Mr. A.G.J. TACON
1. INTRODUCTION
1.1 Context and Purpose of Report
Four different live planktonic food organisms, (diatoms, flagellates, rotifers and Artemia nauplii) are commonly used for the mass propagation of penaeid shrimp larvae from protozoea substage 1, through metamorphosis, to the post-larval substage. Modern penaeid shrimp hatcheries are therefore dependent on highly trained and skilled labour, and sophisticated live food production systems which require high capital, investment in terms of space, facilities and services, and even expensive feed procurement, such as Artemia cysts.
A modern, well-managed, shrimp hatchery using live food feeding strategies can be economically efficient. However, there is a need to develop a simple and inexpensive feeding strategy which can be readily adopted by the owner-operator or rural farmer with limited resources. This report is an evolution of a simple and inexpensive hatchery feeding strategy originally developed in India. Based on the exclusive use of a crustacean tissue suspension throughout the hatchery cycle, this feeding strategy was tested on Penaeus monodon in a series of trial at the Regional Lead Centre in the Philippines, based at the Southeast Asian Fisheries Development Centre's (SEAFDEC) Aquaculture Department
1.2 The Mission
The purpose of the mission was: (a) to collect published information on the use of crustacean wet tissue suspension for larval feeding, and determine the relative merits and demerits of the feeding strategy; (b) to discuss recent developments in larval shrimp feeding with research staff at the Central Marine Fisheries Research Institute (CMFRI) in Cochin (February , 1985); (c) to travel to the Regional Lead Centre in the Philippines (part of the Network of Aquaculture Centres in Asia, NACA) and assist Mr. P. Kungvankij, NACA Aquaculturist (Research) in planning and executing larval feeding trials incorporating the suspension with P. monodon. The work was to be performed at the Leganes Hatchery, SEAFDEC Aquaculture Department, Iloilo, Philippines, (March–April, 1985).
1.3 Acknowledgements
The author would like to thank Dr. T. V. R. Pillay (former Programme Leader, ADCP) and Mr. P.G. Padlan (Senior Aquaculturist, ADCP), for identifying the mission. Valuable experimental information was provided by Dr. E. G. Silas (Director, CMFRI) and his research staff at Cochin, India. The larval feeding trials conducted at SEAFDEC Aquaculture Department were achieved with the enthusiastic cooperation and sustained hard work of Mr. P. Kungvankij, NACA Aquaculturist (Research) and his SEAFDEC research colleagues at the Leganes Hatchery, including Mr. E. Borlongan, Ms. K. G. Corre, Ms. L. F. Gustilo, M. I. O. Potestas, Mr. B. J. Pudadera, Ms. G. A. Taleon, and Mr. A. Unggi. The author is also grateful to many other private persons and officials of NACA and SEAFDEC, and especially to Dr. F. P. Pascual (Nutritionist) of the latter agency for her untiring support and cooperation as a friend and fellow nutritionist.
2. BACKGROUND
Almost all commercial penaeid shrimp hatchery operations rely on the exclusive use of a succession of live food organisms (commonly diatoms, algae, rotifers and Artemia nauplii) for the larval culture cycle (for review see CRC, 1983; Liao, 1984). This practice is due to the lack of a suitable alternative feeding method. Until recently, previous attemps to replace live food organisms completely with artificial dry diets have generally led to poor larval survival and delayed larval development (New, 1976).
Two new feeding systems have been introduced recently as ‘viable’ alternatives to the live food production system: (1) the exclusive use of a rehydratable microencapsulated/microbound larval shrimp diet (Jones, 1984; Scura, Fischer and Yunker, 1984; Kanazawa, 1983), and (2) the exclusive use of a crustacem tissue suspension (Hameed Ali, Dwivedi and Alikunhi, 1982). Although both methods rely on feeding a single, non-living food for the entire larval culture phase, they differ in the feed resource used and the degree of sophistication of feed preparation. This report is concerned with the evaluation of the crustacean tissue suspension feeding strategy.
3. CRUSTACEAN TISSUE SUSPENSION - REVIEW OF PAST INDIAN STUDIES
A novel and inexpensive hatchery feeding system has been used for the mass rearing of penaeid shrimp larvae in India (Hameed Ali, Dwivedi and Alikunhi, 1982). The system is based on the exclusive use of a crustacean wet tissue suspension as a feed for all larval and early post-larval (PL) stages. It has been developed from a series of feeding trials conducted at the Central Institute of Fisheries Education (Bombay), the Brackishwater Aquaculture Development Centre in Jepara (Indonesia), the Regional Shrimp Hatchery at Azhicode (Kerala), and the Mundra Experimental Shrimp Hatchery, Gujarat (Alikunhi et al., 1980, 1982; Hammed Ali, 1980; Hameed Ali and Dwivedi, 1977; Hameed Ali, Dwivedi and Alikunhi, 1982).
Crustaceans which have been successfully processed into a wet tissue suspension for the trials include the Jinga shrimp, Metapenaeus affinis; Kadal shrimp, M. dobsoni (‘Thelli'’); Kiddi shrimp, Parapenaeopsis stylifera; Jawla paste shrimp, Acetes indicus; Spider prawn, Nematopalaemon tenipes; mysids Mesopodopsis spp; and the stomatopod crustacean, Oratosquilla nepa ‘Chelly’. The choice of crustacean is based on local availability, ease of capture by fishermen and on market cost. The commonest crustaceans used have been the Jawla paste shrimp, the stomatopod and the Kadal shrimp. Table 1 shows the feeding regime recommended by Hameed Ali, Dwivedi and Alikunhi (1982) for the individual larval stages of Penaeus spp., Metapenaeus spp., and Parapenaeopsis spp., using a crustacean wet tissue suspension.
Feeding trials in India have been conducted with nine shrimp species: Penaeus monodon, P. merguiensis, P. indicus, P. semisulcatus, Metapenaeus affinis, M. monoceros, M. brevicornis, M. dobsoni and Parapenaeus stylifera (for review see Hameed Ali, Dwivedi and Alikunhi, 1982). All the nine shrimp species were reared successfully on the wet tissue suspension from Z stage, through metamorphosis, to the early PL stage. Of the different suspensions evaluated, ‘Chelly’ (O. nepa) gave higher larval survival than ‘Thelli’ (M. dobsoni) with P. indicus and P. monodon (Alikunhi et al., 1980). Hameed Ali, Dwivedi and Alikunhi (1982) estimate that over 150 million penaeid larvae have been reared using this feeding system, with the production of over 50 million early penaeid larvae. This is an overall survival rate from N6 to PL1 of about 33 percent. Alikunhi et al., (1980) obtained a mean larval survival from N6 to PL1 of 43.8% for P. indicus (78 separate culture runs), 25.3% for P. monodon (x7), 72.0% for P. semisulcatus (x2), 32.9% for M. monoceros (x2), 62.5% for M. dobsoni (x3) and 30.8% for P. stylifera (x2) using either a ‘Chelly’ or “Thelli” suspension between 1979 and 1980. However, although many individual cultures had over 90% survival, with larval PL productions exceeding 200 PL1/1, 18 from a total of 114 cultures successfully implemented at the N6 substage had to be discarded during the larval growth phase due to the development of intense diatom blooms within the tanks. As these failures invariably occurred within rearing tanks exposed to direct sunlight, they were not assumed to be caused by the feeding regime itself, and consequently were not included by Alikunhi et al., (1980) in their final estimations of larval survival. However, these failures represent a 15.8% loss of all cultures. Hameed Ali (1980) and Hameed Ali, Dwivedi and Alikunhi (1982) also reported the development of algal and diatom blooms within the culture tanks during larval production.
Although Alikunhi et al., (1980) and Hameed Ali (1980) state that the shrimp larvae are fed exclusively on a non-living diet, the fact that algal/diatom blooms occur in the culture tank indicates the presence of potential live food organisms for the shrimp. Similarly, although they state that no larval mortalities occurred due to overfeeding with the tissue suspension, they do not comment on the fact that the diatom blooms present may have resulted directly from the nutrients provided in the feeding system used (fertilizing effect). If this is the case, culture losses attributed by Alikunhi et al., (1980) to excessive diatom blooms may have been due to over-feeding and insufficient water exchange.
4. CRUSTACEAN TISSUE SUSPENSION - LEGANES FEEDING TRIALS
4.1 Introduction
On the basis of the very encouraging results obtained by Hameed Ali and co-workers in India with a crustacean tissue suspension for larval feeding, and after discussion with Dr. E.G. Silas and Mr. P. Kungvankij, three experimental feeding trials were conducted with first feeding P. monodon larvae at the Leganes Hatchery, SEAFDEC Aquaculture Department, Iloilo (10 March to 4 April, 1985).1 In addition to the ‘wet’ crustacean tissue suspension feeding system used by Hameed Ali, Dwivedi and Alikunhi (1982), a new ‘dry’ crustacean tissue suspension feeding system was also tested.
