Nature and causes of environmental problems
Impacts of blending on water usability and pollution
Soil degradation (salinization and waterlogging)
Water pollution
Ecosystem disturbances
Water-borne diseases
The world's natural resource base for food production has already been weakened and the likely additional strain of the expected increase in population and agricultural activity needed to feed it are posing a threat to the prospects of sustainable development in many countries (UN 1990). It is pertinent at this stage to define sustainable agricultural development: "Sustainable agriculture is the management and conservation of the natural resource base, and the orientation of technological and institutional change in such a manner as to ensure the attainment and continued satisfaction of human needs for present and future generations. Such sustainable development in the agriculture, forestry and fisheries sectors conserves land, water, plant and animal genetic resources, is environmentally non-degrading, technically appropriate, economically viable and socially acceptable" (FAO 1989). Environmental stress is often the result of the excessive demand for scarce natural resources and the related pollution of the land and water generated by over-development and by poverty. The latter occurs when the poor degrade and destroy their immediate environment as they over-use marginal lands for agriculture and dispose of wastes without treatment to common water supplies in order to meet their living needs. Hence an objective of soil and water conservation must also be to create an economic base which makes it more profitable to conserve and protect resources than to destroy them.
There are a number of potentially undesirable impacts on the environment, as well as on the economic and social components of society, caused by improper irrigation which must be considered if agricultural production is to be sustained, even more so if it is to be expanded by the use of saline waters. These impacts can potentially have far-reaching consequences on present as well as future generations and, hence, can affect the very sustainability of irrigated agriculture. In this chapter, some of the concerns about the environment (within and beyond the farm boundaries), the ecology and the long-term viability of irrigation are discussed.
Figure 13 represents a typical irrigation project and its surrounding area and can be used to help portray the various environmental and ecological problems associated with irrigation (Kandiah 1990). Water is diverted from the source and transported through a system of canals to irrigate the cropland. Part of the resulting drainage water is collected and discharged into a nearby stream by means of a system of collector and disposal drains. In this particular project, the irrigation water is low in salinity, crop yields are good and the farmers are profiting. No immediate threat of salinization or waterlogging is evident within the project itself. However, as a result of project activities:
FIGURE 13 Schematic representation of a typical irrigation system and its environment (Kandiah 1990)
the area immediately below the project, which is a nature reserve, has become waterlogged and salinized due to the build-up of a shallow water table there caused by excessive on-farm deep-percolation and seepage of drainage water from the collector and disposal drains within the project;the stream into which the drainage from the project is discharged has become polluted with salts and agrochemicals to the point that is no longer suitable for drinking and other domestic purposes by a community in the downstream area;
the groundwater beneath the project has also become polluted because the subsurface drains do not fully intercept the downward flow of percolated water from the irrigated land. This drainage water is high in salts, nitrates, selenium, boron, pesticides and some other agrochemicals and is a potential health hazard to the people who are using the groundwater for domestic purposes;
the natural vegetation of the reserve land has undergone undesirable changes in its extent and composition caused by waterlogging and salinization of the area and, as a consequence, the wildlife population has been diminished and altered in its makeup;
the water birds which were attracted to the wetland habitat are dying due to selenium toxicity;
fishermen and hunters who have consumed the fish and game of this wetland and preserve are suffering chronic health problems due to excessive consumption of high selenium (and other trace elements);
the drainage canals and associated wetlands have become breeding sites for mosquitoes; as a result malaria outbreaks are occurring in the project area.
This hypothetical example, albeit an exaggerated one, illustrates the multitude of potential environmental, ecological, health and social problems that can and do sometimes arise as a result of improperly planned and managed irrigation and drainage systems. The use of saline waters for irrigation can either accentuate or help mitigate these problems. Most of the problems depicted in this hypothetical situation can be prevented or greatly minimized with proper design and operation of the irrigation and drainage systems. Implementing an appropriate means of disposing of the saline drainage effluent resulting from irrigation is very important in this regard.
There are at least four major environmentally-related potential hazards associated with irrigation in general and with the use of more saline waters in particular. They are: loss in soil productivity due to salinity and waterlogging, pollution of associated water resources with salts and toxicants by drainage, damage to the associated ecosystems and increased risk to public health resulting from water pollution and waterlogging.
Large and increasing proportions of the world's irrigated land are deleteriously affected by waterlogging and excessive salinity. While the exact area affected is not known, it is estimated that approximately 25 percent of the world's irrigated land is damaged by salinization (Postel 1989; see Table 33). Some claim that up to 50 percent of the world's irrigated land may be affected by salt (Adams and Hughes 1990). Certainly no continent is free from salt-affected soils (see Figure 14). Serious salt-related problems occur within the boundaries of at least seventy-five countries (Rhoades 1988b). Countries with notable salinity problems include Australia, China, Egypt, India, Iraq, Mexico, Pakistan, the republics of the ex-Soviet Union, Syria, Turkey, and USA.
FIGURE 14 Global distribution of salt-affected soils (after Szabolcs 1985)
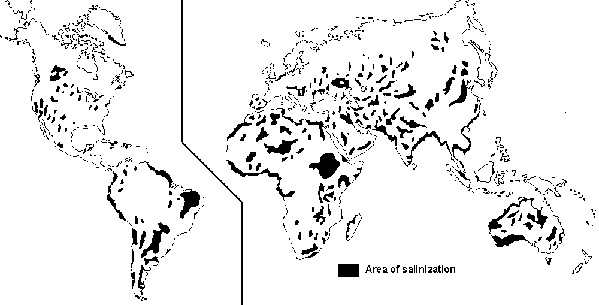
TABLE 33 Irrigated land damaged by salinization, top five irrigators and world estimate, mid-1980s (after Postel 1989)
|
Country |
Area damaged (million hectares) |
Share of irrigated land damaged (%) |
|
India |
20.0 |
36 |
|
China |
7.0 |
15 |
|
United States |
5.2 |
27 |
|
Pakistan |
3.2 |
20 |
|
ex-Soviet Union |
2.5 |
12 |
|
Total |
37.9 |
24 |
|
WORLD |
60.2 |
24 |
A close relationship exists between the depth and salinity of the shallow groundwaters, the soil hydraulic properties and the extent of salt accumulation in soils, especially in natural, semi-arid regions. The major saline regions of the world are generally found in semi-arid and arid and relatively low-lying, poorly drained lands. This is the result of the mobilization of large quantities of salts by excessive irrigation and leaching and the subsequent accumulation of the salt in localized areas with restricted drainage. Such areas are often found in lower-lying regions of the landscape where the water table is at or near the soil surface, and where the salts have ascended into the soil due to evaporation-driven processes. Restricted drainage may be due to low permeability of the fine-textured soils or to the presence of a shallow groundwater. Shallow groundwaters are often related to topographic position. The drainage of waters from the higher-elevation regions of valleys and basins may raise the water table in the lower-lying lands so that it is close (within 2 m) to the soil surface. Permeability of the soils is typically lower in these basin positions because of the higher content of alluvial clays generally found in basin soils, which impedes the downward movement of water and results in poor drainage. Many irrigation projects are located in these lower lying alluvial- and basin-position areas because of their favourable slopes (more level conditions) and closer proximities to easily accessible water supplies.
