Massive occurrence of floating aquatic plants is a common feature on tropical and subtropical water bodies, and it is commonly the result of an expansive growth of non-endemic aquatic macrophytes. In a 1989 report to FAO, Hartley (CSIRO, Brisbane, Australia) estimated that two million people in Nigeria, including more than 24 000 fishermen, and approximately 100 000 persons living in riverine communities in Benin, who rely solely on fishing for their livelihood, are negatively affected by floating aquatic weeds (water hyacinth, salvinia, and water lettuce - Pistia stratiotes). Fishermen in Malawi and Ghana may be hampered by water lettuce. In the Niger, the more recent explosive growth of water hyacinth has interfered with fishing on floodplains of the Niger River. In lakes Kyoga and Victoria in East Africa, until very recently, thousands of fishermen were trying to keep open access to landing and fishing sites blocked by water hyacinth. Water hyacinth and salvinia are also widespread in the tropical zone of Asia and Latin America, representing a major problem for fisheries, irrigation, hydropower production and water transport.
Both the floating and submersed aquatic macrophytes can become nuisance (noxious) plants, especially where they appear as exotics. The problematic submersed plants are: Myriophyllum spicatum, Hydrilla verticillata, Ceratophyllum demersum, Potamogeton crispus, Potamogeton pectinatus. The reason for their explosive growth is often the favourable nutrient environment, and also human impact through eutrophication of some, especially small water bodies.
The rapid expansion of the exotic water hyacinth in Sudan reduced the number of fish and fishing intensity (Davies, 1958/59). The periodical annual invasions of the Kerala (India) backwaters with salvinia renders fishing and boating impossible during certain months (Thomas, 1979). In the Sepik River in Papua New Guinea, where in the late 1970s and early 1980s salvinia spread over numerous floodplain lakes, eventually covering over 200 km2, the tilapia (Oreochromis mossambicus) fishery collapsed (Mitchell et al., 1980). The disruption of traditional subsistence lifestyle became so great that entire villages were abandoned (Anon., 1985). Fortunatelly, biological control using the beetle Cyrtobagous salviniae, which was released over the infested areas, was so effective that within only a few years salvinia virtually disappeared from all backwaters.
Not always the presence of invasive plants becomes a nuisance. On Rawa Pening reservoir in Java, Indonesia, where all fish species are captured for food, control of water hyacinth led to overfishing, and only expansion of aquatic weeds due to the neglect of control measures led to rehabilitation of fish stocks (Carlander, 1980). Later on, a certain balance has been achieved among the water hyacinth area cover, capture fishery, and fish cage fish production.
The impact of invasive plants on fish stocks and fisheries is poorly documented, especially for the tropical and subtropical countries. Long-term monitoring is mostly lacking. Records available for the freshwater Lake Naivasha in Kenya, are an example of well-documented changes in a lake ecosystem under the impact of changes in aquatic macrophytes, but also of the impact of human interference with this freshwater lake (Fig. 26).
Over the last 30 years, Lake Naivasha, situated at an altitude of 1 890 m, has experienced considerable changes in its plant as well as animal communities. Salvinia molesta appeared in the early 1960s, and toward the end of the 1960s it covered 2–3 km2. Its appearance, combined with heavy fishing pressure on tilapia stocks, resulted in a decline to a level that the local frozen fillet plant had to be closed (Gaudet, 1976). Dissolved oxygen concentration underneath the mats was only 10 % saturation as compared to 64–85 % in the open water (Kongere, 1979). On Lake Kariba, Donnelly (1969) observed that tilapias established nurseries in shallow water only if it was free of Salvinia. Where salvinia mats shaded the water, phytoplankton production declined and the young tilapias moved out of the shallows into the deepwater Potamogeton and Ceratophyllum beds, where they were much more susceptible to predation. In Lake Naivasha the large mat of Salvinia almost disappeared by 1987, when it became limited to the river inflow along the northern shore (Harper et al., 1990) Since 1991 the introduction of the biological control agent, the beetle Cyrtobagous salviniae, has kept salvinia under control (Abiya, 1996).
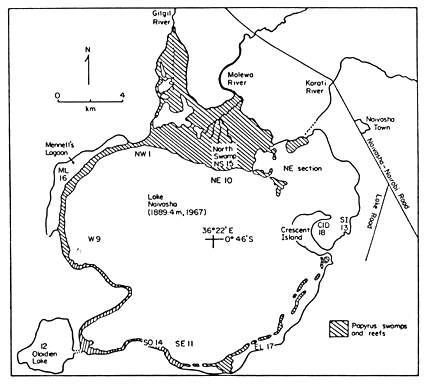
Fig. 26. Lake Naivasha, Kenya.
