A consultancy report
by
Lamont-Doherty Geological Observatorym
of Columbia University
Palisades, New York, USA
The Government of Malaysia, assisted by the United Nations Development Programme and the Food and Agriculture Organization of the United Nations, are engaged in the Coastal Aquaculture Demonstration and Training Project (FI:DP/MAL/77/008), with the main objective to assist the Government in introducing coastal fish farming.
As part of the operation of the project, FAO assigned the Lamont-Doherty Geological Observatory of Columbia University (LDGO) of New York to provide consultant services in geochemistry and microbiology to the project at Gelang Patah, Johore Bahru, Malaysia. Three members of the LDGO staff, Dr James Simpson (geochemist), Dr Bruce Deck (geochemist) and Dr Hugh Ducklow (microbiologist) worked at the Gelang Patah facility during the last week of November and the first ten days of December 1981. Two other aquaculture facilities in the region were also visited briefly, a new private project in southeastern Johore State and a government research station in Thailand near Bangkok, to obtain soil samples for laboratory comparison with samples from Gelang Patah. Summarized in this report are the findings of the field chemical and microbiological studies, as well as subsequent laboratory work at LDGO.
The primary purpose of the consultants' work was to elucidate the geochemical and microbiological factors which contribute to the low yields of shrimp and fish at the Gelang Patah facility which has been in operation for three to four years.
The Coastal Aquaculture Demonstration and Training Project at Gelang Patah is a major investment of resources and personnel to provide guidance for brackishwater aquaculture in Malaysia. As such, one of its crucial roles is to define the most important problems in brackishwater aquaculture development and establish workable solutions to these problems. The staff and facilities at Gelang Patah seem very well suited to address these tasks. It is clear that one of the major problems confronted at the project is acid production from the dike soils and other associated chemical and biological phenomena. This problem is quite a general one in Southeast Asia, and can be expected to occur in many locations which would otherwise be very desirable for brackishwater aquaculture in Malaysia. Thus it is probably good in the long run that the research facility at Gelang Patah has to face this problem early in the management of aquaculture. It is not known what aspects of acid soils are most detrimental to shrimp and fish aquaculture but there are a number of likely candidates, including (a) low pH (perhaps due to interference in Ca transport and storage); (b) high dissolved iron; (c) high dissolved aluminum; (d) iron particles clogging gill surfaces and (e) low primary productivity due to stripping of dissolved phosphate by cycling of iron through oxidation-reduction reactions. It is important to collect good, systematic data on chemical properties in the water, soil and sediments, in conjunction with documentation of shrimp and fish yields, to help define what factors associated with acid soil are most important in limiting aquaculture yields.
With this in mind, some of the steps which would benefit collection of such data are:
Provide dependable 24-hour line electricity to the facility as soon as feasible. This would permit better operation of analytical equipment and improved storage of perishable chemicals and samples, as well as other benefits.
Establish air-conditioned space for crucial analytical equipment. Many electronic instruments are quite difficult to maintain and operate in high humidity environments. Even if only a small room with good insulation were available, the operation of electronic equipment would probably be significantly improved.
Acquire a better spectrophotometer for colorimetric measurements of parameters such as dissolved phosphate and iron.
Obtain several spare electrodes for the pH meter and microburets and more magnetic stirring equipment to simplify alkalinity titrations.
Improve the quality of distilled water, either by upgrading the feed water or employing a pre-deionization step before the still.
Obtain a small programmable calculator for computation of alkalinity data.
Collect pH and alkalinity data from a representative set of ponds on a regular basis (at least twice a week), with samples collected near the gate after the discharge flow from each pond to the canal has been well established, to obtain water which is as representative as possible of the whole pond volume. Data of this type should be obtained over at least the next several years, especially in conjunction with monitoring the effects of any major attempts at leaching of pyrite acidity from the dike soils.
The above recommendations relate to the ability to collect data in support of the main activity of enhancing shrimp and fish productivity. How to effect significant improvement in that productivity appears to be quite a difficult problem. Without some quite major changes in direction, one would expect such improvement to be unlikely. Any of the suggestions which follow would require some major policy decision changes and obviously should not be approached without careful consideration.
The total amount of pyrite in the pond and dike network is so large that it will probably take many years for the acidity problems to dissipate by the leaching processes under present conditions. The most practical way to accelerate leaching of pyrite from the dikes appears to be flooding the entire system, or major portions of it, with brackish water, and draining away the resultant acidic water. This would entail some risk from erosion to water control structures. The consultants cannot judge how serious that risk would be, but it should be considered. Their calculations suggest it would be necessary to repeat such flooding many times, at intervals long enough to permit further pyrite oxidation in the dike soils between flooding episodes (1 week-1 month?). If such a course were chosen, it would be important to collect data on water chemistry before, during and after flooding to document how effective the leaching had been, with special attention to getting good alkalinity data, as the most reliable chemical indicator of the total amount of acid which has been produced.
The consultants do not think application of crushed carbonate rock or ash would provide much benefit on the scale of the entire pond network until the pyrite burden had been substantially reduced. It might be productive to try such applications on a small scale, but the consultants are not optimistic that they would be very effective. Some measurable chemical changes in the sediments and interstitial waters of Pond 29 remain approximately a year after limestone application but significant increase in shrimp yields has not occurred.
Another experiment which could be done on a small scale would be air bubbling. Although this could raise brackish water pH in the range of 6.5 to 7.5 by stripping CO2 from the water down to a value in equilibrium with the air, the consultants can see no way this would significantly improve the chemical conditions for the aquaculture organisms (if the chemical conditions are indeed the main problem).
The scientific findings (see sections 3 and 7 below) generally suggest two principal conclusions. First, the reduced sediments of the ponds do not contribute to low alkalinity and pH of the pond waters and may even keep the water buffered at intermediate alkalinity levels. Second, inwelling of water through the dikes during filling and draining operations may contribute as much iron and acid to the ponds as is contributed during rainstorm-related leaching of the dikes. Thus the consultants suggest increasing the amount of sediment surface area and volume in the dikes, while at the same time reducing the surface area and volume of oxidized, acid-containing dike soil, by raising pond water levels. This could have a beneficial effect on water quality and is unlikely to harm water quality. An experiment designed to test this hypothesis could be performed as follows: select a group of nine ponds (e.g. ponds 15–17, 20–22, 25–27); while maintaining normal filling/ draining schedules, raise the level of three ponds by 1.0 m, and raise three other ponds by 2.0 m; this would allow testing of alkalinity in a set of ponds maintained at three different water levels. The experiment could be continued over an entire shrimp growth season in order to test the effect of sediment volume dike volume on shrimp growth. In any event, it would be necessary to continue the experiment for 2–3 months, making biweekly alkalinity determinations on each pond.
If culturing of shrimp is to remain a major research activity at Gelang Patah, the consultants would recommend that serious considerations be given to construction of additional ponds which would be as free as possible of acid soil conditions. To accomplish this, a good survey of soil chemistry (for potential acidity and/or pyrite mineralization) prior to site selection and construction would be essential. This should include borings, which are analysed at different depths, as well as over all the potential pond areas. It should be feasible for much of the sampling and analysis to be guided and executed by the staff at Gelang Patah. The new ponds should have a little exposed dike soil as possible. This could be accomplished by building the dikes from sediment only up to the low-water level and then edging the ponds with other construction materials (such as bricks?) and then filling the space between pond edges with a very low dike sloping away from the pond water toward a trough in the center. The entire construction plan should be built around minimizing the amount of exposed soil to prevent oxidation of pyrite. Because shrimp are so sensitive to acid conditions, it may not be possible to achieve good yields in a practical length of time without going to such extremes in design of the ponds.
With the present pond system and no major acceleration of pyrite leaching, it is thought unlikely that a significant improvement in shrimp productivity could be achieved in the near future. Because of the area and height of the dike system, pyrite leaching following precipitation will continue to be a substantial problem, even if less were occurring due to a change in water exchange practices to minimize dike leaching during pond water renewal. Thus, a third possible major policy change would be to shift emphasis from crustacean culture to identify species of fish which are least sensitive to the water conditions typical of acid sulfate soil environments. Obviously, such a shift could greatly alter the overall strategy for development of aquaculture in Malaysia but, given the investment already made at Gelang Patah, it is a possible alternative to maximize the research benefit of the facility.
