SCS/82/SBTC/LEC. 21
by
T. Yokokawa2
The water conditions for aquaculture is not special. Almost all of aquatic organisms cultivated require normal water conditions. The meaning of normal water conditions is the same as the conditions where the fish live in nature.
For making good water conditions for aquaculture, we need to know the following various factors:
(1) Physical Factors
(2) Chemical Factors
2 JICA Coordinator and Expert on Aquaculture, NICA, Songkhla, Thailand
| Items | |||||
|---|---|---|---|---|---|
| Rank | pH | BOD | SS3 | DO | E. coli |
| AA | 6.5–8.5 | Less than 1 ppm | Less than 25 ppm | More than 7.5 ppm | Less than 50 MPN4100 ml |
| A | 6.5–8.5 | Less than 2 ppm | Less than 25 ppm | More than 7.5 ppm | Less than 1 000 MPN/100 ml |
| B | 6.5–8.5 | Less than 3 ppm | Less than 25 ppm | More than 5 ppm | Less than 5 000 MPN/100 ml |
| C | 6.5–8.5 | Less than 5 ppm | Less than 50 ppm | More than 2 ppm | -- |
| D | 6.0–8.5 | Less than 8 ppm | Less than 100 ppm | More than 2 ppm | -- |
| E | 6.0–8.5 | Less than 10 ppm | No floating dust | More than 2 ppm | -- |
NOTE: 1. The values are monthly averages
2. For agriculture, pH 6.5-7.5, DO more than 5 ppm
3 SS stands for suspended solids.
4 MPN stands for most probable number (of colonies).
| Items | |||||
|---|---|---|---|---|---|
| Rank | pH | COD | SS | DO | E. coli |
| AA | 6.5–8.5 | Less than 1 ppm | Less than 1 ppm | More than 7.5 ppm | Less than 50 MPN/100 ml |
| A | 6.5–8.5 | Less than 3 ppm | Less than 5 ppm | More than 7.5 ppm | Less than 1 000 MPN/100 ml |
| B | 6.5–8.5 | Less than 5 ppm | Less than 15 ppm | More than 5 ppm | - |
| C | 6.5–8.5 | Less than 8 ppm | No suspended solids | More than 2 ppm | - |
NOTE: The SS value is not applied for fisheries (AA to B).
| Item | |||||
|---|---|---|---|---|---|
| Rank | pH | COD | DO | E. coli | Oil |
| A | 7.8–8.3 | Less than 2 ppm | More than 7.5 ppm | Less than 1 000 MPN/100 ml | None |
| B | 7.8–8.3 | Less than 3 ppm | More than 5 ppm | - | None |
| C | 7.0–8.3 | Less than 8 ppm | More than 2 ppm | - | - |
| - | - | - | - | - | - |
1. For oyster culture, E. coli is less than 70 MPN/100 ml, and use only A rank water
| 1. BOD | 20°C, 5 days, less than 5 ppm | |||
| Less than 3 ppm for salmon and “ayu” (smelt) | ||||
| 2. DO | More than 5 ppm | |||
| More than 5 ppm more than 16 hours per day | ||||
| Always more than 3 ppm | ||||
| 3. pH | In freshwater area 6.5 to 8.5 | |||
| 4. Turbidity | Less than 10 ppm | |||
| In seawater, can keep enough brightness for growing the seaweed. In strong colour | ||||
| 5. Smell | Less than 0.01 ppm, mineral oil | |||
| Less than 0.01 ppm, phenol | ||||
| No smell for edible fisheries products | ||||
| 6. Temperature | No effect for growing aquatic life | |||
| 7. Toxic | Less than following (ppm) | |||
| substance | Hg | 0.004 | Cu | 0.01 |
| Cd | 0.03 | Zn | 0.1 | |
| Pb | 0.1 | Al | 0.1 | |
| Ni | 0.1 | Cr | 1.0 | |
| Mn | 1.0 | Sn | 1.0 | |
| Fe | 1.0 | CN | 0.01 | |
| Cl (Free) | 0.02 | Br | 1.0 | |
| F (Complex) | 1.5 | |||
| S (Complex) | 0.3 in pH 6.5 | |||
| NH3 | 1.0 in pH 8.0 | |||
| Milkfish (Chanos chanos) | Shrimp (Penaeus monodon) | |
|---|---|---|
| Water temp. | 24–32°C | 25–30°C |
| Salinity | 10–30 ppt | 20–30 ppt |
| pH | 7.0–9.0 | 7.5–8.5 |
| DO | More than 3 ppm | More than 4 ppm |
| NH3 | Less than 1 ppm | 0 |
| H2S | Less than 0.3 ppm | 0 |
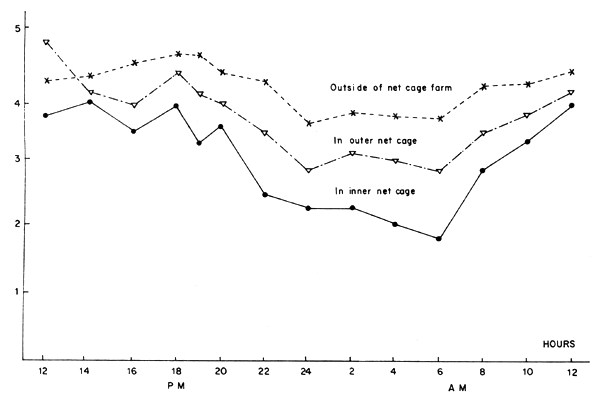
A sample of daily variation of DO value in seabass net-cage form in Songkhla lake
SCS/82/SBTC/LEC. 5/6
by
L. Ruangpan2
1. INTRODUCTION
With recent emphasis on intensive fish culture, there is an increasing awareness of the problems associated with pathogenic agents that cause diseases. The diseases, whether by slow continuous attrition or by sudden catastrophic epizootics, can cause fish mortalities often resulting in great losses of the stocked fish. Generally, infectious diseases of fish and other aquatic animals are caused by parasites, bacteria, fungi and virus. But frequently, diseases and abnormalities due to environmental stresses and nutritional dificiencies have also been recognized. These will continue to be important until adequate and refined diets, as well as effective control of water quality, are fully realized. These latter causes of mortality are not infectious diseases but they can be harmful to the fish and can result in secondary infection by pathogenic agents.
The technology of reducing the impact of diseases is developing, but most disease specialists feel at times like sorcerers or apprentices, as newer and often more difficult problems emerge to replace the solved problems of yesterday. Therefore, the biologists who have more experience and more interest in this work will get better results.
2. VIRUS (Figure 1)
Viral diseases have not been considered to play a dominant role in their effects on marine and brackishwater culture, but there are many reports indicating the existence of viral diseases in oysters and crustaceans which cause mortalities to their populations. So far, no viral disease has been clearly identified as a major problem in seabass culture. But with marine and anadromous fish, such diseases as lymphocytis and cauliflower

Fig.1 Smear of liver and kidney of viral diseased fish
disease such as in eels have become significant. At present however, no facilities for the study are available so that the procedures or techniques of its diagnosis will not be discussed in this paper.
3. BACTERIA (Figure 2)
Bacterial diseases in fish generally do not develop simply as the result of exposing a host to an infectious agent. Mostly, bacterial disease will occur as a result of the complex interactions between pathogen, fish and environmental stress which will affect the susceptibility of the host to diseases. Environmental stresses can affect the homeostatic mechanism of fish, thus reducing their resistance to pathogenic organisms.
Fish reared in intensive culture conditions are exposed to extreme environmental fluctuations, and they may be more sensitive to stress than wild populations.
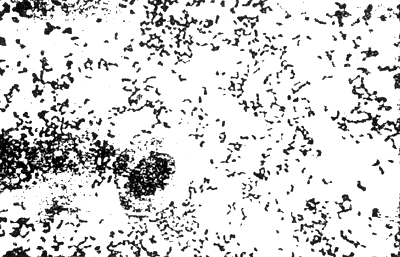
Fig.2 Comparative of red-blood cell and pathogenic bacteria in gram stain
3.1 Aeromonas
Aeromonas sp is a common water-borne bacterium which may be present in the tissue of normal young or adult fishes. Whenever fishes are exposed to environmental stress or injury. it causes serious outbreaks of hemorrhagic disease with high mortalities. Temperature, pH, high CO2 and O2 depletion, decomposition products, and free ammonia in the water, all of these can be considered as possible factors for Aeromonas infection.
3.2 Vibrio species
Diseases caused by Vibrio sp characteristically manifest themselves as an ulcerative haemorrhagic septicaemia. The typical symptons of vibrio disease include congestion of the fins, eccymoses and petechiae on the body surface, and frequently, haemorrhages and ulceration of the skin and muscle tissue. Surrounding the infected anus (the vent) is usually reddened and inflamed. Internally, there is congestion and haemorrhagia of the liver, spleen and kidney, frequently accompanied by the presence of necrotic lesions. The gut and particularly the rectum may be distended and filled with a clear viscous fluid.
