Trypanosomiasis - The clinical picture
Requirements for diagnosis of trypanosomiasis
Concluding remarks
Bibliography
A.G. LuckinsDr A. G. Luckins is Senior Research Fellow, Centre for Tropical Veterinary
Medicine, East Bush, Roslin, Midlothian EH25 9RG, UK.
Pathogenic trypanosomes cause disease in all species of domesticated livestock throughout many of the tropical and subtropical regions of the world. In Africa, Trypanosoma brucei, T. vivax and T. congolense occur wherever the tsetse fly vector is found. T. evansi, which is transmitted mechanically by several different species of haemotophagous biting flies, is found in North Africa, the Near East, the Far East and Central and South America. T. vivax also occurs in South America where it, too, is transmitted mechanically by biting flies. The clinical signs of disease caused by these organisms vary according to the trypanosome species, the virulence of the particular isolate and the species of host infected. Acute disease is characterized by anaemia, weight loss, abortion and, if not treated, possibly death. Animals that survive are often infertile and of low productivity. In some instances, infected animals show no overt signs of disease but can succumb if stressed, for example, by work, pregnancy, milking or adverse environmental conditions (Lucking, 1988).
A disease may be diagnosed on the basis of the clinical signs and symptoms, by demonstration of the causative organism or by reactions to diagnostic tests. In some situations, the clinical manifestations of trypanosomiasis, particularly anaemia, when taken into consideration with ecological conditions, might provide sufficient grounds for a putative diagnosis. However, the clinical signs are so varied and the ecological conditions under which trypanosomiasis occurs so diverse that, in terms of identifying animals with active infections, clinical diagnosis is too imprecise a procedure to use as a basis for the control of trypanosomiasis, and other means of diagnosis must be employed. In the following account, diagnosis refers to methods for detecting infection, either by identifying the parasites themselves or by interpretation based on the results of immunological tests.
The type of diagnostic test used in the detection of infections caused by the animal trypanosomiases will vary according to the epidemiological characteristics of the disease and the strategy for control. Where tsetse-transmitted trypanosomiases occur and where disease prevalence is high, even tests of low diagnostic sensitivity will suffice if chemotherapy or chemoprophylaxis is administered on a herd basis. However, in many situations where mechanically transmitted trypanosomiasis is found, drugs are often administered therapeutically to individual infected animals and it is essential that more sensitive diagnostic tests are used in order to detect active infections.
Similar considerations also apply after control campaigns. As the disease prevalence declines, the need for individual treatment as opposed to block treatment becomes an important issue. When chemotherapy has been applied in areas where drug resistance is known to exist, it is also necessary to detect rapidly any failure in treatment. In many countries where T. evansi causes disease, little is known about its distribution and the prevalence of infection. Thus, before embarking on a control campaign, epidemiological surveys need to be undertaken using appropriate diagnostic methods to determine the extent of the problem (Lucking, 1988).
Whatever the requirements of the particular situation, the tests themselves need to fulfil a number of criteria to be of practical use. Such criteria include high diagnostic specificity and sensitivity, easy reproducibility, simplicity, economy and ease of interpretation. Ideally, the tests should be able to be used in the field. To improve diagnostic efficiency, many parasitological and serological techniques have been developed. These techniques have been reviewed extensively (Molyneux, 1975; Nantulya, 1990) and comprehensive accounts of the various diagnostic procedures can be found therein. The present article seeks to highlight the problems in interpretation of current diagnostic tests which are still likely to be of practical value when developing an overall trypanosomiasis control strategy that requires an accurate assessment of the infected livestock population.
Parasitological diagnosis
Examination of the blood by light microscopy is the most readily applied method for diagnosis of trypanosomiasis and, more importantly, is a technique which can be easily applied in the field. The basic technique, i.e. examination of fresh or stained blood films, has been modified to improve diagnostic sensitivity by concentrating the blood through centrifugation in a haematocrit tube, namely the haematocrit centrifuge technique (HCT) or the dark ground buffy coat technique (DG) (Paris, Murray and McOdimba, 1982).