The consultants cannot choose between the above three suggestions, since that is essentially a matter of policy to be decided in Malaysia, but they think that unless major change is undertaken, the yield per hectare per year of culture organisms will remain quite low at Gelang Patah for a long time. The Thailand aquaculture facilities the consultants visited had more than an order of magnitude less pyrite than the lowest estimate of pyrite they found for Gelang Patah. Shrimp yields were approximately an order of magnitude greater per unit area than at Gelang Patah even without intensive stocking. The ratio of pond area to dike area was much greater in the private traditional aquaculture ponds in Thailand, the dike heights were much lower, often sloping to a central trough, and the abundance of natural organic materials in the water was apparently considerably greater, based on their limited observations of water turbidity and other properties. The consultants think it is likely the soils of the area they visited in Thailand originally had considerable pyrite, based on their XRF measurements of total sulphur and iron. If that were true, the removal of the original pyrite probably occurred over a period of many years during flooding for salt crystallization and from rain leaching before shrimp aquaculture was initiated.
The Gelang Patah Coastal Aquaculture Demonstration and Training Project facilities include 10–12 ha of brackishwater grow-out ponds, which presently have about half of the water in each pond renewed at two-day intervals, from the tidal stream which bounds the main external dike on the north through manual operation of a set of gates to effect the water exchange. Salinities of 24–25‰ in the network of 33 ponds (Figure 1) and the source stream were essentially constant throughout the period of the consultants' field work. Total precipitation over a period of two-three weeks prior to and including the field work was less than 4 cm (Table 1).
The chemical properties of primary interest in this work were those related
to development of acidic sulfate solutions from leaching of pyrite-bearing soils
in the dike network through precipitation runoff and by brackish water penetrating
fissures in the dike soil during water level changes associated with renewal of
water in the ponds. One of the most reliable indications of the total amount of
acid which has reacted with brackish water is the alkalinity (primarily due to
bicarbonate, HCO3-) of the water. Surface sea water has a salinity of about 35‰
and an alkalinity of @ 2.3 milliequivalents per kg of water. Assuming the fresh
water, which reduces the salinity to about 25°/00 (70 percent of open sea water
salinity of 35 percent, has zero alkalinity, one would expect the tidal stream
source water at Gelang Patah to be about 1.6 meq/ (0.7×2.3). All of the stream
water samples measured during the field work had alkalinities very close to this
expected value. Thus, no appreciable strong acid had been added to the brackish
waters in their transit of approximately 10–15 km from the coast to the Gelang
Patah ponds.
(0.7×2.3). All of the stream
water samples measured during the field work had alkalinities very close to this
expected value. Thus, no appreciable strong acid had been added to the brackish
waters in their transit of approximately 10–15 km from the coast to the Gelang
Patah ponds.
A large number of pond water samples were analysed for alkalinity and iron
during the consultants' field work (Table 2). Alkalinity was measured by sequential
additions of acid followed by pH measurements between pH 4 and pH 3 to provide a
very precise value of titration alkalinity which is not dependent upon the absolute
accuracy of the pH data. Iron was measured by colorimetry using ferrozine to form
a coloured complex with Fe+2. Approximately half of the samples had alkalinities
between 1.5 and 1.8 meq/ , indicating little or no acid impact (Fig. 2). One of
the ponds (Pond 23), which had been left for a number of weeks without water
exchange, consistently had an alkalinity of about 0.6 meq/
, indicating little or no acid impact (Fig. 2). One of
the ponds (Pond 23), which had been left for a number of weeks without water
exchange, consistently had an alkalinity of about 0.6 meq/ , indicating that two
thirds of the alkalinity of water entering the pond network had been lost by reaction
with acid. About half of the other ponds (Ponds 1,3,4,5,6,7,8,9,10,16,21,24,25,
27,28,30,32) and some areas of the discharge canals had alkalinities ranging
between 1.0 and 1.5 meq/
, indicating that two
thirds of the alkalinity of water entering the pond network had been lost by reaction
with acid. About half of the other ponds (Ponds 1,3,4,5,6,7,8,9,10,16,21,24,25,
27,28,30,32) and some areas of the discharge canals had alkalinities ranging
between 1.0 and 1.5 meq/ for at least one sample, indicating 10–40 percent of the
alkalinity has been lost by reaction with acid during transit and storage in the
pond network.
for at least one sample, indicating 10–40 percent of the
alkalinity has been lost by reaction with acid during transit and storage in the
pond network.
Almost all of the pond and canal samples had total iron concentrations greater than the source stream water (Fig. 3), but there was not a strong positive correlation with reduced alkalinity. Even Pond 23, which had substantially more visible iron precipitate near both the water surface and sediment surface than most of the other ponds, did not have unusually high total iron concentration compared with the other ponds except within those discrete layers of relatively large particles.
All of the pond waters had easily measurable NH4+, but very little NO2-, PO4º, HS- or Mn++ (Table 3), and the rust-coloured particulate layers were predominantly made of Fe (oxides and oxyhydroxides?).
Interstitial waters in the sediments of Pond 29 had substantially higher dissolved Fe+2, HS- and NH4+ than in the overlying water, and no good evidence of significant sulfate reduction in the sediments (Tables 4, 5). (Sulfate values reported are only approximate; there was a systematic calibration difference between the two sets of interstitial water samples.) The interstitial water data were obtained by equilibration for almost a week of a series of chambers inserted into the sediment and covered by a membrane permeable to dissolved ions, but not to particles greater than a few tenths of a micron. Elevated total iron and sulfide occurred well below the sediment-water interface, indicating relative little molecular diffusion or other transport of iron from the sediments into the water.
It has been observed previously at Gelang Patah that bubbling air through water from the ponds can raise the pH fairly rapidly and thus could possibly be of value in ameliorating effects of acidification. The consultants conducted bubbling experiments using water from Ponds 23 and 29 and from the stream opposite Main Gate I (Table 6). In all three cases pH increased appreciably, while alkalinity and iron were not affected significantly, despite the visual appearance of improved water clarity. The cause of the pH increase is due to reduction of the partial pressure of carbon dioxide (pCO2) in the initial water samples by about an order of magnitude to reach equilibrium with atmospheric pCO2 (300 to 400 × 10-6 atmospheres). The dissociation constants appropriate for computing pCO2 in Gelang Patah waters are summarized in Table 7, along with calculations for each of three bubbling experiments. The initial pCO2 values are much greater than atmospheric pCO2 because of microbial production of CO2 as organic compounds in sediments and waters are oxidized. Such values are typical of streams and shallow ponds throughout the world.
Samples of soil and sediments from Gelang Patah, as well as a few soil samples from a new commercial aquaculture facility in Malaysia and a government aquaculture research station in Thailand, were shipped to Lamont-Doherty Geological Observatory after the consultants' field work was completed. They ground these samples to a fine powder with a mortar and pestle and added known amounts of pyrite powder (prepared from a large single crystal of FeS2) to subsamples of each large sample. Reaction of hydrogen peroxide (H2O2) with these samples resulted in pH values ranging from 1.5 to 3.8 (Table 8). Titration of the acid solutions back to pH 7 with NaOH was used to measure the total acidity yield from each oxidized sample. In general, samples with the lowest pH values had the highest acidity (Fig. 4), as would be expected. The consultants used several oxidation procedures, varying the proportions of sample and peroxide and the time for reaction to occur. The most practical and reproducible sample weight and peroxide volume for their experiments were 0.5 g (dry weight) and 20 ml (Table 9), although there was substantial variation in replicates analysed by all of their procedures. The total acidity for a dike soil sample near Pond 29 was 10 meq/100 g (M-2a, b, c), while sediment from Pond 29 was 20 meq/100 g (M-3a, b, c), and dike soil from a new area of commercial ponds was 100–150 meq/100 g (M-4, 4'). Dike soil from Thailand (T-1 and T-2), with the exception of one sample which cannot be explained at present, was <0.4 meq/100 g. Yields of acidity from addition of known amounts of pyrite powder to these samples ranged from about 30 to 100 percent, assuming 4 equivalents of H+ are produced from 1 mole of FeS2 (Table 10).
Potential acidity measurements on Gelang Patah dike soil and pond sediments by Mr Rosly ben Hassan in November 1981 ranged from 65 to 150 meq/100 g, using a titration end point pH of 8.5. If these values were decreased in proportion to titration values measured here for pH 7 (Table 11) they would still be substantially higher than the consultants observed. Thus the large samples of soil and sediment they collected from Pond 29 appear to have lower acidity potential than is typical of much of the Gelang Patah pond area.
