J. Richard Arthur
Barriere, British Colombia, Canada
Arthur, J.R.. A historical overview of pathogen introductions and their transboundary spread in Asia. p. 21–39.In: Subasinghe, R.P.;Arthur, J.R. (eds.). Regional workshop on preparedness and response to aquatic animal health emergencies in Asia. Jakarta, Indonesia, 21–23 September 2004. FAO Fisheries Proceedings. No. 4. Rome, FAO. 2005. 178p.
ABSTRACT
This paper briefly reviews the history of some of the major transboundary aquatic animal diseases in the Asia-Pacific Region and summarizes the status of some of the major diseases currently affecting the region (primarily those diseases listed by the Office International des Épizooties (OIE) or included in the Network of Aquaculture Centres in Asia-Pacific (NACA)/Food and Agriculture Organization of the United Nations (FAO)/OIE Quarterly Aquatic Animal Disease Reporting System). Emphasis is placed on those diseases having wide distributions and impacts in the region, with less attention paid to diseases of cold-water species such as salmonids, and those of very limited distribution. Some emerging diseases that could have significant impacts on aquaculture in the region in the near future are also discussed.
Introduction
Although parasites and pathogens are common in wild populations of aquatic animals, reports of serious disease outbreaks and associated mass mortalities in wild stocks due to their normal pathogen fauna are rare. This is because in natural systems the pathogen and the host have generally had a long period of time to adapt to each other, the pathogen often becoming less pathogenic and the host more tolerant of, or resistant to infection.
Serious epizootics of fish, shellfish and molluscs appear to be primarily a modern phenomenon, and are often associated with the intensification of aquaculture, where many animals are confined at high density and under conditions that are often stressful. Such conditions are often optimal for disease transmission, and organisms that are not a serious problem to the host species in the wild may become highly pathogenic. The international movement of aquatic animals for aquaculture purposes, while not entirely a recent development, has accelerated considerably during the past 50 years (see Arthur, 2004; Subasinghe and Bartley, 2004). As a result, exotic pathogens have been moved far outside their natural ranges, where they have gained exposure to new and naive host populations. Thus the vast majority of serious disease outbreaks that have occurred in the past 50 years are either known or believed to have been caused by transboundary aquatic animal pathogens (TAAPs) - exotic pathogens that have been transported between countries and regions into new geographic areas where they have flourished. Their economic impacts on aquaculture are well documented and include direct losses to production and secondary industries, as well as socio-economic hardships on communities (see Bondad-Reantaso, 2004). Impacts of TAAPs on wild populations of aquatic animals are less well-documented;but in at least one case, epizootic ulcerative syndrome (EUS) of freshwater fish, are thought to be considerable (Lilley, Callinan and Khan, 2002; Das, 2002).
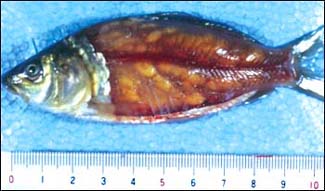
H. YOKOYAMA
Myxobolus artus infection in the skeletal muscle of 0+ carp
This paper briefly reviews the history of some of the major transboundary aquatic animal diseases in the Asia-Pacific Region and summarizes the status of some of the major diseases currently affecting the region (primarily those diseases listed by the Organisation mondiale de la santé animale (OIE) or included in the Network of Aquaculture Centres in Asia-Pacific (NACA)/Food and Agriculture Organization of the United Nations (FAO)/OIE Quarterly Aquatic Animal Disease Reporting System). Emphasis is placed on those diseases having wide distributions and impacts in the region, with less attention paid to diseases of cold-water species such as salmonids, and those of very limited distribution. Some emerging diseases that could have significant impacts on aquaculture in the region in the near future are also discussed.
HISTORICAL ACCOUNTS
Ichthyophthiriasis hits Java
The first recorded disease epizootic in Asia thought to be due to an introduced pathogen was an outbreak of ichthyophthiriasis or “white spot disease”of freshwater fish that occurred on the island of Java, Indonesia in 1932 (see Buschkiel, 1935; Sachlan, 1952; Djajadiredja et al., 1983; Arthur, 1996). The outbreak was reported by Alfred L. Buschkiel (Buschkiel 1935), a German scientist who worked at the Laboratorium voor de Binnenvisscherij in Buitenzorg (now Bogor) during the early 1930s. He reported that ichthyophthiriasis, due to the pathogenic ciliate Ichthyophthirius multifiliis, was particularly prevalent at the eastern border of West Java and that it spread rapidly eastward to Central Java with the transport of infected fish. Java barb (Barbonymus gonionotus), kissing gourami (Helostoma temmincki), common carp (Cyprinus carpio) and giant gourami (Osphronemus goramy) were the species most severely affected. The outbreak was associated with a very long drought that allowed the parasite to develop unchecked in the rather heavily stocked ponds. Although Sachlan (1952) noted that the parasite was first encountered in an aquarium at Bogor in 1928 and was probably introduced with ornamental fish from the United States and Europe, he also stated that many cases had been previously reported from Java and Sumatra. Measures taken to prevent the spread of the disease, which included ordering pond owners to drain infected ponds immediately and prohibiting the sale of infected fish, were ineffective. Losses due to the white spot epidemic in Indonesia were estimated at some 10 000 Dutch guilders, an amount that exceeded losses of salmonids in European countries caused by similar outbreaks between 1919–1928 (see Buschkiel, 1935; Sachlan, 1952).
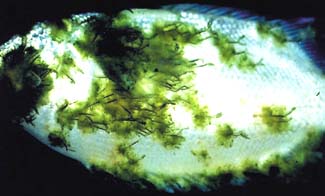
J.R. ARTHUR
Surface parasites, Lerneae cyprinacea infection of giant gouramy
Myobolus and Lernaea problems in Indonesia
In 1951, another pathogen, tentatively identified as Myxobolus pyriformis, was reported to have killed thousands of Java barb fry in a government hatchery in Central Java (Sachlan 1952). Djajadiredja et al. (1983), who considered this myxosporean to have probably been introduced to Indonesia, reported in 1982 that this gill parasite had caused serious losses annually. These authors also noted that two other unidentified species of Myxobolus, suspected to have been introduced to Indonesia from Japan, have caused considerable losses (mortalities of 60–90 percent) of common carp fingerlings in Java since 1974.

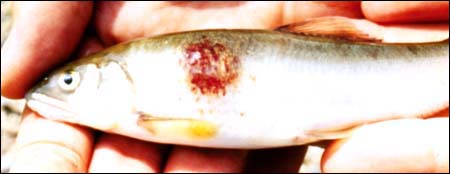
M.G. BONDAD-REANTASO
Wild mullet in philippines (1989) with EUS
Anchor worm (Lernaea cyprinacea) is a parasitic copepod that was introduced into Indonesia from Japan 1953 (Djajadiredja et al., 1983). The parasite caused a serious epizootic in economically important species such as common carp, Java barb, kissing gourami and giant gourami, and quickly spread to other regions of the country. Losses were quite significant, with about 30 percent of hatchery production in the main hatchery centers of Java, northern Sumatra and northern Sulawesi affected. In Java, it was estimated that some 1.48 billion fry were lost, worth some 7.4 billion rupiahs (approximately US$11.4 million).
