PAR NÉJI ALOUI
I- INTRODUCTION
Le succès d'une culture en masse de poissonset de crustaclés dépend surtout de la disponibilité d'une nourriture abondante et adéquate pour les jeunes slades.
Très vite, il s'est avèré que la culture en massedu zooplankton, qui constitue la nourriture naturelle pour les stades larvaires, n'était pas économiquement réalisable.
La décourverte par les chercheurs que les larves d'Artemia constituaient une excellente source de nourriture pour les jeunes alevins, représentatnt une percée importante dans le développement de l'aquaculture.
Cette nourriture vivant peut en effet être produite facilement à partir d'oeufs trouvés sur les berges des lacs salés.
En Tunisie, les recherches sur l'Artemia viennent de voir le jour et ceci après avoir pris connaissance de la nécessité de cet animal pour les activités auquacoles.
II- BIOLOGIE DE L'ARTEMIA
L'Artemia est un animal aquatique, qui fait partie de la classe des crustacés, il appartient à la subclasse des Branchiopodes et à l'ordre des Anostraca.
L'Artemia vit dans eaux salées et hypersalées et peut s'adapter facilement à des variations de salinité, allant de 70% à 300%. Les populations d'Artemia rencontrées dans la nature peuvent être, soit bisexuées, soit parthénogénétiques.
La reproduction est soit ovovivipare (production de nauplii), soit ovipare (production d'oeufs). Dans ce second cas, la femelle pont des oeufs appelés oeufs durables ou cystes de l'embryon peut rester Vivant à l'intérieur de la coque pendant des années quant l'oeuf est bien conservé contre l'humidité. Ces oeufs, une fois incubés dans l'eau de mer préparée aux conditions d'incubation, se gonflent, atteignent le maximum de leur diamètre qui varie entre 100 et 300 um et donnent naissance à des nauplii dont la taille est de l'ordre de 40 um. Le nauplius passe par plusieurs stades de développement pour arriver au stade adulte (0,8 à 2 cm).
III - REPARTITION DE L'ARTEMIA DANS LE MONDE
Les habitats d'Artemia sont distribués partout dans le monde à l'exception de l'Antartique. Actuellement, on compte plus de 300biiotopes d'Artemia (Fig. 1) (SORGELOOS et al, 1986).
D'après cet auteur, 80% des populations étudiées dans l'hémisphère occidnental sont bisexuées, alors que 70% de celles de l'hémisphère oriental sont parthéinogènétiques.
Les biotopes naturel d'Artemia sont limitées aux milieux où les concentrations ioniques sont assez élevées, ce qui exclut les prédateurs vu les prédateurs vu les conditions très difficiles. C'est ainsi que les populations naturelles d'Artemiaso t observées soit dans les eaux athalassohalines. Les sites thalassohalins sont des eaux de mer consentrées en Nacl, alors que les eaux athalassohalines sont caraetérisées par une composition ionique qui diffère beaucoup de l'eau de mer, telle que les eaux riches en carbonates ou en potassium ou en sulfates. (PERSONNE et SORGELOOS,1980).
IV - REOARATION DE L'ARTEMIA EN TUNISIE (Fig. 2)
L'existence d'Artemia en Tunisie a été pour la première fois par (SEURAT, 1921) dans le Chott Ariana. Après cette date ce petit crustacé a été mentionné par (HELDT, 1926) dans les anciens ports de Carthage et par (GAUTHIER, 1928) à Sebklet Sidi-El-Hani.
Recemment, une première étude, prospective a été effectuée par l'INSTOP; cette étude a montré que l'Artemia est présente en Tunisie, elle vit dans les milieux hypersalés qui sont très nombreux et qui peuvent être classés en deux catégorie: les étangs athalassohalins et les étangs thalassohalins. Les permiers sont continentaux et alimentés par l'eau des oueds et des pluies, alors que les seconds sont en communication directe avec la mer et la composition chimique de leur eau est surtout chlorée.
4.1. Les étangs athalassohalins
4.1.1. Sebket ARiana
Cet étang se trouve dans le banlieue de Tunis et est limité au Sud par la Soukra, l'Est par la zone de Rraoued, au Nord par Sidi-Amor-Bou-Khtioua et à l'Ouest par Bourj-Touil. La superficie de cet étang est d'environ 1000 ha. C'est une zone maraicageuse, la salinité de l'eau varie selon l'époque de l'année et selon les endroits.
Des prospections ont été par L'INSTOP dans les parties Sud (Soukra) et est (Raoued). Elle ont parmis de récolter des oeufs d'Artemia dans la zone de la Soukra, mais une bonne partie de ces oeufs étaient vides et morts.
4.1.2. Sebket- Sidi - El - Hani
Située au centre du pays, d'une superficie dis fois plus grande que le Sebklet ARiana, la Sebket Sidi - El - Hani et le village de Khniss; à l'Est, elle longe la route de Sousse-Sfax sur trente Kilomètres; au Sud par, la région de Souassi et à l'ouest par la piste reliant Kairouan à Zmala de Souassi sur trente cinq Kilomètres.
Des prospections ont été faites par l'IMSTOP; elles ont révelé l'existence de l'Artemia dans la partie nord du Sebka.
Figure 1: Réparation de l'Atremia dans le monde (d'après SORGELOS et al., 1986)
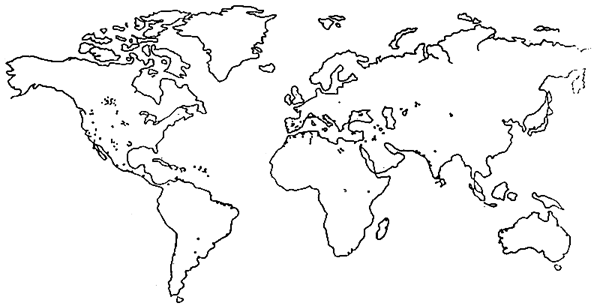
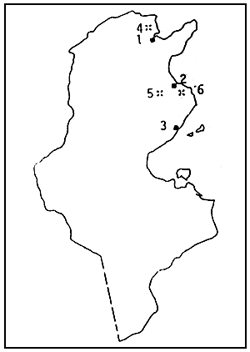
Figure 2: Réparation de l'Artemia en Tunisie
• Salines
* Sebkhas
1 - Saline de Mégrine 2 - Saline de Bekalta 3 - Saline de Sfax
4 - Sebkhet ARiana 5 - Sebkhet Sidi El - Hani 6 - Sebkhet El - Mokhine
4.1.3. Sebket - El - Moknine
Cette sebka se trouve dans la région de Mahdia, elle a une superficie de 3.500 à 4.000 ha et elle est limitée au Nord par Moknine à l'est par la route de Bekalta à Mahdia sur une distance de septKilométres au sud par route Mahdia - Sidi - Bannour et àl'Ouest par la route Moknine - Sidi Bannour.
Les prospections effectuées par l'INSTOP ont révélé l'existence de l'Artemia dans l'eau de la Sebka, alors que les cystes sont presque inexistants.
4.2 Les étangs thalassohalins.
Ces étangs sont des salin, en communication directe avec la mer et servant à fabrication du sel. Ils sont constitués par un ensemble de bassins qui communiquent les uns avec les autres. L'eau arrive de la mer soit par pompage, soit par gravité et stagne dans les premiers bassins où la densité de l'eau est de 3à 8° baumé; de ces bassins l'eau passe dans un autre bassin appelé pièce maîtresse (10° à 15° Be); celle-ci passe par la suite dans le bassin de réserve où celle atteint une densité de 23° Be et elle servira alors à alimenter les tables salins (25° Be) où se fait la fabrication du sel.
Parmi ces milieux salins, il y a lieu de citer: la saline de Sfax, la saline de Bekalta et la saline de Megrine.
4.2.1. La saline de Sfax
Cette saline appartient à la campagnie COTUSAL, entreprise semi-étatique; elle a une superficie de 1485 ha. Les prospections effcetuées par l'INSTOP ont révéla présence de l'Artemia dans certains bassins de la saline.
4.2.2 la saline de Bakalta
C'est une saline privée, dans la région de Bekalta à Sidi El Baghdadi, elle a une superficie d'environ 120 ha. L'eau passe de la mer dans les partenements par gravité puis fait tout son circuit pour arriver dans les cristallisoirs pour la fabrication du sel.
Les prospections effectuées par l'INSTOP ont révélé la présence de l'Artemia dans cette saline.
4.2.3. La saline de Megrine
Cette saline appartient à la compagnie COTUSAL; elle a une superficie de 1000 ha.
Les prospections effcetuées par l'INSTOP ont révéléla présence de l'Artemia dans quelques bassins de la saline.
4.3. Conclusions des prospections
Les prospections menées par l'INSTOP ont permis de révéler les faits suivants:
L'Artemiaest répandue en Tunisie, elle existe dans les milieux athalassohalins et thalassohins;
L'accession et la manipulation de l'Artemia dans les milieux athalassohalins est trés difficile voir même impossible dans la plupart des cas;
Les oeufs d'Artemia se trouvant dans les milieux athalassohalins sont de mauvaise qualité;
L'accession et la manipulation del'Artemia dans les milieux thalassohalins est plus facile que dans les milieux athalassohalins;
Les oeufs d'Artemia se trouvant dans les milieux thalassohalins sont de bonne qualité.
V - TRAVAUX EFFECTUES
A côté de l'étude prospective, paragraphe IV; d'autres études ont été effectuées dont nous citons:
- L'étude effectuée par l'INSTOP portant sur l'Artemia dans la saline de Megrine.
Les résultats de cette étude ont permis de dégager les faits suivants:
sur le plan quantitatif et durant toute la l'étude, la quantité d'oeufs d'Artemia récoltée était de 4 kg de poids sec.
- L'étude effectuée par (VAN BALLET et al ., 1987) et qui a traité des echantillons d'oeufs d'Artemia en provenance des sebkas et des salines. Sur ces échantillons, des analyses relatives à l'identification de l'espèce tunisienne, au sex-ratio et aux caractères biomètriques des oeufs et des nauplii ont été effectuées.
Les résultats de ces analyses ont permis de dégager les faits suivants:
La longuneur du nauplius est de 467,7 um pour la souche de MEgrine, de 482,3 um pour la souche de Bekalta et de 422,2 um pour la souche de Sfax.
- L'étude effectuée par (KHEMAKIIEM, 1988) portant sur l'Artemia dans la saline de Sfax. Ce travail a traité l'hydrologie de la saline d'une part et la biologie de l'Artemia qui y habite d'autre part.
L'hydrologie des bassins représentant tous les circuits de la saline a permis de révéler des fluctuations de certains paramètres physicochimiques, liées aux conditions climatiques d'une part et au système de fonctionnement de la saline d'autre part.
L'étude de l'Artemia a révélé que l'abondance et la distribution saisonnière d'Artemia sont liées aux conditions du milieu. La population d'Artemia dans cette saline est bisexuée. Après fécondation, les femelles peuvent se reproduire soit par ovoviviparité, soit par oviparité, soit par oviparité avec émission dans le premier cas de nauplii et dans le deuxième cas d'oeufs. Sur le plan quantitatif des oeufs d'Artemia et durant toute la durée de l'étude, l'auteur a récolté une quantité très faible.
En conclusion de tous ces travaux, nous pouvons dire que dans les sebkhas, on a découvert l'existance de l'Artemia en très faible densité et de faibles quantités de cystes dispersés sur les berges par l'action du vent. L'étude de la qualité de ces cystes a montré un très faible taux d'éclosion dû au fait que les cystes sont exposés aux changements climatiques et se trouvent alors hydratés et deshydratés plusieurs fois, ce qui diminue la viabilité de l'embryon à l'intérieur du cyste et diminue par conséquent le taux d'éclosion de ces derniers. Dans les salines, le ces se présente différement, c'est à dire que les cystes présentent moins de risques de déterioration et de mortalité, étant donné qu'ils restent baigner dans le milieu salin, par conséquent le taux d'éclosion reste élevé.
