5.1.1. Introduction
5.1.2. Collection from the wild
5.1.3. Collection techniques
5.1.4. Zooplankton grading
5.1.5. Transport and storage of collected zooplankton
Zooplankton is made up of small water invertebrates feeding on phytoplankton. Even though “plankton” means passively floating or drifting, some representatives of zooplankton may be strong swimmers. The yearly plankton cycle consists of various phytoplankton species blooming in response to a particular sequence of changes in temperature, salinity, photoperiod and light intensity, nutrient availability, and a consequent bloom of zooplankton populations. Phytoplankton and zooplankton populations are therefore intimately linked in a continuous cycle of bloom and decline that has evolved and persisted throughout millions of years of evolution.
Studies on the stomach contents of fish larvae caught in their natural environment clearly show that almost no fish species can be regarded as strongly stenophagic (specialized in feeding on only a few or just one zooplankton species), though some specialization may occur (i.e. due to size limitations for ingestion).
There are three obvious advantages of using wild zooplankton as a live food source for the cultivation of the early larval stages of shrimp or fish species:
· As it is the natural food source, it may be expected that its nutritional composition maximally covers the nutritional requirements of the predator larvae, especially with respect to essential fatty acids and free amino acids (Tables 5.1, 5.2 and 5.3).· The diversified composition of wild zooplankton in terms of species variety as well as ontogenetic stages assures that optimal sizes of prey organisms will be available and efficient uptake by the predator is possible at any time during the larval rearing.
· Depending on the harvesting potential nearby the hatchery facility, there might be a low cost involved in the harvest of this live food compared to the infrastructure and production costs of the live food items discussed earlier.
On the other hand, there are also major drawbacks in the use of zooplankton, including: (1) irregular supply due to dependence on natural (in lakes or oceans) or induced (in ponds) phytoplankton blooms; and (2) the introduction of diseases and parasites in the fish culture tanks through infested wild zooplankton, (e.g., fishflea Argulus foliaceus and Livoneca sp. etc.), parasitic copepods (Lernaea sp. and Lernaeascus sp., etc.).
Table 5.1. Biochemical composition of wild zooplankton collected at Maizura Bay, Japan (modified from Kuroshima et al., 1987).
|
|
October |
May |
June |
July |
August |
|
Moisture (%) |
- |
89.7 |
87.0 |
91.1 |
91.2 |
|
Crude protein (%)* |
63.2 |
74.2 |
68.7 |
65.5 |
66.8 |
|
Crude lipid (%)* |
9.4 |
9.8 |
12.1 |
12.6 |
17.2 |
|
Crude ash (%)* |
11.1 |
8.8 |
9.5 |
9.9 |
9.2 |
|
16:0 |
23.8 |
25.5 |
24.2 |
21.3 |
20.1 |
|
16:1n-7 |
8.8 |
10.8 |
11.4 |
8.2 |
8.6 |
|
18:0 |
8.9 |
5.6 |
6.2 |
9.4 |
6.7 |
|
18:1n-9 |
13.7 |
12.9 |
8.5 |
7.1 |
7.4 |
|
18:2n-6 |
2.2 |
5.0 |
3.6 |
5.1 |
3.9 |
|
18:3n-3 |
5.7 |
1.8 |
1.3 |
7.9 |
9.3 |
|
18:4n-3 |
4.2 |
2.0 |
3.4 |
6.3 |
8.7 |
|
20:1 |
2.1 |
0.6 |
1.0 |
2.0 |
2.2 |
|
20:4n-3 |
0.8 |
0.1 |
0.4 |
1.1 |
0.5 |
|
20:5n-3 |
8.6 |
9.5 |
8.4 |
8.7 |
8.6 |
|
22:5n-3 |
1.0 |
Tr. |
0.6 |
0.3 |
0.8 |
|
22:6n-3 |
10.6 |
14.1 |
11.8 |
10.0 |
7.0 |
* dry weight basis
Table 5.2. Free fatty acid composition (FFA; area% of total lipid) of wild zooplankton compared to freshly-hatched Artemia nauplii (AF grade) (modified from Naess and Bergh, 1994).
