6.1 Guidelines for predicting dietary intake
6.2 Estimation of supervised trial median values
Joint Meetings are held in order to evaluate the possible risk to consumers arising from the occurrence of residues of pesticides in foods. As long as the residues of a certain pesticide, taken up by the consumer, do not exceed the ADI the safety of the consumer is considered to be adequately protected. The JMPR combines available residue data with cultural dietary information to make predictions of the residue intake.
The JMPR has, from the beginning, tried to arrive at predictions about the intake of pesticide residues on the basis of available data. In taking the MRL as the residue level and using the dietary patterns for the quantity of food consumed, the JMPR arrived at the Theoretical Maximum Daily Intake or TMDI. The JMPR was well aware of the fact that TMDI calculations result in a gross overestimation of the intake. On the other hand, existing uses of the pesticide not brought to the attention of the JMPR could result in a minor underestimation of the residue intake.
Until recently the dietary intake calculations have been carried out according to the Guidelines for predicting dietary intake of pesticide residues published by WHO in 1989. The dietary intake of any particular pesticide residue is obtained by multiplying the residue level in the food by the amount of commodity intakes from a "global" and five "cultural" diets, also called "regional" diets. Total intake of the pesticide residue in each of the diet groups is then obtained by summing the intakes from all commodities containing the residue concerned. The correction factors include the residue level in the edible portion of the commodity, the reduction or increase of the residues on commercial processing such as canning and milling, and the reduction or increase in the level of residue on preparation or cooking of the food. This leads to the Estimated Maximum Daily Intake or EMDI. According to the Guidelines, EMDI calculations should only be carried out when the TMDI exceeds the ADI for a pesticide. However, the EMDI is still based on the assumption that all crops have been treated with the pesticide in question and with residues in the raw commodity at a level corresponding to the MRL, and this will also give rise to an overestimate of the intake.
The guidelines also described a more realistic intake calculation, the so-called Estimated Daily Intake or EDI. In this calculation, several reduction factors are taken into account which are only available at national levels. EDI calculations were therefore only to be carried out at a national level by those with adequate information on food consumption, the use of a given pesticide locally, and the nature and the amount of imported food.
Based on the request of the CCPR a Joint FAO/WHO Consultation on Guidelines for predicting the Dietary Intake of Pesticide Residues was held in York, United Kingdom, 2-6 May 1995. Its main objectives were to review the existing guidelines and to recommend feasible approaches for improving the reliability and accuracy of methods for predicting the dietary intake of pesticide residues to promote a greater acceptance of Codex MRLs by governments and, most importantly, by consumers. The report of the consultation (WHO/FNU/FOS/95.11) contained recommendations for improving estimates of dietary intake, most notably the use of supervised trials median residue (STMR) levels in lieu of MRLs in the calculation of International Estimated Daily Intakes (IEDIs) and National Estimated Daily Intakes (NEDIs).
The IEDI incorporates those factors which can be applied at international level and which comprise a subset of the factors that might be considered at national level. The factors to be considered for IEDI calculations are:
· Median residue data from supervised trials (not on MRLs);· Residue definitions, which include all metabolites and degradation products of toxicological concern;
· For residues at or below the limit of determination (indicated with *), the median residue should be estimated to be the LOD except when evidence from trials and supporting studies suggests that that residues are essentially zero;
· The edible portion;
· Effects on residue levels due to storage, processing or cooking practices;
· Other known uses of the pesticide.
The National Estimated Daily Intake (NEDI) should be based on the same factors as for the IEDI, but the following additional factors based on national use pattern of the pesticides and food consumption data should also be taken in consideration, which would result in a refinement of NEDI:
· Proportion of crop or food commodity treated;· Proportion of crop domestically produced and imported;
· Compliance monitoring and surveillance data;
· Total diet (market basket) studies;
· Food consumption data, including that of subgroups of the population.
The revised guidelines for intake calculations are under preparation by the WHO Secretariat.
According to those recommendations where TMDI calculations are carried out by the JMPR, they should be based on "cultural" diets rather than the "global" diet, and some changes were proposed in the calculation system. However, the Consultation emphasised that the best use should be made of all available data and recommended that calculations of EMDIs should be discontinued to promote calculations of International Estimated Daily Intake (IEDI) and National Estimated Daily Intake (NEDI). The recommended procedure is illustrated in Figure 6.1.
A further Joint FAO/WHO Consultation on Food Consumption and Exposure Assessment of Chemicals will be held in 1997 at WHO Headquarters in Geneva. The Consultation will follow up certain recommendations of the York Consultation, particularly in the development of regional diets and in addressing issues related to implementation of the recommendation on intake assessment for acute hazards.
