Ms. N. DEVAUCHELLE
INTRODUCTION
The idea itself of developing commercial hatcheries for marine fish in Europe is recent (GIRIN, 1980). But it has now been established, save exceptions, that for sea-bass gilthead sea-bream, sole and turbot, development depends on having under control the production of eggs. Thus it is important for the aquaculturist to know the answers to the questions, where, how, how long and for what results must one, as soon as a hatchery has been created, envisage the construction of a unit for spawners.
From the beginning of the Century. specialists in rearing have remarked that the turbot (ANTHONY, 1910 ; BUCKLAND in MALARD, 1899) and the sole (BUTLER, 1895) could reproduce in captivity. But the reproductibility of the phenomenon has still to be demonstrated. For this, around the years 1960 – 1970, most of the works were aimed at obtaining, as quickly as possible, fish caught at sea, to spawn. Then hormonal induction was commonly employed for maturation followed by spawning and manual fecundations (BARNABE, 1976 a ; BRASOLA, 1974 ; FLUICHTER, 1972). On parallel, the IFREMER Center in BREST concentrated on the possibilities of fish reproduction in captivity, in conditions similar to those found in natural environments. At first, all manipulation was avoided (GIRIN 1979). Later on, so as to simplify the management of the broodstocks and to optimize that of hatcheries, operations were developed, such as the alteration of the seasonal productions of eggs, the use of spawners born in hatcheries, the modification of food diets and artificial spawnings.
Today, all the results obtained permit a comparison to be made between the many techniques employed to obtain eggs.
1. GENERALITIES
1.1 Stock constituting (Tables 1 – 2 – 3)
The first operation to carry out when a hatchery has been created, is to obtain good size spawners from the sea. This is what we have done so as to constitute the first stocks. But little by little, long term management proved more efficient. The principles to employ are as following : acquisition of 3 to 5 age groups of each species, within the limits as shown in table 1 ; substitution, every 1 to 2 years, of the older age groups by younger ones. Their number is calculated from specific productivities (Table 6) and the latency period, usually observed between their capture at sea and their first spawning in captivity is taken into account ; this being, one year for sea-bass, 1 to 2 years for gilthead sea-bream and 2 years for sole and turbot. Sea-bass and gilthead sea-bream are transported in tanks of 1,5 to 2 m3, by road. Loads never exceed 100 kg/m3. For distances requiring, at maximum 4 hours, it is not necessary to oxygenate the water. For the same length of time of transport, flat fish are placed into plastic bags with oxygen, at a rate of 1 volume of fish for Two volumes of water. When the journey is less than 2 hours, turbot are transported without harm, in open plastic bags without water.
When fished, the animals are sorted out according to their sex (DEVAUCHELLE, 1984) so as to adjust the choice of fish to the needs of the station. The males are immediately marked and separated from females. Immediately, on their arrival at the reproduction stations, they are treated with green malachite formol so as to avoid the eventual propagation of parasites to the native stock (Table 3). If the animals should show wounds, they are treated with antibiotics. The lowering of the salinity to 20 ‰ for 2 – 3 days, also permits the rapid recovery of weak sea-bream. Nevertheless, strict vigilence is from then onwards, the rule to abide by : indeed, the first signs of weakness or parasitosis require quick curative treatments. As well as the treatments shown in Table 2, it is henceforth recommended to give intraperitoneal injections of vitamin C to male turbot.
The doses employed are 100 mg/kg of fish every 15 to 30 days until complete recovery has been obtained (MESSAGER, pers. comm., 1985). Generally, the Frequence of treatments is increased when manipulations are performed and when there are important thermic variations of the sea-water.
The week following their transfer, the healthy fish are marked (Table 3); the others are marked after a few weeks of acclimatization to captivity. The ideal marking for the breeders is that which permits to individually identify, in situ, the fish according to their sex and age group. For turbot, the technique employed is a branding iron which has been cooled in liquid nitrogen. The operation takes 8 to 12 seconds and must be reperformed, according to the growth speed of the fish, every 6 to 12 months. For the sea-bass and gilthead sea-bream, this method is not sufficient. Also, the labelled brands proposed on the market have been dropped., They are too small and also cause wounds and necrosis. The magnetic brand, less traumatic, is still costly and does not permit to distinguish the animals in the tank. In the particular case of spawners, it has thus been confirmed as inefficient.
Therefore up to the present moment, sea-bass and gilthead sea-bream are indentified by simply injecting Indian ink into the bottom of the pectoral fins (SUQUET, 1986). The absence of individual marking then calls for, before any hormonal induction for maturation can take place, the control of the state of advancement of the gametogenesis by means of a catheter (BEDIER, 1979) or by performing a biopsy (DEVAUCHELLE, 1984).
The fish (sea-bass, gilthead sea-bream, turbot and sole) sorted out and marked according to their sex, are placed into the tanks for 4 to 6 years, the characteristics of which can been seen in Table 2. But, sometimes, there is not enough space. Certain batches whose spawning season has not been altered are then kept in cages when it is not their period for spawning. This practice, rarely employed, refers to turbot which, in appearance, seem less fragile than the other three species. In all cases, the adaptation of the fish to captivity is facilitated by the presence in the new stock of some “veterans” who incite the others to take inert food while at the same time reducing the fasting period which normally follows, when fish are captured (15 to 45 days depending on the season).
1.2. The storing tanks
They contain 5 to 40 m3 of sea-water. The depth of the water varies from 0.7 to 1.7 m. The materials employed for the construction of the tanks are not standardized. If circular, of subsquare or rectangular section, in polyester. cement, treated wood with PVC covering, it is important, for the flat fish caught at sea, that the tanks be lined with drained sand (DEVAUCHELLE. 1980). In this case, the upkeep charges increase. The sand must be, in fact, purified regularly by means of a drain or by chemical treatment (formol 38 %, 1 000 ppm) so as to avoid the development of parasites with resistant spores. In summer, the tanks are covered individually which limits the development of algae. The average light intensity at the surface is 1 500 to 2 000 lux.
The water distributed to the tanks by means of PVC pipes, is not recycled. Filtration (*a) is necessary with the use of thermic exchangers, (*b) but when there is no regulation available unfiltered water may be used.
*b = Exchangers with titanium plates
With the exception of temperature, the characteristics of the sea-water produce natural variations in our region (Table 4). The oxygen saturation rate is however liable to vary within the alarm limits (50–130 %) depending on the rates of renewal and the induced temperature changes of the water especially.
Regular controls are necessary. The dissolved nitrogen gas tenor can also be surprising, and create perturbations : filtration of air at pump level, turbulences in the pipes and the heating of the water are factors which can bring about the abrupt increase of dissolved gas tenors. This is remarked, depending on the species, by the apparition of exophthalmia (sea-bass) or gassy bubbles which accumulate under the skin or at the extremities of the fins (sole, gilthead sea-bream). In general, intense bubbling in the tanks will avoid the incidental mortalities and favour the rapid resorption (in around 24 hours) of the gas bubbles. The phenomenon is more insidious in sand bottom tanks : the air lift which ensures the drainage also creates air pockets. In burying themselves, the fish (sole) then die quickly of gassy embolism.
Normally, the characteristics of the sea-water are controlled each day by means of 3 classical devices ; the thermograph, the refractometer and the oxymeter.
1.3. The food
The food is distributed ad libitum 2 or 3 times per week, in a fresh or frozen form (less than 3 months of conservation). Except in particular tests (cf § 2.5), no vitamins are added. The quantities distributed vary according to the seasonal temperatures and the gametogenesis. They are situated between 4 and 24 % of the daily averages (fresh weight of the food/fresh weight of the spawner × 100). The quality of the food depends on local fishing. But more often, fat and lean fish are distributed alternatively, to turbot, sea-bass and gilthead sea-bream. Also , depending on the supply stock possibilities, mussels, clams or crabs are given to sea-bass and gilthead sea-bream.
Sole have a different food diet : molluscs (Challysta chione, Glycimeris glycimeris, Laevicardium crassum) et polychaeta (Nereis diversicolor et Nephtys hombergii).
1.4. Spawning techniques
In captivity, the four species can reproduce, without human intervention, naturally fecundated eggs. But in certain circumstances, artificial spawning techniques are employed. This involves spawning by hormonal induction of sea-bass and gilthead sea-bream. HCG (200 – 500 U. l./kg O) and LHRH (1 u g/kg) are then used by following the indications by SUQUET (1986). These hormones synchronize the female ovulations and can be considered as a technique to employ for short period alteration. It is effective only with advanced vitellogenesis :-ovocytes of 650 u or more, for Mediterranean sea-bass (SUQUET, 1986)- Whatever, the quantity of hormones employed, the injections are then followed by spawnings and natural fecundations.
Artificial spawning for turbot consists of “stripping” operations and of dry or wet artificial fecundations without the use of hormones (BARTON, 1981).
The sole neither reponds well to spawning by hormonal induction nor to abdominal pressures.
Also, artificial fecundation, without the need of killing the male, is difficult to envisage due to the size of the testicules (RGS maximum 0.2 % - DENIEL, 1981). with this species, artificial reproduction is not usually employed.