Epizootic ulcerative syndrome - a disease impacts wild fish stocks
Epizootic ulcerative syndrome (EUS) is a fungal-caused disease that has affected cultured and wild fresh and brackish-water fish species throughout much of the Asia-Pacific. It is now endemic in Southeast and South Asia and has recently expanded its range into West Asia (see FAO, 2001).
EUS is indistinguishable from mycotic granulomatosis of Japan, which was first recorded in 1971, red spot disease of eastern Australia, reported in 1972, and the ulcerative mycosis disease affecting Atlantic menhaden in the United States since 1978. Although more than 100 species of fish have been reported to be affected (see Chinabut & Roberts, 1999; Lilley, Phillips and Tonguthai, 1992), populations of some species are much more severely impacted than others.
Some idea of the impacts of EUS on the relative abundances of wild fish is given by Das (1994), who studied the landings at the Jorhat Fish Assembly Centre in Assam for species of EUS-affected fish from the capture fishery in the Brahmaputra River system before (1987–1988) and during the initial (1988–1991) three years in which outbreaks occurred in India (see Table 1). His data showed declines in catches of highly susceptible species by as much as 97 percent. Among the species most severely affected were snakeheads (Channidae - Channa punctata, C. striata) and native cyprinids (Puntius spp., Labeo rohita, L. bata and Cirrhinus cirrhosus).
K. HATAI
Ayu, Plecoglatus altivelis, infected with mycotic granulomatosis
TABLE 1
Impact of EUS on local abundance of highly susceptible species (modified from Das 1994)
| Species | Decline in Landings (%) |
| Channa punctata | 85–94% |
| C. striata | 82–88% |
| Nandus nandus | 82–97% |
| Puntius spp. | 64–90% |
| Amblypharyngodon mola | 37–71% |
| Labeo rohita | 49–63% |
| L. bata | 54–87% |
| Cirrhinus cirrhosus | 28–66% |
THE DISTRIBUTION OF SERIOUS TRANSBOUNDARY DISEASES IN THE ASIA-PACIFIC REGION
The geographic distributions of the major serious transboundary diseases affecting finfish, crustaceans and molluscs in the Asia-Pacific Region (excluding West Asia) are presented in Tables 2–4. The information given in these tables is drawn mainly from the International Database on Aquatic Animal Diseases (www.collabcen.net/toWeb/aq2.asp) and the NACA/FAO Quarterly Aquatic Animal Disease Reports (Asia and Pacific Region) (www.enaca.org/Health/QAAD), which summarize data submitted by the national authorities of participating countries to the NACA/FAO/OIE Quarterly Aquatic Animal Disease (QAAD) Reporting System. These tables represent a “best guess”as to the distribution of the various pathogens, and do not include a number of reports for countries such as India, Laos, Malaysia and Myanmar that are considered to be erroneous or highly suspect.
A number of the diseases of finfish listed by the OIE affect only Salmonidae or other cold or temperate-water fishes and thus have existing or potential ranges in the Asia-Pacific that are limited to countries having temperate climates, such as Japan, the Republic of Korea, northern China, southern Australia and New Zealand. These include infectious haematopoietic necrosis (IHN), viral haemorrhagic septicaemia (VHS), Oncorhynchus masou virus disease (OMVD), infectious pancreatic necrosis (IPN), bacterial kidney disease (BKD), epizootic haematopoietic necrosis (EHN) and red seabream iridoviral disease (RSBID). Although not reported from South Asia, those diseases affecting salmonids could potentially occur in a few mountainous regions of India, Sri Lanka and Nepal where trout (primarily rainbow trout, Oncorhynchus mykiss) have been introduced to support limited sport fisheries. However, any impacts in these areas would be quite localized.
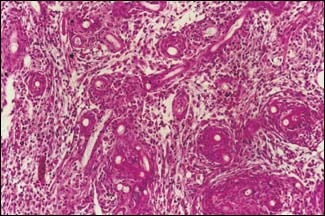
M.G. BONDAD-REANTASO
Typical severe mycotic granulomas from muscle section of EUS fish (H & E)
Several of the OIE-listed diseases that are widespread across the Asia-Pacific Region have been present in the region for more than a decade. These include EUS of fresh and brackish-water fishes, viral encephalopathy and retinopathy (VER) of marine fishes, and white spot disease (WSD), yellowhead disease (YHD) and Penaeus monodon baculovirosis (PMV) of marine shrimp. Some diseases, such as VER, have probably always occurred throughout much on the region in wild populations of aquatic organisms but have only been reported to cause disease problems when their hosts have begun to be raised in aquaculture facilities. Two other diseases recently added to the OIE list that are poorly studied in Asia-Pacific, enteric septicaemia of catfish (ESC) and grouper iridoviral disease (GID), may also fall into this category. Other diseases, such as WSD, YHD and PMV probably originated within the Asia-Pacific and were thus initially of very limited geographic distribution. However, they have now become widely distributed through the movement of infected hosts for aquaculture development. These diseases are now probably endemic in wild populations of aquatic animals throughout much of the region. In many cases, the problem that governments now face is not “How do we prevent the entry of these pathogens into our national territory?”, but rather “How do we prevent their spread to those areas of the country that are still uninfected?”, and “What can we do to prevent their introduction into individual aquaculture facilities, or to manage or eradicate these diseases in infected facilities?”