Other modifications suggested, but not widely applied, include the separation or removal of blood cells prior to centrifugation by anion exchange chromatography or hypotonic lysis (Nantulya, 1990). Freshly collected blood can also be inoculated into laboratory rodents which can then be examined for periods of 30 to 60 days to determine if they have developed trypanosome infections. The evaluation of some of these techniques under experimental conditions has given an indication of their detection limits in relation to the numbers of different species of trypanosomes in a blood sample. In order of decreasing sensitivity, the results were as follows: DG>HCT>thick film>thin film>wet film (Paris, Murray and McOdimba, 1982). Even using the most sensitive technique there were differences among the tsetse-transmitted trypanosome in the lowest number of organisms detected. Using the DG technique, the lowest numbers of trypanosomes that could be detected were 2.5 x 102 T. congolense, 5 x 102 T. vivax and 5 x 103 T. brucei (Paris, Murray and McOdimba, 1982). Similar differences were shown using other procedures.
In the field, practical considerations determine which technique can be used, i.e. remoteness of location could prevent the maintenance of rodents while a lack of generators and centrifuges may preclude the HCT and DG techniques. However, where a number of different diagnostic procedures have been applied in field surveys, their various limitations can be seen.
Microscopic examination of blood detects only a low proportion of T. brucei, as few as 5 percent in the case of a study in the Lambwe Valley in Kenya (Robson and Ashkar, 1972), whereas mouse inoculation picked up 95 percent of infections. More than 80 percent of all T. congolense infections were diagnosed by blood examination and, although mouse inoculation was less efficient, 12 percent of infections would have been missed without its use (Table 1).
In contrast, mouse inoculation was of no use for detection of infections with T. vivax and blood film examination identified only 20 percent of all infected cattle. Examination of lymph from prescapular lymph nodes detected nearly 80 percent of infection (Robson and Ashkar, 1972). These authors considered it essential to examine gland juice (Table 2) but others disagree (Zwart et al., 1973) and, in practice, it is little used in the field. The relative insensitivity of blood film examination is also shown by other members of the Trypanozoon subgenus (Table 1). In camels infected with T. evansi, blood examination failed to detect 50 to 60 percent of infected animals (Godfrey and Killick-Kendrick, 1962; Pegram and Scott, 1976; Nantulya et al., 1989). In horses infected with T. evansi, mouse inoculation gave a sensitivity of 88 percent compared with 53 percent for wet blood film examination (Monzon, Mancebo and Roux, 1990). Several parasitological tests were employed in this survey but none was capable of detecting all infected animals (Monzon, Mancebo and Roux, 1990). Studies carried out over two years in goats and cattle infected with T. congolense and T. brucei showed that HCT was positive on only 10 percent and 19 percent, respectively, of the occasions that the animals were tested (Masake and Nantulya, 1991). Varying sensitivity of the tests and the failure to detect trypanosomes if the number of parasites is too low, as is the case with chronic infections (Masake and Nantulya, 1991), illustrate the limitations of parasitological diagnosis and confirm the need for more reliable methods.
1 Sensitivity of different parasitological detection methods in the diagnosis of trypanosomiasis in domesticated livestock
Sensibilité des différentes méthodes de détection parasitologique dans le diagnostic de la trypanosomiase du bétail
Sensibilidad de distintos métodos de detección parasitológica en el diagnóstico de la tripanosomiasis en ganado doméstico
