Mr. C. KITAJIMA
Red sea bream, or Madai in japanese, Pagrus major (TEMMINCK et. SCHLEGEL) is distributed from the coastal waters around Japan down to the East China Sea and Southeast Asian waters. It grows up to about one meter TL, and is one of the most important and expensive fish in Japan for its delicate flavour and elegant appearance, and often used in various festival occasions such as weddings, birthdays, new year parties and festivals.
The annual catch of red sea-bream in the coastal waters around Japan is about 20 000 ton, but recently its natural stock reveals a tendency to decline.
Culture of this fish using the wild fry started in around 1965 and soon became very popular in the southwestern waters of Japan. Since about 1975, the seed production of this species in hatchery has developed rapidly, and the number of fry produced at hatcheries in the whole country reached 40 millions in 1983 (Fig. 1). At present, the greater part of the seed for culture is taken up by hatchery-produced ones.
On the other hand, the experimental projects to stock with fry the coastal waters in order to promote the coastal fishery started in 1975.
At present, in Japan, in addition to 13 national hatcheries and 40 prefectural hatcheries, several scores of hatcheries are run by private sectors. The fry for stock is mainly produced in the governmental hatcheries, while the seed for culture in private hatcheries. The numbers of fry stocked and cultured in 1983 were 23 millions (at 39 hatcheries) and 16 millions (at 32 hatcheries), respectively. (Fig. 1 and 2)
Although the initial trial of seed production of this fish was carried out by KAJIYAMA and his coworkers in 1917, the full-scale studies started in the late 1950 s. Therefore, the history of fry production dates only 25 years back and many problems still remain to be resolved.
At the early stage in the development of culture techniques, eggs and sperm were taken from wild female and male by means of the so-called “stripping method”. Nowadays, the fertilized eggs are collected from a tank, in which parent fish spawn.
In captivity, this fish sexually matures in the second or third year of age. The biological minimum of female is about 30 cm in total length and 500 g in body weight (KITAJIMA,1978), but most suitable as parent fish are those of 1 to 3 kg (3 to 5 years).
The culturing methods of parent fish is similar to those of ordinal commercial culture ; they are put in a net cage attached to a raft in a bay and fed artificial feed fresh or frozen fish such as sardine, anchovy, horse mackerel and krill.
The spawning season of this species extends from April to July, when the water temperature ranges from 15 to 22° C.
The parent fish, which are ready to spawn, are transferred from a net cage to a spawning tank on land. A density of parent fish in a spawning tank is one fish per ton, with females and males in the same number. It is easy to determine the sex as a male becomes partly black in spawning season.
A female of 1 kg in body weight lays 50 000 to 100 000 eggs at about sunset everyday for 50 days or more. The total number spawned by a female of 1, 1,5 or 2 kg in a season reaches 3 000 000, 5 000 000 and 9 000 000 respectively (KITAJIMA, 1978) (Fig. 3, fig. 4).
The eggs are collected by a small-meshed net which has been fixed at an overflow outlet of the tank. We can estimate the number of eggs collected from the weight, one gram contains about 1 800 eggs.
Normal eggs are buoyant, while unfertilized and undeveloped eggs are settled like in incubation. So, the rate of buoyant eggs against the total number indicates roughly the quality of eggs spawned.
The weight of all the eggs spawned by a female for a season reaches 1.5 times its own body weight, accordingly it is necessary to feed sufficiently parent fish everyday.
Recent studies reveals a close relation between the nutrients of a diet for parent fish and the quality and quantity of eggs spawned. Further studies in this field is required (WATANABE et al., 1984 a, b, c ; 1985 a, b).
There are several trials so as to speed up or lengthen the spawning season by controlling the water temperature of the rearing tank.
Early growth of red sea bream is as following (KITAJIMA, 1978):
| Developmental stage | Age in day | Total length |
| Larval stage | ||
Prelarval stage | 0 -- 3 to 5 | 2.3 -- 3.0 mm |
Postlarval Stage | -- 25 | -- 10 |
| Juvenile stage | -- 60 | -- 40 |
The fish of late juvenile stage, 40 mm in size is suitable for both culture and restocking.
Larvae fish are planktonic and scatter all over the tank, and they are reared at as high a density as 20 000 to 30 000 per ton. At juvenile stage, they settle down to the bottom of the tank and their swimming ability increases remarkably. The inclination to bite one another and cannibalism also begin at this stage. Therefore, it is necessary to rear juveniles is a lower density and in a wider space area.
Larvae are mainly fed on live organisms such as rotifers and Artemia nauplii, while juveniles are generally given inert feed, such as artificial feed and minced fish meal, so the contamination of rearing water becomes often serious at the stage. Accordingly, it is generally adopted that larval fish are reared in a tank, and they are transferred to a net cage in the sea at juvenile stage of 12 to 13 mm in size.
The fertilized eggs are put into a rearing tank which contains 50 to 100 tons of seawater, at a rate of 20 000 to 30 000 eggs per ton. The eggs are incubated in this tank under gentle aeration with 10 to 15 airstones.
Hatching occurs in 50 hours at 18° C and in 40 hours at 20° C after spawning. The newly hatched larvae are about 2.3 mm TL and live on the yolk for 4 to 5 days at a temperature of 18° C.
During the first week or ten days after hatching, we do not change the water in a rearing tank. Thereafter, the filtered seawater is supplied to a tank. Towards the end of larval rearing, the water supplied is that of the capacity of the tank or a little less per day.
In the case of an outdoor tank, it is covered with a layer of shade netting to reduce the maximum light intensity to about 5 000 lux at the water surface.
At a conveniently located hatchery, larvae are siphoned out from the tanks to net cages. A cage size is ordinally 5 by 5 by 3 m. The 12 – 14 mm juveniles are stocked at a density of 2 000 to 3 000 per cubic meter in a 2 mm-mesh-cage. As the juveniles grow, their density is reduced by transferring them to new cages of larger mesh every 7 to 10 days.
The growth of larvae and juveniles is closely related to the water temperature. For example, at 20 – 23° C, the newly hatched larva is 2.3 mm TL ; 4.4 mm in 10 days ; 8.1 mm in 20 days ; 14.8 mm in 35 days ; 23.7 mm in 42 days ; 41.3 mm in 56 days (Fig. 5 KITAJIMA, 1978).
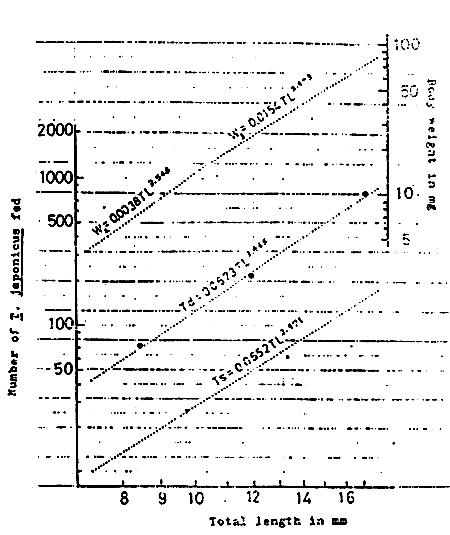
The relationship between the total length (L mm) and wet body weight (W mg) is shown in the following equations (KITAJIMA, 1978)
W1 = 0.0013 L4.1045 (3.75 mm < L < 6.75 mm)
W2 = 0.0038 L3.5486 (6.75 mm < L < 10.25 mm)
W3 = 0.0154 L2.9735 (10.25 mm < L < 42.5 mm)
W4 = 0.0091 L3.4078 (42.5 mm < L < 85 mm)
Although the survival rate is nearly 100 %, 10 days after hatching, about 5.5 mm in size, after that the mortality increases remarkably and continues for 30 days, 12–13 mm TL. Thereafter, the mortality drops gradually and after 50 days, around 25 mm TL, the survival rate is stable at 80 % or more (Fig 5, KITAJIMA, 1978).