|
|
Wild zooplankton |
Artemia |
|
14:0 |
3.4 |
0.8 |
|
16:0 |
16.9 |
12.6 |
|
16:1n-9 |
0.7 |
0.9 |
|
16:1n-7 |
1.7 |
4.0 |
|
16:2n-4 |
0.3 |
0.2 |
|
18:0 |
3.7 |
7.4 |
|
18:1n-9 |
2.9 |
22.5 |
|
18:1n-7 |
3.3 |
10.6 |
|
18:2n-6 |
2.0 |
6.8 |
|
18:3n-3 |
1.5 |
20.3 |
|
18:4n-3 |
1.5 |
2.3 |
|
20:1n-9 |
0.2 |
0.7 |
|
20:1n-7 |
0.6 |
0.1 |
|
20:4n-6 |
0.8 |
2.3 |
|
20:4n-3 |
0.5 |
0.6 |
|
20:5n-3 |
21.1 |
3.6 |
|
22:0 |
0.5 |
1.1 |
|
22:1n-11 |
0.0 |
Tr. |
|
22:5n-3 |
0.8 |
0.1 |
|
22:6n-3 |
32.9 |
0.2 |
|
Sum (n-3)PUFA |
58.3 |
27.1 |
|
Sum (n-6)PUFA |
2.8 |
9.1 |
|
n-6/n-3 PUFA |
0.0 |
0.3 |
|
22:6n-3/20:5n-3 |
1.6 |
0.1 |
|
Total lipid (µg.mg-1 WW) |
13.0 |
13.0 |
|
|
Wild zooplankton |
Artemia |
|
FAA |
|
|
|
Aspartic |
2.1 |
1.2 |
|
Glutamic |
2.0 |
3.6 |
|
Asparagine |
1.5 |
1.3 |
|
Serine |
3.8 |
2.3 |
|
Histidine |
1.3 |
0.7 |
|
Glutamine |
2.8 |
2.8 |
|
Glycine |
23.0 |
2.0 |
|
Threonine |
2.1 |
1.3 |
|
Arginine |
9.9 |
3.6 |
|
Alanine |
9.1 |
4.4 |
|
Taurine |
32.7 |
7.6 |
|
Tyrosine |
1.5 |
1.1 |
|
Valine |
3.8 |
2.1 |
|
Methionine |
4.7 |
2.2 |
|
Tryptophan |
0.6 |
0.3 |
|
Phenylalanine |
2.1 |
1.5 |
|
Isoleucine |
2.4 |
1.5 |
|
Leucine |
4.5 |
2.5 |
|
Lysine |
6.6 |
3.9 |
|
Total FAA |
116.6 |
45.9 |
Zooplankton can be collected from seawater bodies as well as freshwater lakes or ponds. For aquaculture purposes, approximately 80% is of marine origin. Around 25 species of copepods, mysids and euphausids are commercially harvested. Leading countries in using wild zooplankton in industrial aquaculture are Norway (annual catch ranges between 20 to 50 tonnes), Canada and Japan. The global annual catch of planktonic crustaceans (essentially krill) is around 210,000 tonnes, but only a small percentage is used as a direct food source in aquaculture (live or deep frozen).
On the Mediterranean and Atlantic coasts of France, densities of copepods (which make up 85% of the zooplankton) may range from 500 copepods per m3 in winter (November-February) to more than 10,000 per m3 in spring and summer. On average 1,000 copepods per m³ are found in the littoral zone; this figure may, however, be higher in lagoons and estuaries. In some eutrophic brackish water fjords in Norway, for instance, abundant numbers of the copepod Eurytemora may be found, including 6 to 30.106 adults, 15 to 25.106 copepodites, and 25 to 50.106 nauplii per 100 m3 of water. This is roughly equivalent to 100 to 300 g (1-3 mg.l-1) biomass dry weight for the different ontogenetic stages of this copepod.