Artificial spawning techniques alone are employed in some hatcheries. But as they are not standardized, important differences are obtained in the interpretation of the results. To understand more clearly the possibilities of reproduction in captivity, we have willingly left aside the principal objectives of research. The comparison of natural and artificial spawning will thus depend on the data obtained outside the IFREMER Centre in BREST.
1.5. Collection and Treatment of eggs
Systematic measures : Each batch of eggs (or spawnings) collected at the outlet of the tank or after artificial spawnings, are subjected systematically to aliquot measures, for diameter and viability rates. The hatching and bone malformation rates of the larvae with swim bladders are estimated after the standard incubations have been performed in volumes of 0.1 liter (DEVAUCHELLE, 1980).
Particular measures : On the other hand, the weight of the eggs has been estimated for 13 spawnings of sole, 17 spawnings of sea-bass and 48 spawnings of turbot, involving a large range of egg diameter (DEVAUCHELLE, CLADAS, 1983 ; DEVAUCHELLE, in press).
The optimal conditions of temperature and of salinity for hatching, along with the duration for incubation have also been established in an experimental incubation unit under strict control (DEVAUCHELLE et al., in press). On the other hand, measures of the biochemical composition on the eggs have been carried out between 1979 and 1982 (DEVAUCHELLE et al., 1982).
On parallel with these tests, routine incubations are carried out in incubators of 25 to 40 liters (DEVAUCHELLE, 1983). The mobile incubator is operated by the rotation of an electric motor having a frequence of 1 rotation/minute.
Whatever the volume be in the incubator, the embryogenesis takes place in natural photoperiod conditions. The temperature can be regulated as desired, between 7 and 25° C. The water is filtered but no chemical treatment is performed. Loads do not exceed 7 g of eggs per liter which in 5 000 to 10 000 eggs per liter depending on the species. These values have been defined as optimal superior limits for quality hatching.
1.6. Principle of the reproduction/incubation facilities (Fig. 1)
The facilities are separated from those for larvae rearing, on account of the following reasons : the volumes and quality of the water required by these two stages are very different from the others. On the other hand, incubation in small volumes permits, if necessary, to individualize the incubation, to control more adequatly the abnormal mortality of eggs and the consequent development of diseases. Also the estimation of malformations and the hatching rates are facilitated, thus enabling a better interpretation of the results for larvae rearing.
2. RESULTS
2.1. Maturation - Natural spawning and fecundation with neither control of the
temperature nor photoperiod
2.1.1. Spawners
The annual mortality rate (Table 2) recorded, is feeble for sea-bass and gilthead sea-bream : 2 – 5 %. It is around 5 and 10 % for sole and turbot. But although sporadic, the losses with the turbot can be spectacular and destroy more than 50 % of the stock. This happened twice in 10 years, at periods, when there were sudden rises in temperature. The origin of these mortalities has not yet been defined but they are connected with those provoked by bacterial diseases such as vibriosis (LIEWES, 1984). Both males and females, at advanced mature stage, are the most affected. For the present, it has been remarked that simple intraperitoneal injections of vitamin C at rates of : 100 mg/kg of fish reduce these mortalities.
The growth of the wild spawners in captivity seems on general, superior to that remarked at sea (figure 2), with however the exception of the gilthead sea-bream. There are two explanations for this. The data of reference for wild gildhead sea-bream concerns the Golfe du Lion, where the average annual temperatures are superior to those found in the Brittany region. On the other hand, captivity, by interfering with the Winter migrations, permits the gilthead sea-bream to sustain lower temperatures than it could normally, at sea.
2.1.2. The tank volumes
We haven't remarked a clear difference in the productions of eggs in 10–12 m3 and in 40 m3, with maximum loads of 7 kg/m3 for sea-bass, turbot and gilthead sea-bream. On the other hand, the results obtained for sole are comparable, in 5 and 17 m3. Smaller volumes have not been tested, although FONDS (1979) remarked natural fecundations of sole in tanks of 1 500 liters. However, at experimental level and a fortiori, egg production tanks, no smaller than 5 m3 are, as far as we are concerned, the lowest possible limit advisable : In smaller tanks, the results obtained from a very small amount of fish, should not be of statistic signification.
Finally, the experiment proves that groups of tanks of tanks of 10 to 15 m3, with shallow water (0.7 – 1 m) are more suitable for hatcheries whose principal aim is profitability : the state of the population can, in this way, be easily controlled. Also, the upkeep of the tanks, the manipulations and egg retrievals are more easily performed.
2.1.3. The gametogenesis of females and the spawning season (Table 5)
The recent use of biopsies performed on market fish has permitted to define when the gametogenesis begins with the different species : In September/October, which is 3 to 5 months before sea-bass and gilthead sea-bream begin their spawning period ; in January which is 4 to 6 months before turbot begin to spawn. For sole, the sexual rest period has not been clearly defined. As at sea, the gonads of sole contain several generations of ovocytes all year long (LAHAYE, 1972). For the other three species, the time lapse between the first growth of the ovocytes and the beginning of the spawning is still not precise, as this depends especially on the seasonal thermic rates.
The temperature indeed, has an effect on the speed of the vitellogenesis and acts as a minimum/maximum threshold for the oviposition. It has such an effect, that it can reduce by around 25 %, the duration time for the gametogenesis of each of the species concerned, totally suspend the spawning season or advance or delay its date : with the sea-bass for example, the gonads contain, from the beginning of December ovocytes, the diameter of which (800 to 1 000 um) predicts future ovulation, as is remarked in the Mediterranean. While at this period, the temperatures of the sea-water (7 – 8 °C) are at the lowest limits for spawning. Although there exists an advanced ovogenesis, we must wait until Spring when the water is at 9 – 10° C or more, to collect the first sea-bass spawnings. It is evident that the latency period can differ from year to year. Consequently the apparent duration for maturation will also vary. However, as the threshold temperatures for the gametogenesis and spawning are known (Table 5) we can, by means of thermoregulation, be prepared for the effects of seasonal conditions which can be exceptionally unfavourable.
On the contrary, if necessary, the variations in temperature permit blocking the production of eggs, at certain periods. For delicate fish, such as the gilthead sea-bream, this ensures a synchronization of the spawnings, without the needs of manipulations nor hormone injections. With this species, the variations of 2 to 5° C in 24 hours are well tolerated and now practised in certain Centres for the production of juveniles. But more often, when there exists neither thermic nor photoperiod controls, spawning will begin a little later in tanks than at sea : end of February/beginning of march for sole and sea-bass, end of April to the end of June for turbot. Gilthead sea-bream spawn in Winter time if the temperature of the water reaches more than 13° C, but this has never happened naturally in the Brittany region in tanks having a non thermoregulation of the surface water supply. The spawning seasons continue until May (sole), June (sea-bass) and August (turbot). On the other hand, the sexual rest periods, solely associated with the presence of ovogonies founds in the gonads, lasts at most for two months : in Summer, for the sea-bass and gilthead sea-bream, at the end of November/December for the turbot.
Finally, it has been remarked, that males at least, produce sperm throughout the whole spawning season, from November to August, for the sea-bass, from December to October, for the turbot.
2.1.4. Fecundity and quality of the eggs (Table 6)
In non limiting conditions of temperature and photoperiod, the relative fecundity (*a) is inferior by 20 to 50 %, throughout the first two spawning years, for fish at the beginning of their reproduction activity. The adults caught at sea do not recover a normal level of fecundity until the second cycle of spawning takes place in captivity. The fecundity is then stabilized as shown in table 6. We then remark that our data corresponds quite definetely to fecundities estimated for mature sea-bass (table 6). They are however 2 to 3 times inferior for turbot, sole and gilthead sea-bream.
*a = Number of eggs collected per kg of Female
Fish spawn from one to twelve times depending on the species. The eggs of the female sea-bass are all set free at a few days of interval. The spawning period lasts longer for the gilthead sea-bream, sole and turbot : a female turbot can for example produce eggs for 7 to 8 weeks, every 3 – 5 days.
The viability rates are different with round fish than with flat fish. They are very high for gilthead sea-bream and sea-bass as only 10 to 20 % of the spawnings have a viability rate of less than 70 %, more of ten remarked at the beginning and end of the season. for sole, although the viability rate of the eggs is on average less than 10 % than that obatined with sea-bass and gilthead sea-bream, results can vary from year to year.
With a 33% viability rate, the turbot is the most complex species. This figure has been obtained on averages estimated over several years. But the detailed study of the results shows important variations of the annual average rates 0 to 81%. In bad seasons, the cause is the absence of fecundation rather than the quality of the ovules. Neither the adaptation of tank volumes (GIRIN, 1979) nor the necessity of sand bottoms (DEVAUCHELLE, 1980) permit the explanation of this phenomenon. In fact, once again certain physical parameters of the sea-water seem to perturb the process of fecundation.