While salt-affected soils occur extensively under natural conditions, the salt problems of greatest importance to agriculture arise when previously productive cultivated soil becomes salinized as a result of irrigation (so-called secondary salinization). Human activities have modified (likely have increased) the extent of salt-affected areas considerably by the redistribution of water (hence salt) through irrigation. The development of large-scale irrigation projects, which involves diversions of rivers, construction of large reservoirs and the irrigation of large landscapes, causes large changes in the natural water and salt balances of entire hydrogeologic systems. The impact of irrigation often extends well beyond that of the immediate irrigated area; even neighbouring nations can be affected. Water infiltrated into the soil in excess of that used by the agricultural crops passes beyond the rootzone. This water often dissolves salts of geologic origin from the soils and underlying substrata and causes waterlogging in lower areas where it accumulates. When this occurs, soluble salts present in the ground are mobilized and transported to the lower areas where they accumulate and over time salinize the groundwaters and the soils in the areas where the water tables approach ground level.
The problems of waterlogging and secondary salinity prevalent in most irrigated lands have resulted from the excessive use of water for irrigation (resulting from inefficient irrigation systems, poor distribution systems and poor on-farm management practices), from inadequate and inappropriate drainage management, and from the discharge of "spent" drainage water into good-quality water supplies which are used elsewhere for crop production. It is not unusual to find that less than 60 percent of the water diverted for irrigation is used in crop transpiration (Jensen et al. 1990; Biswas 1990). It is important to note that these problems have occurred even where low-salinity waters have been used for irrigation. Thus it might be argued that the use of saline waters for irrigation can only increase these problems, since more salt will be added to the soils with such waters and relatively more leaching (hence drainage) is required in this case for salinity control of the rootzone. However, paradoxically, such need not be the case.
It should also be understood that some soil and water salination is inevitable with irrigation. Typical irrigation waters may contain from 0.1 to 4 kg of salts per m3 and are generally applied at annual rates of 1.0 to 1.5m. Thus, from 1 to 60 metric tonnes of salt per hectare may be added to irrigated soils annually. As discussed earlier, the salt contained in the irrigation water is left in the soil as the pure water passes back to the atmosphere through the processes of evaporation and plant transpiration. Therefore, water in excess of evapotranspiration must be applied with irrigation to achieve leaching and to prevent excess salt accumulation in the rootzone. This water must drain from the rootzone. Seepage from delivery canals occurs in many irrigation projects. These drainage and seepage waters typically percolate through the underlying strata (often dissolving additional salts in the process), flow to lower elevation lands or waters and frequently cause problems of waterlogging and salt-loading there. Saline soils are formed in such areas through the processes of evaporation. Ground- and surface-waters receiving these drainage and seepage waters typically are increased in salt concentration. Thus the problems of waterlogging and secondary salinization are related to inefficient irrigation and/or inadequate drainage.
The primary sources of drainage from an irrigation project are bypass water, canal seepage, deep percolation and surface (tailwater) runoff. Bypass water is often required to maintain hydraulic head and adequate flow through gravity-controlled canal systems. It is usually returned directly to the surface water supply and few pollutants, if any, are picked up in this route. Evaporation losses from canals commonly amount to only a small percentage of the diverted water. However, seepage from unlined canals is often substantial. Such seepage typically contributes significantly to high water tables, increases groundwater salinity and phreatophyte growth, and generally increases the amount of the required drainage (and its salinity) from irrigated areas. Biswas (1990) estimated that 57 percent of the total water diverted for irrigation in the world is lost from conveyance and distribution canals. If the water passes through salt-laden substrata or displaces saline groundwater, the salt pickup from these sources can be substantial.
From the above it is concluded that the majority of the soil degradation (salinity and waterlogging) problems related to irrigated agriculture occurring throughout the world are caused by inefficiencies in the distribution and application of irrigation water, the resulting mobilization and accumulation of excess water and salts in local regions related to hydrogeologic conditions and the return of saline drainage waters to fresh water supplies. The use of saline waters of the levels advocated herein should not result in excessively saline soils nor cause waterlogging with proper management. In fact, the interception of drainage waters percolating below rootzones and their reuse for irrigation should reduce the soil degradational processes associated with excessive deep percolation, salt mobilization, waterlogging and secondary salinization that typically operate in irrigated lands. It should also reduce the water pollution problems associated with drainage discharge to good-quality water supplies. An integrated irrigation and drainage management system for facilitating the use of saline drainage waters for irrigation, while minimizing the soil degradational and water pollution problems associated with drainage, is presented in Chapter 6.
The role of irrigated agriculture in soil salinization has been well recognized for hundreds of years. However, it is of relatively recent recognition that salinization of water resources from agricultural activities is a major and widespread phenomenon of possibly even greater concern to the sustainability of irrigation than is that of the salinization of soils, per se. Indeed, only in the past few years has it become apparent that trace toxic constituents, such as Se, Mo and As, in agricultural drainage waters may cause pollutional problems that threaten the continuation of irrigation in some projects.