The consultants analysed the same set of samples used for the peroxide experiments for major element composition by x-ray fluorescence (Tables 12, 13), as well as some addition samples of dike materials and ash from a brick manufacturing plant (Table 14). The dike soils and sediments from Gelang Patah had relatively high concentrations of total sulphur and iron, with the sediments higher than the dike soil, suggesting some net transport of iron and sulfide minerals into the ponds in addition to leaching of acidic solutions from the dikes. Both calcium and phosphorus were very low in the Gelang Patah soil and sediments, although the sediments appear to still contain some of the Ca delivered to Pond 29 by limestone applications in January, 1981. The commercial pond area dike soil in southeastern Johore State had even higher sulphur and iron concentrations but the Thailand soils were lower in sulphur than those at Gelang Patah. The quantities of total sulphur in the Thailand soils are still considerably greater than would be found in areas not affected by marine sulphur mineralization. Red minerals from Gelang Patah dike soils were predominantly iron (Table 14), as would be expected, while some yellowish solids with a strong resinous odor were primarily organic materials, rather than mineral. Ash from wood burning was very high in calcium, potassium and phosphorus (Table 14), suggesting its potential as a fertilizer for depleted soils, such as those of the Gelang Patah dikes. All of the soils the consultants analysed had total organic matter of 5–6 percent by weight, except for the new commercial ponds area which was 32 percent by weight organic matter. If all the potential acidity from the peroxide oxidation experiments were derived quantitatively from FeS2 (4 meq of H+ per mM FeS2), the pyrite weight percentages would range from 0.3 to 4 percent in the soil and sediments analysed, except for the Thailand soils which had <0.01 percent equivalent pyrite (Table 15). Thus, the Thailand soils had essentially no potential acidity, despite a significant amount of sulphur and quite high iron levels. These data are interpreted as indicating the soils in this area of Thailand have been extensively leached of pyrite from a long history of use for sea salt crystallization and later for aquaculture over periods of decades to centuries.
Estimations of potential acidity by peroxide oxidation and total pyrite by sulphur
and iron measurements (Table 15) indicate the magnitude of total acid production which
might take place, but indicate relatively little about rates of reaction. The
consultants did a series of short-term leaching experiments (Table 16) using dike
soil and sediment, held on filter paper in large glass funnels. Distilled water or
brackish stream water was poured through ground dry soil or sediment and collected
in beakers. Distilled water leached most of the readily available acid from dike soil
with a quantity of water comparable to the sediment weight. Thus, a second leach
removed substantially less acid than the first. The yield of acid was about 0.4 meq
per 100 g of dry dike soil, or about 4 percent of the potential acidity measured by
peroxide oxidation. Stream water leached a much smaller quantity of acid from
freshly collected wet sediments (<1 percent of the total available). Distilled water
leach of ash produced a strongly basic solution (@pH 9), but the titration alkalinity
of the leach solution was only 1.7 meq/ . Thus, a mixture of equal volumes of ash
leach and dike soil distilled water leach was still fairly acidic (pH 4.8). The
total base-yielding potential of the ash is probably comparable to the values obtained
from the short-term leaching experiment, whereas the total acidity potential of dike
soil is more than an order of magnitude greater than from the short-term leaching
experiments. Leaching of dike soil with distilled water which had been used
previously to leach ash yielded an even greater quantity of acid (alkalinity = -10.6
meq/
. Thus, a mixture of equal volumes of ash
leach and dike soil distilled water leach was still fairly acidic (pH 4.8). The
total base-yielding potential of the ash is probably comparable to the values obtained
from the short-term leaching experiment, whereas the total acidity potential of dike
soil is more than an order of magnitude greater than from the short-term leaching
experiments. Leaching of dike soil with distilled water which had been used
previously to leach ash yielded an even greater quantity of acid (alkalinity = -10.6
meq/ ) than with distilled water alone (alkalinity = -6.2 meq/
) than with distilled water alone (alkalinity = -6.2 meq/ ).
).
Water collected less than half an hour after a rain shower from standing puddles
on the dikes had even higher total acid (alkalinities of -12 and -15 meq/ ) than from
the consultants' laboratory distilled water leaching experiments with dike soil
(Table 17). Surface water from Pond 20 after the same rain shower had an alkalinity
of +1.30 meq/
) than from
the consultants' laboratory distilled water leaching experiments with dike soil
(Table 17). Surface water from Pond 20 after the same rain shower had an alkalinity
of +1.30 meq/ , whereas alkalinity in this pond five days earlier was +1.71 meq/
, whereas alkalinity in this pond five days earlier was +1.71 meq/ .
.
Brackish water (salinity = 25‰) from ponds at a new private aquaculture project
in southeastern Johore State (Santee Estates) also had significant loss of alkalinity
(Table 17) from expected values (1.6 meq/ ), with essentially no alkalinity in a pond
left without water exchange for six months.
), with essentially no alkalinity in a pond
left without water exchange for six months.
The primary mechanism which leads to decreasing alkalinity and pH in the ponds is probably abiotic pyrite oxidation and acid export from dike soil during rainstorms. Experiments were performed to simulate this process in the laboratory, using dike soil, pond sediment and pond water from Pond 29. In view of the documented ability of bacteria to catalyze pyrite oxidation, the consultants also examined pond waters, pond surface scum, and their experimental materials for filamentous bacterial forms which could signify the presence and growth of iron-oxidizing bacteria. They performed time-course measurements of alkalinity and pH in their experiments to follow the oxidation process after the initial leaching event. This stimulates pond conditions in the days following a rainstorm and could indicate bacterial activity.
Rainwater leaching of dike soil was simulated by making 1:1 (wt:wt) extractions of dike soil with distilled water. This primary leachate was then used at 100 percent strength and diluted to 20 and 50 percent strength with Pond 29 water. A 100 percent pond water ( 0 percent leachate) control was also employed. These leachate/pond water mixtures were incubated in triplicate for eight days, both alone and over pond sediment. Alkalinity, pH, and detailed microscopic examinations were made on days 0, 3, and 8 following leachate preparations.
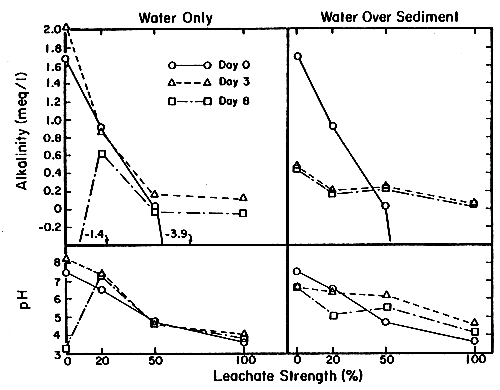
Experiment results are shown in Fig. 7. Initial (day 0) results show the alkalinity and pH levels for the freshly prepared 0, 20, 50 and 100 percent strength leachate solutions. As expected, a straightforward negative correlation between leachate strength, pH, and alkalinity was observed.
No changes were observed after three days at the 20 and 50 percent leachate dilutions but there was a considerable disappearance of acid (or production of alkalinity) in the 100 percent leachate sample by day 3. After another 5 days, no further changes were observed at the 20, 50, or 100 percent leachate strengths. These are puzzling results and the consultants have no good explanation for the large changes they observed at day 3 in 100 percent leachate and at day 8 in 0 percent leachate. However since these are extreme cases which would not be encountered in the ponds, they focus on the intermediate dilutions. These results suggest that most or all of the alkalinity and pH decrease happens during or directly after a rainstrom, and that further biologically mediated processes did not contribute further alkalinity destruction processes in the absence of sediments to any significant extent. By day 8, the full strength pond water had lost all its alkalinity and showed a precipitous pH decline to 3.21. The cause of this surprising result is unclear. It does indicate the poor buffering capacity of the pond water and the fragile poising of acceptable water quality in the pond. This sample did not develop a prominent surface mineral slick or yellowish-brown granular precipitates on the sides, so pyrite oxidation is not suspected as a cause of the alkalinity destruction in this “unpolluted” sample. Fine yellow biofilms precipitates did develop in the 20, 50 and 100 percent strength leachate samples by day 2–3. These were examined microscopically (see below). The films all contained refractile gold or brown granules of amorphous ferric hydroxide, suggesting that biological pyrite oxidation was occurring in these samples.
These results from more realistic experiments show a very different pattern from the water-only results. At 0 and 20 percent leachate strengths, marked deterioration from the initial conditions are apparent by day 3. No changes were noted after day 3, indicating that the process was rapid and complete by that time. Since alkalinity destruction also took place in untreated pond water without sediment by day 8, not all of this effect can be ascribed to the sediment alone. However, the alkalinity decrease in the 20 percent leachate shows that the sediment does contribute to pyrite oxidation, on biological time scales (days rather than minutes to hours). At leachate concentrations of 50 and 100 percent some ameliorating effect of the sediment is apparent. Alkalinity values are higher at days 3 and 8 than on day 0. Apparently the sediment can buffer the water system near 0.2 megl-1 and pH 4–6, which is still quite far from optimal conditions. All the sediment-containing samples developed heavy mineral slicks on the water surface, reddish-brown precipitates on the container sides and filamentous, flocculent, filmy deposits on the sediment surface (see below). The sediment water interface, in these ponds as in many other aquatic systems, is a zone of intensive biological activity. In this particular case, it appears to be a zone of complex pyrite oxidation which contributes to water quality deterioration.