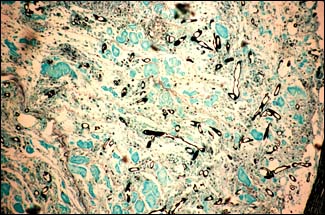
M.G. BONDAD-REANTASO
Mycotic granulomas showing fungal hyphae (stained black) using Grocotts stain
TABLE 2
Historical data on the occurrence of serious transboundary diseases of finfish in the Asia-Pacific Region.1
(+=reported,
?=suspected,
[?]=report of uncertain status
(primary sources:www.colabcen.net/toWeb/aq2.asp;www.oie-jp.org);www.enaca.org/Health/QAAD;FAO 2001
| OIE-listed Diseases1 | Other Diseases Monitored | ||||||||||||||
| Country | EHN | IHN | SVC | OMVD | VHS | VER | IPN | EUS | BKD | RSBID | ESC | KHV | Epi | GID | |
| East Asia | |||||||||||||||
| China P.R. | + | + | + | + | ? | + | ? | ||||||||
| Japan | + | + | + | + | + | + | + | + | + | ||||||
| Taipei China | + | + | + | ? | + | + | |||||||||
| Hong Kong China | + | + | + | + | + | ||||||||||
| Korea Rep. | + | ? | + | + | + | + | ? | ||||||||
| Southeast Asia | |||||||||||||||
| Cambodia | + | ||||||||||||||
| Indonesia | + | + | + | + | + | ||||||||||
| Laos2 | + | ||||||||||||||
| Malaysia2 | + | + | + | + | + | ||||||||||
| Myanmar2 | + | ||||||||||||||
| Philippines | + | + | |||||||||||||
| Singapore | + | + | + | ||||||||||||
| Thailand | + | [?] | + | +[?] | + | + | + | ||||||||
| Viet Nam | + | + | + | + | |||||||||||
| South Asia | |||||||||||||||
| Bangladesh | + | ||||||||||||||
| India2 | + | ||||||||||||||
| Nepal | + | ||||||||||||||
| Sri Lanka | + | ||||||||||||||
| Australia/South Pacific | |||||||||||||||
| Australia | + | + | + | + | |||||||||||
| French Polynesia | + | ||||||||||||||
| New Caledonia | |||||||||||||||
| New Zealand | + | ||||||||||||||
TABLE 3
Historical data on the occurrence of serious transboundary diseases of crustaceans in the Asia-Pacific Region.1
(+=reported,
?=suspected,
[?]=report of uncertain status)
(primary sources:www.colabcen.net/toWeb/aq2.asp;www.oie-jp.org;www.enaca.org/Health/QAAD;FAO 2001
| OIE-listed Diseases | Other Diseases Monitored | |||||||||
| Country | IHHN | TS | WSD | SB (PMB) | TB (BP) | YHD/GAV | SMVD | NHP | BVMGN | |
| East Asia | ||||||||||
| China PR | + | + | + | |||||||
| Japan | + | + | ||||||||
| Taipei China | + | + | + | + | + | |||||
| Hong Kong China | + | |||||||||
| Korea Rep | + | [?] | ||||||||
| Southeast Asia | ||||||||||
| Cambodia | ||||||||||
| Indonesia | + | + | + | + | ? | |||||
| Laos | ||||||||||
| Malaysia | + | ? | + | + | [?] | + | ||||
| Myanmar | + | + | ||||||||
| Philippines | + | + | + | + | + | [?] | ||||
| Singapore | + | + | + | |||||||
| Thailand | + | + | + | + | + | |||||
| Viet Nam | ? | + | + | + | + | |||||
| South Asia | ||||||||||
| Bangladesh | + | + | ||||||||
| India | + | + | + | + | [?] | |||||
| Nepal | ||||||||||
| Sri Lanka | + | + | + | |||||||
| Australia/South Pacific | ||||||||||
| Australia | + | + | +(GAV) | + | ? | |||||
| French Polynesia | + | |||||||||
| New Caledonia | + | |||||||||
| New Zealand | ||||||||||
TABLE 4
Historical data on the occurrence of serious transboundary
diseases of molluscs in the Asia-Pacific Region.
(+=reported)
(primary sources:www.colabcen.net/toWeb/aq2.asp;www.oie-jp.org;www.enaca.org/Health/QAAD;FAO 2001)
| OIE-listed Diseases | Other Diseases Monitored | ||||||||
| Country | Bonamia exitiosa | Marteilia sydneyi | Mikrocytos roughleyi | Perkinsis olseni/atlanticus | Haplosporidium nelsoni | Marteilioides chungmuensis | Akoya Oyster Disease | Abalone Mortality Virus | |
| East Asia | |||||||||
| China PR | |||||||||
| Japan | + | + | + | ||||||
| Taipei China | |||||||||
| Hong Kong China | |||||||||
| Korea Rep. | + | + | + | ||||||
| Southeast Asia | |||||||||
| Cambodia | |||||||||
| Indonesia | |||||||||
| Laos | |||||||||
| Malaysia | |||||||||
| Myanmar | |||||||||
| Philippines | |||||||||
| Singapore | |||||||||
| Thailand | |||||||||
| Viet Nam | |||||||||
| South Asia | |||||||||
| Bangladesh | |||||||||
| India | |||||||||
| Nepal | |||||||||
| Sri Lanka | |||||||||
| Australia/South Pacific | |||||||||
| Australia | + | + | + | ||||||
| French Polynesia | |||||||||
| New Caledonia | |||||||||
| New Zealand | + | + | |||||||
A few diseases, particularly some of those affecting marine shrimp (Penaeidae) are truly exotic to the Asia-Pacific, having recently been translocated into the region along with the introduction of infected exotic shrimp species, primarily white shrimp (Litopenaeus vannamei) and blue shrimp (L. stylirostris). These include infectious hypodermal and haematopoietic necrosis (IHHN), which has now widely distributed in Southeast Asia and the South Pacific, but apparently not in South Asia, and Taura syndrome (TS), which has recently been recorded from several East and Southeast Asian countries.
Unfortunately, the distribution of serious transboundary diseases in some countries in some parts of the Asia-Pacific Region remains unclear due to limited capacity for disease diagnosis, an absence of disease surveillance and monitoring programmes, and/or problems with accurate disease reporting. This is particularly true for diseases of molluscs throughout much of Asia, and for diseases of finfishes in developing countries such as Cambodia, Laos, Myanmar, Bangladesh, Nepal etc. In some countries, an additional problem is the apparent lack of awareness by the veterinary and/or fisheries authorities as to their country's national disease status. Thus in some cases, recent reports to NACA/FAO/OIE for some countries indicate that a given disease is either “never reported”or that there is “no information available”when reports of disease occurrence exist, either in the official reports to OIE, or elsewhere in the scientific literature.
THE CURRENT TRANSBOUNDARY DISEASE SITUATION IN THE ASIA-PACIFIC REGION
Tables 5–7 present summaries of the diseases listed or monitored by the NACA/FAO/OIE QAAD Reporting System for Asia-Pacific for the period 1 July 2003 – 31 June 2004, and include the most recent data available. It should be noted that these tables list only the reports of disease occurrence, and typically do not provide any indication of the seriousness or magnitude of the outbreaks. Nevertheless, the fact that a disease has recently been reported to occur in many countries gives some indication that the disease may still be a major problem in the region.
