Mr. G. ROMAN
1. INTRODUCTION
Bivalve mollusc culture con be started in two different ways: collecting the seed naturally or by hatchery rearing. Some of the advantages and disadvantages of both methods are shown in the table below.
| Natural collection | |||
| Advantages | Disadvantages | ||
| - | Low price | - | Environmental conditions not controlled |
| - | Spat ongrowing on collectors | - | Seasonal and short settlement period |
| - | Skilled labour not required | - | Heavy broodstock in the area is essential |
| Hatchery rearing | |||
| Advantages | Disadvantages | ||
| - | Environmental conditions controlled | - | Expensive building and maintenance |
| - | Settlements during most of the year | - | Spat on-growing in nursery |
| - | Parental stock small | - | Skilled labour required |
Larval rearing in big ponds is halfway between the two methods, and not employed very often.
The choice between hatchery rearing or natural collection depend on the characteristics of the area. As a general rule, natural collection is suggested if working with cheap molluscs or in areas with rich stocks.
Hatcheries developed as a consequence of a decrease in oyster catches, owing to poor recruitments. The causes of the shortage of juvenile oysters have been attributed to the depletion of Fishery grounds, diseases, natural catastrophes and estuarine pollution. As oyster industry required juvenile oysters in numbers that natural settlements could not meet, hatcheries were developed to supply this industry with oyster seed.
Another reason for the development of hatcheries has been the possibility of obtaining genetically selected stocks.
Most of the Atlantic European hatcheries have been working with oyster and clam, formerly Ostrea edulis and Venerupis decussata, nowadays with the easy culture and faster growing Crassostrea gigas and Venerupis semidecussata ( = Ruditapes philippinarum).
However, oysters and clams are not the only commercially important molluscs, and some effort is devoted to the culture of pectinids (Pecten maximus, Chlamys varia, C. opercularis), with promising results both in hatcheries natural collection.
Hatchery bivalve rearing on a commercial basis started in the 60's, and in some way this can be related to the publishing of the following documents: LOOSANOFF & DAVIS (1963), WALNE (1963, 1964, 1965, 1966) and IMAI (1966), describing culture techniques, water processing, conditioning, phytoplankton production, etc…
Nevertheless, in previous years, it was necessary to find out about larval food requirements. Also to this effect, LOOSANOFF's discovery (1945) that sexual ripeness could be brought about by increasing the temperature, allowed a longer working period and a faster development of hatchery techniques.
However, even now, there are more unknown factors in larval rearing leading to sporadic and intermittent failures.
2. REPRODUCTIVE CYCLE OF BIVALVE MOLLUSCS
When culturing bivalve larvae it is convenient to know something about the sexuality of molluscs. It is necessary to known when and how the molluscs you are going to culture reproduce.
Most of the species have separate sexes, that is, some individuals are always males, some of them are always females. However, some commercially important species are hermaphrodites. COE's (1943, 1945) categories of hermaphroditism are as follows:
- Functional hermaphroditism: spermatozoa and ova are produced simultaneously, and self-fertilization may occur : Pecten maximus, Chlamys opercularis.
- Consecutive hermaphrodites, where there is a single sex reversal in life. Usually, the sex change is from a younger male to and older female phase : Mercenaria mercenaria.
- Rhytmic consecutive hermaphroditism: a male phase is followed by a female one and then a male phase and so on : Ostrea edulis.
- Alternative sexuality : in this case, a sex change occurs in adults that function seasonally as separate sexes. This sex change is erratic and not predictive: Crassostrea virginica.
However, regardless of the difference in sexual behaviour, all the commercially important bivalves release their sexual products in the sea water, where fecundation takes place (except the genus Ostrea).
The reproductive cycle in pelecypods can be divided into three main stages:
- Gonad development
- Spawning and fertilization
- Development and growth.
Hatchery management involves these stages as follows:
| Gonad development | Recovering | Conditioning |
| Ripening | ||
| Stimulating | ||
| Spawning and Fertilization | ||
| Ova+Spermatozoa | ||
| Incubating | ||
| Embryogenesis | ||
| Trochophore larvae | ||
| Development and growth | Veliger larvae | Larval rearing |
| Metaporphosis | Settlement |
| Juveniles | Nursery & Planting |
| Growth | |
| Planting |
The right hand side of this table shows the characteristical procedures to be carried out in a hatchery. We must add to them the selection of the broodstock, food culture and water processing.
2.1. Conditioning
In temperate waters the reproductive cycles of bivalves usually occur on a yearly and sometimes bi-annual basis, and the spawning period is very short. Before the experiments of LOOSANOFF & DAVIS (1963) larval rearing was limited to the very short natural periods of spawning, but the conditioning techniques they developed allowed a longer period of spwaning, at least for some species.
The conditioning method involves placing the molluscs in water whose temperature is increased daily until it reaches the required level. In this way, gametogenesis can be induced after one month in animals remaining in a resting phase. If the animals are undergoing the gametogenesis, this will be accelerated. Unless the animals overcome the recovery phase, the conditioning techniques won't work. During this period the animals must store nutrient reserves, consisting mainly of glycogen. That is the reason why we must have a thorough knowledge of the reproductive cycles in the animals we are working with.
2.1.1. Broodstock selection
Origin, aspect and meat quality are factors to be considered when selecting the broodstosk. Animals coming from polluted areas or infested with parasites must be rejected. Animals that appear very old, that are slow growers or that have a bad appearance should be discarded.
Some animals must be opened, and the batches with a higher condition index selected.
When working with animals that change their sex with age, different ages must be conditioned, to be sure both sexes will be present.
Before introducing the animals in the conditioning system, they must be cleaned and brushed carefully.
2.1.2. Number of animals to be conditioned
This number will depend on the number of larvae required. If we can determine the mean number of larvae produced by a Female, and the mean number of females who will spawn in each period of the year, it is easy to determine the required number of animals to be conditioned.
The table below shows the percentage of oysters (Ostrea edulis) that spawned after a three month conditioning period in different seasons of the year.
| Conditioning Start | Percentage of spawnings (swarmings)* | ||
| 1st month | 2nd month | 3rd month | |
| Autumn | 4.0% | 0.0% | 14.9% |
| Winter | 2.0% | 12.1% | 10.5% |
| Spring | 8.0% | 10.6% | 8.8% |
| Summer | 6.7% | 6.8% | 3.5% |
* The mean number of larvae produced at each swarming is 0.96 ± 0.54 × 106
2.1.3. Conditioning systems
There are two types of conditioning system, a flow-to-waste system or a recirculating one. Probably the best results are obtained with the flow-to-waste system, although it is more expensive, owing to the waste of food and heated water.
The water Flow will depend on time volume filtered paranimal, but usually less than this volume is employed to reduce the cost of heating the water and the food demands. When working with Ostrea edulis, high flows can result in larval losses, due to clogging of the filters used to retain the larvae.
We usually give a flow rate of 1.2 l/oyster/hour. WILSON (1981) gives 1 – 2 l/oyster/hour. DUPUY et al., (1977), however, use a flow rate of 10 l/oyster/hour.
Food ration : The energy requirements for building up the gametes must be provided to the animals as food. The incoming water may or may not be Filtered. In the last case some food can be present in the water, but usually this is not enough for the molluscs requirements; so, some food must be added.
In most cases, the food given is five unicellular algae, but sometimes corn-starch is added.
WILSON (1981) adds an amount of dried algal weight equivalent to 6% of the oyster dry meat weight. For 1 g of dry oyster, this represents 60 mg of dried algal weight (= 2.64 × 108 cells of tetraselmis suecica or 1.74 × 109 cells of lsochrysis galbana).
HELM et al., (1973) add 10 Tetraselmis/ul, and FLASSCH et al., (1973) 2.16 × 108 cells tetraselmis/oyster/day.
DUPUY et al., (1977) recommend 2.0 mg/l of corn starch.
ROMAN (1985) feeds oysters on a mixture of 2.4 × 108 cells of Tetraselmis suecica plus 3.1 × 108 cells of Skeletonema costatum per oyster and day (= 8.3 cells of T. suecica plus 108 of S. costatum per microlitre.
The system must be cleaned periodically, mainly if corn starch is added.
When working with species other than oysters (Ostrea), frequent ripeness controls must be performed. This is done to avoid spontaneous spawnings in the system owing to the temperature increase in the water where the animals are held. When the animals are nearly ripe, a slight decrease in the temperature is recommended and we must bear in mind that males reach ripeness before females.
Oysters (Ostrea edulis) have a very characteristic reproductive behaviour, so I will now describe the system we use for conditions and collecting the larvae. A diagram of the tanks where the oysters are held is shown in figure in these plastic tanks (60 × 54 × 40 cm) 25 oysters are placed. The outflow must pass through two filters, 200 and 80 μm mesh size. When a swarming or larval release occurs, the larvae have a tendency to swim at the very surface of the water. Then they are carried away with the out-flowing water and retained in the 85 μm filter.
2.2. Stimulating
On natural conditions when the gonad reaches its maximum development and the gametes are completely mature, spawning occurs, probably as a response to environmental changes.
There can be spontaneous spawnings in the conditioning system. When this happens, most of the gametes are not fit for culture, and they must be rejected.
To avoid this problem, when animals are considered to be ripe, they must be stimulated under controlled conditions, to pick up the gametes when spawned. if stimulation is not possible at the date, water temperature in the conditioning system must be decreased; however, stimulation is recommended as soon as possible, because old gametes can result in bad larvae.
Before stimulating, the animals must be cleaned, and detritus and faeces removed, as well as other organism settling on and in the shell, as can spawn as the same time as the molluscs.
Methods used in stimulating molluscs to spawn
In a recent paper, LE PENNEC (1981) reviews the methods employed to get the molluscs to span. the methods most often employed are thermical changes, gamete addition and physical and chemical shocks. It must be made clear that these methods will only succed if the animals are ripe.
- Thermical changes : By far the system used most often. Usually, the rature is raised 5 – 10° c. Once the required temperature is reached, it can be kept at the level, or there can be increase and decreases for some time.
- Addition of gametes : This system is usually employed together with temperature increases. In this way, the results are improved.
- Chemical stimulation : The latest trend is the use of serotonine injections. In this way, fantastically rapid spawnings can be obtained, but usually only the males spawn.
When warming the water for stimulating, as the gas solubility decreases small bubbles are formed. When animals to be spawned fit inside these small bubbles, the spawning can be prevented. The presence of bubbles is easily noticed by the appearance of floating faeces. The warmed water must be aerated to avoid this.
The time required for spawning after starting to stimulate will vary according to the species, ripeness, the system employed for stimulation, etc… Spawning can start a few minutes later, or it can be delayed several hours. It may also not take place at all. In fact, in our experiments with Pecten maximus, the release of gametes can be delayed 2 – 3 weeks.
When animals start to spawn, they must be placed separately in flasks filled with water at the same temperature as those used in the spawning trays. When a female happens to be a heavy spawner, she must be changed to another flask again.
When working with functional hermaphrodites, the spawners must be watched continuously. Usually, as in the case of molluscs having separate sexes, the first spawners are males, and when the tests is empty, eggs are released. A watchful and steady survey of the flasks where the animals are spawning allows you to detect when the sex reversal takes place. At this moment the animal must be seized and washed inside and out, to remove as much sperm as possible and then placed in a new container, to allow it to release the eggs. The survey must continue, because in some cases, when testis are not empty, new sperm releases can occur.
In this way, we obtain eggs and sperm from different animals in separate containers. Then, we may or may not; put all the eggs together in a bigger container depending on our interests. In any case, and after adding some drops of a pooled sperm suspension, they should be examined microscopically, to ascertain the percentage of fertilization. If necessary, more sperm must be added. Fertilization must be performed as quickly as possible, and no later then one hour after spawning. When working with P. maximus it is almost impossible to prevent a variable and uncontrolled percentage of the eggs to be fertilized before sperm addition).
Next, eggs are passed through a 100 μm mesh, to remove detritus, and retained either on a 20 μm mesh or in a container. Immediatly after, they should be counted and placed in the incubations tanks.
All these operations must be done as quickly as possible. A delay in adding the sperm can result in a low rate of fertilization. If eggs are filtered late after fertilization, when the cleavage is in progress, a high amount of abnormal larvae can appear.
The number of eggs that a female can release depends on the species and the size of the animal. We have counted 7 to 14 × 106 eggs, 68 μm diameter, in 10 cm length scallops (ROMAN & PEREZ, 1976), and 1 to 2 × 106 eggs, 70 μm diameter, in clams (Venerupis decussata and V. pullastra) (PEREZ et al., 1977). Crassostrea virginica can produce 25 to 100 million eggs (DUPUY et al., 1977).
2.3. Incubating
After spawning, the germinal vesicle in the spherical egg is visible. Later on, the germinal vesicle breaks down and the egg remains in this stage until it is fertilized. Then comes the rapid appearance of the first and second polar bodies, the fusion of male and female pronuclei proceeds and cleavage starts. 12 to 24 hours later the trochophore appears, and the next day, that is, 48 hours after fertilizing, the veliger larvae are developed.
These development stages are performed in incubation bins, which must not be disturbed during the 48 hours period. This is a short but important phase in larval culture, and during this time neither food nor aeration is supplied to the larvae.
We usually add 500 to 2 000 eggs per square cm, and get 10 to 68 % of normal larvae (PEREZ et al., 1977, GONZALEZ & ROMAN, 1983 ROMAN & PEREZ, 1976).
The bins we use for incubation purposes are either 100 l volume, 1 900 cm2 bottom surface, or 400 l, 9 500 cm2 bottom surface.
After the 48 hours period, the drums are emptied, and the water is passed through and 100 and a 45 μm mesh size filters, in order to retain the detritus (100 μm) and the larvae (45 μm) in their early veliger stage or straight-hinge stage by this time measuring about 100 μm. The filtering out must be done at a slow rate, to prevent clogging and larval losses. Next, the number of normal and abnormal larvae is counted, and the larvae are placed in the drums used for their culture at the required density.