2.1.2 The eggs : Description and incubation (Table 7)
The impregnated egg is a pelagic sphere which, in an incubation environment, is slightly deformed into the shape of a rugby ball. The different species differ in diameter, weight, and aspect of the lipidic reserves (Photos ). When mature, the egg of the sea-bass contains 1 to 5 lipidic drops which represent 2 to 3% of the total volume. The egg of the sole contains a multitude of diffused lipidic drops. The eggs of gilthead sea-bream and turbot contain one globule with slight variations in daimeter : 180 to 210 um(turbot), 210 to 240 um (gilthead sea-bream). The analysis of the eggs of sea-bass, sole and turbot (Table 12) indicates the water, lipid, protein and ash tenors.
After the fecundation has taken place, the egg hydrates. The rate of humidity increases by 25 % about. The perivitelline space appears 15 to 60 minutes after the emission of the ovule, independantly of the fecundation. The diameters vary from one batch to another : 6 % for sea-bass and gilthead sea-bream and 10 % for sole and turbot. Within these limits, there is no clear correlation between the diameter and the viability rate or bone malformations (DEVAUCHELLE, 1980). However, the dry weight of the egg increases according to the diameter (Figure 3). The duration for incubation depends on the temperature of the sea-water and on the species involved (Figure 4). As an indication, for turbot, the four major phases Morula, Gastrula, Neurula and prehatching represent on average 17, 15, 52 and 16 % of the total duration of embryogenesis. The risks of mortality are reduced when the incubation lasts 3 to 6 days (Table 8), which means at near optimal temperatures defined for spawning.
At the IFREMER/Centre in BREST, the salinity had been subjected to precise experimental tests for turbot and sole. This paragraph deals exclusively thus with these two species. It is important to know at first that salinity has no effect on the duration of incubation but determines, in synergy with the temperature, the hatching and larvae malformation rates. The effect is more pronounced at stages considered as fragile : (Figure 5). However, in all cases, more than 70 % of the viable eggs hatch without malformation at between 25 and 35 ‰, more than 50 % up to 15 ‰. The sole can support better than the turbot low temperatures and salinities. When the eggs tested are at Gastrula and Neurula stages, considered to be more resistant than the Morula stage, the quality of the hatchings is equal to 15 and 35 %. But, In all the cases, 10 % is the limit not to be exceeded for both turbot and sole. In extreme conditions of incubation, the individual characteristics of the different batches of eggs are expressed by the level of the mortality rate ; in optimal conditions, the differences are more remarkable at larvae malformation level.
On general, eggs are fragile at Morula and prehatching stages. With turbot, especially, all stress of mechanical or thermic origin must be avoided during the first and last fifth of incubation. It must be remarked finally that the larvae with air bladders, resist better than the egg at Neurula stage, the variations in temperature and mechanical shocks. The larvae are, in consequence, propice for transport, although at this stage of development, they seem to be more sensitive to chemical treatments, especially chlorine (DEVAUCHELLE, 1980).
The experimental tests, carried out in automatic incubators of 1 liter (Table 6), in optimal temperatures and salinity, lead us to the following conclusions : The hatching rate of viable sea-bass and gilthead sea-bream eggs was 10 % greater than those of turbot and sole. The average rate of malformations was low : 5 %. In these conditions, the differences in results between the batches of eggs of the same species reflects the way in which the rearing was carried out prior to incubation : spawning conditions or genetic factor. Employing the same treatment, 60 % for the hatching rate and 30 % for the malformation rate of sole and turbot eggs have been obtained. For the sea-bass, they are respectfully 20 and 14 % on average. Generally, delayed hatchings are associated with high rates of malformation.
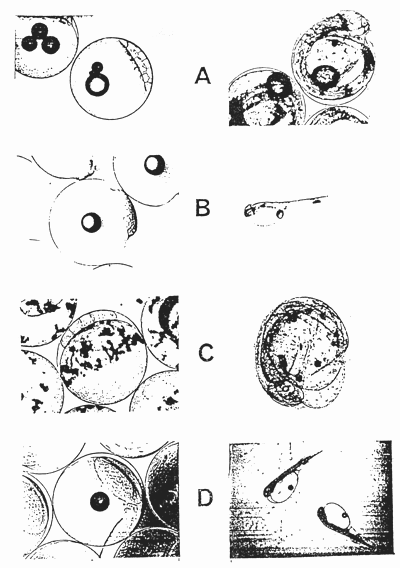
Eggs and larvae which have just been hatched or are about to.
A : Sea-bass - B : Gilthead sea-bream - C : Sole - D : Turbot
The biggest incubators (25 – 40 liters) allow on average a hatching rate of 60 to 70 % and rates of malformation of 5 to 15 %. The differences between the species are less pronounced, as the results reflect essentially the general conditions of incubation, especially mechanical shocks. Other incubation techniques are employed with these species. But in all cases, their effect fulness depends especially on having the same standards : water, incubation densities, temperature levels, salinity and mechanical shocks (DEVAUCHELLE, 1980).
In the actual state of affairs, incubation is not a limiting factor for rearing. The selection of the spawnings depends essentially on the management of the larvae rearings. We could however schedule the sorting out of egg batches according to when they resist less to shocks of thermic or saline origin, at the beginning or end of embryogenesis. The selection can also be obtained by staggering the hatchings.
It can be finally concluded that a big hatchery can reasonably envisage the use of 50 % of the viable eggs collected, by rationalizing the incubation techniques, which represents for 1 kg of females : 100 000 larvae of sea-bass, 400 000 larvae of gilthead sea-bream, 50 000 larvae of sole, 25 to 60 000 larvae of turbot.
2.2. Artificial spawning (Table 6)
In our regions, artificial spawnings are not up to standard for sole. They are often not necessary for gilthead sea-bream which spawn daily a great quantity of eggs, during many months. It is, on the contrary, employed for sea-bass so as to synchronize the spawnings of several females and for turbot so as to compensate for irregular fecundations.
With sea-bass and gilthead sea-bream, hormonal inductions for spawning rises the mortality rates of spawners by about 10 %. On the other hand, it seems to decrease the requirements of the sea-bass concerning environment.
Indeed, the volume of the spawning/fecundation tanks can be reduced to 4 – 5 m3 without causing any disadvantage, while 10 m3 seems to be the minimum volume compatible with natural maturation-spawning-fecundation (BARNABE, 1976 b). The actual progress made in the hormonal induction of spawning guarantees relative fecundities and high rates of viability for both these species. The differences when compared with natural spawnings are all the less evident as the majority of hatcheries carried out alternatively both techniques. It must also be remarked that LHRH is greatly employed which on the contrary to human gonadotropine avoids antigenic reactions (BARNABE, 1985).
With turbot the stripping, less selective than the natural oviposition, doubles the relative fecundity. Nevertheless, the average viability rates are very low. They recall to mind the first artificial fecundations carried out on sea-bass. Indeed, each female turbot has its own rhythm for ovulation (Mc EVOY, 1985) and six hours afterwards, the fecundation levels decrease. Due to the working hours, the hatchery often neglects this rhythm : the difference in the quality of the eggs is evidently remarked. For the moment, the females are rarely selected on account of their rhythm of ovulation. On the other hand, there is still no effective means of synchronizing the spawnings. when fecundation does not take place naturally, the aquaculturist must therefore found his profitability calculations on the maximum figure of 33 % for the viability rates. On the other hand, abdominal pressures, properly carried out do not cause mortality.
2.3. Alterations in the seasonal spawnings
2.3.1. Method
In temperate regions, the process of gametogenesis depends essentially, as already stated, on the temperature and photoperiod, when the salinity rate is at around 30 and 40 ‰. The first alteration in spawnings were thus, naturally based on the simulations of thermic and photoperiod cycles found in our region. The spawnings were either brought forward (GIRIN and DEVAUCHELLE, 1978) or delayed (DEVAUCHELLE, 1983) in report to normal cycles. Later on, we continued little by little, to get as near as possible to the optimal light and temperature conditions remarked at normal spawning season (Table 5). This approach, along with chance (abrupt variation in temperatures) led us to simplify progressively the technique for alteration.
Two objectives were focused :
To reduce to maximum the heating and cooling costs of the sea-water while maintaining a high quality of the eggs.
To know the respective affect of both the temperature and photoperiod factors on the gametogenesis and the spawning processes.
Some results, which were obtained through an experimental approaches have been reproduced over 2 or 3 spawning seasons for each species. On parallel, comparative tests have be conducted on the turbot (DEVAUCHELLE, in process).
2.3.2 Results
The mortality of spawners is comparable to that registered for those which have had no alteration carried out on spawning. On the other hand, only alterations of more than 1.5 – 2 months is taken into account here. Below these values, this is a short term alteration which calls for only simple thermic regulation (cf § 2.1.3).
Generalities: The schematization of the ideal techniques for spawning would lead to excluding numerous possibilities which have not yet been tested. In temperate zones, the results can be resumed as follows : as remarked in normal spawning periods, the light conditions define the beginning of the gametogenesis (previtellogenesis phase). This action is really evident with the turbot. By this fact, it is now common to alter the spawnings according to the photoperiod variation alone. The level of temperature is controlled when necessary, depending on the rearing zone. The spawning for the four species taken into account here, can be altered, at any time of the year by following the indications given in table 9, which are very close to the optimal conditions remarked in natural spawning seasons.