Vibrio disease in young fish especially in juvenile has less well-defined clinical signs. The body is completely covered by a thick layer of mucous, and occasionally small unbroken lesions are present. There may be a reddening of the caudal fins and vent. Internal organs appear normal. Young fish tend to die more rapidly than adults.
The fish pathogenic vibrio which have been described in the literature include Vibrio anguillarum, V. piscium and V. ichthyodermis.
4. PROTOZOA
Protozoans are probably the most important group of animal parasites affecting fish. Many reports from all over the world indicated great losses in fish culture caused by protozoans. Obligate parasites such as the ciliate Ichthyophthirius and certain species of the cnidosporidians are responsible for many of these losses. Many species which are considered as commensal protozoans may become pathogenic under certain conditions. Factors such as environmental influence affect the hosts susceptibility to certain protozoans. Oxygen concentration and temperature are the factors affecting both hosts and parasites. Since many protozoans transfer from fish to fish through the water, therefore fish population density is also an important factor. Tremendous infestation of protozoans can occur in a relatively short time where fish populations are dense. Other factors, such as host size, age, host specificity, immunity, and the aforementioned influences of host condition also play an important part in the host reaction to invasion by protozoans. Most host reactions to invasion by protozoans are directed toward expelling or isolating the parasite (Fig. 3).
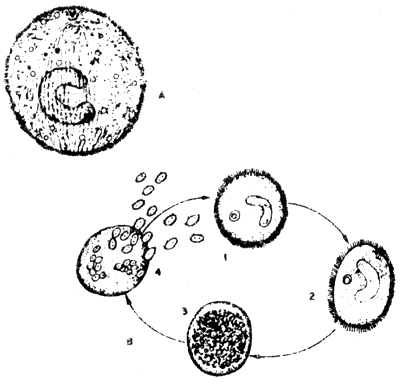
Fig. 3 Ichthyophthirius sp from fish gills
A. Stage which is found in fish
B. Reproduction
Main harm or protozoans to the fish host are mechanical damage, secretion of toxic substance, occlusion of the blood vessels, obtaining nutrition at the expense of the fish host, and rendering the host more susceptible to secondary infections. Some of the most common clinical signs are changes in swimming habits, such as loss of equilibrium, flushing or scraping, loss of appetite, abnormal colouration, tissue erosion, excess mucous production, haemorrhage and swollen body or distended eyes.
4.1 Trichodina
Members of the genus Trichodina with about 60 species described from marine fish and related peritrichous ciliates are the most common parasitic protozoans which are especially harmful to young fish. The species parasitize marine fish by attaching themselves mainly to the gills. Juveniles of seabass observed to be heavily infected with this parasite caused high mortality of over 50 percent of the stock. Trichodina also causes problems to crowded fish reared in cages (Fig. 4).
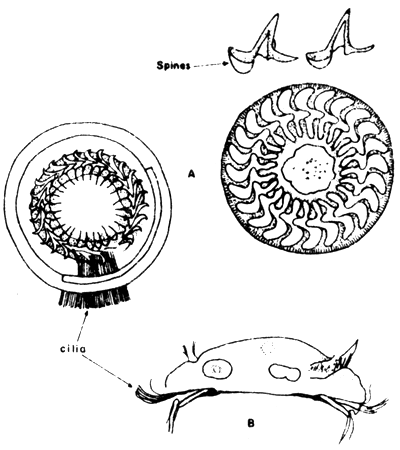
Fig. 4 Parasitic ciliate, Trichodina sp
A. Frontal view
B. Lateral view
Clinical signs of trichodinosis include excess mucous production, flushing, debility and hyperplasia and necrosis of the epidermis. The fin may become badly frayed in heavily infected fish and this may be accompanied by sluggishness and loss of appetite. Excessive numbers on the gills of infected fish may interfere with respiration.
4.2 Cryptocaryon
Cryptocaryon sp is a marine counterpart of the freshwater Ichthyophthirius species and similarly causes the white spot diseases in marine fish. Its morphology and life cycle is quite similar to that of the “Ich”. The surface of invaded fish reveal whitish pustules or numerous minute, greyish vesicles which are nests of ciliates burrowing under the epidermis. They feed on the host's cells, undermine the epithelium and cause heavy irritation resulting at first in excessive production of mucous and finally they can completely destroy the fine respiratory platelets of gill filaments. On the skin, this parasitic protozoan causes considerable lesions resulting in destruction of large areas of the epidermis. Secondary infection may complicate the situation and the host dies. The incidence of Cryptocaryon sp in seabass showed a distinct peak during low water temperature period, with a marked prevalence during February. This ciliated protozoan probably causes more damage to fish populations over the entire world than any other single parasite. Henneguya is another flagellate found in freshwater seabass causing gill diseases while Costia, Epistylis and Zoothamnium can also affect young seabass in freshwater (Figs. 5, 6, 7 and 8).
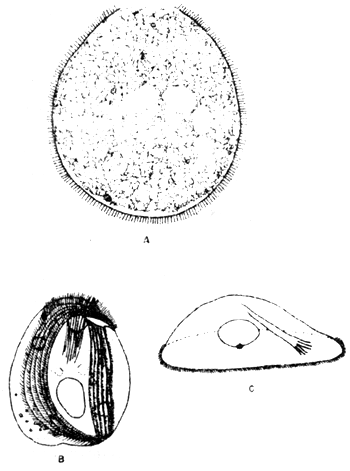
Fig. 5 Ciliate protozoon, Cryptocaryon sp
A. Trophozoite stage
B. Frontal view
C. Lateral view
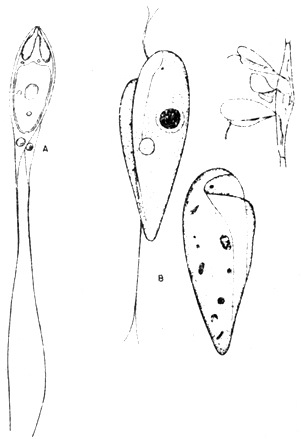
Fig. 6 Parasitic protozoan
A. Henneguya sp
B. Costia sp
Fig. 7 Zoothamnium sp
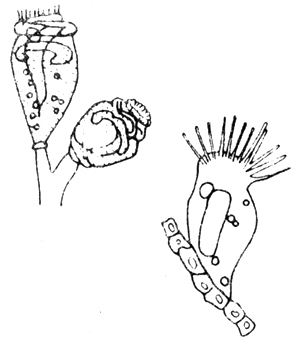
Fig. 8 Epistylis sp
5. PARASITES
5.1 Helminthic parasites
Worm diseases with the possible exception of those produced by monogenetic trematodes, have not yet appeared to be serious problems in seabass culture. This probably due in large part to their complex life cycle and the difficulty in completing such cycles in the culture system. Helminthic parasites which have been found in seabass include monogenetic trematode, digenetic trematode and nematode.
5.1.1 Monogenetic trematodes
Monogenetic trematodes were observed throughout the year. Abundance of these parasites and their seasonal distribution were not studied yet. It has been reported that temperature apparently plays an important role in determining outbreaks of certain parasitic Monogenea. Peak infections of monogenetic trematodes usually occur during the presence of young susceptible fish. Such behaviour is advantageous for the dissemination of the organisms within a fish population.
A monogenetic trematode which is found as dominant species in the gills of seabass is Diplectanum latesi. Dactylogyrus may be present but this has not been fully identified.
5.1.2 Digenetic trematodes
Lecithochirium sp was found in the intestine of seabass especially in wild fish. Incidence of infection was 86.0 percent and average parasite burden was 5.5. Another digenetic trematode which was commonly found in the intestine of wild seabass is Pseudometadena celebesensis. Its incidence of infection and parasite burden were 100 and 9.3. respectively. (Fig. 9).
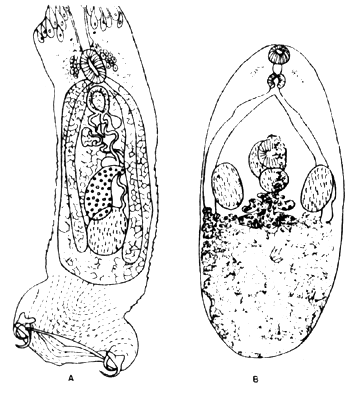
Fig. 9 Parasitic heimenthes
A. Diplectanum lotesi Tripathi, 1957
B. Pseudometadena celebesensis Yamaguti, 1952
5.1.3 Parasitic nematodes
Nematode of the genus Cucullanus was found more common in the gut of larger seabass than that of young fish. Incidence of infection and parasite burden were 1 912 and 1.1, respectively (Figs. 10 and 11).