Abnormal larvae are always present, but in a variable percentage and degree of abnormality. Most of the misshaps in development belong to the following categories:
- After fertilization, no development occurs, and the egg disintegrates.
- Development stops before reaching the trochophora stage.
- The trochophora does not secrete the shell.
- Only a small and abnormal shell is produced.
- The shell looks normal, but either velum can not retract inside or it is deformed.
- The hinge is curved.
- The shell and velum are normal, but the larvae do not feed, and as culture goes on they become whitish.
Most of these abnormalities can be attributed to some of the following causes:
- Poor physical conditions of the spawners.
- Spawning late in the season.
- Strong stimulation, that results in aborted eggs.
- Heavy concentrations of eggs for a long time on the bottom of the containers before counting or adding the sperm, resulting in oxygen depletion.
- Delay in adding the sperm.
- Careless handling when cleavage starts.
- Overcrowded incubation.
- High temperatures while incubating.
- Polyspermy.
As the abnormal larvae do not grow, or their growth rate is slow, they can be discarded after a few days of culture for the appropriate sieving methods.
2.4. Larval culture
As we mentioned earlier, when trochophore larvae held in the incubation containers become veliger larvae, the water in the tanks is filtered through a mesh and the retained veliger are counted and placed in the tanks used for larval culture.
The early veliger or straight-hinge larvae have a D-shaped shell covering all the soft body parts. The most conspicuous larval organ at this stage is the velum, heavily ciliated, used for swimming and feeding. As larvae grow older a more or less prominent umbo develops, and later on a ciliated and retractile foot, with a byssus gland. Some species shoe on eye spot, whose presence announces that settlement can be expected soon.
2.4.1. External factors affecting the larval growth
The larval growth rate is a useful index of the culture quality, and usually, the faster the growth is, the better the survival and the higher the settlement rate will be.
There are several factors influencing the growth rate. Some of them, such as quality and quantity of food, temperature, salinity and larval density, can be easily controlled, but other factors like water quality or disease cannot.
Food
For many years, one of the biggest problems related to larval culture was their food identification. Later on, it was discovered that larvae feed on unicellular algae, the only satisfactory food known to date.
As a consequence, a considerable amount of algal species have been isolated and cultured, to determine its food value.
Some conditions are required for a species considered a good food source:
- The size, 2 – 10 μm being the most advisable.
- Digestibility. In this sense, the presence and thickness of the cellular wall is an important factor.
- Toxic metabolites are produced by some algae, discarding them as food.
- Factors such as easy culture and high yield are also important.
- The alga must fulfill the larvae requirements in carbohydrates, proteins, lipids and vitamins.
- Mobility is not essential, but advisable.
As a rule, mixed food results in a improved growth rate. The sinergical effect can be ascribed to a more complete fulfilment of nutritional requirements.
The food ration, that is, the number of algal cells used for feeding the larvae, regardless of larval density or size, will depend on the algal size. The most commonly employed ration is the equivalent volume of 100 cells of Isochrysis galbana per microlitre.
The food value of a species can change according to age, and even a good species can become toxic owing to bacterial growth.
The most useful species are the following:
Isochrysis galbana
Pavlova (Monochrysis) lutheri
Tetraselmis suecica
Phaeodactylum tricornutum
Pseudoisochrysis paradoxa
Thalassiosira pseudonana (ex : Cyclotella nana)
Chactoceros calcitrans
Skeletonema costatum
Isochrysis tahiti
Rhodomonas baltica
Temperature
usually considered to be the most important factor in larval growth rate. Most of the species living in temperature waters increase their larval growth rate when the temperature rises, but when the temperature is some point above 30° C, survival and growth decreases sharply.
Salinity
As most of the cultured species live in estuarine habitats, it could be suspected that salinity would not affect the larval growth rate excessively as the larvae may be exposed to fluctuating salinities in the environment. In fact, they can withstand a relatively with range of salinity, and there is some evidence that animals living in more estuarine habitats have a broader margin of tolerance for lowered salinities for example,Ostrea edulis has its lower limit at 20 ‰ (DAVIS & ANSELL, 1962), and Crassostrea virginica at about 10 – 12.5‰ depending on the salinity in the area where the parents were living (LOOSANOFF & DAVIS. 1963)
Density of larvae
This factor is related to the larval size. However, there is a wide range of acceptable densities, the growth rate decreasing as density increases.
A good growth rate can be achieved at the following densities (CHANLEY, 1975) ;
| Larval size | Number/ml |
| 50 – 100 μm | 15 |
| 100 – 200 μm | 8 |
| 200 – 300 μm | 5 |
| + 300 μm | 1 |
The decreased growth rate in crowded cultures is supposedly due to accumulation and to an increased rate of collision between larvae. The lack of food can also affect the growth. However, an increased feeding rate can inversely affect the growth rate .
Water quality
The water quality is a problem that is always present when rearing small animals. The high surface-volume ratio of the larval, and their forced exposition to any material present in the water make them extremely succeptible to toxic or disease producing agents (CULLINEY et al., 1975).
Periodically, the sea-water has a harmful effect on the larvae. However, in spite of the importance of this subject, very little information is available on this subject, and failures in growing larvae have been attributed to dinoflagellate blooms, high levels of silt suspension, heavy metals and unidentified filterable components.
As a precaution, and before setting up a hatchery, some environmental considerations must be kept in mind, in order to present water quality problems as much as possible :
- Salinity variations
- Particle matter, and related to this subject, depth and exposure of the shore.
- Pollution.
- Red tides.
2.4.2. Methods of culture
The volume of the containers where larvae are going to be cultured can range from one to several hundred litres.
The culture can be performed in a open-to-flow or a stagnant system, the latter being the most common. In this case, water is changed periodically, usually every other day. Water changes in Monday, Wednesday and Friday is a pattern employed very often.
The water can be either drained through a bottom valve, the larvae and detritus being retained and sieved on filters of suitable size placed underneath or siphoned out by means of a flexible pipe. In this case, either a mesh can be placed in the inner tip of the pipe, preventing the larvae from draining, or the larvae are drained away and retained on sieves in a similar way as when bottom valves are used. They latter system allows us to reject dead or moribund larvae and detritus accumulated on the bottom.
After emptying, the containers must be carefully and thoroughly cleaned, by means of hot fresh water and a non toxic detergent, and then rinsed two-three times with filtered sea water before refilling.
The sieves holding the larvae must be placed in containers filled with filtered sea water before putting them back into the larval culture tanks, and a microcospical survey should be made of a sample picked up means of a PASTEUR pipette for measuring and controlling.
If necessary, selective sieving should be done to remove slow growers or dead shells. After refilling the larval containers. the larvae are replaced inside, and then food and antibiotics (if any) are added. However, as larvae grow older it may be necessary to feed them daily.
Usually, a gentle aeration is supplied.
2.5. Settlement
The larval pelagic period ends when the animal settles. Before settlement there are some changes in the larval behaviour. The larva ready to settle swims with its foot protruding and when it touches some solid surface, the velum is retracted and the larva crawls using the foot.
This behaviour can change suddenly, the foot being retracted, the velum expanded and the swimming restarted.
When the oyster is ready to settle, after crawling and looking for a suitable substrate, the shell twists and with the left valve facing the substrate, it attaches itself definitively using the cementing substance of the byssal gland
However, most of the molluscs settle temporarly by means of filamentous byssus.
Morphological changes from larval to juvenile (metamorphosis) start immediately, involving the disappearance of some larval organs (Velum) and the development of new ones.
Size at settlement
Variable according the species. Usually, between 220 and 320 μm
Timing of settlement
Again variable according to the species. Heavily influenced by external factors. Usually, Ostrea edulis requires 7 – 14 days, Venerupis spp about 3 weeks and Pecten maximus 4 weeks.
Probably this is the most critical phase in larval cultures, as by this time it is normal for larvae to crowd together in a sticky mess of mature crawling larvae, younger swimming larvae, dead shells, faeces, food and detritus.
When arriving at this phase suitable sieving should be done, selecting different development stages by size, and placing them according to their size in different containers.
2.5.2 Settlement techniques
As we said before when settlement is on the point of happening, if is advisable to separate the different sized larvae. The smaller ones will take longer to settle, so they are put back into the larval culture containers, whereas the biggest ones will be ready to settle, so they will be placed in settlement tanks.
We must consider that most of the commercially important species settle in a temporary way whereas oysters settle definitively. Again refering to oysters we can work on cultch or cultchless spat.
The species attaching themselves temporarily do not need any collector, as they settle on the bottom or walls of the settlement containers,from which they can be detached easily, when necessary, by applying a jet of water. However, some filament made collectors can be used for some species like Pecten.
Two main systems are used for oysters : small particles or some kind of tiles or stripping methods. The last technique may or may not involve a detachment a few hours later or when oysters reach a certain size, meanwhile the use of small particles (200 – 350μm) enables us to get oysters without any removing techniques.
Settlement of some species like oyster can be hastened by adding adult oyster extract to the substrate.
2.6 Nursery
Hatchery produced bivalve seed must be grown in a nursery prior to planting or sowing. Small seed planted directly in the sea may suffer high mortalities owing to predators, silting, clogging, adverse weather, etc…
Advisable size of transfer seed to the sea will depend on areas, species and ongrowing techniques.
The aim is to grow the seed, until it reaches a suitable size for planting, as fast as possible, to reduce the expenses involved in feeding, heating and handling but bearing in mind that some seasons are not adequate for planting.
In the last years there have been some improvements in the nursery systems, and the old style tray rearing has been changed to upwelling columns. This system consists of forcing a water rich in phytoplankton through a mesh-made bott container where the seed is held.
Before placing the seed on the upwelling column, the following operations are suggested :
When dealing with animals settling temporarily, change the water every other day. After emptying the settlement containers, a very soft water jet is used Just t rinse the bottom and walls. In this way, only the settled spat. Will remain, attached on the container surfaces. The outflowing water will be passed through a mesh, and the retained larvae inspected, in order to determine if they will be recultured again or not. The byssus-bearing remaining spat will be removed applying a strong water jet, retained on a suitable mesh and placed in the upwelling columns.
- If we are dealing with oysters, we shall remember that two different techniques can be used:
- If a stripping method is employed, just allow the oyster to grow until the required size, and then remove the spat. Next, place them in the upwelling column. This system can damage the spat when detached.
- If oysters are settling on particles, allow them to grow until a size big enough to be sieved is reached. Then sieve, separating the oyster from the particles where settlement did not occur, and place the oysters in the upwelling column.
The use of upwelling columns implies a control of water flow, a number of spat per container and food ration. As these factors are related with size, as the spat grow they must be changed, decreasing the density of spat and increasing the flow and food ration.
WILSON (1981) suggests a minimum water flow of 1 litre per minute per 10 g of spat, and a maximum standing biomass of 200 mg spat per litre.
Some experiments (URBAN et al., 1983) showed the best growth at the highest food ration tested, an algal dry weight equivalent to 5 % oyster live weight.
The increasing food demands of the growing spat becomes a bottle-neck in the culture of molluscs.
2.7 Algal culture
A hatchery requires big volumes of cultured algae, and high cell counts. There are two main ways for culturing algae : monospecific cultures or bloom induced cultures of mixed species. The last one is not advisable for larval feeding, as the mixed species do not always fulfil the larval requirements, and bacterial growth can lead to heavy mortalities. However, it can be the best technique for feeding spat.
There are five major parameters which regulate algal growth : light, pH, temperature, nutrients and turbulence, and they must be controlled when culturing algae.
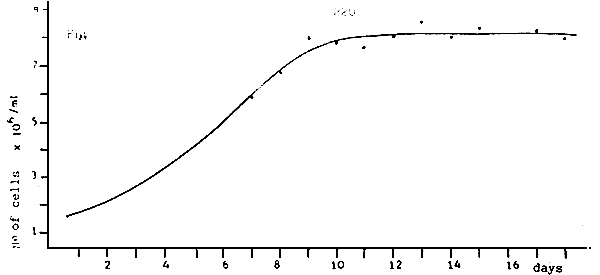
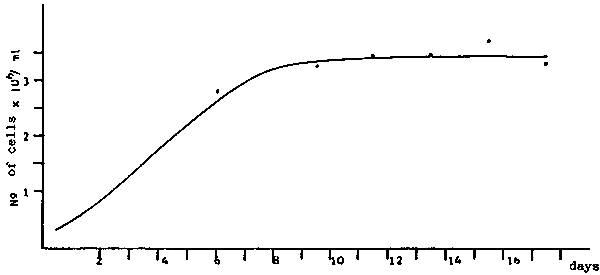
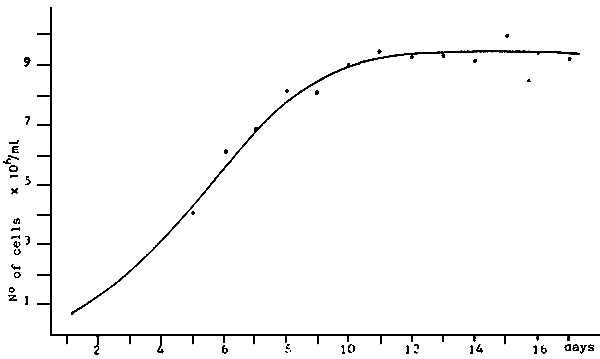
The monospecific culture involves several scaling up levels. In our lab, they are as follows :
- Vial. Primary stock. Obtained from biological laboratories.
- ERLENMEYER flasks, 250 ml. Stock. Plankton perpetuation performed in these stock containers. After a 7day period of growth they are used for inoculating 6 litres carboys. No aeration.