When alteration begins: The groups of fish whose spawning is to be altered for the first time must preferably take place at the end of their sexual rest period. The weight progression must be above all normal, which is according to the bibliographical data, form + 2 to + 20 % depending on the species and age, (Figure 2) in report to the end of the preceding sexual rest. The alterations begin normally with the contraction of the photoperiod cycles and therefore an acceleration of the gametogenesis or with a long continuous light period (turbot). Delayed spawning of more than 1.5 months are less frequent, but possible, by blocking the photoperiod cycle during the sexual rest period.
In any case, the duration of the gametogenesis can be brought to the minimum time length remarked in natural environments, which is 3 – 4 months depending on the temperature. Taking into account the minimum duration of sexual rest (2 months) and average duration of the spawning season, it is possible to provoke the oviposition every 7 months (Table 10), but the decrease in the fecundity and of the quality of the eggs (Table 11) following the more reasonable contractions of the cycles (10 months instead of 12) lead us to consider this forcing as a last resource. The medium results obtained for the second oviposition of sea-bass confirms this more so (SUQUET, 1986).
Ideally, once the alteration is obtained, the advanced or delayed photoperiods must be stabilized at 12 months. The control of the temperature will remain circumstancial, within the limits indicated further above. By this fact, the beginning and end of spawning escapes the seasonal changes in the temperature and the sea-water, more so than in natural conditions. Naturally, the seasons reach their maximum : 5 – 6 months for gilthead sea-bream, 3 –4 months for sea-bass and turbot, 3 months for sole.
On the other hand, the influence of the alteration is not directly remarked, as to concern the diameter of the eggs. Even if the eggs spawned outside the normal season are often smaller, it appears more advisable to correlate these variations to the thermic rates (of Discussion).
The alteration can finally be accompanied by modifications of the global composition of the eggs. The first analysis on sea-bass, turbot and sole, showed increases in the total lipid rates and reductions in the protein rates when compared with wild fish and fish eggs in capativity whose spawning had not be altered (Table 12). The reports of the lipid categories also differ, while the unsaturated fatty acid rates contrast with spawning obtained in captivity and ovules of fish, caught at sea.
In this case, the temperature, on average higher, provoked more regular feeding habits with spawners. Therefore, it can be assumed that the alteration has an indirect action. Taking into account the implications which it can have on the survival of larvae, it must nevertheless be considered. With the improvement of the larvae rearing techniques, especially these for sea-bass (COVES, 1985), there exists ways of verifying the effects of the different quality of eggs for the survival of larvae and juveniles.
2.4. The origin of the spawners
2.4.1. Methods
As there is no data available on the genetic selection, the marine fish spawners born in hatcheries, are often chosen first. To avoid important sex-ratio unbalances, sorting out according to their sex is thus necessary, before they are finally placed into the reproduction unit. Unfortunatly, the sorting out and production of spawners born in hatcheries has only been recently adopted. In Brittany, only data on the growth of sole and turbot is available. On the other hand, a test of comparison was carried out on turbot so as to evaluate the differences in fecundity between fish from hatcheries and those from the “wild”. On parallel, a test on feeding was also carried out (Table 13).
2.4.2. Results
Sole and turbot from hatcheries have a rapid growth phase at high temperatures. But, as soon as they are placed into natural conditions of temperature, their growth rate slows down when compared the that of wild fish, or at least remains the same as the latter (Figure 2). When the first cycle of maturation takes place, they are inferior in weight to wild fish of the same physiological age. Also, in both cases, males become mature a year before females do.
As for the follow up of the fecundity of turbot, it should be noted that, during the test, abnormal mortalities were remarked. They were reduced by intraperitoneal injections of vitamin C (100 mg/kg of fish). In these conditions, the number of eggs spawned by the fish in hatcheries is 36 % less and weight losses following spawning is at 3.3 % against 5.7 % for wild fish.
It must be recalled that the fecundity level is a result of the influence of a great number of parameters which have marked the history, even of long age, of the spawner (STEARNS and CRANDALL, 1984 ; WOOTON, 1982). These results must therefore be interpreted as a consequence of important perturbations during rearing which are still not under control.
With the improvement of larvae and juvenile
2.5. The effect of feeding
On this particular point, there are numerous results available. Their originality make them interesting however.
The artificial spawnings carried out on turbot (table 13) do not allow the definition of the viability rates for the same reasons as those given here above. However, the results show that turbot, weakened for unknown reasons, don't die as much, when their food contains vitamins. This treatment has also brought about a 33 % increase in the relative fecundity of surviving females. This is however not the case when the groups of turbot appear healthy (NOEL, 1985).
For the sole and the sea-bass (Table 14), the changes in food have no clear influence on fecundity. The absence of polycheata for sole or the distribution of artificial food to sea-bass bring about on the other hand a decrease in the viability rate.
With the improvement of artificial food, it appears however that the disadvantage is not so evident with the sea-bass (SUQUET, 1986).
Therefore, these first observations indicate the interest of following attentively the relations between rearing environment-food diet and larvae survival. The improvement in the artificial food for spawners should also be accompanied systematically by larvae rearing test so as to detect their effect at more acute levels, than at fecundity or conformation level of the eggs. The works carried out in fresh water is an example in this sphere (QUANTZ, 1980; LUQUET and WATANABE, 1985).
3. DISCUSSION/CONCLUSION
These results open perspectives for their application in aquaculture, especially for the spawning alteration techniques, the standardization of units for storing spawners and the incubation of eggs. On the other hand, like the observations made in natural environments represent a basis of reflection for the aquaculturist, the artificial techniques employed for spawning can be based on the results obtained form natural reproductions in captivity.
Consequently, today, the adoption of one technique or the other results from the arbitration based on the species, the rearing zone or simply the material constraints. Finally, a better knowledge of the methods of reproduction now permits, to stabilize, increase or stagger as wished (Figure 6) the periods for the production of eggs. But, as in most of the the theoretical or experimental studies carried out on induced reproduction (HOAR, 1969; LILEY. 1980) is to admit that the production of viable embryos results from a delicate compromise which integrates the action of external and intrinsic factors.
The specific differences should be distinguished especially. Thus, the sole and turbot don't adapt as well as the gilthead sea-bream and sea-bass in captivity : they develop parasites more easily ; the annual mortality is on average twice that for sea-bass and gilthead sea-bream; finally the viability rate is weaker. On the other hand, the gametogenesis and the spawning of sole and turbot is suited to low temperatures. As the confinement in tanks does not permit the fish to escape, abrupt increases in temperature is Spring and Summer, their apparent inadaptation to captivity could be linked with unfavourable thermic levels for the species.
Also, gilthead sea-bream, sole and turbot which differ from sea-bass on account of their numerous ovipositions, have in captivity, a less relative fecundity rate that those calculated for mature fish caught at sea. Again, let us give precisions on the methods of estimation: In captivity, fecundity refers to the number of eggs exactly spawned. Due to this, it is difficult to compare it to the fecundity calculated in natural environments from the total number of oogonia and ovocytes found in a gonad (DENIEL, 1981), while taking into account especially that these last waves of ovocytes are often reabsorbed (LAHAYE, 1972). Thus, logically, the differences between the two values increase with the number of ovipositions. Fecundity in captivity cannot be therefore stated as abnormally low.
The age of the animals is also, as is the case for most species, a source for variation in fecundity. Due to this, the good management of the hatchery for marine fish is based on the careful choice of the size, weight and ages of spawners. The experience from salmoniculture (BILLARD, 1986) or of carp breeding (MARCEL) 1986) have also proven this. It is also interesting to discover that the viability rate and the diameter of the eggs vary little with the age of the fish.
As for the environment, its effects on reproduction are remarkable. Thus, on the coast of the La Mancha, from the Atlantic to the Mediterranean, the temperature seems to be the most determinant extrinsic factor. The respect of the inferior/superior limits, indeed influences the good evolution of the gametogenesis and spawning. However, when the thermic control is difficult, hormonal induction of maturation, by LHRH especially, can be of great help (BARNABE and BARNABE-QUEST, 1985). The long term consequences still need precision. On the other hand, certain characteristics of the eggs (viability rate, hatching rate and malformation rate of larvae with air-bladders) are also influenced directly by the temperature. Its indirect effects must also be remarked: by modifying the quantity of food ingested, the temperature can, for example, influenced fecundity, the dates for spawning of the fish (WOOTON, 1982) and as we have suggested, the biochemical composition and the diameter of the eggs. As for this parameter, it is interesting to note its evolution, in function of the temperatures registered at the moment when spawning took place (Figure 7).
Salinity does not need any particular control when within the range 33 – 36 ‰. In zones with lots of fresh water, the gametogenesis can however be blocked, especially with sea-bass (BRUSLE and ROBLIN, 1983 : ZANUY and CARILLO, 1983). On the cotrary, by reproducing in the Baltic sea, at 15 ‰ (KUHLMANN et al, 1980), turbot confirm their euryhaline character.