Although these production figures are high, the required quantities for commercial hatcheries may be enormous. It is calculated that approximately 3000 live prey are needed to produce one European seabass larva. During rearing it is thus necessary to filter 3 m3 water per larval fish or 3.106 m3.month-1 to supply a hatchery with a production capacity of one million fry. This corresponds to an hourly filtering capacity of 4166 m3 for a land-based pumping system. When zooplankton is harvested from a boat, a three minute tow with a 1 m diameter plankton net travelling at a speed of 2.6 km.h-1 would catch about 100 to 300 g dry weight of zooplankton biomass, assuming a 100% filtration rate of the net. If these copepods were fed to 7-day old carp fry weighing 1 mg dry weight and probably eating 100% of their body weight per day it would be sufficient to supply 1 to 3.105 larvae per day on such a short tow.
5.1.3.1. Plankton nets
5.1.3.2. Trawl nets
5.1.3.3. Baleen harvesting system
5.1.3.4. Flow-through harvesting
5.1.3.5. Plankton light trapping
Harvesting techniques depend strongly on the location of the harvesting site and should meet the following criteria:
· capable to operate on a continuous basis without surveillance;
· easy to transport and to set up;
· relatively cheap in purchase and maintenance;
· available on site;
· designed for the required quantities and zooplankton sizes.
The following mesh sizes may be used to collect the various sizes of freshwater zooplankton:
· 80 µm for small species of rotifers and larger infusorians. These are an excellent starter feed especially for the fry of some fishes that need small food in the early stages (tench, grass carp, silver carp, big head, carp);· 160 µm for larger rotifers, nauplius and copepodite stages of copepods;
· 300 and 500 µm for small water fleas and smaller species of cyclopoid copepods;
· 700 µm for adult water fleas of the Daphnia genus, large species of cyclopoid and calanoid copepods, larvae and pupae of Corethra sp., etc.
A multi-purpose plankton net for zooplankton collection is schematically shown in Fig. 5.1. The net is conical shaped, 3-3.5 m long, the inlet opening is 1-1.2 m in diameter and the end hole has a diameter of 0.2-0.5 m. There is a strip of thicker cloth on both ends; the front end is furnished with buoys to allow the net to be fixed to a frame. The rear end may consist of a PVC cylinder (2 l), which can be closed on one side.
Nets for hand collection of zooplankton are of the sac type, 50-60 cm long. The net is fixed to a metallic ring, 40-50 cm in diameter, held on a rod of about 2 m long. Collecting zooplankton with hand nets is rather unefficient: one person can catch about 0.1 to 1.0 kg of plankton per hour, depending on the amount of zooplankton biomass in the reservoir.
Figure 5.1. Conical harvesting net for plankton collection from ponds or lakes
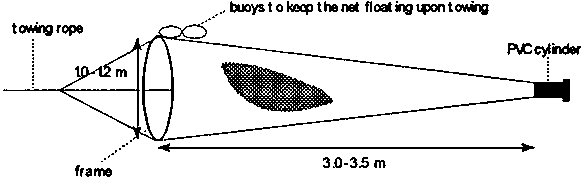
These dimensions of the nets are given just for orientation and can of course be adjusted as needed. However, one should be aware that the greater the surface area of the net the more effective and rapid the filtration. Hence, the upper limit of the dimensions of the nets depends on the ease of handling rather than anything else. The effectiveness of filtering is also influenced by the mesh size of the net: the denser the net the faster it will clog, hence, the smaller its effectiveness. It is therefore necessary to estimate with great accuracy the required size of the food particles with respect to the age and species of the fry and to use an optimal mesh size.