VI - RECHERCHES ACTUELLES
Compte tenu de ce qui précède, nous menons actuellement des recherches relatives à la bio-écologie de l'Artemia dans les salines, à l'utilisation des oeufs d'Artemia récoltés de ces salines, au sevrage et à la maîtrise de l'élevage de l'Artemia en milieu contrôlé.
Les résultats des recherches actuelles ont permis de mettre en évidence les faits suivants:
6.1 Aspect bio-écologique (population de Mégrine). (Fig. 3)
- La souche est bisexuée.
- dominance des jeunes stades fin de l'hiver - début du printemps;
- l'éclosion des oeufs a lieu en automne et fin de l'hiver;
- la souche de Mégrine est tantôt ovovivipare, tantôt ovipare;
- le pourcentage des femelles ovipares s'accroit avec l'arrivée de l'été;
- la production en oeufs est de 20 kg (poids sec).
6.2 Aspect utilisation en milieu d'élevage
L'étude relative à l'utilisation des oeufs d'Artemia en provenance de la saline de Mégrine a donné de bons résultats aussi bien au niveau de la croissance que pour le taux de survie des larves.
6.3 Aspect élevage de l'Artemia à l'échelle du laboratoire
L'élevage de l'Artemia à l'échelle du laboratoire est maîtrisé.
VII - PRESPECTIVES
- poursuivre les études biologiques et écologiques relatives à l'Artemia dans les milieux thalassohalins;
- élever l'Artemia dans des structures plus grandes afin de s'assurer de la maîtrise de son élevage;
- mener des expérimentations sur le larvaire (experiences de sevrage).
- chercher un substitue de l'Artemia.
Figure p 25 à reduire
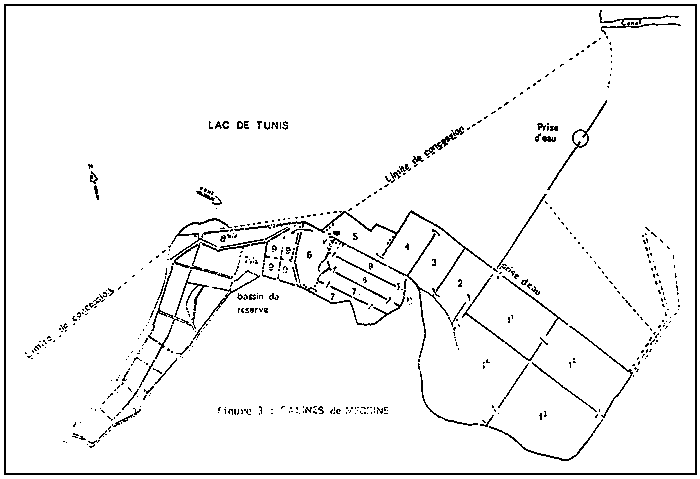
Fig. 3 Salines de megrine
PAR B. MISSAOUI &
N. ABDELKADER
INTRODUCTION
L'activité aquacole a commencé en Tunisie depuis les années 60 avec l'implantation de la première station aquacole “Elevage de coquillage” au nord du pays. Ce n'est qu'au cours du VIème Plan (1982– 86) que les essais de reproduction de poissons ont commencé au niveau de l'écloserie de Ghar El Melh permettant de dégager pour la première fois le problème d'alimentation larvaire.
C'est ainsi qu'un programme de recherche a été mis au point étudier les potentialités d'exploitation de l'Artiémia en Tunisie.
PROSPECTION
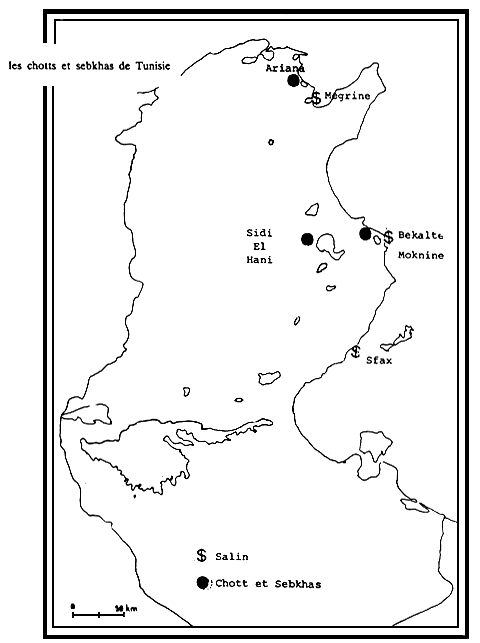
Les prospections (B. Abdelkader, 1985) faites durant les années 1982 et 1983 signalent l'existance de l'Artémia dans les Chotts, Sebkas et Salins suivants (Chott El Jerid, Sebkhra de l'Ariana, Sebkha de Kourzia, Sebka de Moknine, Sebka de Sidi El Heni. Salins de: Megrine, Sahline, Bekalta et Sfax) (voir carte).
1 - Chotts et Sebkhas:
Ce sont des étangs continentaux n'ayant pas de communications avec la mer et qui sont alimentés par l'eau des oueds et des pluies.
Selon l'époque de l'année, ces étangs peuvent être couverts d'eau en hiver ou asséchés en été, et la salinité peut varier de quelques grammes pour mille à plus que 200‰.
Les cystes se présentent sous forme de frange de couleur brunâtre sur les rives de l'étang.
II est à signaler que ces étangs présentent des difficultés d'explotation résultant de:
- La faible hauteur de la colonne d'eau sur ces étangs,
- Les difficultés d'accès à ces étangs,
- L'assèchement de ces plans d'eaux pendant l'été.
2 - Salins:
Il s'agit de bassins communiquants alimentés par l'eau marine; La salinité dans ces bassins varie entre 40‰ et plus de 200‰.
Les salins prospectés appartiennent en majorité à une compagnie semi étatique COTUSAL.
Les prospections dans ces salins ont révélé la présence d'Artémia vivante ainsi que les cystes viables.
Contrairement aux sebkas, les salins sont plus faciles à expoiter en raison de:
- l'existence d'une plus importante hauteur de la colonne d'eau dans des bassins,
- la facilité d'accés aux bassins d'artemia
- la possibilité de gestion de la biomasse d'Atrémia dans les bassins de faible salinité.
RESULTATS DES CAMPAGNES DE PROSEPECTION:
La quantité d'artemia existante dans les salins est faible et ne peut faire l'objet d'une exploitation rentable.
En effet il s'agit d'une récolte de quelque dizaines de Kg dans le salin de Sfax.
L'examen des échantillons de cystes d'Artémia tunisienne a été conjointement par des techniciens
tunisiens et belges.
Les résultats révèlent l'existence de l'existence de l'espèce bisexuelle Artemia tunisiana ainsi que l'espèce Artemia pathenogenetica répandue surtout dans le salin de Mégrine.
La qualité de cystes récoltés en Tunisie varie d'un site à un autre. Les cystes récoltés dans le salin de Sfax sont de bonne qualité (taille dus cystes décapsulé < 220 pm; et présentent un bon taux d'éclosion; et bonne composition en acide gras polynisaturé).
| Taille des cystes | MEGRINE | BEKALTA | SFAX |
|---|---|---|---|
| Cystes non traités | 258,8 | 251,6 | 235,4 |
| Cystes décapsulés | 234,1 | 228,0 | 215,1 |
| Taille des cystes | MEGRINE | BEKALTA | SFAX |
|---|---|---|---|
| Taux d'Ecolosion | MEGRINE | BEKALTA | SFAX |
| Pourcentage (%) | 60,5 | 83,2 | 84,8 |
Bien que ces cystes soient de haute qualité pour leur utilisation en aquaculture, elles se trouvent peu résistantes aux facteurs environnants et présentant une faible productivité.
ESSAIS D'INOCULATION:
Au vue des résultats des prospections et dans le cadre d'un projet de coopération avec “l'Artémai” Référence Center, il nous a paru judicieux d'inoculer une espèce étrangères d'Artémia plus productive et plus résistante que l'espèce locale Artemia tunisiana. C'est le salin de Mégrine qui a été choisi pour réaliser cette expérience d'inoculation.
Les essais ont été faits avec la souche de “Maccau” et celle de “Great Salt Lake” en comparaison avec la souche tunisienne.
D'autres essais ont été faits à plus grande échelle au salin de Sahline moyennant une fertilisation par des déchets de volaille.
Les résultats de ces deux opérations n'étaient pas concluants pour plusieurs raisons
- les opérations de fertilisation et d'inoculation n'étaient pas bien programmées.
- suivi irrégulié de cette expérience,
- faible hauteur de la colonne d'eau dans les salins.
- faible productivité de l'eau dans le salin de Sahline
PERSPECTIVES DE DEVELOPPEMENT
Pour une bonne exploitation de l'Artémai dans les salins tunisiens, une étude a été élaborée dans le cadre du Plan Directeur de l'Aquacuture.
Dans ce travail des recommandations et des suggestions ont été proposées dans le but de la production d'Artémia en Tunisie. Il a été proposè de procéder en 2 étapes:
1 - lère Etape: Vérifier l'hypothèse qu'une souche plus performante donne de plus grandes productions (biomasse et cystes) et une meilleure qualité du produit.
Cette vérification s fait à travers des inoculations.
L'espèce à inoculer doit être performante (A. fransiscana) et résistante à des températue élevées (A. fransiscana produite au Vietnam).
Des suggestion ont été faites pour le choix de l'endroit d'inoculation, (Mégrine bassins d'évaporation №6 ou Sfax Canal à 150g/I).
2 – 2ème Etape: En fonction des résultats de l'expérience d'inoculation procéder à l'intégration de production d'Artémia avec le sel.
Cette action nécessite:
- des aménagements supplémentaries pour augmenter la hauteur de la colonne d'eau dans les circuit de 130 et 200 g/l:
- un programme d'élimination de prédateurs,
- un programme de fertilisation des bassins avec l'urée et la phosphate d'ammonium.
- un programme d'inoculations,
- récolte, conditionnement et traitement des cystes et de la biomasse,
- contrôle de la qualité du produit.
| Production attendue | = 150 T/an. |
| Estimation des revenus | = 375.000 US$/an |
Artemia dans les chotts et sebkhas de Tunisie
Estimation des revenues = 375.000 US$/an
BY S. GHONEIM
Survey for natural artemia available in Egypt: the occurrence of artemia were recorded in different sites. Most of these sites are man made salines located on the Mediterranean coast, while two sites are located in inlands areas
The coastal sites are: Port-Fouad, Balteim, El-Max and Bourg EL-Arab salinas. The inland sites: Malaha El-Salfate in Wady E-Natroun and Fayom salina beside Qaroun lake. Bisexual artemia are found in Malaha El-Salfate (in Wady El-Natroun) port Fouad Salina and Balteim Salina.
Cross breeding tests performed and revealed that artemia from Wady El-Natroun is belonging to artemia tunisiana, while the recent available artemia franciscana. However, parthenogenetic artemia is found in Fayom salina. Artemia from wady El-Natroun possessed the smallest cyst diameter while artemia from Fayom salina possessed the largest cyst diameter.
Artemia franciscana has been inoculated in Port Fouad salina, and it is proved that the production artemia is technically and economically feasible.
BY M. O. MAGSODI
The importance of Artemia increased remarkably in the last years in most countries of the world. This importance stems from expanding its use as life for many species of fine fish and crustacean as well as the development of its use far larvatic stages of the same species especially after the spread of aquaculture on a large scale.
In the Jamahiriya three sites have been known located for the existence of Artemia, two of which are coastal areas and the third is in the desert south Libya as follows:
El Kuwaim sebka in the central region in Brega region; this sebkha is located at a distance of about 770 km from Tripoli.