- 6 litres carboys. used for larval feeding and for inoculation of bigger volumes. They are harvested after 5 – 15 days.
- 100 and 1 000 l tanks. For conditioning and spat feeding. Harvest partial.
2.8. Water processing
Prior to use, sea water must be filtered. The shema below shows the processing we use in our laboratory :
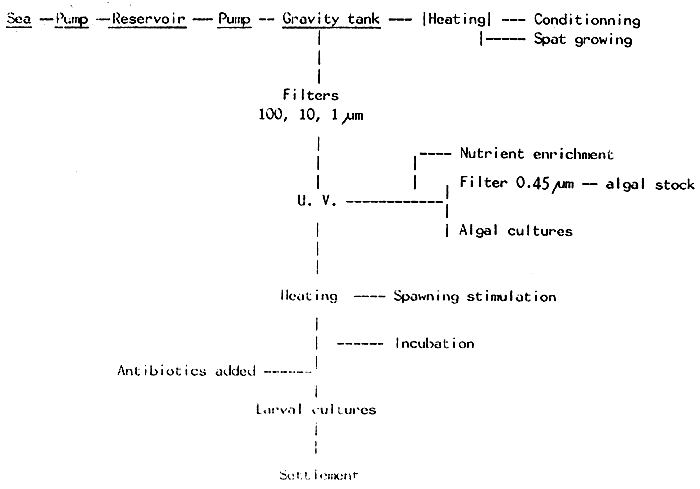
As a rule, sea water must always be filtered, to remove unwanted organisms. After Filtering. Some authors recommend also sterilizing it, using, for example, U.V.. Indeed, the larvae may be cultured without sterilizing the water, but in order to get a better survival, a higher guarantee can be achieved by using U.V. sterilized sea water, its use being on effective measure to prevent fungal or bacterial diseases.
When a volume of water is kept stagnant, the number of bacteria increases so, the larval culture techniques promote bacterial proliferation, sometimes leading to a complete larval mortality. Actually, the sporadic appearance of massive mortalities in hatcheries are not uncommon, and usually they are produced by pathogenic bacteria. The main source of bacterial contamination is due to the algal cultures (PRIEUR & CHARAVAL,1979).
TUBIASH (1975) describes a very common disease, the bacillary necrosis, whose agent is a Vibrio, destroying the cultures in a few hours.
The use of filtered and U.V. sterilized sea water, together with carefull cleaning sometimes are not sufficient measures, and some authors recommend the use of antibiotics. usually, they are employed in a preventive way, owing to the fulminant mortalities produced by pathogenic bacteria, rendering the treatment impossible when the first symptoms appear.
Sanitation is the most important rule to adopt.
Table I : Results from the stimulation experiments carried on Venerupis pullastra
| Stimulation Temperature | Number of experiment | Number of experiment showing positive responses | % | Number of shells stimulated | Number of positive responses | % |
| 28 – 30° c | 8 | 1 | 12,5 | 162 | 16 | 9,9 |
| 23 – 26° c | 12 | 7 | 58,3 | 173 | 43 | 24,9 |
| 18 – 21° c | 11 | 6 | 54,5 | 190 | 43 | 22,6 |
Results obtained with Venerupis decussatus
| Stimulation Temperature | Number of experiment | Number of experiment showing positive responses | % | Number of shells stimulated | Number of positive responses | % |
| 23 – 24° c | 4 | 0 | 0 | 65 | 0 | 0 |
| 28 – 29° C | 8 | 8 | 100 | 194 | 68 | 35 |
Table II : Milligrammes of organic material in 108 cells
| Chaetoceros calcitrans | 1,15 ± 0,39 |
| Isochrysis galbana | 2,45 ± 0,81 |
| Monochrysis lutheri | 2,72 ± 0,60 |
| Skeletonema costatum | 4,04 ± 1,15 |
| Phaeodactylum tricornutum | 4,88 ± 1,36 |
| Rhodomonas baltica | 7,09 ± 1,10 |
| Tetraselmis suecica | 16,23 ± 4,47 |
TABLE III
Effect of different foods on growth rate of the larvae of Pecten maximus. All the values are compared with a mixture of I.galbana + M. lutheri, and this food is considered to be equal to 100.
| Algal Species | Sk | Ph | Rh | I+M+Rh+Ph | Rh+Ph | I+M+Ph | I+M | I+M+Rh | I+M+Ch | I+M+Rh+Ch | ||
| Growth | 24.8 | 49.6 | 70.8 | 73.4 | 82.5 | 82.8 | 100 | 100.3 | 139.8 | 186.1 |
Sk = Skeletonema costatum;
Ph = Phaeodactylum tricornutum;
Rh = Rhodomonas baltica;
I = Isochrysis galbana;
M = Monochrysis lutheri;
Ch = Chaetoceros calcitrans
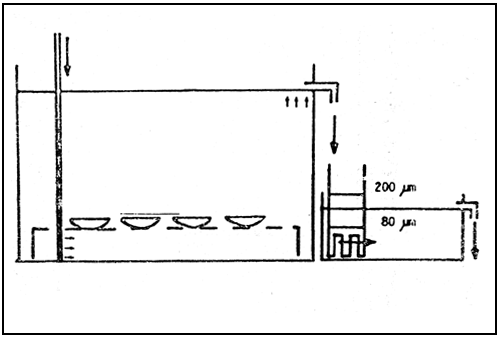
Fig. 1. Diagram of the conditioning system.
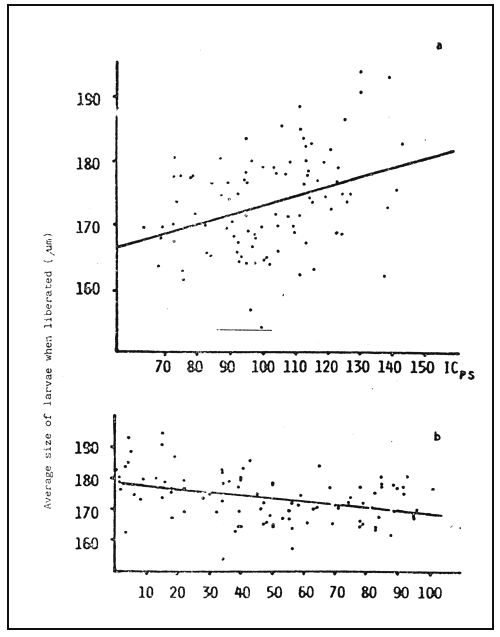
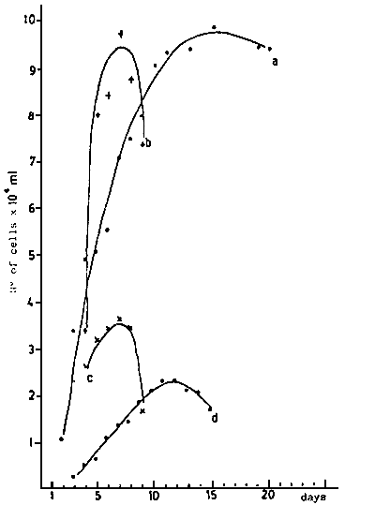
Fig. 2 - Days of conditioning.
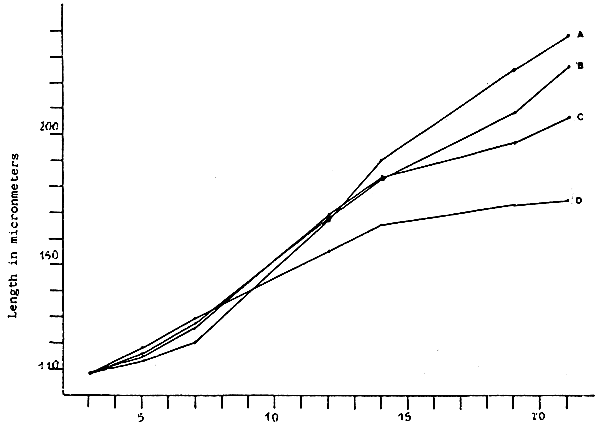
Fig. 3 - Larval growth of V. pullastra which were fed different
concentrations of M. lutheri
A. 100 cells/microliter
B. 75 cells/microliter
C. 50 cells/microliter
D. 25 cells/microliter
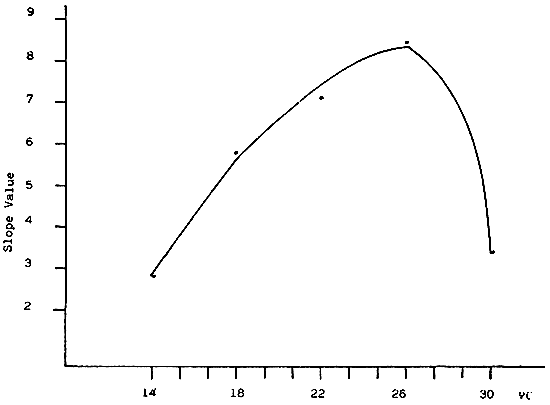
Fig. 4 - Slope of the growth curve of V. pullastra larvae reared at different temperatures
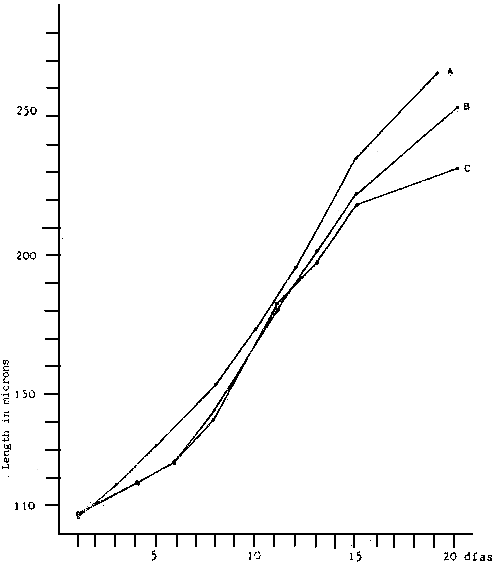
Fig. 5 - Larvae growth of V. pullastra reared at different densities
A. 1 500 larvae/1
B. 3 000 larvae/1
C. 6 000 larvae/1
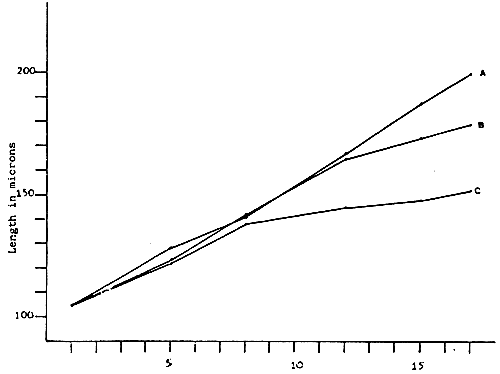
Fig. 6 - Larvae growth of V. pullastra reared at different densities
A. 10,000 larvae/1 - Straight line equation Y = 94.77 + 6.11 × : r2 = 0.99
B. 20,000 larvae/1 - Straight line equation Y = 101.41 + 4.80 × : r2 = 0,99
C. 40,000 larvae/1 - Straight line equation Y = 109.94 + 2.70 × : r2 = 0,90
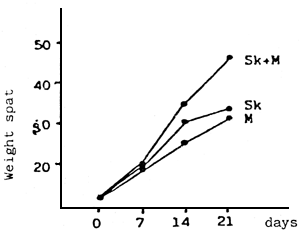 | 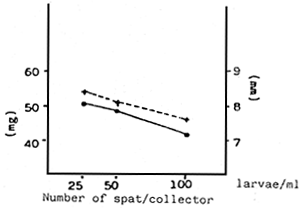 | ||
| Fig. 7 - | a - Effect of two monoalgal diets and a mixture of both on the growth of the larvae (spat) | b - | Effect of the density of the spat culture on the weight increase (mg) and size (μm) |
Fig. 7 - First fattening systems
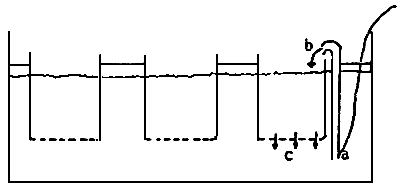
a) Rearing cages
b) “Upwelling” columna flows
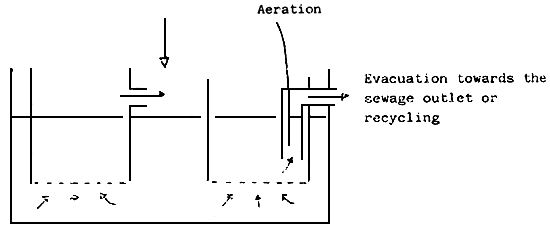
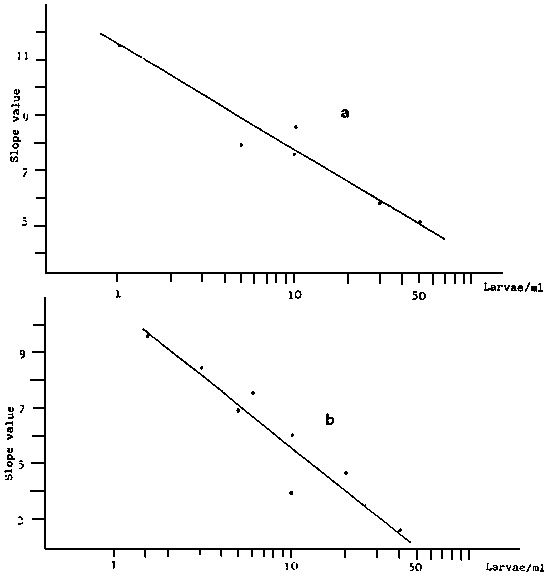
Fig. 8 - Straight regression line between the slope of the growth line Y
and the logarithm of the larvae conentration in culture,
V. decussata (a),V. pullastra (b)
a) Straight line equation : Y = 20.15 - 3.38 × : r2 = 0.94
b) Straight line equation : Y = 25.68 - 4.99 × : r2 = 0.91
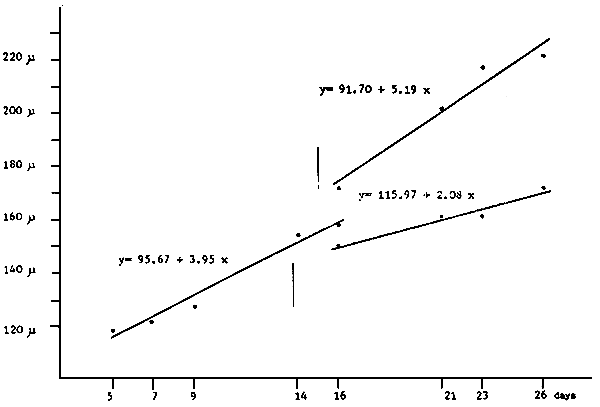
Fig. 9 - Growth curves of Pecten maximus larvae, before and after their separation into two groups of different growth rate.