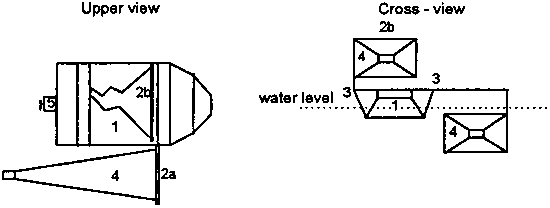
A fishing boat equipped with a frame on which 2-4 plankton nets can be installed on both sides of the boat can be used for this purpose (Fig. 5.2.). Good results have been obtained with a rectangular frame of 1 × 0.6 m and a mesh of 160 µm. When this net is moved at a speed of 1.5 km.h-1 average yields of 40 kg live zooplankton can be harvested in 1 h. In order to minimize the damage to the concentrated plankton, the nets must be emptied every 15-30 min.
Figure 5.2. Boat with plankton nets dragged along. 1) boat; 2) frame with plankton net, a. in working position, b. net lifted; 3) hinge; 4) plankton net; 5) motor (modified from Machacek, 1991).
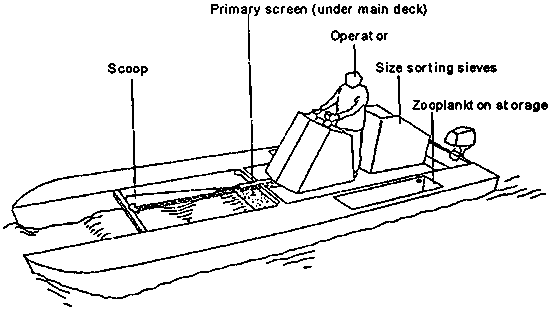
The Baleen harvesting system consists of a boat specifically designed for harvesting zooplankton (Fig. 5.3.). This vessel can filter the surface water at rates up to 400 l.s-1. The zooplankton is scooped onto a primary dewatering screen, after which the organisms are graded through a series of sieves. The stainless-steel mesh of the sieves and primary screen can be changed according to the requirements of the target species. The graded and concentrated zooplankton is stored in wells in the floaters of the vessel and can be unloaded by pumping. The boat can be operated by one person and is powered by an outboard motor and auxiliary petrol engine to drive the pumps and hydraulic rams.
Figure 5.3. The Baleen zooplankton harvesting system (Frish Pty. Ltd., Australia).
· Lake outflows
In reservoirs with a high water flow, a plankton net of adequate size may be placed at the outlet or overflow; in this way the zooplankton present in the water leaving the reservoir can be concentrated. In the case of ponds, the frame of the plankton net may be fixed to the pond gates. The amount of zooplankton collected depends on the zooplankton concentration in the water flowing out of the reservoir and on the volume of the water leaving the reservoir. Again, the nets should be emptied once or twice an hour, depending on prevailing conditions.
This method can be used effectively only in the case where the flow rate of the water at the outlet of the pond is at least 5 to 10 l.s-1. Optimum conditions for this method exist in large eutrophic lakes where the flow rate at the outlet is > 1 m³.s-1 and where several hundred kilos of zooplankton biomass are discharged every day.
· Propeller-induced water flows
Instead of using a motorboat, a propeller can also be actioned from an anchored pontoon, platform, bridge close to the shore, or on a free-floating boat. In all cases, the plankton net needs to be held at a safe distance from the propeller driven by the motor (Fig. 5.4.).
Figure 5.4. Equipment to collect zooplankton with a boat motor (1) with propeller (2), and a plankton net (3) (Modified from Machacek, 1991).
