SCS/82/SBTC/LEC. 11
by
T.Tattanon and S. Maneewongsa
1. INTRODUCTION
Widespread demand for commercial scale culture of seabass is now encouraged by the governments of most Southeast Asian countries including Indonesia, Hong Kong, Taiwan (China), Malaysia, Singapore, and Thailand. Accordingly, the cost of seabass larvae has become very high and is increasing year by year. In Thailand particularly, it is estimated that at least 50 000 000 larvae are needed to supply the needs of the whole country. The advancement of technology on hatchery operation will be required to insure that the supply of fry for growout ponds and cages can be met. The seabass seed supply problem is not only felt in Thailand but also in the whole region mentioned.
Research on the propagation of Lates calcarifer commenced at Songkhla in 1969 under the direction of Mr. Sawasdi Wongsomnuk, who was later joined by Mr. Sujin Maneewongsa. The objective of the project was to develop methods of mass production of fry and to study propagation methods applicable to the physical and economic environment in Thailand.
The first successful mass production technique for seabass fry was achieved in 1973.
2. CARE OF FERTILIZED EGGS AND HATCHING
Fertilized eggs are dipped into 5 ppm of acriflavine solution for 1 minute then washed out 2 to 3 times before placing in hatching-rearing tanks, with size of 3.5 × 4.75 × 1.20 m. Each tank is filled with filtered seawater at the salinity of 28–30 ppt and aerated from the bottom.
Approximately, 800 000–1 000 000 eggs are stocked in each tank. The eggs hatch out in 17 hours at average water temperature of 27°C or at 12 hours at water temperature of 30°C. Various salinities of water give different hatching rates; the best salinity for hatching appears to be between 20–30 ppt (Table 1).
| Salinity (ppt) | Hatching rate (%) |
|---|---|
| 0 | 0 |
| 5 | 2.9 |
| 10 | 58.5 |
| 15 | 75.0 |
| 20 | 82.4 |
| 25 | 83.4 |
| 30 | 80.8 |
| 35 | 49.9 |
3. CARE OF NEWLY HATCHED LARVAE BEFORE FEEDING
After hatching out, aeration is stopped for a few minutes in the tank so that the sediments of undeveloped embryos and other dirt can be siphoned out. During this period, running water system is used in the rearing tank for 2–3 days before feeding.
4. START OF FEEDING AND CARE OF GROWING LARVAE
4.1 Feeding of various age stage
From 3 days old larvae, the yolk is almost completely absorded, the mouth opens and the larvae start their feeding habit. By this time, the rotifer (Brachionus) is required to feed the larvae. Enough rotifer is added or approximately 5–10 rotifers per ml are stocked into the larval tank. Larvae are fed with rotifer up to 10–14 days, later on they are fed with Artemia salina until 20 days. Daphnia or Moina is the last living organism that are fed on by 20–30 days old larvae. After this period, they are trained to change their predatory habit by feeding trash fish which are chopped or minced into bite-sized pieces.
4.2 Nursery tank management
The rearing tank should be cleaned up everytime before using. The rate of water replacement in the rearing tanks depend on feeding period of each age stage. In the period of rotifer feeding to prevent the loss of rotifer through the outlet, approximately 10–20 percent of the water in the rearing tank is drained out only for the replacement of rotifer supply each day. During Artemia feeding period, approximately 50 percent of water is changed while almost complete change is made during trash fish feeding period.
The sediment of dead organisms, larvae or leftover food are siphoned out everyday. The management of seabass nursery is shown in Figure 1.
4.3 Grading techniques
Due to the cannibalistic nature of the fish, size selection or grading or sorting is of prime importance. The first sorting should start at the second week since during this period, the bigger fish can eat the smaller ones. The easiest way of sorting is to use screen with various mesh sizes so that the various sizes of fish can be separated easily. Stocking the same size of fish will reduce the rate of cannibalism, thus the survival rate will be increased and the growth rate of the fish could also be faster and more uniform.
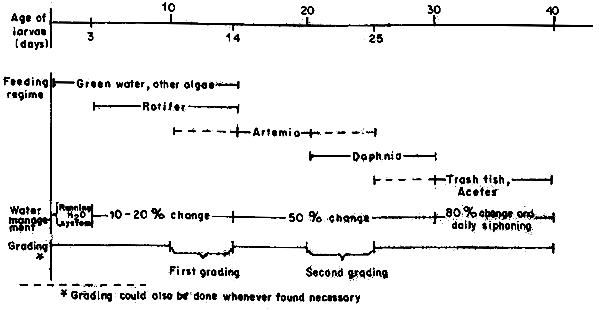
Fig. 1 Chart showing management method for seabass nursery tank within the first 40-day period
4.4 Growth and care of larvae as they develop to fry and juveniles
Up to 40–45 days, the larvae develops to juvenile stage. They are moved from the rearing tanks for culture in netcages. The netcages are of 1 × 2 × 1 m in dimensions and are usually set in open waters. Stock of 2 000–3 000 fry are raised to the fingerling size in these cages.
4.5 Diseases
If hygienic conditions are maintained, the larvae are generally, quite resistant to diseases. However, since the larvae are stocked in the tank for a long period, sometimes they will show their abnormal swimming character, stop feeding, and turn black. These are signs of disease or poor health so that if these occur, they should be treated with 1:2 000 parts of formalin solution for 10–15 minutes for 2–3 days continuously.
4.6 Survival rate
The system of culture outlined above gives about 85 percent hatching rate of eggs and survival rate of 1–7 days old larvae of 30 percent. For 8–15 days old larvae the survival is 80 percent, after which they can be maintained indefinitely with negligible mortality (Table 2).
| Age (days) | No. of larvae* per liter | Survival rate (%) |
|---|---|---|
| 1–7 | 30–40 | 37.2 |
| 8–15 | 15–20 | 80.9 |
| 16–23 | 5–10 | 70.0 |
| 24–30 | 2–5 | 85.3 |
* Normal stocking density used in nursery tanks.
