Worksheet 2.1.: Isolation of pure algal strains by the agar plating technique
Worksheet 2.2.: Determination of cell concentrations using haematocytometer according to Fuchs-Rosenthal and Burker.
Worksheet 2.3.: Cellular dry weight estimation of micro-algae.
|
The following agar plating technique can be used to isolate algal strains from raw seawater and for the maintenance of existing algal strains. · prepare a 0.9% agar medium by weighing out 9 g of agar powder and placing it into a 2 l conical flask to which 1 l of sea water is added |
|
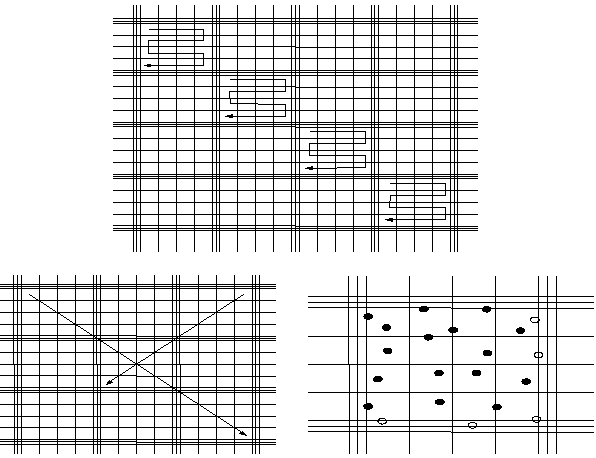
A variety of counting chambers (normally used for blood cell
counts) can be used for cell counts (algae, yeast) (see Table 2.9.). Two types
are most common: Fuchs-Rosenthal and Bürker (Fig. 2.17.). Both types have 2
rafters allowing for 2 subsamples to be examined. They have the following
characteristics: |
||
|
|
Fuchs - Rosenthal |
Bürker |
|
Depth (in mm) |
0.200 |
0.100 |
|
Surface of smallest square (in mm2) |
0.0625 |
0.0400 |
|
minimal cell concentration (in
cells.ml-1) |
103 |
106 |
· dilute sample if needed (use formalin 4% to fixate moving algal cells) clean slide and cover-glass with Kleenex-paper |
||
Figure 2.17. Counting directions (follow arrow) for Fuchs-Rosenthal chamber (upper diagram) and for Bürker (down left corner). For both, count the cells in the square and those which touch the top and left border (·). Do not count the ones touching the right and lower border (o) (see down right corner).
|
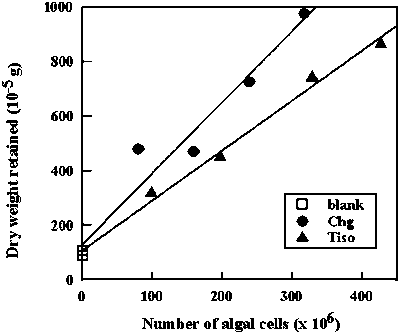
Dry weight of algal cells can be determined by filtering and drying algae from aliquots of culture of known concentration · determine accurately (3 duplicate counts, see Worksheet 2.2.) the concentration of the algal culture to be sampled for dry weight analysis In order to improve the correction for salt residues and the
variation among samples, cellular dry weight can be determined from regression
analysis of DW retained on the filter versus number of algal cells filtered
(Fig. 2.18). |
Figure 2.18. Dry weight analysis of algae by means of linear regression of dry weight retained on the filter versus number of algae cells filtered. Each data set represents the dry weight determination for algae obtained from one culture of, respectively Isochrysis sp. (T-iso) and Chaetoceros neogracile (Chg). Linear regression equators are: T-iso: y = 1.83 × + 107.7 (r2 = 0.99); Chg: y = 2.61 × +130.1 (r2 = 0.95)