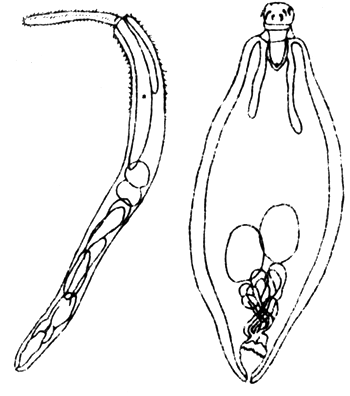
Fig. 10 Acanthocephalid worm

Fig. 11 Nematode worm
5.2 Crustaceans (Figure 12)
Crustacea have long been known as parasites of fish. Especially important among them are Copepoda, commonly parasitic on economically valuable fish species. As parasites, the crustaceans are important to fish in two ways. They can either act as the intermediate hosts for other parasites of fish, or they can parasitize the fish directly. In the former capacity, crustaceans serve as the intermediate hosts of many tapeworms. They also act as the intermediate hosts of nematodes. However, economically more important are those crustaceans which live as direct parasites of fish.
5.2.1 Parasitic copepod
Gills of the young seabass about 3–5 cm were found with Caligus sp attached. The harm they cause is not yet studied.
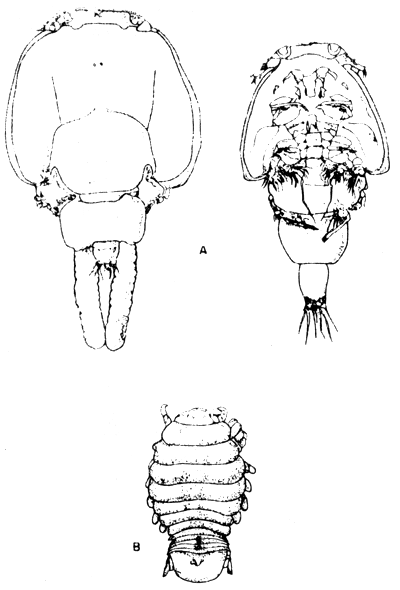
Fig. 12 Parasitic crustacean
A. Caligus sp
B. Aega sp
5.2.2 Parasitic isopod
Isopods which closely resemble Aega sp were found abundant in cage-cultured seabass. The parasites always attach to the gills of its fish host. Comparison of infected and non-infected specimens indicate that young seabass were more than twice as heavily infected as adult fish. Clinical sign of infected seabass showed that fish loss appetite, became anemic and had very low of growth rate. Quick death occurs in 1–2 days.
6. OTHER CAUSES OF MORTALITY
Diseases and abnormalities due to environmental contaminants and nutritional deficiencies have been recognized as important problems in fish culture whenever adequate and defined diets, as well as effective control of water quality, become difficult.
6.1 Nutrition
Nutrition is a vital factor for growth, maturation and reproduction, even for fish life itself. In any environment, nutritional requirements of living organisms must include the various classes of nutrients to be furnished by the ration or available from the environment. Malnourishment or under-nourishment of seabass under culture can result in slow growth. susceptibility to diseases and sometimes death.
6.2 Predators and pest organisms
Cultured fish must be protected from other animals which play important role as pests and predators or sometimes as vectors of diseases such as leeches, water snakes, crabs, snails, water fowls, and of course fish which might be either resident or accidentally introduced into the culture impoundment. The important enemies or predators of seabass in Thailand are Therapon jarbua and Pleuronema tetradactylum. Frequently, the bigger fish in this group by themselves can be predators. So the best way to reduce fish mortality in this cage is size grading. Usually culturists will grade their juvenile every 3 days interval. An important rule of fingerling production in a hatchery is not to rear or stock different age and size groups of fish in the same pond.
To eliminate the predator or other animals in the ponds, this can be done through the use of saponin in the form of teaseed cake (2 kg/rai with reduced depth of water of 10 cm) or rotenone (2–2.5 kg/rai with water depth of 10 cm).
6.3 Unfavourable environment and other stresses
Environmental causes of death also occur. These include lack or very low oxygen content, extremes of salinities and temperature, etc. Other major problems are the stresses that may be applied to fish that are in long-term contact with unsuitable environmental conditions such as temperature, oxygen, pH, salinity, light and other physical or chemical disturbances including various toxic materials.
7. IDENTIFICATION AND TREATMENT OF DISEASES AND PARASITES
Disease control depends on a complex of three factors: diagnosis, preventive measures, and treatment. The best way to control diseases such as in seabass are recommended in the following items.
7.1 Diagnosis and preventive measures
7.1.1 Correct diagnosis (including understanding of life cycle and ecology of the pathogen) is obviously a critical step in any control programme.
7.1.2 Preventive measures constitute the core of disease control programme, and include:
7.2 Treatment
Treatment is usually in the form of chemotherapy, possibly combined with some of the preventive measures listed above. Chemical control should be considered a “last resort” method in disease control.
7.2.1 Chemical prophylaxis
The United States Food and Drug Administration (FDA) rules governing the use of drugs which can serve as useful guidelines are briefly summarized below:
7.2.2 Use of chemicals for pond treatment
To treat the pond accurately, the volume of water in the pond must be known. To determine the volume of a pond, one should multiply the number of surface unit areas of water by average depth of the pond, which gives the value of the volume.
The following chemicals are often used in the treatment of various fish diseases.
7.2.3 How to apply the treatment
For small ponds, dilute the chemical in a bucket of water and distribute evenly over the pond using a dipper. For larger ponds, the chemical should be mixed in a large drum having a faucet connected to about 2 m hose. The chemical should be distributed evenly throughout the pond with a boat and outboard motor, by delivering a small but steady stream of its solution into the wake of the propeller while cris-crossing the pond surface.
7.2.4 Conclusions on disease parasite prevention
7.2.5 General treatments
When treating fish, it is best to know the quality of the water because such things as pH and temperature greatly affect treatment results. Treat a few fish first and see how they react before treating the entire group. Using only the drugs and chemicals which have been cleared by authorized agency for use on food fish. Below are some general treatments for specific groups of pathogens.
Viruses — no treatments are known. Avoidance and prophylactic measures are best for viral disease prevention.
Bacteria — bacteria are treated best by injection of food additives or antibiotics.
Potassium permanganate (KMnO4) used as a wide spectrum treatment at a rate of 2–3 ppm in ponds has given good results against bacterial infections.
External parasites
Formalin is the best treatments for protozoans and gill flukes. Effective rates are 15–25 ppm as a pond treatment and 100–250 ppm for 1 hour as a prolonged treatment. At temperature below 60°F (15.6°C), fish will tolerate a formalin concentration of 250 ppm for 1 hour but a rate of 100 ppm for 1 hour should be used at higher temperatures. Fish should be watched during the treatment period and if they show distress get them back into freshwater. An oxygen depletion may occur a few days after treatment by formalin.
Malachite green at a rate of 0.1 ppm has been used successfully to treat “Ich”. It was recommended that a combination of 25 ppm formalin and 0.1 ppm malachite green to treat “Ich” could have excellent results. Malachite green has been used as a dip treatment at a concentration of 1:15 000 to control fungus on both fish and eggs.
KMnO4 is used for external protozoans at a rate of 2–4 ppm in ponds at a rate of 10 ppm for 20–30 minutes and as a dip treatment at 1:1 000 for 30 minutes.
Acetic acid at a 1:500 concentration has been used for 1–2 minutes to treat external parasites.
Dylox has been shown to be effective as a control for the anchor worm and other crustaceans. It is also effective as a treatment for gill and body flukes but is not effective against protozoans. The concentration used is 0.25 ppm. The treatment should be done with caution at high pH levels.
Copper sulfate has been used to treat certain protozoans at a rate of 1 ppm but the total hardness of the water should be about 25 ppm to be safe.
(d) Internal parasites — most parasites inhabiting the alimentary canal can be controlled by the use of anti-helminthic, Di-N-butyl tin oxide. The tin compound can be mixed in the food at a rate of 1 percent and then fed at 3 percent of BW for 3 days.
The following precautions should be remembered during treatment:
SCS/82/SBTC/LEC.18
by
P. Sungkasem2
1. INTRODUCTION
Seabass (Lates calcarifer) is widely known in parts of the tropical Pacific and Indian Ocean regions. It is kakap in Malaysia and Indonesia, barramundi or barra in Australia, apahap in the Philippines and pla-kapong in Thailand, etc. There are some available data on the economic potentials of seabass in Australia and preliminary studies on the economics of pilot cage culture trials in Phang Nga project in Thailand, but only in grow-out ponds and cages. There has been no available data on the economic aspects of larval production in the world.