SCS/82/SBTC/LEC. 13
by
S. Maneewongsa and T. Tattanon
1. INTRODUCTION
There is no information nor previous studies made on the growth of seabass larvae and juveniles from the wild. It is commonly known that seabass fry when collected from natural areas are big enough so that they can be suitable for stocking growout ponds and cages. Only approximate estimates of their possible age can be made.
As we are now able to spawn the fish and grow the larvae and juveniles under controlled conditions, we have better knowledge on their growth. We are also successful in nursing the seabass larvae and juveniles in controlled conditions at relatively high survival rates.
2. GROWTH OF LARVAE AND JUVENILES
2.1 Growth in relation to food
In the nursery of seabass larvae, the most important factor to consider is to have the right kind and amount of food prepared for the larvae. Various stages of larvae need different kinds of food. The food to be given to the first stage of the larvae when they start to feed is rotifer. Brachionus plicatilis. This kind of food is very suitable for the young larvae making them grow well and giving high survival rate. Therefore, adequate amount of rotifer is very necessary for nursing seabass larvae, the density of rotifer should be at a range of 5–10 rotifers per ml in the nursery tank. Feeding larvae with rotifer is started when the seabass larvae are 3 days old until their 14th day. Artemia is fed to seabass when they get to the 8th day until 20th day. Daphnia or Moina is fed to the larvae at the 16th day until the 30th day. Acetes and minced fish are fed to the fry after the 21st day. These feeding stages are shown in Table 1.
| Percent of food given | ||||||
|---|---|---|---|---|---|---|
| Age (days) | Chlorella | Rotifer | Artemia | Daphnia or Moina | Acetes | Minced fish |
| 3–7 | 10 | 90 | - | - | - | - |
| 8–15 | 10 | 75 | 15 | - | - | - |
| 16–20 | 50 | 50 | - | - | ||
| 21–30 | - | - | - | 80 | 10 | 10 |
| 31–40 | - | - | - | 50 | 25 | 25 |
| 41 | 100 | |||||
Experiments were conducted on nursing of seabass larvae with 3 kinds of zooplankton namely: rotifer, Artemia and Moina. The larvae were reared during their 3 to 15 days of age. The results are shown in Table 2.
| Type of food | Number of larvae | Number survived | Survival rate |
|---|---|---|---|
| Rotifer | 2 000 | 808 | 40.40 |
| Rotifer + Artemia | 2 000 | 951 | 47.55 |
| Rotifer + Moina | 2 000 | 381 | 19.05 |
The results showed that rotifer and combination of rotifer and Artemia were the more suitable food of seabass larvae at this age stage.
2.2 Growth in relation to space
The tanks used as nursery for the seabass larvae measure 3.50 m in width × 4.75 m in length × 1.20 m in depth. This is filled with good fresh seawater 1.10 m deep and aerated at 4 points of the tank bottom. The density of stocking of this size of nursery facility ranges from 600 000 to 1 000 000 larvae/tank. Larval densities that are usually used in nursing tanks per ton are shown in Table 3.
| Age (days) | Density of larvae/ton | Survival rate (%) |
|---|---|---|
| 1–7 | 60 000–100 000 | 37.23 |
| 8–15 | 15 000–20 000 | 80.91 |
| 16–23 | 5 000–10 000 | 70.05 |
| 24–30 | 2 000–5 000 | 85.33 |
2.3 Growth in relation to water quality
Good fresh seawater have to be filtered by phytoplankton net to prevent predators from entering the nursery tank. The salinity of water should be maintained at between 10–30 ppt.
Experiments were conducted using various salinities of water for nursing larvae of 1–30 days of age. The results reveal that the salinity of 20 ppt gave highest survival rate as shown in Table 4.
| Salinity (ppt) | Survival rate (%) |
|---|---|
| 0 | 0 |
| 5 | 24.0 |
| 10 | 28.0 |
| 15 | 28.0 |
| 20 | 68.0 |
| 25 | 22.0 |
| 30 | 18.0 |
| 35 | 10.0 |
The effects of other water quality factors (temperature. pH. DO, etc.) have not been fully studies.
3. GROWTH RATE OF SEABASS LARVAE
By nursing seabass with enough food of the kind described above within 30 days, the larvae should normally attain a length of 1.205 cm. The normal growth within the first 30 days is shown in Table 5.
| Age (days) | Total length (mm) | Remarks |
|---|---|---|
| Fertilized egg | 0.870 | Diameter of fertilized egg |
| 0 | 1.5175 | |
| 1 | 2.1850 | Beginning to hatch out* |
| 7 | 3.5916 | |
| 14 | 4.3650 | |
| 20 | 8.10 | |
| 30 | 12.05 |
* Hatching is at 12th to 17th hour depending on temperature.
SCS/82/SBTC/LEC. 14
by
T. Tattanon and S. Maneewongsa
1. COLLECTION AND CONDITIONING OF FRY BEFORE TRANSPORT
Fry are collected out from the rearing tanks and placed into smaller receptacles.
Fry are treated with 5 ppm of acriflavine solution or 0.5 ppm of copper sulfate solution for 5–10 minutes.
There should be no feeding within 1–2 hours before packing.