Thus, the stage of high mortality, the critical period is 10 to 50 days old of 5.5 to 25 mm TL, especially it is higher in the period between 10 and 30 days, 5.5 mm and 13 mm, and this period corresponds to the metamorphic stage from larval to juvenile stage.
In the recent hatchery-production, the survival rate is 40 to 50 % during larval rearing in tanks, 50 to 60 % in the juvenile rearing in net cages and 20 to 25% through the whole process, although there is a little difference among hatcheries.
For ten years, from the mid fifties to 1965, research work was carried out to find the suitable diet for larval fish, and many species of micro-crustacea, larvae of the coastal invertebrates and artificial diets had been tested for their dietary value for larval fish.
After passing through this feed-research period, at present, a series of generally adequate diets for larval and juvenile fish of most species is almost fixed as follows :
The post-larvae from 4 to 30 days after hatching are fed on masscultured rotifers.
The metamorphic stage larvae are fed on rotifers combined with crustacean plankton such as Artemia nauplii, cultured Tigriopus japonicus, and copepods collected in the natural environment.
juvenile fish are fed on inert feed such as minced fish, shellfish and shrimp meat, or artificial feed.
It is important to know the amount of live feed consumed by a single larva per day at each stage of the sequential growth in order to mass-produce and supply systematically the suitable amount of live feed.
If the number of larvae and the density of the feed organisms in the tank were known, the daily feed consumption by a larva could be calculated through a sequential decrease of the density of feed organisms.
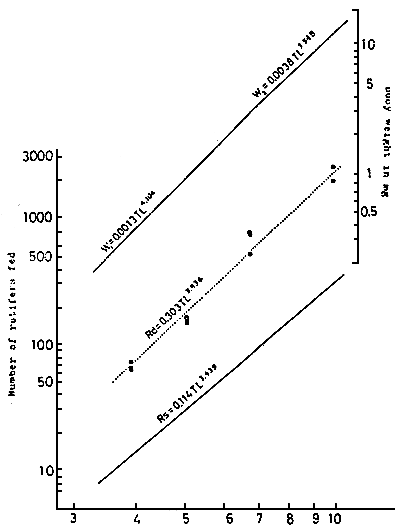
The relations between the number of rotifers (R) or Tigriopus japonicus (T) eaten by a larva per day and the larval total length (L mm) are shown in the following formula (KITAJIMA, 1976 ; KITAJIM et al, 1976).
R = 0.3927 L3.675 and T = 0.06728 L3.466 (Fig. 6, 7)
The average weight of an individual rotifer was 0.003 mg and that of a T. japonicus was 0.034 mg, in these experiments, therefore the consumed amount is roughly 60 % of the larval body weight in common wet basis, at each stage.
Furthermore, the rotifer density in a rearing tank has to be maintened always at least at 5 individ./ml to avoid larval starvation and to make fewer deviations in larval growth. It is estimated that the necessary feed amount of live feed is 1.3 to 1.5 times that of the amount eaten by larvae when taking into consideration the outflow of organisms with the circulation of water. FUSHIMI (1977) found that the growth and the survival rate of red sea-bream larvae were unchangeable even if the daily amount of rotifer given was more than 80 % of body weight.
At the early development stage of the culture techniques before 1972, only marine Chlorella was used as a culture diet for rotifer. With the increase of production of red sea-bream seed, the amount of rotifers required has become enormous and baker's yeast was subtituted instead of marine Chlorella. As a result, the culture density of rotifers increased from 30 – 50 to 100 individ./ml or more.
Although the red sea-bream larvae with the rotifers reared on Chlorella (Chlorella-rotifer) grew normally, serious mortality was observed in the larvae fed on the rotifers reared on baker's yeast (yeast-rotifer) alone for 10 – 15 days, It was then found possible to prevent this mortality by feeding yeast-rotifer with marine Chlorella secondarily for several hours or rotifers reared on baker's yeast in combination with marine Chlorella (KITAJIMA and KODA, 976).
From the results, chemical analyses on both yeast Chlorella-rotifer were conducted by our coworkers in order to clarify their nutritional value. As there was little difference between the amino-acid and mineral composition of both rotifers, it seemed that they were not principal factors in the dietary value (WATANABE et al., 1978 a, 1978 b). On the other hand, it was found that there were marked differences in the fatty acid compositions of them, i.e. yeast-rotifer was quite low in the content of ω 3 highly unsaturated fatty acids (ω 3 HUFA), such as 20 : 5ω3 (eichosapentaenoic acid) and 22 : 6ω3 (dochosahexaenoic acid), which are essential fatty acids (EFA) for marine fish (YONE and FUJII, 1975 a, 1975 b; COWEY et al., 1976), whereas Chlorella rotifer was found to contain a high amount of 20 : 5ω3. The difference in the concentration of 20 : 5ω3 was also found to be attribuable to the difference of the fatty acid composition between yeast and Chlorella (WATANABE et al., 1978 c) (Table 1).
A trial was conducted in order to improve the low dietary value of yeast rotifer by feeding them marine Chlorella for 10 min. over 24 hours at regular intervals before being given to red sea-bream larvae. The dietary value of yeastrotifers was found to be improved effectively by the secondary culture with Chlorella. The larvae fed on yeast-rotifers cultured secondarily for more than 6 hours showed good results and a high survival rate, comparable to those obtained with the larvae fed on Chlorella rotifers (Table 2, KITAJIMA et al., 1979 ; WATANABE et al., 1979).
When marine Chlorella was used as a culture medium, the quite low content ofω3 HUFA in rotifers reared on baker's yeast increased in proportion to the culture period by the incorporation of 20 : 5ω3 from marine Chlorella, and reached a maximum at 27 % in a two-day-feeding WATANABE et al., 1979).
These results indicated that the content of ω 3 in the rotifers was the principal factor in the dietary value of them and that the high mortality observed frequently in red sea-bream larvae induced by feeding them yeast-rotifers as their sole feed was due to EFA deficiency in the fish.
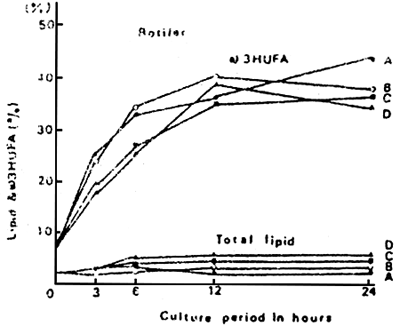
With these results, a new type of yeast (designated as ω-yeast) was produced by adding fish oil or cuttlefish liver oil as a supplement to the culture medium of baker's yeast, resulting in a high content of lipids andω3 HUFA (IMADA et al., 1979). The rotifers cultured with ω-yeast had high lipid content and ω3 HUFA. The incorporation of ω3 HUFA from ω-yeast was observed to reach its maximum after 12 h-feeding. The dietary value of the rotifers for fish larvae was found to be significantly improved, comparable to that of chlorella-rotifers (KITAJIMA et al., 1980 a) (Table 3,4 ; Fig. 8, 9).
The same results as obtained in red sea-bream were found in several species of marine fish larvae, such as rock bream, Oplegnathus fasciatus (FUKUSHO et al., 1978), puffer, Takifugu rubripes (ARAKAWA et al., 1978) and ayu, Plecoglossus altivelis (KITAJIMA et al., 1980 b).
Furthermore, the content of ω3 HUFA is the most important factor in the dietary value of not only rotifers but also other live feeds such as Artemia nauplii and copepods. Especially, a single dosage of Artemia frequently caused a weakening of larvae, and a consequent high mortality of many species of marine fishes. The occurence of this phenomenon varied with the species as well as with the place of production of Artemia. Artermia eggs from different localities were analyzed for fatty acid composition in order to clarify the nutritional value of Artemia for marine fish larvae (WATANABE et al., 1978 d, 1980 ; FUJITA et al., 1980). From the results shown in Table 5, Artemia eggs can be classified in two types according to the fatty acid composition (WATANABE et al., 1978 d). One type contains a large amount of 18: 3ω3 (linolenic acid), which is the EFA for fresh-water fish, whereas the second type is high in 20 : 5ω3, which is one of the EFA for marine fish.