Considering the capture production of seabass in Southeast Asia in 1978, it is recorded that there was a total production of 10 192 metric tons valued at US$5 362 000 (SEAFDEC, 1980). In Thailand, a group of seabass related species grouped together as pla-kapong were recorded with total catch production figures from 1973–1978 as follows: 4 871; 5 442; 6 000; 6 316; 9 472; 8 353 tons, respectively (The Marine Fisheries Statistics 1978. Based on the Sample Survey, Department of Fisheries, Ministry of Agriculture and Cooperatives No. 6/1981). The Lates calcarifer portion of these figures is assumed to be about 30 percent of the above quantities. This would mean seabass landings ranging from about 1 400 t (1973) to 2 800 t (1977) during this 6-year period.
Presently, the importance of the economic aspects of the industry should be studied to provide information for commercial scale ventures and for the culture of the species for both the government and the private sector projects. These studies would be very useful at present, specially as this industry is fast expanding within Thailand as well as in Hong Kong, Taiwan (China), Malaysia and other Southeast Asian countries.
Investment and nursery costs in this report are based on the basic direction developed and modified for the mass production method developed by the National Institute of Coastal Aquaculture (NICA), Songkhla, Thailand. The modification of techniques and difference in standard of living of each country will produce differences in costs. The market prices and demand should also be observed and studied before providing the culturist advice on making a decision for investment.
2. ECONOMICS OF HATCHERY PRODUCTION
The capital or investment costs for small-scale, medium and large-scale projects to produce the larvae were based on the assumption of 20 percent per year depreciation on fixed costs. The operational or running costs added are the following:
Besides the assumptions above, consideration of economic aspects of seed production for small-scale projects is being made on standard of 1 ton capacity for larval rearing with a capacity ratio for the components as follows: Chlorella (C): Rotifer (R): Moina (M): Larvae (L) = 3:2:2:1.
This latter assumption may also be represented diagrammatically as shown below (Numbers represent tank number).
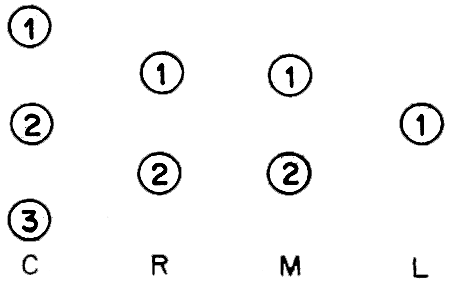
2.1 Fixed costs for 1-ton production capacity of seabass larval rearing receptacles/tanks
| Materials and equipment | No. of units | Cost/unit (Bht)3 | Sum of costs (Bht) |
|---|---|---|---|
| Panlite tanks, 30 1 | 4 | 660 | 2 640 |
| Panlite tanks, 500 l | 2 | 4 600 | 9 200 |
| Panlite tanks, 1 000 1 | 8 | 6 600 | 52 800 |
| Plastic hose, 1½" | 8m | 50 | 400 |
| Plastic hose, 1" | 10m | 30 | 300 |
| Submersible pump. ½" | 1 | 3 000 | 3 000 |
| Sub-total | 68 340 | ||
| Depreciation at 20% per year | 13 668 | ||
| Total | 82 008 | ||
2 Senior Fisheries Biologist (Head, Administrative Division), NICA, Songkhla. Thailand.
3 Baht 22.7 = US$1 at Songkhla in June 1982.
| Materials and equipment | No. of units | Cost/unit (Bht) | Sum of costs (Bht) |
|---|---|---|---|
| Panlite tanks, 30 l | 2 | 660 | 1 320 |
| Panlite tanks, 500 l | 2 | 4 600 | 9 200 |
| Concrete tank. 1 000 l | 8 | 1 000 | 8 000 |
| (ton) | |||
| Plastic hose, 1½" | 8m | 50 | 400 |
| Plastic hose, 1" | 10m | 30 | 300 |
| Submersible pump. 1Z½" | 1 | 3 000 | 3 000 |
| Sub-total | 22 220 | ||
| Depreciation cost at 20% per year | 4 444 | ||
| Total | 26 664 |
2.2 Operations costs per 1 ton larval production capacity
The operations costs per 1 ton larval production capacity per crop consist of the following components:
2.2.1 Cost of Chlorella production per crop
| Items of cost | Amount (Bht) | |
|---|---|---|
| Ammonium sulfate (NH4)2SO4 | 300 g | 12.0 |
| Phosphate | 45 g | 22.5 |
| Urea | 15 g | 0.15 |
| Sum of cost | 34.20 | |
| Plus 20% for contingencies, price fluctuations, etc. | 6.84 | |
| Total | 41.04 | |
2.2.2 Cost of Artemia production
As Artemia is an imported item, the cost is rather expensive; hatching rate of each brand should be considered before choosing which kind to use. For seabass fry production method, use of Artemia for feeding the seabass larvae is being done with corresponding costs as follows (Table 4).
| Age of larvae (day) | Feeding period (days) | Amount of Artemia per day (g) | Total amount of Artemia (g) | Cost (Bht) |
|---|---|---|---|---|
| 8–151 | 8 | 150 | 1 200 | 1 866.00 |
| 16–212 | 6 | 240 | 1 440 | 2 239.20 |
| 22–25 | 4 | 500 | 2 000 | 3 110.00 |
| Sub-total of costs | 7 215.20 | |||
| Plus 20% of sum of cost for contingencies | 1 443.04 | |||
| Total | 8 658.24 | |||
Note: The price of Artemia is 1 555 baht/kg (700 Bht/pound).
1 maintained at 15 000 larvae/ton
2 maintained at 12 000 larvae/ton
The cost of using Artemia was also determined by the consumption by the larvae. It could be based on the rate of 5–10 Artemia per ml per day in the nursery tank of 15–25 days old seabass larvae.
One gram of Artemia (brine shrimp eggs) is about 200 000 eggs, at 70 percent hatching rate, in 1 g we can get 140 000 hatched brine shrimp.
| Age of larvae (days) | Feeding period (days) | No. of Artemia ton/day | No. of Artemia period | Amount (g) | Cost (Bht) |
|---|---|---|---|---|---|
| 8–15 | 8 | 8 × 106 | 64 × 106 | 457 | 710.6 |
| 16–21 | 6 | 16 × 106 | 96 × 106 | 685 | 1 065.2 |
| 22–25 | 4 | 24 × 106 | 96 × 106 | 685 | 1 065.2 |
| Sub-total of costs | 2 841.0 | ||||
| Plus 20% of sum of costs for contingencies | 568.2 | ||||
| Total | 3 409.2 | ||||
| Age of larvae (days) | No. of larvae | Body weight (g) | Total weight (g) | Feeding period (days) | Cost of minced fish (Bht)1 |
|---|---|---|---|---|---|
| 25–30 | 12 000 | 0.1 | 1 200 | 6 | 3.6 |
| 31–35 | 10 000 | 0.15 | 1 500 | 5 | 3.75 |
| Sub-total of cost | 7.35 | ||||
| Plus 20% of sum of cost for contingencies | 1.40 | ||||
| Total cost | 8.75 | ||||
1 Cost of trash fish is Bht 8/kg: trash fish: minced fish = 100:40
2.2.3 Cost of Moina or Daphnia production
Cost of Moina or Daphnia production is determined by the cost of cowdung, ricebran, cowblood for 1 ton larval production capacity per crop; the cost is about Bht 15.
2.2.4 Cost of minced trash fish
Minced fish will be used for 25–35 days during the larval rearing period. The rate of use is based on 10–15 percent of body weight. At this stage, the weight of fry averages 0.15 gram. To produce 10 000 larvae per ton production capacity for 25 days old larvae, 12 000 individuals can be kept. The estimated cost of minced fish is shown in Table 6.
On the determinations above, the cost per 1 ton capacity for larval production per year (4 crops, without labour cost) are as enumerated below:
| a) | Using panlite receptacles | Amount (Bht) |
| Depreciation cost (Table 1) | 13 668 | |
| Cost of Chlorella production = (41.04 × 4) (Table 3) | 164.60 | |
| Cost of Artemia (Table 4) = (8 658.24 × 4) | 34 632.96 | |
| Cost of Moina production = (15 × 4) | 60.00 | |
| Cost of minced fish (Table 6) = (8.75 × 4) | 35.00 | |
| Total cost per year | 48 560.56 | |
| b) | Using concrete tanks | |
| Depreciation cost (Table 2) | 4 444 | |
| Cost of Chlorella production = (41.04 × 4) | 164.50 | |
| Cost of Artemia (Table 3) = (8 583 × 4) | 34 632.96 | |
| Cost of Moina production = (15 × 4) | 60.00 | |
| Cost of minced fish = (8.75 × 4) | 35.00 | |
| Total cost per year | 39 336.46 |
The government price of 35 days old fry (1.5–2.0 cm) is 1 Bht per piece one ton of larval production capacity can produce about 40 000 fry a year or equivalent to Bht 40 000.
