3.1 Balancing nutrient import and export
3.2 Manure production
3.3 Ammonia emission
3.4 Surface water pollution
3.5 Nitrate leaching
3.6 Phosphorus leaching
3.7 Agricultural value
As mentioned in Chapter 1, the effect on the environment of the manure produced in a particular agricultural system should be assessed by considering its role in the total nutrient management of the system. If the import and export of nutrients in the system is in balance, and animal manure is to play a positive role, it implies that losses from animal manure must be minimal. It also implies that efficient use is made of the manure in crop production, i.e. a large fraction of the nutrients from the manure is taken up by the crop. An overview of possible losses is given in Figure 1. If, for example, NH3 volatilization from the stables is high, a large proportion of the N imported into the system through the feed is lost. This is an indication of unbalanced N management of the farming system. During storage of manure in the open, NH3 volatilization and NO3, P and K leaching occurs particularly when rainfall is high. This, again means nutrient losses. Surface spreading of manure without working in during periods of precipitation surplus on fallow land, may lead to NH3 volatilization, NO3 and P leaching, and surface runoff of manure. Eventually, the agricultural value of animal manure will appear to be low. Thus, the effects of manure management decisions early in the nutrient cycle will have a bearing on the later phases.
<???> Image
Van der Meer (1991) gives an example of an unbalanced system in the Netherlands. Of an intensive dairy farm,where the annual imports of N into the system largely exceed the exports. The total import of N per hectare is 533 kg through mineral fertilizers (383), imported feed (127) and atmospheric deposition (23), while removal of N amounts to only 84 kg through milk (72) and cattle weight gain (12).Thus, the annual N surplus of the system is, 449 kg N per hectare.
The extreme opposite of this system is presented in Sub-Saharan Africa, where Van der Pol (1992) estimates the annual nutrient depletion per hectare in a farming system in Southern Mali at 25 kg N, 20 kg K and 5 kg Mg. Stoorvogel et al. (1993) indicate an annual average nutrient loss per hectare from agricultural land in sub-Saharan Africa of 22 kg N, 2.5 kg P and 15 kg K for 1982-84, and estimate these values to be 26, 3 and 19 kg respectively in 2000. Here farmers derive a large part of their income from soil nutrient depletion or soil mining (Van der Pol 1992).
Animal densities in the Netherlands and Southern Mali differ considerably. An average Dutch dairy farm supports two to three highly productive livestock units (LU) per ha.
The overall animal density in the Netherlands, including intensive pig and poultry production and arable land, is over 4 LU per ha.
For Southern Mali, Van der Pol (1992) gives an overall animal density of 1.2 tropical livestock units (TLU) or 0.5 LU per ha of agricultural land.
Studying, the surplus and deficit situations in the above examples it is obvious that, manure management should aim at reducing the negative effects (lower nutrient losses) and maximizing the positive effects (plant nutrient supply and organic matter supply to the soil) of manure. A more balanced nutrient management will result with less burden on the environment.
Before an attempt can be made to assess the manure management in an agricultural system, at least the following key indicators should be explained:
- manure production, expressed as manure N or manure P production;The following sections illustrate some methods or rules of thumb for quantification of these indicators from literature data. The aim is to enable the livestock system specialists of this study to assess the impact of the manure produced in their respective systems.- NH3 emission, expressed as fraction volatilized N from mineral N in the manure;
- surface water pollution by manure, expressed as biological oxygen demand (BOD) and N and P load per kg manure discharged;
- NO3 leaching, expressed as fraction N leached from the soil of total N in manure;
- P leaching, for which the P balance per hectare will be used as an indicator;
- agricultural value of the manure, expressed as fertilizer equivalents.
3.2.1 Manure production per animal
3.2.2 Nutrient excretion per animal
3.2.3 Nutrient distribution between faeces and urine
The amount of manure produced by animals is very variable, even within species, partly due to differences in dry matter content of the manure. As water is not a very interesting component, we will be dealing mainly with the dry matter (DM), the organic matter (OM) and the nutrients N and P.
The little data on DM excretion reported in literature is difficult to interpret, partly because the amount of bedding included often varies, while the weight of the animals and the feeding situation is often poorly described. Müller (1980) gives manure production specifications for different animal species in different regions of the world but the basis for these estimates is unclear.
|
Ruminants |
30-55 |
|
Pigs |
10-35 |
|
Poultry |
10-35 |
Source: recalculated from common intake and digestibility values; in agreement with values given by Fleming and Mordenti (1993), Müller (1980), Romney et al. (1994) and WUMM (1994).On the other hand, faeces production (without bedding) is measured in every in vivo digestibility trial, as the DM in faeces equal the indigestible component of the diet. Thus, if feed intake and apparent digestibility are known, faeces DM production can be calculated.
Although the prediction of ad lib feed intake is complicated (Tolkamp and Ketelaars, 1992) and in practice animals are often not allowed to eat up to their maximum capacity, reasonable guesstimates can be made for most practical situations. Some indications for faeces DM production per species are given in Table II. Values are given in g per kg0.75, as feed intake and consequently manure production is more close by related to metabolic weight to bodyweight. Lowest values are most likely to be associated with rations of very low and high nutritive quality (ruminants) or with rations of very high quality only (monogastrics with restricted feeding), though manure production is also influenced by palatability of the feed, genetic differences in feed intake capacity, and feed management. More accurate estimates can be made for specific situations if feed intake and digestibility are reasonably well known.
The values in table II refer to the quantity of dry matter excreted by animals and not to the collectable amount. During storage, decomposition of organic matter starts, thereby reducing the quantity of collectable manure. The rate of decomposition is dependent on both environmental conditions and manure characteristics. Manure with a high content of easily degradable organic matter compounds, particularly poultry manure, may have high decomposition rates: up to 10% after one week and 40% after 3 months (Müller, 1980).
