by
W. DALL
Department of Zoology
University of Guelph
Guelph, Ontario, Canada
Abstract
Food of the Australian commercial penaeid shrimps, Penaeus esculentus, P. merguiensis, P. plebejus, Metapenaeus bennettae, and M. macleayi, was found to consist of the remains of small animals and a large amount of unrecognizable material. It is suggested that the latter forms the main component of the diet, and that shrimps derive this by browsing on the micro-organisms (bacteria, algae, and micro-fauna) which grow on the surface of the substrata. Gross structure and function of the gut are described. Digestion and assimilation were found to be largely completed within six hours at 20°C, and this is attributed to the finely divided nature of the food. The need for detailed study of the productivity of the micro-organisms of the estuarine and inshore substrata is discussed.
NOURRITURE ET HABITUDES ALIMENTAIRES DE CERTAINS PENAEIDAE D'AUSTRALIE
Résumé
On s'est aperçu que la nourriture des Penaeidae d'Australie présentant un intérêt commercial (Penaeus esculentus, P. merguiensis, P. plebejus, Metapenaeus bennettae, et M. macleayi) consistait en des restes de petits animaux et une forte quantité de matériaux non identifiés. On suppose que ces derniers forment le principal élément du régime alimentaire et que les crevettes se les procurent en broutant les micro-organismes (bactéries, algues et micro-faune) qui poussent à la surface du substrat. La communication contient une description sommaire de la structure et des fonctions du tube digestif. L'expérience montre que la digestion et l'assimilation sont en grande partie terminées au bout de six heures à 20°C, phénomène que l'on attribue au fait que la nourriture est constituée de fines particules. L'auteur examine la nécessité d'entreprendre une étude détaillée de la productivité des micro-organismes rencontrés dans le substrat des estuaires et des zones côtières.
LOS ALIMENTOS Y LA ALIMENTACION DE ALGUNOS PENEIDOS AUSTRALIANOS
Extracto
Se ha comprobado que la alimentación de los camarones comerciales Penaeus esculentus, P. merguiensis, P. plebejus, Metapenaeus bennettae, y M. macleayi consiste en residuos de pequeños animales y en una gran cantidad de material irreconocible. Se supone que este último forma el componente principal de su alimentación, y que los camarones lo obtienen buscándolo cuidadosamente entre los microorganismos (bacterias, algas y microfauna) que crecen sobre la superficie de los substratos. Se describen la estructura general y la función del intestino. Se ha comprobado que la digestión y la asimilación se hacen en general en menos de seis horas a 20°C, atribuyéndose esto a la naturaleza muy dividida de los alimentos. Se discute la necesidad de realizar un estudio detallado de la productividad de los microorganismos de los substratos de los estuarios y de aguas cercanas a la costa.
Until recently, the Natantia (shrimps) as a group have been neglected by crustacean physiologists, though the shallow-water Penaeidae comprise the bulk of the shrimp resources of the world. Most of the research has been carried out in temperate latitudes where the population of Natantia is small, or individuals themselves are small. Yet shallow-water Penaeidae are not difficult to maintain in tanks or ponds for experiments, and, apart from their great economic importance, are intrinsically interesting as the most primitive decapod family (Calman, 1909). Early investigations on the Paneaidae dealt with systematics and field biology, though much still remains to be done in these fields in many countries.
Study of food, feeding and assimilation are of fundamental importance in understanding the rate of growth, population concentrations, gonadial maturation and other metabolic activities. This paper is a consideration of the food of penaeids, their feeding, and some aspects of digestive-tract functions and assimilation.
This study was carried out in Moreton Bay and adjacent waters in the vicinity of Brisbane, Australia. The following species were studied: P. esculentus (tiger prawn) P. merguiensis (banana prawn), Penaeus plebejus (king prawn), Metapenaeus bennettae (greasy-back or green-tail prawn) and M. macleayi (school prawn). Investigations showed that there were no important generic or specific differences in food preferences, feeding, or digestive tract function within this group, and most attention was therefore devoted to M. bennettae.
All observations were made on freshly caught animals under laboratory conditions. Some of these need to be confirmed by field observations as noted by Fuss (1964). Standard glass aquaria 30 cm × 30 cm × 60 cm were used to contain the shrimp, with substrata obtained from shrimp grounds in Moreton Bay. Temperature was 20 – 25°C, and the water salinity 35 parts per mille. Experiments on rate of digestion were made at 20°C.
Dissections were made on freshly killed shrimp, and material for sectioning was fixed directly from living animals. For micro-anatomy formal-saline at 5°C gave the most satisfactory fixation. Sections were stained either with Mayer's haemalum or Mallory's triple stain.
Food was labelled with finely divided silver - 110m (average particle size 5) and counted in a well-type crystal in an “EKCO” scintillation counter.