These examinations were carried out using transmitted light Hoffman modulation interference contrast microscopy. This optical system facilitates visualization of delicate microorganisms in mineral matrices such as iron oxidizing filamentous bacteria surrounded by amorphous ferric hydroxide precipitates. Refractile brownishgold granules were ubiquitous in surface slicks and sediment-water interfaces from ponds and experimental samples and also in the discoloured gills from dead shrimps. These granules indicate active pyrite oxidation in the ponds. Shrimp gills accumulate and may be clogged by these iron precipitates.
Heavy reddish granular deposits from Pond 23 (30 November 1981) contained abundant filamentous forms in intimate association with the granules. These organisms could generally only be seen following treatment with IN HCI, which dissolved the Fe(OH)3. Although the iron-oxidizing bacteria cannot be identified on morphological grounds alone, the clear association of these filamentous forms with iron hydroxides deposited over several days, strongly suggests that they did precipitate the iron through pyrite oxidation. Similar filamentous forms were also seen in the yellow biofilms covering the walls of the experimental vessels and in all other samples examined. The sediment surfaces in the experimental systems all developed heavy rust-red flocculent deposits with mucus-laden, thread-like projections, which contained abundant filamentous organisms. No filamentous bacteria were observed in any shrimp gills examined.
In conclusion the consultants' experiments indicated a clear capability for continued alkalinity destruction, probably from bacterial pyrite oxidation in the water and at the sediment-water interface. The most rapid and significant process of water quality deterioration, however, was simple leaching of dike soil with distilled water (or rainwater) and probably also with brackish water flushed through fissures in the dikes during water stage changes.
It is clear from several kinds of evidence that the dike soils and pond sediments at Gelang Patah contain substantial amounts of pyrite dispersed throughout the system. Some indication of the total burden of pyrite in the pond network can be derived from the peroxide oxidation experiments. The dike soil sample analysed from Pond 29 had 10 meq/100 g of potential acidity. The dike soils analysed by Rosly ben Hassan in November 1981 had some samples with about an order of magnitude greater potential acidity. Using this range of values as representative of dike soils at Gelang Patah, the total quantity of acid available in a 1 m thickness of soil with an area of 10 ha is 107–108 moles (Table 19).
From the XRF analysis of sulphur and iron, the sample of Pond 29 dike soil analysed probably has at least 1 percent pyrite by weight. Using a 1 m soil layer of 10 ha area, the total pyrite burden is at least 103 t. If 4 moles of H+ are produced per mole of pyrite, the total acid available is 3 × 107 moles (Table 19).
Removal of acidity from the dike soils and pond sediments is presently occurring by runoff from precipitation and exchange of brackish water in the ponds with the source stream. Exchange of brackish water with the present management practice of draining about half of the volume of the ponds every two days should remove about 2 × 106 moles of acid per year, assuming the alkalinity data the consultants collected in November-December 1981 are representative of brackish water leaching (very little rain fell during the period of their sampling) and neutralization of acid with bicarbonate from the source water (Table 20). Leaching by precipitation runoff probably extracts a comparable amount of acid each year, assuming the precipitation runoff acidities they measured following one rain shower are representative (Table 20).
Filling of the entire pond system to the top of the dikes with brackish water could possible remove a substantial quantity of pyrite acidity. Some indication of the maximum amount of acid a single filling episode could remove can be made by extrapolating the acidity achieved in the short-term laboratory leaching experiments (Table 20). If the pond system were filled to a mean depth of @ 2 m (water above or near the top of the interior dikes), and the water drained through the dike soils during draw-down, it might be possible to remove @106 moles of acid. This is comparable to the estimates made above for the amount of acid removed during normal operations in 2 to 4 months. This would suggest that many cycles of filling would be required to significantly reduce the total pyrite burden in the dike soils.
If neutralization of all of the pyrite acidity were to be attempted by direct application of limestone, magnesium limestone or ash, very large quantities of material would be required (Table 20). The amounts of crushed carbonate rock would be comparable to the total pyrite mass (103 t), and ash would require orders of magnitude more material.
The purpose of the above budget calculations is to provide first order indications of the rates that pyrite acidity at Gelang Patah is now being or could be alleviated. All of the calculations have large uncertainties (at least a factor of two), and especially the one involving purging of the dike soils with brackish water, because of a lack of knowledge of how efficiently water would flow through and equilibrate with the dike soils in an episode of flooding. With these limitations in mind, simply dividing the estimated rates of leaching (Table 20) into the estimated total pyrite burden (Table 19) gives removal times of: (1) 5 to 50 years for brackish water leaching by present water exchange practices; (2) 3 to 30 years for precipitation runoff leaching, and (3) 1 to 10 years for flooding to near the top of the dikes with brackish water, assuming 10 flooding episodes per year. Each of these processes should help to leach acidity from dike soils, but accomplishing sufficient pyrite removal to approximate the very low acidity potential the consultants measured for the soils of aquaculture systems in Thailand will clearly require years, even if the most optimistic assumptions proved to be valid.
The consultants wish to thank the staff members of the Coastal Aquaculture Demonstration and Training Project at Gelang Patah for their hospitality and help during the consultants' assignment in Malaysia, in particular, Mr Harry Cook, Mr Umpal Pongsuwana, Mr Somnuk Wechasit, En. Ti Teou Loon and En. Rosly ben Hassan. Thanks are also extended to their driver, Ahmad, for his transport and friendship during their stay in Johore Bahru. They wish to thank Ms Lilia, Mr Suppachai Summawuthi and Dr Yont Musig of the Department of Fisheries of Thailand for helping them to learn more about shrimp aquaculture. Mr Soo Man Heng of Agaliem SDN. BHD was host for a visit to the new private aquaculture facility east of Johore Bahru.
Table 1
PRECIPITATION AT GELANG PATAH
(29 Nov. 81–9 Dec.81)
| Date* | Time | Vol.(ml)† | Precipitation (mm) |
| 29 Nov.81 | start | - | - |
| 30 Nov.81 | 08.30 | 7 | 1.4 |
| 1 Dec.81 | 08.30 | 0 | 0 |
| 2 Dec.81 | 10.00 | 27 | 5.4 |
| 3 Dec.81 4 Dec.81 5 Dec.81 | 08.00 | 0 | 0 |
| 6 Dec.81 | 08.00 | 76 | 15.2 |
| 7 Dec.81 | 08.00 | 0 | 0 |
| 8 Dec.81 9 Dec.81 | 08.00 | 32 | 6.4 |
* Collector usually left for 24 hours before emptying, except for2.12–5.12.81 (70 h) and 7.12–9.12.81 (48 h).
