J.C.NAVRRO, F. AMAT AND J.R. SARGENT
ABSTRACT
The lipid class composition of Artemia cysts from several Spanish populations was analysed by highperformance thin-layer chromatography (HP-TLC). A total of 11 lipid classes was detected, of which six were phospholipids and five neutral lipids. The chromatograms were quantified by single-wavelengh scanning densitometry. In general, triacylglycerides were the major component, comprising = 50% of the total lipids, cholesterol accounted for 10% and cholesterol esters fluctuated from 3 to 10%. Phospholipids, mainly phosphatidylcholine and phosphatidylethanolaminc in a ratio 2 : 1, accounted for 10–20% of the total lipids. Pigements and free fatty acids were found to be very variable.
INDRODUCTION
Fatty acid compositions of Artemia have been frequently studied. Artemia nauplii can be deficient
in certain essential fatty acids necessary for fish and crustacean marine larvae (Watanabe et al., 1978,
1983). Determination of fatty acid composition is important to assess the nutritional quality of a
source of Artemia. However, specific lipid classes can also be important in fish and crustacean nutrition
(Kanazawa et al., 1971; D'abramo et al., 1981), but information on the lipid class composition
of Artemia is relatively scarce. Some analysis have been carried out in adults (Enzler et al., 1974;
Gallagher & Brown. 1975; Sasaki & Capuzzo, 1984), Whereas other analysis have been reported for
nauplii, either as information complementary to the main aim of the work (Seikai et al., 1987; Fox,
1990; Reza Ahmadi et. al., 1990; Webster & Lowell, 1990), or as part of broaader biochemical characterisation
of the lipovitellin of a single strain (De Chaffoy & Kondo, 1980; De Chaffoy et al.,
1980). Rudneva & Shchepkina (1990) have recently reported some values for the polar lipids, triacylglycerides
and cholesterol of Artemia cysts.
The aim of this work was to define and quantify the main classes of Artemia cysts and to analyse
their variability using from several Artemia populations of the Iberian peninsula.
MATERIALS AND METHODS
Cysts from the following coastal and inland populations of Artemia were analysed:
Coastal:
Los Hermanos: bisexual diploid strain from the Los Hermanos saline, San Fernando, Cadiz.
Inland:
Gerri: parthenogenetic diploid strain from Gerri, Lérida. The locations of these populations are shown in Fig. 1.
The cyst samples used in this study were personally harvested. Once harvested from the shore of the salines, cysts were kept in saturated brine transported to the laboratory where they were cleaned by differential flotation in freshwater and saturated brine as described elsewhere (Sorgeloos et al., 1977; Amat; Navarro, 1985). The cysts were then dried and stored under nitrogen in tightly sealed contrainers prior to analyses.
Prior to lipid extraction, cyst samples were hydrated in seawater (salinity 34 g/l-I) until the cysts were observed to be spherical under a dissecting microscope. They were then decapsulated according to the procedures described in Bruggeman et al. (1979). After a through wash in distilled water, the cysts were blotted over soft paper tissue and divided into six aliquots, three of which were dried for 24 h at 110°C to calculate the moisture content, the remaining three being used for lipid extraction. Preliminary studies showed no differences between the lipid classes of decapsulated and whole cysts. Decapsulated cysts were routinely analysed here because their lipids are easier to extract. Lipids were extracted using the method of Folch et al. (1957), quantified gravimetrically suing an analytical balance (Mettler H64) and stored at -20°C at a concentration of 10 mg ml-1 in chloroform : methanol (2 : 1, v:v) containing 0.01% (w : v) of butylated hydroxytoluene (BHT) as antioxidant. Nitrogen was flushed through the vial before being scaled. Extractions were carried out in triplicate.
HPTLC (10 × 10 cm) plates of silica gel 60 (E. Merck, Darmstadt, FRG) were pre-run as described in Olsen & Henderson (1989) and activited for 30 min at 110°C. Five separate 1, 5- ul lipid samples were applied to cach plate with a micro-syringe. A sixth sample containing only BHT and the solvent was also applied as a sovent blank.
Plates were run at room temperature following the double development procedures described in Olsen & Henderson (1989). The first solvent system developed the polar lipids to a distance of 5 cm from the origin. The neutral lipids were developed in the second solvent system up to 0.5 cm of the top of the plate. The polar system was a mixtue of methyl acctate: isopropanol : chloroform : methanol: 0.25% KCI (12.5 : 12.5 : 4.5 by vol.); the neutral system was : hexane : diethyl cither: acctic acid (42.5 : 7.5 : 0.75 by vol.).
The individual lipid classes were detected after charring (110°C for 20 min) plates sprayed with 3%
cupric acctate in 8% phosphoric acid (Fewster et al. 1969). Quantification of the resulting chromatograms
was performed on a Shimadzu dual wavelength TLC scanner CS-9000 linked to a Shimadzu
data recorder DR-13. The background created the solvent fronts was also quantified from the blank.
run and subtracted from those zones coinciding with the solvents fronts. Individual lipid classes were
identified by comparison with known standards.
The means of the lipid classes of the coastal and inland cysts were compared with a Student test
(Sokal & Rohlf, 1981), after aresine-transforming the data (Zar, 1984).
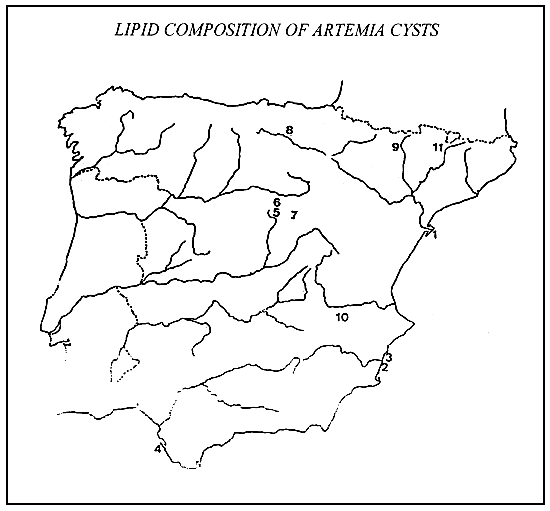
Fig. 1. Location of sampling areas : 1, La Trinidad; 2, La Mata; 3, Bonmati; 4 Los Hermanos; 5, Olmeda; 6, Imon; 7,Medinaccli; 8, afiana; 9, Rolda; 10, Petrola; 11, Gerri.
RESULTS
A total of 11 lipid classes was consistently detected, consisting of six phospholipids: sphingomyclin (SM), phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidic acid/cardiolipid (PA/CL) and phosphatidylethanolamine (PE): and five neutral lipids: pigments, cholesterol (CHOL), free fatty acids (FFA). triacylglycerides (TAG) and cholesterol-esters (CE). Tables I and II summarize the results. The analytical method empolyed did not separate PA and CL. In some samples trace amounts (<0.1%) of lyso-phosphatidylcholien were noticed (Los Hamanos) and in other samples (Imon, Rolda, Petrola), SM could not be quantified because it nigrated very close to PC.
Table 1 : Lipid classes, polar (PL), natural (NL) and total lipids (TL) of cysts from coastal Artemia population. Data for individual lipids are expressed as % of total lipids. Data for total lipids are expressed as % of dry weight off cysts. All data are × ± SD values. Other abbreviations are defined in text.
| Populations | ||||
| Bonmati | La Mata | La Trinidad | Los Hermanos | |
| SM | 0.12 ± 0.02 | 0.31 ± 0.06 | 0.22 ± 0.03 | 0.37 ± 0.03 |
| PC | 0.12 ± 0.41 | 8.01 ± 0.16 | 12.38 ± 0.15 | 5.54 ± 0.34 |
| PS | 0.18 ± 0.03 | 0.24 ± 0.04 | 0.56 ± 0.04 | 0.35 ± 0.03 |
| PI | 1.37 ± 0.06 | 1.48 ± 0.09 | 2.57 ± 0.11 | 1.16 ± 0.09 |
| PA/CL | 0.61 ± 0.03 | 0.72 ± 0.02 | 1.50 ± 0.08 | 0.50 ± 0.01 |
| PE | 4.04 ± 0.16 | 2.26 ± 0.15 | 6.32 ± 0.28 | 3.14 ± 0.11 |
| Pigments | 2.30 ± 0.22 | 10.63 ± 0.08 | 1.66 ± 0.30 | 3.82 ± 0.06 |
| CHOL | 10.21 ± 0.08 | 9.90 ± 0.35 | 12.54 ± 0.93 | 12.30 ± 0.24 |
| FFA | 7.05 ± 0.14 | 4.98 ± 1.11 | 8.63 ± 0.77 | 16.92 ± 0.53 |
| TAG | 60.61 ± 0.87 | 56.34 ± 1.76 | 50.83 ± 1.02 | 48.92 ± 0.49 |
| CE | 6.91 ± 0.51 | 4.73 ± 0.52 | 2.87 ± 0.53 | 6.98 ± 0.31 |
| PL | 12.92 ± 0.60 | 13.41 ± 0.31 | 23.48 ± 0.64 | 11.06 ± 0.61 |
| NL | 87.08 ± 0.060 | 86.59 ± 0.31 | 23.48 ± 0.64 | 11.06 ± 0.61 |
| TL | 19.42 ± 0.14 | 17.80 ± 0.52 | 11.48 ± 0.68 | 15.57 ± 0.82 |
The sterol ester zone of Artemia, named here as cholesterol esters (CE), has been reported as cholesterol esters by other authors (Seikai et al.,1987 Liou & Simpson 1989; Rudneva & Shchepkina, 1990). However, the technique used here did not allow us to identify esterified sterol present, The same applies for the sterols, identified as cholesterol by Teshima & Kanazawa (1971) and named here as this compound (CHOL), It should be noted that hydrocarbons, reported to be present in Artemia in trace amounts by Seikai et al. (1987), co-migrate with CE in the solvent systems used here.
The neutral lipids accounted for 75–89% of the total lipids and exhibited a substantial variation from
9.5% in Rolda to 19.4% in Bonmati.
Fig.2 is a graphic representation of the mean values obtained after pooling the data of the different
lipid classes. The least abundant lipid classes were SM, PS and PA whereas TAG was the most abundant
accounting for = 50% of the total lipids, most lipid classes, particularly FFA and pigments, were
found to be highly variable among samples. CHOL on the other hand, was very constant at = 10%
of total lipids. Despite the extensive variations, some patterns can be identified, particularly among
the phospholipids. Thus SM accounted for <5,% of total lipids, the ratio PC; PE was consistently
= 2 : 1, PT comprised 7–10% of total phosopholipids, PA together with CL accounted for 5–6% of total
phospholipids and PS constituted 2–3% of total phospholipids.
With the exception of CHOL, the neutral lipids showed a less fixed pattern CE was found to be more abundent (P<0,0021) among the cysts of the inland (x = 11.1%, SD = 2.2%) than the coastal populations (x = 5.4%, SD = 2%). The remaining lipid classes did not show significant (P<0.05) differences.
Table II : Lipid classes, polar (PL), neutral (NL) and total lipids (TL) of cysts from inland Artemia populations. Data for individual lipids are expressed as % of
total lipids. Data for total lipids are expressed as % of dry weight of cysts. All data are × ± SD values.