Crustacean tissue suspensions were prepared from the sergestid shrimp, Acetes sp., known locally in the Philippines as ‘Alamang’. This species was chosen because of its local market availability in Iloilo at the time of the Mission and because this shrimp family constituted on of the original feed sources used by Hameed Ali and co-workers for the preparation of a crustacean wet tissue suspension (Jawla paste shrimp - Acetes indicus; Hameed Ali, et al. 1982)
Table 1
Feed Particle Size and Feeding Regime Recommended for the Larval Rearing of Shrimp using a Crustacean Wet Tissue Preparation
| Shrimp Species Reared | |||||||
| Penaeus spp. | Metapenaeus spp. | Parapenaeopsis spp. | |||||
| Feed particle size | (μm) | ||||||
| during Protozoea- | Z1 | 160 | 50 | 50 | |||
| Z2 | 160 | 50 | 50 | ||||
| Z3 | 200 | 160 | 160 | ||||
| Mysis | M1 | 250–300 | 160 | 160 | |||
| M2 | 300–400 | 250–300 | 250–300 | ||||
| M3 | 300–400 | 250–300 | 250–300 | ||||
| Post Larval | PL1 | 400–500 | 300 | 300 | |||
| Optimum feed dosage | |||||||
| (g raw material/1 000 larvae/day) | Squilla* | Others | Squilla | Others | Squilla | Others | |
| during Protozoea- | Z1 | 1.0 | 0.5 | 0.5 | 0.3 | 0.5 | 0.3 |
| Z2 | 1.25 | 0.75 | 0.75 | 0.5 | 0.75 | 0.5 | |
| Z3 | 1.5 | 0.75 | 1.0 | 0.6 | 1.0 | 0.6 | |
| Mysis | M1 | 1.75 | 1.0 | 1.25 | 0.75 | 1.25 | 0.75 |
| M2 | 1.75 | 1.25 | 1.25 | 1.0 | 1.25 | 1.0 | |
| M3 | 2.0 | 1.5 | 1.5 | 1.25 | 1.5 | 1.25 | |
| Post Larval | PL1 | 2.0 | 2.0 | 1.75 | 1.5 | 1.75 | 1.5 |
Source: Hameed Ali et. al., 1982
4.2 Materials and Methods
Three successive feeding trials, code-named A, B and C, were performed with first feeding P. monedon larvae obtained from wild-caught spawners. The feeding trials were conducted in either outdoor larval rearing tanks (experiments A and C) or in indoor larval rearing tanks (experiment B). In experiment A six different feeding regimes were tested (I-VI, Fig. 1); four crustacean tissue feeding options, including the original frozen crustacean feeding strategy described by Hameed Ali, et al., (1982) and two ‘control’ live food feeding options similar to those normally employed at the Leganes Hatchery. The three most successful feeding options observed during experiment A were then evaluated within indoor tanks in experiment B and again in outdoor tanks in experiment C, although in the latter case an additional ‘control’ group receiving no exogenous live or artificial feed input was also introduced.
4.2.1 Experimental Larval Rearing Tanks and Water Management
Two experimental rearing facilities were used for the three larval feeding trials. In experiments A and C larval rearing was conducted in twelve 1 000 1 circular fibreglass phytoplankton production tanks situated outdoors. These tanks were covered by a translucent tarpalion from midday until the following morning so as to minimize fluctuations in water temperature. In experiments B larval rearing was conducted in nine, 250 1 circular fibreglass spawning tanks situated indoors within the hatchery building at Leganes. These tanks were positioned adjacent to a frosted glass window and received only natural lighting. A diagramatic representation of the two experimental tanks systems is shown in Fig. 2.
A non - continuous water management system was adopted within the larval rearing tanks throughout the culture cycle; a pre-determined percentage of the total volume of water within the tank being exchanged once daily at 08.00 h by siphoning (using a box filter) and then replenishing with an equal volume of fresh filtered (15 μm) sea water. The proportion of water replaced daily was dependent upon the developmental status of the larvae present. The water management procedures adopted during the three feeding trials are shown in Table 2.
Throughout the larval feeding trials continuous aeration and water upwelling was maintained within the culture tanks by means of a single aerator placed centrally at the bottom of each tank. Each aerator consisted of a bottom weighted, open ended, PVC air distribution line connected to a central Roots air blower. Water circulation and agitation was essential so as to maintain the shrimp larvae and food organisms tested in constant suspension within the water column.
4.2.2 Experimental Animals
Mature, ready-to-spawn, p.monodon broodstock were collected daily from local fishermen and individually placed (when tank space permitted) into 250 1 circular fibreglass spawning tanks within the hatchery building. Each spawning tank contained around 200 1 of fresh seawater (26–29°/oo salinity, previously filtered through a 15 mm bag net filter), was aerated by a single PVC air distribution line, and was covered by a removable plywood lid. On spawning, which usually occurred at night, the spent female was removed and the fertilized eggs siphoned from the water column into a plastic egg collector consisting of two successive sieves; firstly a 250 μm sieve for retaining unwanted debris, and secondly a 80 μm sieve for retaining the fertilized eggs. Eggs from single spawnings collected in this way were subsequently washed with filtered seawater and then immersed in a bath of methylene blue (2 mg/l), before being re-suspended in fresh filtered sea water within the spawning tank (or hatching tank as it was now called). On hatching, the shrimp nauplii were counted by taking replicate 100 ml water samples, and then transferred to the experimental rearing tanks for the commencement of the different larval feeding trials. Initial nauplii stocking density within the larval rearing tanks varied between 60–100 nauplii/1 (experiments A and C) and 150–200 nauplii/1 (experiment B). During each experiment the larvae were reared from the nauplius stage (N1) to the M3/P1 stage for a total of 10–12 days using one of six different feeding regimes.
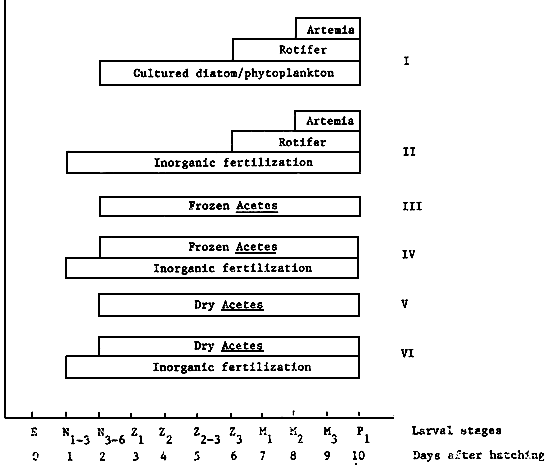
Fig. 1 - Feeding Schemes tested with P. monodon larvae
a.
b.
Fig. 2 - Larval rearing tanks employed during Experiments A and C (a) and B (b)
Table 2
Water management Procedures employed during1 Experiments A, B and C
| Larval Stage | Experiment | ||
| A | B | C | |
| N1–3 | 700 | 200 | 700 |
| N3–6 | 700 | 200 | 700 |
| Z1 | 700 + 200 | 200 ± 60 | 700 + 150 |
| Z2 | 900 + 300 | 200 ± 160 | 850 ± 250 |
| Z2–3 | 900 ± 400 | 200 ± 160 | 850 ± 350 |
| Z3 | 900 ± 400 | 200 ± 160 | 850 ± 350 |
| M1 | 900 ± 400 | 200 ± 160 | 850 ± 400 |
| M2 | 900 ± 400 | 200 ± 160 | 850 ± 500 |
| M3 | 900 ± 400 | 200 ± 160 | 850 ± 450 |
4.2.3 Food and Feeding Regimes
Experiment A
Six different feeding regimes were tested (I–VI; Fig. 1).
A-I Culture live food feeding option:
Tetraselmis sp. (Batan strain) fed so as to maintain a concentration of around 10 000 cells/ml throughout the culture cycle from day 2 after hatching (N3–6) to M6/P1. Brachionus plicatilis fed to maintain a concentration of five organisms/ml from 23 to M3/P1. Artemia salina nauplii fed to maintain a concentration of two organisms/ml from M2 to M3/P1.