Abukamash sebka, this coastal sebka is located at a distance of about 150 km West of Tripoli, in Abukamash area near the tunsian boarders.
Lakes located in the Southern, if can be said that the existence of Artemia in the Jamahiriya and common use as food for human beeings started before now and the importance of this creature as food for fish and crustacean is increasing with farming activities.
Therefore, there is need to point out that:
Just now, no classification and to identification of Artemia have been done in Libya.
No produce of Artemia in Libya.
A few cysts collected from the different sites to run experiments and get some studies going on.
BY M.A. ABI SAAD
L'absence d'une intense activité aquacole au Liban fait que les besions en Artemia ne sont pas du tout ressentis et que son utilisation se fait seulement en petite quantité dans la nourriture du poisson d'ornementation.
D'autre part, eu égard à l'inexistance des lacs salés types sebkha, les populations d'Artémia ne sont pas rencontrées dans les eaux Libanaises. Toutefois, la présence au Liban des marais salants abandonnés, pourraient inciter à y établir une activité d'élevage d'Artémia à petite échelle et qui serait intégrée à celle de la protection du sel.
PAR M.H. AMANE
La production naturelle d'artémia au Maroc est très faible; il s'agit d'une souche parthénogénétique (artemia parthenogenetica) qu'on rencontre dans les marais salants au nord du Maroc du coté de la Méditerranée et à l'ouest sur les cotes atlantiques.
Deux grandes sociétés d'aquaculture implantées au Maroc depuis 1986 consomment des grandes quantités d'artémia (5 tones/an).
Cependant, aucune décision visant la production des cystes d'artémia n'a été à ce jour envisagée sur le plan public our privé.
BY N. GOKGOZ
There is no production of artemia in Turkey which imported yearly about 400 tonnes of dry artemia cysts for larvae culture from Belgium and other countries. While natural production is represented by the Artemia parthenogenetica met in the only salt lake existing in Turkey and quality and size seem to be well adapted in feeding larvae, the presence of that Artemia is not too great.
PAR I. SAMIA
L'étude prospective dans certains sites favorable à la production naturelle de l'Artemia fut établie en 1988 en Algérie. Elle revela la présence d'une population d'Artémia composée de larves, de cystes et d'adultes bisexuels dans la saline d'Arzew.
L'étude biologique et systèmatique ont permis d'identifier l'espèce comme appartenant au genre Artemia tunisiana.
La saline d'Arzew assure une production de 87 000 T de sels par an soit 65% de la production nationale.
La souche d'Artémia d'Arzew est d'une qualité comparable à celle des souches qui sont commercialisées de nos jours (San Francisco Bay et Great sali lake).
La collecte de l'Artemia produite naturellement se fait facilement dans la saline d'Arzew et l'Algérie pense dans l'avenir y entamer la culture de cette artémia.
BY M.S. ROMDHANE
La Tunisie, avec ses 1300 km de côtes présente, à part les lagunes côtières, trois catégories de plan d'eau salées: les salines, les sebkhas et les chotts.
Les salines Tunisiennes sont réparties en 2 groupes, les grandes salines avec une grande superficie et une grande capacité de production, et les petites salines moins équipées et d'activités irregulières.
Les sebkhas et les chotts couvrent environ 1 million ha et leurs superficies trés variables; l'Artémia est observés dans les sebkhas d'Ariana, de Komzia, de Moknine, de Bekalta, de Sidi El Hani et d'El Melah, ainsi que dans 8 chotts El Jerid, El fjaj et El Gharsa.
L'Artemia présente en Tunisie est idenifiée comme souche bisexuelle d'espèce Artemia tunisiana. L'Artémia parthénogénica est observée seulement dans les salines de Mégrine.
Presented by
Mr.Belkhir
Aquaculture activities in Tunisia was enhanced in large scale since 1982 and still being supported to now by the government.
During the period 1982 – 86 trials on fish artificial reproduction showed for the first time several problems in the fish larvae stage.
Then a research program was conceived; it was dealing particularly with the Artemia investigation in the field studies of Artemia characteristics in lakes;
That program was held in the framework of cooperation between the scientific institution (INSTOP) in Tunisia and Ghent University.
Results were very useful and indicated relative and spontaneous richness of Artemia within salt water and some coastal waters.
Ecological and biological characteristics of Artemia were presented to the participants during the first session of the Artemia training course.
Briefly there is need to remind that the artemia cysts found in the south of Tunisia seemed to be better than the northern ones.
Results indicated that Artemia is the bisexuel called : Artemia tunisiana; and other one which is the Artemia parthenogenica was found essentially in the northern part of Tunisia.
From these conclusions, a project was foreseen to develop inoculation trials using the Maccau strains and the great salt lake one.
Activities were held in a very roughly and preliminary way using several fertilisers
Unfortunately, this project fell down and obtained results, even if they were interesting they could not be very indicative because we notify a lack in the efficiency and the regularity of the investigation enterprised during the preliminary phase.
Therefore specialised institutions are according actually an important interest to Artemia exploitation and the guideline of the forthcoming activities on Artemia are conceived in the framework of the national marter plan for the development of aquaculture:
- to develop a more performant strain which gives greater productivity and better products quality for that, Artemia franciscana and the resistant specy to temperature such as the Artemia franciscana produced in Vietnam will be inoculated and tried for production.
- in the second phase, effort will be undertaken to integrate Artemia exploitation and production within the salt production activities.
Before undertaking any pilote activities the following points should be taken into consideration:
- the conception of ponds management, salt waters and their exploitation;
- set up a methodolgy for reducing predators;
- control and follow up cysts production in terms of quality and quantity.
The foreseen production is: 150 tones/year.
The foreseen flow back is: 375 000 US $/year.
BY M.A.S.D.AHMED
Artemia was used for the first time in Egypt in Macrobrachium feeding at Saft Khaled hatchery.
following the studies on artemia done by the university of Suez Canal a commercial Unit of Artemia production was implemented at El Nasr Salines company
the production of Artemia salina cysts and Artemia franciscana reached 500 kg per year covering a total area of 100 ha
culture conditions are : 20–30° C, and 2000 Lux
BY GILBERT VAN STAPPEN
AND PATRICK SORGELOOS
A tiny crustacean, adapted to living in highly saline waters, has become the most widely used live food item in aquaculture. Improved harvesting and processing techniques, as well as culture of the organism itself, have helped make the Artemia virtually indispensable to the industry.
Salt has, always been very precious to man, not only because of its dietary value, but also as an indispensable tool for preserving food. In ancient times salt was a means of payment. This explains the etymology of the word salary, used in so many languages. Modem industry is using sodium chloride salt as a basic ingredient for numerous chemical processes and consumes more than 90% of the annual production of more than 200 million mt.
All over the world local populations have developed methods for solar salt production, ie the extraction of salt from seawater. Management techniques were developed to maximise evaporation, to allow sequential precipitation of caronates and sulphates so as to eventually collect the sodium chloride.
What man didn't realize, until very recent times, was the fact that these very salty waters, often considered lifeless, were the habitat of a remarkable invertebrate organism, the 1-cm long brine shrimp Artemia. Its high food value for aquaculture and aquarium pet organisms was soon appreciated and in less than two decades commercial harvests reached over 1 000 mt of cysts and over 10000 mt of biomass annually. Worldwide sales in 1992 of Artemia cysts and biomass are estimated at over US$50 million.
The selective cosmopolitan
The small crustacean Artemia is found all over the world in a wide array of hypersaline biotopes, eg coastal salt pan, inland salt lakes and chloride, sulphate and carbonate waters. The only factors limiting its presence are the salinity has to be sufficiently high (mostly above 100 g/l total salts) to exclude the presence of predators (fish and arthropods), and the water temperature, that must allow development and reproduction. Depending on these factors, populations are seasonal or are present on a year-round basis.
In an optimal environment the habitat is colonised at an astonishing rate: mature females, reproducing ovoviviparously, can produce 200 – 300 free-swimming nauplii every four days. These nauplii grow into adults in less than two weeks. In a combination of adverse conditions like oxygen stress, high salinity, changes it temperature, food limitation, etc the reproductive mode is switched to oviparity : fertilised eggs develop into a late gastrula, at which moment the metabolism is reversiby interrupted and each individual embryo, now called a cyst, is enveloped by a protective shell. Mother Artemia release these ametabolic cysts which float at the water surface, are aggregated by the wind, and eventually accumulate in a dehydrated form on the shores.
Since the Artemia species has no means of active dispersal, its natural occurence is determined by the factors of wind and migrating water fowl acting upon the dry cysts; hence its geographic distribution shows a discontinuous pattern. The ecological diversity of these isolated biotopes and the genetic flexibility of the species have led to the evolution of more than 350 geographical strains. As could be expected with the exploration of new habitats of Artemia (eg in Central Asia and China), the list is still growing.

An adult male Artemia approximately one cm long
Artemia strains
Strain Characteriastion is a crucial item in Artemia study, however, a dubious reputation among Artemia specialists. Indeed, as more and more natural habitats of this organism were discovered in the late 1970s and 1980s, it soon appeared that all these different populations were displaying a considerable variability in all kinds of characteristics.
This necessitated elaborate morphological, biometrical, and genetic research, making Artemia the favourite of tenacious taxonomists, subdivision of the genus into species, occurence of pathenogeneticand bisexual strains, and generally the entire problem of genetic flexibility and speciation are, however, not merely discussions to be carried out in the confines of ivory towers: practice proves that these strain differences have implications in the field of aquaculture that can hardly be underestimated on one hand, that provide unique opportunities for later selection work on the other.
Tapping nature's resources
The discovery and study of several brine shrimp habitats resulted in a better knowledge of the complex Artemia biology. At the same time, the commercial exploitation of these biotopes has definitely and probably definitely rebalanced the very uneven situation of the 1960s and early 1970s, when a few US companies exploited the hudge Artemia resources of Great Salt Lake, Utah, and San Francisco Bay, California. They owned the world monopoly on cysts and eventually increased prices to levels almost unbearable for many developing countries.
Presently a large part of the cyst market is still supplied by harvests from location, the Great Salt Lake. This situation makes the market still extremely vulnerable to eventual climatological and/or ecological evolutions in this lake.
 |  |
| An Aremia couple in precopulation | An Artemia cysts hatching |
 |  |
| A seabass (Lates calcarifer) Larvae eating enriched Artemia nauplii | Havesting newly-hatched Artemia napulii in a shrimp hatchery in Ecuador |
Interest in the commercial harvesting of other Artemia biotopes is, however, steadily growing, brought about by local import restrictions and/or the inreasing demand for Artemia products as a valuable source of live feed in aquaculture. The quality of Artemia differs from strain to strain and from location as a result of genotypic variation (ie cyst size, cyst hatching characteristics, caloric content and fatty acid composition of the nauplii) determine if a particuler cyst product is suitable for use in commercial hatcheries of fish or shrimp as a larval food source. As a result, it is imperative to determine the nutritional quality of the adult artemia and/or its cysts for specific aquaculture purposes prior to considering commercial use of natural Artemia biotopes. Techniques for cyst and biomass harvesting, storing and processing, once a matter of trial and error, are now well established and guarantee, if properly applied, a high quality product.