* Not detected. Other abbreviations are defined in text.
| Populations | |||||||
| Olmeda | Medinaceli | Gerri | Ariana | Imon | Rolda | Petrola | |
| SM | 0.30 ± 0.05 | 0.36 ± 0.07 | 0.34 ± 0.04 | 0.28 ± 0.11 | * | * | * |
| PC | 8.55 ± 0.29 | 9.68 ± 0.05 | 8.48 ± 0.63 | 13.02 ± 0.70 | 13.78 ± 0.46 | 12.44 ± 0.55 | 12.94 ± 0.30 |
| PS | 0.22 ± 0.01 | 0.35 ± 0.06 | 0.37 ± 0.08 | 0.75 ± 0.04 | 0.69 ± 0.18 | 0.72 ± 0.15 | 0.46 ± 0.05 |
| PI | 1.45 ± 0.19 | 1.40 ± 0.09 | 1.07 ± 0.00 | 2.21 ± 0.05 | 2.00 ± 0.19 | 2.09 ± 0.11 | 1.63 ± 0.19 |
| PA/CL | 1.03 ± 0.01 | 0.97 ± 0.03 | 0.58 ± 0.03 | 1.36 ± 0.16 | 1.39 ± 0.31 | 1.44 ± 0.07 | 1.04 ± 0.07 |
| PE | 4.49 ± 0.20 | 4.68 ± 0.06 | 3.15 ± 0.18 | 5.58 ± 0.40 | 6.75 ± 0.25 | 5.68 ± 0.27 | 5.85 ± 0.06 |
| Pigments | 6.63 ± 0.38 | 1.03 ± 0.03 | 1.57 ± 0.19 | 0.68 ± 0.16 | 2.73 ± 0.14 | 1.35 ± 0.06 | 0.43 ± 0.01 |
| CHOL | 11.34 ± 0.09 | 8.81 ± 0.89 | 10.45 ± 0.86 | 12.48 ± 0.27 | 13.56 ± 1.05 | 10.88 ± 0.24 | 12.02 ± 0.44 |
| FFA | 20.61 ± 0.14 | 0.53 ± 0.05 | 2.25 ± 0.68 | 3.95 ± 0.67 | 3.15 ± 0.18 | 2.08 ± 0.25 | 3.14 ± 0.17 |
| TAG | 37.49 ± 0.88 | 63.84 ± 0.76 | 59.54 ± 0.72 | 46.73 ± 1.09 | 43.45 ± 1.46 | 53.16 ± 11.72 | 50.24 ± 0.57 |
| CE | 8.01 ± 0.69 | 8.41 ± 0.10 | 12.72 ± 0.15 | 13.48 ± 0.94 | 12.50 ± 0.22 | 10.17 ± 0.14 | 12.26 ± 0.14 |
| PL | 15.92 ± 0.84 | 17.45 ± 0.14 | 17.45 ± 0.14 | 13.79 ± 1.07 | 22.68 ± 1.75 | 24.61 ± 0.50 | 22.36 ± 1.02 |
| NL | 84.08 ± 0.84 | 82.55 ± 0.14 | 82.55 ± 0.14 | 86.21 ± 1.07 | 77.32 ± 1.75 | 75.39 ± 0.50 | 77.64 ± 1.02 |
| TL | 12.23 ± 0.56 | 12.72 ± 0.30 | 16.70 ± 0.42 | 13.49 ± 0.20 | 9.63 ± 0.30 | 9.52 ± 0.18 | 11.07 ± 0.42 |
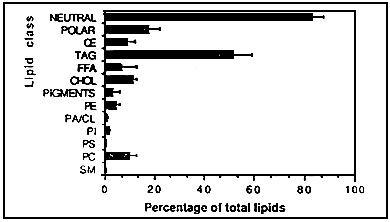
Fig 2. Graphic representation of × values of polar lipids, neutral lipids, and different lipid classes detected in cysts of Artemia population. Error bars are SD values
DISCUSSION
The total lipid contents of Artemia cysts reported in this study agree with data from other authors (Schauer et al.,) 1980; Leger at al 1987) and the proportion of polar/neutral lipids,although subject to high variability, agrees with values reported by other authors for Artemia nauplii (Scikai et al, 1987; Webstar & Lovell, 1990) and cysts (Rudneva & Shchepkina, 1990). However, values of polar lipids of = 50% of total lipids have been reported for some nauplii (Fox, 1990), mainly due to very high values of PE (24.3%). Higher values of all the phospholipid classes were reported by Sasaki & Capuzzo (1984) in cultured adults of a Brazilian strain, using the latroscan technique (TLC-flame ionization detector).
Rudneva & Shchepkina (1990) found values of CHOL in Artemia cysts ranging between 2.2 and 10.6% of total lipids, whereas the CE varied between 2.9 and 12.1%. The content of free CHOL and CE in Artemia nauplii has also been studied by Liou % Simpson (1689) who found much lower values than those reported in this work. Scikai et al. (1987) found even lower values of CHOL, but the total lipid content of their nauplii (5–6%) is very low compared with the values reported in the literature (Leger et al., 1987) raising doubts about the efficiency of their lipid extractions.
The difference in the CHOL content of Artemia lipids reported in the literature may be explained either by the use of different techniques for the detection and quantification of the compound, or by the analyses having been performed on different life history stages. The latter explanation is questionalbe (Navarro et al., 1991). The former, however, does have validity in that, using the methodology here reported, some interference of the CHOL zone with zones of monoacylglycerides and diacylglycerides can be expected (Olsen & Henderson, 1989). However, the latter two lipid classes, if present, generally occur in very small amounts in marine zooplankton (Olsen & Henderson, 1989).
The difference found between the CE contents of the coastal and inland population suggests a phenotypic influence. The pigments of Artemia cysts, identified by Czeezuga (1971, 1980) mainly as a mixture of astaxanthin and B-carotene and by Soejima et al. (1987) as a mixture of echineone (0.5–5.5%) and canthaxanthin (94.5–99.5%), are known to be strongly influenced by the diet. This could account for the high variability in pigment levels found among the different populations analysed in the present work.
Some cyst samples in this study presented high levels of FFA (particularly, Olmeda and Los Hermanos). In general, FFA do not exist naturally in substantial amounts in biological samples because of their market affinity for many proteins resulting in an inhibitory action on many enzyme activities. Where FFA are reported as major constituents they are usually artefacts due to cell damage (Gurr & James, 190), with polar lipid classes likely to be the dominant sources of FFA because they are the most suceptible to hydrolysis by lipases (Sasaki & Capuzzo, 1984). From the data reported in this study, it can be deduced that the FFA are more inversely correlated with the TAG (r = 0.5936; p<0.0542) than with the polar lipids (r = 0.4137; p<0.2059). A possible explanation for this could be that fatty acids are mobilized from the TAG as source of energy for cellular development and that, in those samples where FFA are prominent, hydration of the cysts prior to the decapsulations had triggered TAG metabolism. The fact that lyso-phosphatidylcholine was detected in a population (1.0s Hermanos) with low levels of polar lipids and, and particular, PC points to limited phospholipid hydrolysis (Sasaki & Capuzzo,1984).
In conclusion, the liquid classes of the cysts from the different Artemia populations showed substantial variability, but some patterns can be established. From the point of view gross nutritional value of the cyst lipid, TAG is the major component at = 50% of the lipids, CHOL and CE account for 10 and 3–10%, respectively. Pigment levels can be very variable and are clearly subjected to phenotypic influence, Phospholipids, mostly PC and PE in a ratio of 2 : 1, account for = 17% of total lipids.
ACKNOWLEDGEMENTS
Antonio Rodriguez kindly provided the Los Hermanos sample. J.C.Navarro is a Post-Doctoral Fellow of the «Plan para el Perfeccionamiento de Doctores y Techlogos» from the Spanish Education and Science Ministry Special thanks are given to all colleagues who helped in the sampling of Artemia cysts and to R.J.Henderson, M.V.Bell, J.G.Bell and R. Wilson who kindly answered J.C.Navarro's everyday questions.
REFERENCES
AMAT, F., 1985, Utilization de Artemia en acuicultura, Inf, Tech, Inst, Inv, Pesq., Vol. 128–129,pp 1–59. Bruggeman, E.,M.Bacza-Mesa,E Bossuyt & P.Sorgeloos, 1979, Improvements in the decapsulation of Atremia cysts. In, Cultivation of fish fry and its food, edited by E.Styezynska-Jurewiez et al., Eur.
MARICULT SOC SPEC. PUBL., No. 4, pp. 309–315.
CZEEZUGA, B., 1971 Studies on the carotenoids inArtemia salina L. egg Comp, Biochem. Physiol., Vol 40B, pp 47–52.
CZEEZUGA, B., 1980 Carotenoid content in Artemia salina I eggs and vitality of the young specimens of this crustaccan. In The brine shrimp Artemia. Physiology, biochemistry Molecular biology, edited by G. Persoone et al., Universa Press, Wetteren, Belgium, 607pp.
D'ABRAMO.L.R., C.E. BORDNER.D.E.CONKLIN & N.A. BAUM,1981, Essentiality of dietary phosphatidylcholine for the survival of juvenile lobsters J.Nutr., Vol 11, pp 425–431.
DE CHAFFOY, D., HELP L. MOENS & M.KONDO, 1980. Artemia lipovitellin in. The brine shrimp Artemia Physiology, biochemistry, molecular biology, edited by G.Persoone et as., Universa Press, Wetteren, Belgium, pp. 379–394.
DE CHAFFOY, D & M ,KONDO, 1980 Lipovitellin from the crustaccan, Artemia salina J.Biol. Chem., Vol 225 pp. 6727–6733.
ENZLER L., SMITH, J.S.LIN & H.S.OLCOTT. 1974. The lipids of Mono lake, California, brine shrimp (Artemia salina). J.Agr. Food Chem., Vol 22 pp 330–331.
FEWSTER, M.B., B.J.BURNS & J.F. MEAD,1969, Quantitative densitiometric thin layer chromatography of lipids using copper acetate reagent, J.Chromatogr.,Vol 43 pp 120–126.
FOLCH, J.,N LESS & G.H. SLOANE STANLEY,1957. A simple method for the isolation and purification of total lipids from animal tissues. J.Biol Chem Vol 226 pp.497–509.
FOX,C. 1991. Studies on polyunsaturated fatty acid nutrition in the larvae of a marine fish - the herring. Clupea harengus L Ph. D. thesis. University of Stirling, 196 pp.
GALLAGHER, M & W.D.BROWN, 1975, Composition of San Francisco Bay brine shrimp (Artemia salina). J.Agric, Food Chem., Vol 23. pp.630–632.
Gurr, M.I. & A T.James, 1980 Lipid biochemistry: an introduction Chapman and Hall, London, 247 pp.
BY Paul S. SCHAUER, D. Michael JOHNS,
Charles E. OLNEY, and Kenneth L. SIMPSON
U S A
ABSTRACT.
Artemia cyst and newly hatched nauplii from Australia, Brazil, Italy and the United States (California and Utah) were analyzed for their total lipid level, total fatty acid level and composition, and their energy content in an effort to evaluate their lipid nutritional value as diets of marine organisms. Results are compared to biological data from a nutrtional evaluation of these five Artemia strains on various marine organisms.
The total lipid, fatty acid methyl ester and energy levels of all strains appeared to be adequate to promote good growth and survival of the marine organisms. The fatty acid spectrum of the cysts and nauplii were nearly identical, indicating that the cyst shell contains little fatty acid-type lipids. However, significant differences were found in the fatty acid composition between the various strains.
Artemia were classified into two groups based on their 003 polyunsaturated fatty acid composition. The major difference between the two major difference between the two groups was that one group contained predominantly 18 : 3003, while the other group contained chiefly 20 : 5003. Considering the importance of 20 : 5003 to marine organism nutrition, the Australia, Italy, Brazil, and San Francisco Bay 321 strains which contained the higher level of this fatty acid would provide the best nutrition of the five strains; The San Pablo Bay 1628, San Francisco Bay 313 and Utah strains would probably be of less nutritional value due to the low level of 20 : 5003 and/ or the excess amount of 18 : 3003. It is possible, however, that there is an interaction between an essential fatty acid (20 : 5003) deficiency and a dietary contaminant. This possibility is discussed with reference to biological results obtained when these five Artemia strains were fed to three different marine organisms.
INTRODUCTION
Artemia (brine shrimp) is a widely used food source in laboratory and commercial rearing of many marine organisms. Several new geographical Artemia strains have recently become available and a subsequent need has developed for their analysis as potential supplements of replacements for the present commercial sources.
An important determinant of the overall nutritional value of any food stuff is its lipid content. Triglyceride-type lipids are a major source of a diet's metabolizable energy and are directly linked to the growth of the consumer organism (Pandian, 1975). The dietary fatty acid composition ultimately determines the fatty acid composition of the structural phospholipids (castell et al. 1972 ab; Norred and Wade, 1972). Phospholipids are functionally active in maintaining proper membrane fluidity and cellular transport mechanisms. Recent research has demonstrated that the ω3 polyunsaturated fatty acids (PUFA) are required for lobsters (Castell and Covey, 1976), prawns (Guary et al., 1976; Kanazawa et al., 1979) and for several marine fish, including plaice (Owen et al., 1972), red sea bream (Yone and Fujii, 1975 a) and turbot (Cowey et al., 1976).