† Collector had a diameter of 8 cm (500 m glass beaker) and a crosssectional area of 50 cm2
glass beaker) and a crosssectional area of 50 cm2
Table 2(a)
GELANG PATAH - WATER ALKALINITIES AND TOTAL IRON
(meq/ : 28.11.81–6.12.81)
: 28.11.81–6.12.81)
| Date | Sample | pH | Alkalinity (meq/ ) ) | Iron (mg/l) |
| 28.11.81 | P-29 (draining) | 7.50 | 1.58 | - |
| 28.11.81 | P-29 (draining) | 7.35 | 1.53 | - |
| 28.11.81 | P-29 (draining) | 7.33 | 1.52 | |
| 29.11.81 | P-29 (empty) | 7.28 | 3.68 | |
| 29.11.81 | P-1 | 7.03 | 1.05 | 2.0 |
| 29.11.81 | P-2 | 7.10 | 1.63 | 0.5 |
| 29.11.81 | P-3 | 7.27 | 1.55 | 0.7 |
| 29.11.81 | P-4 | 6.80 | 1.26 | 0.6 |
| 29.11.81 | P-5 | 7.02 | 1.20 | 1.0 |
| 29.11.81 | P-6 | 6.99 | 1.15 | 1.4 |
| 29.11.81 | P-7 | 6.65 | 1.08 | 1.5 |
| 29.11.81 | P-8 | 6.93 | 1.14 | 2.0 |
| 29.11.81 | P-9 | 7.01 | 1.30 | 2.0 |
| 29.11.81 | P-10 | 7.23 | 1.50 | 1.3 |
| 29.11.81 | P-11 | 7.25 | 1.62 | 1.3 |
| 29.11.81 | P-10 | 6.98 | 1.61 | - |
| 29.11.81 | P-23 | 6.65 | 0.66 | 4.1, 2.8 |
| 29.11.81 | P-25 | 6.53 | 0.54 | 2.4 |
| 29.11.81 | P-26 | 7.30 | 1.53 | 0.6 |
| 29.11.81 | P-27 | 6.93 | 1.01 | 1.2 |
| 29.11.81 | P-28 | 6.97 | 1.43 | 1.7 |
| 29.11.81 | P-29 | 7.50 | 1.53 | 2.1 |
| 29.11.81 | P-29 (PM) | 7.50 | 1.83 | - |
| 29.11.81 | P-33 | 6.93 | 1.55 | 2.0 |
| 29.11.81 | SG-2 | 7.30 | 1.38 | 0.7 |
| 29.11.81 | PG-8 | 6.83 | 1.21 | - |
| 29.11.81 | PG-25 | 7.31 | 1.40 | 1.3 |
| 29.11.81 | Canal opp P-1 | 7.17 | 1.66 | 0.1 |
| 29.11.81 | Stream opp MG-1 | 7.10 | 1.64 | 0.2 |
| 30.11.81 | P-1 | 6.22 | 0.68 | 2.4 |
| 30.11.81 | P-3 | 6.68 | 1.17 | 2.4 |
| 30.11.81 | P-10 | 6.54 | 1.11 | 9.2 |
| 30.11.81 | P-11 | 6.80 | 1.53 | 2.4 |
| 30.11.81 | P-14 | 7.33 | 1.67 | - |
| 30.11.81 | P-18 | 7.13 | 1.60 | 1.6 |
| 30.11.81 | P-19 | 7.45 | 1.65 | 1.2 |
| 30.11.81 | P-23 | 6.72 | 0.67 | 1.2 |
| 30.11.81 | P-24 | 7.22 | 1.46 | 1.4 |
| 30.11.81 | P-25 | 7.22 | 1.47 | 0.8 |
| 30.11.81 | P-28 | 7.51 | 1.62 | 5.0 |
| 30.11.81 | P-29 | 7.39 | 1.85 | 0.7 |
| 30.11.81 | P-30 | 7.23 | 1.38 | 2.4 |
| 30.11.81 | P-32 | 6.92 | 1.39 | 1.0 |
| 30.11.81 | P-33 | 7.23 | 1.66 | 3.6 |
| 30.11.81 | Stream opp PG 30 | 7.35 | 1.67 | - |
Table 2(b)
GELANG PATAH - WATER ALKALINITIES (Cont.d)
(meq/ : 28.11.81–6.12.81)
: 28.11.81–6.12.81)
| Date | Sample | pH | Alkalinity (meq/ ) ) | Iron (mg/l) |
| 1.12.81 | P-1 (North end) | 7.53 | 1.12 | |
| 1.12.81 | P-1 (South end) | ⋍7.5 | 1.15 | |
| 1.12.81 | P-10 | 7.02 | 1.54 | 1.6 |
| 1.12.81 | P-23 | 6.90 | 0.83 | (0.5) |
| 1.12.81 | P-25 | 7.20 | 1.74 | 0.5 |
| 1.12.81 | P-28 | 7.12 | 1.51 | 1.4 |
| 1.12.81 | P-29 | 7.51 | 1.78 | 0.8 |
| 1.12.81 | Stream | 7.42 | 1.70 | 0.4 |
| 2.12.81 | P-1 (AM) | 7.45 | 1.15 | 1.1 |
| 2.12.81 | P-3 (AM) | 7.43 | 1.56 | 0.6 |
| 2.12.81 | P-10 (AM) | 7.47 | 1.57 | 0.9 |
| 2.12.81 | P-18 (AM) | 7.48 | 1.68 | 0.5 |
| 2.12.81 | P-20 (PM) | 7.40 | 1.71 | - |
| 2.12.81 | P-21 (PM) | 7.58 | 1.52 | - |
| 2.12.81 | P-23 (AM) | 6.73 | 0.62 | - |
| 2.12.81 | P-23 (PM)(bubbled) | 7.08 | 0.69 | - |
| 2.12.81 | P-23 (PM)(bubbled) | 7.88 | 0.72 | - |
| 2.12.81 | P-24 (AM) | 7.40 | 1.64 | 0.9 |
| 2.12.81 | P-25 (AM) | 7.28 | 1.62 | 0.6 |
| 2.12.81 | P-28 (PM) | 7.36 | 1.65 | - |
| 2.12.81 | P-29 (PM) | 7.50 | 1.72 | - |
| 2.12.81 | P-32 (PM) | 7.46 | 1.58 | - |
| 2.12.81 | P-33 (AM) | 7.34 | 1.66 | 0.7 |
| 2.12.81 | P-33 (PM) | 7.42 | 1.70 | - |
| 5.12.81 | P-3 | 7.51 | 1.59 | 0.6 |
| 5.12.81 | P-10 | 7.39 | 1.57 | 0.6 |
| 5.12.81 | P-11 | 7.20 | 1.65 | 2.4 |
| 5.12.81 | P-14 | 7.42 | 1.71 | 0.4 |
| 5.12.81 | P-14 | 7.36 | 1.70 | 0.8 |
| 5.12.81 | P-15 | 7.10 | 1.52 | 0.7 |
| 5.12.81 | P-16 | 6.74 | 1.40 | 4.6 |
| 5.12.81 | P-21 | 6.56 | 1.34 | 5.1 |
| 5.12.81 | P-28 | 7.02 | 1.53 | 1.1 |
| 5.12.81 | P-30 | 6.55 | 1.18 | - |
| 5.12.81 | P-30 | 7.33 | 1.44 | - |
| 5.12.81 | Stream | 7.50 | 1.60 | 0.5 |
| 5.12.81 | Stream | 7.50 | 1.74 | - |
Table 2(c)
GELANG PATAH - WATER ALKALINITIES (Cont.d)
(meq/ : 28.11.81–6.12.81)
: 28.11.81–6.12.81)
| Date | Sample | pH | Alkalinity (meq/ ) ) | Iron (mg/l) |
| 6.12.81 | P-1 | 7.70 | 1.15 | 0.3 |
| 6.12.81 | P-7 | 7.20 | 1.16 | 1.9 |
| 6.12.81 | P-18 | 7.39 | 1.56 | 0.8 |
| 6.12.81 | P-19 | 7.49 | 1.60 | 0.2 |
| 6.12.81 | P-23 | 6.82 | 0.67 | 2.0 |
| 6.12.81 | P-23 | - | 0.43 | 0.8 |
| 6.12.81 | P-23 (bubbled) | 7.58 | 0.68 | 0.7 |
| 6.12.81 | P-23 (bubbled) | 7.83 | 0.69 | 0.6 |
| 6.12.81 | P-24 | 7.35 | 1.43 | 7.2 |
| 6.12.81 | P-25 | 7.23 | 1.35 | 0.2 |
| 6.12.81 | P-29 | 7.62 | 1.54 | 1.0 |
| 6.12.81 | P-29 | 7.58 | 1.58 | 1.0 |
| 6.12.81 | P-29 (bubbled) | 8.12 | 1.56 | - |
| 6.12.81 | P-32 | 7.01 | 1.39 | 0.3 |
| 6.12.81 | P-33 | 7.46 | 1.60 | 2.0 |
| 6.12.81 | Stream | 7.46 | 1.63 | - |
Table 3
SUMMARY OF GELANG PATAH POND WATER NUTRIENT, SULFIDE
AND MANGANESE MEASUREMENTS-NOVEMBER, DECEMBER 1981
| Chemical Species | Concentration | |
| NH4 | P-10 | 24 μM |
| P-23 | 49 μM | |
| P-25 | 31 μM | |
| P-28 | 15 μM | |
| P-29 | 11 μM | |
| Stream | 14 μM | |
| NO2- | Several ponds | <0.1 μM |
| PO4 | Several ponds | <0.3 μM |
| HS- | Several ponds | <0.01 μM |
| Mn | Several ponds | <0.05 ppm |
Rust colored flocculant precipitate in Pond 23 (0.15 gm from 50 ml volume): 63% Fe, by weight.