|
Trypanosome species infected |
Number of animals infected |
Animals affected |
Proportion of animals positive |
|
|
Blood examination (%) |
Mouse inoculation (%) |
|||
|
T. brucei |
191 |
|
5.2 |
94.8 |
|
T. congolense |
409 |
Cattle sheep |
87.5 |
43.3 |
|
T. vivax |
256 |
|
20.2 |
0.0 |
|
T. evansi |
46 |
Camels2 |
45.0 |
100 |
|
T. evansi |
38 |
Camels3 |
26.0 |
100 |
|
T. evansi |
20 |
Camels4 |
50.0 |
100 |
1 Based on data from Robson and Ashkar (1972).
2 Based on data from Godfrey and Killik-Kendrick (1962).
3 Based on data from Pegram and Scott (1976).
4 Based on data from Nantulya et al. (1989).
2 Sensitivity of blood and lymph gland examination in the diagnosis of Trypanosoma congolense and T. vivax infections in cattle1
Sensibilité de l'examen du sang et des ganglions lymphatiques dans le diagnostic des infections par Trypanosoma congolense et T vivax du bétail
Sensibilidad del examen de la sangre y las glándulas linfáticas en el diagnóstico de infecciones por Trypanosoma congolense y T. vivax en los vacunos
|
Trypanosome species |
Number of cattle infected
|
Proportion of cattle found positive |
||
|
Blood examination (%) |
Lymph gland examination |
Blood and lymph gland examination (%) |
||
|
T. congolense |
83 |
42.2 |
7.2 |
50.6 |
|
T. vivax |
54 |
33.3 |
31.5 |
35.3 |
|
All infections |
137 |
38.7 |
16.8 |
44.5 |
1 Based on data from Robson and Ashkar (1972).
Immunodiagnostic techniques
Although direct demonstration of trypanosomes in the infected animal gives conclusive proof of infection, the limitations of parasitological diagnosis has been the driving force for a great deal of research into alternative techniques that provide indirect evidence of infection, namely immunodiagnostic techniques. There are many reports of the use of immunodiagnostic techniques for diagnosis but, invariably, most of them have been retrospective surveys, intended to add further information rather than play an integral part in a control programme. The one exception to this generalization is in the application of the complement fixation (CF) test to the diagnosis of T. equiperdum, the cause of dourine in horses. Serology has always played a major role in diagnosis of this disease since trypanosomes are rarely found in blood or other body fluids. The CF test was used successfully in the control and eradication of dourine in North America (Watson, 1920) and was also used in the diagnosis of surra in buffalo in the Philippines (Randall and Schwartz, 1936). This assay, little changed, is still in use today in testing sera before the import and export of horses between different countries. The test has not been used extensively for the other animal trypanosomiases because of problems with antigen preparation, standardization of the assay and interference by anticomplementary activity in sera from several animal species. Problems in the control and standardization of another sensitive test, the indirect haemagglutination (IHA) test, have precluded its general use although it was used in the diagnosis of T. evansi in camels (Jatkar and Singh, 1971) and in a control programme for buffalo and cattle (Shen, 1974). In tests with T. vivax it was considered too unreliable (Clarkson, Cottrell and Enayat, 1971).
The breakthrough in immunological diagnosis came with the introduction of primary binding assays for the detection of trypanosomal antibodies. These tests directly measure the interaction between antigen and antibody rather than relying on a secondary reaction consequent upon the initial binding. The indirect fluorescent antibody test (IFAT) has been used extensively in the detection of trypanosomal antibodies in animals and humans. Antigens are usually prepared from blood smears which are fixed in acetone and then stored at a low temperature. The IFAT has proven to be both specific and sensitive in detecting trypanosomal antibodies in infected cattle (Wilson, 1969; Luckins and Mehlitz, 1978) and camels (Lucking et al., 1979).
However, cross-reactions between different trypanosome species do occur. Ashkar and Ochilo (1972) found that more than 85 percent of cattle infected with T. vivax or T. congolense reacted with T. brucei antigen in the IFAT (Table 3). When sera were tested against all three pathogenic trypanosome species, between 45 and 66 percent of sera reacted in the assay, and only by combining all the results did the test detect 94 percent of infected animals (Table 3). Hence, although there is considerable cross-reactivity, these results indicate a degree of species specificity that requires the use of all three antigens in order to obtain maximum efficiency. Modifications in the preparation of antigens involving fixation of the parasites in acetone and formalin (Katende et al., 1987) have provided antigens which are stable even at 4°C, can be kept in suspension until required and are capable of discriminating between different trypanosome species.