The purpose of this research was to determine the total lipid content, fatty acid composition and energy content of cysts and newly-hatched nauplii of Artemia from five geographical regions.
MATERIALS AND METHODS
Sources and culturing of Artemia
Deshydrated cysts from five geogrphical locations were provided by the Artemia Reference Center (Ghent, Belgium). The origin of these cysts were Shark Bay, Australia (World Ocean, lot no; 113); Macau, Brazil (Companhia Industrial do Rio Grande to Norte, CIRNE-Brand, harvested 1978); Margherita di Savoia, Italy (harvested 1977): Great Salt Lake, Utah, USA (harvested 1977); San Pablo Bay, California, USA (Living World, San Francisco Bay Brand, Inc., lot no. 1628) and two samples from San Francisco Bay, California, USA (San Francisco Bay Brand, Inc, lot no. 313/3006 and lot no. 321995). The latter three Californian samples will be referred to by their respective full or abbreviated lot numbers : SP 1628, SF 313 SF 321.
Stage one Artemia nauplii were hatched from cysts incubated at 25°C in 30‰ filtered (0.45 um) seawater for a specific period of time that was dependent on the particular strain (Johns et al., 1980).
Samples were either held at-20°C until analysis or dried at 60°C to a constant weigh for determination of the energy content.
LIPID EXTRACTION AND ANALYSIS
Total lipids
Cysts were ground in mortar and pestle and extracted twice with chloroform/methanol/water (20 ml/40 ml/16 ml) (Bligh and Dyer, 1989). The remaining solids were repeatedly extracted in acetone until the supernatant locked pigmentation. The lipid in the acetone solution was transferred to petroleum either (PE) and along with the chloroform fraction from the Bligh and Dyer extraction was evaporated to dryness at 30°C. Total lipid weight was determined gravimetrically after which the lipids were dissolved in benzene and stored at -20°C until analysis. Total lipid weight of the nauplii was determined in a similar manner. Lipid weights are presented as mg lipid/g dry weight sample.
FATTY ACIDS
The fatty acid composition of each sample was determined by gas-liquid chromatography on two independent columns as described by Schauer and Simpson (1978). Results are presented here as fatty acid methyl esters (FAME) weight percent of total lipid. Quantification of total FAME weights were mad by co-injecting the FAME 20 : 2ω6 as an internal standard and are presented as mg FAME/g lipid of the dry weight samples.
DETERMINATION OF ENERGY CONTENT
Energy content of newly hatched nauplii was determined using wet oxidation in the presence of an acid-dichromate mixture (Maciolik, 1962). These values are reported as Joule/ gram ash-free dry weight.
DATA ANALYSIS
One-way analysis of variance was computed for total lipids, FAME and energy content. If significant differences (P<0.05) were found, a Student-Neuman-Kuels posterior comparison was used to determine where the difference lay (Snedecor and Gochran, 1967).
RESULTS
The Australian cysts' lipid level was statistically greater than the other four strains while the means of the Utah, SP 1628 and Brazilian strains were greater than that of the Italian cyst strain (Talbe I). The nauplii lipid levels, however, were all statistically different from one another except for the SF 321 and SP 1928 strains. The highest level of lipid in the nauplii was found in the Utah strain, and in descending order strains were ranked as follows: Brazil, Australia, SF 313, SP 1628, SF 321 and ltaly.
Table I : The amount of total lipid and fatty acid methyl esters in the cysts and newly hatched nauplii of five strains of Artemia
| Artemia sources | |||||||
| Australia | Brazil | SF 313 | SF 321 | SP 1628 | Italy | Utah | |
| mg total lipids2 | |||||||
| Cysts | 157a ± 8 | 134b ± 8 | N.A3 | N.A. | 134b ± 22 | 19.0c ± 6 | 136b ± 1 |
| Nauplii | 185c ± 9 | 202b ± 8 | 174d ± 4 | 159e ± 13 | 160e ± 3 | 156f ± 2 | 224a ± 14 |
| mg fatty acid methyl esters4 | |||||||
| Cysts | 510b ± 18 | 502b ± 12 | N.A. | N.A. | 573b ± 39 | 704a ± 34 | 490b ± 19 |
| Nauplii | 751a,b ± 41 | 854a ± 26 | 716a,b ± 30 | 602b ± 15 | 711a ± 58 | 739a ± 12 | 742 a, b ± 10 |
1 Values within each row which bear the same letter are note significantly différent at P < 0.05.
2 Per gram dry weight of sample.
3 N.A. = not analyzed.
4 Per gram lipid.
Although the total lipid level of the Italian strain was the lowest in both cysts and nauplii, it contained significantly greater levels of FAME (mg/g lipid) than the other statistically similar cyst strains. The FAME levels for the nauplii of the Brazil, Australia, Utah, Italy, SP 1628 and SF 313 strains were all statistically similar as were the Australia, Utah, SF 313 and SF 321 nauplii, but the Brazilian, Italian and SP 1628 strains were significantly greater than the SF 321 strain.
The fatty acid composition of cysts and nauplii is presented in Table II and Table III, respectively. The relative proportion of fatty acids in cysts and nauplii remain the same, indicating that the fatty acid content of the chorion is small.
Table II : Fatty acid compostion of whole cysts of five strains of Artenia
| FAME | Aust. | Brazil | SP 1628 | Italy | Utah |
| 14:0 | 1.80 | 2.04 | 0.65 | 1.79 | 1.20 |
| 14:1 | 2.11 | 1.03 | 2.88 | 3.55 | 1.94 |
| 15:0 | 1.02 | 0.95 | 0.22 | 0.14 | ----1 |
| 16:0 | 15.1116.35 | 9.80 | 14.15 | 12.39 | |
| 16:1 | 10.66 | 12.88 | 6.49 | 13.05 | 6.00 |
| 16:2ω7 | 0.58 | 1.68 | 1.67 | 2.04 | 4.68 |
| 16:3ω417:1ω8 | 3.98 | 3.93 | 2.69 | 3.47 | 1.47 |
| 18:0 | 2.66 | 2.34 | 2.38 | 3.31 | 3.55 |
| 18:1ω9 | 26.71 | 33.50 | 27.43 | 26.05 | 28.03 |
| 18:3ω6 | 6.229.17 | 5.30 | 7.08 | 5.58 | |
| 18:3ω3 | 13.19 | 4.39 | 31.85 | 6.24 | 28.16 |
| 18:4ω3 | 4.41 | 0.97 | 5.15 | 1.55 | 3.52 |
| 20:1ω9 | 0.27 | 0.49 | 0.46 | 0.31 | 0.21 |
| 20:2ω6/ω9 | 0.08 0.29 | 0.15 | 0.62 | 0.16 | |
| 20:3ω6 | 0.77 | 2.30 | 0.04 | 1.35 | 0.27 |
| 20:5ω3 | 9.32 | 8.35 | 1.66 | 12.61 | 3.23 |
| 22:6ω3 | 0.26 0.11 | trace | ----1 | trace |
1 No value (----) means the fatty acid was not found
The major fatty acides in all Artemia strains tested were 16 : 0,
16 : 1, and 18 : 1ω9. In addition, levels of 18 : 3ω3 and/ or 20 : 5ω3 were substantial in various strains.
Docosahexaenoic acid (22 : 6ω3) was found in only small amounts in the Australian and Brazilian
Artemia nauplii. The Artemia strains were divided into two groups based on their most predominant
long chain ω3 PUFA. Strains that contained mostly 18 : 3ω3 and 18 : 4ω3 included the SF 313, SP
1628, and Utah strains, while the group that contained predominantly 20 : 5ω3 included SF 321,
Italian and Brazilian strains. The Australian strain contained substantial amounts of both 18: 3ω3
and 20 : 5ω3 and therefore could be included in either of the two groups.
Table III : Fatty acid composition of newly hatched Artemia
| FAME | Aust. | Btazil | SF 313 | SF 321 | SP 1628 | Italy | Utah |
| 14:0 | 1.34 | 1.57 | 0.99 | 1.57 | 0.43 | 1.53 | 0.93 |
| 14:1 | 2.23 | 0.81 | 1.27 | 0.74 | 2.26 | 3.30 | 1.45 |
| 15:0 | 1.34 | 0.67 | 0.16 | 0.58 | 0.25 | 0.11 | 0.11 |
| 15:1 | 0.15 | 0.24 | 0.20 | 0.13 | 0.46 | 0.54 | 0.37 |
| 16:0 | 13.45 | 5.42 | 10.33 | 12.13 | 7.79 | 15.23 | 11.78 |
| 16:1 | 9.97 | 10.79 | 13.27 | 19.52 | 5.24 | 10.38 | 5.64 |
| 16:2ω7 | ----1 | ----1 | ----1 | ----1 | 1.51 | 2.94 | ----1 |
| 16:3ω4/17:1ω8 | 3.87 | 3.88 | 2.09 | 2.32 | 2.44 | 3.28 | 2.90 |
| 18:0 | 3.07 | 2.79 | 6.83 | 2.90 | 3.08 | 3.17 | 4.07 |
| 18:1ω9 | 28.23 | 35.86 | 26.97 | 31.20 | 29.15 | 29.05 | 28.58 |
| 18:2ω6 | 5.78 | 9.59 | 9.35 | 3.69 | 4.60 | 6.79 | 4.60 |
| 18:3ω3 | 14.77 | 4.87 | 17.33 | 5.16 | 33.59 | 6.35 | 31.46 |
| 20:4ω3 | 4.37 | 0.96 | 3.26 | 1.28 | 4.88 | 1.01 | 3.10 |
| 20:1ω9 | 0.37 | 0.52 | 0.41 | 0.35 | 0.35 | 0.42 | 0.37 |
| 20:3ω6/ω9 | 0.12 | 0.06 | 0.06 | ----1 | 0.24 | 0.20 | 0.09 |
| 20:3ω6 | 0.79 | 2.76 | 1.01 | 2.23 | 0.05 | 1.47 | 0.48 |
| 20:3ω3/20:4ω6 | ----1 | ----1 | 1.48 | 2.69 | 1.48 | ----1 | ----1 |
| 22:5ω3 | 10.50 | 8.98 | 4.06 | 12.44 | 1.68 | 13.63 | 3.55 |
| 22:6ω3 | 0.26 | 0.06 | ----1 | ----1 | ----1 | ----1 | ----1 |
1 No value (---) means fatty acid was note found.
Energy content for the geographical stains is presented in Table IV. Using the same statistical tests as in the lipid and FAME evaluations, differences in energy content between the various strains are significant (P<0.05). The Australian strain contained the most energy with 2.50 × 104 J/g ash-free dry weight (5.961 Kcal/g); the Italian strain contained the least with 2.24 × 104 J/g ash-free dry weight (5.370 Kcal/g).
TABLE IV : The energy content 1, 2 (X104) of five strains of Artemia nauplii
| Australia | Brazil | SP 1628 | Italy | Utah |
| 2.50a+0.16 | 2.35a,b+0.04 | 2.35a,b+0.11 | 2.24b+0.06 | 2.34a,b+0.8 |
1 J/g ash -free dry weight (ash content 5.4% dry weight).
2 Values which bear the same letter subscript are not significantly at P<0.05.
DISCUSSION
Major differences were found in the lipid level, FAME levels, fatty acid composition and the energy content of the cysts and nauplii of the five geographically different strains. There differences could have been caused by variations in the genetic make up or the previous dietary history of the parental stock which produced the cysts. Clark and Bowen (1976) have isolated six separate species from 27 different geographical strains of Artemia and Bowen et al. (1978) have shown a genetic variation in the hemolymph proteins of various strains. More recently, Abreu-Grobois and Beardmore (1980) found evidence for speciation between population of Artemia and Seidel et al. (1980) showed a variation in the total protein electrophoretic patterns of the same five strains analyzed here.
The total FAME analysis provided an indication of the type of lipids associated with the various strains. A disproportionately high level of FAME for the Italian and a low value for the Utah strains indicated the two extremes. The Italian strain must have contained a higher level of triglyceride lipids per gram sample whereas the Utahan strain probably contains a greater proportion of phospholipids and/or sterol-types lipids.
