has aeration or slight water movements.
The period from fertilization to egg hatching of the seabass egg can be affected by temperature. As mentioned earlier, at 27°C the eggs can hatch in about 17 hours while if the temperature were 30–32°C, the eggs hatch in 12–14 hours. Salinity on the other hand can affect the rate of hatching. This is shown in the following table (Table 2).
| Salinity (ppt) | Rate of hatching (percent) |
|---|---|
| 0 | 0 |
| 5 | 2.86 |
| 10 | 58.56 |
| 15 | 75.03 |
| 20 | 82.35 |
| 25 | 83.36 |
| 30 | 80.78 |
| 35 | 46.90 |
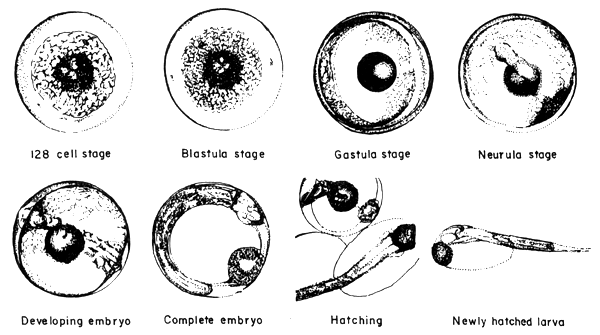
Fig.2 Advance embryonic stages, hatching and newly hatched larva of seabass
3. THE SEABASS LARVAE
3.1 Development of hatchling with egg yolk
The early hatchling or newly hatched larva is 1.5 mm in length with a big yolk sac (Fig. 2). The yolk sac has one big oil globule at its anterior. This enables the larvae to be set in the water with head raised up at 45° to 90° angle of the water surface. The body is slender and pale in colour with a distribution of pigments. The eyes, digestive tract, anus and caudal fins are distinctly seen but the mouth remains closed for a while or a period of about three days.
Under normal conditions, the rate of absorption of the yolk have been observed to be as follows (Table 3).
| Diameter* of yolk in yolk sac (mm) | Time (days) |
|---|---|
| 0.8800 | 0 |
| 0.3525 | 1 |
| 0.2752 | 2 |
| 0.1530 | 3** |
| 0.0050 | 4 |
| 0.00 | 5*** |
* As the yolk is actually elongated, this is a measurementof length from anterior to posterior
** Mouth opens
*** Yoik completely absorbed
3.2 Development of larvae, fry and juveniles
The mouth opens when the larvae get to about 3 days old and the yolk has been almost completely absorbed. This is a sign that the fry can start to feed. Up to seven days old, the larvae are pale in colour and from the age of seven days to metamorphosis at 18–20 days, they appear dark with distinct vertical stripes on certain parts of the body. After the 18th to 20th days, the larvae again assume pale brownish colour. This time, the vertical stripes can be more clearly distinguished. The stripes are three, one at the caudal peduncle, another at the level between the first or spinous dorsal fin and the second or soft dorsal fin, and a third over the head, all of which are particularly distinct.
In one month, the larvae metamorphose into the fry stage which has the appearance very close to the parent fish. The fry measures 1.5–2.0 cm. These further grow and develop into juveniles after the third to the fifth month when they attain 8–15 cm.
SCS/82/SBTC/LEC.3
by
S. Maneewongsa and T. Tattanon
1. INTRODUCTION
Food and feeding are two of the most important factors affecting the survival rate of seabass larvae. The larvae cannot survive if there is inadequate supply of food, which comprises various live organisms, and which varies with the development of the larvae.
2. FOOD USED AND SOURCES
Most of the food that seabass larvae feed on is composed of live zooplankton. The larvae first begin to feed on rotifer. Other kinds of food have also been tried with the early larvae but without success. The supply of live zooplankton is expensive and sometimes causes problems because zoo-plankton culture needs time, facilities and skills. Further, the different kinds of live food required must be prepared in time to satisfy the need of the fast-growing larvae. To maintain a high survival rate, the feeding schedule for the larvae must be closely adhered to.
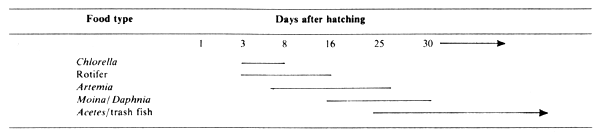
2.1 Live food used at different age stage
The live food used for the different developmental stages of the seabass larvae are shown in Table 1.