Table 4
SEDIMENTINTERSTITIAL WATER
COMPOSITION - POND 29 - CENTER
| Cell No.* | Fe+2,+3 (ppm) | HS- (μM) | SO4= (ppm) | NH4+ (μM) | PO43 (μM) |
| 1 | 1.2 | 1300 | 25 | <1 | |
| 2 | <6 | ||||
| 3 | 1.1 | 1400 | - | <1 | |
| H2O 4 | 6 | ||||
| Sed 5 | 5.3 | 1100 | 66 | <1 | |
| 6 | <6 | ||||
| O2 7 | 13 | 1200 | - | <1 | |
| No 8 | <6 | ||||
| O2 9 | 40 | 1200 | - | <1 | |
| 10 | 6 | ||||
| 11 | 38 | 1200 | 41 | <1 | |
| 12 | 117 | ||||
| 13 | 2.3 | 1300 | - | <1 | |
| 14 | 117 | ||||
| 15 | 9.3 | 1300 | 147 | <1 | |
| 16 | 31 | ||||
| 17 | 1.4 | 1000 | 140 | <1 | |
| 18 | 55 | ||||
| 19 | 3.2 | 900 | 487 | <1 |
Table 5
SEDIMENT INTERSTITIAL WATER
COMPOSITION - POND 29 - MARGIN
| Cell No.* | Fe+2,+3 (ppm) | HS- (μM) | SO4= (ppm) | NH4+ (μM) |
| 1 | 1 | 2000 | 16 | |
| 2 | ||||
| H2O 3 | 2 | 2200 | 82 | |
| Sed 4 | <6 | |||
| 5 | 5 | 2000 | 31 | |
| 6 | <6 | |||
| 7 | 6 | 1900 | 38 | |
| 8 | <6 | |||
| 9 | 7 | 1900 | 62 | |
| 10 | <6 | |||
| 11 | 7 | - | 82 | |
| 12 | <6 | |||
| 13 | 7 | 1900 | 78 | |
| 14 | <6 | |||
| 15 | 29 | 1900 | 88 | |
| 16 | <6 | |||
| 17 | 11 | 1800 | 140 | |
| 18 | <6 | |||
| 19 | 14 | 1900 | 160 | |
| 20 | ⋍6 | |||
| 21 | 12 | 1900 | 160 | |
| 22 | ⋍6 | |||
| 23 | 13 | 1900 | 150 | |
| 24 | <6 | |||
| 25 | <1 | 1900 | ||
| 26 | <6 | |||
| 27 | <1 | |||
| 28 | <6 |
Table 6
AIR BUBBLING EXPERIMENTS
GATE I, POND 23 (2.12.81)
| Time (h) | Control | Bubbled | ||||
| Fe (ppm) | pH | Alkalinity (meq/  ) ) | Fe (ppm) | pH | Alakalinity (meq/  ) ) | |
| 0 | - | - | - | 4.9 | 6.81 | 0.67 |
| - | - | - | 2.6(F) | - | - | |
| 2 | 4.3 | - | - | 3.0 | - | - |
| 2.6(F)* | - | - | 0.7(F) | - | - | |
| 4.3 | 5.6 | 6.80 | - | 3.0 | 7.80 | 0.68 |
| 1.6(F) | - | - | 0.7(F) | - | - | |
| Pump off overnight | ||||||
| 0 | 3.4 | 6.85 | - | 1.5 | 7.35 | - |
| 1.5(F) | - | - | 0.4(F) | - | - | |
| 2 | 3.3 | 6.96 | - | 3.1 | 7.79 | 0.69 |
| 0.5(F) | - | - | 0.3(F) | - | - | |
| II Stream Water (3.12.81) | ||||||
| 0 | 0.5 | 6.95 | - | 0.5 | 6.96 | ≃1.6 |
| 0.1(F) | - | - | 0.1(F) | - | - | |
| 0.5 | - | 7.10 | - | - | 8.21 | - |
| 1 | - | 7.13 | - | - | 8.25 | - |
| 2 | 0.3 | 7.14 | - | 0.3 | 8.26 | - |
| 0.0(F) | - | - | 0.2(F) | - | - | |
| 2.5 | 0.2 | 7.20 | - | 0.5 | 8.26 | ≃1.6 |
| 0.1(F) | - | - | 0.3(F) | - | - | |
| III Pond 29 (6.12.81) | ||||||
| 0 | 1.1 | 7.49 | - | 0.8 | 7.49 | 1.58 |
| 0.1(F) | - | - | 0.2(F) | - | - | |
| 0.6 | 1.1 | 7.51 | - | 0.7 | 8.11 | - |
| 0.1(F) | - | - | 0.1(F) | - | - | |
| 2.5 | 0.8 | 7.55 | - | 0.6 | 8.12 | - |
| 0.1(F) | - | - | 0.1(F) | - | - | |
| 3 | 0.8 | 7.59 | - | 0.6 | 8.18 | 1.56 |
| 0.2(F) | - | - | 0.1 | - | - | |
* Sample filtered before analysis for iron.
Table 7
CARBON DIOXIDE PARTIAL PRESSURE CALCULATIONS
| I | [H+] × [HCO3-] = [H2CO3] × K1 |
| II | [H2CO3] = pCO2 × KCO2 |
| III | pCO2 = [H+] × [HCO3-]/K1 × KCO2 |
| at S = 25‰ (Chlorinity = 13.8‰) | |
| T = 25°C | |
| K1 = 10-6.052 moles/liter | |
| KCO2 = 3.12 × 10-2 moles/liter-atmosphere |
| Bubbling Experiment | Water Source | Alkalinity (meq/  ) ) | pH | pCO2 (x10-6 atm) |
| I-Begin | Pond 23 | 0.67 | 6.81 | 3700 |
| I-End | Pond 23 | 0.69 | 7.79 | 400 |
| II-Begin | Stream | ≃1.6 | 7.0–7.5 | 1800–5800 |
| II-End | Stream | ≃1.6 | 8.26 | 320 |
| III-Begin | Pond 29 | 1.58 | 7.49 | 1800 |
| III-End | Pond 29 | 1.56 | 8.18 | 370 |
Table 8
SOIL AND SEDIMENT POTENTIAL ACIDITY
AFTER H2 O2 OXIDATION VS. pH*
(PLOTTED AS “x's” in FIGURE 6)
| Sample | meq/100g | Initial pH |
| T-1 | (10)† | 3.29 |
| M-2a | 10 | 3.22 |
| M-3a | 20 | 3.75 |
| T-1 + 4.76% FeS2 | 70 | 2.00 |
| M-4' | 105 | 2.24 |
| M-3a + 4.76% FeS2 | 139 | 1.82 |
| M-4'+ 4.76% FeS2 | 156 | 1.89 |
| T-1 + 9.09% FeS2 | 164 | 1.68 |
| M-2a + 4.76% FeS2 | 167 | 1.70 |
| M-3a + 9.09% FeS2 | 237 | 1.57 |
| M-2a + 9.09% FeS2 | 255 | 1.49 |
* Sample weights of 0.5g were oxidized with 20 ml of 30% H2O2, transferred tocentrifuge tubes with a final liquid volume of ≃25 ml: a subsample of 10 ml ofclear solution was titrated to pH 7 within a few hours.
† All other samples of T-1 which were analyzed previously had potentialacidities of <1 meq/100g.
Table 9
PEROXIDE OXIDATION EXPERIMENTS
| Sample | Sample weight (g) | Final Solution volume (m ) ) | pH | Acidity (pH 7) (meq/100g) |
| M-2a* | 5 | 40 | 3.02 | 10 |
| M-2a* | 1 | 26.2 | 3.13 | 9.6 |
| M-2a* | 0.5 | 24.9 | 3.22 | 10 |
| M-2b* | 5 | 40 | 2.82 | 8.6 |
| M-2b* | 5 | 22.4 | 2.35 | 14 |
| M-2c* | 5 | 40 | 2.85 | 9.0 |
| M-2c* | 1 | 25.9 | 3.13 | 9.5 |
| M-3a+ | 5 | 40 | 3.89 | 6.2 |
| M-3a+ | 1 | 38.3 | 3.92 | 18 |
| M-3a+ | 0.5 | 25.4 | 3.75 | 20 |
| M-3b+ | 1 | 37.2 | 3.79 | 17 |
| M-3c+ | 5 | 40 | 3.83 | 7 |
| M-3c+ | 1 | 24.6 | 3.89 | 16 |
| M-3e§ | 5 | 40 | 4.48 | 4.6 |
| M-4† | 5 | 40 | 2.23 | 30 |
| M-4† | 1 | 21.6 | 2.08 | 151 |
| M-4† | 0.5 | 31.4 | 2.51 | 136 |
| M-4'† | 0.5 | 20.4 | 2.24 | 105 |
| T-1Δ | 5 | 40 | 4.99 | 0 |
| T-1Δ | 1 | 18.1 | 5.40 | 0.4 |
| T-1Δ | 0.5 | 28.2 | 3.29 | 10 |
T-2 | 5 | 40 | 6.71 | 0 |
T-2 | 1 | 19.6 | 6.10 | 0.3 |
T-2 | 1 | 28.4 | 6.60 | 0.1 |
* Replicate soil samples from a large sample collected on the south dike of Pond29.
+ Replicate sediment samples from a large sample collected near the south end ofPond 29.
§ Sample source as for other M-3 samples, but only particles >400 microns insize included.
† Dike soil from a new private pond facility in southeastern Johore State.
Δ Dike soil from a government aquaculture research station in Thailand.
 Dike soil from a traditional aquaculture pond in Thailand.
Dike soil from a traditional aquaculture pond in Thailand.