The loss of the chorion, upon hatching, resulted in a greater amount of lipid material per unit weight nauplii, except in the Italian strain. This would indicate that the chorion of the Italian strain contained more lipids than the shells of the other strains. The relative proportion of fatty acids of the cysts and nauplii remained the same before and after hatching and, when related to the increased lipid and FAME levels in the nauplii, indicate that the shell contains practically no fatty acids, with a possible exception of the Italian strain.
The nutrional quality of the five geographical strains used in this study has been recently determined for several species of marine larvae (John et al.,1978; Beck et al., 1980; klein-MacPhee et al., 1980). John et al. (1980), Working with the larval stages of the mud crab Rhithropanopeus harrisii and the rock crab Cancer irroratus found marked differences in the ability of the geographical strains of brine shrimp nauplii to promote high survival and growth. Crab larvae did not complete larval development when fed SP 1628 or the Utah strain but did so when fed the Brazilian, Australian or Italian strains. Klein-MacPhee et al. (1980) found similar results when winter flounder Pseudopleuronectes americanus larvae were reared using the five geographical strains as food sources. Survival through metamorphosis was high in fish larvae fed Australian, Bruzilian or Italian strains while it was markedly lower for larvae fed SP 1629 or Utah brine shrimp.
Beck et al. (1980), also working with the larvae of a marine fish (Menidia mandia), found varying results. Survival and growth of the fish larvae were dependent on several factors including the previous dietary history of the fish larvae. Differences in survival between the fish fed the various geographical strains were less prominent when all larvae were fed the Brazilian strain rather than the SP 1628 strain prior to the start of the experiment. In an earlier study, Johns et al, (1978) had found that both SF 313 and SF 321 promoted good survival throughout larval development of the mud crab. Beck et al. (1980) also showed that these two strains promoted higher (but not significant) survival in Menidia than menidia the SP 1628 strain.
The relatievely good survival and growth of the marine organisms fed the Italian strain indicates that the lipid levels and energy content are probably adequate. The total values of the five strains were in close agreement with those of Paffenhöfer (1967) who found a level of 5953 calories (2,49 x 104 J) per gram organic substance for an unidentified brine shrimp strain. Because the lipid levels and total energy values for the SP 1628 and Utah strains were greater, all strains were considered sufficient in energy and are probably not a significant contributor to the poorer growth and survival of the SP 1628 and Utah fed organisms.
The fatty acid composition of the nauplii appeared to be much closely related to the biological effects of the five Artemia strains. The fatty acid composition of the strains evaluated here may have been different enough to affect their potential nutritional value. Strains that are higher in 20 5003 (Italy, SF 321, Brazil, and Australia) would presumably be better diets for marine organisms (Owen et al., 1972; Yone and Fujii, 1975; Guary et al., 1976; Watanabe et al., 1978). Furthermore, the high level of 18 : 3ω3 (> 30% of the total FAME) and low level of 20 : 5ω3 in the Utah and SP 1628 nauplii may have induced a nutritional stress in marine consumer organisms.
Watanbe et al. (1978) stated « that the calss of EFA (essential fatty acids) contained in Artemia (principally 20 : 5ω3 and 22 : 6ω3) is the principle factor in the food value of Artemia to fish. This was determined by modifying the fatty acid composition of a Francisco Bay Brand Artemia (1976), deficient in 20 : 5ω3 and 22 : 6ω3, by feeding them either a marine chlorella or a yeast-supplemented squid liver oil, both of which are rich in these long chain ω3 PUFA. The enriched Artemia induced better growth and survival of their test organism, the red sea bream Chrysophys major.
Artemia have generally been shown to contain either a fatty acid predominance of 18 : 3ω3 or 20: 5ω3 (Enzler et al., 1974; Benijts et al., 1976; Gallagher and Brown, 1975; Claus et al., 1977, 1979; Watanable et al., 1978; Fujita et al., 1980). Similar results were obtained in this study. Also, a variation was found in the fatty acid composition of cysts collected within the same year at a similar location (Table III., SF 313 versus SF 321) (personal communication, A Schmidt, 1978).
One other important factor which relates to the lipid character of a food organism is the interaction of lipids with contaminants. This is important because a number of cyst strains are collected from salinas in locations near commercial and agricultural regions (e.g, San Francisco Bay, San Pablo Bay, and Great Salt Lake). An analysis of the five geographical strains for chlorinated hydrocarbons revealed that all are contaminated to some extent; the Italian strain contained more of the DDT family of pesticides, the Utah and SP 1628 contained highe3r levels of dieldrin and the SP 1628 contained substantially higher levels of dieldrin and much higher chlordane levels than the other strains (Onley et al., 1980).
The lipid content of a diet affect the accumulation of pesticides which can thereby alter lipid metabolism. For example, Phillips and Buhler (1979) found that rainbow fed dieldrin-contaminated tubificid worms (15% Lipid) experienced a decrease in lipid accumulation while fish fed an artificial diet (10% lipid) containing dieldrin exhibited norma rates of lipid accumulation. These authors suggested that the increased dietary lipid level created a greater reservoir for pesticide accumulation which, in turn, may have altered lipid matabolism. Further, Durham (1967) has shown that dieldrin affects the metabolism of unsaturated fatty acids and accentuates the symptoms of a deficiency of essential fatty acids.
Considering the biological results of the mud crabs (Johns et al., 1980) and winter flounder (Klein-MacPhee et al., 1980) that were fed SP 1628 and Utahan strains of Artemia, it is possible that the excess 18 : 3ω3 and/or minimal quantities of 20 :5ω3 could result in a nutritional stress which, in the presence of a dietary contaminant, could be manifested in a synergistic fashion. Both a nutrional stress and/or a pesticide contaminant could potentially exert a physiological response which may be elevated to a critical point at the time of metamorphosis. This may explain why the results of the five strains as diet on survival of Atlantic silversides (Beck et al., 1980), which has a much less distinct metamorphosis, was not as dramatic as in mud crab and winter flounder. However, specific investigations will be required to evaluate these conditions.
ACKNOWLEDGEMENTS
This publication is a results of research sponsored by NOAA Office of Sea Grant, Department of Commerce, under Grant no NA 798 AA-D-00096, and the University of Rhode Island Agricultural Experiment Station, contribution no.1921
The authors thank Mr Allan Beck and the marine culture tram at the EPA-Environmental Research Laboratory - Narragansett for suppling the newly hatch nauplii.
LITTERATURE CITED
ABREU-GROBOIS F,A, and J.A, BEARDMORE. 1980. International Study on ArtemiaII. Genetic characterization of Artemia populations - an electrophoretic approach. P. 133 – 146. In : The brine shrimp Artemia Vol. 1, Morphology, Genetics, Radiobiology, Toxicology, Persoone G.P. Sorgeloos, O. Roels, and E.Jaspers (Eds). Universa Press, Wetteren, Belgium . 345 p.
BECK A. D., D.A. BENGTSON ,and W.H.HOWELL. 1980. International Study on Artemia. V. Nutrional value of five geographical strains of Artemia : effects on survival and growth of larval Atlantic silverside Mendia mendia p. 249–259, In : The brine shrimp Artemia Vol. 3, Ecology, Culturing, Use in Aquaculture. Persoone G, P. Sorgeloos, O, Roels and E Jaspers (Eds). Universa Press, Wetteren, Belguim. 456 p.
BENIJTS F., E. VANVOORDEN, and P. SORGELOOS. 1976. Changes in the biochemical composition of the early larval stages of the brine shrine, Artemia salina L.p. 1–9 In : Proc. 10th European Symp. Marine Biology. vol.1. Mariculture, Persoone G. and E. Jaspers (Eds). Universa Press, Wetteren, Belgium. 620 p.
BLIGH E. G. and W.J DYER. 1959. A rapid method of total lipid extraction and purification. Can J. Biochem. Physiol. 37 : 911–917.
BOWEN S. T., J. P. DURKIN, G. STERLING. and L,S; CLARK. 1978 Artemia hemoglobins : genetic variation in parthenogenetic and zygogenetic populations, Biol Bul. 155 : 237–287.
CASTELL. J.D, and J.F. Covey. 1976. Dietary lipid requirements of adults lobsters (Homarus americanus M,E.) J. Nutr, 106 : 1159–1165.
CASTELL J,D,J. LEE , and R.O.SINNHUBER. 1972a . essential fatty acids in the diet rainbow trout (Salmo gairdneri) : lipid metabolism and fatty acid composition. J. Nutr. 102 : 93–99.
CASTELL J.D., R.O SINNHUBER,J.H,WALES, AND D.J.LEE 1972b. Essential fatty acids in the diet of rainbow trout (Salmo gairdneri) : growth, feed conversion and some gross deficiency symptoms. J.Nutr. 102 : 77–86.
CLARK L.S, and S.T. BOWEN. 1976. The genetics of Artemia salina. VII. Reproductive ioslation J,Hered. 67 : 385–388.
CLAUS C.,F.BENIJTS. and P.SORGELOOS. 1977. Comparative study of different geographic strains of the brine shrimp Artemia salina, P. 91–105. In : Fundamental and applied research on the brine shrimp Artemia salina (L) in Belgium. persoone G.and E, Jaspers (Eds). European Mariculture Society Special Publication No.2, EMS. Bredene, Belgium. 110 p.
CLAUS C.F.BENIJTS,G,VANDEPUTTE, and W, GARDNER. 1979. The biochemical composition of the larvae of two strains of Artemia salina (L) reared on two different algal foods J. Exp. mar. Biol, Ecol; 36 : 171–183.
COWEY C.B., J.M. Owen. J,W, ADRON, and C.MIDDELETON. 1976. Studies on the nutrition of marine flatfish. The effect of different dietary acids on the growth and fatty acid composition of turbot (Scophthalmus maximus). Br. J. Nutr. 36 : 479–486.
DURHAM W.F. 1967. The interaction of pesticides with other factors. Residue Review 18: 21–103.
ENZLER L.,V. SMITH.J.S. LINN, and H.S.OLCOTT. 1974. The lipids of Mono Lake brine shrimp (Artemia salina), J, Agr. Food Chem. 22 : 330–331.
FUJITA S.,T. WATANABE, and C. KITAJIMA. 1980 Nutrional quality of Artemia from different localities as a living feed for marine fish from the viewpoint of essential fatty acids. P. 277–290. In: The brine shrimp Artemia, Vol. 3.
C.R. SEIDEL, D.M. JOHNS, P.S. SCHAUER and C.E. OLNEY1
ABSTRACT.:
Nauplii from 4 commercially available geographical collections of Artemia and nauplii hatched from the Reference Artemia Cysts were compared for their effects on survival and growth of Rhithropanopeus harrisii larvae. In addition, nauplii from these sources were analyzed for their fatty acid and chlorinated hydrocarbon contents. Despite differences in the amounts of a few important polyunsaturated fatty acids (18 :3ω3, 20 :5ω3), as well as in the chlorinated hydrocarbon content, there was little variation in the survival and development rates of R. harrisii fed these Artemia sources as food. However, growth of R. harrisit from hatching to megalopa was significantly higher on the strain from France, intermediate in the Reference, Brazil and Chinese strains, and poorest on the Chaplin Lake (Canada) strain. The Reference strain is shown to be one of the better sources of Artemia nauplii with regard to their use in crab culture and therefore represent a good standard for future research studies.
The brine shrimp Artemia is extensively used as food source in the culture of larval fish and crustaceans. Although relatively expensive, Artemia is convenient to use and supports better larval development and survival than other live artificial diets tested (Sulkin and Norman, 1976; Manzi and Maddox, 1980). There are, however, differences in Artemia composition that may alter the nutritional effectiveness of some commercially available sources of Artemia (Olney et al., 1980; Schauer et al., 1980)
The research reported here is a continuation of the effort initiated by the International Study on Artemia (Sorgeloos, 1980a) to characterize the biochemical composition and nutritional performance of commercially available sources of Artemia. Collections of Artemia tested were obtained from the following geographic ares: (1) Lavalduc, France, harvested 1979; (2) Tientsin, People's Republic of China, harvested 1979; (3) Chaplin Lake, Canada, harvested 1979; (4) Macau, Brazil, harvested 1978; (5) Reference Artemia Cysts (RAC), povided by the Artemia Reference Center, Ghent, Belgium. The RAC have been proposed as intercalibration material in studies using Artemia nauplii as food source (Simpson et al., 1980 Sorgeloos, 1980b).