2. PACKING AND AMOUNTS THAT CAN BE TRANSPORTED
Plastic bags of 40 × 60 cm of proper gauge are filled with 6–7 liters of fresh seawater and saturated with oxygen besides 10–12 liters of oxygen gas are used for packing. The amount of transportable fry depends on size of fry, water temperature in plastic bags and duration of transporting and handling until the fry reach destination.
| Age stage (days) | Size (TL) (cm) | No. of fry per bag | Water temperature | Duration (hours) | Survival rate(%) |
|---|---|---|---|---|---|
| 7–15 | 0.2–0.3 | 10 000 | 19–23°C | 16 | 90 |
| 20–22 | 0.5 | 5 000 | -do- | 16 | 90 |
| 1 month | 1–1.5 | 1 000 | -do- | 16 | 90 |
| 2 months | 2–3 | 500 | -do- | 16 | 90 |
3. TRANSPORT
In case of transportation by truck, a mixture of crashed ice and sawdust is needed for controlling the water temperature in the plastic bags during transport. The mixture is spread uniformly on the floor of the truck before the plastic bags are laid upon it. The proportion of crashed ice and sawdust is 1:1 for long period transport (12–16 hrs) and 1:2 for short period (4–5 hrs). Transportation should be carried out at night time. By this method, it is possible to control the water temperature between 19–23°C.
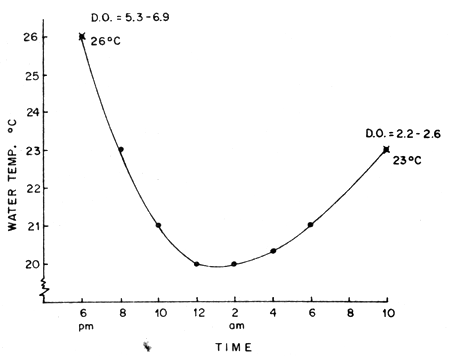
Fig. 1 Fluctuation of water temperature under transportation
Figure 1 shows the observed fluctuation in temperature of the water in the plastic bags during transport. It was also observed that the dissolved oxygen starting initially at 5.3 to 6.0 ppm will drop to 2.3–2.6 ppm at destination.
However, another and more convenient way for land transport of live fish is by using refrigerated truck or air-conditioned bus where temperature of 20–22°C can be controlled during transport.
Air transport is of course a very convenient and fast way but it could be expensive. In this case, the plastic bags are required to be placed in a receptacle. The bags can be covered with some crashed ice before loading. This method takes about 3–4 hours duration while by truck it takes 13–16 hours for transporting in same distance. The price of air transportation can vary from one country to another.
4. DISTRIBUTION
Usually 7–14 days larvae are distributed to the fish farmers who have their own nursery to continue nursing the larvae. After the larvae attain fry sizes of 1.5–2 cm they are distributed to the local fishermen to be reared in grow-out cages or ponds.
SCS/82/SBTC/LEC. 15
by
William L. Chan2
1. INTRODUCTION
In a culture medium, the fish under confinement is invariably deprived of the essentials necessary for its optimal survival. At the same time, it is also subject to the adverse effects of overcrowding and the associated ecological problems inherent in the culture system involved. As environmental parameters fluctuate and other factors extend its adaptive responses, the fish continuously attempts to maintain or re-establish its normal physiological balance. The end result of this process is as often as not, stressful to the fish, and in extreme cases, the chance of survival of the fish is greatly reduced.
The fish under culture is thus subject to a diverse variety of stresses. The dynamics of the relationship between the environment and the fish is however, complicated and little known. When compared with inland culture systems, the need for information for the development of the coastal aquaculture systems practised in the South China Sea region is especially urgent.
In the case of seabass (Lates calcarifer) culture, for example, considerable losses of fry in the nursery phase are being commonly experienced. From marine fishcage culture trials conducted in the sea, as high as 95 percent mortality from fry (2.0–2.2 cm TL) to fingerlings (6.0–8.0 cm TL) has been found to be common under sub-standard stock management conditions. Even for well-managed trials, mortalities though reduced, have remained at 30–50 percent. For pond culture systems, a similar order of mortalities has also been recorded.
The observed high mortalities can therefore, affect the economics of seabass culture. To countries where the fry have to be imported, and for areas remote from fry supply centres, the high cost of fingerlings for grow-out operations can thus be seen as a potential major constraint to confront the future development of seabass culture in the region.
The purpose of this document is therefore, to consider the causes of nursery mortality and the appropriate stock management measures to be taken to overcome them. Although the information used is derived from marine fishcage culture experience, the principles behind the observations and discussions should also be applicable to fishpond culture situations.
2. CAUSES OF NURSERY MORTALITY
2.1 Cannibalism
Typical of serranoids, the seabass is highly cannibalistic especially in the early phases of its development when the individuals of the species tend to congregate more so than for later stages. This offers a convenient opportunity for the stronger fry to take their prey. Upon its first success to prey on its kind, a seabass fry will continue to exhibit increasing vigor and urge to feed mainly through its cannibalistic behaviour beginning from a size smaller than 2.0 cm TL.
From observation made on a total stock of one million 2.0–2.2 cm TL (initial stocking size), it can be concluded that while the bigger readily takes the smaller fry, it is also not uncommon for one to take another of the same size. This phenomenon is most pronounced at dawn and dusk when light intensity is low, and also during slack tide periods when seawater transport is minimal. During each feeding when the confined fry sporadically congregate at high density fighting for the administered feed, the cannibalistic behaviour of the species is perhaps the most pronounced. Lurching over the bottom of a fishcage, the stronger fry readily take the smaller fry when the latter make their typical dive after each successful feeding plunge. Thus, a prey is normally taken from its head.
This explains the distribution of the various size groups of fry within a fishcage with the largest fish lurching over the cage bottom, the normal fry usually of a medium size remaining in midwater, and the weak and always the smallest fry staying just beneath the water surface next to the corners of a cage.
Cannibalistic rate increases with increasing stocking density, clarity of water, and the number of feeding sessions per day. It also increases with decreasing percentage satiation per feeding, the number of feeding sessions per day, and the intensity of light.