A number of serious diseases of finfish that are widely distributed in the Asia-Pacific were reported during the 12 months for which data are available. Viral encephalopathy and retinopathy (VER) of marine fish (groupers, seabass etc.) was reported from East Asia (Japan, Hong Kong and Taipei), Southeast Asia (Indonesia (?), Philippines, Thailand and Viet Nam), Australia and the South Pacific (French Polynesia), while epizootic ulcerative syndrome (EUS) was recorded from Southeast Asia (Cambodia, Thailand and Viet Nam), South Asia (Bangladesh, Nepal and Sri Lanka) and Australia. Red seabream iridovirus disease (RSBID) was reported from three countries in East Asia (Japan, Hong Kong and the Republic of Korea). Several diseases of salmonids continue to be reported from East Asia (primarily Japan). These include IHN, OMVD, VHS, IPN and BKD. Epitheliocystis was reported from East Asia (Japan and Republic of Korea) and New Zealand, while epizootic haematopoietic necrosis (EHN) was detected in redfin perch (Perca fluviatilis) following a fish kill in Australia. Two finfish diseases, Koi herpes virus (KHV) and grouper iridoviral disease (GID), appear to be spreading in the Asia-Pacific Region and thus will be discussed in detail later. Although the occurrence of spring viraemia of carp (SVC) in the Asia-Pacific Region has not yet been officially reported to the NACA/FAO/OIE Quarterly Aquatic Animal Disease Reporting System, the virus has recently been isolated from koi and common carp in China P.R (see the International Database on Aquatic Animal Diseases, www.collabcen.net/toWeb/aq2.asp). One other disease, enteric septicaemia of catfish (ESC), was not reported during the period in question
TABLE 5
Summary of reports of the occurrence of serious transboundary diseases of finfish in the Asia-Pacific Region, July 2003–June 2004.
(+ = reported or known to be present during period;
+? = serological evidence and/or isolation of causative agent but not clinical disease;
? = suspected but presence not confirmed;
+( )= occurrence limited to certain zones;
*** = no information available;
0000 = never reported;
- = not reported during period but
disease is known to occur
(Data for countries reporting to the NACA/FAO/OIE Regional Quarterly Aquatic Animal Disease (QAAD) Reporting System
are taken from the FAO/NACA Quarterly Aquatic Animal Disease Reports (www.enaca.org/Health/QAAD);that for other countries (Taipei China,
French Polynesia, New Caledonia and New Zealand) are taken from OIE (www.oie-jp.org)
Table 6
Summary of reports of the occurrence of serious transboundary diseases of crustaceans in the Asia-Pacific Region, July 2003–June 2004.
(+ = reported or known to be present during period;
+? = serological evidence and/or isolation of causative agent but not clinical diseases;
? = suspected but presence not confirmed;
+( )= occurrence limited to certain zones;
*** = no information available;
0000 = never reported;
- = not reported during period but disease is known to occur
(Data for countries reporting to the NACA/FAO/OIE Regional Quarterly Aquatic Animal Disease (QAAD) Reporting System are taken from the FAO/NACA Quarterly Aquatic Animal Disease Reports (www.enaca.org/Health/QAAD), that for other countries (Taipei China, French Polynesia, New Caledonia and New Zealand) is taken from OIE (www.oie-jp.org)
| OIE-listed Diseases1 | Other Diseases Monitored | |||||||||
| Country | TS | WSD | YHD/GAV | SB (PMB) | IHHN | SMVD | TB (BP) | NHP | BVMGN | |
| East Asia | ||||||||||
| China PR | +( ) | + | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | |
| Japan | 0000 | + | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | |
| Taipei China | - | - | *** | *** | 0000 | *** | *** | *** | *** | |
| Hong Kong China | 0000 | +? | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | |
| Korea Rep. | 0000 | - | 0000 | 0000 | 0000 | |||||
| Southeast Asia | ||||||||||
| Cambodia | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Indonesia | + | + | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | |
| Laos | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Malaysia | ? | +( ) | - | - | - | 0000 | - [?] | 0000 | ||
| Myanmar | 0000 | +( ) | 0000 | 0000 | +( ) | 0000 | 0000 | 0000 | ||
| Philippines | 0000 | + | *** | *** | *** | - | *** | *** | *** | |
| Singapore | *** | - | *** | - | *** | *** | *** | *** | *** | |
| Thailand | + | + | + | ? | + | *** | *** | *** | *** | |
| Viet Nam | +( ) | + | + | + | ? | 0000 | 0000 | 0000 | ||
| South Asia | ||||||||||
| Bangladesh | *** | + | *** | *** | *** | *** | *** | *** | *** | |
| India | *** | +( ) | *** | *** | 0000 | |||||
| Nepal | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Sri Lanka | 0000 | + | ? | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | |
| Australia/South Pacific | ||||||||||
| Australia | 0000 | 0000 | + | - | +? | -(?) | 0000 | 0000 | 0000 | |
| French Polynesia | *** | *** | *** | *** | *** | *** | *** | *** | ||
| New Caledonia | 0000 | 0000 | 0000 | 0000 | + | 0000 | 0000 | 0000 | 0000 | |
| New Zealand | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | 0000 | |
TABLE 7.
Summary of reports of the occurrence of serious transboundary diseases of finfish in the Asia-Pacific Region, July 2003–June 2004.
(+ = reported or known to be present during period;
+? = serological evidence and/or isolation of causative agent but not clinical diseases;
? = suspected but presence not confirmed;
+( )= occurrence limited to certain zones;
*** = no information available;
0000 = never reported;
- = not reported during period but disease is known to occur
(Data for countries reporting to the NACA/FAO/OIE Regional Quarterly Aquatic Animal Disease (QAAD) Reporting System are taken from the FAO/NACA Quarterly Aquatic Animal Disease Reports (www.enaca.org/Health/QAAD), that for other countries (Taipei China, French Polynesia, New Caledonia and New Zealand) is taken from OIE (www.oie-jp.org)
Several diseases of Crustacea were widely reported from the Asia-Pacific during the most recent 12 months of reporting to NACA/FAO/OIE. Whitespot disease (WSD) continues to plague shrimp production in much of East Asia (China P.R., Japan and Hong Kong), Southeast Asia (Indonesia, Malaysia, Myanmar, Philippines, Thailand and Viet Nam) and South Asia (Bangladesh, India and Sri Lanka). Yellowhead disease (YHD) was recorded from Southeast Asia (Thailand and Viet Nam) and South Asia (Sri Lanka), while the closely related gill-associated virus (GAV) was reported from Australia. Infectious hypodermal and haematopoietic necrosis (IHHN) was reported from Southeast Asia (Myanmar, Thailand and Viet Nam), Australia and the South Pacific (New Caledonia), while spherical baculovirosis (SB) was recorded from Southeast Asia (Thailand and Viet Nam). One exotic viral disease of penaeid shrimp (Taura syndrome, TS) is an emerging disease in Asia, and will be discussed in more detail later. Other diseases listed by NACA/FAO/OIE were either not reported during the period in question (spawner-isolated mortality virus disease, SMVD) and baculoviral midgut gland necrosis, BVMGN) or have never been confirmed or reported from the region (tetrahedral baculovirosis, TB, and necrotising hepatopancreatitis, NHP).