The Lake Naivasha level fluctuations, with a high level lasting from 1960 to 1976, followed by a drop, which was followed in 1982 by a sharp rise in water level, has had considerable impact on other aquatic plants. In 1983 the water level started to decline again and by 1985 Kenya experienced a severe drought. By 1987 the lake level returned to the state of the 1950s. Papyrus (Cyperus papyrus) plants, formerly floating on the surface, became restricted to margins, but water-lilies (Nymphaea caerulea) started germinating, and extensive beds of submersed plants such as Ceratophyllum demersum and Potamogeton spp reappeared (Fig. 27). The impact of these changes on fish and crayfish revealed that Tilapia zillii and Oreochromis leucostictus (both introduced species) showed resistance to the changes in lake level fluctuations and subsequent changes in aquatic plants. Both fish feed largely on detritus, which has always been in abundant supply. In T. zillii macrophytes constituted less than 10% of the total food taken, while there were none eaten by O. leucostictus. Decline in tilapia fishery resulted from intensification of the fishery and the use of small-meshed nets. But whenever the lake water level rose, the flooding of terrestrial vegetation and shallows provided good nursery areas and this led to the recovery of fish stocks (Abiya, 1996). Crayfish Procambarus clarkii, introduced in the lake in 1970 and another important component of the lake fishery, is a major consumer of aquatic plants. In the early 1980s it reached a density of about 4 crayfish m-2 in the littoral (Muchiri et al., 1995). The species is known to reduce aquatic vegetation in lakes in the USA at densities over 3 m-2 (Magnuson et al., 1975). The crayfish fishery in Lake Naivasha crashed in 1983, this being followed by the recovery of rooted aquatics. This provides a circumstantial evidence that the decline and subsequent recovery of the native rooted submersed and floating aquatic plants was due to the heavy crayfish herbivory until 1983 and its relaxation thereafter (Harper, 1992). By 1987 the crayfish density was only 0.25 ind. m-2.
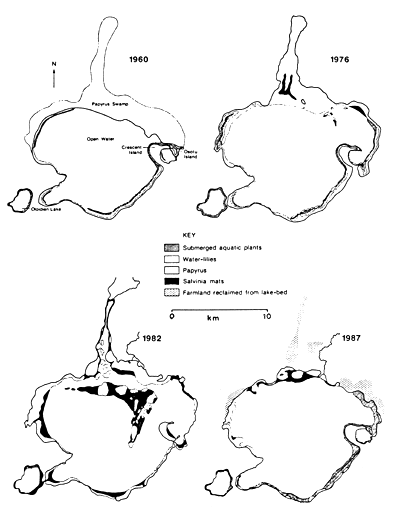
Fig. 27. Changes in the aquatic vegetation of Lake Naivasha, 1960–1987. (From Harper et al., 1990).
Water hyacinth is now widely spread throughout tropical and subtropical countries. Gopal and Sharma (1981) reviewed the available information on this plant for the years preceding their publication. They also provided maps on the global water hyacinth distribution to that date.
Rzoska (1976) described the invasion of Eichhornia crassipes in the Sudanese White Nile. The upper Nile swamps (the Sudd) have become a ‘perennial’ centre of infestation for the downstream Nile. In many water bodies of the Nile floodplains water hyacinth has fringed the shores in a belt of variable width, and it has taken over the function of Pistia. In fringing plants, up to a width of about 6 m, Bailey and Litterick (1993) found a rich community of aquatic macroinvertebrates inhabiting the root of this floating plant, and Hickley and Bailey (1986, 1987) found rich fish communities in water hyacinth fringes. Similarly, Green et al. (1976) found also rich fish fauna in water hyacinth fringes of Lake Lamorgan (Java, Indonesia). However, Davies (1958/59) and Rzoska (1976) noted that fisheries were impeded, specifically in areas inhabited by Shilluks, immediately north of the Sudd in Sudan, and on the Sobat River, also in Sudan. Gopal and Sharma (1981) estimated that in the 1950s about 45 000 t of fish were lost in West Bengal (India) due to the infestation of waters with water hyacinth. Referring to literature, they reported that fish production declined from 905 kg ha-1 at zero percent cover to 281 kg ha-1 at 25% cover, possibly due to low densities of phytoplankton as a result of shading by water hyacinth, and also the uptake of phosphorus by this plant. One observation reported suppression of the phytoplankton production underneath 10% and 15% cover to 2.25 g m-2 and 1.97 g m-2, respectively, as compared to 2.58 g m-2 at 5% cover (Gopal and Sharma, 1981). In experimental ponds in Alabama the production of the phytoplankton-feeding Oreochromis aureus was reduced by about 50% at 10–25% water cover by water hyacinth (McVea and Boyd, 1975).
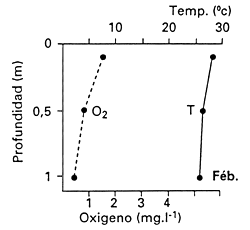
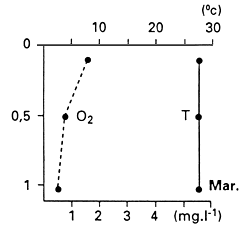
In other situations, thick mats of water hyacinth cause complete depletion of dissolved oxyxgen content and consequently fish kills. The presence of water hyacinth in shallows makes such areas unsuitable for fish spawning. In the Sudd of Sudan, Bishai (1960/61) measured 1.8 mg L-1 of dissolved oxygen 30 cm below the mat when there was no water current, and he also reported high CO2 concentrations, which increase the susceptibility of fish to low dissolved oxygen concentrations. Poi de Neiff and Solis (1994), who measured dissolved oxygen concentrations among the roots of water hyacinth floating islands on the Parana River floodplain in Argentina, recorded a maximum of 2.3 mg L-1 within the first 1 m depth, but the usual concentrations were close to 1 mg L-1, declining with depth (Fig. 28).