As explained above, water infiltrated into the soil in excess of that used by the agricultural crops passes beyond the rootzone. This water, together with that deep percolating from canal seepage, often dissolves additional salts (over and above those present in the irrigation water) from the soils and underlying substrata. Such mobilized salts, when transported to receiving waters, are a source of pollution, as are the salts applied in the irrigation water which have become concentrated in the drainage water through evapotranspiration. These saline drainage waters pollute good-quality receiving waters when they are allowed to mingle with them. Addition potential sources of pollutants from irrigation are the agrochemicals (fertilizers and pesticides) applied to the soils which may also be, in part, mobilized (by leaching) and discharged in the drainage water.
Representative compositions of drainage waters leaving cropped rootzones at steady-state in a controlled lysimeter experiment when irrigated with a range of irrigation waters (see Table 34) are shown in Table 35 for three different leaching fractions. The salt loads of these irrigation (ViwCiw,) and drainage (VdwCdw) waters and their differences (VdwCdw - ViwCiw) are shown in Table 36. Note that the total salt-load discharged from the irrigated rootzone was reduced by about 2 to 12 metric tons/ha/year as the leaching fraction was reduced from 0.3 to 0.1.
The reduction in salt return shown in Table 36 is achieved in three ways. Less salt is discharged with reduced leaching because less irrigation water, and hence salt, is applied. The percent reduction in salt discharge due to reduced application is 100 (VH - VL)/VL, where V^ and V^ are volumes of irrigation water applied with high and low leaching, respectively. Reduced leaching reduces salt discharge still further because the fraction of applied salt that precipitates in the soil increases. A further benefit of reduced leaching is that fewer additional salts are picked up from the weathering and dissolution of soil minerals, because the through-put of drainage water is reduced and the "solvent" capacity of the more saline water is likewise reduced. The latter two benefits are demonstrated in Table 37 where the net effects of soil minerals weathering and dissolution (Sm) and salt precipitation (Sp), as determined in the lysimeter experiment, are given in terms of percentage of the salt load of the irrigation waters (Viw, Ciw,). These data show that weathering and dissolution are less and salt precipitation is greater as the leaching fraction decreases. They also serve to illustrate the following important points. As compared to high leaching, minimized leaching increases the concentration of the drainage water; it reduces the amount of salt added to the soil and discharged from irrigated root-zones because it maximizes the precipitation of applied Ca, HCO3 and SO4 salts as carbonates and gypsum minerals in the soil, and it minimizes the "pick-up" of weathered and dissolved salts from the soil.
TABLE 34 Compositions of river waters used for irrigation (after Rhoades et al. 1974)
* EC = Electrical conductivity (dS/m)
** SAR = Na+/[(Ca++ + Mg++)/2] ½, where all concentrations are expressed in mmolc/l.
* LF = leaching fraction** EC = electrical conductivity dS/m
*** SAR = Na+/[(Ca++ + Mg++) /]½, where all concentrations are expressed in mmolc/l.
* Total concentration (mmolc/l).** The difference in salt output in drainage water between that achieved with leaching fractions of 0.3 and 0.1 assuming a consumptive use requirement of 91 cm/year.
The experimental data of Tables 35 to 37 agree with those calculated using Watsuit (Oster and Rhoades 1975; 1990; Rhoades and Merrill 1976). Thus, it is concluded that salt precipitation and dissolution reactions of such minerals can be modelled and the compositions of a soil and drainage water can be adequately predicted for different irrigation waters and leaching fractions using this model. An example of the use of Watsuit for such purposes was given earlier (Tables 26 and 27).
TABLE 37 Net effect of LF on (Sm-Sp) for six representative river types expressed as percentage of salt input (from Rhoades et al. 1974; on mmolc/l basis)
|
River |
100 (Sm - Sp)/ViwCiw |
||
|
0.1 LF |
0.2 LF |
0.3 LF |
|
|
Feather |
+180 |
+271 |
+348 |
|
Missouri |
-9 |
+5 |
+13 |
|
Colorado |
-24 |
-3 |
+5 |
|
Salt |
-10 |
+6 |
+12 |
|
Sevier |
-25 |
-8 |
-3 |
|
Pecos |
-33 |
-21 |
-10 |
TABLE 38 Predicted effect of reduced leaching fraction on salt and water balance of the Wellton-Mohawk project1 (after Rhoades and Suarez 1977)
|
Item |
Unit |
High LF (0.42) |
Low LF (0.10) |
|
(Sm-Sp)2 |
% |
+8 |
-25 |
|
Viw |
m3 |
638 × 106 |
411 × 106 |
|
Vdw3 |
m3 |
286 x106 |
40.7 × 106 |
|
Salt load |
metric tons |
586 000 |
262 000 |
|
Concentration |
mg/l |
2170 |
6375 |
1 Colorado River water containing 158 metric tons of salt/100 m3 is applied annually to 26 305 ha to meet the estimated consumptive use of 370 × 106 m3.2 (Sm-Sp) is the net effect of mineral weathering or dissolution (Sm) and salt precipitation (Sp) on the salt load of the drainage water relative to that of the irrigation water (ViwCiw)
3 Viw and Vdw are volume of infiltrated irrigation and subsurface drainage water, respectively.
The preceding data clearly demonstrate that decreasing the leaching fraction can significantly decrease the volume and the salt load of drainage waters discharged from rootzones. Where the drainage waters can be intercepted before being returned to surface or groundwater bodies, such reductions are of substantial benefit when they are to be treated to prevent water pollution. Illustrative of such a situation is the Wellton-Mohawk Project in Arizona where the drainage water is collected by pumps and conveyed in discharge canals to a plant for desalinization (see Table 38). With reduced leaching, water diversion into the project can be reduced by 227 × 106 m3, salt return can be reduced by 324 000 metric tons, drainage return-flow can be reduced by 227 × 106 m3, and the drainage water can be concentrated to the point that it would have nearly no remaining value for irrigation.
