by
M. J. GEORGE, S. K. BANERJI and K. H. MOHAMED
Central Marine Fisheries Research Institute1
Abstract
A detailed study of the distribution pattern of size and abundance of the commercial penaeid prawns of the southwest coast of India, Metapenaeus dobsoni, M. affinis, M. monoceros, Penaeus indicus and Parapenaeopsis stylifera, has been carried out based on the catches of the exploratory vessels belonging to the Government of India offshore Fishing Station and the Indo-Norwegian Project, Cochin. The movements of these prawns in different depth zones of the fishing grounds, as seen from the analyses of data, are size-oriented. With the exception of M. dobsoni, all the species studied were seen to enter the fishing grounds from deeper zones by September, which is the beginning of the fishing season. They move out of the fishing grounds into the deeper zones from February to March onwards. M. dobsoni, however, seems to exhibit this movement in the opposite direction. The role of monsoon and upwelling in causing these movements, is discussed.
1 Present address: Central Marine Fisheries Research Substation Ernakulam 6, India
REPARTITION PAR TAILLE ET DEPLACEMENTS DES CREVETTES D'INTERET COMMERCIAL DE LA COTE SUD-OUEST DE L'INDE
Résumé
Sur la base des prises effectuées par les bateaux de pêche d'exploration appartenant au Offshore Fishing Station (Gouvernment de l'Inde) et au Projet Indo-Norvégien de Cochin, on a entrepris une étude détaillée de la répartition par taille et par degré d'abondance des crevettes penéides pêchées commercialement sur la côte sud-ouest de l'Inde: Metapenaeus dobsoni, M. affinis, M. monoceros, Penaeus indicus et Parapenaeopsis stylifera. L'analyse des données montre que les déplacements de ces crevettes dans les différentes zones de profondeur des aires de pêche sont fonction de leur taille. On s'est aperçu qu'à l'exception de M. dobsoni toutes les espèces étudiées quittaient les eaux profondes pour pénétrer dans les terrains de pêche en septembre, c'est-à-dire au début de la saison de pêche. Elles regagnent ensuite les zones profondes à partir de février-mars. M. dobsoni toutefois semble effectuer le mouvement inverse. Les auteurs de la communication examinent le rôle de la mousson et des remontées d'eau dans ces déplacements.
DISTRIBUCION POR TALLAS Y MOVIMIENTO DE LOS LANGOSTINOS COMERCIALES DE LA COSTA SUDOCCIDENTAL DE LA INDIA
Extracto
Basándose en las capturas de los barcos de exploración pertenecientes a la estación de pesca de altura del Gobierno de la India y al Proyecto Indo-Noruego de Cochin, se ha realizado un detallado estudio del tipo de distribución por tallas y de la abundancia de los peneidos comerciales de la costa sudoccidental de la India, Metapenaeus dobsoni, M. affinis, M. monoceros, Penaeus indicus y Parapenaeopsis stylifera. Los movimientos de estos langostinos en diferentes zonas profundas de los caladeros, como se puede observar en los análisis de los datos, tienen relación con el tamaño. Con la excepción de M. dobsoni, pudo verse, hacia el mes de septiembre, que es cuando comienza la temporada de la pesca, que todas las especies estudiadas entraban en los caladeros procedentes de zonas más profundas. A partir de febrero-marzo salen de los caladeros hacia las zonas más profundas. Sin embargo, M. dobsoni parece realizar este movimiento en dirección opuesta. Como causa de estos movimientos se examina la función desempeñada por los monzones y las aguas de afloramiento.
Prawn populations inhabiting the coastal waters around Cochin, on the southwest coast of India, support an intensive and valuable fishery. Detailed biological investigations on these commercial prawns have been initiated in the Central Marine Fisheries Research Institute ever since the fishery commenced on a large scale in 1956–57; and some of the results on the species and size compositions, distribution and their fluctuation from year to year have been reported (George et al., in press) The present contribution is the result of comprehensive studies conducted on abundance and size distribution of the different species during the period September 1961 to August 1964.
An important problem confronted by researchers is to determine whether primary prawn stocks are homogeneous over their ranges or whether they constitute discrete subpopulations overlapping in space, time, or both. The question is prompted mostly by the unique life history of the common penaeid prawns which support the fishery. In general, the eggs are spawned in the offshore habitat of the parent prawn. The hatched larvae, becoming a component of the zooplankton, are soon transported shoreward and into estuaries and backwaters. Here the prawn, now a postlarva, settles to the bottom and undergoes rapid growth during the ensuing few months. As maturation approaches it leaves these ‘nursery’ grounds to return to the parental offshore habitat, where the life cycle is completed. This being the set pattern of life cycle, the fact that most of the species spawn throughout the year with two or three peak spawning periods (George, 1962a; Rao, MS*) brings us to the possibility of regular ingress and egress of juveniles and adults of the different species in the fishing grounds. With the expansion of frozen shrimp exports from the country the demand for large sized prawns has increased considerably. Consequently, the need for locating grounds where bigger sizes of prawns are available and determining the time of their occurrence has become a necessity. In this connection an attempt is made to study the size oriented movement of the prawns in the fishing grounds off Cochin.
The total prawn catches of the trawlers operated from Cochin by the Indo-Norwegian Project and the Offshore Fishing Station of the Government of India are taken into account in computing the abundance factor. The area of operation of the boats and the depth zones are shown in Fig. 1. The size composition of the prawns is determined by regular analyses of samples of catches from all the trawlers. The measurements of prawns are given as total lengths in millimetres, from tip of the rostrum to the tip of the telson.
Throughout this paper the unit of abundance, as expressed by the catch in numbers per trawling hour, is denoted by u, mean size by m (in mm) and size range by r (in mm). These parameters are analyzed for five depth zones as under:
| Depth zone I | 0 to 5 fm | (0 to 9.1 m) |
| Depth zone II | 5 to 10 fm | (9.1 to 18.3 m) |
| Depth zone III | 10 to 15 fm | (18.3 to 27.4 m) |
| Depth zone IV | 15 to 20 fm | (27.4 to 36.6 m) |
| Depth zone V | 20 to 25 fm | (36.6 to 45.7 m) |
* This reference is now in press. (Ed.).