Fig. 10
a) Average growth of Monochrysis lutheri in erlenmeyers of 250 cc.
b) Average growth of Tetraselmis suecica in erlenmeyers of 250 cc.
c) Average growth of Isochrysis galbana in erlenmeyers of 250 cc.
 | 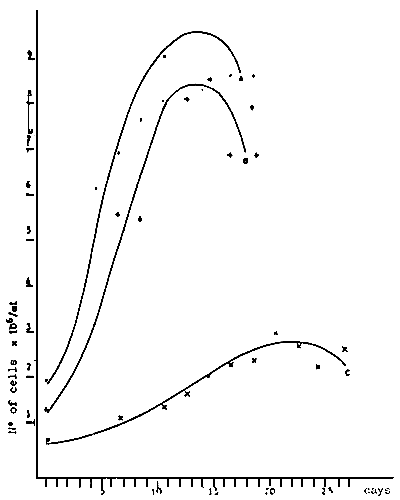 | ||
| - | Average growth of algae cultivated in 6 l recipients | - | Average growth of three algae cultivated in 6 l recipients. |
| a) Ph. tricornutum: b) Ch. calcitrans; c) S. costatum; d) Rh. baltica. | a) M. lutheri; b) I. galbana; c) I. suecica. | ||
| Fig. 11 - Algae growth in 6 l. recipients. (PERES et al., 1977) | |||
REFERENCES
COE, W.R. 1943. Sexual differentiation in mollusks. I. Pelecypods. Q. Rev. Biol. 18 : 154 – 164
COE, W.R. 1945. Development of the reproductive system and variations in sexuality in Pecten and other pelecypod mollusks. Trans; Conn. Acad. Sci. 36 : 673 – 700
CHANLEY, P. 1975. Laboratory cultivation of assorted bivalve mollusks, P 297 – 318 in - W.L. SMITH & M.H. CHANLEY (edt) : Culture of marine invertebrate animals, Plenum Press, N.Y.
CULLLINEY, J.L., P.J. BOYLE & R.D. TURNER, 1975. New approaches and techniques for studying bivalve larvae, p 257 – 271. In W.L. SMITH & M.H. CHANLEY (edt) : Culture of marine invertebrate animals. Plenum Press. N. Y.
DAVIS, H.C. & A. D. ANSELL, 1962. Survival and growth of larvae of the European oyster Ostrea edulis at lowered salinities. Biol. Bull., 122 : 33 – 39.
DUPUY, J.L., N.T. WINDSOR & C. E. SUTTON, 1977. Manual for design and operation of an oyster seed hatchery for the American oyster Crassostrea virginica. Special report № 142, Virginia lNst. Mar. Sci. 104 p.
FLASSCH, J.P., Y. KOIKE & C. AVELINE, 1973. Production de naissain de bivalves à moyenne échelle : but, perspectives. Colloque sur l'aquaculture, 22 24 Oct. 1973, BREST. Publication du CNEXO. Ed. L. LAUBIER & D. REYSS.
GONZALEZ, G & G. ROMAN, 1983. Larval culture of the scallop (Pecten maximus). 4th international Pectinid Workshop, ABERDEEN, Scotland, May 1983. 10 p. Mimeo
HELM, M.M., D.L. HOLLAND & R.R. STEPHENSON, 1973. The effect of supplementary algal feeding of a hatchery breeding stock of Ostrea edulis on larval vigour. J. mar. biol. Assu. U. K., 53: 673 – 684
IMAI, T. 1966. Mass production of molluscs by means of rearing the larvae in tanks. 7th Pac. Sci. Congr. TOKIO, 1966. Venus : 159 – 167
LE PENNEC, M. 1981. Less méthodes expérimentales induisant la ponte chez les mollusques bivalve marins. Haliotis, 11 : 139 – 155
LOOSANOFF. V.L. 1945. Precocious gonad development in oysters induced in mid-Winter by high temperature. Science, 102: 124 – 125
LOOSANOFF, V.L. & H.C. DAVIS, 1963. Rearing of bivalve mollusks. In F.S. RUSELL (edt) : Advances in Marine Biology, Vol. 1. pp 1 – 136. Academic Press LONDON.
PEREZ CAMACHO, A., G., ROMAN & M. TORRE, 1977. Experiencias en cultivos de larvas de tres especies de moluscos bivalvos: Venerupis pullastra (Montagu), Venerupis decussata (Linnaeus) y Ostrea edulis (Linnaeus). Bol. Inst. Esp. Ocean., 235 : 7 – 62.
PRIEUR, D. & J.P. CARVAL, 1979. Bacteriological and physico-chemical analysis in a bivalve hatchery : techniques and preliminary results. Aquaculture, 17: 359 – 374.
ROMAN, G. 1985. Estudio sobre el acondicionamiento de la ostra plana (Ostrea edulis L) I Congreso Nacional de Acuicultura. O. GROVE, 8 – 11 Oct. 1985. In press.
ROMAN, G. & A. PEREZ CAMACHO, 1979. Cultivo de larvas de vieira Pecten maximus (L) en laboratorio. Bol. Inst. Esp. Oceano.№ : 17 p.
TUBIASH, H. S. 1975. Bacterial pathogens of bivalve mollusc larvae. pp : 61 – 71. In : Culture of marine invertebrate animals. W.L. SMITH & M.H. CHANLEY (Edt). Plenum Press. N.Y.
URBAN, E. R., Jr, G.D. PRUDDER & C. J. LANGDON. 1983. Effect of ration on growth and growth efficiency of juveniles of Crassostrea virginica (Gmelin). Jour. Shellf. Res. 3. (1) : 51 – 57.
WALNE, P.R., 1963. Observations on the food value of seven species of algae to the larvae of Ostrea edulis. 1 : Feeding experiments. J. mar. biol. Ass. U. K. 43 : 767 – 784.
WALNE, P.R., 1964. The culture of bivalve larvae, pp 197 – 210. In : Physiology of Mollusca. K.M. WILBUR & C.M. YONGE (Edt). Academic Press. N. Y. Vol. 1.
WALNE, P.R. 1965. Observations on the influence of food supply and temperature on the feeding and growth of the larvae of Ostrea edulis. L. Fish. Invest. LONDON. Ser. 2. 24. (1) : 45 pp
WALNE, P.R. 1966. Experiments in the large scale culture of the larvae of Ostrea edulis L. Fish. Invest. LONDON. Ser. II. 15 (4) : 53 pp.
WILSON, J. 1981. Hatchery rearing of Ostrea edulis and Crassostrea gigas. Aquaculture Tech. Bull., Ireland (4) : pp : 1 – 34.
C.E.NASH & R.M.KONINGSBERGER
History of mullet culture
Farming of the Mugilidae has been practised for centuries but generally the cultivation of this potentially invaluable source of animal protein for man has been small and non-intensive. Subsistence farming has been a tradition in the Mediterranean region, south-east Asia, Taiwan, Japan and Hawaii within fenced lagoons, creeks and swamps, and in man-made ponds.
Experimental work in intensive mullet culture, aimed at finding ways to optimize production which is a necessary forerunner of any large scale operation is more recent. This increased effort has developed in approaches differing from region to region because of traditional needs and practices.
In the Mediterrancan region, especially in Italy, the traditional valliculture methods employed in raising mullet are now advanced (D'Ancona, 1955; De Angelis, 1969). In Egypt a successful stocking experiment was carried out at Lake Qarun in 1921 (Faouzi, 1936) using Mugil cephalus and Mugil capito No effort was made to optimize growth of the transplanted juveniles by supplemental feeding. A second transplanatation was made of M. cephalus, M. capito and Mugil saliens. The latter was reported to spawn successfully in the lake, but the stock of the other two species had to be replenished with juveniles from the estuaries (El-Zarka, 1968).
In the Soviet Union experiments with mullet were carried out in the regions of the Black Sea and the Caspian Sea. In 1930 and 1934 M.Cephalus, Liza saliens and L. auratus were introduced into the Caspian Sea (Thomson 1966). The transplantation of L. saliens and L. auratus was successful and their acclimatization, development and reproduction were studied in later years (Perceva-Ostroumova, 1951; Babaian, 1958) The work was followed by suggestions for further improvements in the region (Babaian, 1960; Chepurnov & Dmitriyev, 1962) and Apyekin & Tronina (1972) subsequently published the tentative results of experiments on the stimulation of the maturing and spawning of M.cephalus, M. saliens and Mugil auratus
The mullet were introduced for a seconary crop in carp ponds in Israel in the 1950s (Perlmutter, Bograd & Pruginin, 1957 ; Pruginin & Kitai 1957) Considerable research work was then performed on artificial feeding in ponds (Erman, 1958), seasonal and regional variations in the spawning season (Abraham, Blanc & Yashouv, 1966), histological studies of ovarian development in captivity (Abraham, Blanc & Yashouv, 1968), culture at different stock densities (Yashouv, 1966), and breeding and growth in captivity (Yashouv & Ben-Shachar, 1967).
In south-east Asia and the Far East efforts to intensify mullet culture were centred in Taiwan, India and Hong Kong. Farming of mullet was also practised in Japan, the Philippines, Indonesia and mainland China. but only as a seconary crop in the culture of other species. Research in these countries has been sporadic. There are two incidences of mullet rearing experiments in Japan. Between 1906 and 1909 at the Aichi Prefecture Fisheries Experiment Station Feeding experiments with mullet juveniles were conducted and these were repeated forty years later (Tamura. 1966)
In the Philippines the possibility of raising mullet juveniles together with milkfish in the brackish-water ponds was first suggested by Adams, Montalban & martin (1931). Although mullet were raised successfully with the milkfish for some time a scientifically supervised rearing experiment was not conducted until 1953 (Blanco & Acosta 1958). Polyculture of mullet with milkfish and carp was again proposed but has yet to be intensified. In 1971 preliminary experiments on induced spawning of Mugil dussumieri were carried out at the Marjan Sabalo Hatchery Experimental Station (Angelos, 1971) indicating that research on intensive mullet culture in the Philippines had not been abandoned.
In Hong Kong intensive culture of mullet was imposed successfully on the traditional practice of carp culture (Bromhall, 1954). The culturing method developed empirically used two pond systems stocked with M. cephalus as both the primary and the secondary crop (Lin, 1940). The ponds were fertilized with organic matter and the fish given artificial food. Culling started when the fish were about seven months old. Chow (1958) conducted growth studies on pond reared mullet and found that their growth rate compared favourably with that of natural stocks.
In India, with its extensive estuarine waters in Kerala, Bengal and Madras, mullet have been farmed from very ancient times. Research in intensive culture began in the 1920s when rearing experiments with young mullet were conducted in Madras at the Fisheries Department farm at Ippur (Campbell, 1921; Hornell, 1922) and at the Chingulpet Fort moat fish farm (Gravely, 1929). In the 1940s two further aspects were emphasized namely studying the feasibility of acclimating mullet juveniles to fresh water, and developing practices in polyculture. The first acclimation experiments were undertaken in Madras with Mugil troschelli and Mugil wagiensis (Devanesan & Chacko, 1943; Job & Chacko, 1947). This was followed by acclimatization studies of Mugil parsia in Bengal (Mookerjee, Ganguly & Sircar, 1946) and of M. cephalus and Mugil seheli in Madras (Ganapati & Alikunhi, 1949).
Several other reports on existing farming practices with their problems were published at the time. Improvements for the brackish-water farming of mullet in the Ganges delta were suggested (Hora & Nair, 1944). Basu (1946) advanced the adaptation of Chinese and Philippine practices for Bengal farms, and Pillay (1947) published his extensive examination of culture in Bengal, Madras and Kerala. The acclimation experiments led Panikkar (1951) to suggest that temperature and salinity tolerance of the mullet species be studied in detail, but little appeared in the literature until the report of Mohanty (1973). He recorded that juveniles of M. cephalus acclimated to fresh water were more tolerant of changes in conditions and that tolerance varied with length.
The most significant development in India has been the undertaking recently of the artificial propagation studies at Kerala. Ovulation but not fertilization was achieved with M. cephalus (Sebastian & Nair, 1973). However successful spawning and larval rearing were achieved by Sebastian and Nair (1974) with Mugil macrolepis.
Although the pond culture of Mugilidae is not practised on a large scale in Korea, the grey mullet is one of the important food fishes in its south-west region. One instance of research deserves mention. Yang & Kim (1962) described an experiment to obtain and hatch eggs of the grey mullet and to rear the larvae, but the work does not seem to have been continued.
In Taiwan, at about the same time, the knowledge gained from centuries of pond culture was utilized to begin an intensive programme on mullet culture. About 39% of the commercial catch of M.cephalus consisted of pond reared fish, cultured in combination with carp in fresh-water ponds and with milkfish in brackish-water ponds. Tang (1964) estimated that ten million juveniles were required to support the pond culture of mullet each year. He pioneered work on the induced spawning of M.cephalus as he knew that these resources were not infinite.