Methods used for the detection of chlorinated hydrocarbons, as well as results of lipid analyses have been reported elsewhere (Olney et al., 1980 Schauer et al., 1980). For both of these analyses samples were run in triplicate.
Newly-hatched zoeae of the mud crab Rhithropanopeus harrisii were used as nutritional bioassay material. Methods for laboratory maintenance of gravid adults, procurement of newly-hatched zoeae and the experimental design used in this have been described in detail by Johns et al. (1980).
The present study provides further information on variability in the biochemical compositions and food value between different geographical sources of Artemia. Differences were found in fatty acid composition, total lipid content and chlorinated hydrocarbon contamination levels. The most noteworthy differences between the 5 Artemia sources were the higher level of 18 : 3ω3 in the Canadian and French strains and the high level of 20 : 5ω3 in the Chinese strain (Table 1). All strains contained substantial levels of 20 : 5ω3. (>8%). The total lipid levels of RAC, Brazilian and Chinese nauplii were higher (>200 mg g-1, dry wt sample) than the level found in the French and Canadian population (< mg g-l, dry wt sample).
All values for chlorinated hydrocarbons were below 100 ppb, except for the total DDT's in the Chinses sample (172 ppb; Table 2). Lowest levels of CHC were found in RAC and Brezilian collections; French and Chinese Artemia were approximately 4 to 6 times more contaminated. Despite such chemical variation no significant differences were found in the ability of the various Artemia sources to support survival and development rate of R.harrisii larvae (Table 3).
Growth rates, however, were significantly higher in crab larvae fed nauplii from the French source; the poorest growth was found in crab larvae fed Canadian brine shrimp. Growth was fastest on the French strain which contained one of the lowest lipid levels, therefore it'appears that lipid levels were adequate in all Artemia strains. In addition, growth did not appear to be affected by the moderately high levels of contaminants found in the French strain. Although no significant differences were found in survival and developmental rates, the lowest mean values for survival and growth and the slowest development rate occured with mud crab larvae fed Canadian brine shrimp nauplii These trends suggest that the Canadian sources of Artemia may be less effective in culturing larvae of R. harrisii
The value of studies such as this would be of little interest if a single, reliable source of Artemia was commercially available. This has not been the case (Sorgeloos, 1980a). For example, Artemia from Macau, Brazil, which had been identified as one of the better food sources for a large variety of marine fish and crustacean larvae (Beck et al., 1980; Johns et al., 1980, 1981; Klein-MacPhee et al., 1980) are presently not commercially available (P,Sorgeloos, pers, comm.) The ISA series of studies, however, have highlighted major differences in the nutritional effectiveness of other commercially available sources of this indispensible source of food for marine freshwater organisms (Sorgeloos et al., 1980a).
The use of Artemia of inferior quality could be an unexpected and confounding sources of variation in experimental results. Although there is no ready solution to this problem, the use of RAC in experiments can give researches a relative indication of the nutritional effectiveness of other Artemia sources they are using in the laboratory. Nauplii of the RAC have been tested for their nutritional performance in the culture of larvae fish (Klein-MacPhee et al., in press) and crabs and have been found to be one of the better Artemia source. Thus far tested by the ISA. The use of RAC as an intercalibration food source in experiments could reduce number of incorrect inferences caused by the poor nutritive value of an uncharacterized laboratory diet.
Aknowledgement. We acknowlege the technial support of W. Berry for maintenance of crab larvae and Artemia
Table 1. Artemia ssp. weight percent fatty acid composition of various geographical colections of newly-hatched nauplii nd:
| FAME | RAC | Brazil 1 | Canada | China | France |
| 14:0 | 1.79 | 1.57 | 0.83 | 1.80 | 1.73 |
| 14:1 | 2.92 | 0.81 | 1.67 | 2.24 | 3.03 |
| 16:0 | 12.70 | 15.42 | 9.99 | 11.40 | 11.90 |
| 16:1ω7 | 16.78 | 10.79 | 9.03 | 19.06 | 11.34 |
| 16:3ω4/17:1ω8 | 4.33 | 3.88 | 1.47 | 2.54 | 2.20 |
| 18:0 | 4.07 | 2.79 | 5.12 | 3.99 | 4.21 |
| 18:1ω9 | 30.37 | 35.89 | 28.24 | 26.81 | 24.73 |
| 18:2ω6 | 9.62 | 9.59 | 7.95 | 4.68 | 6.14 |
| 18.3ω3 | 2.55 | 4.87 | 19.87 | 7.38 | 20.90 |
| 18:4ω3 | nd | 0.96 | 1.60 | 1.26 | 2.04 |
| 20:2ω6/20:3ω6 | 0.20 | 2.82 | 0.44 | 0.15 | 1.13 |
| 20:3ω3/20:4ω6 | 5.82 | nd | 4.21 | 3.34 | 2.45 |
| 20:5ω3 | 8.45 | 8.98 | 9.52 | 15.35 | 8.01 |
| Total % | 99.60 | 98.37 | 99.94 | 100.00 | 99.81 |
| Total lipid | |||||
| mg g-1 dry wt. | 209.4 ± 24.0 | 2020 ± 8.0 | 142.9 ± 34.0 | 201.7 ± 0.3 | 152.1 ± 29.0 |
| 1 Data from schauer et al. (1980); 0.67% | 15:0 and 0.52% | 20:1 9 were also present | |||
Table 2. Artemia ssp. chlorinated hydrocarbon content of verious geographical collections of newly-hatched nauplii. results expressed as ng g-1 wet weight (ppb) nd not deteceted
| RAC | Brazil1 | Canada | France | China | |
| HCB | 0.3 | 0.1 | 0.3 | 1.8 | 97.0 |
| PCB1016 | 1.0 | 5.3 | 6.2 | 8.6 | 6.3 |
| PCB1254/1260 | 0.2 | 1.6 | 5.6 | 32.0 | 43.0 |
| ΣPCB's | 1.2 | 6.9 | 12.0 | 41.0 | 49.0 |
| ppDDE | 1.4 | 1.2 | 3.0 | 14.0 | 85.0 |
| ppDDD | 0.4 | 0.4 | 0.4 | 3.8 | 22.0 |
| opDDT | nd | 0.4 | nd | nd | 0.9 |
| ppDDT | 0.3 | 1.9 | nd | 7.1 | 64.0 |
| ΣDDT's | 2.1 | 4.3 | 3.4 | 25.0 | 172.0 |
| αBHC | 0.2 | 1.1 | 1.6 | 0.3 | 23.0 |
| γBHC | nd | 0.8 | nd | 2.2 | 16.0 |
| t-Chlorodane | 0.1 | nd | 0.1 | 0.3 | 0.4 |
| c-Chlorodane | nd | 0.1 | nd | 0.4 | 0.2 |
| 1 Data from Olney et al (1980) | |||||
Table 3. Rithropanopeus harrissii. Summary of culture data for larvae fed various geografical collections of newly-hatched Artemia ssp. Data presented as mean± one standard deviation. Means having the same grouping letter are not significantly different (P> 0.05) (n) sample size
| Artemia source | Survival to megalopa (%) | (n) | Grouping | Developpement time to megalopa (d) | (n) | Grouping | Megalpa dry weight (μg) | (n) | Grouping |
| Frech | 89 ± 13 | 60 | A | 11.1 ± 02 | 53 | A | 181 ± 10 | 53 | A |
| Reference | 89 ± 10 | 60 | A | 10.9 ± 0.2 | 50 | A | 166 ± 13 | 50 | B |
| Brazilian | 85 ± 10 | 60 | A | 10.7 ± 0.3 | 53 | A | 161 ± 13 | 53 | B |
| Chinese | 84 ± 17 | 60 | A | 10.9 ± 0.3 | 51 | A | 168 ± 27 | 51 | B |
| Canadian | 72 ± 25 | 60 | A | 11.6 ± 0.6 | 43 | A | 144 ± 18 | 43 | C |
LITTERATURE CITED
BECK, A.D., BENGTSON, D. A., HOWELL, W.H. (1980). International Study on Artemia V. Nutritional value of five geographic strains of Artemia: effects of survival and growth of larval Atlantic silversides, Menidia menidia In : persoone,G., Sorgeloos, P., Roels, O., Jaspers, E.(eds) The brine shrimp Artemia, Vol. 3, Ecology, culturing, use in aquaculture, Universa Press, Wetteren, Belguim, pp, 249–259
JOHNS, D.M., BERRY., W. J. WALTON, W. (1981). International Study on Artemia XVI. Survival, growth and reproductive potential of the mysid, Mysidopsis bahia Molennock fed various geographical collections of the brine shrimp, Artemia J, exp. mar. Biol. Ecol, 53: 209–219
JOHNS, D.M., PETERS; M.E., BECK, A.D. (1980). International study on Artemia VI. Nutritional value of geographical and temporal strains of Artemia effect on survival and growth of two species of brachyuran larvae. In: Persoone, G., Sorgeloos, P., Roels, O., Jaspers, E. (eds). The brine shrimp Artemia, Vol. 3, Ecology, culturing, use in aquaculture Universa Presse, Wetteren, Belgium, pp. 291–304
KLEIN-MACPHEE, G., HOWELL., W.H., BECK, A.D. (1980). International Study on Artemia VII Nutritional value of five geographical strains of Artemia to winter flounder ( Pseudopleuronectes americanus), In; Persoone, G., Sorgeloos, P., Roles, O. Jaspers, E. (eds) The brune shrimp Artemia, Vol. 3, Fcology, culturing use in aquaculture Universa Press, Wetteren, BNelgium, pp. 305–312
KLEIN-MACPHEE, G., HOWELL, W.II., BECK., A.D. (In press). Comparison of a reference strain and four geographical stains of Artemia as food for winter flounder (Pseudopleronectes americanus) larvae Aquaculture.
MANZI, J.J., MADDOX, M.B.(1980). Requirements of Artemia nauplii in Macrobrechium rosenbergii (de Man) larviculture, In; Persoone, G., Sorgeloos, P., Roels, O., Jaspers, E.(eds) The brine shrimp Artemia, Vol. 3, Ecology, culturing, use in aquaculture. Universa Press, Wetteren, Belgium, pp. 313–330
OLNEY, C.E, SCHAUER, P. S., MCLEAN, S., LU, Y., SIMPSON, K.L. (1980). International Study on Artemia VIII. Comparison of the chlorinated hydrocarbons and heavy metals in five different strains of newly hatched Artemia and a laboratory-reared marine fish In: Persoone, G., Sorgeloos, P., Rocls, O,. Jaspers, E, (eds). The brine shrimps Artemia, Vol, Ecology, culturing, use in aquaculture, Universa Press, Wettern, Belgium, pp. 343–352
SCHAUER, P, S., JOHNS, D.M., OLNEY, C.E., SIMPSON, K.L. (1980). International Study on Artemia IX, Lipid level energy content and fatty acid composition of the cysts and newly hatched nauplii from five geographical strains of Artemia In; Persoone, G., Sorgcloos, P., Roels, O., jaspers, E(eds.) The brine shrimp Artemia, Vol, 3, Ecology, culturing, use un aquaculture. Universa Press, Wetteren, belgium, pp. 365–373
SIMPSON, K.L., NECK, A. D., SORGELOOS, P. (1980), Workshop I. Characterization of Artemia strains for application in
BY E. J. S. PILLA
HOW DO WE DEFINE AN ARTEMIA BISEXUAL SPECIES IN GENETIC TERMS ?