The dietary value of Artemia of the fresh-water type was found to be improved effectively by giving them feeds with a high amount of ω3 HUFA such as Chlorella and yeast in the same manner as the notifer mentioned above. Furthermore, it is possible to improve the dietary value by giving it directly to Artemia in the form of emulsified lipid with a large amount of ω3 HUFA (WATANABE et al., 1982 Such copepods as T. japonicus and Acartia spp have a large amount of ω3 HUFA and are excellent feed for larval marine fish.
Although the main cause of mortality seems to be infectious diseases and microparasites, the studies on them, have been just started and there are many problems to be solved. The bathing or oral administration of antibiotics are conducted for bacterial disease in the larval and juvenile stages.
With the growth of seed-production of red sea-bream, such deformities as short-tail, pughead, incomplete development of gill cover, skeletal abnormalities, etc.. are observed more or less in the juveniles produced at hatcheries and laboratories. They become a big problem when they occur in high percentages.
Since around 1973, a high prevalence rate of lordosis, V-shape curvature of column, had been frequently observed in almost all the laboratories and hatcheries in Japan. As this happened frequently, 20 to 50 %, it became a severe problem (Table 6).
We had studied the cause of this deformity from 1975 to 1980, and succeeded in almost clarifying the cause of the deformity.
The lordosis is usually found in juvenile of about 20 mm in size. After that the occurence and the degree of curvature increase gradually with the growth. After attaining the length of 120 to 130 mm, both the occurence and the degree of deformation are roughly fixed (KITAJIMA et al., 1975 ; KITAJIMA, 1977) (Fig. 11).
In 1977, it had been revealed that there was a close relation between the lordosis and undeveloped swim bladder (KITAJIMA et al., 1977) (Fig. 12, 13).
Larval red sea-bream gulps atmospheric gas at the water surface, for initial swim bladder inflation for about a week between the initial feeding (ages 3 to 7 days) and growth to about 4,5 mm (10 to 12 day old) (KITAJIMA et al., 1981). YAMASHITA (1966) observed histologically that the first gas appeared in the swim bladder of larval red sea-stream at the age of around 8 days and that the larval pneumatic duct was then closed and gradually degenerated by the 10 to 12 day of age. Accordingly, the period with the functional pneumatic duct in the larval stage corresponds with the stage of gulping air and the resulting initial swim bladder inflation (Table 7 – 8, Fig. 14).
The fish which had failed to gulp air at the water surface for some reason or other during the stage with the larval pneumatic duct, 3.5 to 4.5 mm in total length, grew without swim bladder inflation, and as a result the majority of them became lordotic when they grew to the juvenile or young stage (Fig. 15).
The specific gravity of the individuals with a normal swim bladder is about 1.04, while that with an uninflated one is 1.07 or so (KITAJIMA, 1978). So, the fish with uninflated swim bladder (Fig. 16) displayed difficulty in keeping themselves in the upper or middle layer of the water. They swim upward in an oblique manner with a rapid fin stroke. The lordosis seemed to be induced when they try to compensate for the oblique direction of the body axis thus causing a distortion of the spinal column.
The feeding the rotifers with a low content of ω-3 HUFA to larvae showed a tendency to increase the appearance of fish with uninflated swim bladder (KITAJIMA, 1978 ; WATANABE, 1978), (Fig. 17). Moreover, ISEDA et al., (1978) discovered that the proportion of fish with uninflated swim bladders had a tendency to increase in the rearing tanks with too much aeration (Table 9).
From these results, it is possible to draw conclusions concerning the gulping of air in larvae as following :
As the larval red sea-bream fed on the rotifers with low content of ω-3 HUFA showed low activity, shortage of endurance and lack of reflex responses, it might be difficult for them to gulp air after attaining the water surface while at the same time, having to withstand the current of water caused by the aeration.
In addition to red sea-bream, the same kind of lordosis also occurs in the fish with uninflated swim bladders in hatchery-reared species such as black sea-bream, Acanthopagrus schlegeli (KITAJIMA, 1979), common sea-bass Lateo labrax japonicus (HAYASHIDA et al., 1984) and silver bream, Sparus sarba (KITAJIMA, unpublished), and their causes and mechanisms seem to be the same as those of red sea-bream.
Twenty years have passed since the rotifer, Brachionus plicatilis, was introduced as a food organism for marine larval fishes by ITO (1960). Thereafter, the technique of fry production has rapidly progressed together with the advancement of its culture techniques.
There are two types of rotifers cultured all over Japan. One called S-type, which is relatively small (150 um in lorica length) having its lorica with pointed anterior spines more curved in shape, while the other, called L-type is larger than S-type (250 um) and has a slender lorica with obtuse anterior spines. The former grows actively at higher temperatures of 20 to 27° C, while the suitable temperature for the growth of the latter is lower, between 10 to 17° C.
Recent studies have shown that both rotifers are of different genetic strains, and classified as sub-species ; Brachionus plicatilis rotundiformis (S-type) and B. plicatilis typicus (L-type) (SUZUKI, 1983).
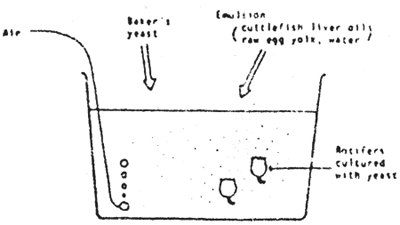
The maximum amount of rotifer produced daily at a hatchery reaches several billions. So, recently large tanks of 50 to 150 ton are usually used for the culture of rotifers. The maximum culture density of rotifers is usually 150 – 300 individuals/ml of S-type and 100 – 150 individuals/ml of L-type, respectively.
There are two methods for mass-culture of rotifers ; the thinning method and the subculture method. In the former method, rotifers are harvested according to the daily increase, 10 to 25 % of all the rotifers everyday. After harvesting rotifers from the tanks by filtering the water through a fine-meshed net, the same volume of sea-water containing Chlorella in concentrations of 1 × 107 to 2 × 107 cells/ml is added to the tank. Usually thinning harvests are carried out for 1 – 2 weeks, and then the whole crop is harvested.
In the latter method, all the rotifers are harvested at the maximum density for several days after the initial inoculation (about 100 individuals/ml). And part of them is used as seed to inoculate a new culture tank With this method, several tanks are used in regular rotation. The method is of great advantage for the systematic production of rotifers.
Marine Chlorella, baker's yeast and ω-yeast are generally used as the feeds for rotifer. Chlorella is the most excellent feed, but a large size tank is necessary for its culture. If Chlorella is used as a sole feed for rotifer, the required total volume of the culture tank will reach 1.5 to 2 times that of the tank for rotifer-culture. Although the production of rotifer has increased remarkably in substituting Chlorella by yeast, Chlorella is still necessary to stabilize the rotifer-culture, it is especially essential for the initial feed, 1 to 2 days in a course of the culture.
The following agricultural fertilizers are usually added to the culture medium of Chlorella : ammonium sulphate 100 g ; superphosphate of lime 10 – 20 g ; urea 10 g and a chelating agent 5 – 10 g per ton of sea-water. But the latter can be omitted.
Results obtained by the HIROSHIMA Prefectural Fish Farming Center from April to July 1982 give an example of a practical rotifer production. The Center used eight outdoor 150 ton tanks (1 200 ton in total) for rotifer culture and twelve outdoor 200 ton tanks (2 400 ton in total) for Chlorella culture. The period for the whole harvest after inoculation was five days. Chlorella was used only at inoculation and the next day. On the following days yeast alone was given. The crops were mixtures of L-type and S-type rotifers and the former was dominant in May and June, the latter was in late June and July. Total harvest was 1 200 × 109 (about 2.5 ton), the harvest per day was 19.2 × 109 individuals and they ensured the production of 6.3 × 106 sea-bream juveniles and 4 × 106 blue crabs, Portunus trituberculatus (FUSHIMI, 1982).