Through cannibalistic behaviour, an ecological stress on the smaller and therefore weaker fry is thus created as witnessed from the uneven growth of the stock and the low-condition factor of the smaller fry. If this is not checked, a reduction in the number of fry in a 1.6 m3 cage from 2 500 to 250 has been observed within a matter of 25 days after stocking at a size of 2.0–2.2 cm TL. This number has been found to comprise 5 percent of 7 cm, 40 percent of 3–5 cm, 40 percent of 2.5 cm and 5 percent of 2.0–2.2 cm fry. Fry of the 2.0–2.2 cm size group are uniformly dark grey to black; the 2.5 cm size group exhibits a dark and light banded and mottled colour pattern with the dorso-medial light predorsal band vividly shown at all times; the 3–5 cm size group also shows a similar colour pattern but with a much less contrast of dark and light pattern; and the 7 cm fingerlings show a silvery colour unless they are disturbed.
Thus, while the cannibalistic behaviour of this species is undoubtedly one of the major causes of high nursery mortality, it would appear to be dependent upon a number of factors. In particular, stocking density, disease infection and stock management have been observed to be closely associated with the observed high rate of mortality.
2.2 Stocking density
High stocking density is another common cause of high nursery mortality especially in the absence of stock management measures. From fishcage culture trials, it has been observed that the initial stocking density, using fry 2.0–2.2 cm TL, at 375 fry/m3 or the equivalent of 429 fry/m2, would appear to be a reasonable figure. In the absence of stock management, this number has been reduced to about 200 fish, or by 47 percent, after 25 days of culture. The remaining stock is much more even in size, comprising roughly 2 percent of 6–7 cm TL, 88 percent of 4–5 cm TL and 10 percent of 2.5–3.0 cm TL. Of this remaining stock, the lowest size group similarly exhibits a contrasting colour pattern signifying a state of stress while the highest size group is also silvery except when disturbed.
As stocking density increases above this level, the percentage of mortality accordingly increases for the same culture period. Thus, high stocking density is definitely another common cause of high nursery mortality.
2.3 Uneven growth
Under confinement conditions, the observed uneven growth of the fish promotes competition among the individuals for feed, space and other essentials for survival. The resulting additive effects of stresses on the smaller and weaker fry as witnessed by the dark to black colour, making them much more susceptible to being preyed and the contract of disease.
If such a stock is not properly managed, uneven growth as an indirect cause of nursery mortality can be rather significant. It can be generalized that uneven growth could be due to cannibalistic behaviour of the species. This generalization would however, appear invalid despite the observed differences in the growth of the trial stocks. In this consideration, it is important to note that even properly graded fry kept in separate confinements, have also shown pronounced differences in growth rates. It would thus appear that in each brood of artificially bred fry there are the normal and the sub-normal fry in terms of growth and other biological characteristics which cannot be detected until sometime later. Dietary and environmental factors may also be responsible.
2.4 Disease infection
Disease infection has been known to be responsible for mass mortalities of fry in seabass nursery operations. Unfortunately, there have been few concerted investigations to establish treatment criteria; and the present knowledge and experience on seabass diseases are varied and fragmentary. Disease infection is no doubt one of the major causes of nursery mortality posing the principal constraint to confront the future development of seabass culture.
The term “disease” is herein generally referred to embrace viruses, bacteria, fungi, protozoans and other harmful pathogens including helminths. In the case of the seabass disease infection often misses the attention of the culturist until in later or secondary stages when the symptoms are more easily discernible, but by which time treatment then becomes ineffective. Based on visual and incomplete laboratory observations, the most common symptoms of diseased seabass fry are:
loss of desire to feed;
immediately followed by increasing production of melanin cells making the fry almost uniformly dark grey to, in extreme cases, black, and coupled with complete loss of desire to feed;
simultaneously, the ventral profile of the fry becomes concave, and the body sub-normally compressed suggesting a noticeable decline in condition factor;
concurrent with (b) and (c) fry show loss of skin typically dorsal on the frontal-supraoccipital area and on the side of the body, sometimes skin flaps still attached to these parts;
either before of after (d), a very long string of faeces typically whitish grey attached to the anal opening trailing behind the fry as it swims sluggishly with head up and tail down; and
initial fungal infection on the skinless parts becoming thick white patches.
As the number of these symptoms increases, the fry show a distinctly poor condition factor, a complete loss of appetite, a uniformly black colour, and a gradual loss of orientation. Death normally begins two to three days after the appearance of symptom (e). The rate at which these symptoms develop from (a) to (f) varies from four to six days. Generally, the higher the stocking density, the faster will be the rate of advancement of these symptoms.
Examinations of such disease-affected fry have shown that fish suffering symptoms (a) and (b) are affected by concentrations of protozoans on the gills. On immersion of the fry in a medium at a reduced salinity at 15–20 ppt with a concentration of 2 ppm formalin for several hours, the expression of the other symptoms can be deterred. On immersion in a medium of reduced salinity at 10–15 ppt with a concentration of 2 ppm of terramycin for more than 2 hours with aeration in a closed or recirculating system, nearly all fry resume the normal intensity of their ground colour and also their desire to feed.
Although these external treatments would appear to have certain positive effect on the sick fish, they are not practical when administered to fishcage and fishpond situations. The only cause of action is to isolate the sick fry and in many cases, it would be prudent to eradicate the disease-affected batch of a stock when such symptoms are first noted, and at the same time reduce stocking density and to ensure a high dissolved oxygen level in the culture medium at all times. Upon the resumption of feed, regular feeding using a suitable diet will also help suppress the additive nature of stresses.
There are no doubt other symptoms involving other forms of disease infections. The fragmentary nature of information does not enable further discussion of this problem.
3. NURSERY MANAGEMENT
The design of any functional nursery systems for the seabass must take into consideration the manageability of the aforesaid causes of mortality. From a management standpoint, priority must be given to the control of the cannibalistic behaviour of the species. If to maintain a uniform size of fry is an effective control measure, then the grading of fry to size groups at regular and frequent intervals must be given priority. To facilitate the effectiveness of this standing control measure, the standardization of stocking density for each size group, the design of a suitable diet and feeding regime for various size groups, the separation of normal from sub-normal fish, and the frequent maintenance of culture facilities and water qualities, will also need to be effectively integrated into the activities of a functional nursery management system.