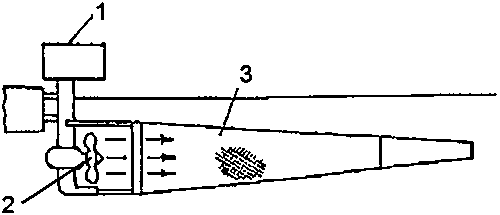
If the distance from the propeller to the net is short, the inlet opening of the net can be reduced and the length of the net increased in order to ensure adequate filtration and prevent losses due to the narrow and strong back current. The longer the distance between the propeller and the net, the wider and shorter the net can be. The distance between the propeller and the net generally ranges from 0.3 to 1.5 m. When equipments of this type are used in shallow reservoirs (below 1 m), care should be taken not to disturb the sediments from the bottom which would clog the net. Therefore, the propeller should be installed close to the water surface. A propeller rotating at 5600 rpm placed at a distance of 1 m of a small plankton net (inlet 30 × 30 cm, mesh size 200 µm), may collect up to 10 kg of zooplankton per hour.
The damage caused by the propeller to the zooplankton is relatively low, but considerable losses may be caused by combustion engines whose exhausts are blown under the water surface.
For rotifers special collecting equipment has been constructed to avoid the rapid clogging of the filter bag due to the accumulation of the small-sized zooplankton (< 100 µm). The collecting apparatus is provided with an automatic cleaning equipment of the filter bag. A propeller is obliquely mounted upstream of a partly submerged cylindrical sieve, that rotates at 15 rpm. Water passes through the cylinder and plankton accumulates on the filter wall.
When part of the filter with attached plankton comes out of the water, the plankton is rinsed from the filter wall by water jets, and collected into a central gutter (Fig. 5.5.).
Figure 5.5. Collecting apparatus for rotifers. A. Profile of the self-cleaning plankton harvester. 1)Propeller; 2) Inlet tube; 3) Electro motor (12 V, 24 W and 100 rpm); this motor can also operate the rotating sieve; 4) Intermediate conical gear system; 5) Electro motor to drive the rotating sieve (12 V, 24 W and 20 rpm; 6) Submerged pump for the spray washing system (15 V and 60 W) with feed pipe to jets; 7) Recovery trough for washing water and plankton; 8) Filter sack for storage of concentrated plankton; 9) Water level; Floaters are not shown. B. Cross-section of the apparatus. 1) Lateral floats; 2) Casing around the apparatus; 3) Microsieve; 4) Recovery trough; 5) Spray bar offset from centre (Barnabé, 1990).
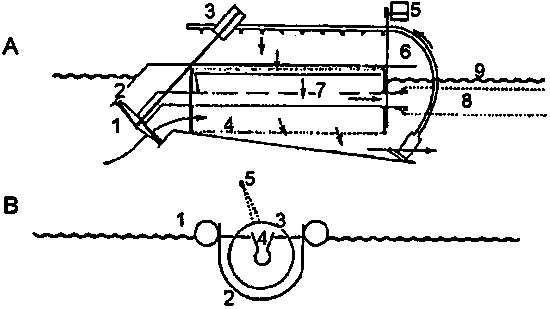
With these devices it is necessary to replace the batteries and to harvest the plankton once or twice a day to reduce mechanical damage of the plankton. The transport of the zooplankton can be carried out in water in a 50 l reservoir and must be carried out very quickly, since the viability of the harvested plankton is low (1h after harvesting already 5% mortality is observed).
· Pump-induced water flows
Another method of collecting zooplankton is to use pumps to pump the water into a plankton net. The plankton net may be located at some distance from the outlet of the pump or may be tightened with a string or rubber band straight to the outlet pipe of the pump. The latter method is better because no plankton can escape by back flushing from the net, but needs more frequent emptying of the net as denser nets are prone to clogging. Using an electric pump with a capacity of 5 l.s-1, as much as 0.5 to 5 kg of zooplankton (depending on zooplankton biomass in the reservoir) may be collected in a net with a mesh size of 160 µm in 1 h (Fig. 5.6.).