A-II Fertilization/live food feeding option:
Inorganic fertilization of water throughout the culture cycle to encourage the growth of a mixed diatom population - daily dosage of fertilizer included 4/0.4 mg/l of NaNO3/NaH2PO6 respectively at day 1 (N1–3) and thereafter at 3/0.3 mg/l until M3/P1. The technical grade fertilizers commonly used at Leganes are KNO3/Na2HPO4, however no stocks were available at the time of this Mission. In addition to inorganic fertilization, B. plicatilis and A. salina nauplii were fed at 5 organisms/ml from 23 to M3/P1 and 2 organisms/ml from M2 to M3/P1 respectively. The algae, rotifer and Artemia culture methods employed have been described previously by Kungvankij et al., (1984).
A-III Frozen Acetes feeding option:
Fresh Acetes sp. was obtained from local fishermen and stored at -20°C. The feeding regime employed was based on a initial feeding rate of 0.5 mg frozen Acetes/larvae/day at N3–6 and Z1 (day 2 and 3 after hatching), thereafter increasing the feeding rate by 207/day until M3/P1. Feeding rates were calculated assuming 100% larval survival so as to ensure a minimum feed particle concentration within the water column. The feed an appropriate volume of sea water in an electric blender, and passing the homogenate through appropriate mesh sieves to obtain the required particle size range for the larvae; <125 μm N6-Z2/3; 125<350 mμ M3-P1.
A-IV Fertilization/frozen Acetes feeding option:
As above but also including the inorganic fertilization regime described under A-II.
A-V Dry Acetes feeding option:
Air/sun-dried Acetes was obtained from the local food market in Iloilo City and ground to a free-flowing powder of appropriate particle size (50<125 mμ N6-Z2/3; 125<250 μm Z3-M2; 250<350 μ M3-P1<) by using a hammermill. An initial feeding rate of 0.10 mg dry Acetes/larvae/day (≡ 0.50 mg frozen Acetes/larvae/day) was employed at N– and Z (days 2 and 3 after hatching), thereafter increasing by 20%/day until M3/P1. The proximate composition of the Acetes used is shown in Table 3.
Table 3
Proximate Composition of Air/Sun-Dried Acetes sp. sample used in Experiment A and over the period March 1977–July 19821
| Component (%) | Acetes sample | |||
| Experiment A | March 1977 – July 1982 | |||
| Range | Mean2 | |||
| Moisture | 14.09 | 3.5 – 14.0 | 7.94 | |
| Dry Matter Basis (%) | ||||
| Crude protein (N x 6.25) | 54.46 | 55.45– 72.78 | 67.59 | |
| Crude lipid | 3.74 | 4.13– 5.77 | 5.04 | |
| Crude fibre | 4.88 | 2.67– 8.09 | 4.97 | |
| Ash | 15.20 | 7.34– 25.42 | 16.29 | |
| NFE3 | 21.72 | NA4 | 6.11 | |
| Calcium | 3.44 | NA | NA | |
| Phosphorus | 1.25 | NA | NA | |
1 Analyses provided by the Analytical Laboratory Division of SEAFDEC Aquaculture Department
3 Nitrogen free extractives = 100-(Moisture + crude protein + Lipid + ash + crude fibre)
A-VI Fertilization/dry Acetes feeding option:
As A-V but including the inorganic fertilization regime described under A-II.
Experiment B
Three different feeding options were tested:
B-I Cultured live food feeding option:
As for experiment A, but also including the feeding of the diatom Chaetoceros calcitrans so as to maintain in cell concentration of around 50 000/ml throughout the aquaculture cycle from N3–6 (day 2 after hatching) to M3/P1 (in addition to the flagellate Tetraselmis sp.)
B-II Frozen Acetes feeding option:
As for experiment A, but feeding rate adjusted to actual number of larvae present/day until Z3, thereafter feeding rate estimated on basis of original larvae stocked at N3–6 (i.e. feeding rates from N3–6-Z3 were not based on 100% survival, but on actual numbers observed).
B-III Dry Acetes feeding option:
As for experiment A, but feeding rate adjusted as above,
Experiment C
Four different feeding options were tested:
C-I Cultured live food feeding option:
As for experiment B
C-II Frozen Acetes feeding option:
As for experiment A
C-III Dry Acetes feeding option:
As for experiment A
C-IV Control bank feeding option:
No cultured algae or exogenous dry/live food input; larval growth being totally dependent on the natural plankton community within filtered sea water.
4.2.4 Management
In experiment A the study consisted of six diet
Feeding commenced immediately after water management in all cases (4.2.1), once daily at 0830 h for the cultured algae, live food and inorganic fertilization feeding options, and four times daily for the frozen/dry Acetes feeding options (0830, 1200, 1700 and 2400 h; the daily feed allowance being divided into four equal parts).
Plankton counts, water temperature, pH and salinity readings were performed in each culture tank before water management (0800 h) and in the afternoon (1500 h) on a daily basis. Since there was little or no variation observed between individual tanks on the basis of the chemical water quality parameters monitored, the ranges and mean values recorded over the entire experimental culture cycle are shown in Table 4.
Table 4
Temperature, pH and Salinity of Seawater within Culture Tanks during Experiments A, B and C
| Experiment | Temperature (°C) | pH | Salinity (°/00) | |||
| Mean | Range | Mean | Range | Mean | Range | |
| A | 28.5 | 24–32.5 | 8.0 | 7.5–8.7 | 34.6 | 34–36 |
| B | 27.0 | 25.6–28.4 | 8.2 | 7.9–8.5 | 35.3 | 34–37 |
| C1 | 30.1 | 28–31.5 | 8.4 | 8.2–8.6 | 36.1 | 34–37 |
1 Data based only up to Z3 stage or day 6 after hatching
Replicate 1 litre water samples were also collected immediately after water management for the estimation of larval numbers and stage of development. In addition to the above routine activities, plankton counts were also monitored in one control tank containing no shrimp larvae and receiving no feed input during experiment A so as to ascertain background plankton levels.
4.3 Results
4.3.1 Experiment A
The larval count, growth and survival rate of P. monodon larvae over the 10-day culture cycle is shown in Table 5 and Fig. 3. The highest mean survival rate was recorded for the dry Acetes feeding option - 46.7%, followed by frozen Acetes - 29.4%, cultured live food - 24.7%, fertilization/dry Acetes - 16.6%, fertilization/live food and fertilization/frozen Acetes - 16.0%. Larval development was similar in all treatments, with the exception of feeding regimes utilizing frozen Acetes, which by day 10 (0600 h) had still not metamcrphosed from the third mysis substage to the first post larval substage (Fig. 3).
A histogram representing the phytoplankton community within the larval rearing tanks is shown in Fig. 4. Although it was not possible to monitor the phytoplankton community throughout the entire 10-day larval culture cycle, all treatments displayed a higher phytoplankton community than the control tank containing no larvae and receiving no feed input. Highest phytoplankton levels were generally observed before the start of the water management programme (Table 2), i.e., on day 3 or the morning of day 4 after hatching. The most dominant species present within all treatment was the solitary or chained form of the diatom Chaetoceros calcitrans. Surprisingly, no beneficial effect of inorganic fertilization was evident in those treatments receiving Acetes inputs.