3.1 Grading of fry
To sort thousands of fry to different size groups using manual methods is both time-consuming and harmful to the fry because of prolonged exposure to handling stresses. Graders have therefore, been designed for this purpose.
A grader for the sorting of seabass fry is either a plastic bowl with uniform round holes, or a frame with netting material affixed to one of its ends. The holes whether round, square or rhomboid are for the escapement of the fry from the inside of a grader. Since the holes of both these types of graders possess rough edges, injuries to the fry are invariably incurred during each grading to such an extent that a 1–2 percent handling mortality is quite a common phenomenon.
Figure 1 shows an improved version of the graders previously adapted by the author. It comprises an easy to handle wooden frame with a piece of soft plastic (plastic bag material) affixed to one of its ends. In this, plastic sheet are made round holes with a steel hole puncher. The diameter of the holes determines the size of fry to be graded. This grader has been thoroughly tested and zero handling mortality has been recorded.
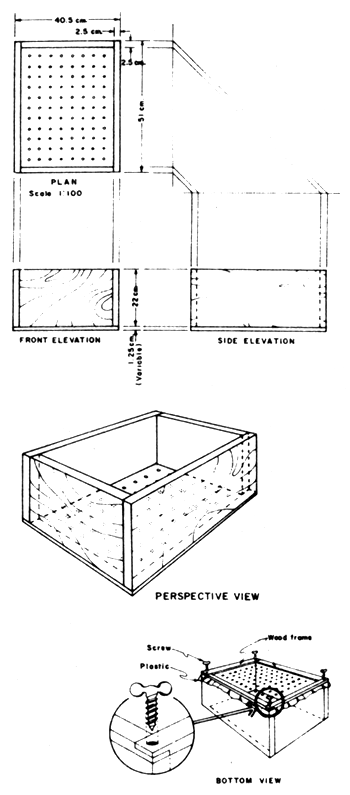
Fig. 1 An improved grader for the grading of seabass fry
The fast growth of the fry necessitates grading to be carried out at three-day intervals. After each grading, there should be a reduction in stocking density and also separation of dark-coloured individuals from the normal fry. All dark-coloured fry can be assumed to be adversely affected by stresses, or suffering from disease infection.
3.2 Stocking density
Coupled with frequent grading, the adoption of a suitable stocking density for each size group is also important in checking losses due to cannibalism. It would appear that investigations should be made to ascertain what may be acceptable density for each size group cultivated under differing facilities. This line of study should be concerted also with the environmental conditions required for optimal growth and survival of the species.
3.3 Water quality management
A stock of disease susceptible fry exposed to inadequate environmental conditions can readily become “diseased” as manifested by symptoms ranging from reduced growth to high mortality rate.
It is therefore, pertinent to maintain a high level of dissolved oxygen at all times as an indicator of having the harmful parameters (such as ammonia, metabolic wastes, etc.) under control. To approach this, it may be necessary to consider improvement on facility management, water exchange rate of a culture medium, feed and feeding method and any other activities which play a direct or indirect role in the attainment of this target.
3.4 Feed and feeding method
Dietary deficiency similarly makes a stock of fry disease susceptible. The trash fish diet presently used for the nursery of the seabass may in all probability require investigation. Although Acetes shrimp is also used, the degree of spoilage of the materials used at least suggests deficiency of vitamins and trace nutrients, and also unhygienic conditions, in these diets.
The application of feed is also another operational activity that warrants priority investigaton as inappropriate feeding method is known to be widely practised in the region. This often results in the contamination of the culture medium and also wastage of feeds.
3.5 Fry selection and disease quarantine
In the acquisition of fry, it is always prudent to examine fry from the same brood before selecting those of the largest size group showing the normal colour of the species. All dark-coloured fry must be rejected. Using external chemical treatment, the selected fry should be put through a quarantine procedure with the purpose of ridding or thinning out harmful pathogens. This involves the immersion of the selected fry in prescribed medicated solutions.
3.6 Disease problems
Little is known about the diseases presently affecting seabass fry. This is largely due to the lack of well-concerted investigations into mortality cases, and also the fact that the monoculture of the seabass has only been a recent introduction. Before scientific information is forthcoming, disease problems should better be controlled through the development and management of dietary and environmental conditions of culture systems, with a view to minimizing the adverse effects of stresses and maximizing the percentage of healthy fry.
SCS/82/SBTC/LEC. 17
by
B. Sirikul
1. INTRODUCTION
The main objective of fish culture is to get the highest crop yield of marketable-sized fish per unit area in the most economical means possible. Total production of seabass culture has increased from small amount in the past to more than 300 metric tons in 1981. There are two main methods of culture for growing marketable size seabass, namely pond culture and netcage culture. The main seabass production came from netcage culture.
2. THE STOCK FOR PONDS AND CAGES
Most of the hatcheries distribute seabass fry to fish farmers at the size of 1.5–2.0 cm in length or at the age of 35–45 days. So, it is necessary for the fish farmers to subject them to nursery rearing in nylon netcages until the fry reach 8–10 cm in length or about 3–4 months in age. If they are collected fry from natural waters, the size of fry is between 5–10 cm in total length.
The size of seabass for stocking in ponds is rather big, 10–15 cm in total length, in the juvenile stage and at the age of about 3–4 months.
For the grow-out netcage, the stock to use can be smaller, 5–10 cm in length. These may be obtained by transfer from the nursery netcages after 2–3 months of culture.
3. GROW-OUT CULTURE OF SEABASS
3.1 Site, layout and construction of grow-out ponds
3.1.1 Selection of suitable site
In the construction of fishponds, the following must be considered in the selection of a suitable site:
Water supply. Water supply in good condition for aquaculture should be adequate all year round. For fishponds along the coastal area, the source of good quality water is the tide which brings in either marine or brackishwater. To avoid the harmful effects of wave action and tidal movement the ponds should be located at some distance, say one kilometer from the seashore. The tidal river or a canal system can serve as waterway.