Two Amazon River floodplain fish species, Colossoma macropomum and Schizodon fasciatum, have shown considerable resilience to hypoxic conditions. Experimental investigations demonstrated that if the hypoxic conditions persist, these species return to the zones of plant cover and remain among the roots of floating plants, where the oxygen availability among the roots seems to be better than in the deeper water. In experiments with Eichhornia crassipes and Pistia stratiotes, conducted by Jedicke et al. (1989), the roots of these plants released oxygen into the water. The input of oxygen beneath a layer of E. crassipes was nearly double that beneath an open water surface. The input from water lettuce (P. stratiotes), however, was only slightly greater than that determined in open water (Fig. 29). Jedicke et al. (1989) has pointed out the advantages for the hypoxia-tolerant fish species to remain among the plant roots: reduced predatory pressure; utilization of food supply when other species cannot access it; lower danger to fish from birds and other terrestrial predators, to which fish species displaying emergency respiratory behaviour at the surface of open water zone are exposed.
 |  |
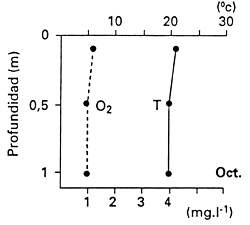 | 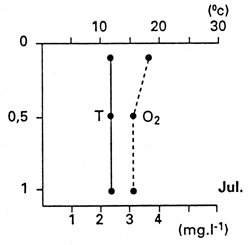 |
Fig. 28. Vertical profiles of temperature (T) and dissolved oxygen (O2) among the roots of water hyacinth. (From Poi de Neiff and Solis, 1994).
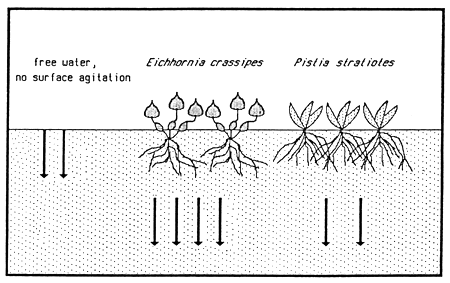
Fig. 29. Diffusion input of dissolved oxygen into surrounding water from water hyacinth and water lettuce. (From Jedicke et al., 1989).
Bailey and Litterick (1993) found markedly less aquatic macroinvertebrates inhabiting the roots of water hyacinth when appearing as small floating mats, as compared with those of the fixed fringe, and attributed this reduction partly to the feeding activity of fish. It is probable that if the plant appears in broken small-sized mats, which allow to maintain dissolved oxygen concentration underneath the mat at reasonably high levels, its is sought by fish to feed on its periphyton and the associated aquatic invertebrates. The plant also provides a habitat to invertebrate hosts and vectors of parasitic diseases such as malaria, schistosomiasis, encephalitis and elephantiasis.
In the 1970s and 1980s in East Africa water hyacinth invaded the lakes Kyoga, Kwania and Victoria. In Lake Victoria water hyacinth was noticed for the first time in 1988, and by 1992 it was already well established (Ogutu-Ohwayo, 1995). In the 1990s it has been recorded also from Lake Albert. It has had a major negative impact on fish catches and fish landings, due to its obstruction of fishing and landing sites (see Section 13.2). It is also believed that its presence along the shores has had a major impact on tilapia nesting, through lowering the dissolved oxygen above such sites and shading. In the early 1990s the worst affected areas have been on Lake Kyoga (FAO, 1993a), but by 1996, in the Uganda sector of Lake Victoria, the weed extended an average of 15 m from the shore, covering almost 80% of the country's 1 000 km of shoreline. Near Kampala, the capital of Uganda, the weed was 30 m wide. Prevailing winds from the south pushed most of the weeds to Uganda, but in 1996 the weed almost encircled the 3 000 km of shoreline (Anderson, 1996). Luckily, the situation has dramatically changed for the better, and by January 2000 it was reported that water hyacinth had been reduced by 80% on Lake Kyoga and 70% on Lake Victoria (Wendo and Ngatya, 2000). In Lake Victoria only 16% of the shoreline remains infested. This reduction is believed to be the result of the success of biological control using two weevil species, i.e. Neochetina bruchi and N. eichhorniae, released on Lake Victoria since 1996, and on Lake Kyoga since 1994.