In 1966 a comprehensive research programme was established at the Taiwan Fishery Research Institute for intensive culture and mass propagation of juveniles. In subsequent years the techniques of induced spawning of mature fish caught during their seasonal migration were refined and improved, and survival of the larvae extended to thirty days. In 1973 the larval survival had increased to 19.35% and the production of juveniles had been established. A summary of a decade of work at the Institute has been made by Liao (1974).
In the United States the earliest mention of mullet farming was made by the US Fish and Wildlife Service (Anon., 1940) and attributed to Prytherch. A general article by Sharpe (1945) about the same farm quoted a yield of 5000 lb of fish per acre. Hiatt (1944) obtained information on the role of mullet in the food cycle of Hawaiian fish ponds. Lunz (1951) described preliminary experiments of mullet culture in brackish-water ponds in South Carolina. In Florida an experiment to raise the pompano, Trachinotus carolinus, inadvertently resulted in a yield of which Mugil curema and M.cephalus constituted the majority of fish (Johnson, 1954).
At present, experimental work with Mugilidae is centred in Hawaii, Texas and Louisiana. In Hawaii, the Oceanic Foundation successfully achieved artificial spawning of M.cephalus following induced breeding of captive broodstock (Shehadeh & Ellis, 1970; Shehadeh, Kuo & Milisen, 1973b). Larval rearing efforts met with mixed success and individual survival figures (up to 25.5%) could not be guaranteed (Kuo, Shehadeh & Milisen, 1973a; Nash, Kuo & McConnell, 1974). In Texas mullet were raised successfully in ponds receiving the heated effluent from a power plant (Linder, Strawn & Luebke, 1974), and in Louisiana acclimatization experiments were conducted with mullet juveniles (Shireman, 1974).
From this brief historical survey it is evident that the direction of the experimental research and development for the culture of the Mugilidae has of necessity, been toward induced spawning and larval rearing. For centuries the farming of mullet as with other species has depended on natural resources for the annual replenishment of stock. However the annual spawning migrations of the adults and the strength of the year-classes are all subject to environmental biological factors about which there is little agreement on importance. Present evidence indicates that these factors differ with locality region and even between populations within the species. The natural availability of juveniles is therefore subject to unpredictable variations in occurrence and abundance. More importantly the juveniles are collected in those coastal and estuarine waters where pollution is threatening or in some cases has already changed the ecosystem. The future availability of resources in such waters is becoming increasingly uncertain. Finally, improvement in fishing techniques, particularly for schooling fish on their spawning migration, is affecting the natural population and hence their progeny.
The literature is full reasons for the depletion of natural populations and weakening year-classes. Yet there is an anomaly for the proponents of culture. Commercial fishermen consider that too many juveniles are removed by pond fishermen thus depleting the resources of the natural fishery. Consequently limits have been imposed on the number of juveniles of certain species which can be removed for intensive culture.
The future of aquaculture is entirely dependent on the success of artificial propagation and larval rearing. Juvenile resources have to be independent of the natural population. Once this is achieved, then the benefits of genetics and selective breeding will make aquaculture the parallel of intensive agriculture with its fast growing strains and specialized breeds. When this occurs, the Mugilidae will really have an advantage over all other species because of the number of related types which can be used to develop particular farming strains for almost any region or specific location.
The need for an intensive effort in mass propagation has been summarized by Oren (1971). He said ‘In order to achieve maximum production of mullets under controlled conditions, one of the axioms is a steady supply of fry independent of conditions in the natural environment, natural spawning and migration of fry inshore. Only when such a supply is available will the most economic and most efficient way of utilization of the mullet, as a consumer of the first trophic level, be channelled for the benefit of man.’
Adult broodstock
The success of an artificial propagation system for any plant or animal species depends primarily on the quality and quantity of broodstock resources. Large numbers of sexually mature individuals must be contained in good health under acceptable environmental conditions in order to reproduce and yield viable offspring. Some essential external conditions for broodstock fish are suitable water quality, a nutritious diet, a high standard of hygiene and limited physical disturbance. But of most importance the broodstock must be exposed to the correct environmental parameters which influence the physiological changes in the pituitary gland and stimulate the gonads to seasonal maturity.
The endocrine system of all vertebrates forms the main link between the reproduction organs and environmental regulators. Rhythmic regulators such as temperature and photoperiod, mediated through the central nervous system, initiate neurosecretions which in turn regulate the activities of the pituitary gland. As one of many target organs the gonads are influenced accordingly. The reproductive cycles are thus regulated intimately by the trophic hormones of the pituitary.
Many experiments with fish have used gonad development to interpret the effects of certain environmental regulators on the reproductive cycle. Among the factors concerned temperature and photoperiod are the two most important which initiate pituitary activity for fish in temperate and sub-temperate regions (Hoar, 1959). The relative importance of each varies with different species of teleosts. Photoperiod has been reported as the dominant factor influencing the reproductive cycle of Enneacanthus obesus, Notropis bifrenatus and Fundulus confluentus (Harrington, 1959),Gasterosteus aculeatus (Baggerman, 1957),Salvelinus fontinalis (Henderson, 1963) and Oryzias latipes (Yoshioka, 1962). Temperature has been shown as dominant for Phoxinus laevis (Bullough, 1940), Apeltes quadracus (Merriman & Schedl, 1941), Gambusia affinis (Medlen, 1951) and Couesius plumbeus (Ahsan, 1966).
Henderson (1963) concluded for Salvelinus fontinalis that the influence of an enviromental regulator varied with the stage of gonad maturation. The period most responsive to a regulator could also vary between males and females of the same species and the gametogenetic process may be independent of environmental regulators at certain stages of maturity.
The reproductive cycle of most vertebrates is under the dual control of an internal physiological rhythm and an external seasonal rhythm. The refractory period or resting stage of the reproductive cycle is considered to be the time during which the two rhythms coincide and reinforce each other. As the fish are exposed to changing environmental conditions such as photoperiod and temperature the external rhythm begins to dominate. Its influence on the reproductive processes is transmitted by changes in the quantity of gonadotropin released from the pituitary gland.
The physical containment and maintenance of adult grey mullet in the most suitable conditions for survival are not difficult problems. The fish readily become domesticated as testified by centuries of pond farming. They grow and are technically mature, although the final stages of gametogenesis are not completed and no incidence of total development and unassisted natural spawning in captivity has been recorded. At present these need the artificial stimulus of hormone injection to complete the breeding cycle.
In order to stimulate the desirable environmental conditions for gametogenesis of captive broodstock it is necessary to examine the conditions at locations where the grey mullet spawn in nature.
Natural spawning locations
The natural spawning locations of grey mullet species, which have been described or deduced by many workers throughout the world, do not indicate specific and similar environmental patterns. The overall picture is confused both by misidentification of generic types observed and the variety of facts on which the spawning record is being made. For example, many reports are based on the collection of adult fish with ripe gonads and usually loose eggs; others describe small numbers of eggs or larvae in plankton tows and make predictions about the spawning location based on tidal movement and stage of development of the samples. Some reports describe inshore schooling and then a migration offshore, presumed to be for spawning; others describe schooling inshore for spawning. The location of spawning grounds for the mullet can only be described as controversial.
Anderson (1958) reviewed the record of many American workers who suggested the time and place of spawning of Mugil cephalus along the South Atlantic and Gulf coasts of the United States. From the evidence of collection of larvae and the occurrence of juveniles on the coast from lower Florida to North Carolina, he believed that the striped or grey mullet spawned offshore over a broad area extending from about the 20 fathom line into the Gulf Stream. In contrast with the spawning of the silver mullet, Mugil curema, which began in early spring when water temperatures were rising over the continental shelf (Anderson, 1957), M. cephalus spawned during late fall or winter when water temperatures were falling. Breder (1940) observed and described an aggregation of adult fish in the shallow creeks of the Florida coast and believed that spawning was taking place. Although he collected no eggs, his detailed description of the movements of the males around the females compares exactly with the observed spawning behaviour of mullet in aquaria, and it must be assumed that the fish were attempting to spawn.
Broadhead (1953), Dekhnik (1953) and Arnold & Thompson (1958) provided authenticated instances of M. cephalus spawning at sea in surface waters, but over deep water (50 fathoms in the Black Sea, and 750 fathoms in the Gulk of Mexico). Fitch (1972) described the capture of a ripe female over 40 miles off the Baja California coast. Other more unusual spawning places have been suggested. Roughley (1916) believed spawning to occur in fresh water. Smith (1935), Breder (1940), Jacob & Krishnamurthi (1948) believed it took place in estuaries and tidal creeks, and Kesteven (1953) in the coastal surf zone of Australian waters.
Demir (1971) reviewed the information available on spawning of grey mullets in the North Atlantic. The spawning of Mugil labrosus was assumed to be during April in British waters, as schools of adults were observed offshore and the juveniles entered tidal pools on the Channel coast and in Southern Ireland in July and August. Kennedy & Fitzmaurice (1969) believed the spawning period to last several weeks in Irish waters with May as the peak spawning month. Hickling (1970), in his contribution to the natural history of the English grey mullets, noted the spawning period of Crenimugil labrosus from January to April and the one of its spawning locations was near the Isles of Scilly. Two ripening Liza ramada he found in autumn indicated a late spawning season. He did not find any Liza aurata with active gonads. Le Dantec (1955) noted that genital activity for C. labrosus lasted from January through until April for fish in the Biscay region.
In the Mediterranean, Caspian and the Black Seas, the spawning periods again varied from region to region. Yashouv & Berner-Samsonov (1970) produced an extensive review of the spawning seasons of five species of Mugilidae in the Black Sea and the Mediterranean. Hamis (1972) stated that the spawning areas of certain species of mullet were those places where the optimum conditions for the young were available. In the Black Sea, Mugil saliens spawned from late June to October, M. cephalus spawned from late May to late October and M. auratus spawned from early June to early November. In the Mediterranean the spawning of M. saliens took place between May and Octomber, M. cephalus from early May to September, M. auratus from early September to late December, L. ramada from early October to late December, and M. labrosus from early December to early April.
Avanesov (1972) established that spawning of the grey mullet in the Caspian Sea occurred in a wide area beginning in the southern part at the end of May and extending northward. He recorded the most intensive spawning in the Turkmenian waters in the south in August, at a temperature of 25–29°C and about seven miles offshore where the depth was 5–40 m. Spawning of the long-finned mullet he noted occurred later at a considerable distance offshore where depths were over 400 m.
Wimpenny & Faouzi (1935) first recorded the shoaling migrations of M. cephalus and M. capito during the spawning period. The schools moving from the Delta Lakes of Egypt to the sea were entirely composed of spawning fish. Thong (1969) observed migrations of Mugil auratus, M. labrosus and M. ramada during egg laying from the coastal regions of NW. France into the open sea.
In the pacific and Indian Oceans little information on spawning of Mugilidae is available except for M. cephalus which spawns in late winter in the tropics and subtropics. Some summer spawners were recorded by Sarojini (1958). In tropical Bengal waters Liza parsia spawned between January and March, and Liza cunnesius from May onward and in the time of the monsoon. Kurian (1974) recorded the maturity of mullet in the ponds and lagoons of the Indian coast. He noted the different types of spawning migrations of M. cephalus, M. tade and Liza marcrolepis, but all were seaward or near to the estuaries.
Wallace (1974) recorded the distribution of M. cephalus in the high saline lake system of St. Lucia on the east coast of South Africa. He observed two seaward migrations in response to the changing hydrological and topographic features of the water system. No spawning was recorded in the lakes. Cervigón & Padrón (1974) observed M. curema in the high saline lagoons along the coast of Venezuela. They concluded that no spawning occurred in the lagoons but the adults migrated to the open sea and possibly for two spawning periods a year. Table 8.1 summarizes the spawning seasons of Mugilidae.
The records deducing the spawning locations from the numbers of eggs and larvae in plankton samples make no reference to the time of day at which spawning might occur. The observations of Deknik (1953) and Arnold & Thompson (1958) indicated that M. cephalus spawned at night at the water surface but over considerable depth. Anderson (1957) reported night spawning of M. curema
Although much reference is made to water depth and location of spawning sites offshore, no workers report that the fish themselves descend deep to spawn. Hotta (1955) recorded very small larvae of M. cephalus in plankton tows from 100 fathoms deep near Japan, and Zviagina (1961) found eggs of Liza haematocheila deep in Peter the Great Bay.
The incidences of mullet eggs and larvae being taken in plankton tows are few. Demir (1971) recorded the occurrence of postlarvae of M. auratus and M. labrosus in British waters for the first time. Previously the eggs and larval stages of Mugil labrosus had only been recorded once by Sanzo (1936), and the eggs only of Mugil auratus by Sanzo (1931) and Vodyanitskii & Kazanova (1954). Although depth may not be important for the adults at spawning it may be important for incubation and larval development (pp. 281 and 289).
From histological examination of adults stenger (1959) believed that M. cephalus spawned more than once a year. Bromhall (1954), on the evidence of the size and distribution of the juveniles, concluded that there were two periods of spawning in the vicinity of Hong Kong with a lunar period apart. However it is generally assumed under normal circumstances that most grey mullet species produce only one brood of eggs each year, and that some females only spawn in alternate years after their first maturity (Thomson, 1955).