The fishery usually commences by September-October and extends till June-July, the fishing activities being almost suspended during the monsoon months. Generally, at the commencement of the season fishing operations are carried out in deeper areas, mostly restricted to the 15 to 25 fm (27.5 to 36.6 m) region, during which time the catches are relatively poor. But as the season advances the fishing activities are confined to lesser depths, mostly depending on the catches.
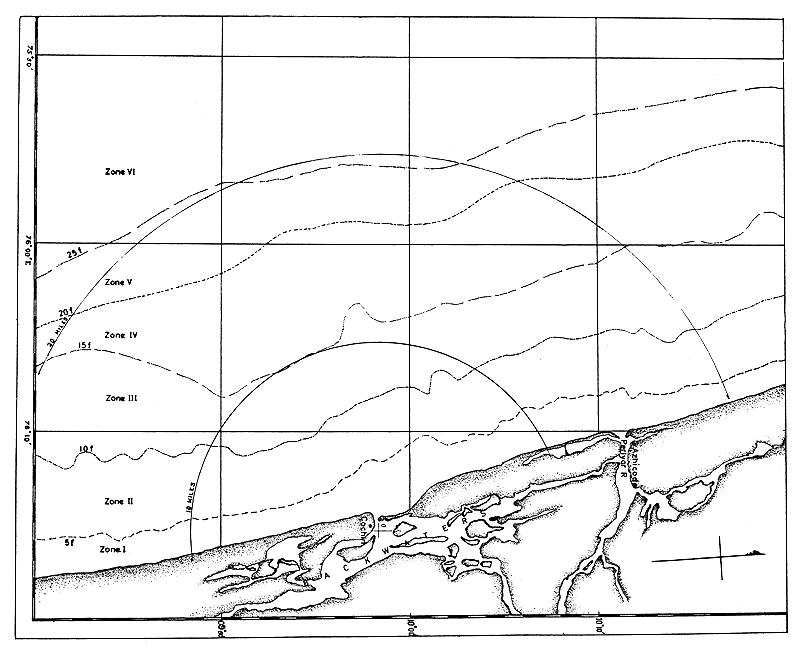
Fig. 1 Area of fishery and depth zones.
Vessels taking part in the shrimping activities of the area comprise mostly shallow draught pablo boats, powered by 10 to 30 bhp engines and owned by private agencies. A few bigger types of shrimp trawlers are also operated which usually concentrate their operations in deeper regions. The conventional two or four seam shrimp trawls are operated by these boats, making daily trips.
Five species of prawns of the family Penaeidae support the fishery. They are:
Metapenaeus dobsoni (Miers) (‘Poovalan Chemmeen’)
Metapenaeus affinis (H. Milne Edwards) (‘Kazhanthan Chemmeen’)
Metapenaeus monoceros (Fabricius) (‘Choodan Chemmeen’)
Penaeus indicus H. Milne Edwards (‘Naran Chemmeen’) and Parapenaeopsis stylifera (H. Milne Edwards) (‘Karikadi Chemmeen’)
The first four species have the typical life cycle referred to earlier, but P. stylifera appears to spend the entire life cycle in the marine environment, there being no evidence of its postlarvae entering estuaries. Hence, some difference in the pattern of behaviour and movements in and out of the fishing grounds can, naturally, be expected in the case of this species.
3.1 Metapenaeus dobsoni
Considerable information on the species from this region is available. The juveniles of the species contribute to a commercial fishery in the backwaters, while the adults form the bulk of the marine catch. According to Menon (1955), three year classes are represented in the fishery. However, George et al. (in press) record only two year classes in the offshore prawn fishery of Cochin. The species breeds throughout the year in these waters, with two definite peak spawning periods; one in June through August and the other in November-December. Rao (MS) records an additional peak spawning period in April. According to George et al. (in press) this is the most important species in the offshore catches as indicated by its percentage in the catches. The normal pattern of size distribution of the species shows the bigger first-year classes dominating early in the season, and later replaced by the smaller late 0-year classes, resulting in a gradual reduction in size of the species composition as the season advances. They have recorded a comparatively higher concentration of the species in the 7 to 8 fm (12.8 to 14.6 m) contour.
3.1.1 Abundance Depthwise distribution of u of the species is shown in Fig. 2. In 1961–62 there was fishing in zone I only during September and October, when u varied from 61 to 66. In zone II there was fishing from October to June. During this period, u varied from 13 to 10918, the highest being noted in March and the lowest in October. In zone III the highest u was also noticed in March, the numerical value being 5630. In zone IV, fishing was done only in February when u was very low and stood at 27 (Fig. 2).
In 1962–63 when there was fishing in zone II from November to June, u varied from 900 to 6031, the maximum occurring in February and the minimum in April. In zone III the operation was mainly in the months January to June and the maximum u was recorded in June.
In 1963–64 in zone II, u ranged from the minimum of 27 in October to the maximum of 4670 in December. During May and June there was no operation in this zone. In zone III, there was operation only in January, May and June with the maximum u of 1717 in June. In zone V there was fishing in May when u was 685.
3.1.2 Size The mean size m and the size range r at different depth zones are shown in Fig. 2. It would be seen that m varies considerably from month to month even in the same depth zone.