A more elegant method for zooplankton collection takes advantage of the positive phototactic behaviour of some zooplankton species. The effectiveness of light to attract the zooplankters is directly dependent on the water transparency and on the intensity of the light source. It is useless to apply this method where the water transparency is below 30 cm. Cladoceran and cyclopoid copepods respond most sensitively to light, rotifers less. The best results of collecting zooplankton with light are obtained in the early night (until about 10 pm); later the effectiveness declines. Though the success of this method may vary, the low expenditure necessary for its application seems to make it an economically viable harvesting system for freshwater species (Nellen, 1986).
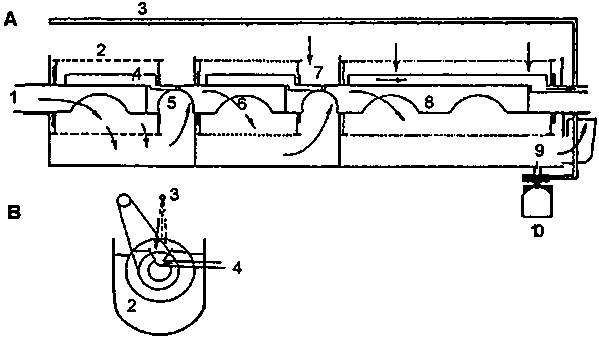
Grading can be accomplished by a set of superimposed sieves with varying mesh sizes. These filters should be submerged so as to minimize mortality. A special device for continuous and automated harvesting and grading has been described by Barnabé (1990) and is schematically outlined in Fig. 5.7. It consists of rotating cylindrical sieves with decreasing mesh size from upstream to downstream.
Figure 5.7. Plankton grader. A. longitudinal section. 1) Inflowing water with high concentration of plankton; 2) First filter drum (500 µm); 3) Spray washing systems with jets; 4) Channel for collecting plankton; 5) Filtered water directed to second filter drum (250 µm); 7) Lateral channel for evaluation of cleaning water and plankton; 8) Third filter drum (71 µm); 9) Outflow of filtered water; 10) Pump for rinsing water. B. Cross-section. The system for driving the drums (not shown in A) is shown here as is the water level and the outflow points for rinsing water (Barnabé, 1990).
Harvest and transport of zooplankton interferes considerably with the survival of these fragile organisms. If it is impossible to convey the material continuously along distribution pipes to the place of consumption, the normal practise is to concentrate and transport the harvested zooplankton in 50 l containers. Under these conditions the survival of the zooplankton depends on the amount of oxygen dissolved in the remaining water. At a concentration of 100 g.l-1, zooplankton can be kept at 10°C without oxygenation for only 15-20 min. At higher temperatures or if the zooplankton is to be kept alive for longer periods, the concentration must be reduced substantially. At a temperature of 18-20°C it can be kept at a concentration of 15-20 g.l-1 without aeration for as long as about 4 - 5 h, although the most sensitive organisms will die. This is certainly the case for Bosmina, Daphnia and others, that are very sensitive to oxygen depletion. Rotifers, cyclopoid copepods and their developmental stages are less sensitive, and some species of the genus Moina, larvae of the genus Corethra, and Daphnia magna are very resistant to low oxygen levels.
When the collected zooplankton is transferred from the net to the transport container, part of the material stays in a layer just above the bottom. These organisms are either mechanically damaged or immobilised and could be administered to the fry first. However, when these organisms die, they will soon start to decay. It is useless to administer these dead animals because the fish will refuse it and their decomposing bodies will spoil the water quality of the rearing system. For this reason, dead zooplankton should always be separated from live zooplankton by decantation.
Preservation of harvested material for long periods is difficult. At present, freezing is the only method used on a large scale. But even at very low freezing temperatures, (i.e. -198°C) one-third of the free and protein-bound amino acids are lost from the plankton samples through sustained proteases activity and leaching. Dehydration has been used successfully on a small scale, while salting causes mortality in fish. Ensilage, using various acids has also been attempted, but needs further investigations.