The major drawback of the IFAT - apart from its requiring sophisticated microscopy - is its subjectivity, which can make comparison of results quite difficult. Undoubtedly, the introduction of enzyme-linked immunosorbent assays (ELISA) for use as diagnostic tests for animal trypanosomiases (Lucking and Mehlitz, 1978; Luckins et al., 1979; Luckins, 1986; Rae et al., 1989; Ferenc, Stopinski and Courtney, 1990) has increased interest in the possibility of a universally applied immunodiagnostic assay, and the modification and refinement of these assays brings field tests a little closer (Nantulya et al., 1989). The tests are carried out in 96-well polystyrene micro-ELISA plates on which trypanosomal antigen is adsorbed. An indirect assay is routinely used in which serum from test cases is reacted with the antigen, followed by incubation of the resulting antigen/antibody complex with an enzyme-conjugated antiglobulin to the IgG fraction of the particular host species. The test is visualized by the addition of enzyme substrate and chromogen, with the resulting colour change allowing a photometric interpretation. Tests using crude sonicated trypanosomal extracts showed that the ELISA had a sensitivity and specificity similar to the IFAT (Table 3). However, where tsetse-transmitted trypanosomiases occurred, cross-reactions were a problem (Table 3). As with the IFAT, to ensure that a high proportion of infected animals were diagnosed, sera had to be screened against all trypanosome antigens in order to obtain the highest diagnostic sensitivity. Fractionation of the crude trypanosomal antigen extracts has identified antigens that are species specific, and this method should enable discrimination between T. brucei, T. vivax and T. congolense infections (Ijagbone, Staak and Reinhard, 1989). In addition, species-specific monoclonal antibodies developed against T. brucei, T. vivax and T. congolense (Nantulya et al., 1989) and T. evansi (Lucking, 1991) will allow isolation and purification of defined specific antigens for use in indirect ELISA.
The tests described above rely on antigen/antibody reactions between common or species-specific antigens but one immunodiagnostic test relies on the presence of a widely distributed variable surface antigen. The card agglutination test (CATT) uses the formalin fixed variable antigen types of T. evansi that are used in the agglutination test. The test, which is simple to perform, has been used for diagnosis of T. evansi (Bajyana-Songa et al., 1987) but is unlikely to be of use for T. vivax or T. congolense because of the difficulty of identifying suitable variable antigens of these species.
A modification of the ELISA, currently of great interest, is based on an antigen capture assay which enables detection of circulating trypanosomal antigen in the blood of infected animals. Antibody against trypanosomal antigen is used to coat ELISA plates and any antigen present in test sera binds. The complex so formed is then incubated with the same antibody, conjugated with enzyme and visualized with a suitable substrate. Early assays using polyclonal antibodies raised against crude trypanosomal antigen preparations were found to detect antigen in animals infected with T. evansi and T. congolense within ten to 14 days of infection, and after trypanocidal drug treatment they disappeared within 21 days (Rae and Luckins, 1984). Later, the species specificity of the assay was improved following the development of monoclonal antibodies as capture antibodies that recognized antigens present in T. brucei, T. vivax and T. congolense. Specific circulating antigens could be detected in cattle from eight to 14 days after infection, but within 14 days of treatment they were no longer detectable (Nantulya and Lindqvist, 1989).