Table 5- Larval count and development of P. monodon larvae on six different feeding regimes in outdoor tanks - Experiment A1
| Days after hatching | Total water volume (1) | Cultured live food (A-I) | Fertilization/live food (A-II) | ||||||||||
| Replicate I | Replicate II | Replicate I | Replicate II | ||||||||||
| Total larval count | Larval stage | Larval density (No./l) | Total larval count | Larval stage | Larval density (No./l) | Total larval count | Larval stage | Larval density (No./1) | Total larval count | Larval stage | Larval density (No./1) | ||
| 2 | 700 | 57 750 | N3–6 | 82 | 43 400 | N3–6 | 62 | 58 450 | N3–6 | 83 | 44 100 | N3–6 | 63 |
| 3 | 900 | 58 500 | Z1 | 65 | 54 000 | Z1 | 60 | 62 100 | Z1 | 69 | 32 850 | Z1 | 36 |
| 4 | 900 | 75 600 | Z2 | 84 | 42 300 | Z2 | 47 | 68 400 | Z2 | 76 | 32 400 | Z2 | 36 |
| 5 | 900 | 48 600 | Z2–3 | 54 | 36 000 | Z2–3 | 40 | 62 100 | Z2–3 | 69 | 28 800 | Z2–3 | 32 |
| 6 | 900 | 54 900 | Z3 | 61 | 28 800 | Z3 | 32 | 40 500 | Z3 | 45 | 18 900 | Z3 | 21 |
| 7 | 900 | 53 100 | M1 | 59 | 30 600 | M1 | 34 | 26 100 | M1 | 29 | 22 500 | M1 | 25 |
| 8 | 900 | 47 175 | M2 | 52 | 28 475 | M2 | 32 | 8 925 | M2 | 10 | 8 500 | M2 | 9 |
| 9 | 900 | 33 575 | M3 | 37 | 25 500 | M3 | 28 | 4 250 | M3 | 5 | 11 475 | M3 | 13 |
| 10 | 900 | 15 000 | P1 | 17 | 16 000 | P1 | 18 | 200 | P1 | 0.2 | 14 000 | P1 | 16 |
| Survival rate (Z) | 19.8 | 29.6 | 0.32 | 31.7 | |||||||||
| Mean survival rate (X) | 24.7 | 16.0 | |||||||||||
| Days after hatching | Total water volume (1) | Cultured live food (A-III) | Fertilization/live food (A-IV) | ||||||||||
| Replicate I | Replicate II | Replicate I | Replicate II | ||||||||||
| Total larval count | Larval stage | Larval density (No./l) | Total larval count | Larval stage | Larval density (No./l) | Total larval count | Larval stage | Larval density (No./1) | Total larval count | Larval stage | Larval density (No./1) | ||
| 2 | 700 | 52 850 | N3–6 | 75 | 51 450 | N3–6 | 73 | 55 300 | N3–6 | 79 | 49 700 | N3–6 | 71 |
| 3 | 900 | 60 750 | Z1 | 67 | 66 800 | Z1 | 74 | 57 600 | Z1 | 64 | 18 900 | Z1 | 21 |
| 4 | 900 | 63 000 | Z2 | 70 | 45 900 | Z2 | 51 | 52 200 | Z2 | 58 | 12 000 | Z2 | 13 |
| 5 | 900 | 45 650 | Z2–3 | 54 | 41 400 | Z2–3 | 46 | 45 900 | Z2–3 | 51 | 9 900 | Z2–3 | 11 |
| 6 | 900 | 49 500 | Z3 | 55 | 42 300 | Z3 | 47 | 45 000 | Z3 | 50 | 9 900 | Z3 | 11 |
| 7 | 900 | 44 100 | M1 | 49 | 37 600 | M1 | 42 | 35 100 | M1 | 39 | 8 000 | M1 | 9 |
| 8 | 900 | 36 875 | M2 | 41 | 41 650 | M2 | 46 | 29 600 | M2 | 33 | 9 775 | M2 | 11 |
| 9 | 900 | 49 725 | M3 | 55 | 39 525 | M3 | 44 | 21 675 | M3 | 24 | 6 800 | M3 | 8 |
| 10 | 900 | 22 000 | M3 | 24 | 16 000 | M3 | 18 | 8 000 | M3/P1 | 9 | 9 000 | P1 | 10 |
| Survival rate (Z) | 34.9 | 24.0 | 13.9 | 18.1 | |||||||||
| Mean survival rate (Z) | 29.4 | 16.0 | |||||||||||
| Days after hatching | Total water volume (1) | Cultured live food (A-V) | Fertilization/live food (A-VI) | ||||||||||
| Replicate I | Replicate II | Replicate I | Replicate II | ||||||||||
| Total larval count | Larval stage | Larval density (No./l) | Total larval count | Larval stage | Larval density (No./l) | Total larval count | Larval stage | Larval density (No./1) | Total larval count | Larval stage | Larval density (No./1) | ||
| 2 | 700 | 47 250 | N3–6 | 67 | 45 850 | N3–6 | 65 | 69 900 | N3–6 | 100 | 52 500 | N3–6 | 75 |
| 3 | 900 | 54 900 | Z1 | 61 | 54 900 | Z1 | 61 | 68 850 | Z1 | 76 | 50 850 | Z1 | 36 |
| 4 | 900 | 62 100 | Z1 | 69 | 62 100 | Z2 | 69 | 60 300 | Z2 | 67 | 47 700 | Z2 | 53 |
| 5 | 900 | 62 100 | Z2–3 | 69 | 46 800 | Z2–3 | 52 | 54 900 | Z2–3 | 61 | 45 000 | Z2–3 | 50 |
| 6 | 900 | 45 900 | Z3 | 51 | 37 800 | Z3 | 42 | 59 500 | Z3 | 66 | 39 600 | Z3 | 44 |
| 7 | 900 | 55 800 | M1 | 62 | 49 500 | M1 | 55 | 47 700 | M1 | 53 | 33 300 | M1 | 37 |
| 8 | 900 | 56 950 | M2 | 63 | 49 300 | M2 | 55 | 31 450 | M2 | 35 | 28 475 | M2 | 32 |
| 9 | 900 | 48 450 | M2 | 54 | 36 975 | M3 | 41 | 37 825 | M3 | 42 | 11 900 | M3 | 13 |
| 10 | 900 | 32 000 | P1 | 36 | 26 000 | P1 | 29 | 10 000 | P1 | 11 | 10 000 | P1 | 11 |
| Survival rate (I) | 51.05 | 41.9 | 14.3 | 19.0 | |||||||||
| Mean survival rate (I) | 46.7 | 16.6 | |||||||||||
1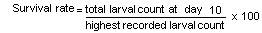
2 Very poor survival due to Vibrio infection at Z3
Note: the discrepancy in total counts during the early stages of development were due to the sampling difficulties involved with counting large numbers of very small larvae
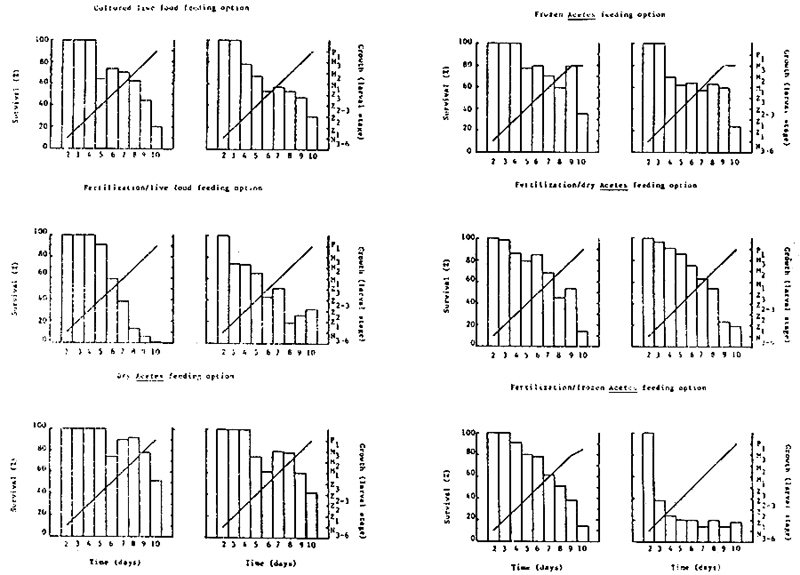
Fig. 3 - Growth and survival rate of P. monodon larvae on six different feeding regimes in outdoor tanks - Experiment A
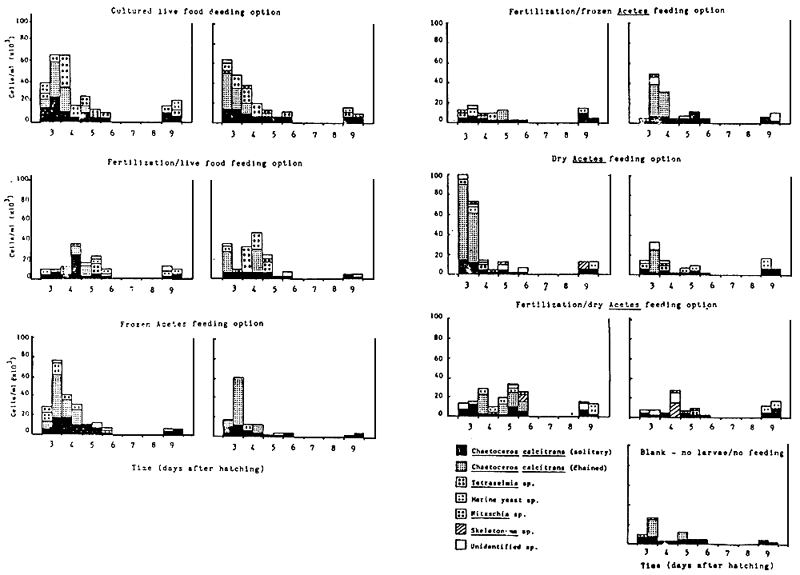
Fig. 4 Histogram representing the phytoplankton community within the larval rearing tanks during Experiment A (each day is sub-divided into a.e. and p.a. counts)
Note: Blank spaces indicate that no samples were taken on these days
4.3.2 Experiment B
The larval count, growth and survival rate of P. monodon larvae over the 11-day culture cycle is shown in Table 6 and Fig. 5. In contrast to Experiment A, all treatments displayed a low survival rate during this culture trial. Although these differences may have resulted from variations in egg quality between individual spawners, this trial was performed in indoor tanks and with double the initial larval stocking density used in experiment A (147–192 larvae/1 as compared with 62–100 larvae/1). The highest mean survival rate (calculated after 10 days of culture) was recorded for the frozen Acetes feeding option - 12.7%, followed by cultured live food - 9.5% and dry Acetes - 8.2%. However, these differences were not significantly different (p<0.05). Within the Acetes feeding options larval mortality was seen to stabilize once the zoea 3 substage had been reached. Interestingly, the Acetes feeding regime employed was changed during the zoea 3 substage from a daily feeding rate based on the actual number of larvae present within the culture thank to a saturation daily feeding rate based on the original number of larvae stocked at day 1 (i.e. so as to maintain a minimum number of feed particles/unit volume of water).