Figure 6.1. Scheme for assessment of dietary intake of pesticide residues for chronic hazards
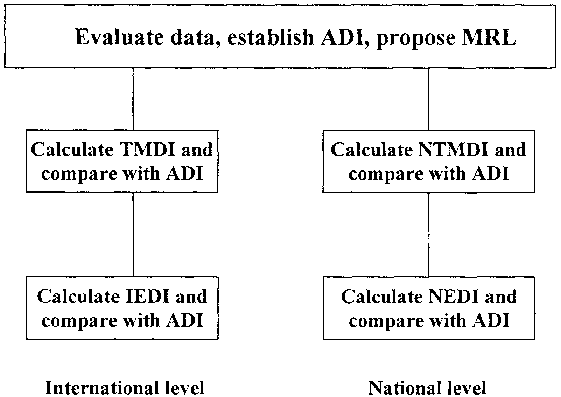
The JMPR will continue the estimation of TMDIs for (old) pesticides where data are not available for estimating IEDIs, but emphasised that when information was available a better estimate of intake should be derived from the IEDI method.
As a consequence of the recommendations made by the Joint FAO/WHO Consultation for Predicting Dietary Intake of Pesticide Residues an informal Workshop was convened in the Hague, Netherlands, in April 1996 to consider the effect of the recommendations on the work of JMPR and especially of those related to the reviews of residue data undertaken by the FAO Panel members. At this Workshop, guidelines were developed in order to give guidance on the implementation of the York Consultation recommendations to the FAO Panel reviewers, and to convert the recommendations into practical methods for evaluating data.
The Workshop focused on the reviews of data undertaken by FAO Panel members and the estimation of supervised trials median residue (STMR) levels. Several general recommendations and 27 specific recommendations for the evaluation of data were made.
Comparability
Residues data from countries are evaluated against the GAP in the country of the trials or a neighbouring country with similar climate and cultural practices.
In identifying the STMR, the trials values selected should be comparable with the maximum registered use (i.e. maximum application rate, maximum number of treatments, minimum PHI) on which the MRL is based.
In establishing comparability of uses in the residue trials to the maximum registered use, the application rates in the trials should generally deviate no more than ±25-30% of the maximum application rate. Deviations from this should be explained in the appraisal. Similarly, maximum deviations of no more than ±25-30% should also be used as a guide for establishing comparability of PHI; however, in this case the latitude of acceptable PHIs will also depend on the rate of decline of residues of the compound under evaluation. Consideration of whether the number of treatments reported in trials is comparable to the registered maximum number of treatments will depend on the persistence of the compound and the interval between applications. Nevertheless, when a large number of treatments are made in the trials (more than 5 or 6), the residue level should be considered very little influenced by further treatments unless the compound is persistent or the treatments are made with unusually short intervals.
In establishing comparability of residue trials data in which more than one parameter (i.e. application rate, number of treatments or PHI) deviate from the maximum registered use, consideration should be given to the combination effect on the residue value which may lead to an underestimation or overestimation of the STMR. For example, a trial result should not normally be selected for the estimation of the STMR if both the application rate is lower (perhaps 0.75 kg/ha in the trial; 1 kg ai/ha GAP) than the maximum rate registered and the PHI is longer (perhaps 18 days in the trial, 14 days GAP) than the minimum registered PHI, since these parameters would combine to underestimate the residue. When results are selected for the estimation of STMRs, despite combination effects, the reasons should be explained in the appraisal.
If the residue value arising from a use considered comparable with the maximum registered use is lower than another residue value from the same trial which is within GAP, then the higher residue value should be selected in identifying the STMR. For example, if the GAP specified a minimum PHI of 21 days and the residue levels in a trial reflecting GAP were 0.7, 0.6 and 0.9 mg/kg at 21, 28 and 35 days respectively, then the residue value of 0.9 mg/kg would be selected.
Trials with more than one residue value
In identifying the STMR only one data point should be taken from each trial (i.e. in this aspect, treatments carried out within a study at the same location with different formulations, or different dosage rates or with the same dosage but at a different time, are considered to be different trials).
Where several residue values have been reported from replicate plots from a single trial (i.e. same dosage at one site location), the highest residue should be selected for the purpose of identifying the STMR.
Where several residue values have been reported from replicate analyses of the same field sample taken from a single trial (i.e. site location), the mean residue should be selected for the purpose of identifying the STMR.
Rounding of results
In identifying the STMR from a residue trial, the actual residue value should be used in the estimation of dietary intake without rounding up or down. This would even be the case where the actual results were below the practical limit of determination considered appropriate for enforcement purposes. Rounding of residue values is inappropriate since the STMRs are used at an intermediate stage in the dietary intake calculation.
Residue definition
The WHO Panel considers and indicates in its evaluations which metabolites should be included in the dietary risk assessment.
If it is recommended that the residue definition for the risk assessment be different from that for enforcement, this is clearly stated in the appraisal.
Close communication should be established prior to the JMPR meeting between the FAO Panel reviewers and the respective reviewers on the Toxicological and Environmental Groups, on questions such as which metabolites are of toxicological significance.
