by
R. MEIXNER
Institut für Hydrobiologie und Fischereiwissenschaft
Hamburg, Federal Republic of Germany
Abstract
An investigation was made on the reproductive cycles of the sand shrimp, Crangon crangon (L.). The shrimps were reared under controlled laboratory conditions from larval to sexually mature stages. Crangon always oviposited after moulting, provided the ovarium had reached the third abdominal somite. Examining freshly caught shrimps against a source of light, one is able to determine this final stage. By use of this technique, estimates on reproductive cycles can be obtained.
REPRODUCTION DE LA CREVETTE DE SABLE Crangon crangon (L.)
Résumé
L'auteur étudie le cycle reproductif de la crevette de sable Crangon crangon (L.). Les crevettes ont été élevées en laboratoire, depuis le stade larvaire jusque' à la maturité sexuelle. Crangon effectue toujours sa ponte après la mue, à condition que l'ovaire ait atteint le troisième somite abdominal. En examinant à contre-jour les crevettes qui viennent d'être prises, on peut déterminer ce stade final. L'utilisation de cette technique permet d'obtenir des estimations relatives aux cycles reproductifs.
LA REPRODUCTION DEL CAMARON Crangon crangon (L.)
Extracto
Se realizó una investigación sobre los ciclos reproductivos del camarón de arena Crangon crangon (L.). Los camarones se criaron en el laboratorio en condiciones controladas desde la fase larval hasta la madurez sexual. Crangon deposita siempre los huevos después de la muda, a condición de que el ovario haya alcanzado el tercer segmento abdominal. Si se examinan camarones recién capturados frente a la luz se puede determinar esta fase final. Empleando esta técnica se pueden obtener estimaciones acerca de los ciclos reproductivos.
The sand shrimp Crangon crangon (L.) sustains an important fishery in Dutch and German waters. The catches are obtained mostly in the tidal zone of the North Sea. In German coastal waters Crangon probably attains sexual maturity at an age of less than one year and a length of about 50 mm. Meyer-Waarden (1935), Tiews (1954) and Kühl (personal communication) agree that Crangon spawns more than once a year. Reproduction shows a distinct seasonal rhythm resulting in a maximum of shrimp larvae in summer and a minimum in winter (Kühl and Mann, 1963; Plett, 1965).
At present there is little information on the number and quantity of ovipositions per female per annum. Berried females are caught in a varying percentage throughout the year. In German coastal waters incubation varies from about 3 mo in the winter to 3 weeks in the summer, depending on the temperature. Rates of reproduction are often under-estimated because it is overlooked that shrimps may moult and spawn again shortly after hatching. To help calculate the number of annual ovipositions per female, the following observation may be of use. At an advanced stage of incubation Crangon eggs show dark spots - the compound eyes of the embryos. At this stage it is possible to determine whether or not the females will spawn again after the next moult (see below).
The earlier-mentioned observations of Meyer-Waarden, Tiews and Kühl regarding reproductive cycles of females, was followed by rearing Crangon under controlled conditions from larval to sexually mature stages by Meixner (1966a).
In the present study the size of the reproductive glands was determined, at weekly intervals, holding the shrimps to an opalescent glass-pane lighted from below. At an age of 8 to 9 mo and a length of less than 50 mm a remarkable enlarging of the female reproductive glands was observed. Females oviposited first at the age of about 10 mo (Fig. 1). Some days after oviposition, renewed growth of the female reproductive gland was noticed. We found that at temperatures simulating those in the field during the period of breeding, optimum rations of food stimulated repeated egg-laying (Table I).