As previously mentioned, the ability of the majority of countries reporting to NACA/FAO/OIE to diagnose diseases of molluscs is very limited and thus the occurrence of outbreaks of disease may go unreported, particularly in South and Southeast Asia. During the 12-month period under consideration, Japan reported cases of Marteilioides and Akoya oyster disease, while the Republic of Korea reported cases of Marteilioides and Perkinsis. Australia reported cases of Marteilia, Mikrocytos and Perkinsis, and New Zealand reported cases of Bonamia and Perkinsis. Two molluscan diseases may be emerging in the region (Akoya oyster disease and abalone mortality virus) and thus will be discussed in more detail below.
Information on the seriousness of the disease outbreaks that occurred in the Asia-Pacific Region during the past 12 months and their social and economic impacts is generally lacking. The impacts of Koi herpes virus on koi and common carp culture in Indonesia and Japan are briefly mentioned below, and are presented in more detail elsewhere (Iida et al., 2004; Sano et al., 2004; Sunarto et al., 2004).
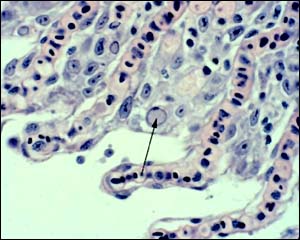
R. HEDRICK, UC DAVIS
KHV pathology. Hyperplasia and fusion of gill lamellae;intranuclear inclusion (arrow) in the branchial epithelium (gill section with H & E).
SOME CURRENT AND POTENTIAL EMERGING DISEASE PROBLEMS
The following section discusses several serious diseases of aquatic animals that are currently expanding their range in the Asia-Pacific or, although of limited distribution in the region, have the potential to expand their ranges and cause significant losses. Of these, white tail disease (WTD) has not been monitored by NACA/FAO/OIE and thus has received little attention. It will thus be discussed in more detail than the other, better known diseases.
White tail disease
White tail disease (WTD) (also reported as “whitish muscle disease”by Chinese workers) has caused epizootic losses of postlarval giant river prawns (Macrobrachium rosenbergii) in southern Taiwan Province of China (Taiwan POC) since 1992. The disease, which can cause mortalities of up to 100 percent of postlarvae (PL) in hatcheries, has also been reported from M. rosenbergii from mainland China, the French West Indies and India (see Arcier et al., 1999; Sri Widada et al., 2003; Qian et al., 2003; Sahul Hameed, 2004). In India, it was first reported in 2003 as affecting many hatcheries at Nellore, Andhra Pradesh and Chennai, and has also been seen in farms in Nellore, Andhra Pradesh (Hameed, 2004; NACA, 2004). WTD appears to spread rapidly, causing heavy economic losses due to high mortalities.
The probable cause of WTD is the Macrobrachium rosenbergii nodavirus (MrNV), also reported as Macrobrachium muscle virus (MMV) by Tung, Wang and Chen (1999). However, as noted by Sri Widada et al. (2003), the virus-host interactions and modes of transmission of this disease are still unknown.
The clinical signs of the disease in severely affected postlarvae (PL), which are similar to those of idiopathic muscle necrosis (IMN) syndrome (see Nash et al., 1987) and thus not sufficient for presumptive diagnosis, include white opaque areas in the abdominal segments, commonly accompanied by lethargy and anorexia (see Tung, Wang and Chen, 1999; Arcier et al., 1999; Sahul Hameed, 2004; NACA, 2004). Severe cases may show degeneration of the telson and uropods. Mortalities of 100 percent may occur within two or three days of the appearance of muscle opacity. Experimental infections indicate that infections may be responsible for branchostegite blister disease (BBD) or “swollen head syndrome”in adults (see Sahul Hameed, 2004; NACA, 2004).
The histopathological changes seen are similar to those described for IMN and include progressive segmental myofibre degeneration of muscle fibres and necrobiotic myopathy with numerous single, rows, aggregations and sheets of hyperchromatic myonuclei. Centrally or eccentrically placed (nuclear internalization) pyknotic nuclei were also frequently observed. However, unlike IMN, cytoplasmic inclusion bodies have been detected in the necrotic muscle of diseased prawns (see Tung, Wang and Chen, 1999). Transmission electron microscopy (TEM) of tissue homogenates from diseased prawns has revealed the presence of numerous non-enveloped virus-like particles of about 30 nm in diameter (Arcier et al., 1999).
Initially, the use of pryonin methyl green was recommended (see Tung, Wang and Chen, 1999) to distinguish the characteristically green-stained MrNV viral inclusions from hemocyte nuclei. More recently Sri Widada et al. (2003) have developed three complimentary genome-based detection methods (dot-blot hybridization, in situ hybridization and reverse transcriptase-polymerase chain reaction (RT-PCR)) for detection of MrNV, dot-blot hybridization being considered the easiest to perform. In addition, Romestand and Bonami (2003) have developed a sandwich enzyme linked immmunosorbent assay (S-ELISA) for detection of this virus.
A second virus, extra small virus (XSV), whose role, if any, in producing WTD is unclear, was first reported in M. rosenbergii postlarvae from mainland China having concurrent infections with MrNV (Qian et al., 2003). Qian et al. (2003) suggested that XSV, which is located in the muscle and connective cells of diseased animals, could be a helper virus for MrNV or a satellite-like virus, possibly acting as a disease modulator. It has also been considered a satellite virus by Widada and Bonami (2004). Although Qian et al. (2003) believed that XSV was always associated with MrNV, results presented by Sri Widada et al. (2004) suggest that this is not always the case. These authors concluded that the cause of WTD may be more complex than previously thought and thus further investigation is required.
Transmission electron microscopy (TEM) has revealed that the XSV viral particle is isocohedral and about 15 nm in diameter. Genome-based diagnostic techniques (dot-blot hybridization and RT-PCR) for XSV have been developed by Sri Widada et al. (2004).
Taura syndrome
Taura syndrome (TS) was first detected in shrimp farms near the Taura River, Ecuador (hence the name of the disease) in 1992. It then spread throughout most shrimp-growing regions of Latin America and the Pacific coasts of Colombia, Costa Rica, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama and Peru. It was also introduced to Hawaii but was successfully eradicated.
Erickson et al. (2002) reported that anecdotal evidence of occurrences of TS epizootics in L. stylirostris since 1999 in Mexico suggests that a closely related virus might have evolved from the original Ecuadorian/Hawaiian isolate characterized by Bonami et al. (1997) and Mari et al. (2002). They noted the possibility that since 1994 a change in Taura syndrome virus (TSV) structural protein may have occurred and that this change is now responsible for the emergence of TS in L. stylirostris, a previously refractive species (Brock et al., 1997).
Survivors of acute TSV infection pass through a brief transitional phase and enter the chronic phase, which may persist for the rest of their lives. This subclinical phase of infection is believed to have contributed to the spread of the disease via carriage of viable virus.