The positive and negative effects of nutrients in manure production, are more important than the dry matter. The nutrients excreted by animals can be derived by subtracting the nutrients converted into livestock (nutrient retention) products from the intake of nutrients via the feed. Table III shows typical nutrient retention values for major animal products, where variation especially in nutrient content per kg of live weight can be seen due to differences in the fat content of animals (fat animals having slightly lower N content per kg of live weight).
|
|
N |
P |
|
Ruminants |
26.0 |
7.5 |
|
Pigs |
22.5 |
5.0 |
|
Poultry |
28.0 |
4.0 |
|
Eggs |
19.2 |
2.1 |
|
Milk (3.4% protein) |
5.4 |
0.9 |
Sources: ARC, 1980; Jongbloed and Everts, 1992; WPSA, 1985; WUMM, 1994.Average nutrient content of almost every feedstuff can be derived from standard feed composition tables. Estimation of feed intake and feed composition is a bigger problem, as the intake is unknown with the exception of some highly advanced year round stall-fed livestock systems However, some reasonable assumptions can be made for practical situations. Table IV gives some examples for nutrient excretion of animals. Owing to the variation in intake and nutrient content of the feeds, these values represent examples and not averages for low or high productive situations.
A distinction between nutrients in faeces and in urine is often highly relevant as management for both types of manure output is often different: collection and utilization rates of urine are often much lower. To assess the quantity of NH3 emitted (Section 3.3) and the agricultural value of manure (Section 3.7) a distinction between N in mineral form (Nm) and N in organic form is highly relevant. Though some Nm may be present in manure, mainly originating from manure decomposition, its concentration is generally negligible. Thus, Nm is in most practical situations equal to the quantity of N in urine.
Digestible protein values, often reported in older literature, are not very accurate for formulating feeding rations, as they do not represent the value of a feed for a particular animal. However, for the estimation of N in faeces, information on digestibility is very useful as the difference between crude protein (CP) intake and digestible crude protein (DCP) intake is the amount of N in the faeces, using the assumption that protein consists for 16% of N. The amount of N in urine can then be estimated as the difference between digestible N intake and N retention in livestock products.
From Table V it is clear that high N excreted via urine is prevalent in high production situations. This is particularly true if rations are unbalanced in terms of rumen degradable energy and rumen degradable protein. In low production situations, N excreted via urine is relatively lower, especially if feed rations are low in N (which is common for many animals in developing countries): e.g. urine from cows on sub-maintenance rations of straw will contain hardly any N as almost all N is recycled to the rumen instead of being excreted via the urine. In low production conditions, N in the urine is also lower, while N in faeces is higher, due to higher amounts of tannin in the feed which reduce rumen degradability of protein (Romney et al., 1994).
|
|
Intake |
Retention |
Excretion |
|||
|
Animal |
N |
P |
N |
P |
N |
P |
|
Dairy cow (1) |
163.7 |
22.6 |
34.1 |
5.9 |
129.6 |
16.7 |
|
Dairy cow (2) |
39.1 |
6.7 |
3.2 |
0.6 |
35.8 |
6.1 |
|
Sow (1) |
46 |
11 |
14 |
3 |
32 |
8 |
|
Sow (2) |
18.3 |
5.4 |
3.2 |
0.7 |
15.1 |
4.7 |
|
Growing pig (1) |
20 |
3.85 |
6 |
1.3 |
14 |
2.5 |
|
Growing pig (2) |
9.8 |
2.9 |
2.7 |
0.6 |
7.1 |
2.3 |
|
Layer hen (1) |
1.23 |
0.26 |
0.36 |
0.04 |
0.87 |
0.22 |
|
Layer hen (2) |
0.55 |
0.15 |
0.05 |
0.006 |
0.50 |
0.14 |
|
Broiler (1) |
1.09 |
0.17 |
0.45 |
0.075 |
0.64 |
0.10 |
|
Broiler (2) |
0.41 |
0.11 |
0.13 |
0.018 |
0.28 |
0.09 |
1) High production situationsTo assess the P excreted by monogastrics the same methodology can be applied, but the digestibility of P is less well established, partly because it is influenced by the supply of P in relation to animal requirements, content of other minerals (especially Ca and vitamins in the feed), etc.
2) Low production situations
In most low production situations, P excreted via urine is low, partly because feeds with low P digestibility are common (see Annex I). In high production situations, P excreted via urine might be considerable (up to 30-40% of total excreted P), especially if most of the feed consists of feedstuffs with a high P digestibility (animal products and mineral P).
Phosphorus excreted via urine by ruminants is usually negligible because a possible surplus of digested P is recirculated to the rumen and from there excreted in faeces instead of in urine.
For most other nutrients, digestibility and retention are not well enough understood to make generalized assessments for the distribution between urine and faeces. It is known, however, that most of the excreted K is in urine (see Annex IV).
|
Species |
% of total excreted N |
|
|
Faeces |
Urine |
|
|
Dairy cow 1) |
31 |
69 |
|
Dairy cow 2) |
50 |
50 |
|
Sow 1) |
27 |
73 |
|
Sow 2) |
36 |
64 |
|
Growing pig 1) |
22 |
78 |
|
Growing pig 2) |
41 |
59 |
|
Laying hen 1) |
18 |
82 |
|
Laying hen 2) |
30 |
70 |
|
Broiler 1) |
17 |
83 |
|
Broiler 2) |
40 |
60 |
1) high production situations
2) low production situations
3.3.1 Adjustment of feeding rations to reduce NH3 emission from manure
3.3.2 Emission from stables and storage
3.3.3 Emission after manure application to land
3.3.4 Emission from grazed pastures
3.3.5 Emission from flooded fields
3.3.6 Threshold value
The mineral N fraction varies from 0.10 for farmyard manure to 0.94 for urine depending on the type of animal and the type of collection and storage system of the manure. Practically all N in fresh urine is in the form of urea, CO(NH2)2, which is hydrolysed into ammonium carbonate in the presence of urease (Eq. 1). This is an unstable compound and decomposes readily into NH3 and CO2 (Eq. 2). Volatilization of NH3, however, is governed by the chemical equilibrium between NH4+ and NH3 (Eq. 3) and the equilibrium between aqueous and gaseous NH3 (Eq. 4).
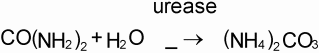
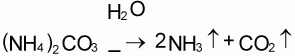
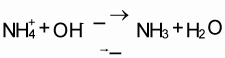
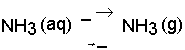
Hydrolysis of urea increases the pH, resulting in higher concentrations of OH in the solution. This forces the equilibrium of Eq. 3 to the right, thus stimulating NH3 production. The rate of volatilization of NH3 as described in Eq. 4 is linearly related to the vapour pressure of NH3 in the solution, which is linearly related to the concentration of NH3 in the solution, and is furthermore temperature-dependent. The rate of volatilization also depends on wind speed (Denmead et al., 1982). The most common method of measuring NH3 emission in the field is a micro meteorological method described by Denmead (1983).