2.2 Food organisms to be cultured
Phytoplankton
Zooplankton
3. METHOD OF FEEDING
Filtered rotifer is administered at a rate of 1 ml for every 5 to 10 larvae. This is followed by a brine shrimp diet starting on the 8th day. Brine shrimp can be hatched in brackish or marine water with salinity ranging from 15 to 30 ppt. Nauplii are feed to the larvae at 1 to 2 tails per ml, one to two times per day. Daphnia or Moina are fed 8 to 10 times per day to avoid over-feeding in the rearing tank which may cause pollution. From 20 days old, the fry can be trained to eat Acetes or minced trash fish at 8 to 10 times per day.
The amount of food to be given has to be adjusted everyday. If the larvae are swimming fast around the tank, more food will be required. On the other hand, if some food remain especially Acetes and trash fish, the amount of food administered would have to be reduced.
Table 1.
Kinds of food and schedule for feeding seabass larvae
SCS/82/SBTC/LEC.4
by
T. Watanabe2
1. INTRODUCTION
The development of seed production of marine fishes in Japan is herein mainly referred to that of the red seabream, Sparus major. The first trial was carried out in 1887, but success was achieved first in 1962 when twenty-two juveniles were obtained by rearing. The mass production of the species became possible since 1965 by using rotifer (Brachionus plicatilis) as food organism. Now many hatcheries can produce more than one million fry per year. It is therefore apparent that the mass production of marine fish seed depends on the mass production of food organisms.
In Japan, the feeding programme for the rearing of marine fish larvae has been standardized: larvae of oyster followed by rotifer, then copepods or Artemia, and a final diet of minced fish. However, rotifer is commonly used as the initial food instead of oyster larvae. It is more effective as an initial food for early fish larvae with smaller mouth size such as species of Sillago, Siganus and Epinephelus.
On the other hand, the feeding sequence has been established in the National Institute of Coastal Aquaculture (NICA), Songkhla, as: rotifer, Artemia, Moina and minced fish. The mass production of rotifer is however still not stable which affects the seed production programme of the seabass. The study of rotifer culture on a large-scale is being carried out to complement the work on the seabass. The purpose of this paper is to present some of the results of trials of the mass production of rotifer to date. The methods of rotifer production in Japan is also presented as general information.
2. GENERAL INFORMATION
2.1 Food for rotifer
In nature, rotifer feeds on minute algae, yeast, bacteria and protozoans. In mass rotifer production, Chlorella and baker's yeast are commonly utilized. However, when fish larvae are fed with rotifer produced by only the baker's yeast (called yeast rotifer), they show low survival, so that usually rotifer is fed with Chlorella together with baker's yeast rotifer is enriched by Chlorella before feeding to fish larvae. Recently, it has been found that yeast rotifer is lacking in highly unsaturated fatty acid (HUFA) which is essential fatty acid for marine fishes. Since squid liver oil contain rich HUFA, baker's yeast enriched by that oil has been developed (called omega-yeast).
In addition, the utilization of bacteria in the by-product of distillery is also under study, and the rotifer produced by photosynthetic bacteria has already been in the market. Recently, marine yeast is practically used in Kuwait.
2.2 Methods for the production of rotifer
The methods for the production of rotifer are classified roughly as follows:
In large-scale hatcheries, the partial harvest system using large tank is adopted commonly. The volume of such a production tank is 10 to 100 tons, with filter equipment in some hatcheries. The procedure for production is shown in Figure 1. The duration for harvest ranges from one to two weeks, although it is shorter under high water temperature conditions. It is possible to harvest continuously for two to three months if equipped with filter apparatus.
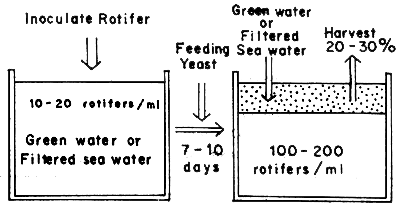
Fig. 1 Partial harvest system using large tank
2 Aquaculture Expert. Japan International Cooperation Agency (JICA), NICA, Songkhla, Thailand.
To small-scale hatcheries, the total harvest system is more effective. The volume of a production tank is 1 to 10 tons, equipped with filter apparatus. The procedure for production is shown in Figure 2. This method is adopted for high density culture. In mass production, however, many tanks will be needed involving considerable amount of hard routine work pertaining to water quality management including the physical labour for cleaning the tank.