Drainage. The site should be drainable whenever necessary. In brackishwater ponds, the land elevation between the mean low tide and lower high tides can serve best to enable drying during most low tides.
Soil. Soil is considered with regard to its water retention properties. Clay, clay loam and sandy clay are the best types of soil suitable for fishponds.
Topography. Level swamplands or tidal flats are the general sites preferred. These would be less costly for development.
Free from floods and strong waves. Sites which are periodically flooded and frequently exposed to strong winds and waves should be avoided. This can lessen the cost of maintenance and avoid wholesale destruction during calamities.
Marketing facilities. The site should be near market outlets and good transportation facilities. If the market is far, additional expenses for handling and transport will reduce possible profits.
Other economic factors. If possible, cheap and trained labour should be available. Training of skilled labour would mean additional costs.
Security. If possible, the ponds should be located where good security conditions exist. This is to avoid poaching of the marketable fish crop.
3.1.2 Layout and construction of ponds
A typical fishpond for grow-out rearing of seabass follows the same general pattern as those of other coastal fishponds. For fishponds along the coastal areas, the soil should consist of a good mixture of silt, sand and clay that are suitable for pond construction. Sometimes, a storm dike or one with riprapping of rock or stones should be built along the exposed part of the project to prevent destruction by big wave action. If the fish farmer cannot build this reinforced dike, the ponds should be constructed on a site with provision of adequate buffer strip from the seashore and have a canal system built to serve as waterway. The size of rearing ponds should be at least 0.4 to 3.0 ha in area with water of 50–60 cm deep. Smaller ponds are relatively more costly to construct and tend to inhibit rapid growth of the seabass. The ponds should have two water gates, one for water inlet and the other for water outlet. The bottom should slop slightly toward the provided passageway which should be 30–40 cm deeper than the average pond bottom to facilitate drainage (Figs. 1 and 2).
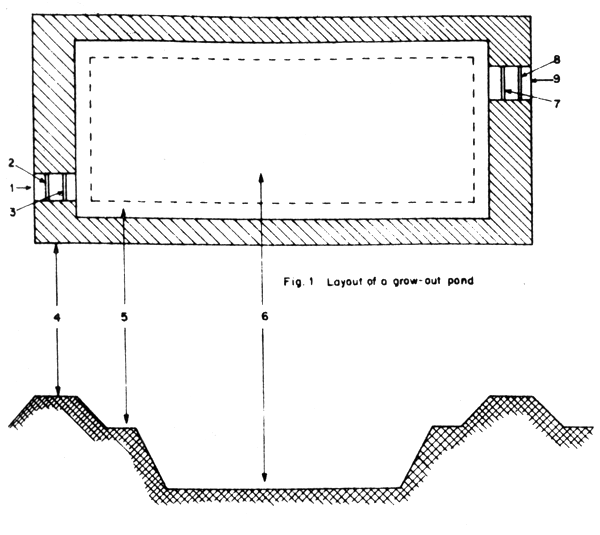
Fig. 2 Cross-section of a grow-out pond
3.2 Rearing in ponds
3.2.1 Stocking and stocking density
The stocking density in the pond ranges from 3–5 juveniles/ m2 with fish of 10–15 cm in total length with feeding. Without feeding 1–2 juveniles/m2 can be stocked in combination with Tilapia.
3.2.2 Care of stock
For feeding seabass in rearing ponds, two methods are used which are described below:
Feeding with trash fish. Fresh trash fish are chopped into small pieces at sizes suitable for the capacity of the mouth of the seabass. The feeding conversion observed is 7–10 kg to 1 kg of marketable seabass.
Feeding by rearing seabass in combination with Tilapia mossambica. First, the broodfish of Tilapia are stocked with the density of 400 fish consisting 200 males and 200 females, in an area of 2 000 m2 before stocking the seabass. Two months later, seabass with 10–15 cm in length are released into the same pond or paddy field at the stocking rate of 3 125–6 250 fish/ha or 500–1 000/rai1. The seabass would feed on the Tilapia fry.
Water management. Along the coastal area, the source of water is usually of good quality. The water has to be partially changed each day by opening the water inlet gate to allow water at high tide to come in. When the tide recedes the water outlet gate is opened to drain water. Water draining should be from the bottom layers of the pond water while water flooding or filling should flow in at the surface. The amount of the water must be sufficient to supply the pond. Water pumps are needed sometimes when it is necessary to let in more water. The nylon net screens used for the gates have to be cleaned everyday. Whatever the source of water, its quality should be carefully checked. It is most desirable that the water will have a pH range between 7 and 8.5, and dissolved oxygen of 5–6 ppm.
3.2.3 Harvesting, handling and marketing
Several methods of harvesting seabass from ponds are available. For catching small quantities, seines, traps and fishing pole can be used. For capturing large quantities of fish to supply fish landings and cold storages and for cleaning out rearing ponds, the best methods to use are seining followed by total dewatering.
The best method of handling fish for the market is by moving the product from the pond to the market in as fresh condition as possible. The fish are mixed with ice and kept in baskets or wooden boxes. The fish are transported by trucks or boats.
Seabass usually are sold fresh and in some areas even live in accordance with regional preference. Most fish are not sold directly to the consumer but through various middlemen that are involved in handling the product.
1 Rai is a Thai measure of land area equal to 1 600 m2 or 0.16 ha; 6.25 rai = one ha.
3.3 Rearing in grow-out cages
This method is suitable for seabass culture in coastal shallow and protected areas. Within recent period, this method of culture has been receiving considerable attention in Japan. This method has been applied by the Songkhla Fisheries Station using Songkhla Lake. Fish can grow well and gave good production of marketable size.
3.3.1 The site for grow-out cages
In the selection of a location for cage culture, the following factors must be considered:
Circulation. There must be sufficient exchange of water through the cage. This washes away feces and uneaten food and insures that dissolved oxygen concentration is at least 3 ppm.