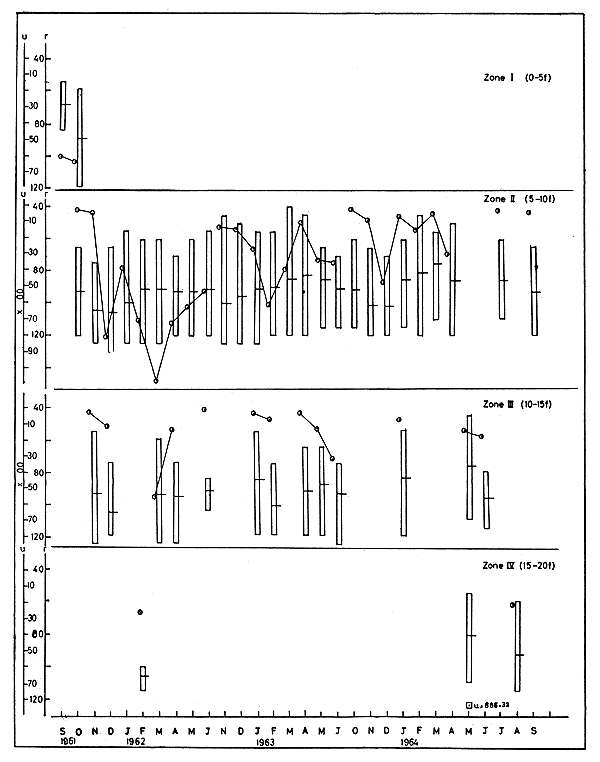
Fig. 2 Abundance and size of M. dobsoni during 1961–62 to 1963–64. Vertical column represents size range (r) and horizontal line on it the mean size in mm. Abundance (u) in number per trawling hour.
In 1961–62 m in zone II during October was 93.0. The highest mean size of 106.2 was reached in December and then declined. In zone III also the highest m, 106.7, was in December and the lowest, 92.4, in June. In zone IV there were fishing operations only in February when m was 106.8. The size ranges varied from 56–85 to 66–130 in different months.
In 1962–63 in zone II, m ranged between 82.5 and 99.7, which was comparatively lower than that of the previous year. The minimum was recorded in April and the maximum in November. In zone III the maximum m of 101.5 was recorded in February and the minimum in May. r varied from 41–120 to 76–125.
In 1963–64 in the lower depth zone, m varied from 75.5 to 101.6, the former in the month of March and the latter in December. In zone III it varied between 76.9 and 96.9 in the three months in which there was operation. r in this year ranged from 46–120 to 81–115.
3.1.3 Movements In a static population which is subjected to exploitation, decreasing abundance will be noticed due to both fishing and natural mortality as the season progresses, and at the same time increasing mean size will be encountered due to growth. But, the fluctuations in mean size and abundance, in the observations recorded above, indicate that the exploited population is dynamic and variable.
Increase in both u and m over any period in a particular zone could be attributed to incursions of large sized prawns into the zone, while a decrease in both these parameters would indicate the egression of these prawns from that area. Fresh recruitment of young prawns into the zone will be manifested in the increase of u and decrease of m and the reverse would be the result of either the movement of the small sized prawns out of the area, or, the effect of fishing and natural mortality, and the effect of natural growth processes.
In the case of M. dobsoni it may be seen from Fig. 2 that in 1961–62 in both zone II and III, u as well as m increased from October to December, indicating that larger prawns of the species were entering this area. In January, both u and m registered a fall in zone II, which might mean that larger size prawns were moving out of this zone. But from January to March, u increased while m decreased showing the incursion of small sized prawns into the area. In April, u dropped while m increased slightly in both these zones. This might have partly been due to excursion of some small prawns from the area and partly due to operation of mortality on the population. From April to June there was a decline in both u as well as m thereby indicating movement of bigger sized prawns out of the areas. Approximately similar trends were noticed in the 1963–64 season also. But in 1962–63, there was slight difference in the pattern of movement of the species. In this year from November to February there was continuous increase of u with corresponding decrease in m indicating incursion of small sized prawns into zone II. In zone III from January to February u doubled together with increase in m showing thereby movement of large prawns into the area. From February to April in both zones u as well as m declined which meant there was movement of big prawns out of the zones. From April to June u increased along with m which indicated an incursion of big prawns into the area.
It is therefore seen that from October to December the offshore migration of bigger sized prawns of the first year class of this species takes place. The fact that big sized prawns occurred in zone I during September-October of 1961–62 shows that these prawns were present very close to the shore towards the beginning of the season just after the monsoon. That this species may be remaining very near to the shore during the monsoon months has been expressed by George et al. (in press) based on the hypothesis of Banse (1959) that during upwelling periods fishes and prawns remain either pressed against the shore or migrate to deeper waters. The presence of this species in large concentrations very near the shore in mud-bank areas in the monsoon period supports this view. After monsoon the offshore movement seen in zone II in October continues to zone III till December-January. The incursions of prawns of smaller size belonging to the late 0-year group into zone II commences in February. These late 0-year class prawns which are recruited from the backwaters may probably belong to the brood of the peak spawning period June-July. During February-March, offshore migration of these small sizes takes place. From April-May not much offshore movement could be noticed.