3.1 Food and feeding
Detailed investigations have been made (Gopalakrishnan, 1952) on the “stomach” (anterior proventriculus) contents of the Indian prawn, Penaeus indicus, the North American white, brown, and pink shrimps (P. setiferus, P. aztecus, P. duorarum, respectively) by Williams (1955), and on P. duorarum by Eldred et al. (1961). In all cases there is close agreement. Indigestible animal remains (chitin fragments, annelid jaws and setae), algal fragments, and sand comprise recognisable dietary components. Williams (1955) notes that unrecognisable debris forms a major component, and suggests that “soft material” may form the bulk of the diet. In general, penaeid shrimps have been described as “omniverous scavengers” or “detritus feeders”.
The Australian species examined conform to the above pattern. In the laboratory, portions of muscle and other body tissues are readily eaten. Shrimps do not appear to be predators, but small sized, disabled or dying animals are readily attacked by starving shrimp. Shrimps may be maintained in captivity for a considerable period on a diet of ground beef liver mixed with vitamin-enriched powdered cereal. In all cases where the food forms a relatively large mass the method of feeding appears to be similar. The food-mass is held by the external maxillipeds, and the mandibles are used to bite or tear portions off, the maxillipeds are then used to push tough food away as it is grasped by the mandibles.
It is unlikely, however, that under natural conditions in a densely populated shrimp-ground, the proportion of larger food-masses would be adequate for full nutrition of the population. It has been tacitly assumed that this deficiency is made good by “detritus”, but Guilcher (1962) points out that estuarine muds usually contain less than 10 per cent organic material. Only a part of this material could be of immediate food value to animals. While it is possible that this could be adequate to maintain a dense population if directly eaten, it is suggested here that the shrimps only browse on the epi-flora and epi-fauna of the mud substrata.
Williams (1958), discussing substratum preferences, notes that post-larvae grow better on a mixed diet of algae and nauplii than on either alone, but that a pure animal diet is better than a pure plant diet. Estuarine muds support a rich growth of bacterial colonies and filamentous and blue-green algae. This flora is inhabited by an abundant micro-fauna of Protozoa, harpacticoid copepods, nematodes and the like (larger algae and animals may, of course, be abundant also). Unfortunately, as Ryther (1963) points out, very little is known of the rate of production of benthic flora, and what is known is restricted to the larger algae. Freshly collected estuarine muddy sand rapidly establishes itself in the laboratory into a superficial aerobic layer and a deeper, black, anaerobic layer. The superficial layer, if undisturbed, soon supports an abundant growth of bacterial colonies, and later algae and micro-fauna appear. A 10 cm Metapenaeus bennettae can survive for over three months on about 300 cm2 of such a substratum. The shrimp feeds by moving slowly over the surface, methodically searching the surface with the three pairs of chelipeds. Each chela, particularly the first pair (Fig. 1) is covered with numerous tufts of setae which appear to be gustatory in function. The tips of each chela meet precisely, so that quite small particles may be picked up and conveyed to the mouth. A row of heavy, tooth-like setae lining the inner edges of the chela enable larger masses to be gripped. Most of the food gathering appears to be done by the tips of the chelae. This browsing behaviour occupies a major proportion of the time when shrimp are active. Estimates were made of the relative volumes of the very fine material in the posterior proventriculus (“filter press”) plus the proximal portions of the digestive gland tubules, and of the contents of the anterior proventriculus (“stomach”) in actively feeding animals. In many cases the fine material was approximately equal in volume to the coarser unsorted material of the “stomach”, and appears to confirm Williams' (1955) suggestion that “unrecognizable material” may comprise a major part of the diet. This material could consist of triturated detritus, but is more likely to be bacterial colonies, small algae and micro-fauna.
3.2 Structure and function of the gut
Young (1959) has published a description of the gut of Penaeus setiferus. The overall structure is shown in Fig. 2 and differs little in the shallow-water penaeids examined, except in details of the gastric mill (Dall, 1957). Penaeus spp. differ from the two Metapenaeus spp. examined, in that the posterior diverticulum of the mid-gut, is compact in the former, while it is a longitudinal, simple structure in the latter.
The oesophagus is short and leads vertically into the anterior chamber of the proventriculus (“stomach”), which serves as a distensible crop, and posteriorly, as a gastric mill. In the floor of the anterior chamber is a system of grooves which enable secretions from the digestive gland to be passed forward and mixed with the food.
The posterior proventriculus is partly embedded in the digestive gland and is divided into a dorsal channel which leads directly into the long simple mid-gut, and a ventral “filter-press” which permits only the finest particles to pass into the digestive gland.