Although marine copepods is an important feed for the rearing of postlarval and early juvenile red sea-bream, a complete technique for the mass-culture of them has not yet been established. However, T. japonicus may be reared successfully in large scale operating tanks for two or three months in combination with rotifer. Using baker's yeast as feed, the density of T. japonicus increases up to 20 to 30 individuals/ml within 3 to 4 weeks after inoculation. We can harvest about 2 kg of this species every-day for 50 days from one 200 ton tank (FUKUSHO, 1978).
Some problems still remain to be solved.
Firstly, it is necessary to systemize the process of fry production with automatic operations in terms of mass-rearing of larvae and juveniles, and mass-culture of live feed. For this purpose, live feed, especially rotifers, must be substituted by artificial feed.
Furthermore, it is important to clarify the cause and counterplan of heavy mortality in larval and juvenile stage of red sea-bream.
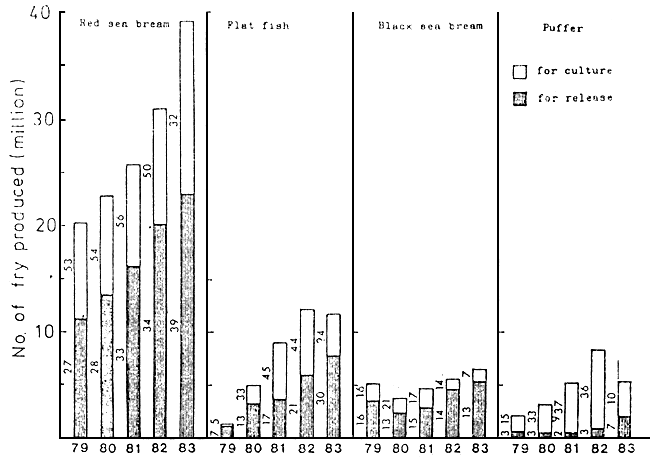
Fig. 1. Number of fry yearly produced at hatcheries in Japan. Numerals showing the number of hatcheries which produced the fry of the species.
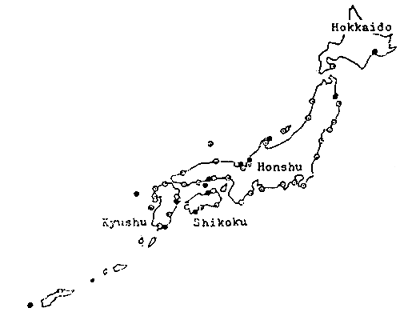 | 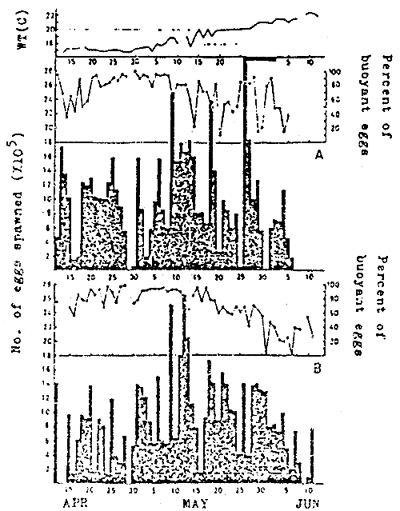 | ||
| Fig. 2 | Sites of national (•) and prefectural (○) hatcheries in Japan | Fig. 3 | Daily changes of number of eggs spawned by red sea bream in outdoor 40 ton tanks, stocked with 20 females and 20 males (A), and 30 females and 31 males (B), respectively. |
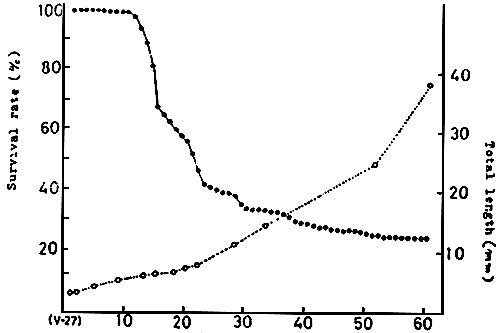
Fig. 5 Growth survival rate of reared red sea bream.
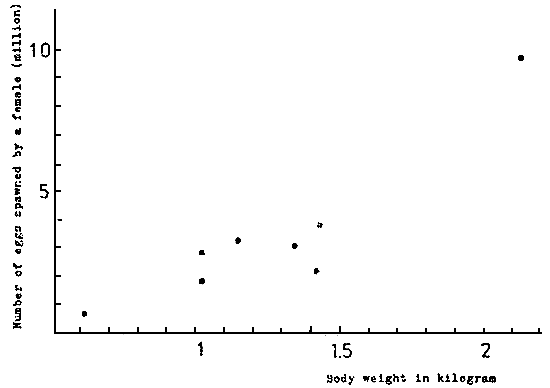
Fig. 4. Relationship between body weight and the number of eggs spawned by a female during spawning season.
 |  | ||
| Fig. 6 | Relationship between total length and satiated amount (Rs) or daily feeding amount (Rd) of rotifer in larva red sea bream | Fig. 7 | Relationship between total length and satiated amount (Ts) or daily feeding amount (Td) of Tigriopus japonicus in larval and juvenile red sea bream |
Table. 1.
Certain fatty acids of total lipids from rotifer Brachlonus pucottils, cultured with baker's yeast,
Saccharomyces cerevislae, and marine Chlorella at Nagasaki Perfectural Institute of Fisheries during
1975 to 1977 (area %)
| Fatty acid | November 1975 | May 1976 | May 1977 | ||||||
| Yeast | Yeast + Chlorella | Chlorella | Yeast | Yeast + Chlorella | Chlorella | Yeast | Yeast + Chlorella | Chlorella | |
| 16:0 | 6.1 | 4.2 | 14.4 | 7.1 | 13.2 | 19.4 | 8.7 | 11.7 | 16.8 |
| 16:1ω7 | 27.2 | 26.7 | 20.4 | 26.5 | 22.6 | 22.4 | 24.2 | 16.6 | 24.3 |
| 18:0 | 3.8 | 4.4 | 2.2 | 4.3 | 3.6 | 1.9 | 4.8 | 6.0 | 1.7 |
| 18:1ω9 | 26.8 | 25.8 | 10.1 | 29.1 | 21.5 | 11.0 | 33.9 | 22.8 | 10.1 |
| 18:2ω6 | 8.9 | 5.1 | 4.7 | 6.9 | 6.3 | 3.4 | 6.8 | 10.4 | 3.2 |
| 18:3ω3 | 0.6 | 0.6 | 0.1 | 0.2 | 0.5 | 0.2 | 0.6 | 2.2 | 0.4 |
| 20:1 | 3.6 | 3.4 | 1.7 | 4.2 | 4.1 | 2.3 | 6.0 | 3.3 | 2.4 |
| 20:3ω3 | |||||||||
| 20:4ω6 | 2.0 | 2.3 | 4.1 | 6.9 | 3.0 | 4.2 | 0.4 | 2.3 | 2.4 |
| 20:4ω3 | 0.4 | 0.6 | 0.2 | 0.4 | 0.4 | tr | 0.5 | 0.6 | 0.2 |
| 20:5ω3 | 1.9 | 11.8 | 27.7 | 1.4 | 11.1 | 22.8 | 1.0 | 8.1 | 24.1 |
| 22:1 | 0.9 | 2.1 | 1.8 | 0.9 | 0.4 | 0.4 | 1.7 | 1.5 | 1.3 |
| 22:5ω3 | 0.3 | 1.8 | 3.0 | tr | 2.9 | 3.4 | 0.2 | 1.7 | 3.8 |
| 22:6ω3 | 0.5 | 0.5 | tr | tr | tr | tr | 0.5 | 0.9 | 0.5 |
| Σω3 HUFA | 3.1 | 14.7 | 30.9 | 2.7 | 14.4 | 26.2 | 2.2 | 11.3 | 28.6 |
| Lipid % | 1.4 | 2.8 | 3.7 | 1.7 | 2.2 | 4.2 | 2.3 | 2.3 | 3.8 |
Table 2. Effect of secondary culture with marine Chlorella on the dietary value for red sea bream larvae of rotifers cultured with baker's yeast and the change of fatty acid distribution of total lipid in these rotifers (Kitajima et al., 1979; watanabe et al., 1979)
| Rotifer* | No. of fish | Total length at the end of feeding (mm) | Rate of survival (%) | Survival at activity test (%) | Content of 20:ω3 (%) | |
| Experiment I | ||||||
| Y-rotifer | 23000 | 5.35+0.53 | 20.2 | 9.2 | ** | 3.2 |
| Y 10m C | 23000 | 6.16+0.49 | 58.7 | 54.3 | - | |
| Y 30m C | 23000 | 7.68+0.81 | 71.7 | 67.8 | - | |
| Y 60m C | 23000 | 7.79+0.76 | 76.1 | 60.7 | - | |
| Y 2h C | 23000 | 8.01+0.54 | 65.4 | 94.3 | 8.7 | |
| C-rotifer | 23000 | 8.76+0.55 | 79.8 | 99.6 | 27.0 | |
| Experiment II | ||||||
| Y-rotifer | 24000 | 4.56+0.30 | 22.1 | 45.6 | *** | 3.2 |
| Y 2h C | 24000 | 5.98+0.52 | 49.5 | 79.2 | 8.7 | |
| Y 6h C | 24000 | 6.31+0.54 | 50.3 | 90.7 | 10.9 | |
| Y 6h DC | 24000 | 5.03+0.45 | 50.6 | 82.4 | 4.8 | |
| Y 12h C | 24000 | 5.76+0.38 | 49.5 | 87.9 | 12.5 | |
| Y 24h C | 24000 | 7.03+0.81 | 60.4 | 96.1 | 16.7 | |
| C-rotifer | 24000 | 7.15+0.52 | 58.1 | 92.7 | 27.0 | |
Table 3 Certain fatty acids of total lipids from baker's yeast, the yeast supplemented with cuttlefish liver oil (ω-yeast) and rotifers cultured with these yeasts.