Minimizing leaching may, or may not, reduce salinity degradation of the receiving water where the drainage water is returned to a surface or groundwater. A reduction of degradation will generally always occur where saline groundwaters with concentrations in excess of those of the recharging rootzone drainage waters are displaced into the receiving water or where additional salts, other than those derived from the irrigation water per se, are encountered and mobilized in the drainage flow-path and brought into solution by weathering and dissolution processes. An example is the Colorado River through Grand Valley, USA. Here, minimizing leaching reduces the salt load in the river downstream of the project by reducing the "pick-up" of geologic salts as the drainage water percolates past the rootzone and displaces highly saline groundwater present in the underlying cobble aquifer into the river, as illustrated in Table 39. The salinity of the Colorado River is increased by 13% (56 mg/l) and its salt load by 541 000 metric tons by irrigation and drainage processes associated with high leaching. For conditions like these, reduced leaching will always reduce the salinity of the river downstream from the project. Similar results will also occur under conditions where the irrigated soils, or underlying substrata, contain gypsum or other forms of mineral salts.
TABLE 39 Effect of reduced leaching on river salinity where highly saline groundwater of independent and constant salt composition is displaced into the river with low and high leaching, simulating Grand Valley, Colorado, conditions (after Rhoades and Suarez 1977)
|
Water |
Composition of water in mmolc/l |
||||||
|
Ca |
Mg |
Na |
K |
CI |
alkalinity |
SO4 |
|
|
Colorado River upstream1 |
2.59 |
0.96 |
2.49 |
0.06 |
1.91 |
2.31 |
1.88 |
|
Groundwater2 |
23.1 |
42.8 |
30.0 |
0.41 |
15.6 |
10.7 |
70.3 |
|
Colorado River downstream (low leaching) |
2.63 |
1.05 |
2.55 |
0.06 |
1.94 |
2.33 |
2.03 |
|
Colorado River downstream (high leaching) |
2.79 |
1.49 |
2.84 |
0.06 |
2.08 |
2.35 |
2.75 |
1 Upstream of irrigation diversion point.
2 In aquifer hydraulically connected to Colorado River.
The above example illustrates well that it is the excess diversion of water for irrigation, concentration of part of this water through evapotranspiration, deep percolation of the concentrated drainage water, mobilization of additional "geologic" salts and return of such waters to surface waters that cause the increase in downstream salinity (pollution) that typifies most river systems used for irrigation and drainage in the world.
For situations where no salts of geologic origin exist in the soils or substrata, the composition of the deeply percolating drainage water is little changed from that leaving the rootzone. For such cases, the composition of the mingled drainage plus receiving water may be the same regardless of leaching fraction, depending upon the saturation status of the receiving water with respect to calcium carbonate and gypsum and fate of water "saved" by reduced leaching. Such cases are more rare than the one described above for the Upper Colorado River; however, the Lower Colorado River is such a case where the "saved" water is passed on downstream and dilutes the returned salts to the same degree regardless of leaching.
As with river systems, degradation of groundwaters receiving irrigation drainage may or may not be benefited by reduced leaching, depending on the hydrogeologic situation. With no sources of recharge other than drainage return flow, the groundwater eventually tends toward the composition of the drainage water, which will be more saline with low leaching. However, reduced leaching slows the arrival time of the leachate. Thus the groundwater salinity will generally be lower for an interim period of time with reduced leaching (Suarez and van Genuchten 1981). Low leaching management can continuously reduce degradation of the groundwater only if other sources of high-quality recharge into the basin exist and if flow out of the basin is high relative to drainage inflow. For more discussion of the effect of drainage management on groundwater pollution see Rhoades and Suarez (1977).
Agricultural drainage is sometimes intentionally returned to common water supplies in order to conserve water and increase water use efficiency. Water quality agencies often deal with agricultural drainage pollution problems by setting allowable concentrations of total salts and specific solutes in the waters that are returned to the water supply system and by blending or diluting the drainage waters with a good-quality water so that the concentration of total salt (or of a specific solute) in the blend does not exceed a value (the so-called safe limit) that is deemed allowable in the water supply. Such practices may be shortsighted, since they do not consider the potential deleterious effect that the discharge of agricultural drainage water to surface and groundwater supplies and such blending - whether it is natural or intentional - can have upon the usability of the total - and the receiving water supplies. The blending process often reduces the maximum practical benefit that can be derived from the total water supply. The return of saline waters to the water supply, even when sufficient dilution occurs to keep the salinity of the mixture within apparently safe limits, reduces the quantity of the total water supply that can be used in consumptive processes which are limited by salt concentration, such as the growth of salt-sensitive crops.
Few data exist on the degree of degradation of associated ecosystems which can be caused by irrigation, especially with saline waters. This deficiency is due to both the lack of effort that has been made to acquire such information for vast areas of the world and the incomplete understanding of how many of the ecological systems are affected by waterlogging and salinity. The task is made more difficult by the absence of a practical means to monitor changes in large irrigated landscapes systems and associated environments in response to developmental factors.
The hypothetical example used to introduce this chapter illustrated some of the ways irrigation and drainage can effect wildlife habit, biological diversity and in-stream use of surface water systems. A real example may serve even better to demonstrate the profound effects irrigation and drainage, especially the effects of saline drainage water disposal, may have upon ecological systems and, in turn, their impact on entire irrigation projects. An example of such a mutual dilemma is the Westside area of the San Joaquin Valley of California and the Kesterson Wildlife Refuge, as summarized by San Joaquin Valley Drainage Program (1990).
Before development, the native habitat of the San Joaquin Valley (this area is the heart of the 4.7 million acres (1.9 million hectares) of irrigated land in California, USA) was a lush patchwork of aquatic wetland, riparian forest and valley savannah and it teemed with an abundance and diversity of fish and wildlife found nowhere else in the USA. Grizzly bear, elk, antelope, deer, wolves, quail, geese and a multitude of species of migratory birds, especially waterfowl and shorebirds, populated the Valley. The streams and rivers abounded with trout, salmon and steelhead. Now after about one hundred and fifty years of settlement and the development of irrigated agriculture in the Valley, the quantity and quality of the ecology has been markedly altered. Dams now block most of the major streams to anadromous fish. Impoundments and diversions of the streams for irrigation have depleted the streams of most of their flow, while lack of recharge and discharges of drainage waters to them have increased the salt concentrations of the remaining flow. The change in habitat has been immense. The Central Valley of California has lost, mostly to agriculture, over 91 percent of its original 4 million plus acres (1.62 million hectares) of marsh land. The two major inland lakes (Tulare and Buena Vista) which were once the largest freshwater lakes in the western USA are now farmland. In the San Francisco Bay, which was the outlet for the San Joaquin River and most of the Valley's streams, the water surface has been reduced by 41 percent. Riparian wetlands have been reduced statewide to less than 2% of their original area.