Salinity and temperature also influence the success of incubation. it has been remarked for the slow and turbot and also for gilthead sea-bream (FREDDI et al, 1981) and probably for sea-bass as applies for all Teleostei (BLAXTER, 1969; HEMPEL, 1979).
Otherwise, all the references (BILLARD, 1979; BYE, 1984) show that in temperate zones, the photoperiod has, along with the temperature, a determinant action on the gametogenesis. However, the combination between light-conditions and temperature levels still remain imprecise. The use of recent techniques for biopsies should help. DEVAUCHELLE (In print) shows also that with turbot, the initiation of the gametogenesis is almost exclusively controlled by the photoperiod, while the temperature regulates, along with the photoperiod, the speed of the vitellogenesis. It is evident that the improvement in the techniques of the spawning alteration requires a progress of knowledge in this sphere. Apart from its effects on maturation, the role of the photoperiod is not clearly understood (WOOTON, 1982) although an evident direct influence on the activity level of the fish is remarked.
As for feeding, most authors (Fontaine and OLIVEREAU, 1962 ; HEMPEL, 1979 ; DABROWSKI, 1984) agree on the impact of food rations on fecundities and spawning dates. The effect on the quality of the eggs, even in extreme conditions is however controversed (LUQUET and WATANABE, 1985). Not having detailed studies at disposal on the “special reproduction” food, the normality of the growth curves and conformation can as far as we are concerned be kept as an indication of good feeding.
On general, the conditions of temperature, salinity or lighting considered as optimal in a rearing environment, are, in fact, close to these found in normal conditions of life in natural environments. This has been verified for the gametogenesis, spawning, incubation, (present study) as well as for other stages of development: for the juvenile stage of turbot especially (SCHERRER, 1985). Depending on the regulation capacities of the species, it is indeed not excluded that the optimal conditions for the development of the parents and in consequence those of the eggs, can vary from one place to another (BLAXTER, 1969). But all the observations made tend to suggest that the choice of the rearing techniques should be based on, for each species, at each stage of development, the previsional effects (Survival, malformations, disease, feeding rate) that are caused by rearing conditions which differ more and more from normal life conditions observed in natural environments.
Apart from the biochemical aspects, the production cost of the larvae with air-bladders can influence the choice of reproduction strategies. It should therefore be precised that out working conditions, considered sophisticated, permit a low costing egg (Table 15) when compared to that of the juvenile. For the moment, the excessive simplification of production techniques of eggs in the actual state of sea-bass, gilthead sea-bream, sole and turbot rearing will have little effect on the production cost of juveniles.
Finally, it should be remarked that only a few of the factors which are liable to bring success to the hatchery reproduction of marine fish have been discussed here. The field of investigation in this sphere still remains open. Thus, the effects of the moon cycles, given phenomena and particular behaviour of each species (LILEY, 1980; COLOMBO et al, 1982; TAYLOR, 1984) offer un deniable interesting subjects of study. But today, so as to better perceive the processes which lead from spawners to commercial size fish, the most promising approach is to associate the study as much as possible to the different rearing phases. The rapid improvement of larval rearing techniques, those for sea-bass especially (COVES, 1985) has recently allowed this.
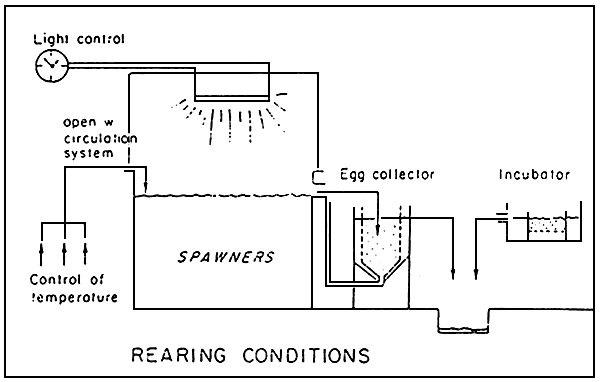
Figure 1: Skeleton diagram of the reproduction tanks, egg collectors
and incubators.
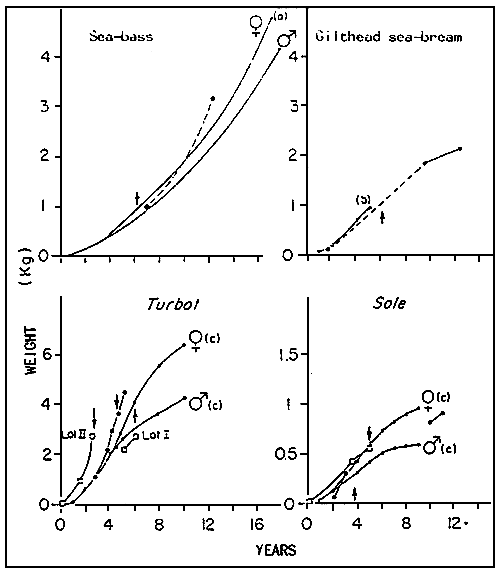
Figure 2 : Growth curves of wild fish in natural environments (0), in captivity (.) and of spawners coming from hatcheries (| |), feeding on fresh or frozen fish for three or more months.
→ = first spawning; ----- = estimated values;  = values
measured
= values
measured
a) BOULINEAU, 1969 ; b) LASSERRE, 1974 ; C) DENIEL, 1981.
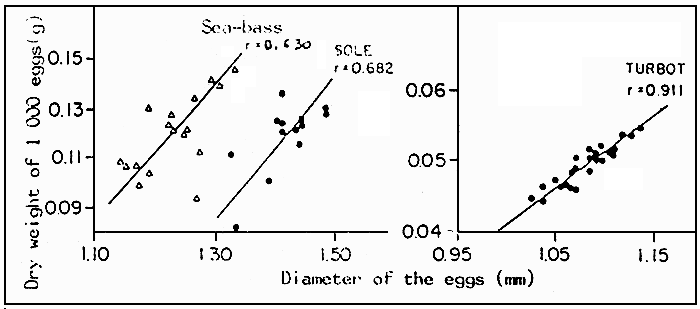
Figure 3 : Size-weight relation of the sea-bass, sole and turbot. The correlations are calculated according to the BRAVAIS-PERSON. coefficient.
Sea-bass : y = 0.1824 × + 0.01037
Sole : y = 0.2427 × + 0.26002
Turbot : y = 0.192 × + 0.149
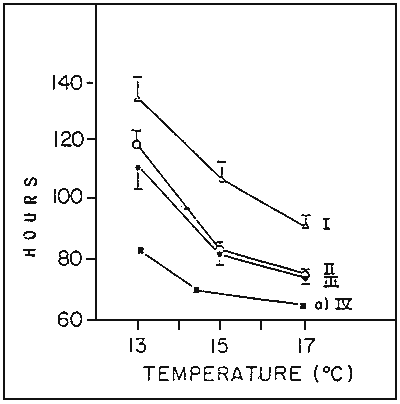
Figure 4 : Duration of egg development of turbot (I), sea-bass (II) and sole (III) (of the fecundation with a 50% hatching rate of viable eggs) and gilthead sea-bream (From stage 4 cells to hatching), depending on the incubation temperature. Between 13 and 17°C, 70 % at least (sea-bass, turbot and gilthead sea-bream) or 50 % (Sole) of viable egg give birth to normal larvae in our incubation conditions
a) CAMUS and KOUTSIKOPOULOS, 1984
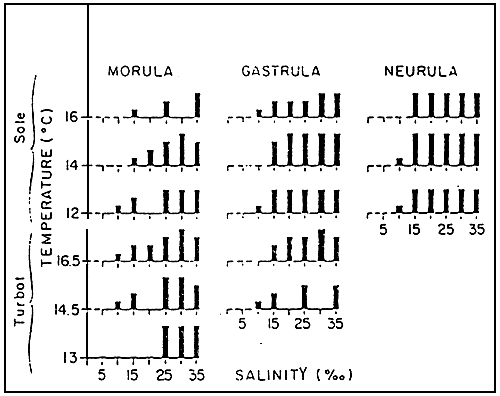
Figure 5 : Percentages of normal larvae coming from viable eggs of turbot and sole at temperatures of 12 to 16.5°C and 0 and 35 ‰ of salinity.
| 0 – 20 % | – – | 20 – 50 % | □ | 50 – 70 % |  |
| 70 – 80 % |  | 80 % |  | Tests not carried out | |
Figure 6 : Evolution between 1976 and 1984 of the total weight (Kg) of spawners, of the viability number and rate of the eggs collected. The objective was, during this period, to maintain a constant level of egg production of sea-bass and sole juring an unique season, the normal spawning season and the annual staggering of spawning for gilthead sea-bream and turbot. In 1984, the sea-bass spawning lasted for a period of 87 days. The unfavourable thermic Cycle for the emission of eggs of sole, not having been abble to be corrected, their spawning season was shortened to 28 days. On the contrary, that same year, two tanks of gilthead sea-bream and three tanks of turbot, having had photoperiod and thermic controls, respectfully reproduced during 218 and 209 days : estimated data.