| Fatty acid | Baker's yeast | ω-Yeast | Rotifers cultured | |
| Baker's yeast | ω-Yeast | |||
| 16:0 | 8.3–20.0 | 13.4–16.9 | 6–7 | 10–12 |
| 16:1ω7 | 14.2–38.2 | 5.0–6.6 | 26–27 | 10–11 |
| 18:0 | 3.4–8.4 | 2.3–2.6 | 3–4 | 2–3 |
| 18:1ω9 | 26.1–43.9 | 15.5–16.4 | 26–30 | 22–24 |
| 18:2ω6 | 2.8–15.1 | 1.0–1.1 | 7–9 | 2–4 |
| 18:3ω3 | 0.5–6.4 | 0.8–0.9 | 0.7–0.8 | |
| 20:1 | tr–1.6 | 8.4–9.2 | 3–4 | 8–10 |
| 20:3ω3 | 3.0–3.4 | 1–2 | 3–4 | |
| 20:5ω3 | 13.4–17.4 | 1–2 | 9–12 | |
| 22:5ω3 | 0.9–1.4 | 0–0.4 | 2–3 | |
| 22:6ω3 | 12.8–15.6 | 7–9 | ||
| Σ ω3 HUFA | 33.5–35.8 | 25–28 | ||
| Lipid % | 1.0–1.6 | 12.3–15.6 | 1.4–1.9 | 3.3–5.4 |
Table 4 Comparison of growth and survival rates of larval red sea bream fed on rotifers cultured with respectively yeast or ω-Yeast.
| Rotifer used | No. of fish | Total length at end (mm) | Rate of survival (%) | Survival at activity test (%) | |
| Expt. I | ω-Yeast | 30 000 | 9.28±0.77 | 73.5 | 86.0 |
| Yeast | 30 000 | 7.10±0.78 | 13.0 | 12.5 | |
| Expt. II | ω-Yeast | 15 000 | 10.11±0.87 | 76.2 | 92.9 |
| Chlorella | 15 000 | 10.21±1.60 | 67.1 | 91.7 | |
| Y 12 h C | 15 000 | 9.11±1.24 | 27.9 | 93.2 | |
| Expt. III | ω-Yeast | 24 000 | 10.32±1.28 | 76.9 | 92.5 |
| Chlorella | 24 000 | 9.78± | 70.1 | 91.5 | |
| Y 3h C | 24 000 | 8.85±1.09 | 27.6 | 55.8 | |
| Expt. IV | ω-Yeast | 10 000 | 10.91±0.94 | 68.9 | 95.5 |
| Yeast | 10 000 | 6.24±0.62 | 3.2 | 45.9 |
Table 5 Certain fatty acids of total lipida inArtemis egg from three localities (Vatanabe et al., 1982)
| Fatty acid | San Fracisco | Brazil | Tien-tsin | ||||||
| A | B | C | A | B | C | A | B | C | |
| 14:0 | 3.6 | 1.3 | 2.1 | 3.3 | 3.4 | 2.1 | 3.0 | 2.8 | 2.0 |
| 15:0 | 25.9 | 14.9 | 23.7 | 16.0 | 18.2 | 13.7 | 12.1 | 12.7 | 12.7 |
| 16:1ω6 | 12.9 | 5.5 | 7.4 | 18.6 | 14.4 | 13.8 | 22.6 | 24.0 | 22.4 |
| 18:0 | 3.7 | 3.5 | 4.1 | 1.9 | 2.9 | 3.2 | 3.6 | 2.9 | 3.3 |
| 18:1ω9 | 19.8 | 20.0 | 23.7 | 21.8 | 23.7 | 28.9 | 26.2 | 26.2 | 28.3 |
| 18:2ω6 | 2.5 | 6.3 | 5.4 | 7.2 | 6.4 | 8.5 | 4.1 | 3.8 | 4.4 |
| 18:3ω3 | 4.8 | 22.4 | 14.7 | 3.3 | 1.1 | 3.2 | 5.5 | 6.0 | 5.1 |
| 18:4ω3 | 0.6 | 0.8 | 0.8 | 2.7 | 3.2 | 4.5 | 0.9 | 1.0 | 0.7 |
| 20:1 | 1.1 | 0.3 | 1.0 | 0.9 | 1.2 | 0.4 | 0.2 | 0.4 | - |
| 20:4ω6 | 0.6 | 0.6 | 0.8 | 2.7 | 3.2 | 4.5 | 1.2 | 1.1 | 1.5 |
| 20:5ω3 | 0.9 | 2.7 | 0.6 | 3.9 | 3.5 | 5.9 | 8.2 | 10.2 | 11.3 |
| 22:1 | 0.3 | 0.6 | 0.3 | 0.7 | 1.0 | 0.4 | - | - | - |
| 22:6ω3 | 0.2 | 0.1 | 0.1 | 0.4 | 0.0 | tr | - | - | - |
| ω3 HUFA | 1.1 | 8.0 | 1.2 | 4.4 | 4.1 | 6.3 | 9.2 | 10.2 | 11.3 |
 | 
|
Fig. 8. The direct method for improving the dietary value of living feeds.
Fig. 9. Incorporation of lipids emulsified with various kinds of reagents (A, B, C and D in rotifers by the direct method. Reproduced with permission from Bull. Jpn. Soc. Sci Fish., Watanabe et al., 1983.
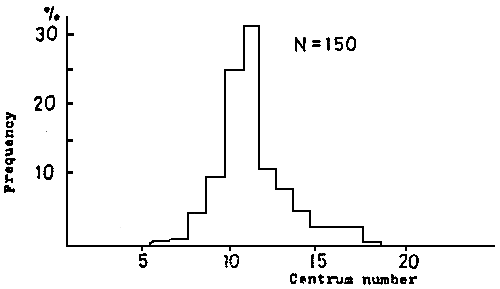
Fig. 10 Skeletal curving point in lordotic deformity of red sea bream
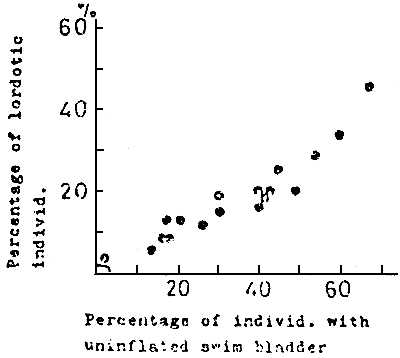
Fig. 12
Relationship between uninflated swim bladder
and lordotic deformity in 20 rearing groups
of young red sea bream of about 50 mm TL.