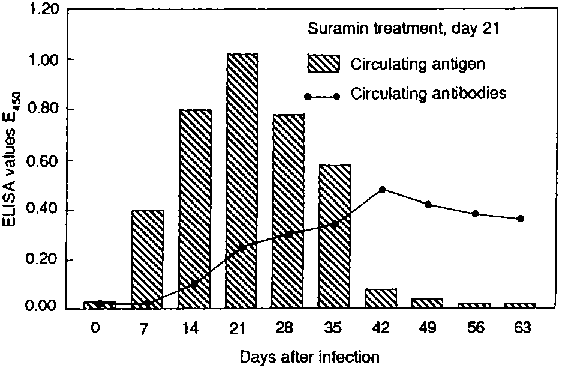
The figure shows the development of antigenaemia and antibodies in rabbits infected with T. evansi. Although antigen levels fall rapidly after treatment, antibody levels continue to rise and are still high some time after treatment. The successful treatment of the animals is confirmed by the rapid disappearance of antigen from the circulation. Antigen ELISA was shown to have a high diagnostic sensitivity; more than 90 percent and 95 percent, respectively, in cattle and camels (Nantulya et al., 1989; Nantulya, 1990). A simplified modification of the assay in polystyrene tubes was found to give similar results to the plate assay (Nantulya et al., 1989). In goats and cattle, experimentally infected with T. congolense over a two-year period, antigens could be detected 94 percent of the time in goats and 82 percent of the time in cattle (Masake and Nantulya, 1991). The false negative results occurred during the early stages of infection, possibly when antigen levels were below the detection limits of the assay.
3 Sensitivity of indirect fluorescent antibody tests and enzyme-linked immunosorbent assays in the diagnosis of trypanosomiasis in infected livestock
Sensibilité des tests d'anticorps fluorescents indirects et des essais d'immuno-absorption enzymatique dans le diagnostic de la trypanosomiase du bétail
Sensibilidad de las pruebas de anticuerpos fluorescentes indirectas y los ensayos de inmunoabsorción enzimática para el diagnóstico de la tripanosomiasis en ganado infectado
|
Animals tested |
Number of active infections |
Proportion of animals serologically positive | ||||
|
|
|
|
IFAT (%) |
ELISA (%) | ||
|
Cattle2 |
Tb |
5 |
Tb |
100 |
|
|
|
|
Tv |
14 |
Tv |
85.7 |
|
|
|
|
Tc |
25 |
Tc |
88.0 |
|
|
|
Total |
|
471 |
|
89.41 |
|
|
|
Cattle3 |
Tb |
2 |
Tb |
46.3 |
Tb |
82.1 |
|
|
Tv |
27 |
Tv |
79.5 |
Tv |
79.5 |
|
|
Tc |
19 |
Tc |
66.7 |
Tc |
82.1 |
|
Total |
|
391 |
|
94.91 |
|
92.31 |
|
Camels4 |
Te |
30 |
Te |
96.7 |
Te |
92.3 |
1 Includes mixed infections with different trypanosome species.
2 Based on data from Ashkar and Ochilo (1972).
3 Based on data from Luckins and Mehlitz (1979).
4 Based on data from Luckins et al. (1979).
Circulating antigens and antibodies in animals infected with Trypanosoma evansi - Antigènes et anticorps circulants dans les animaux infectés par Trypanosoma evansi - Antígenos y anticuerpos circulantes en animales infectados por Trypanosoma evansi
For control of animal trypanosomiasis in Africa, Asia and South America, it is essential that accurate epidemiological information is obtained on the prevalence of disease in different livestock species. Such information depends on the use of sensitive diagnostic tests. The limitations of parasitological diagnosis necessitates the application of immunodiagnostic assays to ensure that this is achieved. However, first it is necessary to decide on a particular assay since, by limiting the choice, the problems of standardization are more readily overcome. For a number of reasons, the leading candidates are clearly ELISA tests. To start with, it is possible to produce defined antigens and monoclonal antibodies. Second, the ELISA is easy to automate in order to screen large numbers of samples and, finally, by appropriate modification it should be possible to adapt the assay for use under field conditions. Antibody assays would enable an overall assessment of the population exposed to infection; antigen assays would enable identification of individuals with active infections, species differentiation and the detection of drug resistance. These tests also have their limitations and, in order to identify animals with acute infections in which antigens are not always detected, sensitive parasitological tests such as HCT or the DG technique should also be employed.