With the decimation of haplochromine stocks by Nile perch (Lates niloticus), especially in Lake Victoria, a potential benefit resulting from the presence of water hyacinth has been observed: this floating plant appears to provide cover for haplochromine stocks which have been on the increase since the appearance of water hyacinth in Lake Kyoga. In the reservoir Rawa Pening in Java, Goeltenboth and Kristyanto (1987) observed that water hyacinth is a valuable habitat for fish as long as it does not form extensive solid mats (Photo 5).
Ogutu-Ohwanyo (1995) in his comments on the potential for controlling water hyacinth in Lake Kyoga implies that reduction in the water hyacinth cover might again expose haplochromines to Nile perch predation. The haplochromine-aquatic macrophyte relationships are far from clear. Witte et al. (1992) suggested that continuous digging by haplochromines in Butimba Bay, Lake Victoria, could have prevented the settling of rooted plants such as Nymphaea spp. With the decline in haplochromines, since the end of the 1980s, the shallows in this bay have been overgrown by Nymphaea spp, Ceratophyllum demersum and invaded by water hyacinth. Witte et al. (1995) believed that water hyacinth invasion of Lake Victoria threatened haplochromines and other fish. It would be interesting to know the result of the presently rapidly diminishing water hyacinth cover on haplochromines and other littoral fish.

PHOTO 5: Fishing along the mat of water hyacinth, reservoir Rawa Pening, Java, Indonesia.
In Ghana, water hyacinth was found for the first time in Tano lagoon, apparently entering this water body from Cote d'Ivoire. More recently fishermen reported a decline in fish catches and it was suggested that this was due to the presence of the water hyacinth. Much of the water hyacinth in Tano lagoon is mobile, being moved by wind or water currents. Holcik (1995), who investigated the situation, thought that the low catches are not due to the decline in fish stocks but rather a result of both the inefficient deployment of nets and of the difficult access to the fishing grounds due to the water hyacinth cover.
In Laguna de Bay, a large lake near Manila, the capital of the Philippines, infestation by water hyacinth has been a major environmental problem in several areas of this lake. Massive proliferation of the plant not only poses a hazard to navigation, but it also leads to poor water quality in this shallow lake, which has considerable fisheries importance. Tilapia and milkfish have been grown in numerous fish pens, the area of which at its maximum expansion reached almost 30 000 ha, representing about one third of the total water surface. Frequent typhoons have been known to drive the floating plants against fish pens (Photo 6), destroying them and releasing the penned fish into the lake. In the late 1980s water hyacinth cover was estimated to be 1 800 ha, and the plant was also present in tributary rivers, creeks and irrigation canals. This resulted in difficult, if not impossible access to fish pens, fishing sites, damage to fish pens, fishing nets and other fishing gear, reduction in fish population due to the low dissolved oxygen levels and increased carbon dioxide concentrations, blockage of water intakes of irrigation pumps, and interference with water transport. The removal of water hyacinth was largely manual, plus using one plant harvester. Monitoring of water hyacinth infestation in Laguna de Bay was done with the help of remote sensing using Landsat imageries (Bina et al., 1978).

PHOTO 6: Water hyacinth fringe on Laguna de Bay, Philippines. Note the numerous fish pens.
Tin-mine lakes, of which about 4 300 exist in Malaysia, are a consequence of abandoned tinmining pits, which have filled with water. Some of them have been used for aquaculture, and these represent 18.9% of the total area used for freshwater fish pond culture in peninsular Malaysia (Arumugam, 1994). While a total of 55 species of aquatic macrophytes have been identified from these lakes, in such lakes which receive a significant nutrient input, drastic changes in macrophyte and other communities take place. Once fish culture has been abandoned, in those lakes which are used as dumping sites for organic waste, Eichhornia crassipes may cover up to 90% of the surface area. Such lakes contain mainly air-breathing fish (Channa spp, Trichogaster pectoralis) which tolerate low levels of dissolved oxygen underneath the mat of floating vegetation. Lakes lacking the dense cover of floating macrophytes support indigenous fish and tilapias.
The widespread abundance of water hyacinth has attracted the attention of fish feed specialists and fishery managers. Water hyacinth has been tested both as a direct food of fish, and as a fish feed component. For example, in experiments carried out in Java, Indonesia, up to 10% of the feed was either composted or non-composted water hyacinth. It was noted that the feed, fed to Puntius javanicus and Cyprinus carpio, did not slow down the growth rate of these fish. Trichogaster pectoralis has shown a significantly slower growth rate when fed higher levels of substitution (Hutabarat et al., 1986). Numerous other experiments have been performed on the use of water hyacinth as an additive to fish feed, but they are not a subject of this review.
In Indonesia, water hyacinth, together with some other floating plants, is used as a fish attracting device. In shallow lakes, an enclosure made of bamboo poles pushed into the bottom mud, and filled with aquatic plants, is called ‘bungka todo’ (Photo 7).

PHOTO 7: Fish attracting using floating aquatic plants (bungka todo, Lake Tempe, Sulawesi, Indonesia.