Maximum sustainable yields of cysts and biomass are influenced by the population dynamics of the local Artemia resource the determination of maximal harvesting rates is, however, complicated by the heterogenous distribution of Artemia, which makes accurate sampling and consequently, precise population estimates very difficult. The recruitement rate of the population may be high in ponds where the dominant reproduction mode is ovoviviparity, and low in cyst-production ponds. Predation by waterbirds is another factor to be taken into consideration. Natural recruitement can eventually be increased by introduction of a more productive strain. In most Artemia habitats population densities are very low as a result of food limitation due to low nutrient contents of the intake waters. Consequently eutrophication of coastal waters by human activity, though reviled by many, is not unwelcome for those involved in the Artemia Business.
 |  |
| An aerial view of large scale cysts harvesting at the Great Solt Lake, utah, USA | Artemia cysts harvesting from an inoculated saltpond in Chachoengsao, Thailand |
Artemia cum grano salis
Over the centuries, hundreds and thousends of hectares of salt pans have been constructed all over the world in tropical and subtropical areas, for so-called solar salt making. Seawater contains salts of every chamical element, in at least trace amounts. Solar salt is normally produced by pumping seawater from one evaporation pond into another, allowing carbonates and gypsum to precipitate, and finally draining NaCl-saturated brine or pickle into crystalliser ponds where sodium chloride precipitates. Before all NaCl has crystalled, the mother liquor, now called bittern, has to be drained off to reduce contamination of the sodium chloride with bromides and other salts that begin to precipitate at these elevated salinities. The techniques of solar salt production thus involves fractional crystallisation of the salts in different ponds to obtain sodium chloride in the purest form possible, eg up to 99.7% on a dry weight basis.
The quantity and quality of salt produced is largely determined by the hydrological activity in a solar salt operation. Algal blooms are generally beneficial since they ensure increased solar heat absorption, resulting in faster evaporation and inceased yields of salt. But if they are not metabolised in time (ie eaten by Artemia), algal excretion and decomposition products, such as dissolved carbohydates, act as chemical trpas and consequently prevent early precipitation of gypsum which will contaminate the sodium chloride in the crystallisers and reduce salt quality. Furthermore, such organic impurities as algal agglomerates, wich turn black on oxydation, may contaminate the salt and reduce the size of the cristals and, hence, the salt quality. In the worst situation, high water viscosities may completely inhibit salt crystal formation and precipitaion.
Artemia gives a helping and by controlling algal blooms and by providing essential nutrients from Artemia metabolites and/or decaying animals as substrate for the proliferation of halobacterium in the crystallisation ponds. High concentrations of these red halophilic bacteria promote heat absorption, thereby accelerating evaporation, and reduce the concentration of dissolved organics. Lower viscosity levels promote the formation of larger salt crystals and, thereby, improve salt quality.
As said before, dispersal of Artemia cysts is a purely passive matter, in many situation the salt farmer cannot rely on this opportunistic dispersal method. In saltworks with short water retention times in the evaporation ponds, a rapid dillution may wash away the existing population; excessive rainfall may eliminate the population or reduce it to such a level that it cannot cope with the algal blooms. Moreover, some saltworks may be completely isolated from natural sources of Artemia dispersal. In other cases the autochthonous strain has poor productivity and the population remains too small to control the algae. in these cases the salt producer has to proceed with the controlled introduction (inoculation) of another strain, better production chatracteristics. However, only general guidelines can be formulated, since each situation has its own requirements: water retention time, food concentration, water temperature and salinity and the fluctuation of these parameters over the year are all factors to be considered when selecting a suitable Artemia strain.
Last but not least, proper Artemia management should lead not only to improved salt production but also provide opportunities for the harvesting of the valuable by-product Artemia, in the form of cysts and biomass. In the large solar saltworks (with individual evaporation ponds of tens to hundreds of hectares) Artemia production yields are limited and can only be influenced by inoculation strategies (eg strain, nauplius density, location and frequency of inoculation). however, harvests of up to 20 kg (dry weight) cysts per ha, or 2000 kg (wet weight) adult biomass per ha per month are achieved in tropical/subtropical regions in Southeast Asia and Central and South America in small artisanal saltworks (mostly operated on a seasonal basis) that are intensively managed for artemia production; ie pond depths are increased, and the low salinity ponds are fertilised twice or more a week with corganic (eg chiken manure, waste of monosodium glutamate processing) and/or inorganic nutrients (eg urea, triple superphosphate). As has been proven in Vietnam, for example this type of integrated, salt, Artemia and shrimp culture, eventually alternated with rice and shrimp in the rainy season, offers interesting socio-economic perspectives for those countries where solar salt production has or is becoming a marginal undertaking.
 |  |
| Drying Artemia cysts in Macau, Brazil | Artemai can be used as a carrier for nutrients or chemotherapeutics |
Artemia : polyvalent and versatile
Among the live diets used in larviculture of marine as well as freshwater fish and crustanceans, brine shrimp (Artemia) nauplii constitute the most widely used food item. Annually over 1000 mt of dry Artemia cysts are marketed worldwide for on-site hatching of the nauplii. Although the use of these cysts appears to be most simple, considerable progress has been made in the past decade in improving and increasing its value as larval diet, eg selection of the most appropriate strains and batches, naw techniques for cyst desinfection and decapsulation, nauplius hatching, enrichment and cold storage.
Using particulate or emulsified products, rich in highly unsaturated fatty acids, the nutritional quality of the Artemia can be further tailored to suit the predators' requirements by bioencapsulating specific amounts of these products in the Artemia metanauplii. Application of this method of bioencapsulation, also called Artemia enrichment or boosting, has had a major impact on improved larviculture outputs, not only in terms of survival, growth and success of metamorphosis of many species of fish and crustaceans, but also with regard to their quality eg reduced incidence of malformations, improved pigmentation and stress resistance. The same bioencapsulation method is now being developed for oral delivery of vitamins, chemotherapeuties and vaccines.
Harvested Artemia biomass may be fed live to the predators, or frozen, freeze-dried or ensiled for later use, or made into a flake diet, with relatively little loss of nutritional composition. Live Artemia biomass is apparently a good food both for the growht of penaeidsbrimp juveniles in nursery ponds and for the maturation of adult broodstock.
In the very extended saltworks locatedalong the Bohai Bay, PR China, in fact the largest solar salt producing area in the world, huge quantities (up to severalthounsand mt per year0 of adult Artemia biomass are harvested and used in local hatcheries, grow-out and maturation facilities of the chinese white shrimp, Penaeus chinesis. Throughout their grow-out cycle, shrimp and fed Artemia biomass at a rate of about 15000 kg/ha, supplemented with artificial feeds, chopped fish and molluse flesh; a quite unique situation -definitely the chinese way - made possible by the abundant availability of Artemia biomass at very low cost from the local salt ponds.
BY F. HONTORIA and F. AMAT
ABSTRACT.
Twelve morphological parameters have been measured in Artemia individuals belonging to 27 different populations located around the Western Mediterranean basin. An analysis, through multivariate discriminant procedures, allows us to establish relationships among different populations. The three different types of population studied (bisexual diploid, parthenogenetic diploid and parthenogenetic tetraploid) are thoroughly characterized by their morphological characteristics. This simple method is shown to be useful in grouping different populations and to have predictive value in assigning new populations to the groups previously analyzed.
INTRODUCTION
The use of Artemia as food for marine fish and crustacean larvae in aquaculture has caused a considerable interest in this organism in the last decades. The variation shown by different populations in many of their biological processes had introduced the need for reliable methods for characterization, in order to understand better the relationships between populations of this complex of species, and to some extent to be able to predict some of their features.
It has only recently been demonstrated that more than one species exist in the genus Artemia (Barigozzi, 1972; 1974; Clark and Bowen, 1976; Bowen et al., 1978). It has been proposed that the specific name A. salina Leach, 1819, formerly used be abandoned, until systematics of this group can be completely understood (Persoone et al., 1980). The present investigation was in mind.
Previous work on the morphology of Artemia (Gilchrist, 1960) has shown that Artemia individuals undergo morphological changes according to the environmental conditions. Furthermore, this study states that wide differences among populations and between males and females can be found, even when the animals were cultured in the same medium, thus demonstrating intrinsic influences as well as environmental ones. Similar results were reported by Amat (1979, 1980) after a complete morphological study on 22 different Mediterranean populations, and comparing these results to those from a population coming from San Francisco (Califronia). He concludes that the variation in these characters observed among Artemia males and females from different populations allows one to classify the different main types of Artemia, but it is difficult to distinguish among populations of the same type. in a recent work Varo (1988) has studied three parthenogenetic diploid populations (two from the Canary Islands and one from the Eastern Spanish coast). Although, she could not clearly distinguish these populations (pertaining to the some type), as Amat (1979, 1980) predicts, she found that the most discriminant variables studied are : length of the furca; diameter of eyes; width of the head; length of the first antenna; and the distance between the eyes.
In the present paper variation of different morphological parameters measured on Artemia females from different populations coming from the Iberian peninsula and adjacent area is studied. Multivariate discriminant analysis has been used in order to maximize the differences among groups of observations and thus to obtain an increase in the sensitivity of the morphological studies, which use only direct observation of the data. Thus, allowing some discrimination among the populations of the same type.
METHOD
Studied populations
We have considered 27 populations, most of them (20) coming from the Iberian Peninsula. The remainder were obtained from cysts collected in the Balearic and Canary Islands, Morocco, France and Italy, and, therefore, very closely related to the former. All of them can be clustered in three different types of populations, which are the most frequently found around the Mediterranean basin : zygogenetic diploid (bisexual); parthenogenetic diploid; and parthenogenetic tetraploid. The different population origins, the abbreviations utilized from now on, the population type to which they belong, and the number of individuals studied in each case are shown in Table I. Figure 1 shows the geographical location of each population.
Culture conditions
All populations studied were raised under standardized conditions in order to minimize the strong
envionmental influences displayed by the body from in Artemia.
Cysts belonging to each population were allowed to hatch in sea water (38‰ salinity) at 28°C under
constant aeration and lighting. The nauplii were transferred to 11 containers with the bottom closed
by a piece of 60 μm plankton net. These containers were then suspended in groups of 10 m bigger
reservoirs (1501) filled with seawater (salinity 30–32‰), thus all sharing the same culture medium.
The water was maintained at 25°C, under summer natural photoperiod (16 h light : 8 h dark), and
supplied with moderate aeration from the bottom, this sets the oxygen concentration to saturation.
The culture densities were never allowed above 50 animals 1–1. Cultures were fed live unicellular
algae (Tetraselmis succia), at an approximate density of 100 000 cells ml-1 (ad libitum). The medium
was completely renewed twice per week with fresh seawater and microalgae cultures. It did not seem
necessary to control the ammonia level because the volume, density and renewal conditions described
the adult state (this was conspicuous when females had their ovisac developed), after 15 – 30
days in tetraploid, different samplings were performed in order to measure them.
Table I. Studied populations, abreviation utilized (numbers in parentheses identify populations in Figure I), number of observations (n), and type of population to which they belong, T, parthenogenetic tetraploid; d; B; bisexual diploid
| Type | Population | Abbreviation | n | |
| T | Alcochete (Portugal) | ALT | (2) | 30 |
| T | Bujaraloz (Spain) | BUJ | (19) | 27 |
| T | Comacchio (Italy) | COM | (22) | 40 |
| T | Delta of Ebro River (Spain) | DTE | (15) | 20 |
| T | Guerande (France) | GND | (21) | 33 |
| T | Larache (Morocco) | LAT | (6) | 33 |
| T | Margherita di Savoia (Italy) | MSV | (23) | 33 |
| T | Medinaceli (Spain) | MED | (18) | 24 |
| T | Saelices (Spain) | SAE | (17) | 27 |
| T | Sanlucar (Spain) | SLT | (3) | 29 |
| T | Tierzo (Spain) | TZO | (16) | 24 |
| D | Bonmati(Spain) | BNM | (12) | 27 |
| D | Bras de Port (Spain) | BPP | (11) | 24 |
| D | Calpe(Spain) | CAL | (13) | 27 |
| D | Cabo de Gata (Spain) | CGT | (7) | 31 |
| D | Gerri de la sal (Spain) | GER | (20) | 21 |
| D | Janubio (Spain) | JAN | (1) | 24 |
| D | Larache (Morocco) | LAR | (6) | 27 |
| D | La Mata (Spain) | LMT | (9) | 17 |
| D | San Fernando (Spain) | SFP | (5) | 25 |
| D | San lùcar (Spain) | SLC | (3) | 27 |
| B | Bras de Port (Spain) | BPB | (11) | 27 |
| B | Ibiza (Spain) | IBZ | (14) | 21 |
| B | Salinera Española (Spain) | SEB | (10) | 24 |
| B | San fernando (Spain) | SFR | (5) | 27 |
| B | San Felix (Spain) | SFX | (4) | 24 |
| B | San Pedro del Pinatar (Spain) | SPP | (8) | 30 |
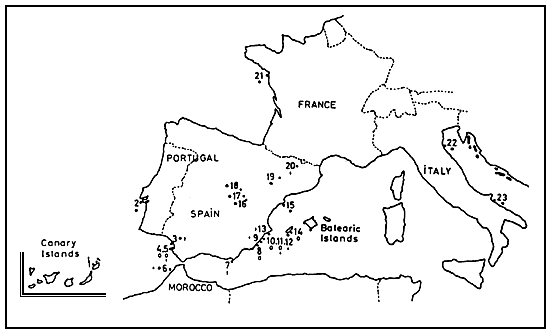
Fig. 1. Geographical location of the studied populations that numbers identify are given in table 1. Parthenogenetic tetraploid (*); Parthenogenetic diploid (+); bisexual (o).