As a consequence of these changes in land use, tremendous losses in native habitat have occurred. Fish and wildlife populations are a fraction of what they were originally. Still substantial populations (about 7-8 million ducks and geese) winter in the Valley. However, where once they found about 105 300 hectares of marsh, they now find only 2025 hectares. Where once they could land on 243 000 hectares of freshwater lakes, they now find only 2835 hectares of saline evaporation ponds.
These drastic reductions in the area of native habitat have resulted in population declines in a number of species and plants endemic to the Valley. Several Valley species have become extinct and others are listed as endangered by the Federal or State Governments. Even though irrigated agriculture has nearly completely altered the original ecology and diversity of the San Joaquin Valley, a new ecological concern has recently emerged to threaten the very existence of continued irrigation in a substantial fraction of the San Joaquin Valley. Because of the occurrence of waterlogging and a lack of a final outlet for drainage water disposal in much of the San Joaquin Valley, evaporation ponds were created as local outlets for "waste" disposal from irrigation. One such pond (the so-called Kesterson Reservoir) was constructed in 1975 to operate as a storage and flow regulating facility as part of a proposed drainage canal planned to discharge ultimately to the San Francisco Bay and to serve simultaneously as a wildlife refuge. Because of concerns about potential environmental impacts (nitrates and pesticides, primarily) of the disposal of this agricultural drainage on the Bay, construction of the canal ceased in 1978 and the Kesterson Reservoir (486 hectares) became the terminus of the drainage canal serving 3240 hectares of irrigated land and, effectively, an evaporation pond. At Kesterson, contaminants in the drainage water, specifically selenium at about 35 parts per billion, built up in the food chain, accumulated in the fish and birds using the "pond" and manifested itself by 1982 in gross deformities, reproduction failures and deaths of waterfowl. As a result, in 1985 the Kesterson Reservoir was closed to drainage and the drainage outlets from the source, the Westland Irrigation District, were sealed. Some 2800 hectares of additional evaporation ponds exist in the Valley and another 11 300 hectares are under consideration. However, because of the concerns about the effects of these ponds on the waterfowl, their future is in doubt.
Based on levels of selenium found in a survey of fish and wildlife in the regions of the ponds, health warnings have been issued to avoid or restrict consumption of wild plants, fish and/or wildlife from several areas of the San Joaquin Valley.
Numerous studies and considerable funds have been dedicated to finding a feasible and acceptable solution to the mutual dilemma of finding a means of drainage water disposal from the irrigated lands of the San Joaquin Valley and of sustaining the 320 000 hectares of irrigated land now being threatened by waterlogging and salinity while simultaneously protecting the water quality of the surface and groundwaters, and remaining associated ecological habitats (largely wildlife refuges) of the region.
This example illustrates the new concern about the environment and ecology that is developing worldwide and the new more holistic approach that must be undertaken to balance developmental, environmental and ecological needs. In the case of the San Joaquin Valley "drainage" problem, the approach being undertaken involves a series of programmes. Firstly, source control through the implementation of more efficient irrigation systems and practices are being undertaken to conserve water and reduce deep percolation. Reuse of the unavoidable drainage waters through a succession of crops of increasing salt tolerance, including eucalyptus and halophyte species, is also being implemented so as to reduce drainage water volumes and conserve water, while producing useful biomass. Conjunctive use of saline groundwater and surface water is being considered to aid in lowering water tables, hence reducing drainage disposal need, and conserving water. Treatment of drainage water and various means of ultimate disposal of the unusable final drainage effluent through deep aquifer injection and ecologically safe evaporation ponds and its release during high stream-flow periods are also under consideration. Lastly, release of freshwater supplies to refuge areas and the retirement of irrigated land deemed the major source of the pollutional problems are also being considered. All of these so-called "in-valley" solutions are being put ahead of the construction of a master drain and ocean disposal in keeping with the philosophy of dealing with the problem at the source and in making the "polluters" pay the costs of pollution that they cause rather than allowing them to discharge their wastes at the expense of others (people, environments and ecological systems).
For more details on the drainage problems and solutions underway in the San Joaquin Valley see Letey et al. (1986), and the books edited by Dinar and San Joaquin Valley Drainage Program (1990), Dinar and Zilberman (1991) and the National Research Council (1989).
California is not the only place which has suffered from ecological effects of irrigation. Each year some 3300 km3 of water are removed from the earth's rivers, streams, and groundwater systems to irrigate crops (Postel 1989). Such diversion and redistribution of water has had a profound impact on the earths ecology. Much wetland habitat has been lost due to reduced river and stream flows, surface water supplies have become contaminated with salts and agri-chemicals, groundwater aquifers have been depleted and overlying lands have subsided due to excessive extraction, and fish and fowl have been poisoned by toxic salts released through irrigation and drainage (Postel 1989). The Aral Sea in the central Asian republics of the ex-Soviet Union is another good example. Fully 95 percent of the ex-Soviet Union Republics' cotton harvest is grown in this region, as well as a third of the country's fruits, a quarter of its vegetables and 40 percent of its rice. Ninety percent of these croplands are irrigated. By 1950, the flows of the rivers (Amu Dar'ya and Syr Dar'ya) replenishing the Aral Sea had been reduced to a trickle, the Sea volume reduced by two-thirds and its salinity increased threefold. All native fish species have disappeared. Winds pick up salt from the dry seabed and annually dump 43 million tons on surrounding cropland. The outlook for the Aral Sea and its associated ecology is bleak. Such visible damage from large-scale irrigation has spawned strong opposition to new dams and diversion projects, even in developing countries where irrigation development remains a high priority (Postel 1989).