Table 6
Yearly occurence of deformities in red sea bream produced at the
Aquaculture Research Laboratory of Nagasaki Prefectural Institution
of Fisheries
| Year | 1974 | 1975 | 1976 | |
| No. of fry produced ×103 | 420 | 270 | 490 | |
| Date of examinations | 12 Aug | 1 Sept | 26 Jul– 30 Aug | |
| No. of fish examined | 497 | 152 | 1024 | |
| Total length mm | 93.3 | 118 | 64.9– 112.3 | |
| Curving angle | ||||
| <10 | 3.4 | 4.6 | 4.5 | |
| Lordosis % | <20 | 20.5 | 13.2 | 8.3 |
| <30 | 9.3 | 7.9 | 2.3 | |
| 30> | 8.2 | 3.3 | 1.9 | |
| Total | 41.4 | 28.9 | 17.0 | |
| Short statue (fused centrums) | 2.8 | 2.0 | 5.7 | |
| Other deformities | 5.0 | 5.9 | 6.5 | |
| Total | 49.2 | 36.8 | 29.0 | |
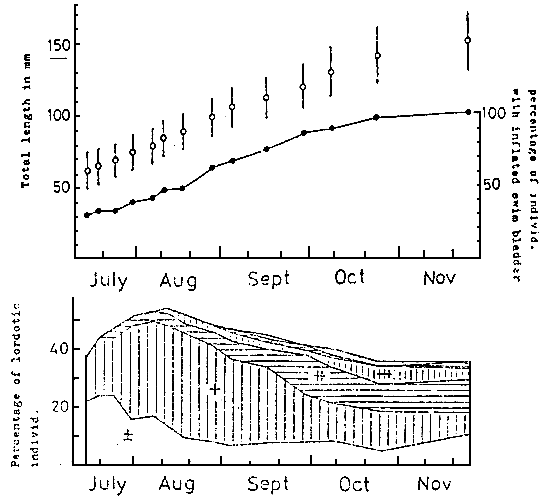
Fig. 11
Changes in percentage of individuals with inflated swim bladder
and lordotic deformity of marked red sea bream.
± : 10°> in skeletal curve
+ : 10°<20°
++ : 20°<30°
+++ : 30°<40°
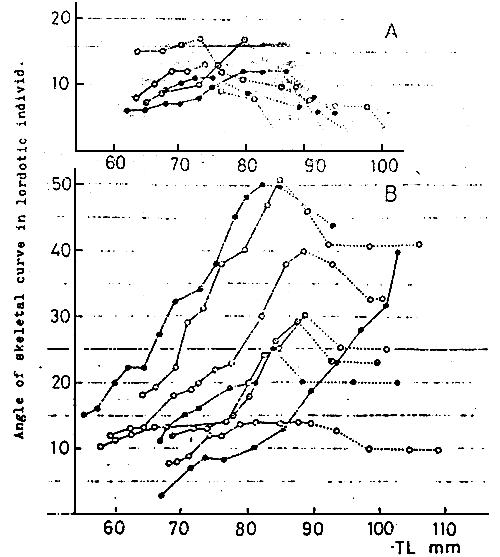
Fig. 13
Change in angle of skeletal curve in lordotic individuals
by repeated observations of particular ones. Solid line
showing the period with depressed swim bladder, and dotted
line the period with normally inflated swim bladder.
A: group of slightly lordotic individ., B: group of seriously
lordotic individ.
Table 7. Comparison of incidence of swim bladder inflation in the larval red sea bream reared in the tank sealed with a layer of liquid paraffin and the open tank (Exp. I)
| Date (Days after hatching) | Sealed tank | Open tank | |||||
| N | TL (X±SD) (mm) | Incidence (%) | N | TL (mm) | Incidence (%) | ||
| May | 6 ( 8) | 9 | 3.61±0.17 | 0 | 9 | 3.61±0.17 | 0 |
| 8 (10) | 28 | 3.95±0.14 | 0 | 23 | 3.98±0.22 | 41.7 | |
| 10 (12) | 30 | 4.12±0.21 | 0 | 30 | 4.29±0.27 | 73.3 | |
Table 8 Comparison of incidence of swim bladder inflation in the larval red sea bream reared in the tanks sealed with a layer of liquid paraffin and the open tank (Exp. II)
| Date (Days after hatching) | Sealed tanks | Open tank (C) | ||||||||
| Non aeration (A) | Aeration (B) | |||||||||
| N | TL (mm) | Incidence (%) | ||||||||
| N | TL (mm) | Incidence (%) | N | TL (mm) | Incidence (%) | |||||
| May | 15 ( 8) | 27 | 3.70±0.20 | 0 | 23 | 3.92±0.40 | 0 | 26 | 3.82±0.26 | 53.3 |
| 17 (10) | 28 | 3.91±0.32 | 0 | 28 | 4.37±0.24 | 0 | 23 | 4.11±0.25 | 76.7 | |
| 19 (12) | 25 | 3.93±0.26 | 0 | 29 | 4.74±0.44 | 0 | 27 | 4.62±0.55 | 65.5 | |
| 21 (14) | 30 | 5.33±0.51 | 0 | 29 | 5.21±0.46 | 70.0 | ||||
| 23 (16) | 30 | 5.75±0.48 | 0 | 30 | 5.49±0.50 | 31.8 | ||||
| 23 (18) | 30 | 6.11±0.48 | 0 | 30 | 6.30±0.56 | 90.0 | ||||
| 29 (22) | 30 | 7.38±0.53 | 0 | 25 | 7.52±0.89 | 92.0 | ||||
Table 9 Comparison of incidence of swim bladder inflation in the larval red sea bream reared in the tank blown at the water surface with an electric fan and in the non-blown tank (Exp. III)
| Date (Days after hatching) | Blown tank | Non-blown tank | |||||
| N | TL (mm) | Incidence (%) | N | TL (mm) | Incidence (%) | ||
| Jan. | 9 ( 4) | 18 | 3.28±0.12 | 0 | 22 | 3.28±0.14 | 9.1 |
| 11 ( 6) | 30 | 3.63±0.18 | 3.3 | 30 | 3.70±0.20 | 46.7 | |
| 13 ( 8) | 23 | 4.06±0.18 | 0 | 29 | 4.08±0.17 | 69.0 | |
| 16 (11) | 30 | 5.08±0.37 | 0 | 28 | 5.24±0.27 | 60.7 | |
| 20 (15) | 30 | 6.84±0.54 | 6.7 | 30 | 6.62±0.60 | 66.7 | |
| 27 (22) | 30 | 11.37±1.50 | 13.3 | 30 | 11.91±1.09 | 71.3 | |
| Jul. | 1 (26) | 30 | 17.34±1.93 | 10.0 | 32 | 17.59±1.50 | 84.4 |
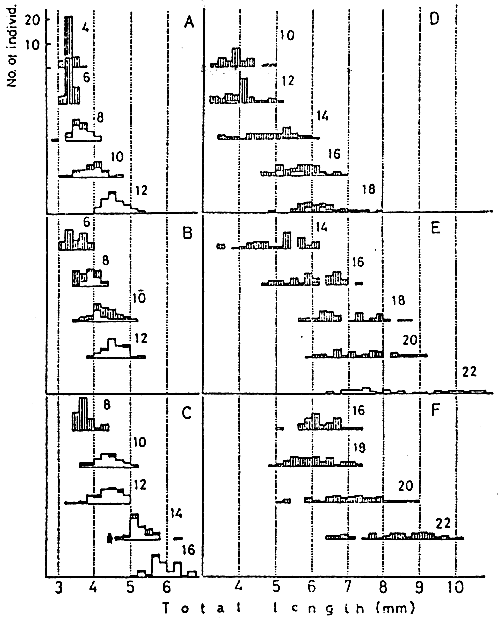
Fig. 14 Comparison of swim bladder inflation among the groups of larval red sea bream transferred to the tanks with open surface, after rearing in the tank sealed with a layer of liquid paraffin for various duration, 2 to 16 days, after hatching (A–F). Numericals in each graph denotes days after hatching, among which the top one shows the day when the day when the larvae were transferred from the sealed tank to each tank with open surface. Shadow area; individuals with uninflated swim bladder, open area; individuals with inflated swim bladder.