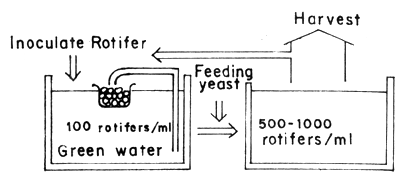
Fig. 2 Total harvest system using small tank
2.3 Feeding methods in rotifer culture
Chlorella is a suitable group of algae to serve as food for the rotifer. The growth rate of Chlorella however, is slower than the consumption rate by rotifer. It is thus difficult to supply enough food for the rotifer by using only Chlorella in mass production. Therefore, commonly, Chlorella and yeast are used together, in which Chlorella is first cultured for feeding the rotifer and after the Chlorella has been consumed, yeast is given to the rotifer.
The feeding rate of Chlorella differs from hatchery to hatchery. It is however common that 10 to 20 million cells per ml of Chlorella water is used for culture media. The feeding rate of yeast is 1 to 2 g (wet weight) per one million rotifer per day, increasing to 2 to 3 g for high temperature conditions (higher than 25°C). In case of using dry yeast, it becomes one-third to half of the amount for wet weight. It is increased in case of low density of rotifer, but decreased in high density.
2.4 Other problems
There are two types of rotifers used in Japan, i.e., small type (S-type) and large type (L-type) (Fig. 3). S-type shows good population growth in higher temperatures (higher than 20°C); whereas, L-type shows this under lower temperature conditions. According to Dr. K. Fukusho, rotifers used in the Philippines, Singapore and Thailand belongs to the S-type, while in Indonesia, both types are used. Usually, it is mentioned that rotifer is fed to fish larvae by consistently maintaining 4 to 5 rotifers per ml in the rearing tank. This rate is however based on the use of the L-type, so that the quantity of rotifer required is two or three times more than when S-type is used.
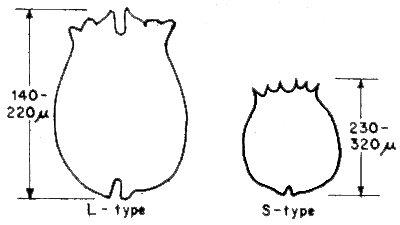
Fig. 3 Two types of Rotifer (Brachionus plicatilis), Large type and small type
The aforesaid nutritional problem is also vital to the management of the rearing of early larvae. Therefore, rotifer fed with baker's yeast must be enriched by feeding with Chlorella or squid liver oil for six to twenty-four hours.
3. TRIALS ON MASS PRODUCTION OF ROTIFER IN NICA FROM MARCH TO MAY 19821
3.1 Production using green water
Three kinds of single-celled green algae are cultured in NICA, viz, Chlorella sp, Tetraselmis sp and mixed blue green algae. Chlorella water is usually contaminated with blue green algae. In April, Tetraselmis was mainly used since it was blooming constantly. However in May, it had to be replaced with Chlorella and blue green algae which bloomed much more readily. The fertilizers for green water culture included: ammonium-sulphate at 400 g and calcium-superphosphate at 20 g per tank (24 tons), for April; and ammonium-sulphate at 1 500 g, 16–20–0 (fertilizer for agriculture) at 150 g and urea at 150 g per tank, for May. The cell density of green water fed to the rotifer was as follows: in April, Chlorella with bluegreen algae at 1–2 million cells per ml and Tetraselmis at 160–100 thousand cells per ml; and in May, Chlorella with bluegreen algae at 4–8 million cells per ml, and Tetraselmis at 40–60 thousand cells per ml.
The procedure for production of rotifer is shown in Figure 4. In this method, maximum density of rotifer was 30–50 per ml in April, and 15–30 per ml in May (Fig. 5).