Salinity. Seabass is a brackishwater fish and can grow well at salinity range of 20–30 ppt.
Pollution. In fish cage culture, the site should be remote from sources of domestic, industrial or agricultural pollution.
Protection from winds and waves. The cage culture site should be located in sheltered water area.
Accessibility. It must be economically feasible to transfer fry and food to farm and ship marketable fish from it.
Security. No thieves and robbers are in the area so that losses through poaching can be minimized.
3.3.2 Size and construction of cages and materials used
The type of grow-out netcages are divided into two types as described below:
Floating netcages. This type of cage has frame made of wood or iron and nylon net with stretch mesh size of 2.5–5.0 cm (1–2 inches) of string No. 15 is used. The cage is suspended in the water by hanging on raft. There are many sizes of the cages such as 1 m long × 1 m wide × 1.5 m deep; 2 m long × 2 m wide × 2 m deep. Fish at the size of 8–10 cm in length are stocked with the density of 25–30 juveniles/m2. The floating netcages are set in the deeper areas along the coast. The water depth in floating cages is maintained at about 1.2–1.5 m so that the stocking rate in this type of netcage is more than those in set nets. After rearing for six months, the yield can get to 50–60 kg/m2 with fish size of 500–600 g in weight. This is the suitable size for restaurant demand. We call this fish size as “table size” or “desk size” fish.
Set netcages. These netcages are set in shallow coastal sites or in mangrove areas. This type of netcage culture is popularly used in Thailand. Netcages are made of nylon net that are made in square or rectangular shape. The size and mesh of the netcage depend on number and size of fish to be cultured.
Nylon netcage with the size of 2 m long × 1 m wide × 0.90 m deep with 0.2 cm mesh size is used for nursing fry at the size length range of 1.5–2 cm. Stocking density of fry is between 300–500 fry/cage. These netcages are anchored by tying them with poles or hanging on rafts. The water depth in the cage is about 50 cm (Fig. 3).
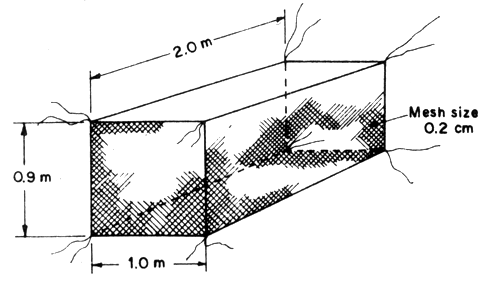
Fig. 3 Fry nursery nylon netcage
Nylon netcage with the size of 2 × 4 × 2 m with 1.5 cm mesh are made of nylon string No. 6. These netcages are used for rearing juveniles and are anchored by tying them to wooden poles. The stocking rate of juveniles at the size average of 5 cm in length is between 300–400 juveniles/cage. Two months later, the juveniles can reach 10 cm in average length and they can be transferred to the big netcages.
Nylon netcage of big size that is 5 m long × 5 m wide and 3 m deep or 10 m × 5 m × 3 m or 10 m × 10 m × 3 m with the mesh size of net of 5–7.5 cm (2–3 inches) made of nylon string No. 15 or 18. These netcages are used for rearing juveniles from average size of 10 cm until the fish reach marketable sizes. The netcages are anchored by tying them with wooden poles with diameter of 15–25 cm. Water depth in the cage ranges from 0.70–1.50 m depending on the state of the tide, but the lowest should not be less than 0.50 m. Stocking density of juveniles with size of 20 cm in length ranges from 8–10 fish/m2. After the first year, the production of seabass can get to 6–8 kg/m2 for 10 × 10 × 3 m cage or 600–800 kg/cage of 100 square meters. The fish could have attained 0.8–1.2 kg in body weight in one year (Fig. 4).
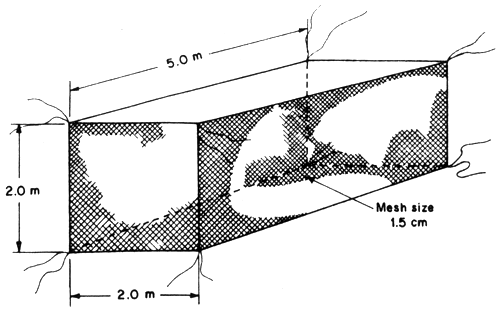
Fig. 4 Juvenile rearing nylon netcage
3.3.3 Care in the cage
Fresh trash fish are used as seabass food. The fresh trash fish are chopped into small pieces suitable for the mouth of the fish. Feeding rates depend on the size of the fish. If the fish weighs less than 100 g, the feeding rate ranges 8–10 percent of the body weight. When the fish is bigger or more than 100 g, feeding rate ranges 3–5 percent of the body weight. The amount of food is shown in Table 1.
| Size of seabass | Body weight (g) | Weight of food per day (kg) | Percent of feeding |
|---|---|---|---|
| Small size | 50 | 0.4 | Feeding 8% of body weight |
| 100 | 0.5 | Feeding 5% of body weight | |
| 200 | 1.0 | ||
| 300 | 1.5 | ||
| 400 | 2.0 | ||
| 500 | 2.5 | ||
| Medium size | 600 | 2.4 | Feeding 4% of body weight |
| 700 | 2.8 | ||
| 800 | 3.2 | ||
| 900 | 3.6 | ||
| 1 000 | 4.0 | ||
| Big size | 1 000 | 3.0 | Feeding 3% of body weight |
| 1 200 | 3.6 | ||
| 1 300 | 3.9 | ||
| 1 400 | 4.2 | ||
| 1 500 | 4.5 |
The practice of feeding in grow-out culture of seabass are as follows:
Diseases and other causes of mortality are not much of a problem in grow-out stage of seabass culture. This subject is discussed in other lectures and practical exercises of this course.