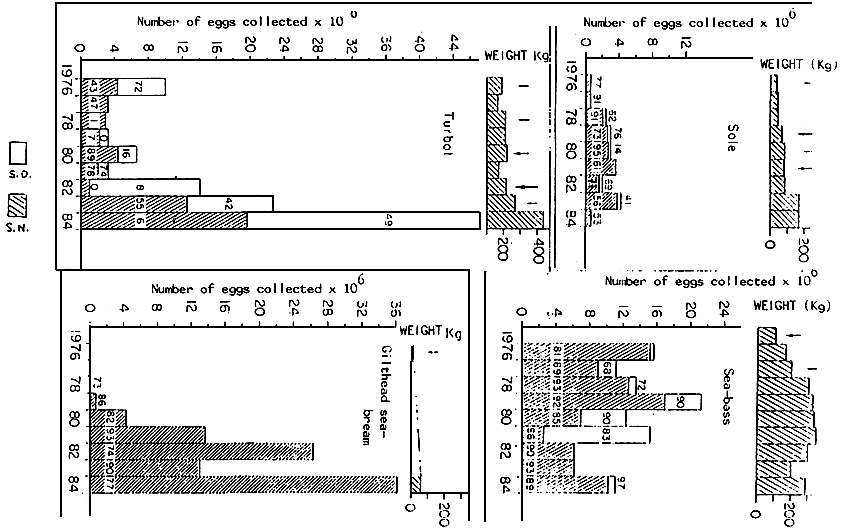
SD : Altered season
SN : Normal season
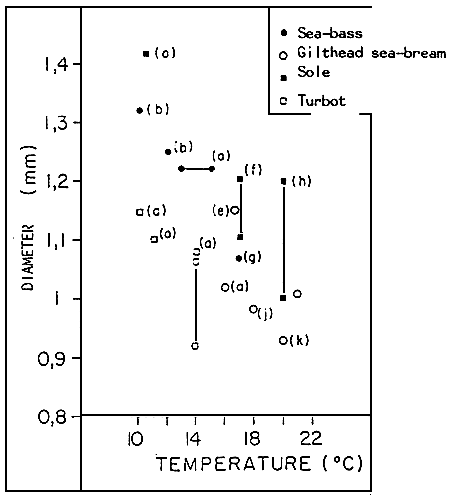
Figure 7 : Relations between the size of the eggs (D) and temperature (T) of the sea-water at the moment of spawning. D and T correspond to the punctual and average values of the data (b to k), from the IFREMER Centre in BREST (a).
a) DEVAUCHELLE, 1980 and our data
b) KENNEDY and FITZMAURICE, 1972
e) BRASOLA, 1974
f) BRASOLA, 1974
g) VILLANI, 1974
h) RAMOS, 1977
j) RAMOS, 1978
k) ALESSIO, 1975
| Conditioning | Reform | ||||||
| Species | Fish caught at sea | Born in hatcheries | |||||
| Kg | Years | Kg | Years | Kg | Year | ||
| Sea-bass | 0 | 0,6 | 5 – 8 | 0,7 | 4 – 7 | 2,5 | 9 – 12 |
| 0 | 0.8 – 1 | " | 1 | " | 3 | 10 – 13 | |
| Gilthead Sea-bream | 0 | 0,5 | 2 – 3 | 0,5a | * | - | |
| 0 | 0,8 – 1 | 3 – 5 | 0,6a, b | 3 – 4 | 10 – 13 | ||
| Sale | 0 | 0,5 | 3 – 4 | - | 1,2 | 15 –20 | |
| 0 | 0,8 – 1 | 4 – 5 | - | 1,5 | 15 – 20 | ||
| Turbot | 0 | 0,5 – 2 | 2 – 4 | 2 | 3 – 4 | 5 | 8 – 10 |
| 0 | " | " | 2,5 | 4 – 5 | 7 – 8 | 10 –12 | |
Table 1 : Choice basis of spawners expressed in weight and age
a : SUQUET, 1986;
b : ZOHAR et al., 1984
* : Recall that the gilthead sea-bream is protandrous hermaphrodic whose sexual reversion rate is at around 80 %
| Species | Fishing techniques | Volume of the tanks | Density Kg/m3 | Food | Mortality %/year | |
| Quality | Quantity %/S | |||||
| Sea-bass | line | 10 – 40 | 1,5 – 7 | Fish | 12 | 2 – 5 |
| Gilthead sea-bream - Seine not | 5 – 40 | 2 – 7 | Fish | 12 | ||
| Crabs | ||||||
| Molluscs | ||||||
| Sole | Seine net | 5 – 17 | 1 – 3 | Molluscs | 10 | 6 |
| Polycheatae | 2 | |||||
| Turbot | Drag net of 15 to 30 mn | 15 – 40 | 0,5 – 7 | Fish | 15 | 5 – 10 |
Table 2 : Fishing conditions of wild spawners : General indications on the storing conditions. The quantity of food concerns the fresh weight of food ingested/weight of the spawners × 100 per week.
| Species | Selecting + biopsy gonads | Stamping | Preventive and curative treatment | ||
| Sea-bass | +a | Indian ink | Copepoda | - | in reproduction tanks 200 ppm f ± 0.5 ppm VM normal water flow 3 days in succession |
| Gilthead sea-bream | +a | " | - | In baths, 500 ppm F - 20 mn Decrease in salinity | |
| Sole | +a | - | Monogenic Endobtella solea | - | In small aered volumes 300 ppm + 1 ppm VM 3 hours |
| Turbot | +a | Nitrogen | Copepoda | - | In an aered reproduction tank without water renewal Neguvon 1 ppm - 2 hours |
| Trichodina | - | In baths : 220 ppm F + 0.6 ppm VM - 20 mn | |||
| Metacercaires | In baths : 200 ppm F + 0.6 ppm VM - 20 mn | ||||
| In big aered tanks with water renewal : 100 ppm F + 0.3 ppm VM - 3 times | |||||
Table 3 : Principal treatments for spawners
a) DEVAUCHELLE, 1984
b) SUQUET, 1986
| SPECIES | Estimations carried out on the day of spawning | Incubation results remarked | N. of Spawners | Hatching of normal larvae | ||||||
| Aliquots taken from the N. of eggs × 106 | Diameter (mn) | Weight | ||||||||
| Average | mini-maxi | N. Spawning | average | -mini-maxi | N. of spawning | Duration of development ° C × hours - at 15 ° C = | ||||
| Sea-bass | 78 | 1.22 | 1,07 1,32 | 17 | 1 030 | 811 –1 240 | 3 | 1 215 ± 45 | 10 | 85 |
| Gilthead Sea-bream | 71 | 1,02 | 0,94 – 1,05 | - | 1 755a | - | 659 ± 18a | 7 | 85 | |
| Sole | 18 | 1,41 | 1,2 – 1,57 | 13 | 690 | 668 – 786 | 3 | 1 390 ± 91 | 12 | 74 |
| Turbot | 91 | 1,08 | 0,98 – 1,18 | 13 | 1 550 | 1 225 – 1772 | 3 | 1 400 ± 46 | 14 | 78 |
Table 7 : Principal characteristics of the eggs : The tests on incubation took place in incubators of “moteur” type described by DEVAUCHELLE, 1984, at optimal temperature and salinity levels.
| SPECIES | Temperature ° C | Duration of | ||
| Spawning | Incubation A | Incubation B | the embryogenesis (days) | |
| Sea-bass | 13 – 15 | 13 – 15 | 13 – 17 | 3,6 – 4,6 |
| Sole | 10,5 – 11 | 11 – 13 | 11 – 15 | 4,6 – 5,4 |
| Turbot | 13 – 15 | 13 – 17 | 13 – 19 | 5,6 – 3,3 |
| Gilthead sea-bream | 15 – 17 | 14,5a | - | 3 |
Table 8 : Comparison of optimal temperature ranges necessary for spawning and incubation. For this, two cases are envisaged :
a) CAMUS and KOUTSIKOPOULOS, 1984
| Spawning | ||||
| SPECIES | N. of Spawning Seasons | Duration(days) mini - maxi (average) | T° C | E (hours) |
| Sea-bass | 7 | 42 – 109 | 9 16 | 8,30 16,00 |
| (71) | (13 – 15) | (10 – 14) | ||
| Gilthead Sea-bream | 6 | 36 – 154 | 12,5/13 24 | 8,30 15,30 |
| (107) | (15 – 17) | (9 – 11) | ||
| Sole | 7 | 13 – 128 | 8 12 | 11 16 |
| (64) | (10,5 – 11) | (11,5 – 12) | ||
| Turbot | 8 | 30 –100 | 9,5 17 | 10 16,30 |
| (62) | (13 – 15) | (15 – 16,30) | ||
Table 9 : Spawners submitted to thermic and photoperiod controls. Duration of the spawning season and corresponding ascending descending and stable – temperature and photoperiod conditions. For the sea-bass, high temperatures (16 – 20 ° C) have been tested: the results obtained indicate that in favourable lighting conditions (11 – 13 hours day) the gametogenesis is blocked when T 21 – 22 ° C. The number of spawning seasons to which the data related is equal to the number of annual cycles.