- FEW DAIGNOSTIC CHARACTERS, WHICH MAY BE NOT UNIVERSAL FOR THE SPECIES AS A WHOLE:
CYTOLOGY
REPRODUCTIVE ISOLATION
MORPHOLOGICAL DIFFERENTIATION
MORPHOMETRIC DIFFERENTIATION
GENETIC DIFFERNTIATION:
- NUCLEAR DNA (ALLOZYMES, REPETITIVE SEQUENCES)
- MITOCHONDRIAL DNA (RFLP, GENE ORGANISATION)
LIFE HISTORY TRAITS (NO OFFSPRING, % CYSTS, CYST SIZE, etc)
BIOCHEMECAL FEATURES (e.g. H.U.F.A; CONTENTS)
PHYSIOLOGICAL FEATURES (e.g ADAPTATION TO SPECIFIC WATER COMPOSITION)
MOST PRUDENT IS TO USE A RANGE OF FACTORS
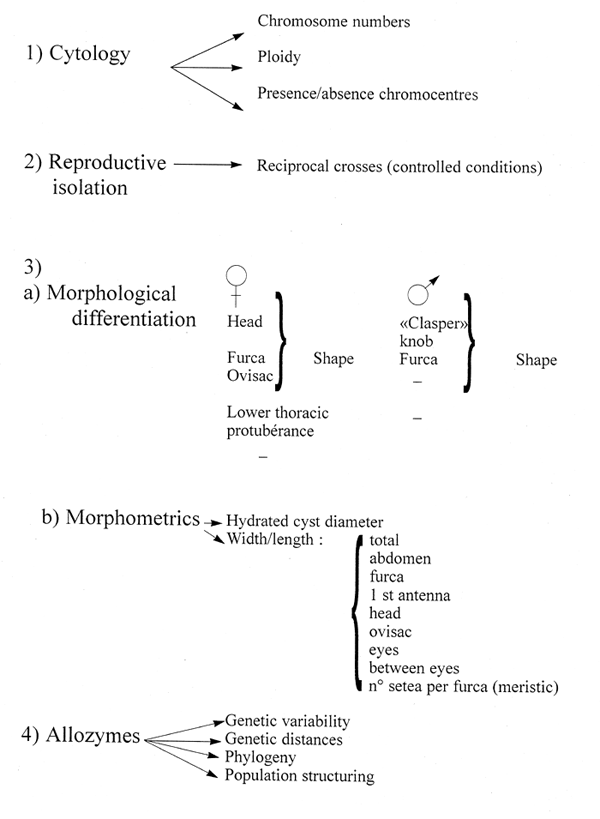
| • | OLD WORLD (EURASIA AND AFRICA): ESTIMATED CIRCA 70% OF POPULATIONS ARE PARTHENOGENETIC, REMAINING (INCLUDING A. TUNISIANA ARE BISEXUAL. |
| • | ARTEMIA TUNISIANA - ANCESTRAL, SPECIES TO OLD WORLD BISEXUAL (e.g.A. URMIANA AND A. SINICA WHICH GAVE RISE TO PARTHENOGENETIC FORMS) |
| • | ONLY BISEXUAL SPECIES, TO DAT, WITH DOCUMENTED CONSPICUOUS NATURAL COEXISTENCE WITH OTHER (PARTHENOGENETIC) SPECIES |
| • | MEDITERRANEAN BASIN: NORTH AFRICA (TUNISIA, ALGERIA, EGYPT, LIBYA?, MOROCCO?), CYPRUS, SPAIN (INCLUDING BALEARIC ISLES) ITALY (INCLUDING SARDINIA AND SICILY), SOUTHERN AFRICA? |
| • | ENDURANCE OF TUNISIANA INTERESTING PHENOMENON. |
| • | NO WIDE COMMERCIAL USE. HOWEVER, |
| • | BIOLOGICAL PROPERTIES OF POTENTIAL USE IN AQUACULTURE. |
| • | POPULATIONS STUDIED TO DATE DISPLAY SOME USEFUL FEATURES FOR PRODUCTION AND USE IN AQUACULTURE: |
| - | WELL ADAPTED TO LOW TEMPERATURES (e.g. 15°C) |
| - | HIGH FEMALE ENCYSTMENT RATES (85–100%) |
| • | DRAWBACKS TO BE ADDRESSED: |
| - | LOW REPRODUCTIVE OUTPUT |
| - | SHORT REPRODUCTIVE CYCLE (BUT ONLY SIGNIFICANTLY SO WHEN COMPARED WITH, IN THE OLD WORLD, POLYPLOID PARTHENOGENS) |
| CYTOLOGY | |
| • | MAJORITY OF POPULATIONS ANALYSED (HOWEVER, ONLY ITALIAN AND SPANISH POPULATIONS LOOKED AT) FOR NAUPLII MITOSES 2N= 42, BUT CONSPICUOUS 2N=44 AND 40,44,46,48 AND 50. |
| • | HETEROPLOIDY/ANEUPLOIDY NOT CONFIRMED IN ADULT POPULATION. PHENOMENON LIMITED TO LARVAL STAGE? |
| • | CHROMOSOME NUMBERS DO SEEM TO INFLUENCE INTRA OF INTRPOPULATION FERTILITY |
| • | LACK OF CHROMOCENTRES (IN COMPARISON WITH A. FRANCISCANA) |
| - | USEFUL DISCRIMINATORY TOOL TO ASSESS EXTENT OF DISSEMINATION OF INTRODUCED A. FRANCISCANA, ESPECIALLY IN NORTH AFRICA |
Summary of the genetic variability in bisexual populations of Artemia
| Population | Mean sample size (s.c.) | Mean № alleles per locus (s.e.) | % polymorphic loci* | H (s.e) | H (s.e) |
| A. franciscana | 65.7 | 1.4 | 30.0 | 0.049 | 0.058 |
| (SFB) | (1.3) | (0.1) | (0.027) | (0.031) | |
| A. tunisiana | 64.7 | 1.6 | 35.0 | 0.057 | 0.075 |
| (TUN) | (2.6) | (0.2) | (0.028) | (0.039) | |
| Artemia sp. | 61.7 | 2.0 | 60.0 | 0.096 | 0.108 |
| (RUS) | (1.4) | (0.2) | (0.033) | (0.037) | |
| Artemia sinica | 66.3 | 2.0 | 55.0 | 0.097 | 0.097 |
| (SIN) | (2.0) | (0.3) | (0.033) | (0.033) | |
| Artemia umiana | 78.9 | 2.3 | 65.0 | 0.080 | 0.096 |
| (URM) | (1.8) | (0.3) | (0.024) | (0.028) | |
| Artemia sp. | 49.0 | 1.9 | 55.0 | 0.084 | 0.095 |
| (YIM) | (0.4) | (0.2) | (0.035) | (0.037) |
*0.99 criterion..
** = unbiased eslimate (see Nei, 1978).
ALLOZYME DATA
GENETIC DIFFERENCTIATION
• MEAN CONSPECIFIC GENETIC DISTANCES
ALLOZYME FREQUENCIES ARE CONVERTED INTO A MEASURE OF DIVERSITY BETWEEN POPULATIONS AND SPECIES:
A. TUNISIANA (7 populations): MEAN D= 0.091 (+0.061)
• Other bisexual spp.:
A. FRANCISCANA (21 pup.): MEAN D= 0.126 (+0.067)
A. SINICA (6 POPULATIONS): MEAN D=0.014 (+0.001)
• MEAN CONGENERIC GENETIC DISTANCES
| OLD WORLD Vs. NEW WORLD | |
| A. TUNISIANA VS. A. FRANCISCANA | D= 1.501 (+0.355) |
| NEW WORLD Vs. NEW WORLD | |
| A. FRANCISCANA Vs NEW WORLD | |
| A. FRANCISCANA Vs. A. MONICA | D = 0.098 (+0.050) |
| A. FRANCISCANA Vs A. PERSIMILIS | D = 1.073 (+0.299) |
| OLD WORLD Vs. OLD WORLD | |
A. TUNISIANA Vs. A. URMIANA | D = 0.664 (+ 0.154) |
A. TUNISIANA Vs. A. SINICA | D = 0.868 (+0.206) |
A. SINICA Vs A. URMIANA | D = 0.355 (+0.045) |
REPROCUCTIVE ISOLATION
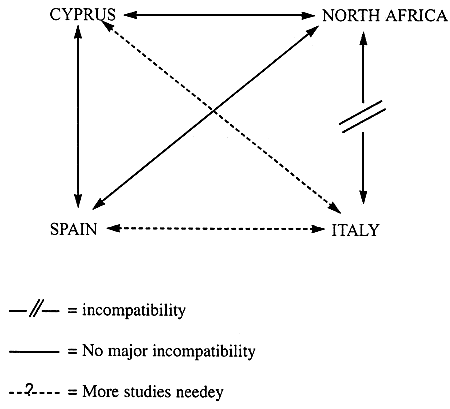
NO COMPREHENSIVE STUDY INTO FERTILITY OF A. TUNISIANA PERFORMED TO DATE
SOME SIGNS OF MINOR AND MAJOR GENETIC INCOMPATIBILITY (e.g. BETWEEN ITALIAN AND TUNISIAN BRINE SHRIMP: SAN BARTOLOMEO + TRAPANI Vs. CHOTT ARIANA + SFAX)
INFERTILITY COULD BE THE RESULT OF «RING SPECIES» PATTERN OF DIFFERENTIATION
MORPHOLOGY AND MORPHOMETRICS
• MORPHOLOGY:
- MORE USEFUL IN CONGENERIC (i.e. BETWEEN SPECIES) TERMS,
ABSENCE OF LOWER THORACIC PROTUBERANCE IN A. TUNISIANA
FEMALES, AND PRESENCE IN FEMALES OF OTHER SPECIES
MORPHOLOGY OF MALE CLASPER KNOB REQUIRES DETAILED (S.B.M.) ANALYSIS
FURCA SHAPE AND NUMBER OF SETAE SUBJECT TO SALINITY CHANGES
• MORPHOMETRICS:
- MORE USBFUL FOR STUDYING VARIATION BETWEEN POPULATIONS OF THE SAME SPECIES (CORRELATING WITH OTHER DATA)
GENETICS
* ALLOZYMZ DATA
GENETICS VARIABILITY
| A. TUNISIANA | |||
| SITE | Alleles/ locus | Polymorphic loci (%) | Heterozygosity (%) |
| Barbarena | 1.41 | 36.4 | 12.6 |
| (Spain) | (± 0.18) | (± 4.9) | |
| Salin di | 1.46 | 27.3 | 9.9 |
| Poetto (italy) | (± 0.24) | (± 4.3) | |
| Chott Ariana | 1.32 | 31.8 | 9.3 |
| (Tunisia) | (± 0.14) | (± 3.7) | |
| Larnaca | 1.17 | 44.4 | 6.3 |
| (Cyprus) | (± 0.17 | (± 3.9) | |
| San Felix | 1.36 | 27.3 | 8.5 |
| (Spain) | (± 0.17) | (± 3.6) | |
| San Pablo | 1.43 | 38.1 | 12.5 |
| (Spain) | (± 0.23) | (± 5.4) | |
| Santa Pola | 1.18 | 22.7 | 7.4 |
| (Spain) | (± 0.10) | (± 3.6) | |
| Quartu | 1.33 | 33.0 | 9.0 |
| (ltaly) | (± 0.18) | (± 4.0) | |
| AVERAGE | 1.60 | 35.0 | 7.5 |
| (± 0.20) | (± 3.7 | (± 3.9) | |
• POPULATION SUBSTRUCTURING
FROM ALLOZYME SURVEY (FEQUENCIES), IT IS POSSIBLE TO ESTIMATE AMOUNT OF GENETIC VARIATION ATTRIBUTABLE TO POPULATION SUBDIVISION (i.e., RELATIVE DIFFERENCES BETWEEN POPULATIONS OF A SPECIES) BY USING A STATISTIC KNOWN AS Fst, AND TO TEST WHETHER IT IS SIGNIFICANTLY DIFFERENT FROM ZERO.
A. TUNISANA (7 POPULATIONS) Fst = 0.117 (+0.118)
In other words, 12% of total genetic variation is due to population substructuring (Not significantly different from 0)
• Other bisexual spp.:
AFRANCISCANA (21 POPULATIONS): Fst = 0.240 (+ 0.045)
24% of total genetic variation is due to differences between populations (P<0.05)
A. SINICA (6 POPULATIONS): Fst = 0.064 (+ 0.010)
Differences between populations account only for 6% of total variations (not significantly different from 0)
ALTHOUGH ARTEMIA POPULATIONS CLEARLY DO NOT FORM CONTINUOUS DEMES, IT IS IMPORTANT TO ESTIMATE HOW MUCH MIXING MAY BE OCCURING BY BIRDS, MAN, WIND, ETC
• GENE FLOW/MIGRATION
Fst FIGURES FOR EACH SPECIES MAY BE CONVERTED INTO MEASURES OF GENE FLOW BETWEEN POPULATIONS (MAKING SOME ASSUMPTIONS ON PATTERNS OF GENE FLOW). Nm = NUMBER OF MIGRANTS PER GENERATIONS.