Tree branches or brush are often added to the aquatic plants. In Kalimantan, such systems are used on inland lakes of the Mahakam River in the east. On the Kapuas River in the west, water hyacinth is rarely found as it does not grow well in black waters of inland floodplain lakes, therefore any water hyacinth which is found is treasured and used as shade to protect fish in cages from direct sunshine, rather than using it as bungka todo (Petr, 1993) (Photo 8).
The plants provide the fish with shade and food, especially periphyton and its associated invertebrate fauna. The floating aquatic plant enclosures are regularly fished. It has been suggested that in lakes threatened by overfishing, such as Lake Tempe in Sulawesi, bungka todo could be an efficient way of fish stock protection and preservation in areas set aside and designed strictly off-limits for fishery. From them, the unprotected areas of the lake would then be naturally recolonized, at least by some fish species.
Pistia stratiotes is a free floating common aquatic plant in tropical Africa, where it occasionally forms dense and large mats. In most situations, such mats are not permanent, and are moved or dispersed by wind. In some water bodies, Pistia may become part of sudd. The plant consists of a rosette of leaves covered with hydrofobic hairs extending above the water surface, and of submersed, finely divided roots reaching to a depth of 15–20 cm. The roots usually harbour a rich community of invertebrate fauna, which in turn is preyed upon by fish and aquatic predatory invertebrates, such as dragonfly nymphs.

PHOTO 8: Water hyacinth used as shade plant for fish cages. River Kapuas, Kalimantan, Indonesia.
In Volta Lake (a reservoir in Ghana, West Africa), large floating mats of Pistia stratiotes developed soon after the reservoir started filling. It became abundant especially where shelter against wind and waves was provided by floating or stranded trunks of dead trees, and inbetween dead trees standing in shallows. In places it was knitted together with Scirpus cubensis. The biggest expansion of Pistia carpets occurred toward the start of rising water level. After the water level stabilized, Pistia became less numerous, with large plants gone, and only small plants remaining. In Pistia plants, situated well inside a mat, roots contained between 5 000 and 16 000 m-2 aquatic macroinvertebrates (5–35 g m-2 wet weight), excluding zooplankton (Petr, 1968). The values are lower than those for the margins of floating meadows of varzea, where Junk (1973) recorded a maximum of 780 000 individuals (62 g m-2). Junk included the very abundant crustaceans (Copepoda and Cladocera) in his samples. Throughout the observation period, in Lake Volta the Pistia roots macroinvertebrate fauna was dominated by the predatory dragonfly nymphs, which although low in numbers exceeded browsers and filter feeders in biomass. The presence of macroinvertebrates was considered to be of importance for fish feeding on periphyton and on it associated invertebrates. In 1965, the first year of the reservoir filling, Pistia of Lake Volta also harboured the snail Bulinus forskalii, which was very numerous at the peak of the dry season, when the water level was slowly declining and the surface water temperatures were at their highest. In 1966 the first Bulinus globosus rohlfsi, a host of schistosome (Schistosoma haematobium) parasites, was recorded from the lake (Paperna, 1970) while B. forskalii became rare. In one area Paperna collected over 50 B. t. rohlfsi from a single Pistia plant. When in some areas of the lake Pistia started disappearing, the snails thrived particularly on Ceratophyllum demersum, which became very common. This change has led to a dramatic increase in the incidence of schistosomiasis among the fisherfolk on this reservoir. (See also Section 14.).
Little is know about the fish feeding on the aquatic fauna and periphyton associated with the submersed parts of Pistia. In Lake George, Uganda, experimental catches within and alongside drifting mats of Pistia, which entered a bay surrounded by papyrus, haplochromids represented 98.3% of the total number, and 98.2% of the total biomass of fish captured during a 24 hour period (Petr, unpublished). Among the haplochromids Haplochromis nigripinnis, H. aeneocolor, and H. angustifrons represented 66.5, 17.8, and 13.2% by number of the total, respectively, and a biomass in a similar proportion. H. nigripinnis is an omnivore feeding predominantly on algae, insect larvae and emerging insects; H. aeneocolor, which is abundant along the papyrus edge, feeds on insect larvae and adult aquatic insects, and the guts contain also plant fragments, suggesting that this species may selectively forage on the food among the roots of papyrus and Pistia and that the plant fragments are roots taken incidentally with the insects; H. angustifrons is predominantly an open lake species feeding on insect larvae, especially chaoborids, and on zooplankton (Dunn, 1975).
Pistia may be used as a shading plant in aquaculture. In tropical Australia, it has been used in a raceway culture of juvenile growout of freshwater crayfish Astacus quadricarinatus. The use of this plant was considered advantageous for several reasons: provision of suitable habitat/substrate for juvenile crayfish; photosynthetic production of oxygen; stabilization of water temperature; absorption of nitrogenous wastes; provision of natural food organisms associated with the plant, i.e. epibionts; shading effect; high carrying capacity for crayfish juveniles with reduced intra-specific competition; enhancing survival and growth of the juveniles (Jones, 1988).