In addition to adjusted manure storage and application techniques, NH3 emission can be limited by feed ration adjustments. Since most of the N in the urine originates from an imbalance between the amount and the quality of digestible protein and animal requirements, the amount of N in the urine can be reduced by adjusting the feed ration.
In intensive ruminant production systems, balancing the intake of rumen degradable protein and rumen degradable energy can produce major effects. In most European countries this would require a substantial reduction in the quantities of grass fed, as grass with a reasonable productivity per hectare, almost inevitably has a high surplus of rumen degradable protein. However, this would imply a significant shift in feeding practices and even in the whole livestock system.
In intensive monogastric production systems, reductions in N losses will be mainly achieved by adjusting the protein content of the feed to the variable requirements of animals of various age and productivity (phase-feeding) and by balancing amino acid requirements and digestible amino acids offered. For the last-mentioned option, addition of synthetic amino acids is an important and increasingly popular strategy. Many commercial compound feeds for monogastrics contain added synthetic amino acids, mainly lysine and methionine as these are often the first limiting amino acids. High costs may prevent broader application of other limiting amino acids (Schutte and Tamminga, 1992).
Much research has been conducted on NH3 volatilization in intensive livestock systems in the Netherlands where the animals are kept indoors for most of the year. Urine and faeces are collected together through the slatted floor in the stables and the manure is stored in liquid form. A considerable part of the total N excreted volatilizes in the stables and during storage. Some of the data collected in the Netherlands are given in Table VI. Depending on the measures taken to reduce NH3 emission, between 5 and 35% of the total N excreted volatilizes even before it is applied to the land. Measures to reduce the NH3 emission are based on one or more of the following strategies:
- minimize the area of contact between manure and air, e.g. by better or more frequent cleaning of the stables and by covering the manure storage;Annex II gives some manure collection systems with limited NH3 emission from pig houses in The Netherlands.- decrease the concentration of NH3 (and thus the vapour pressure) in the manure by dilution with water;
- lower the pH in the manure by addition of acid; and
- stop the formation of NH3 from uric acid in poultry manure by drying until the dry matter content exceeds 70%.
No data can be found for intensive livestock systems in other climatic zones. It can, however, be envisaged that emissions would be higher in climates with higher average annual temperatures because of the effect of the temperature, and because more aeration of the stables would be necessary.
In locations where there is a concentration of cattle (such as in feedlots, kraals and bomas) faeces and urine are trampled by the animals, this leading to increased NH3 emission. No quantitative data for these situations have been found. Minimizing the contact area between manure and air by cleaning and covering reduces emissions.
|
Source |
Emission per type of animal (as fraction of mineral
N in excreta) |
|||||
|
Dairy cattle |
Veal |
Pigs |
Sows |
Layers |
Broilers |
|
|
Stable - normal |
0.18 |
0.19 |
0.23 |
0.27 |
0.11 |
0.13 |
|
Stable with minor adjustments |
|
|
0.18 |
0.15 |
|
|
|
Stable with major adjustments |
0.09 |
|
0.12 |
0.12 |
0.05 |
0.04 |
Adapted from: Van der Hoek, 1994 and Monteny, 1991.The effect on NH3 emission of the use of organic materials with a high C:N ratio as bedding material is also worth investigating as it may cause microbial immobilization of N from excreta. Little quantitative information is available, partly due to measurement problems. In one deep litter stable with straw bedding, NH3 emission was 23% less than the average emission from dairy cattle stables in the Netherlands (Groenestein and Reitsma, 1993). Methane emission from this deep litter stable, however, was three to four times higher than the values mentioned in literature for other stables.
Manure storage outside the stables is another source of NH3 emission. High NH3 emission will occur from lagoons as the major part of the N is in mineral form, though the level is greatly dependent on factors like depth of the lagoon, storage time and mineralization rate. Schulte (1993) mentions losses of 70-80% of the total N.
In the Netherlands, emission from slurry from dairy cattle stored in separate storage tanks is estimated at 7-14% of the mineral N in the excreta and from pig slurry at 10-19%. Covering the manure storage achieves an emission reduction of 80% (Monteny, 1991).
In the introduction to this section it was mentioned that the rate of NH3 volatilization is strongly influenced by NH3 concentration in the manure, temperature and wind speed. These factors, however, do not necessarily influence the total amount of NH3 volatilized during, say, a period of seven days after surface spreading. Hence, the total amount of NH3 volatilized in seven days in warm, dry and windy conditions may not be very different from that in cool and quiet conditions. The difference is that in the first situation 95% may volatilize during the first day after application and the remaining 5% during day 2-7, while in the second situation volatilization is spread more evenly over the week (Figure 2).
According to various sources (e.g. Terman, 1980; Monteny, 1991), NH3 volatilization after surface spreading of liquid cow or pig manure amounts to between 30 and 100% of the total mineral N applied. A substantial reduction in NH3 volatilization after application is possible by using one of the following techniques:
- Dilution of the manure. Experiments with 1:1 and 1:3 water diluted manure have shown substantial reductions in emission (Monteny, 1991).- Rain. An artificial shower of 20 mm produced by means of a sprinkler installation.
- Immediate tillage after manure application in such a way that the manure is worked in.
- Sod manuring in grassland. The liquid manure is applied in very narrow furrows made in the sward.
- Injection. The manure is injected into the soil at a depth of 10-20 cm.
- "Drag-hose machine". This machine applies the manure very close to the surface in such a way that contact between manure and air is minimal at the time of application.
|
Application technique1 |
Average |
Range |
|
Surface spreading |
0.59 |
0.29-0.91 |
|
Diluted manure 1:3 |
0.24 |
0.12-0.35 |
|
Rain 20 mm |
0.15 |
0.11-0.18 |
|
Immediate tillage |
0.17 |
0.14-0.20 |
|
Sod manuring |
0.06 |
0.04-0.08 |
|
Injection |
0.04 |
0.00-0.13 |
|
Drag-hoses |
0.35 |
0.25-0.44 |
1 For an explanation of the application techniques see text and Annex III. Data adapted from Monteny, 1991.<???>Image
Sophisticated machinery has been developed in recent years for the last three options.