Measuring individuals
From each population a random sample was taken. Animals were anesthetized with some droplets of water saturated with chloroform and females, always more than 20 (except LMT, which underwent a high mortality in culture) were separated from the rest. The following morphological parameters were quantified in each female: total length; abdominal length; width of third abdominal segment; width of the ovisac; length of furca; number of setae inserted on each branch of the furca; width of head; maximal diameter and distance between compound eyes; length of first antenna; and the ratio abdominal length × 100/total length. Figure 2 illustrates these above mentioned body measures. In all cases, a similar number of individuals for each length interval was included in order not to bias results through the sampling. Preadult individuals were considered as well.
Statistical analysis
The 12 morphological variables, measured in all individuals (Table 1), were used to establish relationships among these populations through discriminant analysis. This multivariate procedure provides a series of variables (Z1, Z2,…), Which are linear functions of the morphological variables studied, with the form Zn = λ1X2+λ2X2+… (Where λs are the calculated discriminant coefficients and Xs the variables being considered). They maximize the separation among different groups of observations defined a priori (Anderson, 1984). Thus, the first discriminant function is the equation of a line cutting across the intermixed cluster of points representing the different observations. This function is constructed in such a way that the different predefined groups will evaluate it as differently as possible. Obviously, this will not be accomplished if the number of groups is high, and subsequent discriminant functions will be needed. Two analyses were carried out: first, all observations were grouped by the type of population (bisexual diploid, parthenogenetic diploid and parthenogenetic tetraploid); in the second analysis, the separation criterion was the origin of the population. These analyses have been performed using a backward stepwise procedure that allows removing the different variables out of the model separately and ranking them for their relative importance in discriminating Artemia populations. Nevertheless, all described variables were kept in the model. These calculations have been performed with the help of the statistical package Statgraphics v. 3.0 (Statistical Graphics Corp., Rockville, MD) run on an IBM AT personal computer.
RESULTS
In Table II, the results obtained when the type of population was used as a separation factor are displayed. The two functions found give 100% separation, and both are statistically highly significant (P<0.001). Morphological characteristics allow a clear differentiation among the three groups considered (Table II, groups centroids). The morphological characteristics that most significantly contribute to the discrimination among the three groups are : lengh of first antenna, width of head and those related to the form and size of the head, the ratio abdominal length/total length in form of percentage and the width of ovisac and abdomen (Table II).
Results of the second analysis (factor of separation is population of origin) are shown in Table III and Figure 3. In this case, 12 discriminant functions are needed in order to separate thoroughly the 27 populations, but the first five of them give a cummulative separation percentage of 90.66 (the four discriminant functions shown in Table III give a 85.87% cummulative separation). The first eight functions calculated are highly statistically significant (P,0.001), the ninth is also significant (P<0.05) and the last three are not significant. The morphological characteristics that most signifiantly contribute to separate the groups in this case are : distance between eyes, eye diameter, length of the first antenna and all variable related to the shape and size of the head and the length of the furca (Table III).
For clarity, the first two or three discriminant functions solved in the mean points of each group (centroids)
instead of individualized observations are shown in Figures 3 and 4. Centroids can be considered
representative of each group because of the significance levels. Figure 3 shown that the centroids
of the populations belonging to the same type tend to be grouped. Although the goal of the
discriminant analysis is to separate thoroughly the different groups of observations considered, the
relative distance between centroids in a multidimensional space (12 dimensions in this case) allows
us to draw a very precise idea of the mophological relationships among different populations.
Figure 4 shows the same data as Figure 3, with the addition of the third discriminant function. The
increase in amount of information allows us to be more precise describing the different morphological
relationships. This representation reveals some hidden separations in Figure 3. For example,
CAL (13) appears in Figure 4 as markedly different from other parthenogenetic diploid populations,
while in Figure 3 it seems more alike to them.
Table II. Results of the discriminant analysis on the morphometric variables measured in Artemia females from 27 different locations, and grouped by the type of population to which they belong. Non-standardized coefficients of the resulting discriminant functions. F-value contrasting the decrease of separation among groups when each variabel is removed from the model. Group centroids were solved for the discriminant functions obtained.
| Discriminant function | 1 | 2 | F-value |
| Total length | -0.51 | -0.35 | 1.2 |
| Abdominal length | 1.37 | -0.08 | 4.2 |
| Width of ovisac | -0.67 | 1.89 | 44.6 |
| Abdiminal width | 8.92 | 1.56 | 37.5 |
| Length of furca | 0.92 | 7.16 | 21.8 |
| No. setea left branch | 0.01 | 0.13 | 7.9 |
| No. setea right branch | 0.03 | 0.01 | 0.7 |
| Width of head | -5.15 | 12.33 | 47.1 |
| Distance between eyes | -1.59 | -7.18 | 33.2 |
| Diameter of eyes | -23.13 | -0.46 | 23.8 |
| Length of 1st antenna | 7.05 | -3.51 | 107.8 |
| % length abdomen/total length | -0.38 | 0.04 | 44.9 |
| Constant | 18.99 | -1.92 | |
| Group centroids | |||
| Pathenogenetic diploid | 1.67 | 0.68 | |
| pathenogenetic tetraploid | -0.42 | -1.31 | |
| Bisexual | -1.87 | 1.60 |
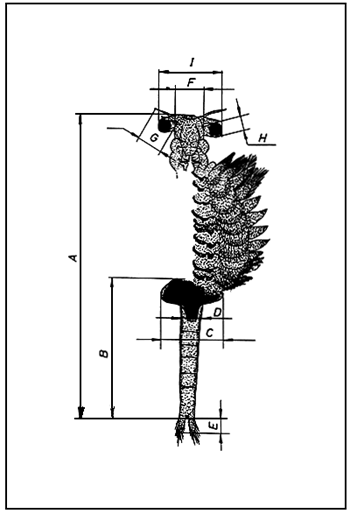
Fig. 2 Shematic illustration of adult female Artemia showing the different body measurements utilised. (A) Total length; (B) abdominal length; (C) width of ovisac; (D) width of third abdominal segment; (E) length of furca; (F) width of head; (G) length of first antenna; (H) maximal diameter complex eye; (I) distance between complex eyes.
Table III. Results of the discriminant analysis on the morphometric variables measured in Artemia females from 27 different locations, and grouped by its origin. Non-standardized coefficients of the first four resulting discriminant functions. F-value contrasting of separation among groups when each variable is removed from the model.
| Discriminant functions | 1 | 2 | 3 | 4 | F-value |
| Total length | -0.40 | 0.02 | -0.59 | -1.73 | 11.6 |
| Abdominal length | 0.68 | 0.06 | -1.94 | 2.98 | 9.1 |
| Width of ovisac | -0.29 | 1.60 | 0.69 | -1.38 | 8.4 |
| Abdominal of furca | 6.11 | 1.53 | 2.57 | 17.69 | 11.8 |
| Length of furca | 6.22 | 8.11 | -6.44 | -14.95 | 18.1 |
| No. setae left branch | 0.07 | 0.18 | 0.07 | 0.16 | 3.7 |
| No. setae right branch | 0.05 | 0.04 | 0.01 | 0.13 | 1.6 |
| Width of head | -8.28 | 6.77 | 5.99 | 0.46 | 12.6 |
| Distance between eyes | 2.47 | -8.13 | 8.79 | -0.66 | 19.2 |
| Diameter of eyes | -26.49 | 1.66 | 22.32 | -8.43 | 35.8 |
| Length of first antenna | 5.93 | -5.07 | -6.10 | -1.54 | 27.3 |
| % length abdomen/total length | -0.41 | 0.20 | 0.11 | -0.03 | 12.0 |
| Constant | 20.16 | -8.15 | -9.82 | 0.93 | - |
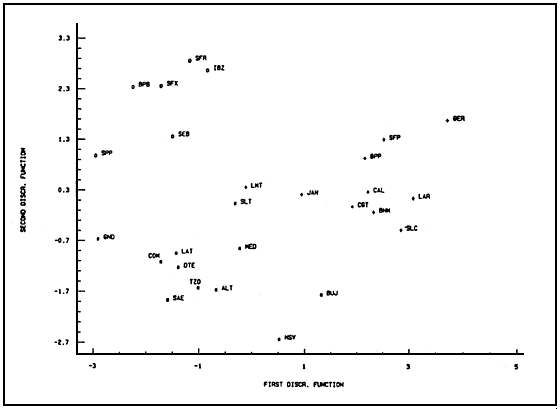
Fig.3. Group centroids solved for the first two functions result ing from the disriminent analysis when using the origin of each individual as separation factor. Bisexual (O); parthenogenetic diploid (+), parthenogenetic tetraploid (*).
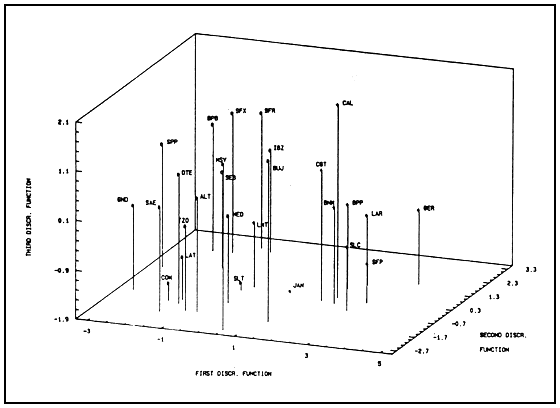
Fig. 4. Group centroids solved for the first three functions result ing from the disriminant analysis when using the origin of each individual as separation factor. Bisexual (O); parthenogenetic diploid (+), parthenogenetic tetraploid (*).
DISCUSSION
This method clearly allows us to differentiate the morphological patterns that characterize at least some different Artemia species. In some cases, it is possible to discern between varieties having a sufficiently. Important characteristic, such as the different ploidies in parthenogenetic populations (Table II). It is not possible to assign a morphological pattern to every population studied with the parameters utilized here, in disagreement with previous work described in the introduction (Gilchrist, 1960). However, this fact should be considered as a validation of the methodology more than a defect, since some of these populations probably have a high genetic closeness. For example, the third discriminant function from the second analysis, makes it possible to discern between some populations that previously seemed closely related (Figure 4). This is the case of LMT (9) and SLT (3). In addition, bigger differences in each of the three main groups can be seen with this procedure.