TS has recently spread to Asia through the introduction of Litopenaeus vannamei and has now been reported from Taiwan POC, Indonesia, Malaysia (suspected), Thailand and Viet Nam (Palanisamy, 2004; Sunarto et al., 2004; de la Peña, 2004;Van, 2004).
In Indonesia, TSV was first discovered in shrimp culture in November 2002 in L. vannamei from East Java (see Sunarto et al., 2004). It is suspected that TSV first occurred in Banyuwangi and Situbono before spreading to other districts of East Java through the movement of infected PL. Samples testing positive by PCR have also been detected from Brebes (central Java), Situbondo (East Java) and the islands of Bali, Sulawesi and Sumbawa. It thus appears that the virus is now widespread in Indonesian shrimp culture where it causes mortalities in 1–2 month old L. vannamei reared in intensive culture systems.
Koi herpes virus (=carp nephritis and gill necrosis virus)
Koi herpes virus disease first appeared in the Asia-Pacific Region in March 2002 in Indonesia and in Taiwan POC in December of the same year, with subsequent outbreaks in Japan beginning in October 2003(see Lio-Po, 2004; Tu et al., 2004, Sano et al., 2004; Sunarto et al., 2004). Earlier reports of infections with Koi herpes virus (KHV) are recorded for the Republic of Korea (1998, suspected - reported as viral infection causing mass mortality of koi and common carp), Malaysia (2000) and Japan (2000).
An interesting characteristic of the disease has been its rapid spread across Indonesia and Japan through the trade in live koi for the ornamental fish trade and common carp for foodfish production. In both cases this has been due to the disease appearing rapidly in major production areas, and its rapid dissemination through the live fish trade. By the end of May 2004, KHV had been reported from 24 of Japan's 47 prefectures (Sano et al., 2004), while in Indonesia, the disease has now spread throughout much of Java and into South Sumatra (see Sunarto et al., 2004).

STUART MILLAR
Koi carp infected with Koi herpesvirus (KHV)
In both Indonesia and Japan, losses have been significant. In Lake Kasumigaura, Japan, mortalities of almost 100 percent occurred in netpen culture, with a total loss of almost 25 percent of the lake's annual production. In Indonesia, the first occurrence of mass mortalities due to KHV occurred in cultured koi in March 2002 in Blitar, East Java. Mortalities of up to 80–95 percent were seen in koi carp of all ages. As Blitar is the center of koi production for Indonesia, diseased fish were rapidly transported throughout the country, with Central Java, West Java and Jakarta being the main markets. Common carp is an important foodfish in Indonesia, and KHV rapidly impacted carp aquaculture production. Epizootics subsequently occurred in Subang Regency (West Java) in April 2002 and then in neighbouring areas. The disease has continued to spread through the transfer of live infected carp, with outbreaks occurring in May–June in carp cultured in netcages in the Citarum River system and by February 2003, it had been translocated to Lubuk Lingau Regency, South Sumatra along with carp moved from West Java by fish traders. It has since continued its spread to neighbouring provinces.
The origins of KHV remain obscure. In Indonesia, the disease is thought to have been introduced with the importation of koi from mainland China (via Hong Kong) in December 2001 and January 2002. Earlier, KHV had been reported from Israel, the United States and the United Kingdom in 1998, with subsequent cases in nine other European countries and in South Africa (see Crane, Sano and Komar, 2004).
The social and economic impacts of KHV in Indonesia are still being felt. In Blitar alone, the disease has affected more than 5 000 carp culturists with economic losses exceeding Rp 5 billion during the first three months of the outbreak. As of December 2003, the total losses due to KHV due to revenue lost to the carp culture sector and the accompanying impact on rural farming communities was estimated at some US$15 million (Sunarto et al., 2004).
Grouper iridoviral disease
Grouper iridoviral disease, also known as “sleepy grouper disease”, is caused by grouper irodovirus (GIV). It affects groupers (Epinephelus spp.) in South and East Asia, having been reported from Taipei, Hong Kong and PR China (suspected), Singapore and Viet Nam (see Nakajima, 2003; Table 2). It is a problem in grouper culture, where it causes losses of fry, juveniles and market-size fish. As “sleeper grouper disease”has also been reported from Malaysia and Thailand (see Kasornchandra, 2002), the virus probably naturally occurs throughout much of Southeast and East Asia where grouper are cultured. Because most grouper fry used for stocking are still wild-caught, it is probably also widely distributed in wild grouper populations.
Spring viraemia of carp
Although spring viraemia of carp (SVC) has traditionally been considered a disease of European countries having low water temperatures during winter (FAO, 2001), SVC virus has recently been isolated from China P.R. As Chinese carps, the principal species affected by SVC, are widely cultured throughout Asia, the disease has the potential to affect carp culture wherever suitably low temperatures (11–17 °C) occur.
Withering syndrome of abalone
Withering syndrome of abalone is caused by an intracellular prokaryote, the rickettsia-like organism Candidatus Xenohaliotis californiensis, which infects the epithelium of the digestive tract (see Bower, 2004). Although its confirmed distribution is the Pacific coast of southern California to Baja, Mexico, a similar disease has been reported from northern China, and rickettisia-like organisms have also been reported from abalone cultured in South Africa. Although the presence of this pathogen in the Asia-Pacific has not yet been confirmed, the disease was recently added to the NACA/ FAO/OIE Quarterly Aquatic Animal Disease (QAAD) Reporting System.
The disease is lethal to all sizes of abalone (genus Haliotis), in which it causes lethargy, retracted visceral tissues and atrophy of the foot muscle, affecting the abalone's ability to adhere to the substrate.
Akoya oyster disease
Akoya oyster disease is as yet known only from Japan, where it first appeared in 1994. It affects Japanese pearl oyster, Pinctata fucata martensii, of at least one year old or older, causing stunting, tissue atrophy and cytopathological changes (see Wada, 2003). It causes significant economic losses to the pearl culture industry in southern Japan, disease outbreaks occurring at high water temperatures (above 20°C). Although a virus has been isolated from diseased oysters, its role in the disease has not been shown, and thus the causative agent is still considered to be unknown. Because the disease is believed to be caused by a biological agent, and causes significant mortalities of pearl oysters, which are widely cultured and have significant economic value in many countries of the Asia-Pacific Region, Akoya oyster disease was recently added to the NACA/FAO/OIE QAAD Reporting System.