The feeding rate for broodfish before spawning should be one percent of body weight per day. Cultured seabass for the market from the size of 30–50 g in weight can grow to 1.0 to 1.5 kg in one year. If seabass is reared from the size of 300 g in weight, fish will attain weight 1.5–2.5 kg in one year as shown in Table 2.
| Period of rearing (month) | Total body length (cm) | Body weight (g) |
|---|---|---|
| start | 15 | 50 |
| 2 | 20 | 100 |
| 4 | 25 | 200 |
| 6 | 30 | 350 |
| 8 | 33 | 500 |
| 10 | 35 | 700 |
| 12 | 38 | 900 |
| 14 | 41 | 1 200 |
| 16 | 44 | 1 500 |
| 18 | 46 | 1 800 |
| 20 | 48 | 2 000 |
| 22 | 51 | 2 200 |
| 24 | 53 | 2 700 |
3.3.4 Yield from cages
Seabass culture in cages can use higher stocking density than in ponds. Fish production from the set netcage with size of 10 m long × 10 m wide × 3 m deep yields 600–800 kg/cage or 6–8 kg/m2 in one year, while in floating netcages the yield is 10–25 kg/m2. From rearing ponds, seabass yields range from 500–600 kg/rai or 3 250–3 750 kg/ha/year.
It has been found that the amount of trash fish feed to seabass to obtain 1 kg is 7–10 kilograms. Harvesting from cages is simple; this is done by merely lifting the net and scooping the fish. The fish are brought to the dealer or to the market in as fresh condition as possible (Fig. 5).
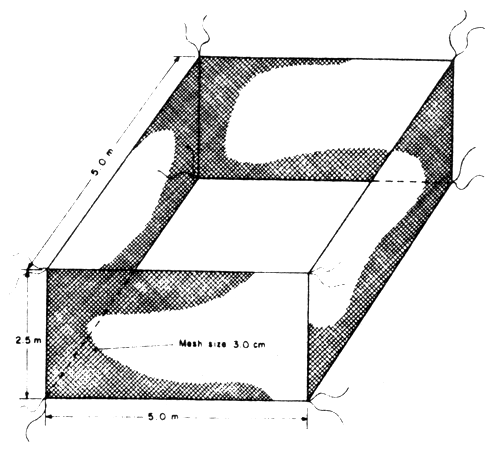
Fig. 5 Grow-out nylon netcage
SCS/82/SBTC/LEC. 12
by
S. Maneewongsa and T. Tattanon
1. INTRODUCTION
The broodstock can be obtained from two sources: (a) broodstock collected from the spawning ground during the spawning; and (b) broodfish obtained from grow-out cultures especially from netcages.
Broodfish collected from the spawning grounds are used only for artificial fertilization method by stripping. The fish taken from grow-out cages are usually used for spawning in captivity.
At present, seabass fry are in demand among the fish farmers in Thailand and also in some other countries such as Malaysia. Singapore and Taiwan (China). The production of seabass seedling should not be conducted without enough broodfish that are properly maintained.
2. CARE OF BROODSTOCK DURING THEIR EARLIER AGE STAGES
Broodfish are raised from juvenile stage in cages. The healthy and fast growing fish with size 1.5–2.0 cm in total length are selected out from the rearing tank to be raised in netcages of 1 × 2 × 1 m in natural open water for use as future spawners.
About one month later, they become 3–3.5 cm in length and again approximately 50 percent of them are selected from those that appear healthy and fast growing to be kept for culture up to two years. After the first year rearing period, when the fish are within their third or fourth month, they are transferred from cage of 1 × 2 × 1 m to a bigger cage of 5 × 5 × 2 m until they are matured. After two years, another selection of 50 percent of this stock is made out and the remaining stock are then raised up to three years. At this time, the fish will have attained 3.5–4 kg in their body weight and are ready to mature. The gradual reduction in number of selected broodstock are shown in Table 1.
| Age stage | Cage dimension (m) | Stock density |
|---|---|---|
| 1 month | 1 × 2 × 1 | 2 000 |
| 2 months | 1 × 2 × 1 | 1 000 |
| 1 year | 5 × 5 × 2 | 500 |
| 2–3 years | 5 × 5 ×2 | 250 |
During this period, the fish are fed approximately 5–10 percent of their body weight daily.
3. CARE OF MATURING AND MATURE BROODSTOCK
One month before the spawning season (April-September), broodfish are transferred from the cagenet to the spawning tank. These are round-concrete tanks with 10 m diameter and 2 m depth, used as spawning tanks. Twenty-four fish are kept in each tank with female to male sex ration of 1:1. The spawners are fed with sardine after the head portion and the intestines are removed. The feeding rate is approximately 1 percent of their body weight, daily.
The water in the spawning tank should be in good quality utilizing the running water system. Everyday, the total amount of water changed by this system is about 30 percent of total volume water in the tank. Every after few days, the tank should also be cleaned and 80 percent of the water in the tank is changed for a new fresh seawater. Enough supply of aeration is always supplied in the spawning tank.
The fish spawn in the tanks without any inducement. After spawning the fertilized eggs are scooped out from the spawning tank to the hatching-rearing tanks. Since the spawners are kept in the tank for a long time, they are sometimes damaged by bacteria causing finrot disease. If this occurs, they should be treated with 1 ppm KMnO4 solution for 10–15 minutes and antibiotics such as tetracycline hydrochloride at 50–60 milligrams per day for 3–4 days continually.
The age of the fish which one can continue to use as spawners each year is under study. However, at NICA the female fish of 6 years old is still usable as good spawner. Each year, some amount of seabass fingerlings should be selected for future spawners in order to replace the ones that would grow old.
After the end of the spawning season, the broodfish are moved back from the tanks to the netcages in natural open water.
4. FURTHER INFORMATION NEEDED ON SEABASS BROODSTOCK
Studies on genetic selection and the use of artificial diets are needed in order to improve the technique and production of seabass fry.