TABLE I
Total number of individual Metapenaeus dobsoni caught, in thousands (C), and catch per trawling hour (C/E)
| Size in mm | 1961–62 | 1962–63 | 1963–64 | |||
| C | C/E | C | C/E | C | C/E | |
| 41 – 45 | - | - | 11 | 3 | - | - |
| 46 – 50 | - | - | 23 | 7 | 3 | 2 |
| 51 – 55 | - | - | 35 | 11 | 13 | 8 |
| 56 – 60 | 9 | 2 | 48 | 14 | 25 | 14 |
| 61 – 65 | 63 | 13 | 105 | 31 | 57 | 33 |
| 66 – 70 | 117 | 23 | 385 | 116 | 132 | 77 |
| 71 – 75 | 730 | 148 | 847 | 254 | 239 | 139 |
| 76 – 80 | 1769 | 358 | 1124 | 338 | 284 | 165 |
| 81 – 85 | 3299 | 667 | 1386 | 416 | 263 | 152 |
| 86 – 90 | 5359 | 1084 | 1300 | 391 | 260 | 151 |
| 91 – 95 | 5429 | 1098 | 1428 | 429 | 251 | 146 |
| 96 – 100 | 6178 | 1250 | 1402 | 421 | 308 | 179 |
| 101 – 105 | 5188 | 1049 | 963 | 289 | 278 | 162 |
| 106 – 110 | 2051 | 415 | 377 | 113 | 108 | 63 |
| 111 – 115 | 2673 | 541 | 349 | 105 | 85 | 49 |
| 116 – 120 | 1352 | 274 | 183 | 55 | 46 | 27 |
| 121 – 125 | 238 | 48 | 14 | 4 | - | - |
| 126 – 130 | 11 | 2 | - | - | - | - |
In 1961–62, m in zone II was 105–106 in November-December and it declined to 92.1 in February and thereafter registered slight increase. In the other years also, more or less the same trend was noticed, indicating a general size-oriented migration which starts in November-December and ceases by March-April. The fact that m was higher in zone III than in zone II in all the months except November, 1961, and January, 1963, further strengthens this view.
3.1.4 Mortality From March to June 1961–62 in zone II it may be seen that u declined when m increased. If we assume that migratory movement has stopped by about that time, then the fall in u might have been due to mortality and increase in size due to growth. Then the total instantaneous mortality could be estimated by the formula
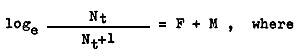
Nt denotes the number of prawns of age t in any month and Nt+1 denotes the number of prawns of age t+1 in the next month. Since the absolute number of prawns is not known, the relative abundance may be used in making this calculation.
Taking the relative abundance of M. dobsoni between March and April 1962, the monthly instantaneous mortality would be
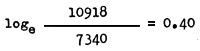
The monthly instantaneous mortality rate between the months of April and May 1962 is given by
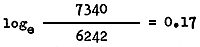
and that between May and June 1962 by
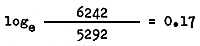
The above calculations show that probably some migration was still taking place in March in zone II, and only from April did the outward migration cease. The estimate of monthly instantaneous mortality rate may be taken as 0.17 corresponding to an annual instantaneous mortality rate of 0.17 × 12 = 2.04.
That this interpretation is correct can be verified by considering the entire fishing area, pooling together all zones, so as to do away with the effect of migration. Table I gives the catch (in numbers) and catch per unit of effort of the different size groups for the three years from 1961–62 to 1963–64.
Banerji and George (1967) have shown that M. dobsoni reaches a size of about 95 mm at the end of one year and about 115 mm at the end of the second year of its life. Taking this as the basis, the relative abundance of the various age groups of M. dobsoni for the three fishing seasons from 1961–62 to 1963–64 are given in the following table:
TABLE II
Relative abundance of various age groups in different seasons
| 0-year | 1-year | 2-year | |
| 1961–62 | 3393 | 3255 | 324 |
| 1962–63 | 2010 | 928 | 59 |
| 1963–64 | 887 | 453 | 27 |
In the above table, the relative abundance of 0-year class may be an underestimate since prawns of small size may have escaped, but for the purpose of this calculation the same may be ignored.
It is well known that
Nt = Noe-(F+M) t
where Nt is the number of fish of any age t and No is the initial number of fish at 0 age and (F+M) is the total annual instantaneous mortality rate. The above equation can be rewritten as
| loge Nt | = loge No - (F+M) t |
| = A+Bt |
showing a linear relation between loge Nt and t. Thus if a linear regression is fitted between loge Nt and t, the slope B = -(F+M) can be determined which will furnish an estimate of (F+M). From Table II, we have the following data:
| t | loge Nt |
| 0 | loge 3393 |
| 1 | loge 928 |
| 2 | loge 27 |
The slope determined for the linear regression furnishes an estimate of 2.41 for the annual instantaneous mortality rate (F+M). This is not very different from the monthly estimate of instantaneous mortality rate determined from the data of abundance in zone II in 1962 on the assumption that outward migration has ceased from April onwards.
3.2 Metapenaeus affinis
This species contributes to a good percentage of the trawl fishery of the area, whereas its percentage representation in the backwater fishery is very low, so much so, it is clear that the species utilizes the backwaters and estuaries as nursery grounds only to a limited extent. George et al. (in press) record two year classes for this species in the offshore fishery. Rao (MS) observed the spawning period from October to March with the peak in November-December. The bigger first-year classes dominate early in the season when the maximum catches are recorded, smaller sizes dominating later in the season (George et al., in press). They have also recorded a concentration of the species in the 10 to 11 fm (18.3 to 20.1 m) contour.
3.2.1 Abundance In 1961–62 u in zone II ranged from 4 to 3018. An increase was observed from October onwards and the maximum was reached in May. In zone III the minimum of 85 was recorded in April, while the maximum of 1823 was in March. In January and February there was no fishing in this zone. In February, when there was fishing in zone IV, u was as high as 1031 (Fig. 3).
In 1962–63, there was fishing in zone I in November only, when u was 210. In zone II u varied between 348 and 1779, the catch being higher in the early half of the season. In zone III in May u was unusually high, being 1233. In zone IV, fishing was carried out in October-November and June and u was quite high in October and June. Fishing was conducted in zone V in September and October. In the latter month u was 223.
In 1963–64 u was quite insignificant in zone I when there was fishing in October-November. In zone II u ranged from 27 to 812, the former in October and the latter in December, and in zone III u varied from 216 to 1183. There was no fishing operation in zone V.
In general there was steady increase in u in the first half of the season with reduction later.
3.2.2 Size In zone II the maximum m ranged between 130.9 and 132.6 in the first half of the season and the minimum between 90.6 and 94.5 in the later half, this being true of the three seasons in 1961–64. In zone III also similar trend was noticed. Here the maximum ranged from 129.9 to 137.0 and minimum from 83.4 to 101.0. In zone IV, where there were operations in a few months, m varied between 113.5 and 125.8. In general, the size increased steadily in the early half of the season and later declined. In these years r ranged between 46–135 and 86–165.