Among diploid parthenogenetic populations, CAL (13), GER (20), JAN(1) and LMT (9) seem clearly different from the remaining populations of this type (Figure 4). For CAL (13), the observations of Barigozzi and Baratelli (1982) about the presence of triploid nauplii among the more frequent diploid could account for some morophological differences. However, these fingings do not agree with Amat (1979, 1980), who could not differentiate both forms through direct observation of individuals; with Abreu-Grobois and Beardmore (1982), who do not find triploid mitosis in the preparations of nauplii from this population; and with Varo (1988), who did not get triploid mitosis in her preparations, nor can separate CAL (13) from JAN (1) through a discriminant analysis similar variables to ours.
The populations of GER (20) has not been studied very extensively, but it holds two characteristics which can be of interest in this context. It is located at a higher latitude, which has forced this population to develop in different climate conditions and to adapt to colder temperatures. This, as stated above, has a very strong influence on the morphology of Artemia. The other characteristic is that the salterns that this populations inhabits are considerably far from the sea. This possibly means a different ionic composition of the brine. Such an environmental characteristic is known to cause Artemia populations living under it to have remarkable differences in their biology. This is the case of some inland athalassic lakes in North America that hold some interesting Artemia populations: Mono Lake, California; Great Salt Lake, Utah; Chaplin Lake, Canada; etc. (Lenz, 1980; 1984).
In the case of JAN (1) and LMT (9), an explanation is more difficult, since the characteristics of the ecosystems where they live are very similar to those of the other populations studied which display a vary close morphological pattern among them.
Bisexual populations show a more homogenous distribution, without remarkable differences among them, this is probably due to the closeness of some of them, making easier an exchange of genetic information through fertile interbreeding. This situation is not possible among parthenogens even in closely located populations.
Tetraploid parthenogenetic populations display greater variability than populations in the two former groups. Nevertheless, there is a group of four populations (DTE -15), ALT -2/, SAE - 17 - and TZO -16-) that are closely related, and MED (18) and GND (21) farther from this nucleus, but still rather morphologically similar to them. Thee are two more different groups: BUJ (19) and MSV (23); and COM (22), LAT (6) and SLT (3). In this case, the possible explanations are not easy to draw, since they share none of the above mentioned characteristics (geographical closeness, environmental or physical traits of their ecosystem, etc.). The only reasonable facts are the similarity between SLT (3) and LAT (6), which are populations located in very close places, on both sides of the Gibraltar Straits; and its separation from more northern populations such as BUJ (19) and MSV (23).
Studying the standardized coefficients of all discriminant functions from these two analyses (data not presented here) and from others performed on different populations as well, and from the results of the stepwise analyses, it is possible to determine which variable have more influence on discriminating different populations. Generally, these variable are the ratio abdominal length/total lengh, which agrees with Gilchrist (1960) and Amat (1979, 1980), but not with Varo (1988); the form of the head (distance between compound eyes, eye diameter and head width), and the length of the first antenna, which are very variable among populations as stated by the above mentioned authors.
The main difficulty in using this technique of characterization is the need for raising an adult population under standardized conditions. It is, thus, not a rapid methodology. In spite of this, it is very simple, since only a few measurements are required instead of more sophisticated, complex or expensive methods.
The discriminant analysis carried out in this study has an important predictive value, in addition to the clustering purposes used here. Thus, the discriminant functions calculated allow one to classify new data sets into the different groups utilized in this study. In addition to this, since standardized culture techniques have to be used, the phenotypic variation is likely to reflect genetic variability; consequently, morphological data could be used through discriminant analysis to study relatedness among Artemia populations. This extent is currently being developed in our laboratory through the morphological study of 37 Spanish populations. These morphological databases as well as other data concerning American populations, by means of the discriminant analysis, offer a powerful tool in order to accomplish a better understanding of the taxonomy of the genus Artemia; particularly, when these results and genetic studies can be contrasted together.
ACKNOWLEDGEMENTS
The authors would like to acknowlege Dr John H. Crowe and Dr Petra H.Lenz for their helpful reviewing of the manuscript and M.Nieves Sanz for her assistance in the measurements. This work has been supported by the grants AC83/0002 funded by the CAICYT and MAR88/0224 by the CICYT. F.Hontoria has been granted by the Exma. Diputacion Provincial de Castellon.
REFERENCES
ABREU-GROBOIS, F.A. and BEARDMORE, J.A. (1982) Genetic differentiation and speciation of the brine shrimp Artemia. In Barigozzi, C. (ed.). Mechanisms of Speciation. Alan Liss Inc., New York, pp. 345–376.
AMAT, F. (1979) Diferencicacion y distribucion de las poblaciones de Artemia (Crustaceo Branquiopodo) de Espana, Ph.D. thesis. Universidad de Barcelona, Spain.
AMAT, F. (1980) Diferenciacion y distribucion de las poblaciones de Artemia (Crustaceo, Branquiopodo) de Espana. I. Analisis morfologico; Estudio alométricos referidos al crecimiento y a la forma. Inv. Pesq., 44,217–240.
ANDERSON, T.W. (1984) An Introduction to Multivariate Statistical Analysis, 2nd edn.J.Wiley & Sons, New York.
BARIGOZZI, C. (1972) Problems of speciation in the genus Artemia, In Battaglia, B. (ed.). Proc. Fifth European Marine Biology Symposium. Piccini Editore, Padua, Italy, pp.61–66.
Barigozzi, C. (1974) Artemia: a survey of its significance in genetic problems. Evol. Biol., 7, 221–252.
BARIGOZZI, C. and BARATELLI, L. (1982) New data on chromosome number of the genus Artemia.
ACADEMIA NAZIONALE DEI LINCEI. Rendiconti della classe di Scienze fisich, matematiche e naturali, 73, 139–143.
BOWEN, S.T., DURKIN, J.P., STERLING, G. and CLARK, L.S. (1978) Artemia hemoglobins: genetic variation in parthenogenetic and zygogenetic populations. Biol. Bull., 155, 273–287.
Theodore ABATZOPOULOS,
GherdA KARAMANLIDIS,
Philippe LEGER & Patrick SORGELOOS
ABSTRACT
Cysts of parthenogenetic Artemia strains collected from the Citros and M. Embolon saltworks in Northern Greece are evaluated for their potential use in aquaculture. The following characterizations were performed: cyst and naupliar biometrics, cyst hatching characteristics, fatty acid profile of the nauplii, caloric content of nauplii stored at 25° C and in a refrigerator (4 – 8° C). The above evaluation reveals that the two Artemia strains studied exhibit good qualities for use in aquaculture, especially in culturing fresh-water species. The biometrical analysis of cysts, nauplii and adults shows a high degree of similarity with other parthenogenetic strains from various geographical sources, but especially with tetraploid Artemia from Spain. The Greek Artemia strains cannot be considered as ‘sources’ for aquacultural uses unless proper management of the saltworks is assured.
INTRODUCTION
Since the brine shrimp Artemia is of high economic importance, it is essential to analyse the ‘commercial’ characteristics of various populations with regard to their potential for application in aquaculture. In previous studies, two Northern Greek Artemia populations found in Citros, Piera and M. Embolon, Thessaloniki, have been characterized as to their mode of reproduction and ploidy. They were found to be parthenogenetic and tetraploid (Abatzopoulos et al., 1986). Data from studies on DNA reassociation Kinetics and on thirteen enzymatic systems of these two populations indicate that they belong to one species (Abatzopoulos et al., 1987).
In this paper we report on the biometrical characteristics of cysts, nauplii and adults, on the hatching characteristics of cysts, i.e. hatching rate, hatching percentage, hatching efficiency and hatching output, on the changes in dry weight and caloric content of instar-I nauplii maintained at room temperature and cold storage conditions, and on the fatty acid profiles of instar-I nauplii.
MATERIALS AND METHODS
The two populations of Northern Greek Artemia evaluated are from the saltworks of Citros, Pieria (C strain), and from the saltworks of M. Embolon, Thessaloniki (ME strain). Cysts were collected in 1983, processed according to Sorgeloos et al. (1978), and stored in sealed plastic bags at -25° C, for future use.
Biometry
Hydrated cysts, and instar-I nauplii were measured according to the technique of Vanhaecke & Sorgeloos (1980)
Adult brine shrimps were anesthezied in chlorofrom-saturated and measured under a dissection microscope using a Leitz micrometer, The following measurements were performed : total length, abdominal length, maximal width of brood pouch, width of third abdomianal segment, width of head, length of first antenna, maximal diameter of complex eye, and distance between complex eyes (Amat, 1980). Measurements were performed on animals - sample size = 50 animals-originating from wild cysts and cultured under standard labouratory conditions (yeast as food, temperature of 25 + 1° C, and seawater of 35% salinity). The data were statistically treated by analysis of covariance (Sokal & Rohlf, 1981).
Hatching characteristics
Hatching analyses were carried out according to Vanhaecke & Sorgeloos (1983). Hatching efficiency, hatching percentage, hatching rate and hatching output were analysed following rate and hatching output were analysed following Sorgeloos et al. (1978), Bruggeman et al. (1980), Vanhaecke & Sorgeloos (1982), and Vanhaecke & Sorgeloos (1983), respectively.
Caloric content
The hatching of the cysts and the separation and collection of the nauplii (instar-l to instar-III) were carried out according to the procedure described in Vanhaecke e al. (1983).
The samples of nauplii were dried in an incubator at 55°C for 48 hrs. For each naupliar stage studied, four to six replicated samples, of approximately 10 mg dry material were burned totally in pure oxygen and their caloric content was measured in a Phillipson microbomb calorimeter.
Fatty acid profiles
Fatty acids were determined on freshly hatched nauplii of both strains by capillary gas chromatography.
Prior to analysis, samples were homogenized in an ultrasonic homogenizer (Sonifier B12) and total lipids were extracted according to the method of Bilgh & Dyer (1959). Saponification and methylation were performed as described by Shauer & Simpson (1978). Fatty acid methylesters were injected in a capillary column (25 m fused silica; inner diameter; 0.32 mm; liquide phase : SILAR 10C, film thickness: 0.3um) installed in a Carlo Erba Mega 2350 gas chromatograph.
Operating conditions were as follows: oncolumn injector, carrier gas : hydrogen, flow rate ,2ml/min, F.I.D. detection, oven temperature programme : 105°C to 150°C at 10°C/min and 150°C to 200°C at 5°C/min. Peak identification and quantification was done with a caliberated plotter-integrator (IIewlett-Packard 3390 A) and reference standards.
Results are expressed in area-percent composition of total fatty acids and mg fatty acid methylesters per gram dry weight.
RESULTS
Biometry
We counted 223 000 + 1563 cysts g-1 and 900 + 2322 cysts g -1 in the C and ME strains, respectively.
The results of the measurements for the calculation of the mean diameter and the mean volume of the cysts are presented in Table 1.
Table 1. Mean diameter and cyst volume of greek strains (harvested from the wild of from standard cultures at 35% salinity).
| Strains | Mean diameter (pm) (sd) | Mean volume (106 pm3) | Number of cysts measured |
| Cytros (wild): | 260.19 ± 0.031 | 9.20 | 500 |
| (from culture): | 270.13 ± 0.048 | 10.30 | 500 |
| M.Fmblon (wild): | 264.7 ± 0.038 | 9.62 | 500 |
| (from culture): | 279.36 ± 0.077 | 11.48 | 500 |
The size frequency distribution of cysts (Fig.1) appears to be similar between the two strains, although a slight increase in cyst diameter is observed in cysts produced in the laboratory. Figure 2 shows the size distribution of cysts from teh C strain in two different havests of the same year, which seems remarkably stable.
There are no significant differences between the nauplier length and the size frequency distribution of the nauplii from the C and ME strains (Table 2, Fig 3). As shown in Fig. 4, ovoviviparous nauplii are significantly larger than those hatched from cysts and show a different size distribution.