Table 8.1. Spawning seasons of Mugilidae according to the literature
| Apr. | May | June | July | Aug. | Sept. | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. | |
| Mugil saliens | Israel | |||||||||||
| Tunisia | ||||||||||||
| Black Sea | ||||||||||||
| Venetian lagoons | ||||||||||||
| Mugil chelo | Israel | |||||||||||
| Tunisia | ||||||||||||
| Creni-mugil labrosus | British waters | British waters | ||||||||||
| Irish waters | ||||||||||||
| Biscay region | Biscay region | |||||||||||
| Mugil labrosus | NW France | NW France | ||||||||||
| N Adriatic | ||||||||||||
| Mugil auratus | Israel | |||||||||||
| Tunisia | ||||||||||||
| Black sea | ||||||||||||
| Messina | ||||||||||||
| Castellón | ||||||||||||
| NW France | ||||||||||||
| Mugil capito | Israel | |||||||||||
| Tunisia | ||||||||||||
| NW France | ||||||||||||
| Mugil cephalus | Gulf of Mexico | |||||||||||
| Formosa | ||||||||||||
| W Florida | ||||||||||||
| E Florida N Carolina | ||||||||||||
| Israel | ||||||||||||
| Corsica | ||||||||||||
| Tunisia | ||||||||||||
| Egypt | ||||||||||||
| Black Sea | ||||||||||||
| Australia (winter) | ||||||||||||
| N Adriatic | ||||||||||||
| SW India | ||||||||||||
| Hawaii | ||||||||||||
The references consulted were: Australia, Thomson (1963): Biscay region, Le Dantec (1955), Black Sea, Hamis (1972); British waters, Hickling (1970): Castellon, Belloc (1938): Corsica. Belloc (1938): Egypt, Paget (1923): Florida east coast North Carolina. Anderson (1958): Florida west coast, Broadhead (1953): Formosa, Tang (1964); Gulf of Mexico, Arnold & Thompson Yashouv & Berner-Samsonov (1970); Messina, Sanzo (1931); N Adriatic, Sanzo (1936). NW France, Thong (1969); SW India, Kurian (1974); Tunisia Heldt (1948); Venetian lagoons, Gandolfi & Orsini (1970).
However it is generally assumed under normal circumstances that most grey mullet species produce only brood of eggs each year, and that some females only spawn in alternate years after their first maturity (Thomson, 1955).
In summary, from the extensive and conflicting data and the possible misidentification by the observers, it is not possible to define a pattern in the environmental conditions necessary for successful natural spawning. Hamis (1972) was probably close to the truth when he stated that the spawning areas of certain species of mullet were those places where the optimum conditions for the young were available. More importantly, he should have included the optimum conditions for the incubation of eggs.
Photoperiod and temperature rhythms time the onset of gametogenesis and these rhythms, which differ from region to region throughtout the world, influence the indigenous population of mullet species. Existing data on the influence of salinity, temperature and dissolved oxygen on the viability of the eggs of M. cephalus (see pp. 282–3) show that incubation and development occur to some degree within a wide range of these parameters. The results also show that there are optimum conditions for both.
Environmental data on the temperature of the water during spawning of M. cephalus indicates some adaptation from region to region, with temperatures recorded between 12 and 24 °C. All records show a strong preference by the fish for oceanic water as the medium for incubation, with salinities of 32–35 ‰. It is interesting to note the observations of Wallace (1974) and Cervigón & Padrón (1974) for fish which lived in hypersaline conditions but which migrated to normal oceanic salinities to spawn.
Kuo, Nash & Shehadeh, (1974b) demonstrated that environmental manipulation of photoperiod and temperature increased the individual spawning frequency of adults, and that the spawning season could be prolonged throughout the year. The manipulations were all made in sea water of salinity 32‰. Their evidence indicated that the spawning behaviour of the grey mullet species was not a strictly controlled and regulated act. In fact the evidence pointed to a loosely controlled behavioral response. Therefore the many controversial and apparently misleading observations on the spawning period and location will be authentic for that respective population at that specific time of year and in that particular region.
It is concluded that the adults move to spawn in the nearest location which will provide the eggs and larvae with the highest chances for survival. specifically to oceanic water of salinity of 32–35‰ but up to 39.5‰ for the eastern Mediterranean and 41‰ for the northern Red Sea have been recorded. The distance of the migration may be short or long, depending on the local conditions of topography and tidal movements. For example, in Hawaii where there is no strong tidal movement but a coastal gyre, the adults move offshore beyond the coastal reef a distance of one or two miles; in Australia the fish move upcurrent many miles on both the west and east coasts before spawning, so that the long natural drift will return the juveniles back to the home estuaries several weeks later. Depth does not appear to be a vital factor for survival. Although the eggs have a natural buoyancy and are capable of surviving great pressures and can be found in deep water, the records do show that eggs and larvae develop predominantly in the upper ocean layers.
Artificial spawning conditions
No record has been made of the Mugilidae spawning unaided in captivity or in artificial conditions. Successful fertilization of hand stripped eggs and milt from adults caught at sea has been claimed by Sanap (1936) and Bollow (1938). Other successes have been reported with fish matured in large ponds. The chances for this practice to become the base of an artificial propagation unit are as yet too small to be considered further. Reliable results have been obtained from fish matured in captivity or captured at sea but induced to complete gametogenesis and spawn by the injection of hormones.
Suitable artificial conditions for holding the broodstock of grey mullet can only be described as those enclosures which permit the female fish to develop their oocytes beyond the tertiary yolk globule stage (stage III as described by Kuo et al., 1974b) and the males to complete spermiogenesis. Without hormone stimulation females will not mature to the ripe stage (stage IV) prior to ovulation, but will undergo atresia (stage V) and degenerate.
The first report on the induced spawning of Mugil cephalus reared in captivity in fresh water ponds was made by Yashouv (1969). Liao (1974) summarized the work in Taiwan between 1963 and 1973 on the propagation of M. cephalus, using spawning fish collected from the sea and from fish contained in large salt-water ponds. For the broodstock the workers in Taiwan relied on strong uninjured male and female specimens selected from the catches of commercial fishermen during the spawning run along the southwest coast of Taiwan. The selected fish were placed in strong plastic bags filled with sea water and inflated with oxygen. Most of the mullet caught were of the IV-year class and measured about 32–50 cm in length and weighed 1.0–2.1 kg each. The specimens were all sexually mature with well developed gonads, but the eggs of the females were never fully ripe for natural spawning.
Pond stock were maintained in Taiwan in fresh water at first, with sea water added slowly over a three-month period prior to spawning. The fish were fed a special diet and also injected periodically with mullet pituitary glands and Synahorin. Pond reared fish were easier to spawn than wild stock as they were more docile and free from injury. Large tanks specially constructed for holding broodstock were made of concrete and measured 5 × 7 × 1.5 m deep Sea water was circulated through each and all were aerated continuously
Shehadeh, Kuo & Nash (1973c) established broodstocks of M. cephalus in small, rubber-lined dirt ponds supplied with circulating sea water. In addition, the ponds contained a substrate of weighted polyethylene strips which increased the internal surface area and provided a stable supply of benthic diatoms, blue-green and filamentous algae. Three-year old fish survived readily in such conditions and matured the following year. They were then successfully induced to spawn by hormone injection. The small ponds were excavated and lined with butyl rubber sheet (1 mm thick) and had a volume of 26 m3. A food supplement was provided in addition to the available natural growth.
Sebastian & Nair (1974) reported on the collection and holding of Mugil macrolepis in preparation for spawning. Mature fish were taken from local brackish waters in Chinese dip-nets and transported to small concrete tanks, 170 × 95 × 70 cm deep. The gravid female fish were 13–23 cm in length and 40–130 g in weight. Water in the tanks was changed intermittently
Kuo et al. (1974b) described at length the holding of M. cephalus subjected to environmental manipulations of temperature and photoperiod. The time of onset of vitellogenesis was determined to be about eight weeks after exposure to a short photoperiod regime (6 L/18 D) at temperatures ranging from 17 to 26 °C. The response of oocyte development to the retarded photoperiod regime was consistent and unrelated to any other preconditioning of photoperiod changes, including a simulated natural light cycle. The data also indicated that development of the oocytes was accelerated by constant exposure to a temperature of 17 °C and a 6 L/18 D photoperiod, but that it was not completed as only limited yolk deposition occurred in the tertiary stage.
For M. cephalus, it is essential to maintain broodstock prior to spawning in full saline conditions (32–35‰ and up to 39‰ for the eastern Mediterranean). There is also evidence that the fish mature readily even after prolonged periods in captivity. Yashouv (1969) reported some success working with M. cephalus in fresh water, but the work of Hines & Yashouv (1971) on the increased activity of the spermatozoa of M. capito in sea water together with the practical experience of others working with M. cephalus indicate a preferential use of sea water of holding broodstock.
Ambient temperature and photoperiod conditions regulate the normal seasonal maturation of captive stocks. But the feasibility of breeding throughout the year or extending the breeding season by manipulation of the photoperiod and temperature regimes has been proved and will produce greater use and increased efficiency of propagation facilities.
Spawning behaviour and fertilization
Breder (1940) and Arnold & Thompson (1958) made some of the first observations on the spawning behaviour of Mugil cephalus in nature. They observed large numbers of fish schooling but scattered into small groups, generally made up of one large female and a varying number of smaller and more active males. The groups remained close together as if attached.
The induced spawning techniques developed by Shehadeh & Ellis (1970) and Shehadeh et al. (1973b) permitted natural spawning behaviour following injection of regulated doses of purified salmon pituitary gonadotropin. The final courtship and spawning behaviour described by them was similar to that observed by the others many years before.
As a consequence, for the final stages of the present induced breeding techniques with natural spawning, two or three males are placed in an aquarium with each recipient female about 2 h before spawning. A female will usually spawn some 12 h after receiving the second and last injection. The males become more active as hydration in the female progresses, indicated by distension of the belly and frequent excretion of calcium deposits.
Spawning is heralded by a violent quivering of the males which are then lying parallel to and facing the same way as the female and touching. The first release of a small number of ripe eggs stimulates the males to liberate spermatozoa. The female then responds with an explosive and continuous release of eggs.
Although male fish were one given exogenous hormone treatment to finalize maturity the practice was found unnecessary for spawning in the natural breeding season. If males are needed for spawning out of season then spermiation can be induced readily by the injection of 17-alpha methyltestosterone (Shehadeh, Madden & Dohl, 1972).
The effectiveness of the hypophysation technique for spawning depends ultimately on the selection of suitable recipient fish at the proper stage of ovarian development. For species of fish which undergo normal gonad development but fail to spawn in captivity, identification of this stage is critical.
To date, selection of recipients has been largely subjective. External anatomical characteristics have been described and used e.g. depth and fullness of belly, colour and state of swelling of the cloaca, softness and resilience of the belly, roughness of pectoral fins or presence of head tubercules. More complex descriptions include the microscopic appearance of oocytes (Sundararaj & Goswami, 1969), histological structures of eggs (Chen, Chow & Sim, 1969), or other histological data. Other physiological parameters associated with sexual maturity, such as elevated plasma proteins and calcium concentration, have been used but are of little practical use. Shehadeh, Kuo & Milisen (1973a) described a method for the assessment of ovarian maturity in vivo, which was accurate and reliable and could replace all subjective methods for Mugilidae.
Kuo, Nash & Shehadeh (1974a) described standard procedures developed and applied regularly to induce spawning of M. cephalus under controlled conditions. Methods for determination of the stages of egg development and required dosage of partially purified salmon gonadotropin (SG-100) for spawning were illustrated and emphasized so that the procedures could be readily used by other culturists. The potency of the gonadotropin was described by Donaldson, Yamazaki, Dye & Philleo (1972) as 1 mg equivalent to 2150 IU human chorionic gonadotropin (HCG).
Liao, Lu, Huang & Lin (1971), from experience gained spawning M. cephalus freshly taken from coastal waters, recommended a first injection within 1 h after stocking in the tanks and again within 24 h. The total dosage for each fish was 2.75–5 mullet pituitary glands mixed with 20–50 Rabbit Units of Synahorin. Vitamin E was also injected into the fish. The fish were later stripped and the eggs fertilized by the dry method after careful observation for the correct spawning time. The best response was judged by distension of the belly after about 10 h, a loose and soft belly, and finally the release of ‘water eggs’ particularly when the spawner was handled.
Anatomical characteristics are not always the most reliable indication of maturity, particularly for fish held captive all year round. Many females with soft and enlarged bellies can be in the early stage of oocyte development even during the natural breeding season. Enlarged abdomens are often the result of engorged intestines and accumulation of visceral fat.
Ovarian maturity, that is the stage of development of intra-ovarian oocytes, is most accurately obtained by the method of Shehadeh et al. (1973a). Intra-ovarian oocytes are removed in vivo from an unanaesthetized female through a polyethylene cannula. The cannula is inserted into the oviduct for a distance of 6–7 cm from the cloaca, and oocytes sucked orally into the tube by the operator as the cannula is withdrawn. Oocyte samples from the mid-portion of the ovary are the most representative and sampling error is minimized by avoiding the extremities.
The oocytes are removed from the cannula and washed and preserved in a solution of 1% formalin in 0.6% sodium chloride solution. They are then placed on a small ‘Plexiglas’ plate and measured with an ocular micrometer. Fine grooves cut in the plate align the oocytes and facilitate measurement. Egg diameters are measured along the horizontal axis and the measurements grouped into 50 μm class intervals. The sexual maturity of the fish is expressed in terms of mean egg diameter. calculated from the egg diameter frequency distribution.
The oocytes of M. cephalus develop in synchrony. Ovarian development therefore is determined accurately and quickly without sacrificing female fish. The method also provides a means to observe and record oocyte development in individual fish and thus precludes variation between females in the broodstock. Furthermore it replaces the need for any histological processing and examination of oocytes.