3.2.3 Movements In the first season from October to January both u and m showed increase in zone II indicating influx of new groups of prawns of bigger sizes into the zone. The data from zone III, which is relatively deeper and adjoining the previous one, also showed similar increase from October to December indicating influx of new populations of bigger sizes into this zone also. The value of m remained above 130 in zone III in all these months, whereas, that size was attained only in December-January in zone II. In the month of February, both u and m fell in zone II, showing movement of bigger sizes out of the area. The r during this month was between 56–145 as compared to 76–160 during the previous month. This would suggest egression of larger sized prawns and incursion of smaller ones in the zone. The direction of movement of these big prawns becomes clear when the simultaneous increase in abundance of the larger prawns in zone IV is taken into account, showing that they were moving offshore. In March, u increased considerably in zone II, while m showed only slight increase; and in zone III, u increased while m decreased, obviously due to the entry of small sized prawns into both these zones. The slight increase in mean size in zone II could have been due to the migration of the very small prawns to zone III, or to natural growth. In the following month both u and m registered decline in both these zones. The r showed that prawns above 135 were not at all seen in these zones. This meant that on the one hand big sized prawns had completely moved away while on the other hand the offshore migration of small prawns continued. In May, however, the same trend continued showing fresh incursion of small sized prawns from the inshore areas into this zone. The smaller prawns which entered zones II and III in May moved out into the deeper zones as evident from the lower values of u and m. This is corroborated by the high value of u and low m recorded in zone III during this month.
The data for the next two seasons also showed the same type of movement with minor differences in the time of commencement and ending of these movements. The general picture that emerges is that shoreward movement of big sized prawns commences in October or even earlier, and from February both big sized as well as small sized prawns begin to move outward into the offshore regions. By about June no big prawns are seen up to 15 fm (27.5 m) area.
3.3 Penaeus indicus
Certain biological aspects of the fishery of this species in the region have been elucidated by the studies of Menon (1957), George (1961; 1962a; 1962b), Menon and Raman (1961), George et al. (in press) and Rao (MS). The juveniles of this species contribute to a good fishery in the backwaters. In the marine environment the spawning period of the species, according to George (1962a) and Rao (MS), is prolonged from October to May with two peaks, one during February to April and the other in November-December. The size composition in the trawl fishery is given by George et al. (in press) and according to them three year classes are represented in the catches.
3.3.1 Abundance In the 1961–62 season zone I was fished only in September-October and the value of u was very low. In zone II u varied from 19 to 732, the minimum in April and maximum in February. From November to January operations in zone III gave values of u between 165 and 376. In zone IV there was fishing only in February when u was recorded as 33 (Fig. 4).
Between November and April in 1962–63, u ranged from 3 to 486 in zone II, the maximum being in December; and in zone III fishing was almost nil except in November when u was quite insignificant. A high value of 1296 u was recorded in June from zone IV.
In the 1963–64 season u remained comparatively low, varying between 5 and 148, in all the months and in all the depth zones.
3.3.2 Size In 1961–62, m ranged between 105.5 and 170.0 in zone II from December to June, the lowest being in April and the highest in January. In zone III from November to January m remained quite high, above 170.0, and in March it was as low as 133.7. In the next deeper area, zone IV, there was fishing only in February, when m was 159. In 1962–63, in zone II the range of m was between 121.7 and 168.0, the latter in November. In a few operations in the deeper zones m varied between 143.0 and 154.3. Range of r was from 96–135 to 151–185.
3.3.3 Movements The data in respect of this species is insufficient to trace the movements fully. However, from February to April in 1961–62, in zone II, both u and m declined, indicating that big prawns of the species were moving out of the area. Similarly, both u and m were seen decreasing in the succeeding years also in the same depth zone. In 1963–64 from January to March, in zone III, both u and m were seen declining, i.e., the big prawns were moving out of this area also. In February 1963–64 no prawns of size larger than 135 mm were found in zone II, but at the same time in zone III prawns up to 190 mm were seen, and by March, prawns larger than 160 mm size were also noticed. But, prawns of 181–190 r were seen in the same month in zone IV. This leaves no doubt that bigger size prawns were moving offshore during December-February. Towards the latter half of the season it was generally seen that smaller sizes were recruited into these zones and they in turn moved out to deeper areas. In 1963–64, during the period February-April, u increased while m decreased in zone II indicating thereby that smaller sized prawns were moving into the zone. In 1962–63 during the same period u decreased while m showed an increase, indicating that small sizes were moving out of the zone. In 1961–62, however, these movements of the smaller sizes took place much later in the season, during April to June.
3.4 Metapenaeus monoceros
Juveniles of this species contribute to the backwater fishery of the area more than to the inshore fishery. Knowledge about the biology and fishery of this species is limited to the studies by George (1959; 1962a), Menon and Raman (1961), George and George (1964) and George et al. (in press). The 0-year class contribute to the backwater fishery. According to George (1959) three year classes are represented in the mechanized fishery around Cochin, the bigger year classes coming into the fishery in November-December and the smaller sizes appearing later. It is noted that in some years the bigger sizes fail to appear in the fishery.
3.4.1. Abundance The species was caught only in a few months in the first two years, in zone II. Value of u was not high in April and May. In zone III u varied from 1 to 297 in these years. In 1962–63, there were comparatively higher values of u ranging from 47 to 1622 in the deeper zones IV and V in September-October (Fig. 5).
In 1963–64 in zone II the maximum u of 203 was recorded in December and in July it was at the minimum of 2. In zone III, u ranged between 24 in February and 467 in May. Generally, an increase in u was noticed in the earlier months with decline later.