• TO PREVENT POPULATIONS FROM DIVERGING COMPLETELY, Nm NEEDS TO BE> 1.
A. TUNISIANA Fst=0.117
Nm=1.88 (i.e. MIGRATION OF THE ORDER OF 2 INDIVIDUALS PER GENERATION)
• Other bisexual spp.:
A. FRANCISCANA Fst = 0.24
Nm = 0.8 (i.e. MIGRATION OF LESS THAN 1 INDIVIDUAL PER GENERATION)
(HENCE INCIPIENT SPECIATION)
A. SINICA Fst=0.064
Nm = 3.65 (i.e. CIRCA 4 INDIVIDUALS PER GENARATION)
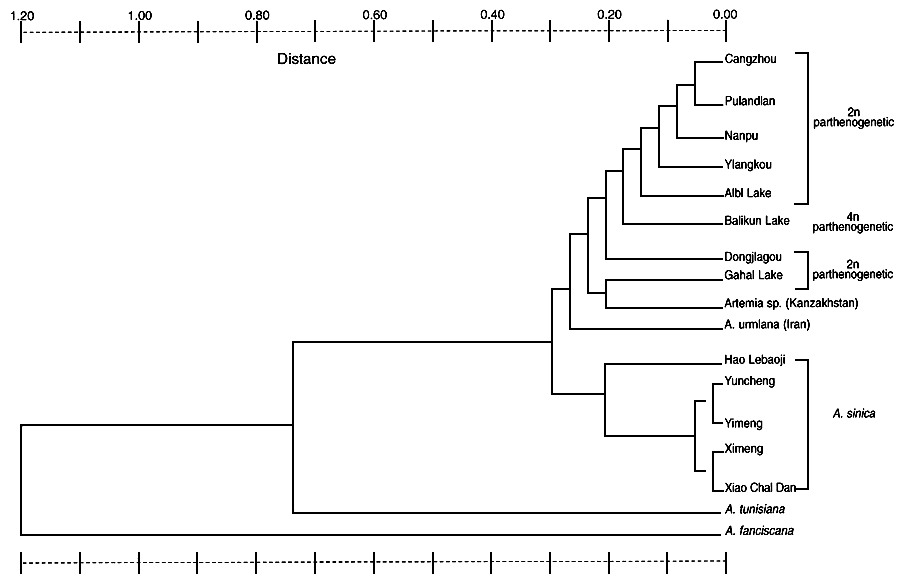
UPGMA DENAOGRAM OF NER'S GENETIC DISTANCE FOR BISEXUAL AND PARTNENOGENETIC SPECIES OF ARTEMIA
P. LEGER, D. A. BENGTSON, P. SORGELOOS,
K.L. SIMPSON and A.D.BEEK
INTRODUCTION
Successful rearing of larval stages of aquatic organisms is aa challenger for aquarium hobbysts, a tool for aquatic ecologists and ectoxicologists, and necessity for the aquculturists. All these people agree the primery problem in any type of larval rearing is that of food. Ideally, one would prefer to feed larvea their natural diet, which is usually characterized bay a wide diversity of nutritious live organisms.
Although not a «natural» food, Artemia have been successfully that such food could be attained with a food from such unusual (i.e., hypersaline) environment. Some recent experiences suggest that the use of Artemia does not absolutely guarantee success (see reviews by Sorgeloos, 1980, and Simpson et al., 1983). Explantions for and remedies to this variable success will be covered in this review through an analysis of the larval organism's requirements for food (see Fig. 1). A more complete review of the nutritional value of Artemia is presented by leger et al.
PRATICAL REQUIREMTNS FOR THE CULTURIST
A food organism must first meet the nutrional needs of the predator. In addition, other practical requirement have to be met to satisfy the culturist. The consistent availability of food organisms is of utmost importance for continuous cultures. In this respect, Artemia is superior to all other live foods since it is available as an off-the -shelf food in the form of dormant cysts. From those cysts, nauplii are obtained through simple hatching procedures (Sorgeloos et al., 1983). Ideally, a food organism should also be hardy and easily cultured. Artemia nauplii fulfill this requirement quite well, since they are very tolerant to various culture environments, resistant to even rough handling and may be disinfected resulting in a biologically uncontaminated live food (Sorgeloos et all., 1983).
The wide size range of Artemia and their different physical forms (Fig. 2) make them very versatile in use. Since they are easily cultured, Artemia nauplii and later stages may be fed according to the changing needs of the predator during its development. Also a smaller food particle may be used in the form of decapsulated cysts, which are some 50% smaller than freshly hatched nauplii and have several other advantages: a) they are disinfected and separated from the cyst shells during the decapsulation process (Sorgeloos et al., 1977, b) hatchability of the embryos is improved (Bruggemann et al., 1980), so that otherwise nonhatchable cysts increase in value, c) the energy content is higher (Vanhaecke et al., 1983), leading to a higher naupliar biomass production per gram of cysts and a smaller, more energy-rich food particle for the larval organism.
A last example of the versatility of Artemia as a food is the possibility of using Artemia (nauplii or adults) as a carrier for components which are otherwise difficult to administer to fish and crustacean larvae. Indeed, essential nutrients, pigments, prophylactics and therapeutics may be bioencapsulated in Artemia and introduced to the consumer organism (see Fig. 3; Leger et al., 1985).
PHYSICAL REQUIREMENTS
Physically, a food organism has to be clean, free from alien organisms and materials and especially free
from contagious diseases. Artemia may be disinfected and fed as a clean food, although cysts are often
heavily loaded with microorganisms. Austin and Allen (1981) did not find an intimate microbial contamination
of the nauplii and they demonstrated that bacteria surrounding the nauplii may be easily removed
by simple washing procedures or even better by disinfecting the cysts prior to hatching incubation
by a dipping procedure. No direct evidence exists for Artemia-borne infections in larvae.
A second physical requirement is that a food organism has to be accepted by the predator.
Acceptablity of food is determined by several factors. The bright color of Artemia nauplii and their
continuous movement make them easily perceptible. Perceptibility may be enhanced further by staining
techniques, as demonstrated for sole larvae (Dendrinosn et al., 1984. Artemia nauplii are easily
caught because they lack an effective escape response. Palatability is apparently adequate, since
Artemia is often used as a gustatory attractant in artificial diets (Barahona-Fernandes et al., 1977;
Gatesoupe And Luquet, 1981/1982). Ingestibility of a palatable food is governed primarily by its size;
Size of Artemia nauplii is therefore the first important consideration. Indeed, most first-feeding marine
fish species and some decapods, such as penaeids, can not ingest (or handle) Artemia nauplli. Even
species which may accept some Artemia nauplii as a first food sometimes face ingestion and preyhandling
problems. Vanhaecke and Sorgeloos (1980) have demonstrated considerable variation in
naupliar size (422 – 517 m) and volume (7.638 – 13.604 • 106 m3) among different geographical
strains. The effect of size in feeding nauplii tofish to fish has been described by Beck and Bengtson
(1982). They fed freshly nauplii from eight different Artemia strains to Atlantic siverside (Menidia
menidia) larvae. The correlation between size of nauplii and mortality of the fish larvae indicated that
at least 20% mortality could be expected when nauplii larger than 480 un were fed as a first food.
NUTRITIONAL REQUIREMENTS
In addition to physical requirements, a food organism also has to meet certain nutritional requisites, including digestibilty. Watanabe et al. (1978a) found high digestibility rates for Artemia fed to carp and rainbow trout and reported high values for net protein utilization and protein efficiency ratio. Enzymes such as amylase and trypsin that are found in Artemia (Samain et al., 1980) may also play an important role in enzymatic autolysis during the transit of the nauplii through the larval gut, ant thus contribute to digestion.
Even when easily digested, a food organism still may not meet the nutrient requirements of the predator. The main problem in evaluating Artemia in this respect is our lack of knowledge of the nutrient requirements of most predators to which artemia are fed. A proximate analysis of Artemia (Table I) reveals an equilibrated high protein diet indicating that macronutrient requirements probably are satisfied for most predators. However, several investigators report considerable variation in larval culture success (review in Leger et al., 1986).
Variation in larval growth rate has been attributed to significant differences in individual energy
content (0.0366–0.0725 J) and dry weight (1.61 – 3.33 ug) of LArtemia nauplii from different geographical
origin (Vanhaecke et al., 1983). Selection of high energy strains is therefore recommended;
Varying growth rates may also result form the use of older unfed instar stages which contain up
to 39% less energy and 34% less dry weight than freshly hatched nauplii (Fig. 3) (Vanhaecke et al.,
1983). This energy and dry weight loss can be avoided by storing instar I nauplii at lower temperatures,
since they survive well for 24 hr at 2–4°C without significant losses in dry weight (Leger et
al., 1983). This cold storage technique further allows a complete automation of feeding permitting
a 24 hr feeding of energy-rich instar I. Starved nauplii not only contain less energy and dry weight,
which may be less able to meet the requirements of the predator, but they are also less visible, larger
and faster swimming, so their acceptablility is reduced. Starved nauplii have a lower free amino
acid content (Dabrowski and Rusiecki, 1983), which may reduce their digestibility. All these negative
factors may be reflected in poor growth of the larval predator.
Decapsulated cysts, on the other hand, constitute the highest energy
Artemia form and are more favorable for use except when a predator feeds only on moving prey.
Besides variable growth rates, other problems have been attributed to the use of Artemia. A realationship has been found between the use of particular geographical strains of Artemia and the appearance of various symptoms in fish and crustacean larvae, such as lethargy, lack of coordination, abnormal development, problems at metamorphosis, abnormal pigmentation, and even mortality (Wickins, 1972; Campillo, 1975; Beck et al., 1980; Johns et al., 1980; Klein-MacPhee et al., 1980, 1982). Several authors have tried to explain these observations and they have formulated diverging and sometimes contradictory explanations. For example, Bookhout and Costlow (1970) suspected that high levels, of DDT caused the problems, but Wickins (1972) research suggested that a nutritional deficiency was involved.
INTERNATIONAL STUDY ON ARTEMIA
The elucidation of the nutritional variability of Artemia was one of the major concerns of the participants in the International Study on Artemia (ISA) (Sorgeloos, 1980). Nutritional bioassays with several larval fish and crustacean species fed various Artemia strains confirmed previous reports of varibility in food value among different strains of Artemia (Table II). However, this variability was not a factor in culture tests with freshwater fish larvae. Major problems seemed to occur only in tests with marine species. Certain Artemia strains guarranteed good culture success for all marine species tested (Brazil, San Francisco Bay and Reference Artemia). The 1978 batch San Pablo Bay (SPB) Artemia nauplii, on the other hand, were consistently poor for all species. Utah Artemia in some cases gave poor culture results, whereas intemediate success was obtained with the Canadian strain. Generally good results were obtained with the Australian, Chinese, French and Italian strains, except for Atlantic silverside larvae, which, as mentioned before, were adversely affected by the large naupliar size. Major problems seemed to occur primarily when SPB and Utah naupliar size. Major problems seemed to occur primarily when SPB and Utah nauplii were fed to marine organisms, especially to those species, such as crab and flatfish, whose larvae undergo a pronounced metamorphosis. In those species almost all mortality was suffered at the onset of matamorphosie (Figs. 4 and 5). This information coincides with numerous reports from authors culturing decapod and flatfish larvae, such as crab, turbot and plaice (review in Leger et al., 1986).