Exotic aquatic fern, the floating Salvinia molesta is spread widely through the tropics. Thick mats of this plant prevent or make difficult boat traffic (Photos 9 and 10), impede or make fishing impossible, and the plants harbour also invertebrate hosts of water-borne diseases.
Solid mats of salvinia depress dissolved oxygen concentration, increase concentration of carbon dioxide, and occasionally may result in formation of hydrogen sulfide in water underneath it. Lake Kariba, a large reservoir on the Zambezi River, was invaded by salvinia shortly after the formation of this reservoir. After some years salvinia, covering at its largest expansion in 1962 approximately 1 000 km2, i.e. 23% of the reservoir surface area (Karenge and Kolding, 1995), declined in the 1970s to about 1% (Mitchell and Rose, 1979), largely due to nutrient deficiencies. Salvinia was replaced by increasing amounts of rooted macrophytes down to a depth of around 10 m (Fig. 30). In parallel with that there was an increase in the benthic macroinvertebrates and in benthic-feeding fish species such as Mormyrus longirostris, Synodontis zambezensis, Serranochromis codringtonii, Serranochromis macrocephalus and Labeo altivelis. Increased diversity within the inshore fish community in Lake Kariba was attributed to the development of a mosaic of new niches among the aquatic macrophytes, periphyton and submersed trees where a variety of benthic gastropods, molluscs, insects and other invertebrates provided a new food resource to fish (Karenge and Kolding, 1995). The lake littoral is, however, not a stable environment due to a drawdown of this reservoir, which serves the production of hydroelectric power for Zambia and Zimbabwe. It was recommended that to promote full development of macrophytes, the drawdown should not exceed 3.5 m. This limit is sometimes exceeded in Lake Kariba, where dry years may irregularly alternate with wet years. In spite of that, the development of a macrophyte belt has enhanced the stocks of juveniles and small fish species such as S. macrocephalus, Brycinus imberi and S. zambezensis which have increased during the 1980s (Karenge and Kolding, 1995). The authors also mentioned that in parallel with the increase of the above fish species there was a decline in small-sized Hydrocynus vittatus, Hippopothamyrus discorhynchus and Marcusenius macrolepidotus. The reason for this decline is not understood.
With respect to aquatic macroinvertebrate populations on Salvinia molesta as compared to those on water hyacinth, Arunachalam et al. (1980) reported a higher diversity from the first plant, where they found 66 species, than on the roots of water hyacinth (about 30 species) from the freshwater lake Veli in India. The authors do not provide information on the biomass of the macroinvertebrates on these two plants, hence it is difficult to assess which plant is more important for fish as a source of invertebrate prey.

PHOTO 9: Boats hindered by Salvinia molesta. Backwaters of the Sepik River, Papua New Guinea.

PHOTO 10: Access to a fishing boat landing site filled with salvinia, Sepik River, Papua New Guinea.
In Lake Kariba, salvinia rootlets were found in stomachs of a great variety of fish species of herbivorous, omnivorous and even piscivorous character. In Tilapia rendalli they were found in 19.6% of fish stomachs, but represented only 6.7% of the total food taken (Mitchell, 1976). Salvinia rootlets, detritus or fragments occurred also in: 9.3% Haplochromis codringtoni, 11.1% Haplochromis darlingi, 19.2% Pseudocrenilabrus philander, 29.6%, Eutropius depressirostris, 8.1% Clarias gariepinus, 24.0% Synodontis zambezensis, 38.2% Micralestes acutidens (where it formed 19.2% of the total content), 12.3% Alestes lateralis, 11.1% Mormyrus longirostris, 5.0% Marcusenius macrolepidotus, 10.2% Hippopotamyrus discorhynchus, 8.1% Mormyrops deliciosus. In the majority of fish species it would appear that salvinia rootlets and detritus were taken when feeding on aquatic macroinvertebrates and plankton residing among the rootlets. For example, out of the 19 fish species investigated for food, 15 contained the crustaceans Cyclestheria and Caridina, 18 contained the nymphs of the mayfly Povilla adusta, 14 contained chironomid larvae, 13 contained trichopteran larvae.
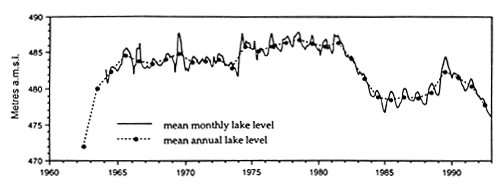
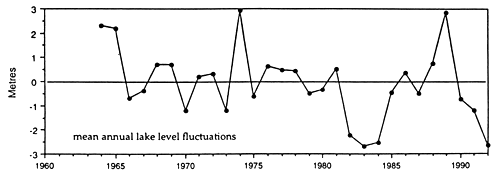
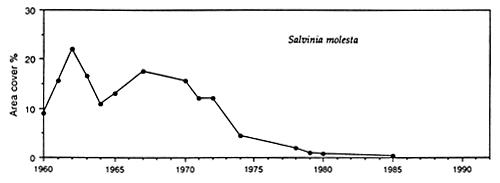
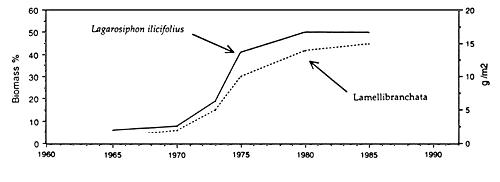
Fig. 30. Lake Kariba: monthly and mean annual lake levels, mean annual lake level fluctuations, coverage of Salvinia molesta, changes in the benthic (Lamellibranchiata) and aquatic macrophyte biomass (Lagarosiphon). (From Karenge and Kolding, 1995).