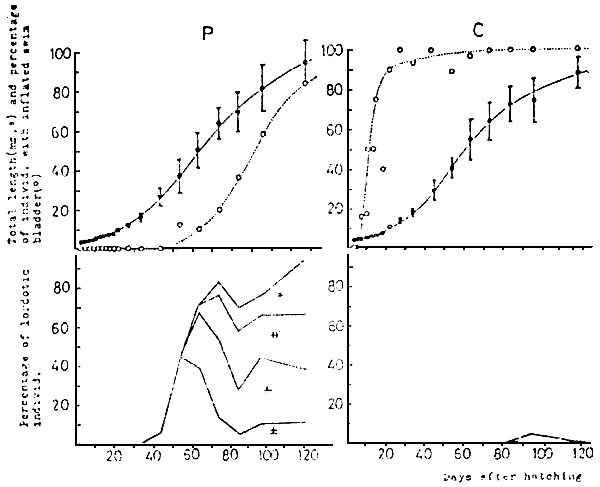
Fig. 15 Comparison of incidence of swim bladder inflation and the lordotic deformity in the larval
red sea bream reared in the tank sealed with a layer of liquid paraffin (P) and the open
tank (C).
The mark of  shows mean ±SD (n = 30). The degrees of lordosis are expressed by the
supplementary angle at the curving point: ±, less tahn 10°; +, 10° to 20°;
shows mean ±SD (n = 30). The degrees of lordosis are expressed by the
supplementary angle at the curving point: ±, less tahn 10°; +, 10° to 20°;  , 20° to 30°;
, 20° to 30°;  ,
30 ° to 40 °
,
30 ° to 40 °
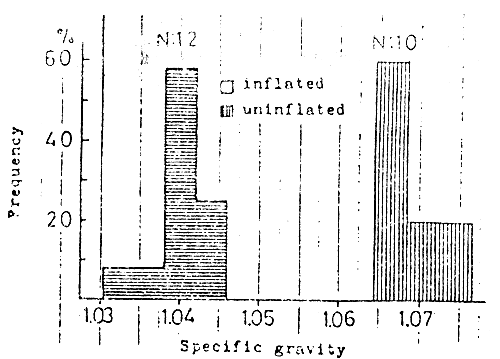
Fig. 16
Comparison of specific gravities in
the young red sea bream with inflated
swim bladder and uninflated one.
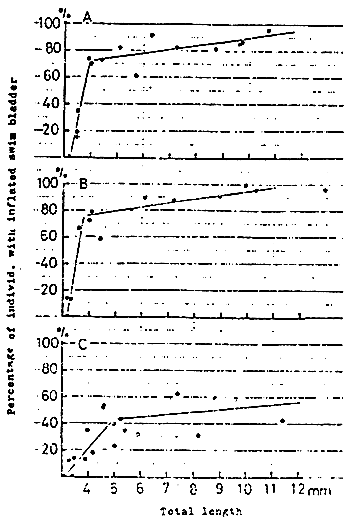
Fig. 17
Comparison of incidence of swim bladder in the
larva) red sea bream fed on Chlorella-rotifer (A),
ω-yeast-rotifer (B) and Y3C-rotifer (C),
reapectively.
LITERATURE CITED
ARAKAWA, T., T. YOGATA, and T. WATANABE, 1979. Rearing of larvae of puffer (Fugu rubripes rubripes) by rotifer (Brachionus plicatilis) cultured with various foods. Bull. NAGASAKI Pref. Inst. Fish. 5 : 5–8 (In Japanese with English synopsis).
COWEY, C.B., J.M. ADRON, and C. MIDDLETON, 1976. Studies on the nutrition of marine flat fish. The effect of different dietary fatty acids on the growth and fatty acid composition of turbot Scophthalmus maximus). Br. J. Nutr. 36 : 479 – 486.
FUJITA, S., T. WATANABE, and C. KITAJIMA, 1988. Nutritional quality of Artemia from different localities as living feed for marine fish from the view point of essential fatty acids. The brine shrimp Artemia. 3. Ecology, culturing, use in aquaculture. PERSOON G., P. SORGELOOS, O. ROELS, and E. JASPERS, Universa Press, Wetteren Belgium : 456 p.
FUKUSHO, Y., H. IWAMOTO, M. MATSUOKA, and M. IMADA, 1978a. The mass production of juveniles of the percoid fish, Oplegnatus fasciatus, using the rotifer Brachionus plicatilis fed on the special yeast. The aquaculture, 26 (2) 71 – 81 (In Japanese with English synopsis).
FUKUSIO, K., H. IWAMOTO, T. SEIKAI, and C. KITAJIMA. 1978b. Effect of initial supply of Chlorella sp. on the copepod Tigriopus japonicus production in combination with the rotifer Brachionus plicatilis, feeding baker's yeast. Bull. NAGASAKI Pref. Inst. Fish., 4 : 47 – 56. In Japanese with English synopsis).
FUSHIMI T. 1975. Foods. Feeding and development of fish larvae and fry (Edited by Japan Soc. Sci. Fish.) KOSEISHA-KOSEIKAKU, TOKYO : 122 (In Japanese).
FUSHIMI, T. 1982. Rotifer culture in HIROSHIMA Prefectural Fish Farming Center (Personal communication).
HAYASHIDA, G., Y. TSUKASIMA, K. MATSUKIYO, and C. KITAJIMA, 1984. Relationship between swim bladder uninflation and lordotic deformity in hatchery reared Japanese sea bass, Lateolabrax japonicus. Bull. NAGASAKI Pref. Inst. Fish., 10 : 35 – 40. (In Japanese with English synopsis).
IMADA, O., Y. KAGEYAMA, T. WATANABE. C. KITAJIMA, S. FUJITA, and Y. YONE, 1979. Development of a new yeast as a culture medium for living feeds used in the production of fish seed. Bull. Japan. Soc. Sci. Fish., 45 (8) 955 – 959. (In Japanese with English synopsis).
ISEDA, H. 1982. Prevention of deformation in the juveniles of red sea-bream, Pagrus major reared in ponds-III. Relationship between the initial conditions of rearing environment and gas content in air bladder. Bull. KUMAMOTO Pref. Fish. Expr. Stn. 2 : 25 – 46. (In Japanese).
ISEDA, H., M. ISHIHARA, S. SUMIDA, M. OWAKI and S. TABATA, 1979. Prevention of deformation in the juveniles of red sea bream, Pagrus major reared in ponds-ll. Relationship between the initial rearing conditions and lordotic deformity. Bull. KUMAMOTO Pref. Fish. Exper. Stn. 1 : 9 – 17 (In Japanese).
ITO, T. 1960. On the culture of maxohaline rotifer Brachionus plicatilis O. F. MULLER in the sea water. Rep. Fac. Fish. Mie Pref. Univ. 3 (3) 708 – 740. (In Japanese with English synopsis).
KITAJIMA C. 1976. Amount of the copepod, Tigriopus japonicus consumed by red sea bream larvae, Pagrus major. Bull. NAGASAKI Pref. Inst. Fish. 2 : 105 – 112. (In Japanese).
KITAJIMA, C. 1978. Acquisition of fertilized eggs and mass-culture of juvenile of red sea bream, Pagrus major. Special Rept. NAGASAKI Pref. Inst. Fish. 5 : 1 – 91. (In Japanese with English synopsis).