Gross estimates of NH3 emission under the various manure application practices are shown in Table VII. As the data collected by Monteny (1991) show large variations, the range in measured emission is also given. These variations are attributed to the variable weather conditions during the period of measurement. More information on the application techniques mentioned in Table VII can be found in Annex III.
Emission of NH3 from grazed pastures can be attributed mainly to volatilization from urine patches. Volatilization from dung pats is less significant. Various authors (Denmead et al., 1974; Galbally et al., 1980; Healy et al., 1970; Vallis et al., 1982; Vertregt and Rutgers, 1987) have reported NH3 volatilization from urine patches ranging from 4 to 29% of the N in the urine. There seems to be a consensus that in temperate climates about 10% of the N in the urine volatilizes and in subtropical and tropical climates the figure is about 25%.
Terman (1980) reports data on NH3 volatilization from flooded rice soils ranging from 3-50% when mineral fertilizers such as ammonium sulphate, ammonium nitrate or urea are applied. No data have been found where in flooded conditions, NH3 volatilization from mineral fertilizers is compared with NH3 volatilization from animal manure. Incorporation of green manure, however, resulted in a cumulative NH3 loss of 3-4% whereas loss from incorporated urea was 10% (IRRI, 1991). One can reason that, under flooded conditions, NH3 loss from animal manure should be lower than loss from mineral fertilizers because (1) the concentration of NH3 is usually lower after use of animal manure; and (2) decomposition of organic material from manure increases the CO2 pressure and the increased acidity created reduces NH3 volatilization (see Eq. 3). This assessment is in agreement with results from Japan (Wolf and van Keulen, 1989) where N losses from inorganic fertilizer plots were much higher than that from organic manure plots (51.4 and 22.3 kg per ha respectively), though no distinction was made between volatilization and denitrification.
The main sources of NH3 in the atmosphere, in countries with intensive livestock systems, are surface spreading of manure and the livestock housing system (Sequi and Voorburg, 1993).
The negative effects of NH3 volatilization are bad odour and, after deposition, nitrogen enrichment and acidification of the soil and surface water. It is impossible to set general threshold values for NH3 emission of livestock because of several reasons:
1) Other sources, like industry, cars and households, also contribute to total acid deposition. In most situations NH3 is less important than SO2 and/or NOx. In Europe (including Russia), for instance, NH3 only contributed to ca. 24% of the total acid emission, compared to more than 50% by SO2. Only in relatively small areas with high animal densities, like the Netherlands, is NH3 the major contributor to total acid deposition (Heij and Schneider, 1995), though there are indications that NH3 is also becoming important in other areas (Van der Eerden, 1995).2) The threshold value is dependent on the type of soil and vegetation, the main problem (vegetation change or N leaching, etc.). In the Netherlands this gives a variation of maximum deposition values of ca. 460% (ranging from 650 to 3000 mol/ha; Heij and Schneider, 1995). Lowest values are mainly related to the increase of nitrophic plants.
3) A threshold value for NH3 deposition is not easily translated into a threshold value for emission as the dispersion patterns are highly dependent on climatic factors, such as prevailing wind speed and direction, rain, etc. However, most of the emitted NH3 is deposited at a short distance from the source (see table VIII).
4) Maximum N deposition is dependent on N uptake via plants. Higher plant uptake reduces the potential acid function of NH3. Maximum deposition values of NH3 all assume a zero nett uptake, which is only relevant in non-productive nature areas.
|
Distance (m) |
Vegetation |
Other |
|
Forest |
||
|
20 |
21000 |
10500 |
|
50 |
7110 |
3570 |
|
100 |
2340 |
1170 |
|
200 |
660 |
330 |
|
500 |
108 |
54 |
|
1000 |
26.7 |
13.2 |
3.4.1 Direct discharge
3.4.2 Run off
3.4.3 Threshold values
In some countries with intensive livestock systems (particularly Eastern Europe), manure is discharged directly into the surface water, often after first having been treated in lagoons. This implies complete loss of nutrients and organic matter in the manure. Direct discharge into surface water causes an-aerobiosis in the water, because the easily decomposable fraction of the manure will start to decompose immediately using all dissolved oxygen and killing the water fauna and flora. In addition, the load of nutrients in the manure will contribute to water eutrophication and heavy metals will cause toxicity. Where grazing animals have free access to surface water, they can urinate and faecate in it or on the slopes leading to it. Direct discharge or runoff into the water will be the result.
To some degree, runoff of manure into surface water causes the same problems as direct discharge. The most important sources of runoff are locations where animals are concentrated such as feedlots, stables, bomas and kraals. The manure in these places is often drained and the liquid allowed to flow, directly or indirectly via the soil, into surface water. Runoff from manure applied to the surface of agricultural land can also occur.
Factors affecting runoff are:
- rate of manure application;- precipitation, total amount as well as intensity;
- infiltration capacity of the soil, which is nil or low for frozen, sun-baked and waterlogged soils; and
- slope of the soil surface.
The values of directly discharged or runoff manure for oxygen demand, nutrient and (some) heavy metal loads all exceed the respective threshold values for effluents into surface water. The EU has set threshold values of effluents into surface water at 25, 15 and 2 mg/l for BOD, N and P, respectively (Council Directive 91/271/EEC, 1991), while typical BOD values for manure and open yard runoff range between 10,000-30,000 and 1,000-2,000 mg/l respectively (Archer and Nicholson, 1992). These practices should, therefore, never be permitted.
3.5.1 Nitrification and denitrification
3.5.2 Factors affecting fate of "N not accounted for": leaching or denitrification
3.5.3 Threshold values
Nitrogen from manure may be subject to many transformation processes (Figure 3). Fresh animal excreta does not contain N in the form of NO3. If manure is not aerated (e.g. by composting), NO3 will not be formed until after application to the soil.
Transformation of NH4 into NO3, called nitrification, is a microbiological process (Eq. 5) that needs oxygen (O2). In well-aerated soil, nitrification of NH4 added to manure may be completed in a few days. In less aerated soil it may take weeks. At low temperatures, the process is very slow. NO3 can also be formed after decomposition of the organic N compounds of manure.