Table 10
PYRITE ADDITION EXPERIMENTS*
(PEROXIDE OXIDATION)
| Sample | Initial Acidity (meq/100g) | Final Acidity (meq/100g) | Δ (meq/100g) measured | Δ (meq/100g) theoretical† | Δ measured/theoretical |
| T-1+4.76% FeS2 | 10 | 70 | 60 | 159 | .38 |
| T-1+9.09% FeS2 | 10 | 164 | 154 | 303 | .51 |
| M-2a+4.76% FeS2 | 10 | 167 | 157 | 159 | .99 |
| M-2a+9.09% FeS2 | 10 | 255 | 245 | 303 | .81 |
| M-3a+4.76% FeS2 | 20 | 139 | 119 | 159 | .75 |
| M-3a+9.09% FeS2 | 20 | 237 | 217 | 303 | .72 |
| M-4'+4.76% FeS2 | 105 | 156 | 51 | 159 | .32 |
* Samples (0.5g dry weight) were oxidized in 30% H2O2 (20ml), with heating to destroyresidual H2O2 and titrated with NaOH (0.1N) to pH 7. Known amounts of FeS2 powderwere added to duplicates of samples T-1, M-2a, M-3a and M-4' and then carriedthrough the same procedure.
† Maximum theoretical production of acidity was assumed to be 4 meq of H+ foreach mM of FeS2.
Table 11
COMPUTED POTENTIAL ACIDITY
AS A FUNCTION OF END POINT pH*
| Sample | Titration volume (m ) pH 7 ) pH 7 | Titration volume (m ) pH 8 ) pH 8 | Titration volume (m ) pH 8.5 ) pH 8.5 | V(pH 8.5)/V(pH 7.0) |
| T-1 | 0.02 | - | 0.034 | - |
| T-2 | 0.004 | 0.02 | 0.05 | - |
| M-2c | 0.366 | - | 0.521 | 1.42 |
| M-2a | 0.368 | - | 0.519 | 1.60 |
| M-4 | 2.17 | 2.67 | 2.87 | 1.32 |
| M-3a | 0.474 | 0.647 | 0.863 | 1.82 |
| M-3b | 0.452 | 0.612 | 0.771 | 1.71 |
| M-3c | 0.651 | 0.895 | >1.0 | >1.54 |
* Base (0.1 N NaOH) was added from a microburet to a samplevolume of 10 ml.
Table 12
GELANG PATAH - POND 29
(WEIGHT PERCENTAGES - XRF)
| Element | Dike Soil | Pond Sediment | |||||
| M-2a* | M-2b* | M-2c* | M-3a† | M-3b† | M-3c† | M-3d† | |
| Si | 34 | 35 | 34 | 29 | 28 | 27 | 27 |
| Al | 11.3 | 12.2 | 11.9 | 10.2 | 10.0 | 9.2 | 9.9 |
| Fe | 3.1 | 3.0 | 3.1 | 5.0 | 4.6 | 4.6 | 4.2 |
| Mg | 0.2 | 0.2 | 0.2 | 0.3 | 0.4 | 0.3 | 0.4 |
| Ca | <0.05 | <0.05 | <0.05 | 0.23 | 0.30 | 0.27 | 0.16 |
| Na | 0.1 | 0.1 | 0.08 | ≃4 | ≃7 | ≃9 | ≃7 |
| K | 1.5 | 1.3 | 1.4 | 1.4 | 1.2 | 1.2 | 1.0 |
| P | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| Mn | - | 0.03 | 0.02 | - | 0.02 | 0.02 | 0.02 |
| S | 1.00 | 1.03 | 1.00 | 1.20 | 1.38 | 1.31 | 1.02 |
| ∑ | 51.3 | 53.0 | 51.8 | 51.3 | 48.3 | 52.9 | 50.7 |
| Organic matter Δ | 5.1 | 5.4 | 5.5 | 6.5 | 5.9 | 5.7 | 5.5 |
* Replicate dike soil samples from a large sample from one site.
† Replicate sediment samples from a large sample from one site.
Δ Organic matter measured by oxidation overnight at 375°C after drying at 130°C.
Table 13
OTHER AQUACULTURE SYSTEM DIKE SOILS
(WEIGHT PERCENTAGES - XRF)
| Santee Estates Dike Soil Johore, Malaysia | Thailand Dike Soil |
| Element | M-4* | M-4'* | T-1† | T-1c§ | T-2+ |
| Si | 24 | 24 | 11 | 7 | 29 |
| Al | 7.7 | 8.2 | 2.6 | 1.8 | 8.4 |
| Fe | 3.7 | 3.3 | ≃7 | 5.6 | 8.2 |
| Mg | 0.8 | 0.7 | 0.4 | 0.2 | 2.5 |
| Ca | 0.82 | 0.81 | 0.15 | 0.12 | 0.96 |
| Na | 0.7 | 0.8 | ≃40 | >30 | 0.3 |
| K | 1.6 | 1.3 | 1.0 | 0.5 | 1.8 |
| P | 0.04 | 0.04 | 0.05 | 0.04 | 0.08 |
| Mn | - | 0.03 | 0.3 | 0.2 | 0.3 |
| S | 4.06 | 4.10 | 0.5 | 0.8 | 1.8 |
| Organic MatterΔ | 32.7 | 31.8 | 4.8 | - | 5.2 |
* Replicate dike soil samples from one site.
† Aquaculture station soil particles ground to fine powder.
§ Aquaculture station soil particles <400 μ.
+ Dike soil from traditional aquaculture farm near aquacultureresearch station.
Δ Organic matter measured by oxidation overnight at 375°C after drying at 130°C.
Table 14
GELANG PATAH - MISCELLANEOUS SAMPLES
(WEIGHT PERCENTAGES - XRF)
| Element | Resinous Yellow dike materials | Red dike materials | Pond water hydroxide precipitate | Ash from Johore brick kilns | ||
| M-1 | M-6 | M-7 | M-8 | M5a | M5b | |
| Si | 2 | 0.7 | 6 | 0.5 | 11 | 9 |
| Al | 0.7 | 0.2 | 3.8 | 0.03 | 2.7 | 4 |
| Fe | 0.6 | 0.6 | 28 | 0.1 | 1.5 | 1.2 |
| Mg | <0.1 | <0.1 | <0.1 | 14 | 6 | 5.7 |
| Ca | 0.04 | <0.05 | 0.07 | 0.5 | 19 | 19 |
| Na | 0.03 | 0.02 | 0.03 | 27 | 0.03 | 0.04 |
| K | 0.1 | 0.06 | 0.1 | 0.07 | 3.7 | 5.6 |
| P | 0.005 | 0.005 | 0.01 | 0.005 | 1.2 | 1.2 |
| Mn | 0.2 | 0.3 | <.01 | 0.01 | 0.1 | 0.1 |
| S | 0.6 | 0.3 | 0.9 | ≃4 | 3.1 | 5.2 |
| ∑ | 4.4 | 2.2 | 39 | 46 | 49 | 51 |
Table 15
EXCESS ACIDITY, SULPHUR AND IRON IN SOILS AND SEDIMENTS
| Potential Excess Acidity (meq/100g) | Equivalent* FeS2 (wt %) | ∑S by XRF (wt %) | ∑Fe by XRF (wt %) | |
| M-2† | 10 | 0.30 | 1.0 | 3.0 |
| M-3§ | 20 | 0.61 | 1.3 | 4.6 |
| M-4Δ | 130 | 3.9 | 4.1 | 3.5 |
T-1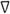 | 0.4 | 0.01 | 0.5 | 7 |
| T-2+ | 0.2 | <0.01 | 1.8 | 8 |
* Assuming 4 meq H+ for each mM FeS2, 1% FeS2 = 33 meq H+/100g ofsample.
† Gelang Patah dike soil (P-29).
§ Gelang Patah sediment (P-29).
Δ Santee Estates dike soil.
 Thailand aquaculture research station dike soil.
Thailand aquaculture research station dike soil.
+ Thailand traditional aquaculture dike soil.
Table 16
LEACHING EXPERIMENTS*
| Sample | pH | Alkalinity (meq/ ) ) |
| Distilled H2O leach of P-29 dike soil - First | 3.64 | -6.2 |
| Distilled H2O leach of P-29 dike soil - Second | 3.98 | -2.5 |
| Distilled H2O leach of P-29 dike soil - First & Second† | 3.90 | -3.9 |
| Distilled H2O leach of P-29 dike soil - large particles | 3.93 | -3.4 |
| Stream H2O leach of P-29 sediment | 4.78 | +0.1 |
| Distilled H2O leach of Ash | 9.23 | +1.7 |
| Distilled H2O leach of Ash mixed with distilled H2O leach P-29 dike soil - large particles | 4.78 | -1.4 |
| Distilled H2O leach of Ash, followed by leach of P-29 dike soil (two step leach experiment) | 4.02 | -10.6 |
| Stream H2O stored with P-31 dike soil for 1 week | 4.36 | -0.1 |
* All experiments involved equal weights of solids and liquids.