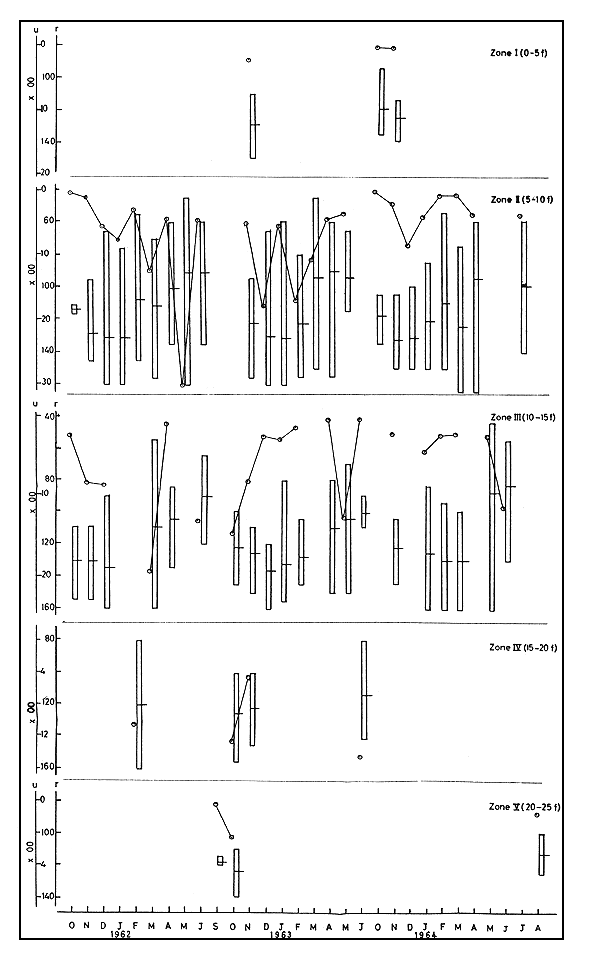
Fig. 3 Abundance and size of M. affinis during 1961–62 to 1963–64. Vertical column represents size range (r) and horizontal line on it the mean size in mm. Abundance (u) in number per trawling hour.
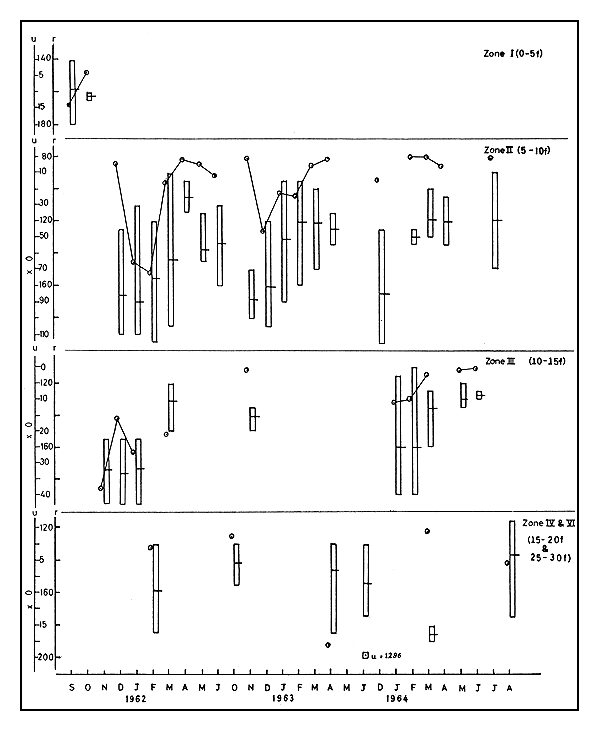
Fig. 4 Abundance and size of P. indicus during 1961–62 to 1963–64. Vertical column represents size range (r) and horizontal line on it the mean size in mm. Abundance (u) in number per trawling hour.
3.4.2 Size In the limited catches of zone II, m ranged between 74.6 and 120.5 in the first two years with mostly the smaller sizes being caught in April and May. In zone III, m varied between 88 and 143 and in zone IV, the maximum m of 168.0 was recorded in November. In 1963–64, in zone II, m was recorded at the maximum of 128.0 in November and the minimum of 63.0 in February. In zone III, m was at the maximum value of 135.0 in January and the minimum of 78.0 in June. In zone IV, there was catch only in March when m was 150.5. The size range, r, was between 51–90 and 166–175.
3.4.3 Movements Taking the data of the first year into consideration, in zone II, from January to May there was slow increase in u along with a fall in m, indicating that during these months smaller sized prawns entered the zone though not in large numbers. In zone III, between January and April, u was declining along with m, showing gradual exodus of bigger prawns from the zone to deeper regions, and in zone IV, prawns of sizes above 86 mm only were seen in February. The migration pattern seems to be size related. By about May only prawns up to 90 mm size remained in zones II and III, while bigger prawns moved further offshore. In 1962–63, although not much data were available, it could be seen that in September-November big sized specimens were present in zones IV and V, and in April-May small sized specimens were found in zones II and III. In November-December of the following year, in zone II, u increased while m fell slightly, indicating influx of new groups of smaller sizes. The size ranges showed that prawns of 80 to 95 mm size have entered this zone. Next month both u and m declined as bigger prawns above 150 mm moved out of zone II. By February prawns bigger than 65 mm have all left the area as indicated by r. But for a slight increase of m in March, during the remaining months only small sized prawns were present in this zone. In zone III both u and m increased from November to January showing influx of bigger prawns. It is already seen that sizes above 150 mm left zone II in January, obviously for zone III. In February, u and m decreased, showing departures of bigger specimens from here. In the next month, u was noticed to increase while m fell, indicating the entrance of some smaller prawns into zone III; and by about June, prawns bigger than 95 mm had left this zone.
In general, the offshore migration commenced from November and by about April all prawns greater than 80 mm size moved out of zone II. By June the prawns above 95 mm size moved out of the deeper zone III also. In still deeper waters prawns of larger sizes only were encountered.