Eggs
Fecundity
Thomson (1963) reviewed a number of papers which reported the fecundity of Mugil cephalus in terms of total egg numbers. He quoted estimates of 1.2–2.8 million eggs per fish. Sebastian & Nair (1974) estimated the fecundity of Mugil marcrolepis 1.2–4.0 million eggs per fish depending on size. Hickling (1970) listed the facundity of several species of grey mullet as follwos:
| Species | No. of eggs (thousands/kg) | Reference | ||
| Mugil cunnensis | 15–57 | After Sarojini(1958) | ||
| Aldrachetta forsteri | 126–650 | Thomson (1957) | ||
| Mugil parsia | 200–600 | Sarojini (1957) | ||
| Crenimugil labrosus | 372–745 | Hickling (1970) | ||
| Liza ramada | 581–1243 | Hickling (1970) | ||
| Mugil cephalüs | 1200–2800 | Thomson (1963) | ||
| Mugil cephalus | 3600–7200 | Nikolskii (1954) |
Kuo, Shehadeh & Milisen (1973a) determined the fecundity of M. cephalus to be about 648 eggs/g body weight of three-year old fish. Nash et al. (1974) quoted 849 eggs/g for older individuals. Liao, Cheng, Tseng, Lim, Hsieh & Chen (1972) reported 0.7–1.9 million/fish.
In Hawaii and Taiwan, where induced spawning of M. cephalus is regularly performed, IV-group females weighing between 1 and 2 kg each are preferred for breeding. Consequently over 1 million eggs per female fish are released for fertilization following the induced hormone treatment.
Morphology and quality
The morphology of eggs before fertilization was described for several of the Mugilidae by the early naturalists, most of whom made reference to the characteristic large oil globule. The availability of eggs at all stages of development during the induced breeding procedure has resulted in full descriptions for Mugil cephalus by Sanzo (1930), Tang (1964), Yashouv (1969), Liao et al. (1971), Kuo et al. (1973a) and Tung (1973): by Sanzo for M. chelo (1936) and M. labeo (1937): by Anderson (1957) for M. curema by Perceva-Osroumova (1951) and Dekhnik (1954) for M. saliens
Eggs of the Mugilidae are spherical and transparent. The surface of the egg shell is smooth and unsculptured. The yolk appears unsegmented and there is predominantly one large oil globule making the eggs extremely buoyant. The eggs are not adhesive
Eggs from M. cephalus and M. capito were observed by Yashouv & Berner-Samsonov (1970) to have more than one oil globule and these subsequently developed and hatched. During development they observed the droplets to merge. On hatching the larvae had one oil globule (rarely two) located in the yolk sac.
Sanzo (1936) described eggs of M. chelo with one large and several smaller globules, and Perceva-Ostroumova (1951) noted the same for M. saliens. Kuo et al. (1973a) stated that the frequency of multiple oil droplets in eggs of M. cephalus increased with the manual pressure of artificial stripping. Spontaneous release of the eggs by the females produced eggs with a single oil globule and Nash et al. (1974) considered that to be normal and desirable. Although the small oil globules were observed to coalesce during development the survival of eggs which initially contained multiple oil droplets was always low.
Yashouv & Berner-Samsonov (1970), in an extensive contribution to the knowledge of eggs and early larval stages of Mugilidae, reviewed data of egg and oil globule diameters in samples from Mugil saliens, M. cephalus M. capito, M. auratus and M. chelo at a variety of locations. They included some data from the synopsis on M. cephalus prepared by Thomson (1963). The comprehensive data revealed a wide range of diameters reported for the same species in different locations.
Kuo et al. (1973a) reported the mean egg diameter of fertilized eggs of M. cephalus as 930 μm, with a range of 880–980 μm. The single large oil globule had a uniform diameter of 330 μm. Tung (1973) quoted a mean egg diameter of 0.89 mm for the same species, and oil globule diameter of 0.39 mm. Nash et al. (1974) specified a mean egg diameter of 0.93 mm.
A question posed by many workers culturing either Mugilidae or other species by induced breeding is whether the quality of individual oocytes is inferior to those produced in nature. Induced breeding does accelerate final development and therefore the egg and subsequent embryo may be deficient in certain biochemical constituents necessary for total development.
Bromhall (1954). Sarojini (1958), Erman (1961) and Hickling (1970) examined the gonads of several species of mullet including M. cephalus and observed that their state of development was often consistent with a seasonal production of more than one batch of eggs, possibly within a period of a month. Kuo, Shehadeh & Nash (1973b) demonstrated that M. cephalus could be induced to spawn more than once a year. It is possible that artifically induced spawning, with its positive climax and total release of oocytes from the ovary, may be an unnatural forced reaction impeding complete development of the embryonic stages.
Little work has been accomplished on the biochemical composition of the oocytes before and after spawning. Kuo (unpublished data) examined changes in the biochemistry of eggs of M. cephalus during hydration and changes in mean egg diameter, water content and osmolarity. He showed that both soluble (glucose) and insoluble (glycogen) carbohydrates gradually decreased through hydration with a distinct drop prior to spawning. Total lipids increased from 16 to 25%. The major polar lipid was lethicin with decreasing amounts of phosphatidyl/ethanolamine and lysolecithin, plus other unidentified trace components. The main nonpolar lipids were cholesterylesters and triglycerides. The proportion of free fatty acids was small. Palmitoleic acid (C16:1), palmitic acid (C16:0) and oleic acid (C18:1) were the major fatty acids, with decreasing amounts of myristic acid (C18:0), linoleic acid (C18:2) and some higher fatty acids containing 20, 22 and 24 carbon atoms. He recorded a conspicuous increase in the amount of soluble nitrogen-containing ninhydrin compounds. There was a general increase in amino acids particularly the neutral ones such as alamine, serine, leucine and isoleucine. There was some change in the level of total protein but less pronounced than that of the soluble nitrogen containing compounds.
Kuo (unpublished) also showed the pre-ovulatory eggs were isotonic with sea water and the ovulatory eggs became hypotonic due to the rapid intake of external water during hydration. In the 4 h postovulatory period a remarkable increase in the osmolarity adjusted the osmotic pressure from hypotonic to isotonic to sea water at the time of spawning. A change of the main individual electrolytes sodium, potassium, calcium and magnesium ions was found variable due to the increase in water content. No comparable data exist for the analysis of eggs from M. cephalus developing naturally without induced treatment.
Although the need to identify the biochemical composition of normal eggs of Mugilidae still exists, artificial propagation of certain species following induced breeding has been reasonably successful in Taiwan, Hawaii, lsrael and India. It is improbable that the quality of eggs produced by external hormone stimulation is inferior. The failure of the larvae to survive in large numbers is probably due to subsequent mishandling because of poor techniques.
Behaviour
The vertical migratory behaviour of the eggs of Mugilidae has been variously described. Sanzo (1936) observed that all fertile eggs of Mugil chelo sank soon after fertilization, while Yashouv (1969) reported sinking towards the end of incubation for eggs of M. cephalus. Kuo et al. (1973a) recorded that the majority of eggs of M. cephalus which sank within the first twelve hours were undeveloped or unfertilized (absence of perivitelline space). They noted that when eggs were prevented from settling by strong aeration in small vessels survival was increased, and the incubation period reduced by two hours at the same temperature. They concluded that water temperature and turbulence had a decided effect on incubation time, and this was supported by Tung (1973)
Tang (1964) noted that eggs of M. cephalus sank in standing water and so incubated them in suspension by circulating water. Yashouv & Berner-Samsonov (1970) described the development of M. capito eggs floating on the surface. Eggs of M. cephalus lost their buoyancy after 20 h and sank, but hatched successfully. Sebastian & Nair (1974) observed that eggs of M. marcrolepis floated in shallow hatching trays, and Liao (1974) reported that the fertile eggs of M. cephalus were buoyant and floated near the surface. He maintained them in suspension during incubation by aeration as he noted that some did sink later. The rearing practices of Nash et al. (1974) attempted to maintain the fertilized eggs of M. cephalus in suspension by circulating water, particularly when incubating at a density of 250 eggs/l.
Although incubation is apparently continued for eggs of M. cephalus which sink below the surface the artificial environment of a container for hatching obviously introduces problems not normally encountered by eggs liberated in the sea. Density of eggs during incubation is a key factor influencing hatching rates and overcrowding produces problems of agglutination, bacterial contamination, oxygen depletion and increased metabolite production. Suspension of the eggs by water movement counters these problems, and few workers attempt to incubate fish eggs without the minimum of circulation or water movement through aeration, particularly when operating at high density.
Environmental conditions for incubation
Egg development and hatching time are both temperature dependent. Tang (1964) reported that hatching of M. cephalus took place in 59–64 h at temperatures ranging from 20.0 to 24.5 °C and salinity from 24.39 to 35.29‰. Fertilization was low (32%) and the rate of hatching was below 10%. Yashouv & Berner-Samsonov (1970) noted that under laboratory conditions the eggs of M. cephalus and M. capito developed and hatched within 36–44 h at 22–32 °C. Kuo et al. (1973a) stated that hatching of M. cephalus eggs was evident 36–38 h after fertilization at 24 °C, and 48–50 h at 22° C, Total length of the newly hatched larvae was 2.65±0.23 mm. Salinity was 32‰.
Liao (1974) stated that hatching of M. cephalus eggs took place in 34–38 h at 23–24 °C, and at 49–54 h in 22.5–23.7 °C, with salinities of 30.1–33.8‰. Tung (1973) described the relationships between mean water temperature (θ) and duration of incubation period (T) as Te0.133 θ = 1262 in still water, and as Te0.037 θ = 106 in running water. Finally, sebastian & Nair (1974) recorded the incubation time of M. marcrolepis as 23 h at 26–29°C and 29–31% salinity.
Nash et al. (1974). Nash & Sylvester (unpublished data) and Nash & Kuo (1975) report the survival of eggs of M. cephalus within broad ranges of temperature salinity and dissolved oxygen. Minimal mortalities of eggs occurred at 22 °C for normal sea water (32‰) and an effective temperature range for incubation was 11–24°C.
Most workers prefer a working temperature range of 18–24°C for M. cephalus. Above 25 °C incubation is inhibited although some eggs will hatch at 30 °C; the mortality beyond 25 °C is usually above 90% and often total. Optimal salinities for incubation are 30–32‰ under ambient temperature conditions (19.5–20.5 °C), and significant decreases in egg survival occur with eggs incubated in mean oxygen concentrations below 5.0 ppm.
Good temperature control during incubation is essential. Present work on the propagation of M. cephalus is conducted within the most desirable temperature range and mostly at the optimum level. Although individual daily temperature fluctuations may be responsible for some egg mortality, it is believed that temperature per se is not influencing the high mortalities experienced in the early stages of development. However the working range of 18–24 °C is suitable for rapid bacterial growth and therefore indirectly a causative factor of environmental instability during incubation.
Kuo (unpublished data) recorded the change in osmolarity of the eggs prior to spawning and that they were isotonic to sea water at the time of spawning. This fact together with the migratory behaviour of the adults at breeding time moving out into oceanic water indicate that salinity level is most important for egg development, and incubation should be conducted in full sea water.
Incubation facilities
Liao (1974) reported that fertilized eggs of Mugil cephalus prior to 1968 were incubated and hatched in two types of hatching equipment. The first was a flowing water type. It consisted of a fine mesh net hanging in the water and provided with a continuous slow exchange of water. It was similar in design to that used for hatching eggs of Chinese carp. The second was a static system of simple containers each with aerated sea water. After hatching the larvae were transferred to other rearing tanks. The systems were later modified to avoid loss of larvae during transfer. All development stages were then completed in either plastic or large concrete tanks indoors with good environmental control.
Kuo et al. (1973a) incubated eggs in well aerated static sea water (32‰) in 140 l fibreglass tanks. Incubation temperatures were 22–24°C. They worked on a series of improvements and with Nash et al. (1974) developed a modified circular kreisel for incubating eggs of M. cephalus. The advantages of the kreisel were that the eggs could be pretreated with antibiotics to reduce bacterial growth and the density of eggs was high for the size of the apparatus. Also separation of the emergent larvae from empty shells and inviable eggs could be made during transfer of the larvae to the rearing containers. This reduced potential fouling in the rearing tanks.
The kreisel has proved to be an effective rearing apparatus and can be constructed in various dimensions. Aeration and circulation of the water are maintained by an airlift pump located in the centre column. The water is drawn by the airlift pump into the column through a large filter stone, aerated and passed back down the outside of the pump and redirected into the container. A connection on one of the discharge arms of the column directs a fraction of the water to waste, and that volume is replaced by an incoming supply located above the column.
Nash et al. (1974) used ambient sea water (32‰) in the kreisel. Before use it was treated by filtering, irradiating and treating with antibiotics. Both penicillin (10 IU/ml) and streptomycin (0.01 mg/ml) were added daily and effectively reduced marine bacterial growths. The eggs were suspended in sea water until after hatching and the larvae safely transferred by siphon into larger prepared rearing containers.
Yashouv (1969) used specially designed incubators to prevent the washing out of eggs with the changing sea water. Sebastian & Nair (1974) used shallow trays similar to those used for the incubation of salmonid eggs.
Oppenheimer (1955) demonstrated bacterial growth on fish egg shells by photomicrographs and found that the percentage hatch of a number of marine species increased by controlling marine bacteria with a mixture of penicillin and streptomycin. Shelbourne (1964) credited the successful culture of marine flatfish to the use of antibiotics. Using controlled amounts of the two he demonstrated that the survival of young flatfish was significantly increased from 40% to 60% at metamorphosis.
The antibiotic treatment in incubation tanks reduces bacterial activity around the shell and prevents agglutination of the eggs. Physical damage to the eggs shell, or the shell with bacterial slime or mucus, breaks down the osmotic balance between the eggs and the surrounding sea water. The osmotic regulation between the two appears to be a key factor in successful propagation of marine and brackish water species, and needs a great deal of further attention and research.