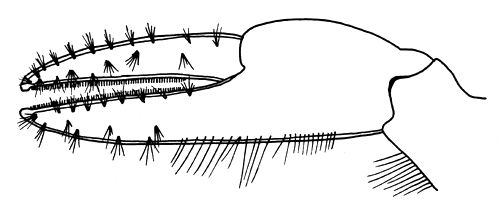
Fig. 1 First chela of Metapenaeus bennettae showing tufts of sensory (gustatory) setae, and inner rows of peg-like setae.
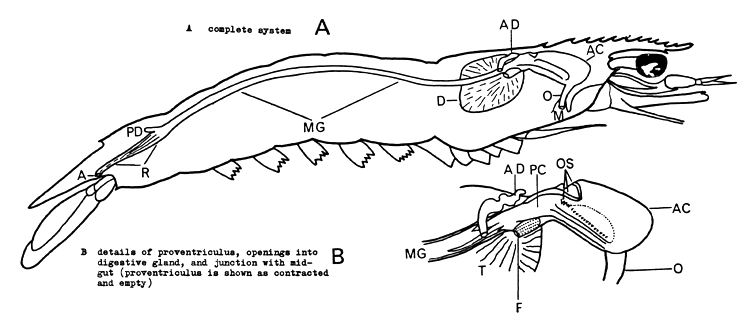
A anus
AC anterior chamber of proventriculus (“stomach”)
AD anterior diverticulum of mid-gut (this is a paired structure)
D digestive gland
F opening (paired) from “filter press” of posterior diverticulum into digestive gland
M mouth
MG mid-gut
O oesophagus
OS ossicles of gastric mill
PC posterior chamber of proventriculus
PD posterior diverticulum of mid-gut
R rectum
T tubules of digestive gland
Fig. 2 The digestive tract of a penaeid shrimp (slightly diagrammatic).
Paired openings from the ventral “filter-press” lead into the digestive gland. This organ is compact in shape and forms 3 to 4 percent of the total weight of the shrimp. The glands constitute a significantly larger proportion of the body weight in females than in males. The openings from the proventriculus lead directly into the simple tubules which comprise the gland. The tubule walls are a single layer of epithelium in which two cell types may be distinguished: a secretory type, and a “storage” type which contains polysaccharides (Dall, 1965).
Fujii et al. (1963) report powerful proteinases in the digestive gland of Penaeus orientalis. It is likely that all shallow-water penaeids are similar in this respect, and the very rapid post-mortem autolysis of digestive glands, characteristic of shrimps, is probably due to these enzymes. An amylase, equivalent to approximately 40 units per gram (wet weight) was found to be present (activity of human salivary amylase usually 100 – 150 units per ml). Digestive gland secretions also emulsify fats and it is probable that lipases are present, though these were not specifically looked for. As noted long ago by Yonge (1924) omnivorous Decapoda appear to possess a “full complement” of enzymes, and penaeid shrimps appear to be no exception.
By the time the food particles have reached the digestive gland, digestion is well under way, and is completed in the proximal half of the digestive gland tubules. No food particles can ordinarily be detected in the distal portions of the tubules, and presumably digestion is complete by the time the food has reached this level. It is not clear how the digestive gland is filled, nor if indigestible residue is ejected from the gland. Dall (1967) suggests that filling is accomplished by a combination of water ingestion and action of the proventriculus muscles. Any emptying may occur in much the same way. The fate of large indigestible particles is much clearer. These pass through the dorsal part of the posteríor proventriculus into the mid-gut which secretes a peritrophic membrane. When the mid-gut is filled with particles the peritrophic membrane is grasped by longitudinal “pads” in the rectum and expelled. Thus fresh faecal pellets of penaeids consist of lengths of peritrophic membrane approximately equal to the mid-gut in length.
The anterior and posterior diverticula of the mid-gut appear to be concerned with osmotic and ionic regulation (Dall, 1967), and have no role in digestion.
3.3 Rate of digestion
The anterior chamber of the proventriculus is filled to capacity within two minutes when a starved shrimp is provided with abundant soft food. Ingestion then ceases until some food has passed through the gastric mill. Shrimp from “rich” feeding grounds have the proventriculus at least partly filled while they are active. Pre-moult animals, in common with other Decapoda, do not feed (Dall, 1965). During “browsing” the rates of ingestion and digestion are probably equal, so that the relatively small “stomach” is not a major disadvantage. Dall (1964) has shown that Metapenaeus bennettae (as M. mastersii) has little in the way of food reserves, and that regular feeding is essential for survival. This may not apply to species such as Penaeus merguiensis and Metapenaeus macleayi which migrate to a certain extent (Racek, 1959).