These problems along with the loss of free-flowing rivers, the destruction of fisheries and damage to riverine and other wildlife habitat must be recognized. Efforts to restore and protect natural ecosystems may require the shifting of some water away from agriculture. The implementation of management practices to conserve water, to reduce deep percolation and the disposal of drainage wastes into good water supplies will go a long way towards sustaining ecology. The reuse of drainage water and the use of saline waters for irrigation will aid appreciably in these matters.
The above examples illustrate the ecological problems and mitigation costs and complexities associated with irrigation and drainage and the potential benefit that the use of saline drainage waters can have as part of the solution to the disposal issue.
Irrigation creates an environment that is conducive to the breeding of many vectors of water-borne diseases. Vectors are organisms which transport pathogens from one person (or animal) to another and also provide within themselves an environment for the pathogen to complete part of its life-cycle. The long and unfortunate record of increases in diseases, which are associated with water development in general and irrigation in particular, demonstrates the increased disease vulnerability of a region following the establishment of irrigation schemes. While there is agreement on the potential water-borne disease hazards associated with irrigation developments, it is important to recognize the complementarity of health and irrigation development. Improved nutrition, provision of a good and adequate water supply for domestic use, rural infrastructure, and housing and health facilities, which many irrigation projects bring to rural communities, contribute significantly to good health. Many of the health hazards associated with irrigation development could well be eliminated if the development is approached in a well-planned and integrated manner and environmental management measures are incorporated in the design and management of irrigation projects to safeguard the populations from health hazards.
In this publication, discussion is limited to two important vector transmitted water-borne diseases, mainly malaria and schistosomiasis and their relationships to water quality.
Malaria is by far the most important. At the global level more than two thousand million people are estimated to be at risk; some 240 million are estimated actually to carry the parasite at any given time, and annually an estimated 100 million cases of clinical illness resulting from the infection take place. Vectors of malaria are mosquitoes belonging to the genus Anopheles which generally speaking require stagnant or slow-flowing, clean fresh water for their larval development. Exceptionally some species breed by preference in organically polluted or in brackish water.
Schistosomiasis (bilharziasis) is endemic in 76 countries, where about 200 million people are infected with the schistosome parasites. Perhaps more than malaria, which has a rather patchy distribution over time and space, schistosomiasis is generally perceived as directly linked to irrigation schemes and other water resources development projects. The intermediate hosts of the schistosome parasites are aquatic or amphibious freshwater snails with a remarkable tolerance to a number of environmental parameters, but particularly thriving in waters infested by aquatic weeds (which they use as a substrate) and with organic matter.
Physical, chemical and biological parameters of water quality may all influence the suitability of certain water bodies for mosquito and snail breeding. In theory, possible physical parameters include temperature, clarity, viscosity, conductivity, surface tension and, though perhaps not really a physical quality, water current speed. Chemical parameters include the concentrations of various anions and cations, overall salt concentration, pH and the concentration of synthetic compounds. Biological parameters include organic matter, bacterial/fungal/algal contamination of aquatic weeds. Any of the abiotic water quality factors may also indirectly affect vector breeding by favouring certain types of aquatic vegetation (Bos 1991).
As a rule of thumb. Anopheles mosquitoes breed in fairly clear, and oxygen rich water. Turbidity, due to organic pollution, results in a diminished light penetration, and at a certain depth anaerobic processes may take over. This, together with eutrophication will considerably lower the oxygen pressure and make the water unsuitable for anopheline breeding. Nevertheless, there are a number of exceptions: A. kochi, A. vagus, A. barbirostris, A. gambiae and A. pharoensis are all rice field associated mosquitoes that have been observed to breed in turbid water (Lacey and Lacey 1990). For A. stephensi in India and A. arabiensis in Nigeria similar observations have been made in other habitats (WHO 1982).
The ionic composition and overall salt concentration of water bodies is a crucial chemical parameter for mosquito vectors of malaria. Most anophelines prefer fresh water, but there are some notable exceptions of species with a preference for brackish water: Anopheles sundaicus (in South and South East Asia) and A. aquasalis (in South America).
There are some notorious malaria epidemics related to sudden changes in salt concentrations in water bodies. An outbreak in the Indonesian village of Brengkok (East Java) in 1933 was attributed to a combination of saline soils and a year with exceptionally low rainfall. The normally rainfed cultivated fields were left fallow and because of the lack of rain the pools turned brackish. This led, in turn, to a population explosion of the malaria vector and an outbreak of malaria (Snellen 1988).
Tidal changes and seasonally varying flow volumes of rivers result in fluctuating salt concentrations in coastal lagoons. This may give rise to seasonal malaria outbreaks, either because one of the brackish water breeding mosquitoes is favoured when salt concentrations are high, or because a freshwater species is temporarily favoured when they are low (e.g. Anopheles albimanus in coastal lagoons in El Salvador).
Water chemistry may also have an indirect effect on mosquito populations, when it favours organisms on which larvae feed, or when it affects potential biological control agents of mosquitoes. A study by Pitcairn et al. (1987) showed that in Californian rice fields hard (calcium-rich) water favoured the growth of a macrophytic alga, Chara, whose presence is positively correlated with the abundance of Anopheles freeborni and Culex tarsalis larvae.
Mather (FAO 1985) reported that water quality factors may intensify a vector problem or create physical conditions resulting in the problem. He summarized four ways in which water quality may affect the size and species composition of disease vectors and nuisance insects:
· by creating soil conditions which extend water surfaces in area or in duration;· by requiring irrigation practices which result in the extension of water surfaces in area and duration;
· by modification of aquatic flora and fauna;
· by direct influence on the vector.
In many irrigation schemes, lack of or inadequate surface drainage was found to be a major cause of vector multiplication. Badly constructed drains, as well as poorly maintained ones, create ideal breeding conditions for mosquitoes and aquatic snails. Adoption of good irrigation water management practices and appropriate environmental management measures such as efficient water conveyance, proper irrigation scheduling, improved on-farm irrigation methods, and unimpeded drainage result in a minimum of unnecessary water surface and standing water and thus provide little opportunity for breeding of vectors. In conclusion, it may be said that proper use of saline water for crop production is not likely to contribute any significant increase in the incidence of water-borne diseases.