† 1254 ml of H2O were added to 1254g of dry soil yielding 465ml of leach-first;then 1489 ml of H2O were added to the same wet soil yielding 660 ml of leach-second; the two leach H2O's were pooled after 50 ml of each portion was with-drawn to measure alkalinity.
Table 1
OTHER WATER SAMPLE KALINITIES
| Date | Sample | pH | Alkalinity (meq/ ) ) |
| 12/7/81 | Puddle in laterite following rain shower | 4.46 | +0.1 |
| 12/7/81 | Puddle on dike near P-30, 31 following rain shower | 2.87 | -11.8 |
| 12/7/81 | Runoff from dike following rain shower | 2.74 | -15.3 |
| 12/7/81 | P-20 following rain shower, prior to stocking with shrimp | 7.30 | +1.30 |
| 12/8/81 | Pond filled for 6 months at Sante Estates* | 5.12 | 0.01 |
| 12/8/81 | Pond A filled for 1 week at Sante Estates* | 6.88 | 0.83 |
| 12/8/81 | Pond B filled for 1 week at Sante Estates* | 7.06 | 0.74 |
Table 18
ALKALINITY (meq/ -1) and pH of INCUBATED POND WATER/SOIL
-1) and pH of INCUBATED POND WATER/SOIL
LEACHATE MIXTURES
| Day | Leachate strength | Water only alkalinity | pH | Water over sediment alkalinity | Sediment pH |
| 0 | 0 | 1.83 | 7.50 | 1.83 | 7.50 |
| 20 | 0.92 | 6.55 | 0.92 | 6.55 | |
| 50 | 0.03 | 4.77 | 0.03 | 4.77 | |
| 100 | -3.91 | 3.90 | -3.91 | 3.90 | |
| 3 | 0 | 2.10 | 8.29 | 0.49 | 6.70 |
| 20 | 0.87 | 7.42 | 0.22 | 6.40 | |
| 50 | 0.16 | 4.70 | 0.25 | 6.20 | |
| 100 | - 0.13 | 4.03 | 0.07 | 4.66 | |
| 8 | 0 | -1.44 | 3.21 | 0.42 | 6.64 |
| 20 | 0.63 | 7.39 | 0.19 | 5.08 | |
| 50 | -0.01 | 4.69 | 0.21 | 5.59 | |
| 100 | -0.02 | 3.88 | 0.06 | 9.17 |
Table 19
ESTIMATES OF TOTAL POTENTIAL ACIDITY IN DIKES OF
GELANG PATAH AQUACULTURE SYSTEM
I. Area = 10 ha = 105 m2
Soil Depth = 1 m (assumed thickness which can react with the water).
∑ Volume = 105 m3
∑ Mass = 105t (assumed density = 1g/1m )
)
If potential acidity = 10 meq/100g
then total moles of acid available = 107
If potential acidity = 100 meq/100g
then total moles of acid available = 108
II. Use same ∑ mass as above,
assume 1% by weight pyrite
then total pyrite present = 103t
If 4 moles of H+ produced per mole of pyrite
then total moles of acid available = 3 × 107
Total Acid Potential: 107 – 108 moles
Table 20
ESTIMATES OF RATES OF REMOVAL OF ACIDITY
AT GELANG PATAH AQUACULTURE SYSTEM
Exchange of Pond Water by Present Practices
10 ha × (102m)2/ha × 1 m/4 days = ≃ 2 ×104 m3/day
If 20% of alkalinity in water is removed (1.6 meq/ →1.3 meq/
→1.3 meq/ )
)
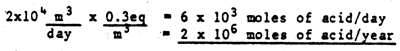
Leaching of Dike Soils by Precipitation

Maximum Leaching of Dike Soils by Filling Entire System with Stream
Water-Acidity from leaching experiments (-6 meq/ of alkalinity)
≃ 20 × as effective per unit volume of water as present pond water
exchange
of alkalinity)
≃ 20 × as effective per unit volume of water as present pond water
exchange
If filled to 2 meters depth (8 × daily exchange depth)
20 × 8 = 160
160 × 6×103 moles of acid ≃ 106 moles of acid per fill
1 ton of Limestone = 2 × 104 moles of alkalinity
1 ton of Magnesium Limestone = 2.2 × 104 moles of alkalinity
1 ton of Ash = 2 moles of alkalinity
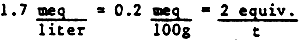
Figure 1
Gelang Patah Pond Layout
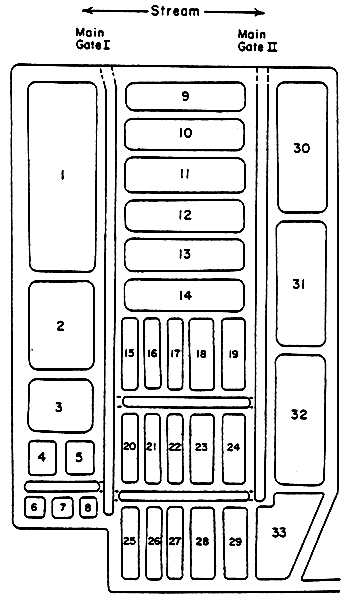
Figure 2
Gelang Patah Water Alkalinities
(meq/ )
)
[11/28/81-12/6/81]
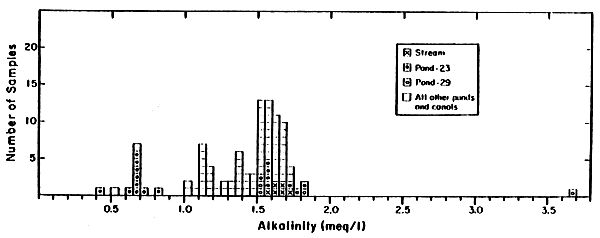
Figure 3
Gelang Patah Water Iron Concentrations (Total)
(meq/ )
)
[1/28/81-12/6/81]
Figure 4
Sediment Interstitial Water
Pond 29-Center
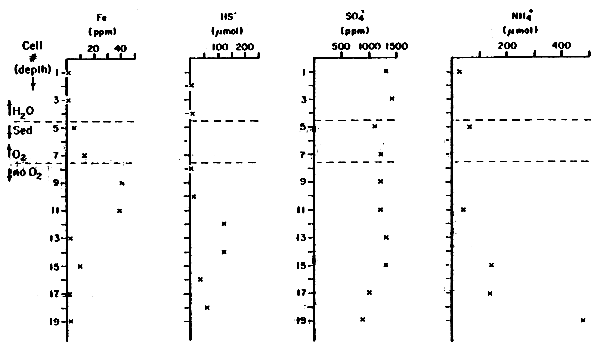
Figure 5
Sediment Interstitial Water
Pond 29-Margin
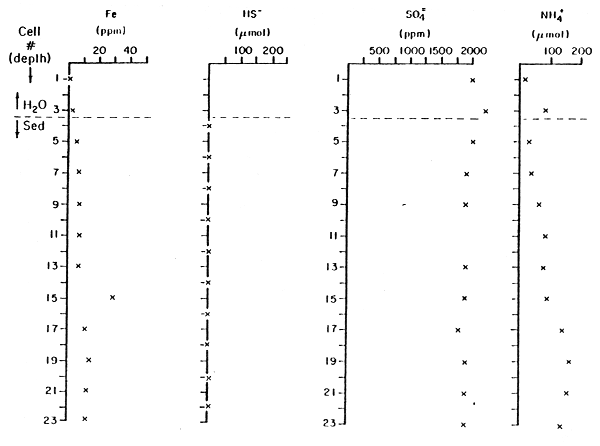
Figure 6
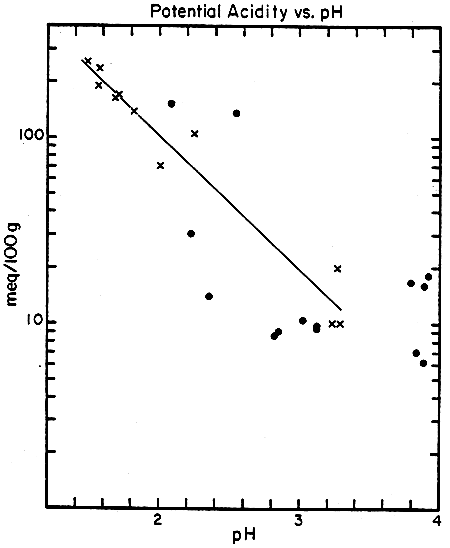
Figure 7
GELANG PATAH WATER-SEDIMENT INCUBATION EXPERIMENTS