Although the effectiveness of antibiotics can be replaced by ultraviolet sterilization and bacterial filters in the water system of a hatchery, the usefulness of antibiotic treatments cannot be underestimated (Struhsaker et al. 1973). Nash et al. (1974) utilized antibiotic washes and prolonged treatments during egg incubation. Buoyant viable eggs at the gastrula stage were removed from the spawning tank, washed under irradiated and filtered sea water, and then dipped for one minute in à sea water bath containing potassium penicillin G (80 IU/ml) and streptomycin sulphate (0.05 mg/ml). They were then distributed into the kreisels at a density of about 250 eggs/l and incubation was continued for the remainder of the period. Low levels of antibiotic were added daily and reduced all bacterial contamination.
Oppenheimer and Shelbourne theorized on the ways that bacterial activity affected and damaged eggs (Costlow, 1969). Penicillin is effective against Gram-positive organisms, and streptomycin against Gram-negative organisms. The combination therefore sterilizes sea water if there are no resistant organisms present. Antibiotics have also proved effective in the culture of shellfish (Walne, 1958). However they are known to unbalance the mechanisms for calcium transfer in some species and could not be used for the culture of Haliotis tuberculata (Nash, unpublished data). Although the work of Nash et al. (1974) did not demonstrate fully the need for antibiotic treatment in the culture of Mugil cephalus, the need for bacterial control is vital for successful incubation of many marine and brackish water species.
Little mention is made in the literature on construction materials used for incubation tanks. Shelbourne (1964) recommended the use of inert materials and the incubators used in the first flatfish hatchery were made of black polyethylene. The colour was important to provide a nonreflective surface against which the larvae could clearly see living food particles. Other workers mentioned the use of fine mesh net containers of terylene or nylon, but omitted to specify the materials of the larger container in which the net bag was suspended. Both cement and fibreglass incubators have been used after prolonged leaching of metallic ions or resins but plasticizers have proved damaging. A great deal of information is lacking on the suitability of certain constructional materials. Until good data are available most workers practise prolonged leaching of containers either in a heated atmosphere or submerged under water.
Larvae and larval culture
Hubbs (1943) defined the terminology for the young stages of fishes and separated prelarval, postlarval and juvenile stages on observed criteria. He considered that the postlarval stage began immediately on absorption of the yolk sac, and lasted as long as the structure and form were unlike that of the juvenile. The juvenile he considered to be the young stage similar to the adults in all essentials.
For most teleosts the formation of the scales signifies the end of the postlarval stage. The Mugilidae however develop scales in the early postlarval stages (at 8–10 mm in length) and are soon well developed (at 12–14 mm in length). Hubbs criteria do not therefore apply.
Roule (1917) divided the postlarval stage of Mugilidae into two successive periods. The first had rudimentary scales as the diagnostic criterion followed by the second stage with true scales. Anderson (1958) regarded the formation of the third spice of the anal fin as signifying completion of the postlarval stage and classed individuals as juveniles if the third anal ray had fused into a spine. Tung (1973) described five stages of larval development and morphology.
Young mullet which first appear in small schools along the coasts and in the estuaries are fully scaled. They measure 18–28 mm in length. Extrapolated growth data for the species indicate that young of this size are between 30 and 45 days old. The transition stage from postlarvae to juveniles used by Anderson (1958) does not occur until the young are 35–45 mm in length or 45–60 days old. Thomson (1963) regarded the transition complete at about 50 mm when the third anal spine formed from the anterior ray and the adipose eyelied started to form.
For the purpose of this text the artificial propagation of the mullet must include the culture of individuals to a stage of development when they can withstand relocation from hatchery to nursery pond. Heavy losses will occur if the young fish are mishandled or transferred too soon. This treatise considers young mullet up to 50 days old as the responsibility of the hatchery. They are technically larvae until that time. Young fish will be designated as juveniles when they are transferred from the hatchery to small ponds and they should be at least 50 days old. This arbitrary classification closely fits the morphological definition used by Anderson (1958). The use of the term ‘fry’ for young fish is mostly avoided in the text. It is commonly used to describe all the resources of postlarvae and juveniles collected along the coastlines and transferred to nursery ponds.
Morphology
Some of the first descriptions on the morphology of the larvae of Mugilidae were made by Sanzo. He observed and illustrated the early stages of Mugil chelo and Mugil cephalus (1936) and Mugil labeo (1937). He considered their prolarvae to be poorly developed. They measured only 2.2–2.5 mm in length. The mouths were closed and there were no traces of a branchial skeleton. Characteristic of the larvae were the voluminous yolk sac and large oil globule often accompanied by smaller oil droplets.
The first complete morphologic descriptions of larvae of Mugilidae were made by those workers involved in the induced spawning of the adults by hormone injection. Mostly they described the development of M. cephalus. Among them were Tang (1964), Yashouv(1969), Yashouv & Berner-Samsonov (1970), Liao et al. (1971), Kuo et al. (1973a) and Tung (1973). Sebastian & Nair (1974) described the development of Mugil macrolepis.
Newly hatched larvae of M. cephalus vary in length between 2.2 and 3.5 mm. The oil globule (and any additional small droplets) is situated in the posterior part of the yolk. Tung (1973) recorded 24 myotomes. He described the anterior half of the body bent on the yolk sac. The larvae had five or six pairs of cupulae on the body side, from eye to tail, and several pairs on the front of the head. Feeding began three to five days after hatching. Yashouv & Berner-Samsonov (1970) gave full descriptions of the keys to the eggs and larvae of five mullet species: M. cephalus, M. capito, M. saliens, M. chelo and M. auratus.
Thomson (1963) reviewed work on M. cephalus and quoted detail the embryonic development described by Sanzo (1936) and Anderson (1958) A full and accurate description of development and behaviour of M. cephalus was made by Liao (1974), and is reprinted in Table 8.2
Behaviour
Liao (1974) described the larvae of Mugil cephalus as having weak swimming activity with the posture of belly up and head down, sometimes moving with a jerky motion up and down. Kuo et al. (1973a) reported that newly hatched larvae were inactive and usually remained upside down suspended in the water column in an inclined position with the ventral side oriented towards the water surface. Occasionally each larva would go into a jerky motion and right us position. It would then dart upward rapidly then sink passively back to its resting position. They noted that sustained larval activity increased after the second day.
The presence of the oil globule influences the activity of the larvae during early development. Kuo et al. (1973a) dealt at length with the vertical distribution of the larvae and changes in specific gravity. They noted that larvae gained sustained swimming powers between the tenth and twelfth days after hatching and documented vertical distribution and measured specific gravity before that period. They observed that during the first two days the larvae were passively suspended in the water column and tended to become evenly distributed in depth at their specific gravity increased from 1.0263 g/ml at 12 h to 1.0339 g/ml at 36 h after hatching.
They described a vertical migration of the larvae between the second and third days. At the end of 60 h over 87% of the larvae came to rest on the bottom of the 80 cm experimental column, with the remainder suspended in the lower 50 cm of the column. After 72 h the pattern was reversed with over 85% of the larvae aggregated at the surface. During this migratory period the larval specific gravity decreased from 1.0310 g/ml at 60 h to 1.0264 g/ml at 96 h by which time all the larvae were back at the surface. The larvae remained at the surface until the sixth or seventh day.
Between the sixth and seventh day after hatching a second migration occurred. It was accompanied by a similar increase in the specific gravity of the larvae on the seventh day. Al the larvae were on the bottom of the tank on the eighth day, with a complete reversal on the ninth day. Conditions at the time were full sea water (32‰) and ambient temperature (24 °C).
Table 8.2 Development of mullet larvae and their behaviour (after Liao, 1974)
| Days after hatching | Total length (mm) | Development and behaviour |
| 1 | 2.56–3.52 | Newly hatched larvae had a large yolk and oil globule. The front part of notochord being curved along the yolk sac and the curve degree related to the duration of hatching. At low temperature, this duration was long and the curve more distinct. Weak swimming activity with the posture of the belly up and head down, sometimes with jerky motion slightly up and down. Pigmentation dependended on individual. Eye not coloured. Mouth not developed. Digestive tube not well-developed. |
| 2 | 2.64–3.28 | Formation of organs was in progress. More pigmentation was found in eye and body. The total length was shorter than before. Mouth was under development. Bud of pectoral fin appeared. Nostrils were obvious. |
| 3–4 | 3.11–3.53 | Opening of mouth. Good development of upper and lower jaws. lrregular peristalsis of stomach and intestines, able to take food. Yolk was diminished, being ¼ of original size. Oil globule was also reduced. It was the first critical period, always accompanied by serious numbers of deaths. Gill clefts appeared. Being attracted by and tending to concentrate at 600–1400 lux area. Distributed at upper level during night-time. |
| 5–7 | 3.06–3.40 | Digestive tube was well developed. Movement up and down individually both during day and night. Feeding was easy to observe but only limited in the day-time. Formation of abnormalities in the bladder and swelling of hyoid could be inhibited by freshening of rearing water. Formation of stomach, intestine, gall bladder, pancreas, gas bladder and continued reduction of oil globule. |
| × | 3.35–3.80 | Complete disappearance of oil globule. Formation of gill filament. This is the flexing point of the growth curve, with the growth starting to accelerate. |
| 10–13 | 3.45–5.10 | Finfold moved backward. Gill filament well developed. Body surface became dark in colour. Strong phototaxis. Formation of hypural bone. It was the the second critical period with very low survival rate. |
| 14–15 | 3.85–5.70 | Commencement of swimming in schools. Formation of urostyle. 7–9 ray bases were found in each of anal fin and second dorsal fin. Gill lamellae formed on gill filament. |
| 16–19 | 5.40–6.60 | Seventeen soft rays in caudal fin. Black spots scattered on the whole body. Shiny silver white complexion appeared from gill cover along ventral part of anus. Schooling in upper levels, sometimes into middle level. |
| 20–21 | 6.00–7.65 | Showing phototaxis during day-time while floating during night-time. Appearance of brown colour sometimes and silver green at other times, higher variety found in healthy larvae. Four soft rays on the first dorsal fin. |
| 22–24 | 8.25–10.9 | Formation of complete 20 soft rays in caudal fin, 11 rays in anal fin, 6 rays in pelvic fin, 15 rays in pectoral fin. Fin membrane of dorsal fin and pelvic fin almost entirely degenerated. Appearance of scales with 1–3 ridged circles. The size of largest scales reached 400 × 250 μm. Silver-white complexion. In day-time larvae swam in upper levels in schools and against aeration and stream. At night they floated and scattered on surface of water and liked to gather under the light. |
| 25–28 | 8.80–15.0 | All scales and fin rays were well formed. Silver-green complexion. Appearance of teeth. Two nostrils separated. |
| 29–32 | 16.6–20.7 | Very sensitive. Gathering in small schools. In day-time, swam in middle and lower levels at night-time, continued to be floating but easily startled. |
| 34–35 | 22.2–26.2 | In day-time larvae swam in large schools along the circle of rearing tank in the middle and lower levels. In night-time, floating individually. Grass-green in colour, sometimes silver-white on the dorsal part. Some diseased fish were found with ‘pop-eye’ symptom. |
| 37–40 | 23.1–29.3 | Some changes in feeding behaviour, feeding at late afternoon. Sensitive to light, and no longer gathering under light. |
| 45 | 27.5–32.8 | Strongly resistant to environment. Suitable for stocking. |
Kuo et al. (1973a) concluded that the first sinking was probably related to the rapid absorption of the yolk sac and the resulting change in specific gravity. Planktonic larvae are normally able to distribute themselves through the surface waters. This ability may be more pronounced in Mugilidae because of the continuous references to the spawning of the adults over deep water (see pp. 270–5) and the rarity of eggs and larvae taken in plankton tows.
The second descent could not be fully explained. Although the larvae were not capable of sustained swimming at the time, they were capable of swimming to the surface of the water column. Kuo et al. (1973a) could not account for the sudden and transient increase in specific gravity of the larvae on the seventh day. Morphological observations of histologic sections of the larvae revealed that the pneumatic duct of the air bladder was occluded between the sixth and seventh days, but the relevance of this was not understood. The migration was known not to be a phototropic response.
During the mass propagation of the larvae Kuo et al. (1973a) experienced heavy mortality at the critical stages associated with the two migratory periods. They attributed the mortality to mechanical and physical damage during prolonged contact with the bottom of the container as the larvae had no control over their ability to escape. The mortality was reduced slightly by using deeper containers (1.5 m) for rearing.
Nash et al. (1974) used a rearing kreisel and increased survival significantly during the first migration. The kreisel provided mechanical circulation but the larvae were protected from the damaging effects of strong aeration by a simple central device. Summarizing the migratory behaviour they believed that the larvae were responding first to changes in utilization of the yolk and sank to the bottom. After ascending the larvae had improved musculature control and lost the indiscriminate floating activity much earlier than previously stated.
The second vertical migration was associated again by Nash et al. (1974) with the changes in specific gravity of the larvae and suggested that two factors were concerned. The first was associated with the physiological and morphological changes, the second with nutrition. They observed that there were distinct differences between feeding and nonfeeding larvae as early as the seventh day after hatching. Established larvae were longer and more active, and were intensely pigmented. Unestablished or nonfeeding larvae remained in the surface water layers and used the increased surface tension along the sides of the container to support themselves. Development of the pigment was slow and little or no growth was evident. Such larvae died between day 7 and day 10 depending on the water temperature. Coincidental with the migratory movement of the larvae to the bottom, many undeveloped individuals sank because they were unable to sustain themselves further and were moribund. Hence the earlier association of heavy mortality with the migratory period. Developed larvae also had to undergo the changes associated with the migration which must be critical to further development. As with other larval forms undergoing metamorphosis the mullet larvae became inactive for a period and sank.
All workers now believe that those larvae which survive the second migration are capable of full development, providing that the conditions and food are suitable and if they are not mishandled. Established larvae after the second migration soon develop scales and then school together.