| SPECIES | Minimum duration (months) | T (° C) | E (hours) |
| Sea-boss | - 2,5 | 20 16 | 11 12 |
| Gilthead sea-bream | - 3 | 12 – 14 | 16 14 |
| Sole | - 3 | 11 – 15 | 11 – 15 |
| Turbot | - 3 | 14 | 16,30 |
| -3,6 | 10 | 16,30 |
Table 10 : Temperature T and lighting E conditions tested so as to reduce the gametofenesis time length.
| SPECIES | N. of eggs observed S. N/S. D × 106 | N. of eggs/spawning % | Survival % | Hatching of viable eggs % |
| Sea-bass | 17,6/3,4 | - 84 | - 17 | - 13 |
| Sole | 4,4/1,2 | - 82 | - 13 | - 12 |
| Turbot | 4,2/8,8 | - 55 | - 22 | - 30 |
S.N : Unaltered season
S.D : Altered season
Table 11 : Maximum reduction observed (%) of the spawning volumes, viability and hatching of eggs when the reproduction is altered by contraction of the photoperiod cycles
| WATER | PROTEINS | LIPIDS % dry weight | ASBES | ||
| Sea-bass | I | 65,2 | 63,9 | 26,2 | 4,2 |
| II | 88,4 | 54,2 | 33,1 | 6,2 | |
| III | 89,1 | 52,6 | 26,1 | 6,6 | |
| Sole | I | 66,8 | 77,8 | 19,1 | 5,6 |
| II | 92 | 62,3 | 15,7 | 8,1 | |
| III | 92,1 | 67,8 | 13,1 | 9,8 | |
| Turbot | I | 66,1 | 74,7 | 17,8 | 4,2 |
| II | 91,4 | 76 | 17,3 | 10,6 | |
| III | 91,6 | 62,9 | 15,6 | 11,3 |
Table 12 : Global composition of mature ovules taken from females caught at sea
(I), eggs from gametogenesis, natural spawnings and fecundations, with
(II) or without (III) the control of the temperature or photoperiod.
BY DEVAUCHELLE et al, 1982.
| TEST 1 (a) | ||||||
| Origin | Treatment | N. of fish | Density Kg/m3 | P.M. (kg) | N. of dead fish | N. of eggs kg ° × 106 |
| SAUVAGE | I | 12 | 4.1 | 5.15 ± 1.43 | 1 | 408 |
(S) | II | 12 | 3.2 | 4.56 ± 1.62 | 7 | 278 |
| ECLOSERIE | I | 12 | 3 | 4.19 ± 1 | 2 | 299 |
(E) | II | 12 | 2.7 | 3.86 ± 0.83 | 1 | 163 |
| TEST 2 (b) | |||||
| Origin | Treatment | N. of fish | P.M. Kg | N. of dead fish | N. of eggs/Kg of larvae ° X |
| S + E | I | 32 | 3.3 | 0 | 12.7 |
| S + E | II | 32 | 3.3 | 0 | 22.8 |
Table 13 : Effect of the origin (Test 1) and of feeding (Test 1 and 2) of turbot on the production of larvae and eggs, per Kg of female.
- The food consists of pieces of fish distributed ad libitum with (I) or without (II) vitamins (C, E. 8, B6, Biotine and Inositol) at a rate of 1 mg/Kg of fish/week (METAILLER, comm. pers., 1985). The average weekly consumption (wet weight of the Food/weight of spawners) is 2% (test 1) and 4% (test 2).
- The treatment I also involves an intraperitoneal injection of vitamin C (100 mg/Kg of fish) 3 months before spawning takes place. The storing tanks have a volume of 20 3 (test 1) and 30 m3 (test 2).
| SPECIES | Period | Food | N. of fish at the beginning | Density Kg/m3 | Mortality/Year % | N. of eggs collected × 106 | Average viability rate considered |
| Sea-bass | 1976 | I | 20 | 1.2 – 1.5 | 0 | 81 | 89 |
| 1977 | II | 30 | " | 0 | 143 | 57 | |
| III | 30 | " | 0 | 52 | 86 | ||
| Sole | 1976 | IV | 60 | 2.5 – 3.1 | 5.7 | 9 600 | 77 |
| 1982 | |||||||
| 1983 | V | 66 | " | 5.3 | 5 200 | 54 | |
| 1984 |
Table 14: Effect of food given to sea-bass and sole on the quality of the eggs.
Sea-bass : I = pieces of fish - II = dry food - III = mixed food (fish + dry food), DEVAUCHELLE, 1980.
Sole : IV = molluscs (M) and polychaeta (P)
Average net weight of the food ingested/Kg of fish/week (C) = 10 % (M) + 2% (P): V = Molluscs, C = 12%
| SPECIES | Production of 107viable eggs (F.F) | Annual cost Upkeep of a 10 m3 tank (F.F.) | Pumping | Food | Group | |
| Sea-bass | 21 000 | 6 700 | 35 | 36 | 29 | |
| Gilthead sea-bream | 5 500 | 7 000 | 21 | 21 | 58 | |
| Sole | a | 46 000 | 3 500 | 38 | 44 | 17 |
| b | 140 000 | 10 500 | 11 | 83 | 5 | |
| Turbot | 8 300 | 9 200 | 45 | 46 | 9 | |
Table 15 : The cost price of eggs, not counting salaries nor depreciation, in normal spawning seasons, calculated on the basis of 3 Kg of spawners per m3 of sea-water. The cost of the eggs of sole varies in function of the food : molluscs alone (a) or molluscs and polychaeta (b).
The 10 m3 tanks represent an interesting unit for the hatchery, its upkeep price is indicated. In the Brittany region, the spawning alteration based on a thermic and photoperiod control doubles the price of the egg.
BIBLIOGRAPHY
ALESSIO, G., 1975. Riproduzione artificiale di orata, Sparus aurata (L) (Osteichtyes Sparidae). 5° Primi risultati sull'allevamento ed alimentazione delle larve a degli avanotti. Bull. Pesca Piscic. ldrobiol., 30(1) 71 – 92
ANTHONY, R., 1910. The cultivation of the turbot. Proceedings of the fourth international fishery congress, WASHINGTON, 1908. U.S. Bureau of fisheries Bull., 28 (2): 859 – 870.
BARNABE, G., 1976a. Rapport technique sur la ponte induite et l'élevage des larves du loup Dicentrarchus labrax et de le dorade Sparus aurata. In: Conseil général des pêches pour la Méditerranće, 55 : 63 – 116.
BARNABE, G., 1976b. contribution à la connaissance de la biologie du loup (Dicentrarchus labrax. Poisson Serranidae. Thèse d'état. Fac. Sciences, MONTPELLIER: 426 PP.
BARNABE, G., et R. BARNABE - QUEST, 1985. Avancement et amélioration de la ponte induite chez le loup Dicentrarchus labrax (L) à l'aide d'un analogue de LHRH injecté. Aquaculture, 49 (2): 125 – 132.
BARTON, A.L., 1981. Egg quality of turbot (Scophthalmus maximus) (L) kept in captive conditions. Thèse PHD présectée à l'Université de LIVERPOOL, Grande Bretagne: 129 pp.
BEDIER, E. 1979. Production à l'échelle pilote d'alevins de loup (Dicentrarchus labrax - L) presented at the Early life history of fish. Symposium hold at Woods Hole, USA, 2 – 5 avril 1979 : 17 pp.
BILLARD, R., 1979. La gamétogénèse, le cycle sexuel et le contrôle de la reproduction chez les poissons téléostéens. Bull. Fr. Pisc., 273: 117 – 136.
BILLARD, R., 1986. La salmoniculture on eau douce. In: Aquaculture, Tome 2. Ed. Lavoisier. PARIS: 525 – 569.
BLAXTER, J.H.S., 1969. Development: eggs and larvae. Fish Physiology, 3. Academic Press Inc., NEW YORK, 178 – 271.
BONDARI, K., G.O. WARE, B.G., MULLINIX, and J.A. JOYCE, 1985. Influence of brood fish size on the breeding performance of channel catfish. Prog. Fish. Cult., 47 (1) : 21 – 27.
BOULINEAU, F., 1969. Contribution à l'étude boilogique du bar Dicentrarchus labrax (Linné). Thèse de 3ème cycle, Fac. Sciences PARIS, Ronéo, 176 pp.
BRASOLA, V., 1974. Riproduzione artificiale della sogliola (Solea solea) effetuata con sucesso preso la laguna di Orbetello. Riv. Ital. Piscic. Ittio. Patol., 9 (4): 99–101.
BRUSLE, J. et C. ROBLIN, 1984. Sexualité du loup Dicentrarchus labrax en condition d'élevage contrôlé. In: l'Aquaculture du bar et des sparidés. Actes du colloque sur l'aquaculture du bar (loup) et des sparidés tenu à SETE (France) les 15 – 16 – 17 mars 1983: 33 – 44.