In Sri Lanka, salvinia is often removed from water reservoirs manually, but biocontrol using the beetle Cyrtobagous salviniae is more efficient (see Section 13) (Photo 11).

PHOTO 11: Manual removal of Salvinia molesta from a tank (reservoir) near Polonnaruwa, Sri Lanka).
Papyrus rhizomes are typically rooted in loose detritus in a floating mat. Papyrus may occur as free-floating or stranded, or forming fringe swamp. Occasionally, the lake-side edge of the fringe papyrus swamp will float if the lake level permits (Fig. 31).
The extent of papyrus swamplands in East and Central Africa is enormous. In southern Sudan the Upper Nile forms large swamps called the Sudd, which covers approximately 40 000 km2 (Rzoska, 1974). (For the Sudd see Section 8.1.). In Uganda, papyrus encircles completely Lake Kyoga, and is well developed in Lake Victoria basin (Thompson, 1976) (Fig. 32, Photos 12 and 13).
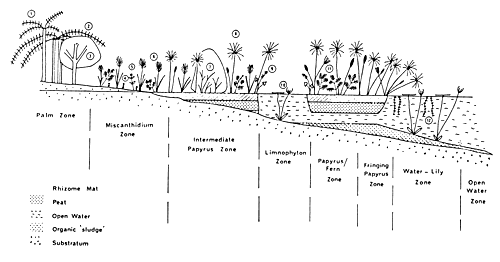
Fig. 31. Vegetation zonation of the fringe of Lake Victoria. (From Thompson, 1976).

PHOTO 12: Papyrus in Lake Victoria margins, Jinja, Uganda.
Fig. 32. Wetlands in the White Nile upper catchment, with large papyrus areas. (From Thompson, 1976).

PHOTO 13: Papyrus fringing Lake George, Uganda.
In Lake George, Uganda, where it forms large fringing floating swamps, papyrus flourishes in water with a conductivity of 200 μS, while in Lake Edward (850 μS) it is restricted to river mouths (of much lower conductivities) on the Uganda shore of the lake (Lock, 1974). Gaudet (1980), in summarizing the significance of the papyrus for the aquatic fringe ecosystem, pointed out that papyrus swamps supply large amounts of fixed nitrogen to tropical lakes and rivers every year thus allowing an increase in animal and plant production at the swamp edge. Papyrus fringes act to moderate changes along a river system by regulating nutrient flow and recycling. For the most part this is accomplished by production of an organic nutrient output at the expense of an inorganic nutrient input. Papyrus plants effectively extract dissolved nutrients from tropical river systems, but such nutrients are later exported as organic particulate matter or adsorbed to particles which are carried into a lake by through-flow (Gaudet, 1979) (Fig. 33). In practical terms, papyrus swamps may be effective in removal of organic nutrients from sewage effluents into these swamps (Gaudet, 1978), since papyrus seems to be quite tolerant to pollution. The organic nutrients released from the organic particulate matter or adsorbed to particles represent an energy source to a very diverse tropical fauna and flora, which in turn supports a profitable shallow water fish industry in many parts of Africa (Gaudet, 1980). Summarizing the importance of papyrus swamps along the major rivers in Africa, such as Congo and White Nile, Gaudet characterized them as an ecological buffer zone, which moderates changes along the river system by regulating nutrient flow and recycling. For the most part this is accomplished by production of an organic nutrient input at the expense of an inorganic nutrient input. The organic nutrients serve as an energy source to a very diverse tropical fauna and flora, which in turn supports a profitable shallow water fish industry in many parts of Africa. At lake edges papyrus swamps are often sites of intensive fishing and thus a resource worthy conservation.
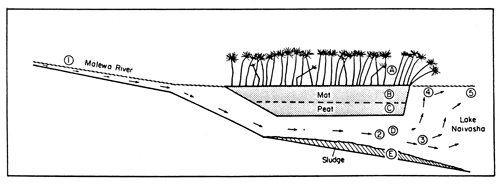
Fig. 33. Malewa River discharge through papyrus swamp into Lake Naivasha. (From Gaudet, 1979).