<???>Image
Transformation of organic N into mineral N is a microbial process called N mineralization. Following NO3 formation, complete or partial denitrification can take place during which N2 and N2O are emitted (Eqs. 6 and 7).
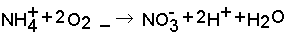
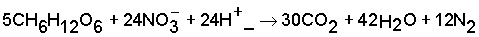
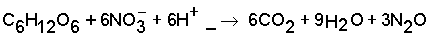
As in soils usually aerobic and anaerobic patches or conditions may occur concurrently or alternately (rain showers, irrigation runs), both nitrification and denitrification processes may take place both at the same time and alternately. This makes it extremely difficult to predict the fate of applied nitrogen that is not volatilized or added to the soil N, or recovered by the crop: leached as NO3 or emitted as N2/N2O. An example of an estimate of this ‘N not accounted for’ is given in Box 1.
|
In a temperate climate, grassland is used for fodder
production without grazing. In spring, 60 ton/ha cattle slurry is applied by
surface spreading. Total N applied with the slurry is 312 kg. NH3
volatilization accounts for a loss of 50% of the mineral N: 78 kg. Additional N
fertilization with mineral fertilizer was 350 kg N. Total N removed by harvested
grass is 400 kg. The mineral N content in the soil in the next spring is the
same as in the previous year. The nitrogen balance for the mineral N in the soil
is: |
|
Input: |
|
mineral fertilizers 350 kg N |
|
|
mineral N from slurry |
78 kg N |
|
|
N mineralized from slurry (30% from organic N, see Table
XI) |
47 kg N |
|
Total |
|
475 kg N |
|
Output: |
|
N in harvested grass 400 kg N |
|
Balance: |
|
75 kg N |
If it is assumed that no immobilization of the remaining N occurs, the balance of 75 kg N must be either leached as NO3 or denitrified as N2 or N2O. This balance is sometimes referred to as "N not accounted for".
The level of N fertilization the major factor determining the amount of N available for NO3 leaching or denitrification. It may be assumed that with moderate N doses (0-150 kg/ha for arable crops or 0-300 kg/ha for grassland in temperate zones) no NO3 is present in the soil at the end of the growing season (Van der Meer, 1991). Only higher N doses would result in residual NO3, as illustrated in Figure 4 for grassland in a cut-and-carry system on clay soil in the Netherlands. High N doses may be applied in situations where animal manure is seen as a waste or where the level of mineral N fertilization is determined without taking into consideration the N effect from animal manure if both nutrient sources are combined.
Under grazed pastures, NO3 leaching can be considerable even at moderate rates of N fertilization because of leaching from urine patches, which is up to 5 times higher than under grassland without grazing (Garwood and Ryden, 1986). Van der Meer and Meeuwissen (1989) present the results of a study on the effect of grazing on nitrogen emissions. In Table IX, their results have been summarized. It is clear that on a well-drained, sandy soil in the Netherlands, NO3 leaching is much higher under grazed pastures than under a cut-and-carry system. Similar data for other soil or types or climates have not been found.
|
|
N fertilization (kg/ha/year) |
|||||
|
0 |
100 |
200 |
300 |
400 |
500 |
|
|
N load in urine patches (kg/ha) |
225 |
323 |
454 |
595 |
717 |
858 |
|
NO3 leaching without urine |
15 |
18 |
22 |
28 |
57 |
103 |
|
Net effect of urine on leaching |
2 |
4 |
22 |
58 |
89 |
116 |
|
Total leaching |
17 |
22 |
44 |
86 |
146 |
219 |
Source: Van der Meer and Meeuwissen, 1989.Not only the level of N fertilization, but also the timing of application affects NO3 leaching. Nitrate can accumulate in the soil after removal of the crop through N mineralization from soil organic matter and easily decomposable organic N from crop residues or manure (Ne). The "N not accounted for" must be considered a loss. Many factors influence the fate of the residual N between the end of the growing season and the subsequent crop. The following crop can be sown immediately after or even before harvest of the main crop, thus acting as an "interceptor" of NO3 that otherwise would have leached or been emitted as N2/N2O after denitrification. Without this so-called cover crop, the partitioning between leaching and denitrification is influenced by the rate of NO3 formation, the balance of precipitation and evaporation, the amount of organic matter in the soil and its drainage conditions.
Leaching from unsealed kraals, feedlots and solid manure storage heaps can be considerable, as the conditions are similar to very high application rates. In general, leaching from lagoons should be less than from solid manure storage heaps as its anaerobic conditions will hardly allow for nitrification. No appropriate quantitative data have been found on this subject, but the level of leaching is highly dependent on the precipitation surplus, in case of uncovered kraals, feedlots and solid manure storage heaps, and on soil conditions (permeability).
A maximum concentration of 50 mg NO3 per litre is allowed for drinking water by the World Health Organisation (WHO) of the United Nations. At higher NO3 concentrations, public health is at stake through the formation of toxic nitrite from NO3 in the human body. Babies are particularly vulnerable to nitrite if drinks are made of water exceeding the threshold value. To reach this objective, less than 34 kg N/ha may leach annually, given a precipitation surplus of 300 mm/y (50 mg NO3 = 11.3 * 10-6 kg N; 300 mm precipitation surplus = 3 * 106 l/ha; 11.3 * 3 = 34 kg N). A translation into a maximum N surplus per ha, however, is impossible as quantities N emitted via denitrification, ammonia emission, etc. vary widely.
3.6.1 Phosphorus balance
3.6.2 Phosphorus balance in relation to other environmental problems of manure application
3.6.3 Adjustment of feeding rations to reduce P excretion
3.6.4 Threshold values
Leaching of P from agricultural land into surface water is an important contribution to its eutrophication. This may lead to excessive growth of algae and eventually to anaerobic water. The water becomes smelly and water life is seriously disturbed.
Due to the complex sorption and fixation behaviour of soils with regard to P, no direct relation has been found between P fertilization and P leaching. For sandy soils in the Netherlands, however, a relation can be given between P in the soil solution, P saturation level of the soil and P leaching. The P saturation level indicates to what extent the soil's P retention capacity has been utilized. When P retention capacity of the soil body above the highest ground water level exceeds 25%, it becomes P saturated and P-leaching to ground water and surface water is almost inevitable even if fertilization is reduced. This is one of the reasons why governments place much emphasis on the containment of P2O5 over-fertilization.