Larval development was found to be delayed by at least one day for all treatments receiving Acetes as a feed input; metamorphosis from the zoea to the mysis substage requiring one additional day (Fig. 5). Furthermore, all larvae fed with Acetes (frozen or dry) were noticeably smaller than the corresponding larvae fed culture live food.
A histogram representing the phytoplankton community within the larval rearing tanks is shown in Fig. 6. In contrast to experiment A, the phytoplankton community within tanks receiving cultured live Food was many times greater (i.e. cells/ml) than the levels observed within the Acetes treatments, due to the introduction of the diatom C. calcitrans into the cultured live food feeding option. Although larval tanks were not exposed to direct sunlight, the phytoplankton levels present were of the same order as those observed during experiment A for tanks receiving dry or frozen Acetes inputs (Fig. 4). The most dominant species present within all treatments was the solitary diatom C. calcitrans and not the chained form. Two new species were also present; the food diatom Navicula sp. and an unidentified blue green algae (although absent within treatments receiving frozen Acetes, Fig. 6).
4.3.3 Experiment C
The larval count, growth and survival rate of P. monodon larvae over the 9-day culture cycle is shown in Table 7 and Fig. 7. As observed during experiment A, the highest mean survival rate was recorded for the dry Acetes feeding option - 40.6%, followed by frozen Acetes - 10.3% and cultured live food - 7.8%. No mean survival data are available for the control - no feeding treatments as all tanks were discarded on day 9. The survival rate for two replicates at day 8 being 5.5 and 17.12 respectively at the mysis 2 substage (Table 7). However, all non-fed larvae were visibly small and weak during the mysis substage. Despite the high survival rate of larvae fed the dry Acetes feeding option, larval development was one day behind (for two out of the three replicates) that of larvae fed the cultured live food feeding option which reached the mysis 2 substage after only 7 days from hatching (Table 7). Unfortunately, no phytoplankton data are available for comment.
Table 6 - Larval count and development of P. monodon larvae on three different feeding regimes in indoor tanks - Experiment B 1
Feeding option tested: cultured live food (a-I)
| Days after hatching | Total water volume (1) | |||||||||
| Replicate I | Replicate II | Replicate III | ||||||||
| Total larval count | Larval stage | Larval density (No./l) | Total larval count | Larval stage | Larval density (No./l) | Total larval count | Larval stage | Larval density (No./1) | ||
| 1 | 200 | 29 500 | N1–3 | 147 | 38 500 | N1–3 | 192 | 32 000 | N1–3 | 160 |
| 2 | 200 | 30 000 | N3–6 | 150 | 30 000 | N3–6 | 150 | 31 500 | N3–6 | 157 |
| 3 | 200 | 25 000 | Z1 | 125 | 21 000 | Z1 | 105 | 15 500 | Z1 | 77 |
| 4 | 200 | 19 000 | Z2 | 95 | 20 000 | Z2 | 100 | 11 000 | Z2 | 55 |
| 5 | 200 | 10 000 | Z2–3 | 50 | 17 000 | Z2–3 | 85 | 13 000 | Z2–3 | 65 |
| 6 | 200 | 9 700 | Z3 | 48 | 11 200 | Z3 | 56 | 10 700 | Z3 | 53 |
| 7 | 200 | 2 600 | M1 | 13 | 9 600 | M1 | 48 | 9 400 | M1 | 47 |
| 8 | 200 | 3 600 | M2 | 18 | 9 600 | M2 | 48 | 9 200 | M2 | 46 |
| 9 | 200 | 2 300 | M3 | 14 | 6 300 | M3 | 31 | 4 400 | M3 | 22 |
| 10 | 200 | 2 500* | P1 | 12 | 4 200* | P1 | 21 | 3 000* | P1 | 15 |
| 11 | 200 | *Transferred (0730) to outdoor tanks | *Transferred (0730) to outdoor tanks | *Transferred (0730) to outdoor tanks | ||||||
| Survival rate (Z) | 8.3 | 10.9 | 9.4 | |||||||
| Mean survival rate (X) | 9.5 | |||||||||
| Feeding option tested: frozen Acetes(B-II) | ||||||||||
| 1 | 200 | 35 500 | N1–3 | 177 | 38 000 | N1–3 | 190 | 33 000 | N1–3 | 165 |
| 2 | 200 | 32 000 | N3–6 | 160 | 34 200 | N3–6 | 171 | 32 000 | N3–6 | 160 |
| 3 | 200 | 23 000 | Z1 | 115 | 18 000 | Z1 | 90 | 22 500 | Z1 | 112 |
| 4 | 200 | 7 000 | Z2 | 35 | 9 500 | Z2 | 47 | 13 500 | Z2 | 67 |
| 5 | 200 | 6 000 | Z2–3 | 30 | 4 500 | Z2–3 | 22 | 10 000 | Z2–3 | 50 |
| 6 | 200 | 3 400 | Z3 | 17 | 4 700 | Z3 | 23 | 7 400 | Z3 | 37 |
| 7 | 200 | 3 600 | Z3-H3 | 18 | 2 800 | Z3 | 14 | 6 200 | Z3 | 31 |
| 8 | 200 | 3 200 | M1 | 16 | 3 600 | M1 | 18 | 6 100 | M1 | 30 |
| 9 | 200 | 3 600 | M2 | 18 | 2 600 | M2 | 13 | 4 200 | M2 | 21 |
| 10 | 200 | 3 000 | M3 | 15 | 2 8000 | M3 | 14 | 7 400* | M3 | 37 |
| 11 | 200 | No data available | No data available | No data available | ||||||
| Survival rate (Z) | 8.4 | 7.4 | 22.4 | |||||||
| Mean survival rate (%) | 12.7 | |||||||||
| Feeding option tested: dry Acetes (B-III) | ||||||||||
| 1 | 200 | 35 500 | N1–3 | 171 | 28 500 | N1–3 | 147 | 36 000 | N1–3 | 180 |
| 2 | 200 | 30 300 | N3–6 | 151 | 29 500 | N3–6 | 147 | 32 000 | N3–6 | 160 |
| 3 | 200 | 23 500 | Z1 | 117 | 23 000 | Z1 | 115 | 20 000 | Z1 | 100 |
| 4 | 200 | 18 500 | Z2 | 92 | 10 500 | Z2 | 52 | 12 000 | Z2 | 60 |
| 5 | 200 | 5 000 | Z2–3 | 25 | 3 000 | Z2–3 | 15 | 7 500 | Z2–3 | 37 |
| 6 | 200 | 3 400 | Z3 | 17 | 1 200 | Z3 | 6 | 5 800 | Z3 | 29 |
| 7 | 200 | 4 200 | Z3 | 21 | 1 200 | Z3-M1 | 6 | 6 200 | Z3 | 31 |