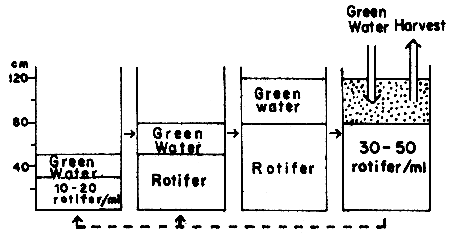
Fig.4 Procedure for production trial of Rotifer using
green water (20 cm depth  2.2 ton)
2.2 ton)
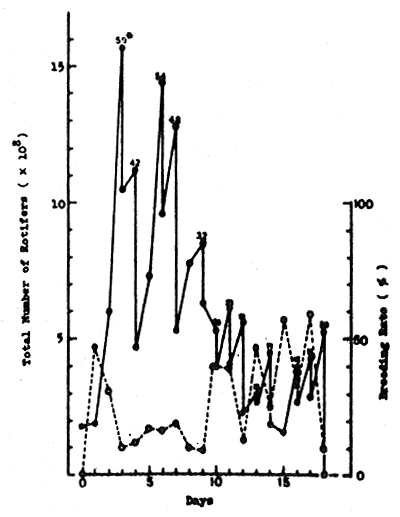
Fig. 5 One example of production using green water during
Apr. 8–26;  total number of Rotifer;
total number of Rotifer;
 breeding rate;
breeding rate; 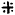 density of Rotifer per ml
density of Rotifer per ml
3.2 Production using baker's yeast and green water
This method was tried in May, a dry baker's yeast (Fermi-pan from Holland)1 was used. Feeding rates were as follows: less than 30 rotifers per ml, 300 g per 18 tons; more than 30 rotifers, per ml, 0.5 g per one million rotifers. The procedure for production is shown in Figure 6. Maximum density of rotifer was 8–20 per ml and the duration for harvest was 7–10 days (Fig. 7).
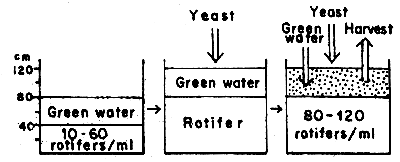
Fig. 6 Procedure for production of Rotifer using baker's yeast and green water
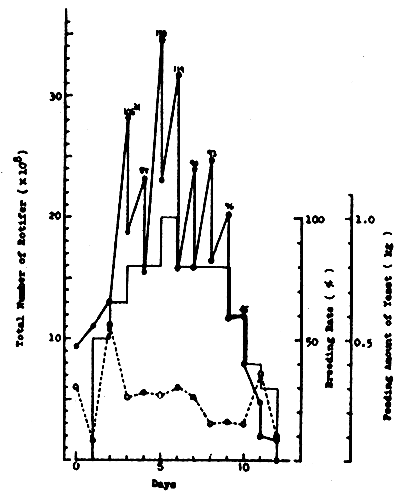
Fig. 7 One example of production using baker's yeast and
green water during May 14–26;  total number
of Rotifer;
total number
of Rotifer;  breeding rate; —feeding amount
of yeast;
breeding rate; —feeding amount
of yeast; 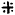 density of Rotifer per ml
density of Rotifer per ml
3.3 Production using marine yeast
This method was tried in March. The seed of marine yeast was obtained from the drainage in the hatchery. In small-scale culture of marine yeast (ml), cell density of yeast was at 33–500 million per ml; however, in large-scale culture 500–1 000 liters), it was only 100 million per ml. The nutrients for yeast culture are as follows: sugar at 10 g, ammonium-sulphate at 1 g, and potassium phosphate at 0.1 g, per 1 liter of seawater. Hydochloric acid is also added for adjustment of pH (about 4). The mass production of marine yeast is still not established, so that trial of rotifer production was carried out at the same time of the trial mass production of marine yeast. The feeding amount was adjusted depending on the breeding rate of the rotifer. The result is shown in Figure 8. The maximum density of rotifer was 80–120 per ml, and the duration of culture was 25 days.
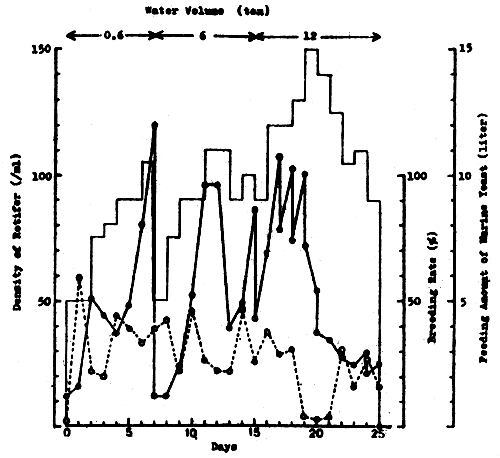
Fig-8. Result on Production of Rotifer Using; Marine Yeast.
 Density of Rotifer,
Density of Rotifer,  Breeding Rate, — Feeding Amount
of Marine Yeast ( scale is in 0.6 ton water volume, ×10 in 6 ton
and ×20 in 12 ton).
Breeding Rate, — Feeding Amount
of Marine Yeast ( scale is in 0.6 ton water volume, ×10 in 6 ton
and ×20 in 12 ton).
Although the mass production of rotifer is still not established in NICA, the trials introduced should highlight some of the pressing problems confronting mass fish seed production programmes of hatcheries.
1 Mention of brand does not suggest indorsement of product.