Table 3 summarizes the mean values for the various variables measured on adult brine shrimps, while Fig. 5 (a–g) prsents the correlation between these variables and total individual length within the same population as well as between population. Analysis of co-variance showed that the regression lines in each case can be replaced by a single regression line and therefore, the values from the two populations for any variable can be considered as values derived from one strain only.
Hatching quality
Artemia cysts collected in the C and the ME saltworks and analysed following standard pocedures appear to have a good hatching quality. The C strain presents slightly better hatching characteristics than the ME (Table 4). Hatching for ME and C cysts ranges between 84–88% (narrow range) and most nauplii occur within 25 hrs of incubation. Figure 6 presents the hatching curves of C and ME cysts.
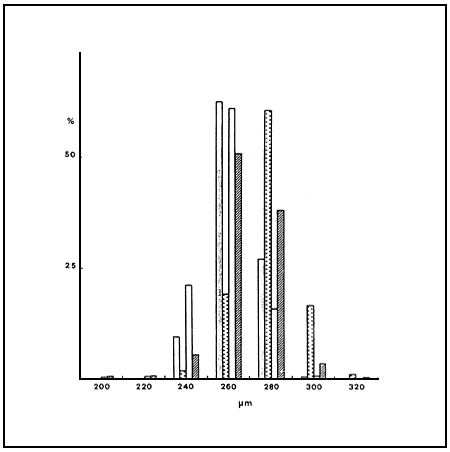
Fig. 1 Size frequency distribution of cysts from the tow Northern Greek strains: : Me (wild population), :ME (from culture), : C (Wild population), : C from culture
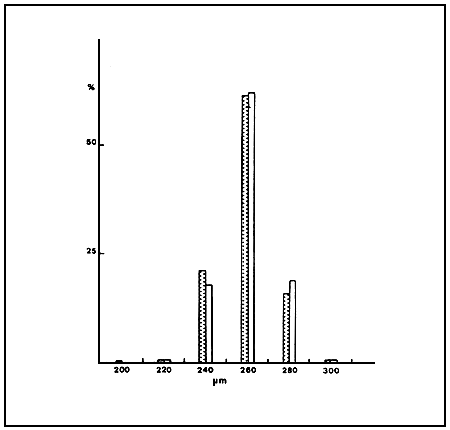
Fig. 2 Size frequency ditribution of cysts from Citros saltworks during June ( ) and September ( )
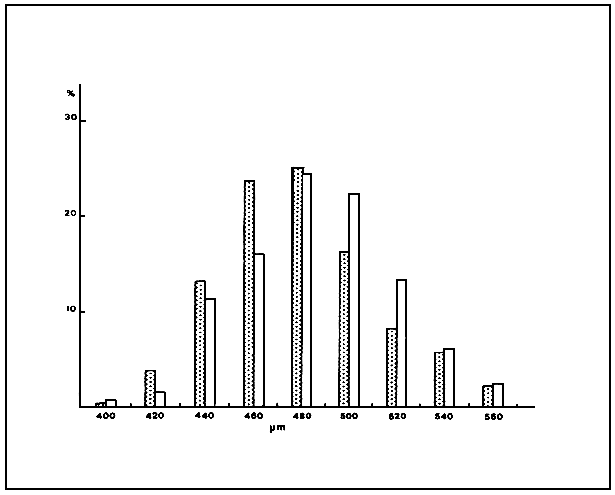
Fig. 3 Size frequency ditribution of nauplii (instar-I) hatched from cysts: : ME strain, : C strain
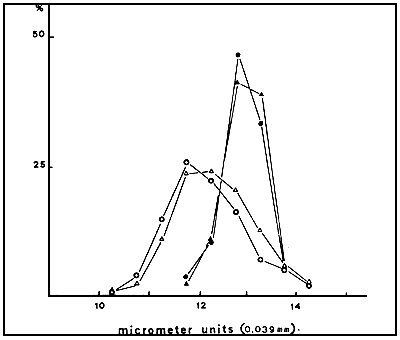
Fig. 4. Size distribution of oviparous and ovoviviparous nauplii from the Greek strains. Oviparous: ME: O,C: ; Ovoviviparous: ME:, C:
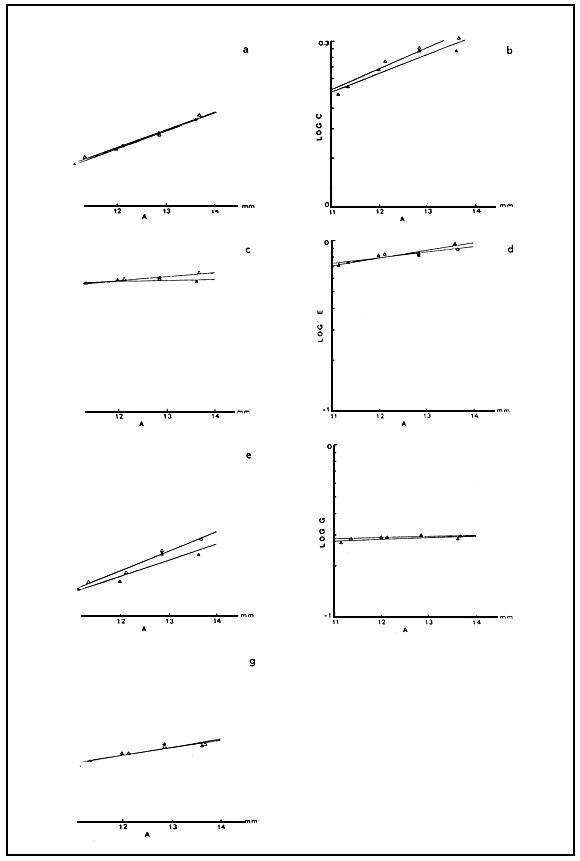
Fig. 5. parameters (B-H) versus total length (A) in the C strain (▲) and the ME (Δ) Artemia cultured under standard conditions (see also Table 3).
Table 3. Mean values for various parameters of adult Artemia shrimps cultured under standard laboratory conditions.
| Citros strain (mm) | M. Embolon strain (mm) | |
| a | 12.428 (+0.0829) | 12.192 (+0.09) |
| b | 6.550 (+0.0468) | 6.435 (+0.049) |
| c | 1.862 (+0.0218) | 1.803 (+0.023) |
| d | 0.573 (+0.0056) | 0.563 (+0.056) |
| e | 0.828 (+0.0102) | 0.809 (+0.011) |
| f | 1.147 (0.015) | 1.121 (+0.011) |
| g | 0.304 (+0.0045) | 0.293 (+0.002) |
| h | 0.628 (+0.0164) | 1.626 (+0.019) |
a : Total lenght,
b : abdominal lenght,
c: maximal width of brood pouch,
d: width of 3rd abdominal segment,
e: width of head,
f: length of 1st antenna,
g: maximal diameter of complex eye, and,
h: distance between complex eyes.
Table 4. Hatching characteristics of cysts from Citros and M. Embolon strains (Greece).
| Citros | M. Emblon | |
| Hatching Percentage hatching medium 35 ppt | 90.5 | 86 |
| Hatching efficiency (nauplii/g cysts) | 198000 | 188000 |
| Hatching rate characteristicsa | ||
| T0 | 16 | 17 |
| T10 | 17.5 | 19 |
| T90 | 25 | 28 |
| Ts | 7.5 | 9 |
| Hatching output | 601 | 590 |
| (mg/dry biomass/g cysts) |
Individual dry weight and caloric content
The naupliar (instar-I) dry weight, the ash content and the individual energy content are remarkably similr in the two strains as can be seen from the data which are summarized in Table 5. Figure 7 shows the energy consumption en early larval stages in correlation with time under normal culture temperatue (25° C+1° C), and under cold storage of live nauplii in a refrigerator at 6° + 2° C.
Fatty acid profiles
The analyses of lipids extracted from freshly hatched nauplii (Table 6) - following the procedure of gas chromatography - show that the fatty acid profiles of C and ME material are very similar to each other. Eicosapentaenoic acid (20 : 5ω3) is present and can be considered as barely sufficient for culturing marine species (Léger et al., 1986) while the other essential fatty acid 22: 6ω3 is found in detectable amounts only in the C strain. Also, the C strain contains high levels of linoleic acid 18: 2ω6.
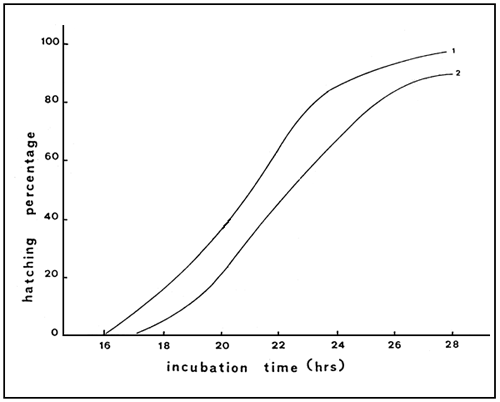
Fig. 6 Hatching curves for the cysts from Citros (1) and M. Embolon (2)
Table 5. Individual dry weight, ash content and energy content of instar-I nauplii from two N. Greek population.
| Strain | Individua dry weight) (in mg) | Ash content (in % of dry weight) | Energy content | Individual energy content (in joules) | |
| Kcal/g | joules/g | ||||
| Citros | |||||
| Hatching medium 35 ppt | 3.04 (± 0.29) | 4.44 | 5.5299 (± 0.05) | 23050 (± 200) | 0.070072 |
| Hatching medium 5 ppt | - | - | 5.9517 (± 0.12) | 24810 (± 500) | - |
| M. Embolon | |||||
| Hatching medium 35 ppt | 3.14 (± 0.32) | 4.56 | 5.3447 (± 0.11) | 22320 (± 450) | 0.070084 |
| Hatching medium 5 ppt | - | - | 5.5226 (± 0.06) | 23040 (± 250) | - |
Table 6. Composition of fatty acid methyl esters (F.A.M.E.) of artemia nauplii hatched from cysts collected in the slatworks of Citros (C) and M. Embolon (ME) Greek, 1983).
| F.A.M.E. | Area % | mg/g DW | ||
| C | ME | C | ME | |
| 14:0 | 2.0 | 1.3 | 3.1 | 1.5 |
| 14:1 | 2.6 | 1.6 | 4.1 | 1.8 |
| ?* | 0.9 | - | 1.4 | - |
| 15:0 | 0.5 | 0.7 | 0.8 | 0.8 |
| 15:1 | 0.7 | 1.1 | 1.1 | 1.2 |
| 16:0 | 11.7 | 12.6 | 18.4 | 13.8 |
| 16:1ω7 | 11.9 | 17.0 | 18.7 | 18.8 |
| 17:0 | 1.0 | 1.3 | 1.6 | 1.5 |
| 16:3ω4 | 3.2 | 3.3 | 5.0 | 3.6 |
| 18:0 | 4.4 | 4.4 | 6.9 | 4.8 |
| 18:1ω9 | 25.4 | 14.9 | 40.0 | 16.4 |
| 18:1ω7 | - | 20.5 | - | 22.6 |
| 18:2ω6 | 11.1 | 6.6 | 17.4 | 7.2 |
| 20:1 | 0.8 | 1.0 | 1.3 | 1.1 |
| 18:3ω3 | 13.2 | 4.8 | 20.7 | 5.2 |
| 18:3ω6 | 0.2 | - | 0.3 | - |
| 18:4ω3 | 1.2 | 0.8 | 1.9 | 0.8 |
| 20:3ω3 | 0.2 | - | 0.3 | - |
| 20:4ω3 | 1.0 | 2.3 | 1.6 | 2.6 |
| 22:1 | 0.4 | 1.0 | 0.6 | 1.1 |
| 20.5ω3 | 3.4 | 2.7 | 5.3 | 3.0 |
| 20:3ω3 | 0.9 | 2.1 | 1.4 | 2.3 |
| 20:5ω3 | 0.2 | - | 0.3 | - |
| 20:6ω3 | 0.5 | - | 0.9 | - |
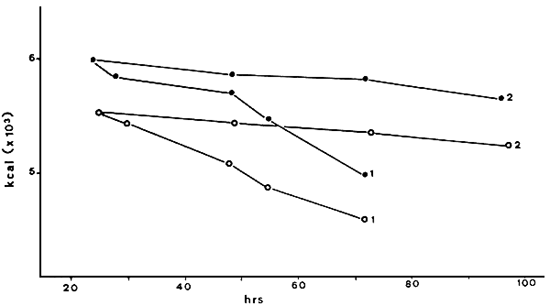
Fig. 7. Energy content of cC (•) and ME (O) nauplii; stored at two temperatures (1) 25°C (+-1°C) (2) 6°C (+- 2°C)
DISCUSSION
The two Greek Artemia strains examined, exhibit no differences in cyst diameters and resemble those of LSpanish parthenogenetic populations (Amat, 1980; Vanhaecke & Sorgeloos, 1980: léger et al, 1986); they are significantly larger than bisexual Artemia-a typical phenomenon for parthenogenetic tetraploid strains but they must be considered quite small among the parthenogenetic and tetraploid strains (Vanhaecke & Sorgeloos, 1980). The inerease in cyst diameter which was observed in cysts produced in standard laboratory cultures (Table 1) might be due to optimal culture conditions-such as food abundance - and lower metabolic costs.