However, the price with the private sector is Bht 2.50 per fry or an equivalent value of Bht 100 000.
2.3 Cost of mass production of seabass larvae
2.3.1 Fixed or construction costs
The fixed or construction costs for mass production of seabass larvae in this report was determined on the costs incurred at NICA for seabass production.
The total cost of construction of NICA is 725 million yen1 (about Bht 72 500 000). The cost of the hatchery construction is estimated to be about 40 percent of this cost or equivalent to about Bht 29 000 000. The hatchery components comprise of the following:
| - Raw seawater reservoir about 500 tons | |
| - Filtered seawater reservoir about 500 tons | |
| - Pump station | |
| - Electrical room | |
| - Generator room | |
| - Workshop | |
| - Blower room | |
| - Tank room | |
| - Gas storage room | |
| - Oil storage room | |
| - Rectangular tanks (with translucent corrugated sheet roofing) 4m (w) × 6m (1) × 1.5m (d). 16 tanks | |
| - Rectangular tanks (without roofing) 4m (w) × 6m (1)× 1.5m (d). 16 tanks | |
| - Circular tanks 10m diameter 2.0m deep | 4 tanks |
| - Circular tanks 5m diameter 5.0m deep | 2 tanks |
| - 3 HP electrical pump for freshwater | 2 sets |
| - 3 HP electrical pump (for city water) | 2 sets |
| - 10 HP electrical pump (for filtered intake) | 2 sets |
| - 15 HP electrical pump (for elevated seawater) | 2 sets |
| - 15 HP electrical pump (for elevated filtered seawater) | 2 sets |
| - 1 generator 150 K V A | 1 set |
| - Air blower, 10 HP | 2 sets |
2.3.2 Running or operations costs
The running or operations costs for seabass larvae production in NICA is based on the expenses incurred in 1981. The details are shown in Table 7.
| Item | Expenses (Bht) | Remarks |
|---|---|---|
| Fertilizers & chemicals | 29 713 | |
| Materials | 244 970 | |
| Artemia | 8 510 | |
| Fish meat | 253 000 | |
| Public utilities | 1 085 506 | About 6 months for seabass work |
| Salary (permanent wages) | 16 200 | |
| Salary (temporary wages) | 50 613 | PVC pipe water intake repairing system, electrical, etc. |
| Maintenance & gasoline | ||
| Total | 1 688 512 |
2.3.3 Production and value of production per year
The production of seabass larvae comprises 3 or 4 sizes. It depends on market demand and the skill of private hatcheries and culturist who operate for the market in order to get the most out of their own investment. The NICA's production of seabass larvae and fry in 1981 was 7 559 300 individuals which was separated as shown in Table 8.
| Age (days) | Size (mm) | No. of larvae or juveniles | Government price per piece (Bht) | Total value (Bht) |
|---|---|---|---|---|
| 8–15 | 3–5 | 7 310 000 | 0.45 | 3 289 500 |
| 15–30 | 6–12 | 42 000 | 1.50 | 63 000 |
| 30–45 | 12–20 | 214 000 | 2.50 | 535 000 |
| 45–60 | 20 | 4 100 | 8.50 | 34 850 |
| Total | 7 559 300 | -- | 1 339 700 | |
2.3.4 Profitability of hatchery enterprise
Considering the investment of government on mass production of seabass larvae and value of the production in 1981 in NICA, it appears that a loss of around 3 million baht a year for the operation in 1981 (including 10 percent depreciation cost per year) was incurred (1 688 512 + 2 900 000) - (1 339 700). However, the hatchery is not only used for seabass production but also for the production of shrimp (penaeid) postlarvae and Macrobrachium prawn juveniles.
On the other hand, the investment of the government was directed mainly to encourage and stimulate the seabass culture industry and larval production industry in Thailand. The cheap price of seabass larvae and fry used by the government was set to induce the private sector to culture and progress rapidly in the hatchery industry. By way of comparison, the price of seabass larvae and fry of the government and the private sector are different (Table 9).
| Age (days) | Size (mm) | Government price (Bht) | Private sector price (Bht) |
|---|---|---|---|
| 8–15 | 3–5 | 0.15 | 0.45 |
| 15–30 | 6–12 | 0.50 | 1.50 |
| 30–45 | 12–20 | 1.00 | 2.50 |
| 45–60 | 20- | 2.00 | 7.00–10.00 |
Based on the selling price of seabass larvae and fry between the government and the private sector, on the same kind of production, the private sector can make about triple income than the government. On the investment aspect, the private sector can further reduce by about half of the government investment since the private operators do not need the large circular tanks for spawners and may not require elaborate water intake system.
| Items | Cost (Bht) | Remarks |
|---|---|---|
| 1. Variable costs (Total cost) | 16 422.00 | |
| a. Spawner | 2 500.00 | Cost of stripped out egg and milt from wild spawner |
| b. Food | 4 250.00 | |
| c. Labour | 1 900.00 | |
| d. Public utilities | 950.00 | |
| e. Gasoline | 3 200.00 | |
| f. Others (sundries) | 1 862.50 | |
| g. Opportunity cost | 1 759.50 | 12% interest |
| 2. Fixed costs (Total cost) | 2 036.16 | |
| a. Depreciation of fixed cost | 1 818.00 | |
| b. Opportunity costs | 218.16 | 12%interest |
| 3. Total costs (variable & fixed costs) | 18 458.16 | |
| 4. Number of larvae produced | 250 000.00 | |
| 5. Cost of production per larva | 0.07 | 8–15 day old |
| 6. Sale price per larva | 0.45 | |
| 7. Value of production | 112 500.00 |
The above table clearly shows that seabass larval production can be very profitable. The income derived far exceeds the annual cost of production, and with modest set-up, the investment costs can be minimal and recoverable within relatively short period.
3. ECONOMICS OF GROW-OUT CULTURE
On the economics of the grow-out culture of seabass, the operations expenses will be determined for cage and pond culture. As the NICA works very closely during extension work with private cage culture farmers in Songkhla and adjacent areas, the data in hand emphasizes on costs of cage culture investment. On the other hand, for culture in ponds, since the fish farmers from the middle part of Thailand such as Samutsakhorn, Samut Songkram, Samutprakarn, etc., come to buy the fry from NICA to culture in ponds in 1981, the NICA was able to gather some data on ponds also.
3.1 Cage culture
The determination of the economics of cage culture covers items shown in Table 11.
| Items | No. of units | Cost (Bht) | Remarks |
|---|---|---|---|
| 1. Fixed costs (Total costs) | 2 627.00 | 25% depreciation | |
| a. Nylon mosquito net 1 × 2 × 0.8m | 7 | 700.00 | |
| b. Nylon netcage, 1.5cm mesh, size (2×4×2m) | 1 | 900.00 | |
| c. Nylon netcage, 3 cm mesh size | 1 | 4 500.00 | |
| d. Poles and other materials | 1 000.00 | ||
| e. Opportunity cost | 852.00 | ||
| 2. Variable costs …. (Total costs) | 25 088.00 | ||
| a. Cost of fry | 1 000 | 1 000.00 | |
| b. Feeding cost | 12 000.00 | ||
| c. Labour cost | 8 400.00 | ||
| d. Maintenance cost | 1 000.00 | ||
| e. Opportunity cost | 2 688.00 | ||
| TOTAL COST (FIXED AND VARIABLE COSTS) | 27 715.00 | ||
| 3. Production | |||
| a. Total production (kg) | 581.80 | Ave. 1 kg/ fish size | |
| b. cost of production per kg | 47.64 | ||
| c. Sell out price per kg | 65.00 | ||
| TOTAL VALUE OF CROP | 37 817.00 |
| Items | Cost (Bht) | Assessed cost1 | Total |
|---|---|---|---|
| 1. Variable cost | 29 325.00 | 12 981.50 | 42 307 |
| a. Seabass fry | 5 404.00 | -- | 5 404 |
| b. Feeding cost | 19 683.00 | -- | 19 683 |
| c. Labour | 312.00 | 9 462.50 | 9 775 |
| d. Gasoline | 2 047.00 | -- | 2 047 |
| e. Maintenance | 98.00 | -- | 98 |
| f. Interest | 544.00 | -- | 544 |
| g. Other materials | 1 044.00 | -- | 1 044 |
| h. Opportunity cost | -- | 3 519.00 | 3 519 |
| 2. Fixed cost | -- | 4 723.00 | 4 723 |
| a. Depreciation | - | 4 217.00 | 4 217 |
| b. Opportunity cost | - | 506.00 | 506 |
| TOTAL COSTS (VARIABLE AND FIXED COSTS, PER YEAR) | 29 325.50 | 17 704.50 | 47 030 |
| 3. Production | |||
| a. Average production per farm (kg) | 1 421 | ||
| b. Average number of cage per farm | 3 | ||
| c. Average production in kg per cage | 474 | ||
| d. Average cost of production per kg | 33.10 | ||
| TOTAL SELL COST PRICE 60 BHT PER KG FOR AVERAGE FARM | 85 260.00 | ||
1 Projected costs, not actually expended, e.g. family labour, etc.
The above estimates of costs of annual operations and production show that small-scale grow-out culture in cages can be profitable. In the example above, about 180 percent profit can be realized.