The ultimate objective of water quality protection should be to permit the maximum practical benefit (use) to be derived from the available water supply. Broadly speaking, users of a water supply may be classified into two groups: those who consume the water in the process of use, and those who use it without appreciable consumption. The first type of users will suffer disbenefit in the "blending" philosophy of water quality protection.
The purpose of this section is to provide evidence - theoretical and conceptual - that the blending approach typically used for water quality enhancement and protection is often deficient for these purposes and to offer an alternative approach for dealing with the "disposal" of saline drainage waters - one that provides a greater practical benefit from the total water supply than blending does.
In considering the use of a saline water for irrigation and in selecting appropriate drainage management to protect water quality, it is important to recognize that the total volume of a saline water supply cannot be beneficially consumed for irrigation and crop production (transpired); the greater its salinity, the less it can be consumed before the concentration becomes limiting. Plants must have access to water of a quality that permits consumption without the concentration of salts (individually or totally) becoming excessive for adequate growth. In the process of transpiration, plants essentially separate nearly pure water from the salt solutions present in the rootzone and these salts are concentrated in the remaining unused soil water. This water ultimately becomes drainage water. A plant will not grow properly when the salt concentration in the soil water exceeds some limit specific to it under the given conditions of climate and management (Bernstein 1975). Thus, it is obvious that not all of the water in a supply can be consumed by a plant, if the water contains salt. The practice of blending or diluting excessively saline waters with good quality water supplies should only be undertaken after consideration is given to how it affects the volumes of consumable water in the combined and separate supplies.
Three case examples are given to illustrate some of the preceding conclusions. In these examples, the factor limiting crop growth is assumed to be the presence of excessive total dissolved salts, but an analogous case could also be made for boron or any other constituent that is specifically toxic to plants. Calculations of the salinity of the soil water resulting within the rootzone were made from knowledge of the salinity of the irrigation water (ECiw,) and leaching fraction (LF) using the non-computer version of Watsuit. The leaching requirement, Lr, was taken to be that value of LF needed to keep the average salinity of the rootzone from exceeding the threshold tolerance level of the crop (the maximum level that the crop can tolerate without loss of yield, ECe; a higher value could be used, if some loss of yield can be tolerated). Relative crop yield was calculated from the predicted average soil water salinity, knowledge of the plant tolerance to salinity and the assumption that crops respond to the average salinity within their rootzone. The values of ECe used were those given in the crop tolerance tables (9 and 10). The fraction of the irrigation water that was consumed in evapotranspiration without yield loss was determined by Vet/Viw, which was calculated from Lr, using the following relation:
Vet/Viw = (1-Lr (9)
In the case examples, the volumes of Vet, were normalized by expressing them relative to Vet, i.e. for the case where Vet is taken to be equal to 1.
Case 1
The conditions: use of a "good-quality" water of ECiw, = 0.5 dS/m for the irrigation of beans (ECe = 1.0 dS/m).
This water is judged suitable for the irrigation of beans, since the product (ECiw,) (Fc) is less than ECe at practical levels of leaching. For example, the predicted level of average salinity within the rootzone resulting from long-term irrigation with this water supply at LF = 0.15 is only 0.75 dS/m (0.5 dS/m × 1.51; the value 1.51 was obtained from Table 27). Beans can tolerate a soil salinity of ECe = 1.0 dS/m without any loss in yield using conventional irrigation management (Table 10). The leaching requirement for this case, as obtained from Figure 8 or 12, is even lower, i.e. 0.09. If beans were irrigated at this latter most-efficient level of leaching, the EC of the drainage water (ECiw) resulting from irrigation would be 5.55 dS/m (0.5/0.09; ECiw /LF). Obviously this latter drainage water could not be used again to grow beans, since the resulting average rootzone salinity could not be kept within acceptable limits at any reasonable level of LF.
Case 2
The conditions: use of the saline drainage water of EC = 5.55 dS/m, as obtained in case 1, for the irrigation of cotton (ECe = 7.7 dS/m).
This water which was judged unsuitable for growing beans (see case 1), is quite acceptable for growing cotton, since the predicted level of average rootzone salinity resulting from its use for irrigation is less than the ECe value of cotton at practical levels of leaching. For example, the average ECe will be less than ECe for any value of LF in excess of 0.17 (see Figure 12 for the case of F'c = 7.7/5.5). When irrigated at LF = 0.17, ECdw Will be 7.7 dS/m and ECiw will be 32 dS/m (5.5/0.17).
Thus it is apparent that the saline drainage water of EC = 5.55 dS/m (that resulted from the irrigation of beans with the "good quality" water) could be used satisfactorily to grow salt-tolerant crops like cotton, barley, sugarbeets, etc. It is also true that the drainage volume needing ultimate disposal from the irrigated area would be greatly reduced through its reuse for irrigation within the area. In this case the percent reduction in volume of drainage water ultimately needing to be discharged from the area is 83 (100 - 17; this value can also be calculated using Equation 10, i.e. 1 - 5.55/32). The secondary saline drainage water of EC = 32 dS/m that resulted from the irrigation of cotton obviously cannot be used again to grow more cotton (or sugarbeets, etc.), since excessive yield losses would result. But this water is in a favorable condition for disposal or desalting, i.e. it is in a relatively small volume and at a relatively high salt-concentration.
Case 3
The conditions: use of a blend of the "good quality" water (EC = 0.5 dS/m) and the secondary saline drainage water (EC = 32 dS/m) achieved in case 2 from the irrigation of cotton with "bean" drainage water. The blend is made up of 40 units of the "good quality" water and 1 unit of the very saline drainage water; the ECiw, of this blend is 1.5 dS/m.
This blended water could be used to grow beans without yield loss since the predicted resulting level of average rootzone salinity can be kept less than ECe (1.0 dS/m), but only by irrigating at a very high and generally impractical level of leaching (Lr,. = 0.6, as obtained from Figure 12). However, the process of blending has reduced the volume of water in the total supply that can be used by the bean crop (or any other salt-sensitive crop) for transpiration, as shown in the following paragraphs.