For air-breathing fish papyrus swamps and other large swampy divides are not likely to be a barrier to dispersal. For water breathers, the use of and dispersal through papyrus swamps is likely to be limited by the availability of oxygen, the open surface area, and efficiency of oxygen uptake in the species (Chapman and Liem, 1995). In a study of a papyrus swamp, which feeds a small river in Uganda, Chapman et al. (1998) found peak of dissolved oxygen concentrations associated with biannual flooding, and they suggested that this provides opportunities for fish to use or move through papyrus. However, peak flood values never reached 50% saturation, and thus may pose barriers for some hypoxia intolerant species. At the lake edge of Lake George in Uganda dissolved oxygen showed a rapid decrease moving inside the papyrus belt (Petr, 1973) (see Fig. 22). Chapman et al. (1998) reported Ctenopoma muriei, Clarias liocephalus and Nothobranchius sp. to be found principally in papyrus swamps and other wetlands of the Lake Victoria Basin. Chapman and Liem (1995) found the small cyprinid Barbus neumayeri to inhabit the dense interior of papyrus swamps, due to the presence of large gills and the use of aquatic respiration at the air-water interface. Chapman et al. (1995) reported that 12 species of East African cichlids are tolerant of low dissolved oxygen concentration and able to use low oxygen habitat along the margins of Lake Victoria as a refugium against the introduced Nile perch.
In lakes with a more pronounced fall in water level, papyrus stands may become drastically reduced. In Lake Naivasha water level declined by just over 4 m between 1980 and 1987, exposing some 6 500 ha of soils, or about 35% of its 1980 area (Harper et al., 1993). During this decline exposed soils were cleared of vegetation, mostly stranded Cyperus papyrus and other emergent plants, and put down to agriculture. Harper et al. (op. cit.) noted that fringing papyrus is now voluntarily kept by some landowners to keep a buffer zone, which assists in reducing the input of high nutrient loads into the lake. The increasing demand for water is seen as a direct threat to the lake in the future (Abiya, 1996).
For details on water chemistry in papyrus swamps and underneath floating papyrus mats of the fringing zone, see Section 4.1, and Thompson (1976).
Myriophyllum spicatum, a native Euroasian plant, spread rapidly across North America in the 20th century, displacing native species in some water bodies and often forming dense stands and surface mats. In Currituck Sound in North Carolina, USA, it was detected for the first time in 1965, when it covered 40 ha. In 1966 it covered 3 200 ha, and by 1974, 32 000 ha were infested (Sheldon, 1997). M. spicatum can withstand a broad range of conditions: it can grow in oligotrophic to eutrophic waters, in 0.5 m to 8 m depth, in sand, in organic substrate, under pH ranging from 5.4 to 10, in brackish water, in northern temperate lakes and rivers, and in subtropical water bodies.
In 1978 in Lake Pontchartrain estuary, this macrophyte has become a dominant species, with a lower abundance of the native macrophytes Vallisneria americana and Ruppia maritima present there. Duffy and Baltz (1998), who in the estuary studied the impact of the invasive Myriophyllum on fish, found that the highest fish species diversity was in Vallisneria, while the lowest in Ruppia and Myriophyllum. The common fish were significantly more abundant in vegetated areas than on adjacent unvegetated substrate, and total abundances were higher in Myriophyllum and Ruppia than in Vallisneria. The authors suggested that the exotic Myriophyllum may not have had a detectable influence on fish assemblages or abundances relative to the native macrophytes because the disturbance of the substrate by waves in more open areas prevent it from growing densely enough to strongly alter the original microhabitat. However, in some other water bodies, M. spicatum may form thick, impenetrable walls of uniform height and leaf form. When the stem density exceeds 250 stem m-2 fish start avoiding the plant (Savino and Stein, 1982). Keast (1984) found a lower fish density in Myriophyllum beds than in native aquatic macrophytes, and Dvorak and Best (1982) found lower densities of aquatic macroinvertebrates in this plant, when compared to native plants.
Sloey et al. (1997), who studied the distribution of aquatic macroinvertebrates in beds of M. spicatum in Fish Lake, Wisconsin, found higher invertebrate biomass, density, and taxa richness in the upper foliated sections of plants versus the lower unfoliated sections. The edges of the beds contained higher biomass, density, and taxa richness than the centre of the bed. They did not study the impact of the differential distribution of macroinvertebrates on fish, but other studies confirmed that the structural complexity of plant species and spatial distribution of plants are among the decisive factors determining which and how many fish are frequenting aquatic macrophyte stands. (See also Section 2.1.1, and 5.)
Hydrilla verticillata is another invasive submersed species, which is present in numerous water bodies of the Americas, Europe, Africa, Asia and Australia. In some water bodies in the USA this plant has led to a considerable increase in fish stocks and fish species of sport fishery importance, while in other water bodies its presence has required a management response to prevent decline in recreational fishery. Apart from grass carp, for which this plants is a preferred food, other fish have also been used for controlling this plant. In Africa, Tilapia zillii and T. rendalli are known to feed readily on it, in India Cirrhinus mrigala and Etroplus suratensis, stocked in nursery tanks with Channa punctatus, efficiently controlled this plant (Raj, 1976), and also in India, Tor tor and Puntius spp feed readily on it. Herbivorous turtles in Bangladesh, and manatee in the USA and some Latin American countries also feed on hydrilla.