| 8 | 200 | 2 800 | M1 | 14 | 1 100 | M1 | 5 | 6 800 | M1 | 34 |
| 9 | 200 | 3 000 | M2 | 15 | 1 600 | M2 | 8 | 5 400 | M2 | 27 |
| 10 | 200 | 2 700 | M3 | 13 | 1 200 | M3 | 6 | 4 600 | M3 | 23 |
| 11 | 200 | No data available | No data available | No data available | ||||||
| Survival rate (2) | 7.6 | 4.1 | 12.8 | |||||||
| Mean survival rate | 8.2 | |||||||||
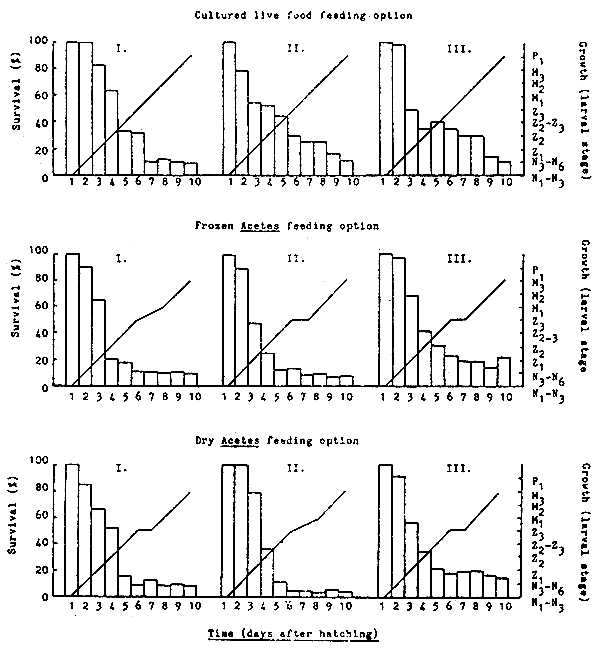
Fig. 5 Growth and survival rate of P. monodon larvae on three different feeding regimes in indoor tanks. Experiment B
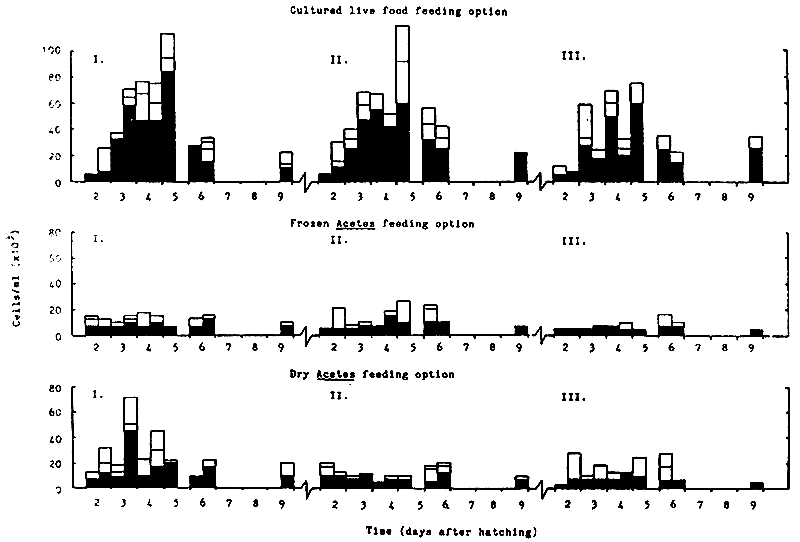
Fig. 6 Histogram representing the phytoplankton community within the larval rearing tanks during Experiment B (each day is sub-divided into a.m. and p.m. counts)
Note: Blank spaces indicate that no samples were taken on these days
Table 7 - Larval count and development et P. monodon larvae on four different feeding regimes in outdoor tanks - Experiment C1
Feeding option tested: cultured live food (C-1)
| Days after hatching | Total water volume (1) | |||||||||
| Replicate I | Replicate II | Replicate III | ||||||||
| Total larval count | Larval stage | Larval density (No./l) | Total larval count | Larval stage | Larval density (No./l) | Total larval count | Larval stage | Larval density (No./1) | ||
| 2 | 700 | 50 400 | R3–6 | 72 | 58 800 | N3–6 | 84 | 58 800 | N3–6 | 84 |
| 3 | 850 | 38 675 | Z1 | 45 | 55 675 | Z1 | 65 | 55 675 | Z1 | 65 |
| 4 | 850 | 27 500 | Z2 | 32 | 49 300 | Z2 | 58 | 51 000 | Z2 | 60 |
| 5 | 850 | 13 600 | Z2–6 | 16 | 22 525 | Z2–3 | 26 | 5 525 | Z2–3 | 6 |
| 6 | 850 | |||||||||
| 7 | 850 | 2 550 | M2 | 3 | 1 700 | M2 | 2 | 5 100 | M2 | 6 |
| 8 | 850 | 2 700 | M3 | 3 | 1 700 | M3 | 2 | 1 700 | M3 | 2 |
| 9 | 850 | 2 500 | 3 | 5 600 | 7 | 5 200 | 6 | |||
| Survival rate (%) | 5.0 | 9.5 | 8.8 | |||||||
| Mean survival rate (%) | 7.8 | |||||||||
| Feeding option tested: frozen Acetes (C-II) | ||||||||||
| 2 | 700 | 56 700 | N3–6 | 81 | 50 400 | N3–6 | 72 | 51 100 | N3–6 | 73 |
| 3 | 850 | 61 200 | Z1 | 72 | 64 600 | Z1 | 76 | 67 150 | Z1 | 79 |
| 4 | 850 | 44 200 | Z2 | 52 | 56 100 | Z2 | 66 | 55 000 | Z2 | 65 |
| 5 | 850 | 14 475 | Z2–3 | 17 | 10 200 | Z2–3 | 12 | 6 800 | Z2–3 | 8 |
| 6 | 850 | |||||||||
| 7 | 850 | 11 050 | M1 | 13 | 8 500 | M1 | 10 | 6 800 | M1 | 8 |
| 8 | 850 | 10 200 | M2 | 12 | 4 675 | M2 | 5 | 6 375 | M2 | 7 |
| 9 | 850 | 5 000 | 6 | 5 000 | 6 | 10 000 | 12 | |||
| Survival rate (%) | 8.2 | 7.7 | 14.9 | |||||||
| Mean survival rate (Z) | 10.3 | |||||||||
| Feeding option rested: dry Acetes (C-III) | ||||||||||
| 2 | 700 | 50 400 | N3–6 | 72 | 63 000 | N 3–6 | 90 | 51 800 | N3–6 | 74 |
| 3 | 850 | 55 675 | Z1 | 65 | 55 675 | Z1 | 65 | 45 900 | Z1 | 54 |
| 4 | 850 | 42 200 | Z2 | 50 | 49 300 | Z2 | 58 | 44 200 | Z2 | 52 |
| 5 | 850 | 33 575 | Z2–3 | 39 | 27 950 | Z2–3 | 27 | 29 750 | Z2–3 | 35 |
| 6 | 850 | |||||||||
| 7 | 850 | 17 000 | M2 | 20 | 47 600 | M1 | 56 | 35 700 | M1 | 42 |
| 8 | 850 | 35 275 | M3 | 41 | 45 050 | M2 | 53 | 32 725 | M2 | 38 |
| 9 | 850 | 10 000 | 12 | 35 000 | 41 | 25 000 | 29 | |||
| Survival rate (Z) | 18.0 | 55.6 | 48.3 | |||||||
| Mean survival rate (Z) | 40.6 | |||||||||
| Feeding option tested: Control - no feeding (C-IV) | ||||||||||
| 2 | 700 | 51 800 | Z3–6 | 74 | 61 600 | N3–6 | 88 | 54 600 | N3–6 | 78 |
| 3 | 850 | 38 250 | Z1 | 45 | 43 775 | Z1 | 51 | 32 300 | Z1 | 38 |
| 4 | 850 | 38 200 | Z2 | 45 | 19 500 | Z2 | 23 | 17 850 | Z2 | 21 |
| 5 | 850 | 36 550 | Z2–3 | 43 | 23 375 | Z2–3 | 27 | 41 225 | Z2–3 | 48 |
| 6 | 850 | |||||||||
| 7 | 850 | 2 570 | M1 | 3 | 32 300 | M1 | 38 | |||
| 8 | 850 | 3 400 | M2 | 4 | 9 350 | M2 | 11 | |||
| 9 | 850 | Discarded | Discarded | Discarded | ||||||
| Survival rate (%) | ||||||||||
| Mean survival rate (%)1 | ||||||||||
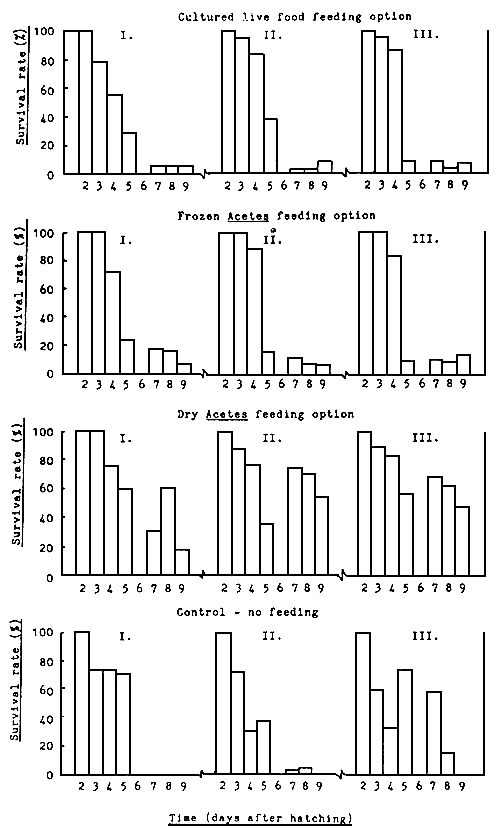
Fig. 7 Survival rate of P. Monodon larvae on four different feeding regimes in outdoor tanks - Experiment C
4.4 Discussion
Although the studies of Hameed Ali and co-workers in India were restricted to the development of a 'frozen' crustacean tissue suspension for larval feeding, the present investigation would suggest that a 'dry' crustacean tissue preparation may hold even greater potential as a larval shrimp feed. For example, in the two outdoor feeding trials conducted (experiments A and C) the highest mean larval survival rate observed was for the dry Acetes feeding option - 46.7% and 40.6% (calculated after 10 and 9 days from hatching, respectively), followed by frozen Acetes - 29.4% and 12.7%, and cultured live food - 24.7% and 9.5%. However, the performance of all three feeding strategies was poor within the indoor rearing tanks (experiment B); highest mean survival rate being recorded for the frozen Acetes feeding option - 12.7% (calculated after 10 days from hatching), followed by cultured live food - 9.5% and dry Acetes - 8.2%. The poor larval survival observed for the cultured live food feeding strategy was particularly surprising, since survival rates of 30–40% from N to P1 are normally reported for this feeding strategy in Leganes (Kungvankij et al., 1984).