Defecation may begin in a starved animal an hour after feeding, reaches a peak in four to six hours, and if feeding is confined to the beginning of the experiment, virtually ceases by eight hours. Food labelled with silver-110m shows, however, that some residue may remain in the gut up to 20 h. The digestive gland begins filling almost immediately food is taken into the proventriculus. Dall (1965) found that labelled glucose was absorbed very rapidly, and that carbon-14 carbon dioxide appeared one hour after introduction into the proventriculus. It seems likely then, that assimilation can be very rapid, and this would be particularly so if the bulk of the food consists of very small particles (micro-organisms or otherwise). This is consistent with indications that penaeids may have a high metabolic rate when active, coupled with low food reserves (Dall 1965). Their dependence on suitable substrate would be partly offset by their being omnivorous with efficient and rapid food assimilation.
From the foregoing, it is obvious that more attention should be paid to the nature of penaeid food and its production. Williams (1958) has shown that there may be a species preference for a particular type of substratum, and suggested food as one cause. If commercial penaeid species are mostly feeders on micro-flora and micro-fauna, then a full study of these organisms and their production is warranted. This would ultimately enable the fisheries biologist to make better estimates of the size of the stock that can be supported in any given area. Factors which cause sudden population fluctuations or migrations could well be bound up with successions of micro-organisms and the smaller algae, for example. Some knowledge of utilization is also desirable in order to ascertain which organisms have the highest nutritive value. The implications of this in pond culture are obvious. Also, a more detailed knowledge of the reasons for shrimp substratum preference might enable exploratory shrimp fishing to be much more effective than it is at present.
Further investigation of digestive gland fuctions is also desirable, apart from actual food digestion. The digestive gland in Decapoda is generally regarded as a storage organ as well as a digestive gland, but histologically there is no evidence of a large volume of storage cells in Penaeidae. Yet the relatively larger size of the female digestive gland is presumably in some way associated with the greater amount of synthesis required for ovarian development and maturation. This is a completely unknown area of shrimp physiology.
Calman, W.T., 1909 Crustacea. - Appendiculata. In A treatise on zoology, edited by Sir E.R. Lankester, London, A. & C. Black
Dall, W., 1957 A revision of the Australian species of Penaeidae (Crustacea Decapoda: Penaeidae). Aust.J.mar.Freshwat.Res., 8:136–231
Dall, W., 1964 Studies on the physiology of a shrimp, Metapenaeus mastersii (Haswell) (Crustacea Decapoda: Penaeidae). I. Blood constituents. Aust.J.mar. Freshwat.Res., 15(2):145–61
Dall, W., 1965 Studies on the physiology of a shrimp, Metapenaeus sp. (Crustacea Decapoda: Penaeidae). 4. Carbohydrate metabolism. Aust.J.mar.Fresh-wat. Res., 16:163–80
Dall, W., 1967 Hypo-osmoregulation in crustacea. Comp.Biochem.Physiol., 21:653–78
Eldred, B., et al., 1961 Biological observations on the commercial shrimp, Penaeus duorarum Burkenroad, in Florida waters. Prof.Pap.Ser.mar.Lab.Fla., 3:1–139
Fujii, M., et al., 1963 Studies on the proteinases of prawn (Penaeus orientalis Kishinouye). 2. Division and activities of proteinases contained in the internal organs of the prawn. J.Shimonoseki Univ.Fish., 12(1):7–11
Fuss, C.M., 1964 Observations on the burrowing behaviour of the pink shrimp, Penaeus duorarum Burkenroad. Bull.mar.Sci.Gulf Caribb., 14:62–73
Gopalakrishnan, V., 1952 Food and feeding habits of Penaeus indicus. J.Madras Univ. (B), 22(1):69–75
Guilcher, A., 1963 Estuaries, deltas, shelf, slope. In The sea, edited by M.N. Hill, New York, John Wiley & Sons, vol.3, pp.620–54
Racek, A.A., 1959 Prawn investigations in Eastern Australia. Res.Bull.St.Fish.N.S.W., (6):1–57
Ryther, J.H., 1963 Geographic variations in productivity. In The sea, edited by M.N. Hill, New York, John Wiley & Sons, vol.2, pp.347–80
Williams, A.B., 1955 A contribution to the life histories of commercial shrimps (Penaeidae) in North Carolina. Bull.mar.Sci.Gulf Caribb., 5(2):116–46
Williams, A.B., 1958 Substrates as a factor in shrimp distribution. Limnol.Oceanogr., 3(3):283–90
Yonge, C.M., 1924 Studies on the comparative physiology of digestion. 2. The mechanism of feeding, digestion, and assimilation in Nephrops norvegicus. Br.J. exp.Biol., 1:343–89
Young, J.H., 1959 Morphology of the white shrimp, Penaeus setiferus (Linnaeus 1758). Fishery Bull.Fish Wildl.Serv.U.S., 59(145):1–168