| Items | Actual costs (Bht) | Assessed costs | Total |
|---|---|---|---|
| 1. Variable cost | 327 515 | 39 302 | 366 817 |
| a. Seabass fry | 40 000 | - | 40 000 |
| b. Feeding cost | 196 000 | - | 196 000 |
| c. Labour | 53 475 | - | 53 475 |
| d. Gasoline | 6 720 | - | 6 720 |
| e. Electric utilized | 720 | - | 720 |
| f. Interest | 9 600 | - | 9 600 |
| g. Other materials | 21 000 | - | 21 000 |
| h. Opportunity cost | - | 39 302 | 39 302 |
| 2. Fixed cost | - | 25 080 | 25 080 |
| a. Depreciation cost | - | 22 393 | 22 393 |
| b. Opportunity cost | - | 2 687 | 2 687 |
| TOTAL OF VARIABLE AND FIXED COSTS/YEAR | 327 515 | 64 382 | 391 897 |
| 3. Production | |||
| a. Total production (kg) | 12 000 | ||
| b. Number of cages | 54 | ||
| c. Average production (kg per cage) | 222 | ||
| d. Cost of producing of marketable seabass per kg | 33 | ||
| TOTAL SELL OUT PRICE OF CROP AT 60 BHT PER KG | 720 000 |
The above data on big-scale grow-out project of seabass in cages also showed profitable business. The estimated annual profit in this case also amounted to about 180 percent.
3.2 Pond culture
Pond culture of seabass is widely practiced along the coastal provinces particularly in the middle and eastern part of Thailand. The ponds are usually rectangular in shape with depth of at least 1.5 m. The size depends on the available land and on the loan and resources of the farmer. Sluice gates are set on both sides of the pond and provided with several mesh sizes of screens. Seabass pond culture may be separated into two sizes.
3.2.1 Small culture pond
Small ponds are used for culturing the seabass fry for 40–60 days (2–3 cm) up to 10–15 cm. The culture area is about 0.5–1 rai (800–1 600 m2), depth about 1 m, and about 1 600 fish is the stocking rate used. Feeding rate in the pond is practiced at 300 g per 100 fish per day in a period of 4–6 months.
3.2.2 Big culture pond
The big culture ponds are used for the seabass juveniles with sizes about 15–25 cm for raising them to marketable size. The sizes of ponds are about 2 rai (3 200 m2) with depth of about 1.5–2.0 m. Stocking rate used is about 800–1 000 individuals per rai (5 000–6 250 per ha). The culture period is about 8–10 months, with feeding rate of 1.5 kg per 100 fish per day.
4. PROFITABILITY
4.1 Profitability of seabass larval production
The cost of seabass larval production if considered on the government side as shown in Tables 7 and 8 showed a loss. In the case of the government however, the profit is not only from the money income but also the chance to extend to the private sector quantities of seabass larvae which they themselves can rear to sizes so that they can sell them and derive profit. This type of promotion by the government (DOF Thailand) can stimulate the private sector to run this activity (some phases of it) to produce crops which has market value and from which they can derive profit. The cost for a private hatchery would be much cheaper in investment than that of the government, not only for not incurring part of the nursery cost but also the lessened cost if the large-sized spawning tanks can be avoided. The cost of production of medium-scale private hatchery (Table 10) shows that the operator will be able to derive good profit. Its annual production of 8–15 day old larvae of about 250 000 can cost only Bht 18 458 from which the owner can get about Bht 112 500. This is based on sell out price Bht 0.45 per larvae.
4.2 Profitability of grow-out production
The grow-out production cost in cage culture listed in Tables 11, 12 and 13 showed that average cost to produce 1 kg of fish were Bht 47.7, 33.10 and 33 in each standard unit of cage culture farm, i.e., one unit, 10 unit and over 10 unit, respectively. The prevailing sale price varied from Bht 60–75 on the average during the years from 1980 to 1982. Based on production costs and sell-out prices, the fish farmer will profit by about Bht 25–30 per kg depending on seasonal variation in price.
4.3 Prospects of the market
Seabass is known as an expensive fish comparing with the price of other species. The demand is rather limited to the middle and upper class of society. In Thailand, recently, some restaurants can serve seabass only at certain seasons but after the extension programme the local consumers are able to have the fish all year round. Presently, since the supply for local market is becoming adequate the prospect for markets abroad are being looked into by local producers. The demand of specific processed types and various sizes of marketable fish will also influence the expansion of the industry and its market abroad. In this respect, the problem of the producers and the technicians are:
How to maintain and/or increase the production so that the foreign market can be adequately supplied
How to process the over production after the local supply is filled so that it could be exported
4.4 Social benefits
According to the National Economic and Social Development Board plan in the field of fisheries development, the Royal Government of Thailand strongly emphasizes coastal aquaculture development. Since the operation of marine capture fisheries has become more crowded and difficult for the Thai fishermen, the government tried to convert them from capture to culture fisheries. The seabass culture development programme is certainly in support of this objective. At present, most of the local fishermen along the coast easily acquire the techniques of cage culture of seabass. The number of cage farmers from agricultural farmers and fishermen are increasing rapidly.
5. REFERENCES
MANEEWONGSA, S., et al., 1981 On the propagation of seabass. Contrib. No. 1 (Thai version) National Institute of Coastal Aquaculture, Songkhla, Thailand, 1981.
SUNGKASEM, P. and C. BOONTAE, The production and (In press) economic aspects of netcage culture of seabass (Lates calcarifer Bloch). Annual Report, NICA, 1978.
ANONYMOUS, 1981 Report on cost and income of seabass culture: 1981 Agriculture and Economics Division Agricultural Economics Bureau, Ministry of Agriculture and Cooperatives (Thai version).
SCS/82/SBTC/LEC. 19
by
P. Brohmanonda and P. Sungkasem
1. INTRODUCTION
Lake Songkhla is a large lagoonal water of Thailand. It is located in the southern part of the country. This water body is surrounded by three provinces: Songkhla, Pataloong and Nakornsrithamraj; it is at a distance of about 1 200 kilometers from Bangkok.
Lake Songkhla is one of the richest grounds for aquatic animal resources. There are so many economic species of fish, prawns, shrimps, crabs and aquatic plants. The capture fishery production has been supplying enough fish protein food to the people of the nearby provinces for a long time. However, at present because of the changing ecosystem of this natural body of water and overfishing, the quantities of these resources have shown a tendency of declining. Moreover, it should also be mentioned that the freshwater of the northern area of Lake Songkhla is a very important resource for agricultural development as it provides the water supply for the Ranort Irrigation Project.
2. GENERAL DESCRIPTION OF THE LAKE
Lake Songkhla has an area of about 986.8 square kilometers or 98 680 ha (616 750 rai). It is one of the largest of inland waters in Thailand. The lake area is a typical lowland watered area receiving water flow from hundreds of streams. The depth of this water body is relatively shallow, usually about 1–2 meters on the average. The type of bottom is muddy and very flat. It is quite a unique area because the environment of the several parts of the lake vary from freshwater to brackishwater and saltwater. The lake itself is of great importance to 15 000 people of 4 500 families (1972) that reside on its shore. At present, this population has increased to about 7 500 families with 25 000 people.
The people who are residing along the lakeshore are paddy farmers and also fishermen at the same time. Most of them depend on the lake for the animal protein mainly on the subsistence category. The total production of economic aquatic animals from this lake is estimated at 6 000 tons annually.
The lake system consists of three different parts of basins which are as follows (Fig. 1).