For the ELISA to be used more widely it will be necessary to provide training programmes providing a standardized assay protocol and reference preparations, including suitable, defined control sera. ELISA assays for antigen detection are currently being evaluated in ten countries in Africa. The programme is organized by the FAD/IAEA Division of Nuclear Techniques in Food and Agriculture, in collaboration with the International Laboratory for Research on Animal Diseases (ILRAD) and the Centre for Tropical Veterinary Medicine (CTVM) and with funds provided by the Government of the Netherlands. Using various laboratories and taking into account different conditions, the programme is providing a considerable amount of information on the practical application of ELISA in the field, and it will be a valuable exercise in determining the logistics of the method's implementation on a larger scale. +
Ashkar, T. & Ochilo, M. 1972. The application of the indirect fluorescent antibody test to samples of dried sera and blood from cattle in the Lambwe Valley, South Nyanza, Kenya. Bull. World Health Organ., 47: 769-772.
Bajyana-Songa, E., Hamers-Casterman, C., Hamers, R., Pholpark, M., Pholpark, S., Leidl, K., Tangchaitrong, S., Chaichanopoonpol, I., Vitoorakool, C. & Thirapataskum, T. 1987. The use of a card agglutination test (Testryp CATT) for use in detection of T. evansi infection: a comparison with other trypanosomiasis diagnostic tests under field conditions in Thailand. Ann. Soc. Belg. Med. Trop., 67: 137-148.
Clarkson, M.J., Cottrell, B.A. & Enayat, M.S. 1971. The indirect haemagglutination test in the study of Trypanosoma vivax infections of sheep. Ann. Trop. Med. Parasitol., 65: 333-340.
Ferenc, S.A., Stopinski, V. & Courtney, C.H. 1990. The development of an enzyme-linked immunosorbent assay for Trypanosoma vivax and its use in a seroepidemiological survey of the Eastern Caribbean Basin. Int. J. Parasitol., 20: 51 -56.
Godfrey, D.G. & Killick-Kendrick, R. 1962. Trypanosoma evansi of camels in Nigeria: a high incidence demonstrated by the inoculation into rats. Ann. Trop. Med. Parasitol., 56: 14-19.
Ijagbone, I.F., Staak, C. & Reinhard, R. 1989. Fractionation of trypanosome antigens for species specific diagnosis. Vet. Parasitol., 32: 293-299.
Jatkar, P.R. & Singh, M. 1971. Diagnosis of surra in camels by the passive haemagglutination test. Br. Vet. J., 127: 283-288.
Katende, J.M., Musoke, A.J., Nantulya, V.M. & Gooderis, B.M. 1987. A new method for fixation and preservation of trypanosomal antigens for use in the indirect immunofluorescence antibody test for diagnosis of bovine trypanosomiasis. Trop. Med. Parasitol., 38: 41-44.
Luckins, A.G. 1986. Antigens and antibodies in fungal and parasitic diseases. Trypanosoma brucei. In R.F. Masseyef, ed. Methods of enzymatic analysis, Volume 11, Antigens and antibodies, p. 351-367. Weinheim, VCH Verlagsgesellschaft mbH.
Luckins, A.G. 1988. Trypanosoma evansi in Asia. Parasitol. Today, 4: 137-142.
Luckins, A.G. 1991. Antigen detection ELISA for Trypanosoma evansi using group-specific monoclonal antibodies. In Improving the diagnosis and control of trypanosomiasis and other vector-borne diseases of African livestock using immunoassay methods, Third Research Co-ordination Meeting, Abidjan, 20-25 May 1991.