KITAJIMA, C. 1979. Swim bladder deformity and lordosis in hatchery reared black sea bream, Acanthopagrus schlegeli. Bull. NAGASAKI Pref. Inst. Fish. 5 : 27 – 32. (In Japanese).
KITAJIMA, C., T. ARAKAWA, F. OOWA, S. FUJITA, O. IMADA, T. WATANABE, and Y. YONE, 1980a. Dietary value for red sea bream larvae of rotifer Brachionus plicatilis cultured with a new type of yeast. Bull. Japan. Soc. Sci. Fish., 46 (1) : 43 – 46. (In Japanese with English synopsis).
KITAJIMA, C. S. FUJITA, F. OOWA, Y. YONE, and T. WATANABE, 1979. Improvement of dietary value for red sea bream larvae of rotifer Brachionus plicatilis cultured with baker's yeast Saccaromysea cerevisiae. Bull. Japan. Soc. Sci. Fish. 45 (4) : 469 – 471. (In Japanese with English synopsis).
KITAJIMA, C., K. FUKUSHO, H. IWAMOTO, and H. YAMAMOTO, 1976. Amount of rotifer, Brachionus plicatilis, consumed by red sea bream larvae, Pagrus major. Bull. NAGASAKI Pref. Inst. Fish., 2 : 105 – 112. (In Japanese).
KITAJIMA, C., H. IWAMOTO, and S. FUJITA, 1977. Relation between curvature of vertebral column and undeveloped swim bladder in hatchery reared red sea bream, Pagrus major. Bull. NAGASAKI Pref. Inst. Fish., 3 – 23 – 32. (In Japanese)
KITAJIMA, C., H. IWAMOTO, and K. MATSUKIYO, 1975. Abnormity of hatchery-reared juvenile of red sea bream, Pagrus major. Bull. NAGASAKI Pref. Inst. fish., 1 : 19 – 27. (In Japanese).
KITAJIMA, C, and K. KODA, 1976. Lethal effects of the rotifer cultured with baking yeast on the larval red sea bream, Pagrus major, and the increase of survival rate using the rotifer recultured with Chlorealla sp. Bull. NAGASAKI Pref. Inst. Fish. 2 : 113 – 116. (In Japanese).
KITAJIMA, C., Y. TSUKASHIMA, S. FUJITA, T. WATANABE, and Y. YONE, 1981. Relationship between uninflated swim bladders and lordotic deformity in hatchery-reared red sea bream Pagrus major. Bull Japan. Soc. Sci., 47 (10) : 1289 – 1294. (In Japanese with English synopsis).
KITAJIMA, C., M. YOSHIDA, and T. WATANABE, 1980b. Dietary value for ayu Plecoglossus altivelis of rotifer Brachionus plicatilis cultured with baker's yeast Saccaromyces cerevisiae supplemented with cuttlefish liver oil. Bull. Japan. Soc. Sci. Fish., 46 (1) : 47 – 50. (In Japanese with English synopsis).
SUZUKI, M., 1983. Taxonomical study on rotifers cultured for fry production. Zool. Mag., 91 (4) : 657. (In Japanese).
WATANABE, T., 1978. In “Dietary lipids in aquaculture” (Ed. by Japan Soc. Sci. Fish.), KOSEISHA - KOSEIKAKU, TOKYO : 93 – 111. (In Japanese).
WATANABE, T., T. ARAKAWA, C. KITAJIMA, S. FUJITA, 1978a. Nutritional evaluation of proteins o living feeds used in seed production of fish. Bull. Japan. Soc. Sci. Fish., 44(9) : 985 – 988. (In Japanese with English synopsis).
WATANABE, T., T. ARAKAWA, C. KITAJIMA, and S. FUJITA, 1984a. Effect of nutritional quality of broodstock diets on reproduction of red sea bream. Bull. Japan. Soc. Sci. Fish., 50 (3) : 495 – 501.
WATANABE, T., T. ARAKAWA, C. KITAJIMA, K. FUKUSHO, and S. FUJITA. 1978b. Proximate and mineral composition of living feeds used in seed production of fish. Bull. Japan. Soc. Sci. Fish., 44 (9) : 979 – 984. (In Japanese with English synopsis).
WATANABE, T., A. ITOH, C. KITAJIMA, and S. FUJITA. 1984b. Effect of dietary protein level on reproduction of red sea bream. Bull. Japan. Soc. Sci. Fish., 50 (6) : 1 015 – 1 022.
WATANABE, T., A. ITOH, A. MURAKAMI, Y. TSUKASHIMA, C. KITAJIMA, and S. FUJITA. 1984c. Effect of nutritional quality of diets given to broodstock on the verge of spawning on reproduction of red sea-bream. Bull. Japan. Soc. Sci. Fish., 50 (6) : 1 023 – 1 028.
WATANABE. T., A. ITOH, S. SATOH, C. KITAJIMA, and S. FUJITA, 1985a. Effect of dietary protein levels and feeding period before spawning on chemical components of eggs produced by red sea bream broodstock. Bull. Japan. Soc. Sci. Fish., 51 (9) : 1 501 – 1 509.
WATANABE, T., C. KITAJIMA, T. ARAKAWA, K. FUKUSHO, and S. FUJITA. 1978c. Nutritional quality of rotifer Brachionus plicatilis as a living feed from the view point of essential fatty acids for fish. Bull. Japan. Soc. Sci. fish., 44 (10) : 1 109 – 1 114. (In Japanese with English synopsis).
WATANABE, T., T. KOIZUMI, H. SUZUKI, S. SATOH, T. TAKEUCHI, N. YOSHIDA, T. KITADA, Y. TSUKASHIMA. 1985b. Improvement of quality of red sea bream eggs by feeding broodstock on a diet containing cuttlefish meal or on raw krill shortly before spawning. Bull. Japan. Soc. Sci. Fish., 51 (9) 1 511 – 1521.
WATANABE, T., F. OOWA, C. KITAJIMA, and S. FUJITA. 1978d. Nutritional quality of brine shrimp, Artemia salina, as a living feed from the view point of essential fatty acids for fish. Bull. Japan. Soc. Sci. Fish., 44 (10) : 1 115 –1521. (In Japanese with English synopsis).
WATANABE, T., F. OOWA, C. KITAJIMA, and S. FUJITA. 1980. Relationship betwwen dietary value of brine shrimp Artemia salina and their content of ω 3 highly unsatured fatty acid. Bull. Japan. Soc. Sci. Fish., 46 (1) : 35 – 41.
WATANABE, T., M. OOWA, C. KITAJIMA, and S. FUJITA, 1982. Improvement of dietary value of brine shrimp Artemia salina fir fish larvae by feeding them on ω 3 highly unsaturated fatty acid. Bull. Japan. Soc. Sci. Fish. 48 (12) : 1 775 – 1 782.
WATANABE, T., F. OOWA, C. KITAJIMA, S. FUJITA, and Y. YONE, 1979. Relationship between the dietary value of rotifers Brachionus plicatilis and their content of ω 3 highly unsaturated fatty acids. Bull. Japan. Soc. Sci. Fish., 45 (7) – 883 – 889. (In Japanese with English synopsis).
YAMASHITA, K. 1966. Fundamental studies for the culture of Chrysophrys major IV. On disease of larval and young fish (2). Abnormal expansion of the swim bladder. Bull. Japan. Soc. Sci. Fish., 32 (12) : 1 006 – 1 014. (In Japanese with English synopsis).
YONE, Y., and M. FUJII, 1975a. Studies on nutrition of red sea bream-XI. Effect of ω 3 fatty acid supplement in a corn oil diet on growth rate and feed efficiency. Bull. Japan. Soc. Sci. Fish., 41 (1) : 73 – 77.
YONE, Y., and M. FUJII, 1975b. Studies on nutrition of red sea bream-XII. Effect of ω 3 fatty acid supplement in a corn oil diet on fatty acid composition of fish. Bull. Japan. Soc. Fish., 41 (1) : 79 – 86.