Despite the high larval survival observed in outdoor rearing tanks with the dry Acetes feeding regime, all Acetes treatments displayed reduced larval development and growth compared with the cultured live food feeding strategy; larval development being delayed by at least one day, with the exception of larvae fed dry Acetes during experiment A. Furthermore, although no high-density phytoplankton blooms and crashes were observed within the Acetes fed culture tanks during water management, the rearing water of culture tanks receiving frozen Acetes was noticeably ‘ frothy’ and ‘odorous’ by the last night feeding (2400 h. or 1600 h after water management). However, apart from the development of a heavy Vibrio infection during experiment A in one of the culture tanks receiving a fertilization /cultured live food feeding strategy, no further bacterial outbreaks were observed. This was surprising, as one may have expected the rapid development of a contaminating bacterial and fungal flora, particularly within the frozen Acetes fed culture tanks.
During the present investigation the frozen Acetes feeding option performed as well as the cultured live food feeding strategy on the basis of larval survival Larval survival was within the range reported by Alikunhi et al. (1980) for P. monodon using a wet suspension - mean survival of 25.3% from N 6 to P1 after seven culture runs.
5. ADVANTAGES AND DISADVANTAGES OF A CRUSTACEAN TISSURE SUSPENSION FEEDING SYSTEM
5.1 Advantages
Compared with conventional live food feeding practices the development of a successful suspension feeding system would after numerous advantages to the shrimp farmers:
It would involve the use of a single ‘non-living’ food item for the entire hatchery culture cycle from N to P1
It is a simple feeding system which can be easily adopted by hatchery staff with little training requirement; thus reducing the level of technical skill required to operate a hatchery (from the live food production viewpoint).
It would totally dispense with the use of sophisticated algal/diatom monoculture rearing facilities and the use of live food organisms such as Brachionus spp. and Artemia nauplii. At present the maintenance of live culture organisms constitutes the major work load in hatcheries.
By eliminating the need for live food production systems, it would simplify hatchery design and more importantly reduce the high ‘start-up’ capital cost requirements for a shrimp hatchery; thus facilitating the development of ‘small-scale’ shrimp hatcheries by the family operator with limited resources. For example, using a conventional live food feeding strategy, Kungvankij et al., (1984) estimate the capital investment and operational cost for a small-scale or ‘backyard’ shrimp hatchery in southeast Asia to be not more than US$ 30 000 and US$ 10 000 respectively (hatchery production capacity of1–5 million post-larvae per annum). At present at least 20% of the total larval. rearing tank capacity of small tank shrimp hatcheries (Satul system) is recommended for algal production (kungvankij, 1982).
It would involve the use of a locally available food item and at a cost below that of conventional ‘imported’ food sources, such as Artemia cysts. In many developing countries the importation of Artemia cysts necessitates import clearances, taxes and the availability of foreign exchange facilities. By contrast, total sergestid shrimp (Acetes) landings from capture fisheries in the Philippines for 1983 was reported to be 26 217 metric tons (FAO, 1984). Although the fishery is seasonal (depending on locality), pre-dried supplies can be purchased and stored until required. The market cost of Acetes and a range of other food items commonly used at Leganes is shown in Table 8. Although it was not possible to calculate the live food feeding costs per PL produced at Leganes, the Centre Océanologique de Bretagne (France) have estimated the dry weight cost of producing Artemia nauplii and Brachionus spp. to be US$ 220/kg and US$ 2 000/kg respectively (Girin, 1979). The estimated dry Acetes cost for producing 1 million p1 larvae is p. ps. 140 or p. ps. 0.00014/p1. This calculation is based on a larval survival rate or 40% from N to p1, and the use of 0.10 mg dry Acetes/larvae at N3–6 and Z1, thereafter increasing the feeding rate by 20% day until P1 (equivalent to the use of 3.5 kg of dry Acetes for an initial stocking of 2.5 million N1 larvae). However, these costs do not include grinding and operating costs such as labour. According to Hameed Ali, Dwivedi and Alikunhi (1982) the operating costs of PL production using a wet crustacean feeding system are at least half that of conventional live food production systems.
The feeding system involves the use of a food item, the nutrient profile of which approximates to the larval shrimp's own dietary requirement; in theory the best food to feed a shrimp larvae is a shrimp larvae!
Table 8
Market Cost of Acetes and some commonly used Food Items 1
| Food Item | Market Cost (P. Ps. /kg)2 | |
| Fresh foods | ||
| Acetes(‘Alamang’) | 10 | |
| Mussel (Perna spp.) | 20 | (excluding shell) |
| Squid | 25 | |
| Trash fish (Tilapia spp.) | 2 | |
| Trash fish (Sardines) | 15–17 | |
| Chicken egg | 1.75 | (per piece) |
| Dry food | ||
| Acetes | 403 | |
| Artemiacysts | 85042 0005 | (imported) |
1 Source - Ms. K.G. Corre (March 1985)
2 Market costs at the time of this mission (March, 1985), P.ps. 18 = US$ 1.00
3 All Acetes costs are exclusive of grinding
4 San Francisco Bay Brand
5 Saunders Brand (Great Salt Lake)
5.2 Disadvantages
There is a risk of disease transmission to the developing larvae when using tissue suspensions prepared form closely related fond organisms (e.g. ‘Thelli’ M. dobsoni). It is essential therefore, that strict attention is paid to the preparation and processing (Boiling/drying of the food organisms used.
The Indian method of storing the prepared crustacean tissue suspension is questioned. At present, appropriately sized feed particles are diluted by 300% by wight with water and stored in a refrigerator for periods of up to 24 hours until fed. The stories of Grabner et al. (1961) on the suitability of frozen and freeze-dries zooplankton (Artemia salina and Moina,,spp.) as a feed for fish larvae have shown that freezing causes considerable call damage which results in extensive leaching of water soluble nutrients on thawing into water. For example. these researchers report that after 10 minutes at 9°c, about 70–75 percent of the activities of proteases and L-lactate dehydrogenase, and an even larger percentage of the free amino acids, have disappeared form the food material and can be recovered in soluble form in the water. nOt only would these soluble nutrients act as a fertilizer for algal/diatom growth, but their loss from the crustacean tissue suspension with time would lead to a progressive decrease in the nutritive value of the feeding suspension to the developing larvae. It would be more advantageous to directly freeze the appropriately sized feed particles and to dispense the frozen material into a feed suspension during each individual feeding interval. This would not only result in an enhancement of the nutritive value of the feed suspension but in would also obviate the necessity to prepare the feed suspension afresh on a daily basis. Alternatively, the feed material could simply be dried and fed as such.
The dependency of the ‘wet’ suspension feeding system on freezing/refrigeration facilities necessitates a 'dependable' power supply with adequate backup.
Potential deleterious effect on water quality. Particularly if uneaten food is allowed to accumulate in the culture tank. Although these spoilage problems can be avoided by keeping strict controls on water management, it may be necessary to introduce antibiotics into the rearing water to check the development of contaminating bacteria and fungi (Lewis et al., 1982; Simon, 1981).
Labour and equipment requirement for ‘wet’ sieving or ‘dry’ grinding.
In view of the potential deterious effect of a crustacean tissue suspension on water quality, the suspension feeding system may only be suited to small or ‘manageable’ hatchery rearing tanks of 1–2 ton capacity, where strict controls can be made on water quality.