BUCKLAND in A. E. MALARD, 1899. Sur le développement et la pisciculture du turbot. C.R. Acad. Sc. PARIS, 129: 181–183.
BUTLER, G.W., 1895. Report on the spawning of the common sole (solea vulgaris in the aquarium of the Marine Biological Association's laboratory at PLIMOUTH, during April and May 1895. J. Mar. Biol. Ass. U. K., 4: 3–9.
BYE, V.J. 1984. The role of environmental factors in the timing of reproductive cycles. In: Fish Reproduction Strategies and tactics Ed. G. W. POTTS, R.J. WOOTON. LONDRES: 187–205.
CAMUS, P and C. KOUTSIKOPOULOS, 1984. Incubation and embryonic development of gilt-head bream Sparus aurata (L) at a range of temperatures. Aquaculture, 42: 117–128.
COLOMBO, L., P. C. BELEVEDERE, A., MARCONATO and F. BENTVEGNA, 1982. Pheromones in telecost fish. In: Reproductive physiology of fish. Proceedings of the International Symposium on reproductive physiology of fish, WAGENINGEN, the Netherlands, 2–6 août 1982.
COVES, D., 1985. Etat actuel de l'élevage du loup en écloserie. AQUA REVUE 3: 26–30
DABROWSKI, K. 1984. The feeding of fish larvae: present “state of art” and perspectives. Reprod. Nutr. Develop., 1984 (6): 807–833.
DEVAUCHELLE, N., 1980. Etude expérimentale sur la reproduction, les oeufs et les larves de bar (Dicentrarchus labrax, daurade (Sparus aurata), mulet (Liza remada), rouget (Mullus surmulatus), sole (Solea solea), turbot (Scophthalmus maximus). Thèse 3ème cycle, UBO BREST: 117 pp.
DEVAUCHELLE, N., 1984. L'incubation des oeufs de bar (Dicentrarchus labrax) et de daurade (Sparus aurata). In: L'aquaculture du bar et des sparidés. Actes du colloque sur l'aquaculture du bar (loup) et des sparidés tenu à SETE (France) les 15 – 16 – 17 mars 1983: 117–124.
DEVAUCHELLE, N., 1984. Identification du sexe et prélèvements d'ovocytes sur turbots (Scophethalmus maximus vivants. Bull. Piscis., 293–294: 65–71
DEVAUCHELLE, N., et Y. CLADAS, 1982. Influence de a taille, du poids et du taux d'humidité d'oeufs de trois espèces de poissons marins sur les taux d'éclosion et d'anomalies des larves. Rapport ICES/CIEM, CM 1982/F: 19, 14 pp.
DEVAUCHELLE, N., G. BRICHON, F. LAMOUR, G. STEPHAN, 1982. Biochemical co,position of ovules and Fecund eggs of sea-bass (Dicentrarchus labrax, Sole, (Solea vulgaris and turbot (Scophthalmus maximus). In: Reproduction physiology of fish. Proceedings of the International Symposium on reproductive physiology of fish: 155–157.
DENIEL, C., 1981. Les poissons plats (Télécostéens, pleuronectiformes) en baie de DOUARNENEZ. Reproduction, croissance et migration des Bothidae, Scophthalmidae, Pleuronectidae, et Soleidae. Thèse d'état, UBO - BREST: 476 pp.
DIVANACH, P., 1985. Contribution à la connaissance de la biologie et de l'élevage de 6 sparidés méditerranéens: Sparus aurata, Diplodus sargus, Diplodus vulgaris, Diplodus annularis, Litognathus mormyrus, Puntazzo puntazzo (poissons téléostéens). Thèse d'état. Univ. Sci. et Tech. Languedoc: 479 pp.
FLUCHTER, J., 1972. Induction of spawning in the turbot (Rhombus maximus L) by injection of hypophyseal suspensions. Aquaculture, 1 (3): 285–287.
FONDS, M., 1979. Laboratory observations on the influence of temperature and salinity on development of the eggs and growth of the larvae of Solea solea (Pisces). Marine Ecology progress series 1 (2): 91–99.
FONTAINE. n. et M. OLIVEREAU, 1962. Nutrition et sexualité chez les poissons. Annales de la nutrition et de l'alimentation ; T. 16 : A 125 – A 152.
FREDDI, A., L. BERG, and M. BILIO, 1981. Optimal salinity temperature combinations For the early life stages of Gilthead breamSparus auratus L., J. World. Maric.Soc., 12 (2), 130–136.
GIRIN, N. et N. DEVUCHELLE, 1978. Décalage de la période de reproduction par raccourcissement des cycles photopériodiques et thermiques chez des poissons marins. Ann. Biol. Anim. Bioch Biophys., 18 (4) : 1059 – 1065.
GIRIN, N. 1979. Méthodes de production des juvéniles chez trois poissons marins, le bar. la sole et turbot. Rapport CNEXO Scientifique et technique, 39 : 202 pp.
GIRIN, N., 1980. L'élevage des poissons marins. La recherche : 11 (107) : 36–44.
HRMPRL, N.S., 1979. Early life history of marine fish. The egg stage : Library of Congress Cataloging in publication data : 71 pp.
HOAR, N. S., 1969. Reproduction. In Fish Physiology. Ed. By N.S. HOAR R.J. RANDALL, Vol. 3 : 1 – 72.
JONES, A., 1974. Sexual maturity, fecundity and growth of the turbot (Scophthalmus maximus) l. J. Mar. Biol. Ass. U. K., 54 : 109 – 125. KENNEDY,N., and P. FITZNAURICE,1972. The biology of the bass, Dicentrarchus labrax, in irish waters. j. Mar. Biol. Ass. U. K. 52 : 55 -597.
KUHLMANN, D., G. QUANTZ, W. NELLEN and J. LENZ, 1980. The development of turbot eggs, Scophthalmus maximus L ., From the Baltic SEa under different temperature and salinity conditions. Presented at the ICES meeting 1980/F ; 31 : 8 pp.
LAIIAYE, j., 1972. Cycles sexuels de quelques poissons plats des côtes bretonnes. Rev. Trav. Inst. Pêches Marit., 36 (2) : 191 – 207.
LASSERRE, G., 1974. Recherches sur la dynamique des populations des daurades royales Sparus auratus L des régions de SETE et d'ARCACHON. Univ. Sci. et Tech, Languedoc : 214 pp.
Le FOLL, A., 1979. Fécondité de la sole Solea solea, et d'un arnoglosse,Arnoglossus laterna. en baie de DOUARRNENE. Rapport de DEA, UBO, BREST : 54 pp.
LIEWES, E.W. 1984. Diseases of commercial flatfish species. Ed. A.A. BALKEMA. P.O. Box 1675. 3 000 Br ROTTERDAM, Netherlands : 103 pp
LILEY, N, R., 1980. Patterns of hormonal control in reproductive behaviour of fish and their redevance to fish management and culture programs. In : Fish behaviour and its use in the capture and culture of fishes. Ed. J.E. BARDACH, J.J. MAGNUSON, R.C. MAY and J.M. REINHART,ICLARM conference proceedings 5 : 210 – 246.
LUQUET, P: and T. WATANABE,1985. Interaction “Nutrition-Reproduction” in fish, Presented at the 7th Conference of the European Society For Comparative Physiology and biochemistry. BARCELONGE, 26 – 28 août 1985 – 23 pp.
MACEL, J., 1986. La pisciculture en étang. In : Aquaculture, Tome 2. Coordonnateur G. BARAABE. Ed. Lavoisier : 1123 pp.
Mc EVOY, L.A., 4985. Double ovalutory cycles in some captive turbot,Scophthalmus maximus l., J. Fish. Biol. 26 : 63 – 66.
NOEL, T., 1985. Bilan trimestriel sur la reproduction du turbot à la SODAB, TREDARZEC, Cotes du Nord, France, 25.07.85 : 3 pp.
GUANTZ, G., 1980. Uber den Einflub von czrotionidreichen trockenfutter auf die Eibefruchtung der regenbogenforelle (Salmo gairdneri R). Arch. Fish. Wiss., 31 (1) : 29–40.
RAMOS, J. 1977. Primeras experiencias de cria del lenguado (SOLEA SOLEA L). Inf. Tech. del inst. de invest. Pesqueras. BARRCELONA, Sept, 1977. 4f8 :16 pp.
ROMOS, J. 1978. Experiencias de cultivo de dorada (Sparus aurata L) en tanques. Informes technicos del Instituto de investigaciones pesqueras.
BARCELONA, 55 : 20 pp.
SCHERRER, P., 1955. Influence de la température et de la salinité sur la croissance et al consommation d'oxygène du juvénile de turbot Scophthalmus maximus L. (phase nurserie). Thèse 3ème cycle, U80 - BREST : 151 pp.
STEARNA, S.C. and R.C. CDRANDALL, 1984. plasticity for age and size at sexual maturity. a life history response to unavoidable stress. In : Fish reproduction. Strategies and tactics. Ed. G. W. POTTS, R. J. WOOTON, LONDRES: 13–33.