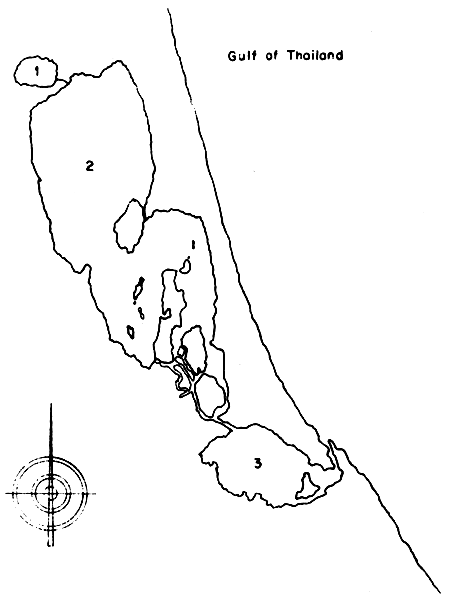
Fig. 1 Map of Songkhla Lake
1. Tale Noi (Inner Lake)
2. Tale Luang (Middle Lake)
3. Tale Sab (Outer Lake)
Inner lake or “Tale Noi” situated at the northern part of Lake Songkhla. It has an area of about 28 square kilometers (17 500 rai). The length of ths shoreline is around 20 kilometers. The deepest portion of this part of the lake is 2.5 meters and it is true freshwater body.
Middle lake or “Take Luang” is situated further south of Tale Noi. It is the largest area of this lake with about 782.8 square kilometers (489 250 rai). The length of its shoreline is about 200 kilometers and and average depth is 2.5 meters. The upper part of this middle portion is connected with Tale Noi by a short canal. The condition of the water at the upper portion of this lake is still freshwater but the lower part of Tale Luang is brackishwater.
Outer lake or “Tale Sab” is located at the southern end of this water body. It has an area about 176 square kilometers (110 000 rai). The water depth averages 4 meters. The deepest area is at the narrow canal (10–12 meters) that connects to the open sea at the lower part of the Gulf of Thailand at Songkhla town.
The shallow water area along the shoreline of Tale Noi and the northern part of Tale Luang are covered by many types of aquatic plants, but further beyond the banks are paddy fields. The shore of the southern portion of Tale Luang and Tale Sab is covered by small mangrove forest; and behind this there are large paddy fields. Koh Yai, Koh Si, Koh Ha, and Koh Nangkam are the big islands located in Tale Luang; Koh Yor is the single island in Tale Sab.
3. PHYSICO-CHEMICAL CHARACTERISTICS OF THE LAKE
3.1 Water quantity
The tidal fluctuation of water in Lake Songkhla is comparatively low similar to the nearby provinces along the seacoast on the Gulf of Thailand. The average tidal range is about 50 centimeters.
The water discharge and flow rate vary greatly seasonally and from year to year. These are very much due to the rainfall in the watershed and lake areas. According to the records of the Harbours Department, the total annual water discharged in 1973 was 12 045 000 cubic meters and the current range is from 0 to 2 545 cubic meters per second. Together with the speed and volume of water, silt of as high as 1 334 925 tons is carried per annum into the sea. This process may help in delaying the filling up of the lake. However, the reverse flow from the open sea into the lake is also high during certain months especially in March, June, September and October. The figures showed 2 164; 154; 1 328; and 958 million cubic meters per month, respectively.
3.2 Water and soil quality
3.2.1 Water temperature
The temperature of the water in Lake Songkhla is not much different than the other shallow water bodies in the country. It has been measured throughout the year at ten designated stations in different parts of the lake. Brohmanonda and Vichiensan (1967) found that the average range of water temperature was between 28.91° to 30.38°C. No considerable seasonal fluctuation of water temperature has been found, but biggest range observed was between 26.51° to 31.72°C as shown by the averages in the months of January and April.
3.2.2 Salinity
The salinity of Lake Songkhla normally fluctuates all the year round and this is one of the most important factors affecting aquatic life. The annual average ranges of salinity in several parts was between 0.13 to 27.57 ppt. In the small lake or Tale Noi and at the northernmost part of Tale Luang, the average range of salinity annually was 0.13 to 0.27 ppt. These parts of Lake Songkhla are considered as the areas of fresh-water.
The salinity of the lower or most of the water area of the middle lake or Tale Luang averages between 1.0 to 4.41 ppt. This is the brackishwater area.
In the water area of the outer lake or Tale Sab, the annual average of salinity is 6.07 to 15.69 ppt while the mouth of the outlet that opens to the sea is 27.58 ppt on the average. These are the saltwater areas of this Lake. However, during period of northeast monsoon rain in the months of November-December to January-February, the salinity of the water becomes rather low throughout the lake area.
3.2.3 Bottom soil
According to the report of Brohmanonda and Vichiensan (1967), the bottom soil of Lake Songkhla varies from part to part. In the northern basin, the soil is clay and clay loam and becomes silty clay, with silty clay loam southward.
The nutrient-content of the bottom soil is remarkably high. The nitrogen, phosphorus and potassium are as high as 0.0999, 4.775 and 236.3 ppm, respectively. In the northern small basin, very high amount of organic matter, 8.15 percent is observed while in the other areas the amount of organic matter ranges from 0.64 to 2.01 percent. The pH of the bottom soil is more on the acidic side, ranging from 3.04 to 5.87 on the average.
3.3 Biological characteristics
3.3.1 Zooplankton
Biological study on three stages of marine shrimps in Lake Songkhla was carried out by Pongsuwana and Brohmanonda (1974). The results showed that salinity was the main factor affecting the distribution of shrimps in their larval and juvenile stages. The larval stages were mostly found in June, with the average of 4 984/1 000 cubic meters of water in 1965 and only 1 049/1 000 cubic meters in 1967 in the estuarine areas outside Lake Songkhla where salinity ranged from 30 to 32 ppt. The largest amount of shrimps in juvenile stages were found in September, about 2 045/rai (12 780/ha) in 1965 and 1 722/rai (10 760/ha) in 1967; and the smallest amount is December and January near the mouth of the lake where the salinity was 21–28 ppt. In addition, seven species of marine shrimps in mature stage were studied for economic reasons. It was found that there was 47.34 percent of Metapenaeus monoceros as the most numerous while 0.17 percent were of Penaeus monodon, the least. From the production point of view, this may serve as basic information for the purpose of shrimp farming extension in the outer part of Lake Songkhla.
The investigation on marine animals has been conducted many times in these waters. It was proved that the outer part of Lake Songkhla was the natural nursery ground of many economic species of fish and shrimps.
Regarding capture fisheries some kinds of fishing gears in the said area of the outer Lake Songkhla are not allowed such as push nets with engines; small mesh size seines and other specified fishing gears.
3.3.2 Benthic fauna
The study on benthic fauna in Lake Songkhla was conducted by Wongsomnuk and Sukawong in 1969. There are representatives of Nematoda, Oligochaeta, Polychaeta, Arthropoda, Mollusca, Chordata under this category. Relative quantities of each were expressed as individuals per square meter of lake bottom and the biomass of each group were estimated and expressed in milligrams dry weight.
The results revealed that, Polychaeta and Arthropoda were dominant in five sampling stations in the lake. The range of dry weight were 7.00–69.85 mg/m2 and 34.87–189.61 mg/m2, respectively. The total dry weight of all benthic forms showing highest abundance occur in the northernmost part of the lake with 377.17 mg/m2. The area of the outer lake near the estuary was 315.85 mg/m2. The other areas ranged from 212.89 to 273.10 mg/m2.
4. FISHERY RESOURCES
Lake Songkhla is a large water body and has varied water conditions. Therefore, there are many species of fish distributed either in freshwater, brackishwater and saltwater seasonally. According to the report of the Songkhla Fisheries Station, in 1970 there were 240 species of fish, 5 species of crabs, 19 species of shrimps and prawns, 7 species of shells, and 49 species of aquatic plants. As mentioned earlier at least 25 000 persons from 7 500 families depend upon the fishery resources of the lake. It is estimated that the annual production of fishery resources is 6 000 tons valued at about 100 million baht.
5. POTENTIALS FOR AQUACULTURE
Lake Songkhla has been surveyed for its general condition many times both for the study of its ecological and geographical aspects. It was revealed that this water body has very high potentials for aquaculture with brief details as follows:
The area in the outer part of Lake Songkhla or Tale Sab with shallow waters of 600 rai (96 ha) near the lake opening are suitable for oyster and sea mussel culture, and for cage culture of seabass, grouper and red snapper. This includes 30 000 rai (4 800 ha) of the shallow water around Koh Yor island. In addition, the shallow water area along the coast of Kao Kieo and Pak-raw villages can be developed for marine shrimp culture in net enclosures, an area of about 14 000 rai (2 240 ha).
The water area of the middle part of Songkhla Lake, Tale Luang, is also suitable for aquaculture. The deeper area along the canal connecting the middle with the outer lake is suitable for seabass culture in netcages, with a total area of about 30 000 rai (4 800 ha). The areas situated along the shoreline both on the land and shallow waters of the inner lake can be developed for freshwater finfish and giant freshwater prawn culture in pens and ponds with an estimated total area of about 60 000 rai or (9 600 ha).
Utilization of the above areas for aquaculture has been started in some places. The full potential of the lake for fisheries production through aquaculture however, still awaits further development.