In tabulating the residue trials data the FAO Panel reviewer should indicate the levels of relevant metabolites separately from those of the parent compound, but in a way which would allow subsequent combination, in order to ensure that changes in the residue definition can be accommodated at the JMPR meeting.
In those cases where it is not possible to finalise the risk assessment at the JMPR (September, year 1), usually because of a change in residue definition, the MRLs would still be recommended to the CCPR (by way of Codex circular letter for comment at step 3) and the compound would be re-discussed at the following year's JMPR meeting (September, year 2). The recommended MRLs together with the conclusion of the risk assessment would be available for the next CCPR (April, year 3).
If two compounds for which STMRs can be calculated produce the same analyte in compliance monitoring (e.g. CS2 for dithiocarbamates), it is possible to separate the intake assessments, if required, because the intake assessment is no longer based on the MRL but is based on residue data specific to the individual compounds.
Combining of populations of data for the calculation of STMRs
In identifying the STMR, residue data reflecting different countries' GAPs would normally be combined. However, if the trials data reflecting different countries' GAPs appear to give rise to different populations of data then these data sets should not be combined. In these cases the STMR should be calculated from the population(s) of data which is (are) driving the MRL. In deciding whether the results of trials reflecting different countries GAPs give rise to different populations of residues data, the size of the database reflecting the different countries' GAPs should be taken into account.
Residues below the limit of determination
As a general rule, where all residue trials data are <LOD, the STMR would be assumed to be at the LOD, unless there is scientific evidence that residues are "essentially zero". Such supporting evidence would include residues from related trials at shorter PHIs, exaggerated, but related, application rates or a greater number of applications, expectations from metabolism studies or data from related commodities.
Where there are two or more sets of trials with different LODs, and no determinable residues have been reported in the trials, the lowest LOD should normally be used for the purpose of STMR selection (unless the residues can be assumed to be essentially zero as given above). The size of the trials database supporting the lowest LOD value should be taken into account in the decision.
Processing, cooking factors and edible portion residue data
In using data on the effects on residue levels of processing or cooking practices, the mean processing factor should be applied to the STMR estimated for the raw agricultural commodity as already described. The STMR value estimated in this way for the processed commodity should be referred to as the STMR-P.
If data are available for the residues in the edible portion of the commodity (e.g. banana pulp), a STMR should be estimated directly using the edible portion residue values from maximum registered use trials (as opposed to using pesticide values for the whole commodity).
Acute dietary intake2
2 The estimation of acute dietary intake and MRLs for products of animal origin are currently under reconsideration by FAO, WHO and the JMPR.
For the purpose of acute risk assessment the MRL, or the highest residue in the edible portion, should be used in estimating dietary intake.
Estimation of MRLs for products of animal origin2
2 The estimation of acute dietary intake and MRLs for products of animal origin are currently under reconsideration by FAO, WHO and the JMPR.
In estimating MRLs for products of animal origin, theoretical feed intakes for domestic animals should be calculated using the STMR for each feed item (derived from supervised trials comparable with the maximum registered use), rather than the MRL, together with the maximum feed incorporation rates. This is in conformity with past JMPR decisions.
Estimation of STMRs for commodity groups
Where there are adequate trials data the STMRs should, in principle, be identified for the individual commodities and these values used for the intake assessment. However, where the MRL has been established for a group of commodities (e.g. pome fruit) a single STMR should be calculated for the group of commodities.
Presentation of STMRs in the JMPR monographs and report
The GAP(s) on which trials data have been selected for the purpose of identifying the STMR should be clearly identified in the monographs.
In tabulating trials data in the monographs the reviewer should ensure that, in addition to the normal underlining of trials data that are within GAP (and therefore have been used for the MRL evaluation), the single residue values selected for the estimation of the STMR are double- underlined.
Information on the residue values on which the STMR is based should not only be identified in the tabulated trials data (see above) but a list of the residue values selected should be included in the appraisal, in numerical order, with the median residue underlined. Where the residue situation is complex (e.g. a number of metabolites to be considered) these data may best be tabulated in the appraisal. In addition, the STMR values should be included in the recommendation table in the appraisal and in Annex 1 of the report.
The range for the rates and PHIs used in the selection of residue values for STMR should be clearly identified in the appraisal (e.g. trials data with application rates from 1.8 - 3.0 kg ai/ha have been selected).
Methods for presenting estimated STMR levels are still being developed. The aim is to communicate the results as clearly and unambiguously as possible; experience may indicate that further changes are necessary. As pesticides are used in a wide variety of situations, methods for evaluating data must be developed to take into account cases that are not already covered by the suggested procedures. For instance, the recommendations on the estimation of STMRs in animal commodities arising from residues in feed, acute dietary intake and ectoparasite treatments of farm animals may require further development. Further guidance will be developed at future meetings of JMPR.