For agricultural purposes, some over-fertilization is necessary, i.e. more P should be added to the soil than removed in crop products to maintain an adequate P condition of the soil. The required overdose of P depends on factors such as crop, soil type and ground water level, but is in any case 25 kg P2O5/ha/year or more in the Netherlands. Legislation in the Netherlands on the use of animal manure in agriculture is based on the addition to the soil of P2O5. Table X shows for the period 1990-2000 the maximum amounts of P2O5 in animal manure that have been and probably will be allowed to be added to the soil for different crops. As it was common practice for many intensive livestock holders to use maize plots as "dumping sites" for their manure surpluses, the law allowed for relatively high amounts of P2O5 on maize during the first years, to give those farmers the opportunity to adjust their manure management to the new legal situation. The objective is that by the year 2000 the estimated P2O5 addition to the amount removed in crops will be restricted to 90 kg/ha for grassland and 65 kg/ha for maize and other field crops averaged over the country. As yields vary among regions and farms, so will crop removal of P2O5. The present idea is, therefore, to identify farm-specific maximum doses of P2O5 application. A compulsory P balance registration system at farm level will be the tool to determine this dose. Whether legislation and law enforcement can manage this system is questionable.
|
|
1990 |
1991-2 |
1993 |
1994 |
1995 |
1996 |
2000 |
|
Grass-land |
250 |
200 |
200 |
200 |
150 |
135 |
90 |
|
Maize |
350 |
250 |
200 |
150 |
110 |
90 |
65 |
|
Field crops |
125 |
125 |
125 |
125 |
110 |
90 |
65 |
Note: Legislation is given in kg P2O5, which is 2.29 x PCurrently, policy makers and specialists are discussing specification of an acceptable overdose on top of the amount of P2O5 removed by the crop.
A large gap, however, exists between the environmentally desirable overdose (<1 kg/ha, Section 2.6.3) and the agriculturally optimal overdose (>25 kg/ha). Discussion on this topic has been going on for some years and is expected to continue. The basic principle of balancing P addition to the soil and P removal in crops will be maintained. Box 2 illustrates this principle for two contrasting situations.
It is sometimes stated that environmental problems like N leaching and heavy metal accumulation will be negligible if P equilibrium is maintained at field level (e.g. Jongbloed, 1991). This statement is obvious for N leaching because the N:P ratio in manure is in all but very exceptional cases lower than the N:P ratio in crops. Thus, N removal in crops will be generally higher than N-application via animal manure (see Box 3).
Box 3 also shows that problems with Cd and Zn are not likely. If Cd accumulation problems will occur, they are mainly related to phosphorus fertilization: P fertilizer often has a relatively high Cd content, e.g. ca. 80 mg per kg P2O5 in the Netherlands (Heidemij, undated), though P fertilizer with less Cd is available.
|
In Southern Mali, it is estimated that 8.5 kg
P2O5/ha is removed via food crops (mainly millets and
pulses). One TLU will produce ± 1.92 kg P2O5 per year
which can be applied on arable land (46% of total P-excretion; van Duivenbooden
et al. 1991). Thus, 8.5/1.92 = 4.4 TLU (» 1.8 LU) per ha of crop land would
result in a P equilibrium, if no other P containing materials were removed from
the land, which is not the case as soil erosion is considerable. |
|
An average crop of silage maize in the Netherlands removes a P
equivalent of 65 kg P2O5/ha (± 13 ton DM/year). One
crop is grown every year. Only silage maize is removed from the land. According
to environmental requirements, a maximum P addition to the soil of 66 kg
P2O5/ha/yr is then allowed. If the annual manure P
production per pig is 5.7 kg P2O5 (Table IV: 2.5 kg P *
2.29), pig slurry of 11.5 pigs per ha maize can be applied. In case of dairy
cattle the maximum animal density is 1.7 LU per ha (66/(16.7*2.29)); Table
IV). |
Continuous application of other types of animal manure will cause even less problems as heavy metal concentrations in the feed of these animals are lower, with the possible exception of Cd in broiler feed (Heidemij, undated). Note, on livestock farms, when buying part of the feed as concentrate, heavy metal accumulation will always be possible as both retention in animal products and leaching are very low, resulting in a gradual build up of heavy metal concentrations in home-grown feed.
|
In Box 2 P equilibrium for a maize crop in The Netherlands was estimated at 11.5 pigs per hectare. Annual N excretion of 11.5 pigs is equal to 161.5 kg (11.5 * 14; Table IV), while with maize ± 183 kg N is removed per year (13 ton DM, CP-content 88 g/kg DM). Annual Cd excretion of 11.5 pigs is ± 1.1 g (assuming 0.13 mg Cd in pig feed; Heidemij, undated). With the maize crop 5.6 - 49 g Cd is removed (0.43 - 3.79 mg per kg DM; Heidemij, undated). Annual Cu excretion by 11.5 pigs is ± 514 g (using feed intake of Annex I, Cu content in starter feed 175 mg/kg and in fattening feed 35 mg/kg, retention is assumed to be negligible). With the maize crop 57 - 105 g Cu is removed (4.4 - 8.1 mg per kg DM; Heidemij, undated). Annual Zn excretion by 11.5 pigs is ± 789 g (using feed
intake of Annex I, Zn content in starter feed 100 mg/kg and in fattening feed 90
mg/kg, retention is assumed to be negligible). With the maize crop 676 - 1397 g
Zn is removed (51.8 - 269 mg per kg DM; Heidemij, undated). |
It is commonly assumed that P digestibility of feedstuffs of plant origin is about 30-35%, but large variations seems to exist, ranging from 20% or lower for maize and rice bran, to ca. 38% for barley and soybean meal and ca. 46% for wheat and peas. Digestibility is highest in feedstuffs of animal origin, ranging from 70 to 90% (Jongbloed and Kemme, 1990).