3.5 Parapenaeopsis stylifera
Among the five species of penaeids contributing to the prawn fishery of the region, the behaviour of this species is unique, as the postlarvae of this alone do not enter the backwaters. The fishery is limited to the marine environment. Some aspects of the biology and fishery of the species have been studied by Menon (1953: 1957) and George (1961). George et al. (in press) found two year classes represented in the trawl fishery off Cochin.
3.5.1 Abundance Very early in the season small quantities of this species with comparatively low values for u were found in zone I in all the three years. In 1961–62, u ranged between 124 and 2609 in zone II, with the maximum in October and the minimum in January. Very high values of u, above 2000, were recorded in May and June. In zone III, u varied from 11 in December to 2845 in June, and it was fairly high in September-October in zones IV and V (Fig. 6).
In the next year, a maximum u of 1036 was recorded in December and the minimum of 76 was in May, in zone II. Very high values of u viz., 4030 and 1598 were obtained in October and November, from zone III. The maximum abundance of 10846 u was recorded in September from the deeper area, zone V. In zone IV, u was very high in June.
In zone II, u varied between 210 and 997 from December to May, 1963–64, and was significantly high in zone III, in June, being 3667. In zone V there was significant catch with u at 1473 in October only.
3.5.2 Size The trend in size variations was quite similar in all the three years. In zone II, in all the years, m increased gradually and reached the maximum of 90.2 to 95.3 by January-February and then declined. In zone III, in 1963–64, a maximum of 113.0 m was recorded in February. In zones IV and V m was comparatively lower, ranging from 77.2 to 90.0.
3.5.3 Movements Although mean size m showed a regular pattern in all the years, abundance did not give any dependable trend. In 1961–62, in zone II, there was decrease in m along with increase in u in April-June which indicated entry of small sized prawns into the area. In 1963–64, from October to December there was increase in u along with increase in m showing incursion of bigger sized prawns into this zone. In 1962–63, from March to May both u and m were noticed declining, indicating movement of bigger sized prawns away from zone II.
Apart from these, no clear pattern of movement could be discerned from the variations in u and m.
The foregoing account provides a general picture of the nature of fluctuations in abundance (u) and mean size (m) of the commercially important penaeids of this region. The pattern of movements of prawns in the fishing grounds is shown to be size-oriented and this is true of all the species dealt with. Soon after the monsoon, when the fishery commences, large sized prawns are seen to move into the fishing grounds which are at that time mostly confined to zones II and III. This movement, from adjoining deeper zones, is probably a sort of recolonization of the grounds, the prawns having deserted them earlier during the physico-chemical disturbances brought about by the monsoon and the upwelling. It is, therefore, apparent that the effect of upwelling during the monsoon (Banse, 1959) is to drive all the species of prawns, except M. dobsoni, into the deeper waters. The available information does not indicate up to what depth these prawns are pushed out, or at what level they remain during this period. This phenomenon, coupled with decrease of fishing effort during the period, results in the increase of mean size which is evident at the commencement of the fishery. The better catches of prawns obtained by a few well equipped trawlers from the deeper waters operating from Cochin during monsoon lend additional support to this view. The offshore movement of these prawns from January onwards, particularly of the larger species, P. indicus, M. affinis and M. monoceros, indicate prospects of better yield if trawling is carried out in deeper waters towards the end of the season also.
While tracing the movements of these prawns in the fishing grounds, it becomes necessary to examine the possibility of their coastwise movement. The pattern of distribution of size and abundance of all the species brought out here clearly indicates movements from one depth zone to the other. In a dynamic population some coastwise movement can naturally be expected although the present data do not seem to give any indication of the same in the depth zones considered. Extensive marking experiments only can bring out conclusive evidence in this respect. The detailed marking projects carried out on the white shrimp Penaeus setiferus showed no indication of coastwise migration along the major portion of the Gulf of Mexico (Lindner and Anderson, 1956).
The distribution of abundance over the three years of observation show a decreasing trend, particularly in zone II, (Figs. 2 – 6) where the maximum exploitation is taking place at present. George et al. (in press) observed decreases in abundance in the same fishery from 1958–59 onwards, but the unusually large catches in 1961–62 made them attribute this to natural fluctuations. Here the trend is continued in the next season also, as shown by the present study, and may well be attributed to natural fluctuations, especially when the rate of instantaneous mortality of the major constituent of the fishery, M. dobsoni, is estimated as being very low (Banerji and George, in press). A more or less similar trend was observed in the yield of shrimps of Sanibel-Tortugas areas by Kutkuhn (1960) during 1956–59. Examination of related data over a protracted period concerning the same fishery enabled Eldred et al. (1961) to attribute this declining tendency to natural fluctuations.
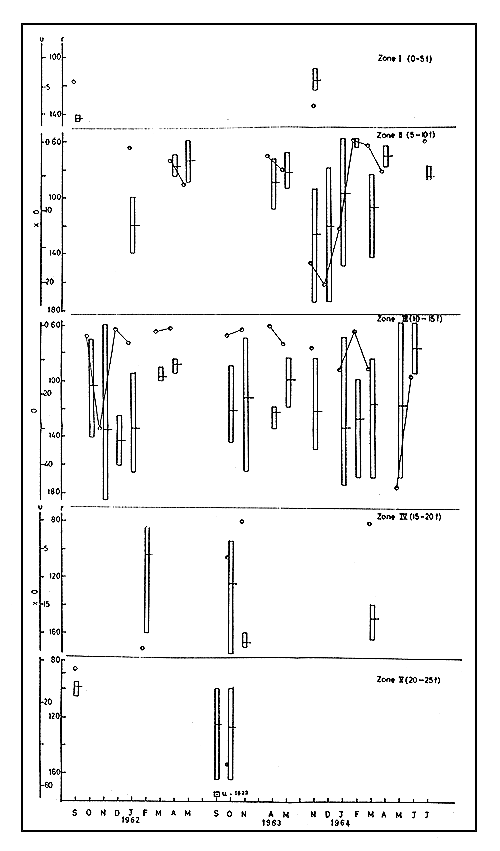
Fig. 5 Abundance and size of M. monoceros during 1961–62 to 1963–64. Vertical column represents size range (r) and horizontal line on it the mean size in mm. Abundance (u) in number per trawling hour.