The size frequency distributions of cysts from the Greek strains are very similar for the different batches (Figs 1 and 2) and again they resemble strains from Spain (Amat, 1980).
The results of the hatching characteristics from Greek Artemiacysts and their comparison to other strains reveal that these Greek Artemia are of high quality (Sorgeloos et al. 1986). The small differences in hatching rate and hatching efficiency (Table 4) between the ME and C strain may be due to the exposure of the cysts to suboptimal conditions - before harvesting - which can result in mortality of some embryos (Sorgeloos et al., 1976, Vanhaecke & Sorgeloos, 1983l; Van Ballaer et al., 1987). The hatching percentage can be slightly improved by incubation of cysts in a 5 ppt hatching medium, where the energy consumption during hatching data the Greek Artemia strains are close to those obtained from French Artemia (Lavalduc) (Vanhaecke & Sorgeloos, 1983). It is interesting to note that the hatching synchrony-expressed by Ts (Table 4) - for the ME and C cysts ranges between 7–9 hrs and therefore it is easy to harvest only instar-I nauplii which bear the highest energy content (Table 5) (Benijts et al., 1976).
The hatching outputs for our strains are among the highest mentioned (Vanbaecke & Sorgeloos, 1983). Of course, the high hatching outputs do not necessarily assure success in using these products as larval food sources because other criteria should be taken into account as well, i.e. the size of newly hatched nauplii (Beck et al., 1980; Vanhaecke & Sorgeloos 1980), the energy content and the fatty acid profile (Schauer et al., 1980). Should be taken into account, as well, i. e. the size of newly hatched nauplii (Beck et al., 1980; Vanhaecke & Sorgeloos 1980), the energy content and the fatty acid profile (Schauer et al., 1980).
The naupliar size of ME and C Artemia approximates the size range of Spanish parthenogenetic tetraploid strains (Amat, 1980) and can be considered relatively large - typical for parthenogenetic strains (Vanhaecke, 1983) - (Vanhaecke & Sorgeloos, 1980: Léger et al., 1986). Among parthenogenetic tetraploid LArtemia the naupliar size of Greek Artemia is certainly one of the smallest, while the naupliar dry weight is quite high and similar to the Artemia strains from Lavalduc (France) and Tiensin (China) (Sorgeloos et al., 1983; Léger et al., 1986). The above combination can be beneficial as long as the size of the nauplius does not interfere with the ingestion mechanism of the predator (Sorgeloos et al., 1983). Since the ME and C nauplii are larger than 480 um than 480 um, they are good for use with predators where Artemia size is not critical, such as freshwater fishes; they are, however, of less value for marine fish and shrimp larvae where they can be used only in later stages (Léger et al., 1986, 1987).
The individual energy content and individual dry weight of newly hatched nauplii appear to be very similar for the ME and C strains. The nonsignificant differences in energy content, on an ash-free dry weight basis, might be due to slightly different metablolic rates during pre-larval development in the uterus, caused by varying environmental conditions.
The energy content can be increased by 3–8% when cysts are hatched in 5 ppt, which is to be expected since less energy is needed for the emergence of the nauplius in a lower external osmotic pressure (Clegg, 1964).
Variation in growth rates of unfed nauplii which results in energy and dry weight losses can be avoided by storing instar-I nauplii at low temperatures (Léger et al. 1983). Nauplii from the Greek strains hatched at 5 ppt and maintained at 25°C lose 17–20% of their initial caloric content after 40 hrs, while the same nauplii stored 6 + 5° C lose only % after nearly 80 hrs (Fig.7).
The comparison of our data on energy content with the results from other parthenogenetic strains of different geographical origin reveals great similarities (Vanhaecke et al. 1983; léger et al., 1986). It is interesting to mention that the drops in energy contents between instar-I and instar-II and III nauplii which have been reported for other Artemia strains (Vanhaecke et al., 1983) appear to be faster than those observed for our material.
In general terms, the fatty acid profiles for these Greek Artemia agree with a ‘normal’ composition (Léger et al., 1986). The 18 : 2ω6 (linoleic acid) content, however, is slightly higher in the C strain than in most other strains; its levels are similar to those that have been reported for Artemia from Colombia, the Bahamas and the Phillipines (Léger et al., 1986).
The levels of the essential fatty acids (F.F.A.) 18 : 3ω3 (linolenic acid) and 20 : 5ω3 may not be sufficient to secure good survival and growth of marine larval organisms (Léger et al., 1986). Our Artemia strains appear to be better food for fresh water organisms which demand high 18 : 3ω3 levels and low 20 : 5ω3 content, and in that respect the Greek strains belong to the so-called ‘freshwater type’ Artemia.
It is interesting to note that the C strain contains detectable levels of another essential fatty acid, 22 : 6ω3, which is rarely found in Artemia nauplii and is known to promote survival and growth especially in crab cultures (Levins & Sulkin, 1984; Léger et al., 1987).
The slight variation observed in the levels of E.F.A. and especially in 20 : 5ω3 between the two Greek Artemia strains can be due to small differences in food conditions in Artemia ponds; this has been demonstrated in culture experiments where it has been found that Artemia cysts reflect different 20 :5 ω3 Levels of the diet available for the parental population (Léger et al, 1987). Variability in E.F.A. content is particularly great in stains produced in solar salt works which are characterized by completely different food composition in the various ponds. Thus, it can be deduced that the food conditions in the two Greek solar saltworks are very similar.
The analysis of all morphometric parameters examined in adult brine shrimps revealed that the two Greek populations exhibit considerable similarities with parthenogenetic tetraploid populations from the lberian peninsula (Amat, 1980) and support the view, we expressed elsewhere, that they belong to the same strain (Abatzopoulos et al., 1986, 1987).
CONCLUSIONS
This study of two N.Greek Artemia populations has shown that their ‘commercial’ characteristics are quite good, The size of cysts and nauplii are among the smallest known for parthenogenetic tetraploid populations, and their nutritional composition is acceptable for use, mainly with freshwater organisms, because their high energy content could result in better and faster growth rates, since less energy will be spent by the predators in hunting and food uptake.
As they stand, the data on the fatty acid profiles of the strains studies are acceptable for possible practical use. However, since the content in 20 : 5ω3 may fluctuate significantly within each strain both between harvests of cysts of the same and/or different years (Van Ballaer et al., 1987) we cannot be certain as to the usefulness of the N. Greek cysts for particular aquacultural demands, unless culture tests are performed with specific predators (Sorgeloos et al., 1983). Surely, the appropriate enrichment (Léger et al., 1986,1987) will improve the quality of this material.
Finally, the biometrical data, the hatching criteria and the nutritional value of the Greek Artemia reveal their good quality for possible commercial use, under the condition, of course, that the solar sultworks ecosystem is properly managed.
ACKNOWLEDGEMENTS
We thank Professor C.D. Kastristsis for help with this manuscript, and for useful discussions throughout this study. Thanks are also due to Drs. M. Lazaridou and G. Stamou for allowing us to use their facilities. This work was supported in part by grants from the Greek Ministry of Agriculture and the Greek Ministry of Industry, Research and Technology.PS is a senior scientist with the Belgian National Science Foundation (NFWO).
REFERENCES
ABATZOPOULOS, TH. J., C,D. KASTRISTSIS & C.D TRIANTAPHYLLIDIS, 1986. A study of Karyotypes and heterochromatic associations in Artemia, with special reference to two N. Greek populations. Genetica 71 : 3–10.
ABATZPOULOS, TH. J., C.D. TRIANTAPHYLLIDIS & C.D. KASTRITSIS, 1987. Preliminary studies on some Artemia populations from northern Greece. In :Sorgeloos, P., Bengtson, D.A., Decleir, W,& Jaspers, E. (eds). Artemia research and its applications, l : Universa Press, Wetteren (Belgium) ; 107–114.
AMAT, D.F., 1980. Differentiation in Artemia strains from Spain, In: Persoone, G., Sorgeloos, P., Roels, O.,& Jaspers, E. (eds). The brine shrimp Artemia, l.Universa Press, Wetteren (Belgium): 19–39.
BECK, A, D., D.A. BENGTSON & W. H.HOWELL, 1980. International study on Artemia. V.Nutritional value of five geographical strains of Artemia: effects on survival and growth of larval LAtlantic silverside Menidia menidia. In: Persoone, G., Sorgeloos, P., Roels, D. & Jaspers, E. (eds). The brine shrimp Artemia, 3. Universa Press, Wetteren (Belgium): 249–259.
BENIJTS, F., E. VANVOORDEN & P. SORGELOOS, 1976. Changes in the biochemical composition of the early larval stages of the brine shrimp Artemia salina L. In: Persoone, G. & Jaspers, E. (eds). Proc. 10th Europ. Symp. on Marine Biology, I. Universa Press, Wetteren (Belgium): 1–9. Bligh, E.G. & W.J. Dyer, 1959. A rapid method of total lipid extraction and purification. Can J. Biochem. Physiol. 37: 911–917.
BRUGGEMAN, E., P. SORGELOOS & P. VANHAECKE, 1980. Improvements in decapsulation technique of Artemia cysts, In: Persoone, G., Sorgeloos, P., Roels; O. & Jaspers, E. (eds). The brine shrimp Artemia, 3. Universa Press, Wetteren (Belgium): 261–269.
CLEGG, J. S., 1964. The control of emergence and metabolism by external osmotic pressure and the role of free glycerol in developing cysts of Artemia salina. J. Exp. Biol. 41: 879–892.
LÉGER. PH., D.A. BENGTSON, K.L. SIMPSON & P. SORGELOOS, 1986. The use and nutritional value of Artemia as a food source, Oceanogr. Mar. Biol. Ann. Rev. 24:521–623.
LÉGER, PH., E. NAESSENS-POUCQUAERT & P. SORGELOOS, 1987. International study on Artemia. xxxv. Techniques to manipulate the fatty acid profile in Artemia nauplii, and effect on its nutritional effectiveness for the marine crustacean Mysidopsis bahia (M.). In: Sorgelos, P., Bengtson, D. A., Decleir, W. & Jaspers, E. (eds). Artemia research and its applications, 3. Universa Press, Wetteren (Belgium): 409–424.
LÉGER, PH., P. VANHAECKE & P. SORGELOOS, 1983. International Study on Artemia XXIV. Cold storage of live Artemia nauplii from various geographical sources: potentials and limits in aquaculture, Aquacult, Engineering 2: 69–78.