Part of the variation in P digestibility of feeds of plant origin can be explained by the content of both phytate P and phytase: phytate P is hardly digested, but if phytase activity is high P digestibility will increase. Phytase of plant origin is present in germinating seeds, but also in grains in rest, particularly rye and wheat. Major efforts have been made to examine the effects of the addition of microbial phytase to pig and poultry feeds to increase P digestibility, and consequently, to reduce the amount of P excretion (Simons et al., 1990). It is estimated that P digestibility can be increased up to 60% via the use of phytase. Important aspects of research were the thermo-resistance of the enzyme, as temperatures above 80 °C occur during pelleting, and its activity under low pH conditions resulting in the identification of micro-organisms producing suitable enzymes for use in the compound feed industry.
An alternative possibility to reduce P excretion is to alter feed rations to include ingredients with higher P digestibility i.e., mainly feedstuffs of animal origin or leguminous feeds. However, the use of more leguminous feeds is at this moment seriously hampered by the occurrence of high levels of anti-nutritional factors in most of these feeds.
The policy in the Netherlands aims at a total P concentration in surface water below 0.15 mg per litre. Research has indicated that at this concentration, no excessive growth of algae will occur. The water will remain sufficiently aerobic to be suitable for multiple use: fish, water recreation, irrigation, etc.
To reach the environmental objective of 0.15 mg P total/l or less in the ground water, the P2O5 overdose in a possible steady state situation in the Netherlands cannot exceed 1 kg P2O5/ha/yr (The annual precipitation surplus in the Netherlands is 300 mm, which is 3*106 l per ha; this volume of water with P concentration of 0.15 mg/l contains 3*106 * 0.15*10-6 = 0.45 kg P; this equals 0.45 * 2.29 = 1.03 kg P2O5). At an annual precipitation surplus of, say, 600 mm, the environmentally maximum allowable overdose would be 2 kg P2O5/ha.
For ground water, a threshold value of 0.10 mg ortho-P/l is suggested for sandy soils at the depth of the mean highest ground water level. At this concentration, flow of ground water to surface water is assumed not to cause the concentration of P in the surface water to exceed the indicated threshold value.
3.7.1 Fertilizing value of nitrogen in manure
3.7.2 Fertilizing value of phosphorus in manure
3.7.3 Fertilizing value of potassium in manure
3.7.4 Contribution of manure to soil organic matter
The fertilizing value of animal manure not only depends on its composition, but also on the crop to which it is applied, the climatic conditions, the type of mineral fertilizer substituted, the method of application and the time of application in relation to crop growth.
<???>Image
The efficiency of a nutrient in animal manure can be expressed in the working coefficient. The working coefficient indicates how much nutrient from a reference mineral fertilizer is used to produce the same yield as an application of animal manure which contains 100 kg of the nutrient. Figure 5 gives an illustration of the concept. For both animal manure and mineral fertilizer, nutrient-yield response curves can be plotted. Usually the crop responds better to nutrients in a mineral fertilizer than to nutrients in animal manure.
In Figure 5 this is q kg, hence the working coefficient is q/100. The mineral fertilizer used for comparison of the animal manure, is called the reference fertilizer. As yield response varies among mineral fertilizers, the working coefficient for a particular type of animal manure depends, among other things, on the choice of the reference fertilizer.
As mentioned earlier (Figure 3), N in manure and in soil can be present in various forms. Furthermore, plants and microorganisms can use N in different ways. Because of this complexity, crop utilization of N is rather unpredictable. It would be best to establish, through experiments, crop response curves under all circumstances relevant to the identified livestock systems and determine the working coefficients as shown above. Only through experimentation can working coefficients be determined as illustrated in Figure 5. Such data, based on experiments, are often not available, so an alternative approach is suggested.
First, the form in which N is present in the manure has a decisive influence on the availability for plants. Higher plant availability of N in the manure implies that it can substitute for more mineral fertilizer, i.e. the working coefficient will be higher. Sluijsmans and Kolenbrander (1977) suggested differentiation between:
- Nm: N present in mineral form;The fractionation depends, among other things, on the type of animal, its diet and the method and duration of storage of the manure (Table XI). The working coefficients can now be estimated with some assumptions (example in Box 4).- Ne: N mineralized within one year after application in temperate zones or within three months in lowland tropics;
- Nr: N mineralized later.
In the example in Box 4, assumptions have been made for the situation of a wheat crop in a temperate climate. These include:
|
|
Nm |
Ne |
Nr |
|
Cattle farmyard manure |
0.10 |
0.45 |
0.45 |
|
Cattle liquid manure |
0.50 |
0.15 |
0.35 |
|
Pigs liquid manure |
0.50 |
0.22 |
0.28 |
|
Poultry liquid manure |
0.70 |
0.20 |
0.10 |
|
Veal liquid manure |
0.80 |
0.09 |
0.11 |
|
Urine |
0.94 |
0.03 |
0.03 |
Sources: Sluijsmans and Kolenbrander (1977) and Van der Meer (pers. comm.)Flooded rice is a special case. Here, the manure mineral N dose will usually be low compared with the urea mineral N dose. In both cases the mineral N is diluted many times by the irrigation water. Decomposition of manure, however, will increase the CO2 concentration in the water and decrease the pH. Therefore, x will be very low. The NH3 volatilized from urea will be more than that from the reference fertilizer (section 3.3.5).1. Ammonia volatilization during and after application: One of the processes determining the working coefficient of Nm is volatilization of NH3. The working coefficient for Nm can be considered as (1-x), where x is the fraction of volatilized Nm. When x=1, all Nm volatilizes as NH3, which may happen when liquid manure is surface applied without being worked in. In Table VII of section 3.3.3 more information on the determination of x for cattle and pig slurry can be found.
2. NH3 volatilization before application: To estimate the mineral N (practically all in the form of NH3) at the time of application Nm, volatilization before application, in the stable and during storage should be taken into account (3.3.2). Table VI, gives the summarized dataFor tropical lowland at an average annual temperature of 27 °C, Nr refers to residual organic N three months after application of the manure.3. NO3 leaching: The wheat crop only utilized half of the N mineralized annually. The remainder is assumed to have leached out or denitrified. Timing of the manure application is important in this respect. A longer period between manure application and crop planting date leads to higher emissions. Autumn application, winter fallow and spring planting, would result in a much lower N working coefficient (Section 3.5).
4. Temperature: Higher temperatures result in more rapid N mineralization. As rule of thumb Janssen (1993) says that the decomposition rate doubles for every 9 °C rise in average annual temperature. Nr as given in Table XI refers to the temperate zone at an average annual temperature of 9 °C. For a climate with an average annual temperature of 18 °C, Nr refers to organic N six months after application.