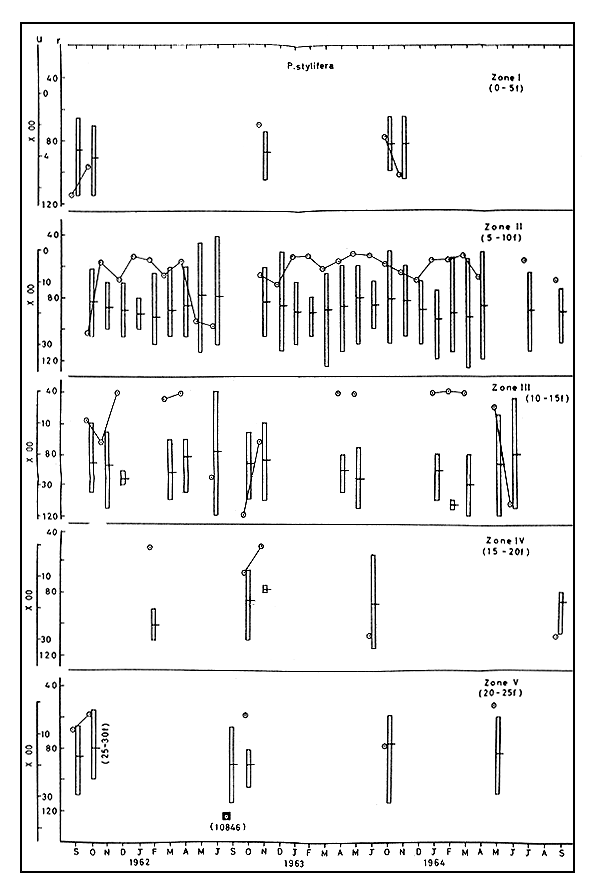
Fig. 6 Abundance and size of P. stylifera during 1961–62 to 1963–64. Vertical column represents size range (r) and horizontal line on it the mean size in mm. Abundance (u) in number per trawling hour.
Nevertheless, this declining trend may cause apprehensions to the industry and has to be very carefully watched. The distribution of fishing effort, which is now concentrated in zone II where this trend is particularly evident, may be advantageously directed to the deeper zones where the possibility of better yield of large sized prawns exists as pointed out here.
Banerji, S. K. and M. J. George, 1967 Size distribution and growth of Metapenaeus dobsoni (Miers) and their effect on the trawler catches off Kerala. Proc.Symp. Crustacea mar.biol.Ass.India, Part 2:634–48
Banse, K., 1959 On the upwelling and bottom trawling off the south west coast of India. J.Mar.biol.Ass.India, 1(1): 33–49
Eldred, B., et al., 1961 Biological observations on the commercial shrimp Penaeus duorarum Burkenroad, in Florida waters. Prof.Pap.Ser.mar.Lab.Fla., 3:1–139
George, M.J., 1959 Notes on the bionomics of the prawn Metapenaeus monoceros (Fabricius). Indian J.Fish., 6(2):268–79
George, M.J., 1961 Studies on the prawn fishery of Cochin and Alleppey coast. Indian J.Fish., 8(1):75–95
George, M.J., 1962a On the breeding of penaeids and the recruitment of their postlarvae into the backwaters of Cochin. Indian J.Fish., 9(1):110–16
George, M.J., 1962b Observations on the size groups of Penaeus indicus Milne Edwards in the commercial catches of different nets from the backwaters of Cochin. Indian J.Fish., 9(2):468–75
George, M. J., K. Raman and P.K. Nair, (in press) Observations on the offshore prawn fishery of Cochin. Indian J.Fish., 10
George, P. C. and M. J. George, 1964 On the location of a possible spawning area for the penaeid prawn Metapenaeus monoceros (Fabricius) off Cochin. Curr.Sci., 33(8):251–52
Kutkuhn, J.H., 1960 The Sanibel-Tortugas shrimp fishery 1956 to 1959 - Annual report, Fishery Research Galveston Biological Laboratory. Circ.Fish Wildl.Serv., Wash., (92):11–5
Lindner, M.J. and W.W. Anderson, 1956 Growth, migrations, spawning and size distribution of shrimp, Penaeus setiferus. Fishery Bull.Fish Wildl.Serv.U.S., 56(106):555–645
Menon, M. K., 1953 Notes on the bionomics and fishery of the prawn Parapenaeopsis stylifera (M. Edw.) on the Malabar coast. J.zool.Soc.India., 5(1):153–62
Menon, M. K., 1955 Notes on the bionomics and fishery of the prawn Metapenaeus dobsoni (Miers) on the south west coast of India. Indian J.Fish., 2(1):41–56
Menon, M. K., 1957 Contributions to the biology of penaeid prawns of the south west coast of India. 1. Sex ratio and movements. Indian J.Fish., 4(1):62–74
Menon, M. K. and K. Raman, 1961 Observations on the prawn fishery of the Cochin backwaters with special reference to the stake net catches. Indian J.Fish., 8(1):1–23
Rao, P. V., (in press) Maturation and spawning of the penaeid prawns of the southwest coast of India. Paper presented to FAO World Scientific Conference on the Biology and Culture of Shrimps and Prawns, Mexico, 12–21 June, 1967.
Acknowlegments
The authors are grateful to Dr. S. Jones for the continued interest shown during the preparation of this account and to Dr. R. Raghu Prasad for critical suggestions offered. They are thankful to Messrs. P. V. Rao, T. S. Krishnan and Thomas Japapalan for help rendered in the preparation of the paper.