CONCLUSIONS
Countries in the Asia-Pacific remain highly vulnerable to transboundary aquatic animal diseases. As the recent introduction and spread of KHV throughout most of Japan and in Indonesia has shown, governments can be easily caught unawares by new diseases. To better protect themselves from exotic diseases, countries in the Asia-Pacific Region should undertake risk analyses to determine which transboundary diseases are most likely to gain entry via their current trading practices, and of these, which are most likely to cause serious damage to their aquaculture industries and capture fisheries. Based on this knowledge, national governments should take steps to reduce the risk of pathogen entry through health certification, quarantine and/or other appropriate risk mitigation measures. They should also initiate contingency planning to develop emergency response plans that will limit the spread and impacts of such diseases. Along with this, the other components of a National Aquatic Animal Health Strategy should be given high priority, so that the necessary legislation, expertise and capacity to deal with a disease emergency is in place before a serious disease outbreak due to an exotic pathogen occurs.
LITERATURE CITED
Arcier J.-M., Herman, F., Lightner, D.V., Redman, R.M., Mari, J. & Bonami, J.-R. 1999. A viral disease associated with mortalities in hatchery-reared postlarvae of the giant freshwater prawn Macrobrachium rosenbergii. Dis. Aquat. Org., 38: 177–181.
Arthur, J.R. 1996. A history of fisheries parasitology in Southeast Asia. In:S.S. De Silva, ed. Perspectives in Asian fisheries - a volume to commemorate the 10th anniversary of the Asian Fisheries Society. pp. 383–407. Manila, Asian Fisheries Society.
Arthur, J.R. 2004. A brief review of international trade in live aquatic animals. In:J.R. Arthur & M.B. Reantaso, eds. Capacity and awareness building on import risk analysis for aquatic animals. Report of the Joint APEC/FAO/NACA/OIE/DoF-Thailand/INP/CONPESCA/SAGARPA Workshops, Bangkok, Thailand, 1–6 April 2002 and Mazatlan, Sinaloa, Mexico, 12–17 August 2002. pp. 1–7. Asia-Pacific Economic Cooperation, APEC Fisheries Working Group.
Bonami, J.R., Hasson, K.W., Mari, J., Poulos, B.T. & Lightner, D.V. 1997. Taura syndrome of marine penaeid shrimp:characterization of the viral agent. J. Gen. Virol., 78: 313–319.
Bondad-Reantaso, M.G. 2004. Trans-boundary aquatic animal diseases/pathogens. In: J.R. Arthur & M.B. Reantaso, eds.. Capacity and awareness building on import risk analysis for aquatic animals. Report of the Joint APEC/FAO/NACA/OIE/DoF-Thailand/INP/CONPESCA/SAGARPA Workshops, Bangkok, Thailand 1–6 April 2002 and Mazatlan, Sinaloa, Mexico, 12–17 August 2002. pp. 9–22. Asia-Pacific Economic Cooperation, APEC Fisheries Working Group.
Bower, S.M. 2004. Synopsis of infectious diseases and parasites of commercially exploited shellfish:withering syndrome of abalone. (www-sci.pac.dfo-mpo.gc.ca/shelldis/pages/fwsab_e.htm)
Brock, J.A., Gose, R.B., Lightner, D.V. & Hasson, K. 1997. Recent developments and an overview of Taura syndrome of farmed shrimp in the Americas, In:T.W. Flegel & I.H. MacRae, eds. Diseases in Asian aquaculture III. pp. 275–283. Manila, Fish Health Section, Asian Fisheries Society.
Buschkiel, A.L. 1935. Neue Beiträge zur Kenntnis des Ichthyophthirius multifiliis Fouquet. Arch. Néerland. Zool., 2:178–224
Crane, M., Sano, M. & Komar, C. 2004. Infection with koi herpesvirus--disease card. In:NACA/FAO. Quarterly aquatic animal disease report (Asia and Pacific Region). April–June 2004, 2004/2. pp. 43–47. Bangkok, NACA.
Chinabut, S. & Roberts, R.J. 1999. Pathology and histopathology of epizootic ulcerative syndrome (EUS). Bangkok, Aquatic Animal Health Research Institute, Department of Fisheries, 33 pp.
Das, M.K. 1994. Outbreak of the fish disease epizootic ulcerative syndrome in India - an overview. In:R.J. Roberts, B. Campbell & I.H. MacRae, eds. ODA Regional Seminar on Epizootic Ulcerative Syndrome at the Aquatic Animal Health Research Institute, Bangkok, Thailand, 25–27 January 1994. pp. 21–38.
Das, M.K. 2002. Social and economic impacts of disease in inland open-water and culture-based fisheries in India. In: J.R. Arthur, M.J. Phillips R.P. Subasinghe, M.B. Reantaso, & I.H. MacRae, eds. Primary aquatic animal health care in rural, small-scale, aquaculture development. pp. 333–344. FAO Fisheries Technical Paper No. 406, Rome.
de la Peña, L. 2004. Transboundary shrimp viral diseases with emphasis on white spot syndrome virus (WSSV) and Taura syndrome virus (TSV). In C.R. Lavilla-Pitogo & K. Nagasawa. Transboundary fish diseases in Southeast Asia:occurrence, surveillance, research and training. pp. 67–69. Tigbauan, Iloilo, Philippines. SEAFDEC Aquaculture Department.
Djajadiredja, R., Panjaitan, T.H., Rukyani, A., Sarono, A., Satyani, D. & Supriyadi, H. 1983 Country Reports:Indonesia. In:F.B. Davy & A. Chouinard, eds. Fish quarantine and fish diseases in Southeast Asia. pp. 19–30. Report of a workshop held in Jakarta, Indonesia, 7–10 December 1982. International Development Research Centre Publication IDRC-210e, Ottawa.
Erickson, H.S., Zarain-Herzberg, M. & Lightner, D.V. 2002. Detection of Taura syndrome virus (TSV) strain differences using selected diagnostic methods:diagnostic implications in penaeid shrimp. Dis. Aquat. Org., 52:1–10.
FAO. 2001. Asia diagnostic guide to aquatic animal diseases. M.G. Bondad-Reantaso, S.E. McGladdery, I. East & R.P. Subasinghe, eds. FAO Fisheries Technical Paper No. 402, Suppl. 2, 240 pp, Rome.
Iida, T., Sano, M., Ito, T., Kurita, J., Yuasa, K. & Miwa, S. 2005. Responses to koi herpesvirus (KHV) outbreaks in Japan In:R.P. Subasinghe & J.R. Arthur, eds. Preparedness and response to aquatic animal health emergencies in Asia. pp. xx-xx. FAO Fisheries Technical Paper No. xxx.
Kasornchandra, J. 2002. Major viral and bacterial diseases of cultured seabass and groupers in Southeast Asia. In:C.R. Lavilla-Pitogo & E.R.. Cruz-Lacierda, eds. Diseases in Asian aquaculture IV. pp. 205–212. Manila, Fish Health Section, Asian Fisheries Society.