TABLE I
Moults and ovipositions of 5 female Crangon crangon under laboratory conditions. Experimental period: April to August 1965. Range of length: 55 to 65 mm. Temperature: 14°C (from 15 July 17° to 18°C). Salinity: 30 ‰. Food: Artemia salina (juvenile and adult)
| Crangon No. | April | May | June | July | August | Total |
| I | + | + | + | + | + | 5 |
| II | + | - | + | - | - | 2 |
| III | + | + | + | - | + | 4 |
| IV | + | + | + | + | - | 4 |
| V | - | + | + | + | - | 3 |
| + = moult and oviposition | - = moult without oviposition | |||||
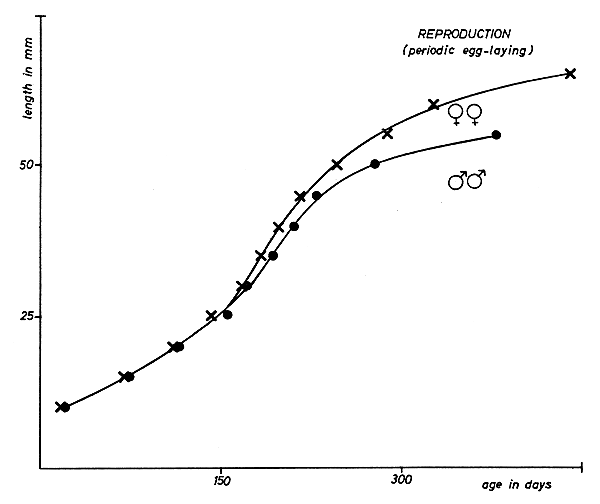
Fig. 1 Growth after metamorphosis and reproduction of 5 female and 20 male Crangon crangon (L.) under laboratory conditions. Temperature: 14°C. Salinity: 30 ‰. Food: larval and postlarval Artemia salina.
At temperatures of 14° to 18°C and at daily rations of food (juvenile and adult Artemia salina) of about 4 mg (dry weight) moulting and oviposition took place at 3 to 4 weekly intervals. Suboptimum rations of food slowed down ovarian growth, and the subsequent moult was not followed by oviposition (Table II). Increased rations of food again stimulated ovarian growth.
TABLE II
Influence of food uptake (mg dry weight) on oviposition of 4 Crangon crangon. Before the experiment all females had successfully spawned. Temperature: 18°C. Salinity: 30 ‰. Food: juvenile and adult Artemia salina
| ♀ I | ♀ II | ♀ III | ♀ IV | |
| Experimental period (weeks) | 3 | 3 | 3 | 3 |
| Ration of food (mg) | 96.7 | 82.8 | 40.2 | 53.4 |
| Spawning after next moult | + | + | - | - |
| + = moult and oviposition | - = moult without oviposition | |||
In all our experiments Crangon oviposited when the continuously enlarging ovaries reached the third abdominal somite. This stage was determined by viewing the individuals against a transmitted light. By this means it was possible to forecast any further oviposition.
It appears that it would be possible to obtain a reliable estimate of the number of ovipositions per female per annum on a basis of observations on the relative abundance of ovigerous shrimps (eye spot stage) and those showing, on examination, ovaries, which fill the body up to the third abdominal somite (terga). Investigations on reproductive cycles should be made at intervals determined by the moulting rhythm of sexually mature females. Some information on moulting rhythm was published by Tiews (1954) and Meixner (1966b), but further studies are needed to corroborate their observations.
Kühl, H. and H. Mann, 1963 Das Vorkommen von Garnelenlarven (Crangon crangon L.) in der Elbmündung. Arch.FischWiss., 14(1–2):1–7
Meixner, R., 1966a Eine Methode zur Aufzucht von Crangon crangon (L.) (Crust.Decap.Natantia). Arch. FischWiss., 17(1):1–4
Meixner, R., 1966b The effects of food supply on moulting, growth and spawning of the shrimp Crangon crangon (L.). ICES Paper Shellfish Comm., C.M. 1966/M:5:7 p.
Meyer-Waarden, P.F., 1935 Ein Beitrag zur Frage der Laichperiodizität bei der Nordseekrabbe (Granat) Crangon vulgaris Fabr. Zool.Anz., 109(1–2):23–32
Plett, A. 1965 Über das Vorkommen von Garnelenlarven (Crangon crangon L.) vor der deutschen Küste in den Jahren 1963 und 1964. Arch.FischWiss., 16(1):54–67
Tiews, K., 1954 Die biologischen Grundlagen der Büsumer Garnelenfischerei. Ber.dt.wiss. Kommn Meeresforsch., 13(3):235–69