Luckins, A.G. & Mehlitz, D. 1978. Evaluation of an indirect fluorescent antibody test, enzyme-linked immunosorbent assay and quantification of immunoglobulins in the diagnosis of bovine trypanosomiasis. Trop. Anim. Health Prod., 10: 149-159.
Luckins, A.G., Boid, R., Rae, P., Mahmoud, M.M., El Malik, K.H. & Gray, A.R. 1979. Serodiagnosis of infection with Trypanosoma evansi in camels in the Sudan. Trop. Anim. Health Prod., 11: 1-12.
Masake, R.A. & Nantulya, V.M. 1991. Sensitivity of an antigen detection enzyme immunoassay for diagnosis of Trypanosoma congolense infection in goats and cattle. J. Parasitol., 77: 231-236.
Molyneux, D.H. 1975. Diagnostic methods in animal trypanosomiasis. Vet. Parasitol., 1: 5-17.
Monzon, C.M., Mancebo, O.A. & Roux, J.P. 1990. Comparison between six parasitological methods for diagnosis of Trypanosoma evansi in the subtropical area of Argentina. Vet. Parasitol., 36: 141-146.
Nantulya, V.M. 1990. Trypanosomiasis in domestic animals: the problems of diagnosis. Rev. Sci. Tech. Off. Int. Epiz., 9: 357-367.
Nantulya, V.M. & Lindqvist, K.J. 1989. Antigen detection enzyme immunoassays for the diagnosis of Trypanosoma vivax, T. congolense and T. brucei infections in cattle. Trop. Med. Parasitol., 40: 267-272.
Nantulya, V.M., Lindqvist, K.J., Diall, O. & Olaho-Mukani. 1989. Two simple antigen detection immunoassays for the diagnosis of Trypanosoma evansi infections in the dromedary camel (Camelus dromedarius). Trop. Med. Parasitol., 40: 415-418.
Paris, J., Murray, M. & McOdimba, F. 1982. A comparative evaluation of the parasitological techniques currently available for the diagnosis of African trypanosomiasis in cattle. Acta Trop., 39: 307-316.
Pegram, R.G. & Scott, J.M. 1976. The prevalence and diagnosis of Trypanosoma evansi in camels in southern Ethiopia. Trop. Anim. Health Prod., 8: 20-27.
Rae, P.F. & Luckins, A.G. 1984. Detection of circulating trypanosomal antigens by enzyme immunoassay. Ann. Trop. Med. Parasitol., 78: 587-596.
Rae, P.F., Thrusfield, M.V., Higgins, A., Aitken, C.G.G., Jones, T.W. & Luckins, A.G. 1989. Evaluation of enzyme immunoassays in the diagnosis of camel (Camelus dromedarius) trypanosomiasis: a preliminary investigation. Epidemiol. Inf., 102: 297-307.
Randall, R. & Schwartz, S.C. 1936. A survey for the incidence of surra in the Philippine islands. Vet. Bull. US Army, 30: 99-108.
Robson, J. & Ashkar, T.S. 1972. Trypanosomiasis in domestic livestock in the Lambwe Valley area and a field evaluation of various diagnostic techniques. Bull. World Health Organ., 47: 727-734.
Shen, Y.S. 1974. Diagnosis of chronic surra in ruminants by the passive haemagglutination test. Taiwan J. Vet. Med. Anim. Husb., 25: 41-46.
Watson, E.A. 1920. Dourine in Canada. History, research, suppression. Dominion of Canada, Department of Agriculture.
Wilson, A.J. 1969. Value of the indirect fluorescent antibody test as a serological aid to diagnosis of Glossina-transmitted bovine trypanosomiasis. Trop. Anim. Health Prod., 1: 89-93.
Zwart, D., Perie, N.M., Keppler, A. & Goedbloed, E. 1973. A comparison of methods for the diagnosis of trypanosomiasis in East African domestic ruminants. Trop. Anim. Health Prod., 5: 79-87.