Lilley, J.H, Callinan, R.B. & Khan, M.H. 2002. Social, economic and biodiversity impacts of epizootic ulcerative syndrome (EUS). In: J.R. Arthur, M.J. Phillips, R.P. Subasinghe, M.B. Reantaso & I.H. MacRae, eds. Primary aquatic animal health care in rural, small-scale, aquaculture development. pp. 127–139. FAO Fisheries Technical Paper No. 406, Rome.
Lilley, J.H., Phillips, M.J. & Tonguthai, K. 1992. A review of epizootic ulcerative syndrome (EUS) in Asia. Bangkok, Aquatic Animal Health Research Institute and Network of Aquaculture Centres in Asia-Pacific, 73 pp.
Lio-Po, G.D. 2004. Summary brief:International Symposium on Koi Herpesvirus Disease. In C.R. Lavilla-Pitogo and K. Nagasawa. Transboundary fish diseases in Southeast Asia:occurrence, surveillance, research and training. pp. 71–73. Tigbauan, Iloilo, Philippines. SEAFDEC Aquaculture Department.
Mari, J., Poulos, B.T., Lightner, D.V. & Bonami, J.R. 2002. Shrimp Taura syndrome virus:genomic characterization and similarity with members of the genus cricket paralysis-like viruses. J. Gen. Virol., 83: 917–928.
NACA. 2004. White tail disease (WTD) of Macrobrachium rosenbergii in India. (www.enaca.org/modules/news/articlephp.storyid=50 (posted 20/11/32003).
Nakajima, K. 2003. Grouper iridoviral disease - disease card. Developed to support the NACA/FAO/OIE Regional Quarterly Aquatic Animal Disease (QAAD) Reporting System in the Asia-Pacific. Bangkok, NACA, 4 pp. (www.enaca.org/Health/DiseaseLibrary/Disease_card_GIV_Nakajima.pdf)
Nash G., Chinabut, S. & Limsuwan, C. 1987. Idiopathic muscle necrosis in the freshwater prawn, Macrobrachium rosenbergii de Man, cultured in Thailand. J. Fish Dis., 10: 109–120.
Palanisamy, V. 2004. Part II:Associated transboundary aquatic animal pathogens. Paper presented at the Workshop On ”Building Capacity to Combat Impacts of Aquatic Invasive Alien Species and Associated Trans-boundary Pathogens”, 12–16 July 2004, Penang, Malaysia, 14 pp.
Qian D., Shi, Z., Zhang, S., Cao, Z., Liu, W., Li, L., Xie, Y., Cambournac, I. & Bonami, J.R. 2003. Extra small virus-like particles (XSV) and nodavirus associated with whitish muscle disease in the giant freshwater prawn, Macrobrachium rosenbergii. J. Fish Dis., 26: 521–527.
Romestand B. & Bonami, J.R.. 2003. A sandwich enzyme linked immunosorbent assay (S-ELISA) for detection of MrNV in the giant freshwater prawn, Macrobrachium rosenbergii (de Man). J Fish Dis., 26:71–75.
Sachlan, M. 1952. Notes on parasites of fresh-water fishes in Indonesia. Contributions of the Inland Fisheries Research Stations, 2:1–60.
Sahul Hameed, A.S. 2004. White tail disease of Macrobrachium rosenbergii. (www.enaca.org).
Sano, M., Ito, T., Kurita, J., Yuasa, K., Miwa, S. & Iida, T. 2004. Experience on common carp mass mortality in Japan. In C.R. Lavilla-Pitogo & K. Nagasawa. Transboundary fish diseases in Southeast Asia:occurrence, surveillance, research and training. pp. 13–19. Tigbauan, Iloilo, Philippines. SEAFDEC Aquaculture Department.
Sri Widada, J., Durand, S., Cambournac, I., Qian, D., Shi, Z., Dejonghe, E., Richard V. & Bonami, J.R. 2003. Genome-based detection methods of Macrobrachium rosenbergii nodavirus, a pathogen of the giant freshwater prawn, Macrobrachium rosenbergii: dot-blot, in situ hybridization and RT-PCR. J. Fish Dis., 26:583–590.
Sri Widada J., Richard, V., Shi, Z., Qian, D. & Bonami. J.R. 2004. Dot-blot hybridization and RT-PCR detection of extra small virus (XSV) associated with white tail disease of prawn Macrobrachium rosenbergii. Dis Aquat Org., 58: 83–87.
Subasinghe, R.P. & Bartley, D. 2004. Risks of species introduction. In:J.R. Arthur, & M.B. Reantaso, eds. Capacity and awareness building on import risk analysis for aquatic animals. Report of the Joint APEC/FAO/NACA/OIE/DoF-Thailand/INP/CONPESCA/SAGARPA Workshops, Bangkok, Thailand 1–6 April 2002 and Mazatlan, Sinaloa, Mexico, 12–17 August 2002. pp.23–31. Asia-Pacific Economic Cooperation, APEC Fisheries Working Group.
Sunarto, A., Widodo, Taukhid, Koesharyani, I., Supriyadi, H., Gardenia, L., Sugianti, B. & Rukmono, D. 2004. In C.R. Lavilla-Pitogo and K. Nagasawa. Transboundary fish diseases in Southeast Asia:occurrence, surveillance, research and training. pp. 91–121. Tigbauan, Iloilo, Philippines. SEAFDEC Aquaculture Department.
Tu, C., Lin, S.-Y. & Sung, H.-T. 2004. Current status of koi herpesvirus in Taiwan. In C.R. Lavilla-Pitogo and K. Nagasawa. Transboundary fish diseases in Southeast Asia:occurrence, surveillance, research and training. pp. 21–24. Tigbauan, Iloilo, Philippines. SEAFDEC Aquaculture Department.
Tung, C.W., Wang, C.S. & Chen, S.N. 1999. Histological and electron microscopic study on Macrobrachium muscle virus (MMV) infection in the giant freshwater prawn, Macrobrachium rosenbergii (de Man), cultured in Taiwan. J. Fish Dis., 22: 319–324.
Van, V.K. 2004. Current status of transboundary fish diseases in Vietnam:occurrence, surveillance, research and training. In C.R. Lavilla-Pitogo and K. Nagasawa. Transboundary fish diseases in Southeast Asia:occurrence, surveillance, research and training. pp. 221–227. Tigbauan, Iloilo, Philippines. SEAFDEC Aquaculture Department.
Wada, K.T. 2003. Akoya oyster disease - disease card. Developed to support the NACA/FAO/OIE Regional Quarterly Aquatic Animal Disease (QAAD) Reporting System in the Asia-Pacific. Bangkok, NACA, 3 pp. (www.enaca.org/Health/DiseaseLibrary/AkoyaDisease.pdf)
Widada, J.S. & J.R. Bonami. 2004. Characteristics of the monocistronic genome of extra small virus, a virus-like particle associated with Macrobrachium rosenbergii nodavirus: possible candidate for a new species of satellite virus. J. Gen. Virol., 85: 643–646.