The relative volume of irrigation water required to meet ET and to achieve Lr in this case is 2.500 units (1/1-L,.). Of this volume, 1.500 units will pass through the rootzone to become drainage water (Vdw = Viw, - Vet). Of the- 2.500 units of blended irrigation water, 2.439 units (40/41 × 2.500) consist of the "good-quality" water of EC = 0.5 dS/m and 0.061 units (1/41 × 2.500) consist of the secondary saline drainage water of EC = 32 dS/m. Thus, at best, only 0.061 units of the 1.50 units of volume of the drainage water that resulted from irrigating this bean crop with the blended water could possibly have come from the drainage water that was put into this blend. Therefore, the rest (i.e. 1.439 units) must have come from the "good-quality" water component of the blend. This amount of drainage water is much higher than that for the case where only the "good-quality" water of EC = 0.5 dS/m was used to grow the beans (see case 1, where Lr was 0.09, Viw, was 1.099 units, and Vdw was 0.099 units). A comparison of the results of cases 1 and 2 shows that 127 percent more of the "good-quality" water had to be used to irrigate the bean crop when it was used in the blend (1.401 units more; 2.50 versus 1.099 units) compared to when it was used solely. This is so because 1.401 units of the good-quality water was made unavailable for transpiration by the bean crop without loss in yield, through the blending process. Also as a result of blending, the volume of required drainage was increased substantially (1.500 versus 0.099 units). Such excessive drainage may cause other problems, such as increase in area affected by waterlogging in the project, in the loss of nutrients through excessive leaching, etc.
Another way to illustrate that a loss of usable water in the total supply has occurred as a consequence of this blending is to contrast the relative fraction of the "good-quality" water supply that could be used to grow beans (i.e. could be used for transpiration) with and without blending. For this purpose, assume that the volume of the good-quality water of EC = 0.5 dS/m is 100 units. Without blending all but 9 units, i.e. 91 units, ((100 - Vdw, or (100) - (100) (.09)) can be consumed in ET. However, when saline drainage water of EC = 32 dS/m is blended with this 100 units of "good-quality" water in the ratio of 40 to 1 to give a larger total supply of 102.5 units (for which Lr. is 0.6 and Vdw is 61.5 units), only 41 units (102.5 - 61.5) are usable for ET by beans without loss of yield. Thus, 50 units (91 - 41) of the original 100 units of "good-quality" water were made unusable for the production of beans by adding saline water of EC = 32 dS/m to it in the ratio of 1: 40.
The results of these case-studies clearly show that adding saline waters to good quality water supplies can reduce the volume of the good-quality water supply that could be consumed by salt-sensitive crops. The amount of such reduction will depend upon the relative volumes and concentrations of the receiving and waste waters and upon the tolerances of the crops to be irrigated. The significance of such losses of usable water through blending will depend upon a number of factors, especially upon the salt sensitivity of the crop to be grown with the blended water and the relative concentrations and volumes of the drainage and receiving waters. Therefore the merits of blending should be evaluated on a case-by-case basis. The case of a hypothetical river system receiving drainage return is discussed elsewhere (Rhoades 1989; Rhoades and Dinar 1990). This case study showed that the pollution of rivers that occurs through the return of drainage waters can be avoided by intercepting the drainage return flows, reusing them for irrigation and isolating the ultimate unusable drainage from any good quality water supply.
In the previously discussed case studies, it was assumed that the fraction of water usable for crop production was limited by ECe. Obviously, more water use can be achieved, if some loss of yield is permitted. When the growth-limiting factor is salinity, the ultimate fraction of water in a supply that can be used in crop growth is:
Fraction of water used in crop growth 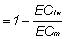 (10)
(10)
where ECiw, is the electrical conductivity (concentration can be used alternatively) of the water supply and ECiw is the maximum electrical conductivity (concentration, etc.) of the water in the rootzone (on a soil water basis; essentially ECdw) the plant can tolerate (i.e. draw water from and still yield about 85 - 100 percent). Values of ECm vary among the crop species, but typically they are (according to Bernstein 1975) about 45 for such tolerant crops as cotton, sugarbeets, barley, 30 for intermediate crops like, tomatoes, wheat and alfalfa, and about 15 for sensitive crops, like beans, clovers and onions. In some cases, it may make economic sense to blend and to bear the consequences of the losses of water usability and of crop yield when the alternative costs of disposal are much more costly.
Sometimes drainage waters are purposely diluted with a "good-quality" water to meet some specified discharge standard (say an EC of 1.5 dS/m, as resulted in case 3) and then returned to a "good-quality" water supply. For example (as in case 3), 1 unit of drainage water of EC = 32 dS/m could be blended with 40 units of water of EC = 0.5 dS/m and then the 41 units of blended water of EC = 1.5 dS/m returned to the major water supply of good quality. But as the above-described results showed, even when such a relatively small volume of such blended water is incorporated into the larger "good-quality" water supply, the net result is that a fraction of this latter water is made unusable for transpiration by salt-sensitive crops (such as beans) without loss of yield. In the case described above, 50 units out of every 100 units in the large supply will be made unusable for each 1 unit of drainage volume added to it. Thus it is concluded that blending or diluting drainage waters with good quality waters in order to increase water supplies or to meet discharge standards may be inappropriate under certain situations. Even though the concentration of the blend may appear to be low enough to be acceptable by conventional standards, the usability of the good-quality water supply for growing salt-sensitive crops (or for other salt-sensitive water uses) may be reduced through the process of blending. Each time the salt content of an agricultural water supply is increased, the degree to which it can be consumed before its concentration becomes excessive and limiting is decreased. More crop production can usually be achieved from the total water supply by keeping the water components separated. Serious consideration should be given to keeping saline drainage waters separate from the "good-quality" water supplies, especially when the latter waters are to be used for irrigation of salt-sensitive crops. The saline drainage waters can be used more effectively by substituting them for "good-quality" water to irrigate certain crops grown in the rotation after seedling establishment. Reuse of drainage water for irrigation of suitably salt-tolerant crops reduces the volume of drainage water needing ultimate disposal and the off-site pollution problems often associated with the discharge of irrigation return flows.