Applying the indicated calculation method and the data from Tables VII and XI it is possible to calculate for a particular livestock system how much fertilizer N can be substituted by N from animal manure, provided manure production is known.
In a very high rainfall situation the recovery of N (kg N in crop/kg N applied) from manure may be higher than from inorganic fertilizer, due to the slow release character of manure resulting in N mineralization which is better synchronized with plant uptake than inorganic fertilizers. This would result in a N working coefficient of manure, in terms of fertilizer equivalents, higher than 1.0 minus 'before application losses'.
Under flooded conditions N recovery is low for both inorganic and organic fertilizer: estimated at 35 and 25% respectively from a long-term experiment in Japan (Wolf and van Keulen, 1989). However, these results were obscured by confounding effects, such as highly reduced biological N fixation and decreasing soil N reserves in the case of inorganic fertilizer. If these effects were included, N utilization from organic manure would be much higher than from inorganic fertilizer. Hence, N working coefficients would again be higher than 1 minus 'before application losses'.
|
On a wheat crop in a temperate region a spring application of
poultry liquid manure is given. The manure is surface applied and almost
immediately ploughed in. Nm is assumed to have the same effect as N
from mineral fertilizer although a part (15%) volatilizes as NH3. The
working coefficient for Nm is therefore 0.85. Of the so-called easily
decomposable organic N, Ne, only 50% mineralizes in the period during
which the wheat crop can utilize it. The remaining 50% mineralizes outside the
period of wheat growth. The working coefficient for Ne is, thus, 0.5.
Nr is the fraction becoming available for plant uptake, leaching or
other processes only a year after application of the manure and is therefore not
available for this year's wheat crop. The working coefficient is 0. The total
working coefficient is 0.85*Nm+0.50*Ne =
0.85*0.70+0.50*0.20 = 0.70. This is the working coefficient for N from a single
application of poultry manure on a wheat crop grown immediately after
application. In this case Nr does not contribute to nutrient supply
to the crop. |
|
What if poultry manure is applied each year? After repeated
annual applications of manure, a steady state will eventually develop, where the
annual addition of N to the soil organic matter equals the annual mineralization
of soil organic N. In other words: in a steady state, a quantity of N equal to
the fraction Nr is mineralized. Only 50% of this Nr can be
utilized by the wheat crop. In addition, it is assumed that the wheat crop can
utilize 10% of the mineralized N that was missed by the previous year's crop,
i.e. 10% of (100-50)% of Ne plus Nr. In a steady state,
then, the working coefficient would be 0.85*Nm + 0.50*Ne +
0.50*Nr + 0.10*0.50*(Ne+Nr) = 0.85*0.70 +
0.50*0.20 + 0.50*0.10 + 0.10*0.50*(0.20+0.10) = 0.70 + 0.06 = 0.76. |
For practical recommendations to Dutch farmers, Noij and Westhoek (1992) differentiate between a single dose and repeated annual doses of P. They assume a working coefficient of P from any type of slurry on grassland of 0.8 after a single dose and of 1.0 after repeated annual doses. For maize, they assume a working coefficient for cattle, pig or poultry slurry of 0.6, 1.0 and 0.7, respectively for a single dose, and again 1.0 if repeated annual doses are applied. For maize, a fraction of the P is released only in later years. Working coefficients for P from farmyard manure higher than 1.0 have been reported from experiments on potatoes over a period of six years in India (Sharma, et al., 1980). Working coefficients for P from farmyard manure of 0.6, 0.4 and 0.5 were from experiments on hyacinth bean (Dolichos lablab L.) for three consecutive seasons Noor et al. (1992),
An indicative value for the working coefficient of P for any type of animal manure is 1.0 with repeated annual applications. For single applications of farmyard manure, the P working coefficient can be assumed to be 0.5.
Temperature influences mineralization of the organic P in manure. It can therefore be assumed that like N, P is mineralized more rapidly in tropical than in temperate conditions. Because the ratio organic P/inorganic P in manure is much lower than organic N/inorganic N, temperature influences the working coefficient of P for single applications much less than that of N.
The sources quoted in the section above suggest a working coefficient of 1.0 for K in animal manure. Noij and Westhoek (1992), however, make an exception for animal manure applied in the Netherlands on sandy soils before February, in which case leaching of K may occur, which will reduce the working coefficient to 0.8 on average.
In clay soils, soil organic matter is important for soil porosity and aeration. On loamy and sandy soils, soil organic matter increases water holding capacity, cation exchange capacity (CEC) and improves soil structure. A higher soil organic matter content, therefore, makes soils less susceptible to erosion. Any addition of organic material, including manure contributes to soil organic matter content. This humification coefficient indicates which fraction of the added organic material remains after a certain period of decomposition as "stable organic matter" and, thus, contributes to soil organic matter. Manure has a relatively high humification coefficient, so the contribution of animal manure to the build up of soil organic matter is, therefore, high. However, for the production of one ton dry matter as manure, more dry matter as fodder is needed. Livestock systems therefore, may not contribute more to soil organic matter than do agricultural systems without livestock. On the other hand, livestock systems often involve cropping patterns with crops that contribute more to soil organic matter content than arable crops. Only in situations where potential feed biomass is wasted can livestock play a positive role in this respect. Box 5 gives an example for the situation of crop residues.
|
In a rice farming system two rice crops are cultivated per
year. Straw production is 2 times 3 tons/ha, giving an annual straw production
of 6 t/ha. Straw is usually burnt by farmers, because it hampers tillage
operations. Thus, the straw contributes little to build-up of soil organic
matter. If animals would be fed with this straw, they would produce, ca. 3.6 t
manure. An annual addition of 3.6 t/ha manure to the soil, will contribute
0.5*3.6 = 1.8 t soil organic matter/ha. This is equal to 0.06% of the topsoil
(0-20 cm), assuming a bulk density of 1.5 kg/dm3. If the organic
matter content of the soil is 2%, and annual decomposition rate of the soil
organic matter in the rice paddy, 4% per year, the added farmyard manure would
compensate for {0.06/(0.04*2)} * 100 = 75% of the annual decomposition of the
soil organic matter present. |