6. LIST OF REFERENCES
BROHMANONDA, P. and S. VICHIENSAN. 1967 Seasonal fluctuations of water temperature and salinity in Songkhla Lake. Annual Report, Songkhla Fisheries Station, Department of Fisheries 1966–1967: 69–95.
PONGSUWANA, U. and P. BROHMANONDA. 1974 Study on some biological aspects of commercial penaeid shrimps in Songkhla Lake. Journal of the National Research Council of Thailand, Vol. 6 Nos. 1/2/3/4. March, June, September, December, 1974: 128.
WONGSOMNUK, S. and S. SUKAWONG. 1969 Study on the abundance and distribution of benthic fauna in Songkhla Lake. Annual Report, Songkhla Fisheries Station, 69–100.
SCS/82/SBTC/LEC. 22
by
V. Soesanto
INTRODUCTION
During the last decade, there has been an increasing interest and increased consideration given to the development of aquaculture in the SCSP region.
Surveys and studies have been undertaken during this period by the countries concerned. As a result, positive and encouraging developments have been achieved. Intensive culture for certain species (milkfish, shrimp) has shown rapid growth.
Seabass (Lates calcarifer) is considered as a species suitable for culture with high potential for development. Studies and experimentation on the possibilities of its culture have been conducted. Culture practices have gradually developed and become more and more established. (Example: Work in Thailand has started on the possibility of induced spawning in 1971 and the first success at mass production of fry was achieved in 1973).
With the availability of suitable environmental conditions, seabass culture development, if properly supported, could considerably contribute its share towards the income-generating source for the fish farmers/fishermen.
Before development programmes could be implemented, consideration should be given to meet certain essential prerequisites (e.g. institutional build-up, legislation, etc.) Attention should be directed towards the application of methodology to the consumers in the field (fish farmers), with the consideration that as the levels of technology advance and progress, so must the farmer's capability to make use of them.
NOTES
We are not trying to formulate a programme for each respective country or for the region. This is not within the scope of this forum and outside our competence.
The following are outlines to give a general idea of a programme for development which may or may not be applicable to each respective country, since each country has developed its own policy on aquaculture, the seabass culture in particular. These notes are to provide a basis for discussion on which further activities could be generated.
The steps or stages in generating a development programme such as that for seabass may follow the lines as shown in Figure 1. These steps are further elaborated briefly in the sections that follow.
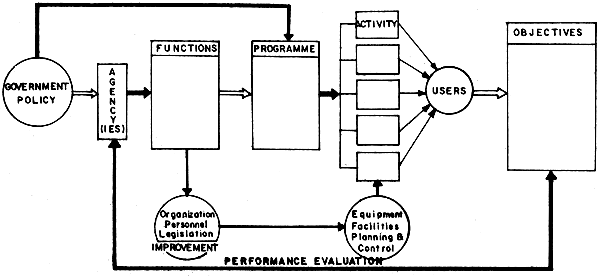
Fig. 1 Steps in generating a development programme
POLICY
Areas which may be common in the region:
FUNCTIONS OF AGENCY (IES)
PROGRAMMES AND ACTIVITIES TO ENCOURAGE SEABASS CULTURE DEVELOPMENT
| Programmes | Activities |
|---|---|
| 1. Resources development (research) | - Surveys on suitable location for seabass culture |
| 2. Appropriate technology development | - Study in natural abundance of fry |
| - Improvement of larval/ juvenile rearing | |
| 3. Entrepreneurship for fish culture (small-scale) | - Fry handling and transportation |
| 4. Training/extension | - Piloting of culture |
| - Socio-economic study | |
| - Credit scheme | |
| ETC. | - On-the-job training |
| (Marketing) | - Extension work, etc. |
| (It may also include extension of earlier activities where concrete results have been achieved). |
REGIONAL
| 1. | Should be based on countries need. | To foster cooperation carrying out programmes common to the countries |
| 2. | Activities on subjects of mutual interest. | |
| 3. | Aim to building up man- agerial technical capabi lities required in the development process. |
USERS/CONSUMERS
Categories
Two distinct sectors:
OBJECTIVES
| Immediate | Development |
|---|---|
| - Developing favourable conditions for industry to prosper and expand (seabass culture) | - To meet increasing demand of fishery products |
| - Export to earn foreign exchange | |
| - Increase of income for fish farmers/raise living standard | |
| - Generating employment |
SCS/82/SBTC/LEC. 23
by
M. Masuo2
1. INTRODUCTION
The total production by fisheries in Japan was about 10 million tons. These fisheries are classified into the following five types of fisheries.
| Types of fisheries | Percentage of production | |
|---|---|---|
| 1. Distant water fisheries | 37 | Capture fisheries = 92% |
| 2. Offshore water fisheries | 35 | |
| 3. Coastal water fisheries | 20 | |
| 4. Culture in shallow sea | 6 | Culture fisheries = 8% |
| 5. Inland water fisheries and culture | 2 | |
Production by culture in shallow sea mainly consists of the following animals and plants:
| Fish: | Yellowtail Red seabream | (Seriola quinqueradiata) (Chrysophrys major) |
| Molluscs: | Oyster Scallop | (Crassostrea gigas) (Patinopecten yessoensis) |
| Seaweed: | Nori Wakame | (Porphyra spp) (Undari spp) |
Quite a few species of aquatic animals and plants are commercially cultivated. The production of yellowtail, oyster and seaweed by culture has been stagnant, inspite of the development of its production techniques because of the narrowing down of the culture grounds due to pollution and reclamation of the foreshore.
But recently, the cultivation of aquatic animals like flounder (Paralichthys olivaceus), puffer (Fugu rubripes), abalone (Haliotis spp) and Kuruma shrimp (Penaeus japonicus) has been increasing and sale of these species will be larger than before.
2. MASS CULTURE OF SEED FOR FISH FARMING
In fish farming of specific species, the basic thing is to rear the seed animals of the species and there are two methods for this:
Natural seed collecting method
For yellowtail, oyster and scallop, their juveniles are collected in the open sea; also seed of seaweed are collected.
Seed culture in tanks
For flounder, abalone and Kuruma shrimp, these seeds cannot be collected in the open sea therefore, their juveniles are artificially reared in a tank. In case (a) the larvae are spawned and grown in the open sea, and collected when they attach themselves to the collectors. In case (b) those are reared in tanks almost fully artificially and then eggs are collected, sometimes helped by the use of ultraviolet rays (abalone) or injection of pituitary hormone; also utilized is temperature shock.
The following table summarizes the main products of culture in shallow seas (Unit: ton)
| Year | Yellowtail | Oyster | Porphyra | Undaria | ||
|---|---|---|---|---|---|---|
| 1966 | 16 875 | 221 139 | 128 440 | 37 809 | ||
| 1967 | 21 169 | 232 200 | 157 550 | 58 080 | ||
| 1968 | 31 777 | 267 388 | 144 969 | 76 698 | ||
| 1969 | 32 722 | 245 458 | 134 320 | 59 821 | ||
| 1970 | 43 354 | 190 799 | 231 464 | 76 358 | ||
| 1971 | 61 855 | 193 846 | 244 946 | 95 155 | ||
| 1972 | 77 059 | 217 373 | 217 906 | 105 795 | ||
| 1973 | 80 439 | 229 899 | 311 410 | 113 211 | ||
| 1974 | 92 946 | 210 583 | 339 314 | 153 762 | ||
| 1975 | 92 407 | 201 173 | 278 127 | 101 937 | ||
| 1976 | 101 786 | 226 278 | 291 050 | 126 701 | ||
| 1977 | 115 098 | 212 779 | 279 031 | 125 798 | ||
| 1978 | 121 956 | 232 068 | 350 471 | 102 665 | ||
| 1979 | 155 053 | - | - | - | ||
| 1980 | 111 730 | 225 253 | 278 180 | 88 544 | Plus: | |
| - scallop | 67 741 | |||||
| - red seabream | 10 970 |
Note: Oysters is in total weight with shell, Porphyra and Undaria is in wet weight.
3. MARINE RANCHING PROGRAMME
Recently the Japanese Government, Fisheries Agency has formulated a new coastal aquaculture development plan which is called “Marine Ranching Programme”. This programme combines the maintenance of seed animals, management of production and control of the environment.
For the establishment of this programme modern equipment such as fish-finder, echo sounder and other remote sensor system will be utilized. The subject animals of this programme were selected, such as tuna, salmon, yellowtail, flounder and molluscs (Haliotis spp, Anadara spp, and Pecten spp).
In the past, Japan is the largest fishing nation in the world, but now we lost many fishing grounds because of the declared 200 miles economic zone by the different countries. Fortunately, our country is an island country surrounded by the sea. We have 3 900 000 km2 area of economic zone. We can therefore, develop more of the coastal resources.