6. CONCLUSIONS
Despite the encouraging results obtained by Hameed Ali and co-workers in India, and during the present NACA/SEAFDEC feeding trials with a ‘wet’ suspension feeding strategy, it may be more profitable in the short term to concentrate research effort on the refinement of a larval feeding system based on the use of a dry crustacean tissue suspension. the results obtained during the present investigation clearly indicate that the dry feed particles are consumed by the larvae and possess a nutritional quality capable of sustaining larval growth and survival. however, at present it is not known the contribution the ‘background’ natural phytoplankton community plays in the nutrition of the developing larvae and in the apparent success o the crustacean tissue suspension feeding strategy. Clearly, long-term feeding trials are required over a complete growing season, and under a variety of climatic and hatchery conditions, before the true potential of a crustacean suspension can be fully assessed. A list of suggested research topics is given in Annex 1.
REFEFECNES
Alikunhi, K. H., 1980 et. al., Observations on mass rearing of penaeid and Macrobrachium larvae, at the Regional Shrimp Hatchery, Azhicode, during 1979 and 1980. Bull. Dept.Fish.Kerala,2(1):68 p.
Alikunhi, K. H., 1982 Report on mass rearing of shrimp larvae at the Regional Shrimp Hatchery, Azhicode, during 1981. Bull.Dept.Fish.Kerala.3(1):40 p.
CRC, CRC Handbook of Mariculture, 1983 Volume 1 crustacean Aquaculture, edited by J.P. McVey, CRC Press Inc., Boca Raton, Florida, 442 p.
FAO, Yearbook of fishery statistics, 1984 1983. Yearb.Fish.Stat., (56):393 p. (trilingual)
Girin, M., 1979 Some solutions to the problem of producing juvenile marine finfishes for aquaculture. European Mariculture Society, Special Publication No. 4, pp. 199–209
Grabner, M., w. 1981 Wieser and R. Lackner, The suitability for frozen and freeze-dried zooplankton as food for fish larvae: A biochemical test program. Aquaculture. 26:85–94
Hameed Ali, K., 1980 A new system for mass rearing of penaeid shrimp larvae. Proceedings of the first National Symposium on Prawn Farming, Bombay, 16–18 August. 1978: 254–62
Hameed Ali, K. and S. N. Dwivedi, 1977 Acceleration of prawn growth by cauterisation of eye stalks and using Acetes indicus as supplementary feed. J. Ind.Fish.Assoc.Bombay. 3–4 (1–2)L:136–38
Hameed Ali, K. S.N. Dwivedi and K.H. Alikunhi, 1982 A new hatchery system for commercial rearing of penaeid prawn larvae. Bull.Central Inst. Fish Education ,Bombay2–3:9 p.
Jones, D.A., 1984 Penaeid Iarval culture using microencapsulated diets. Paper presented at the First International Conference on the Culture of Penaeid Prawns/Shrimps. IUoilso City, Philippines, December 4–7, 1984
Kanazawa, A., 1983 Penaeid nutrition. In proceedings of the Second International Conference on Aquaculture Nutrition; Biochemical and Physiological Approaches to Shellfish Nutrition, edited by C.D. Pruder, C.J. Langdon and D.E. Conklin. Bacon Rouge, Louisiana State University Press, pp. 87–105
Kungvankij, p., 1982 The design and operation of shrimp hatcheries in Thailand In Working party on small-scale shrimp/prawn hatcheries in South East Asia, Semarang, Central Java, Indonesia. 16–21 November 1981. II. Technical Report, SCSFB & C.P., Manila, Philippines, May 1982, pp. 117–20
Kungvankij, P., et al., 1984 Shrimp hatchery design, operation and management. NACA Training Manual Series No. 1, November 1984, in press
Lewis, D.H., J.K. Leong and C. Mock, 1982 Aggregation of penaeid shrimp larvae de to microbial epibionts. Aquaculture, 27:149–55
Liao, I., 1984 A brief review on the larval rearing techniques of penaeid prawns. Paper presented at the first International Conference on the Culture of Penaeid Prawns/Shrimps, Iloilo City, Philippines, December 4–7, 1984, in press
New, M.B., 1976 A review of dietary studies with shrimp and prawns. Aquaculture, 9:101–44
Scura, E.D., J. Fischer and M.P. Yunker, 1984 The use of microencapsulated feeds to replace live food organisms in shrimp hatcheries. Paper presented at the First International Conference on the Culture of Penaeid Prawns/Shrimps, Iloilo City, Philippines, December 4–7, 1984
Simon, C.M. 1981 Design and operation of a large-scale, commercial penaeid shrimp hatchery. J.World Maricult.Soc., 12(2): 322–34
Watanbe, T., C. Kitajima and S. Fujita, 1983 Nutritional value of live food organisms used in Japan for mass propogation of fish: A review. Aquaculture, 34:115–43
ANNEX
SUGGESTIONS FOR FUTURE RESEARCH
Ideally the following trials should be conducted in replicate 1–2 t hatchery rearing tanks using initial nauplii concentrations of 100–150/1. Nauplii should be obtained from individual spawners, and more than one species tested. For example, depending on location: P. monodon, p. indicus, P. merguiensis, P. semisulcatus, P. japonicus, P. aztecus, P. duorarum, P. setiferus and P. vannamei.
A. RESEARCH PROPOSALS INVOLVING THE EXISTING DRY ACETES FEEDING STRATEGY
1. Acetes feed survey
2. Acetes feed quality
Nutrient content
spoilage characteristics/shelf life - lipid oxidation, microbial spoilage
3. Role of natural phytoplankton in the nutrition of Acetes-fed larvae
Using standard hatchery procedures the following observations should be made in conjunction with larval growth and survival:
When monitoring larval growth and survival the following additional data should be collected (if possible):
4. Comparative larval feeding trials with other existing hatchery feeding regimes
These feeding trails should be compared on the basis of larval growth and survival, dependability and cost/unit of production.
B. RESEARCH PROPOSALS INVOLVING MODIFICATIONS TO THE DRY ACETES FEEDING STRATEGY
1. Feeding regime
To determine the optimum feed particle size for each larval stage.
| Present feed particle size | Suggested size ranges for testing | |
| N3–6-Z2 | 50<125 | 10 – 200 |
| Z3-M2 | 125<250 | 100 – 500 |
| M3 - P1 | 250<350 | 200 – 600 |
To determine the optimum feeding level for each larval stage.
Present feeding level is 0.10 mg/larvae/day at N3–6 and Z, thereafter increasing by 20%/day until P1. Feeding levels of 0.05, 0.10, 0.15, and 0.20 mg/larvae/day, and subsequent daily increments of 10, 15, 20, 25 and 30% should be tested. For example, the delayed larval development observed for Acetes fed larvae may have been due to under feeding.
To determine the optimum frequency or feed presentation.
Present feeding frequency is four feeds/day at 0830, 1200, 1700 and 2400 b. In view of the potential lose of soluble nutrients through leaching, the effect of a range of different feeding frequencies should be tested. For large-scale hatchery operations the feasibility of using automatic feed delivery systems should also be tested.
2. Feed preparation and formulation
Drying technique.
Effect of different drying techniques on feed performance: air drying (indoors/outdoors), freeze-drying or drum drying.
Vitamin/lipid fortification.
The effect of adding a vitamin/lipid supplement to the dry (Acetes prior to feeding should be tested. The aim of using such a supplement is to fortify Acetes with essential vitamins and polyunsaturated fatty acids, to make the particles more visible to the larvae (by using carophyll red as a pigment), and to increase the water stability of the feed particles and so reduce nutrient leaching (by emulsification with soy lecithin). Such a diet could also be tested during the nursery stage as a replacement for Artemia nauplii. A suggested vitamin/lipid supplement for testing could be as follows:
| Carophyll red1 | 0.5g |
| Soy lecithin | 1.5g |
| Shrimp head oil 2 | 5.0g |
| Vitamin mix3 | 2.0g |
1 10% suspension of canthaxanthin in an oil base
2 If not available, can be replaced with red fish oil or krill oil
The vitamin/oil premix should be prepared by first dissolving the carophyll red and soy lecithin in the fish or shrimp oil, followed by the vitamin premix. Mix and homogenize well and then apply to the dry basal protein source (i.e., Acetes; using 9 g of vitamin/oil premix for every 91 g of dry pre-ground (Acetes). When using shrimp or fish oil, efforts should be made to procure sources which have been pre-stabilized with 250–500 ppm antioxidant.
3. Suitability of other crustacean preparations for larval feeding
Depending on availability, these could include M. affinis, M. dobsoni, P. stylifera, N. tenipes, O. nepa and Mesopodopsis spp.