The ISA has developed various correlations to try to explain strain differences in nutritional value, based on results of the bioassays and the chemical and biochemical analyses of the strains. Abnormalities, effects on growth, and mortality may be an expression either of nutrient deficiency of toxicity. For this reason a detailed analysis of chlorinated hydrocarbons (CHCs) was carried out on the Artemia strains used in the bioassays (Olney et al., 1980; Seidel et al., 1982). The most heavily contaminated strains, in terms of total CHSs, (the Italian and chinese strains) however gave excellent results in the bioassay. On the other hand, Utah ranked among the cleanest strains. The only similarity between SPB and Utah samples was their higher level of dieldrin. Furthermore, SPB 1628 had the highest level of chlordane and high molecular PCBs. Follow-up studies by Johns et al. (1981) and McLean et al. (1985) have demonstrated that Brazilian nauplii purposely contaminated with the suspected CHCs did not cause mortality in crab larvae or post-metamorphic flounder, although growth was reduced in flounder fed on Artemia with moderate levels of CHSs. CHSs therefore were probably not a principal factor controlling the dietary value of the Artemia strains tested. Heavy metals were also probably not significant factor since no metal common to SPB and Utah was present in dramatically higher levels (Olney et al., 1980). The prescence of toxic materials in Artemia is still a concern, however, because CHS and heavy metal contamination is anthropogenic and thus subject to variations. For example, copper levels have been shown to vary considerably in Utah Artemia (Blust, personal communication) and very high pesticide levels have been reported in a batch of Philippine Artemia (Simpson et al., 1983). Shelbourne (1968) hypothesized that Utah Artemia may have accumulated toxins produced in dinoflagellate blooms, but measurable amounts of paralytic shellfish poison have not been detected in SPB 1628 (Olney et al., 1980). Apparently, differences in nutritional value between the Artemia sources tested are not related to presence of toxic contaminants.
The hypothesis that nutrient deficiency mayt explain nutritional variablility is borne out by the difference in results of feeding Artemia strains to freshwater and marine species, which indeed have different dietary requirements. In-depth biochemical profiles of the different strains tested showed that differences in amino acid profile coud not explain differences in culture results (Seidel et al. 1980). All strains were found to meet the essential amino acid requirements for chinook salmon, though nethionine appeared to be the first limiting amino acid. Similarly, the varying culture success could not be explained by differences in carotenoid (Schauer et al., 1980), mineral (Watanabe et al., 1978a), caloric or lipid content (Schauer et al., 1980). However, pronounced differences were found in fatty acid profiles. As compared to other strains, Utah espcially SPB 1628 Artemia contained high levels of 18 : 3ω3 and particularly low levels of 20 : 5ω3 (Schauer et al., 1980; Seidel et al., 1982). The relative lack of 20 : 5ω3 may explain the poor results in feeding the Utah and SPB strains to marine organisms. The highly unsaturated fatty acid (HUFA) 20 : 5ω3 known to be essential for marine fish and crustacean larvae (Teshima, 1978; Yone, 1978; Kanazawa et al., 1979). Canadian nauplii, in spite of hight levels of 20 : 5ω3, provided intermediate results in the bioassays, which indicates that for this strain other factors, possibly energetic ones, may be involved.
IMPORTANCE OF ESSENTIAL FATTY ACIDS
Leger et al., (1985c) further studied the relationship between 20 : 5ω3 level and nutritional value of Artemia by evaluting different batches of the San Francisco Bay strain together with Reference Artemia and SPB 1628 as, respectively, positive and negative control. Levels of 20 : 5ω3 varied considerably among different batches within the same strain. Batches with high 20 : 5ω3 levels yielded high biomass production in culture tests with mysids, while batches with low 20 : 5ω3 levels consistenly yielded less biomass (Fig. 6). CHC analyses were also performed on these different batches and again considerable differences were found, but could not be correlated with the biomass figures (Fig. 7) from these studies we may conclude that the content of the essential fatty acid 20 : 5 oo3 seems to be most important factor determining the nutritional value of Artemia nauplli to marine organisms. This is supported by the observation that Utah and San Pablo Bay nauplii provided good survival of freshwater fish which do not require highly unsaturated fatty acids such as 20 : 5ω3 in their diet.
Watanabe et al. (1978) classified Artemia strains into marine type Artemia, which contain high levels of 20 : 5ω3, and freshwater type Artemia, which contain low levels of 20 : 5ω13. They obtained good survival of red seabrean larvae fed with the marine type nauplii and poor survival of those fed the freshwater type. However, when the freshwater type nauplii were fed on 20 : 5ω3-rich diets, such as marine Chlorella or oo-yeast, before they were fed to the fish, superior survival rates of the seabream larvae were achieved. Watanabe et al. (1982) have demonstrated that 20 : 5ω3 could be incorporated into Artemia by feeding them for 24 h on 20: 5ω3-rich diets. Nauplii prefed oo-yeast also contained significant levels of 22 :6ω3 and red seabream larvae did best on these nauplli. Similarly, Leger et al. (1985c) have shown that feeding a HUFA-enrichment diet to San Pablo Bay 1628 Artemia increased its levels of 20 : 5ω3 and 22 :6ω3 and markedly enhanced its nutritional value for penaeid shrimp larvae. The nutritional improvement of fattyacid-enriched Utah Artemia has also been demonstrated for mysids, two penaeid species and seabass larvae (Van Ballear et al., 1985; Amat et al., 1985; Leger et al., 1985b).
If the abundance of certain essential fatty acids governs the nutritional value of Artemia nauplii, what exactly determines their respective levels in Artemia? Schauer and Simpson (1985) have demonstrated that Artemia have a limited need to produce their own 20 : 5ω3 and Millamena et al. (1985) reported that Artemia HUFA levels strongly resembled those of the algal diets on which they were fed. Lavens et al. (in preparation) grew nauplii containing about 5% 20: 5ω3 on two different diet, one containing 6.7% and the other only 0.7% 20:5ω3. The resulting adult populations were induced to produce cysts in a controlled cyst production unit (Lavens and Sorgeloos, 1984). The cysts produced by adults grown on the 20 : 5ω3-rich diet contained high levels of 20:5ω3 while the others contained very low levels of this fatty acid. This experiment clearly demonstrated that Artemia cysts reflects 20: 5ω3 levels of the diet available for the parental population.
If these results can be extrapolated to wild populations, different food conditions in Artemia ponds and lakes probably explain differences in fatty acid profile between Artemia stains-and even within the same strain. Compilation of data from literature and our own analyses (Table III) (see review in leger et al., 1986) shows that 20 : 5ω3 levels may indeed vary considerably among and within strains. Variability is particularly great in strains produced in solar salt works, e.g., SFB, Brazilian and Chinese Artemia. Variability is small inCandian and especial Utah Artemia, both of which are produced in inland salt lakes characterized by a more stable environment. Food composition in solar salt ponds is more diverse and often completely different from one pond to another. The nutritional quality of Artemia cysts produced in solar salt ponds is therefore subject to uncontrolled variability and is difficult to predict.
ARTEMIA ENRICHMENT
The technique of feeding Artemia nauplii on HUFA enrichment diets markedly increases the nutritional value of inferior strains and batches and hence reduces strain and batch differences. The application of Artemia enrichment with alage was pioneered by Forster and wicking (1967) and wickins (1972) and further developed by Japanese, French, and Belgian researchers using prepared diets (see review in Leger et al., 1986). Since those methods have been covered elsewhere in this Symposium (Leger et al., 1985b; Robin et al., 1985; Watanabe et al., 1985), we will not review the techniques here, but summarize the advantages of Artemia enrichment. Enriched nauplii have an improved nutritional composition, i.e., they have a higher energy content and contain all essential fatty acids including 22 :6ω3, which is generally absent in nauplii from all strains. Through enrichment techniques other nutrients, prophylactics and therapeutics may be passed to the predator via Artemia nauplii. The application of enriched Artemia is reflected in improved performances in larval culture, in terms of both survival and growth, and consequently improved performances are obtained in later stages (Leger, unpublished data; Chamorro, personal communication). Larvae fed on enriched Artemia are indeed healthier and more resistant to stressful conditions, such as inflections, weaning of fish, or transfer of shrimp from hatchery tanks to nursery ponds. The only disadvantage of enriched Artemia is their larger size, which may be a problem for early larval stages. For those cases where size is a problem, freshly hatched, high quality instar I nauplii may be used as food for the first few days, followed by a gradual switch to enriched metanauplii as soon as the predator's size permits ingestion of larger particles. Optimized enrichment procedures may also reduce the disadvantage of size by obtaining similar enrichment levels in less time (Leger e shrimp larvae. imilar enrichment techniques may also be applied to juvenile and adult Artemia which may be used as a carrier for essential nutrients and other components to be administered to postlarval shrimp, juvenile fish and lobster larvae.
CONCLUSIONS AND RECOMMENDATIONS
To summarize, Artemia is an excellent food for a wide variety of cultured marine and freshwater organisms. The major constraint of Artemia as a food organism for marine predators is its variable nutritional quality. However, we recommend some measures to remedy this problem:
- for the problem of size and variable energetic content between strains and instar stages, one should:
select suitable strains
use freshly hatched first instar nauplii (i.e., though application of optimized hatching procedures, cold storage of nauplii and optimized feeding strategies.
when possible, use decapsulated cysts.
- for the problem of variable nutritional composition, one should, one should:
apply enrichment techniques
select high quality lots for early larval stages and as a reference material in ecological studies, Especially for those where reproducibility of culture is of utmost importance, an urgent need exists for the fully controlled production of standard high quality Artemia cysts.
Finally, realizing that nutritional quality of Artemia may be so poor that complete mortality of certain predators may result, we wonder how mariculture would developed if Seale (1933) and Rollefsen (1939) had used a poor quality Artemia sources when they pioneered the use of Artemia for larval fish culture back in the 1930's. Despite variability among geographical strains in size, caloric content, nutritional compostion and contaminants, Artemia has proven to be the most widely used and successful diet for aquaculture activities.
ACKNOWLEDGEMENTS
Our research contributions for this review have been sponsored by the Belgian National Science Foundation (NFWO) grant FKLO 30,0012,82, the Belgian Institute for the Promotion of Industry and Agriculture (IWONI,), the Belgian Administration for Development Cooperation, the Belgian Company NV Artemia Systems, the United State Environmental Protection Agency, and the United States Sea Grant Program. PS is senior scientist with the Belgian National science foundation.
LITERATURE CITED
AMAT F., IIONTORIA, AND. J.C. NAVARRO.1985. A preliminary nutritional evaluation of different Artemia nauplii as food for marine fish and prawn larvae. Book of Abstracts, Second International Symposium on the Brine Shrimp Artemia, Antwerp (Belgium), Sept, 1–5, 1985, p. 9.
AUSTIN B. and D.A, ALLEN. 1981/1982. Microbiology of laboratory-hatched brine shrimp (Artemia) Aquaculture 26: 369–383.
BARAHONA-FERNANDES M.H., GIRAIN and R. METALLER. 1977. Experiences de conditionnement d'alevins de bar (Pisces, Dicentrarchus labrax) a diiferent aliment composes Aquaculture 10 : 53–63.
BECK A.D. and D.A. BENGTSON. 1982, International Study on Artemia.
XXII Nutrition in aquatic toxicology - diet quality of geographical strains of Artemia p. 161–169. In:
Aquatic Toxicology and Hazard Assessment: Fifth Conference, ASTMSTP 766. Pearson J.G., R.B.
Foster, and W.E. Bishop (Eds.). American Society for Testing and Materials, Philadelphia, USA.
BECK A.D., D.A. BENGTSON. and W.H. HOWELL. 1980. International Study on Artemia. V. Nutritional value of five geographical strains of Artemia : effects on survival and growth of larval Atlantic silverside, Menidia menidia p. 249–259. In: the brine shrimp Artemia, Vol. 3. Ecology, Culturing, Use in Aquaculture, Persoone G., P. Sogcloos, O Roels and F. Jaspers (Eds). Universa Press, Wetteren, Belgium. 456 p.
BOOKHOUT C.G. and J.D. COSTLOW, Jr. 1970. Nutritional effects of Artemia from different locations on larval development of crabs. Helgol, Wiss; Meeresunters. 20 : 453–442.
BRUGGEMANN E., P. SORGELOOS and P, VANHAECKS. 1980. Improvement in the decapsulation technique of Artemia cysts. p. 261–269, In; The brine shrimp Artemia Vo. 3. Ecololgy, Culturing, Use in Aquaculture. Persoone G., P. Sorgeloos, O Roels and E,Jaspers (Eds). Universa press., Wetteren, Belgium. 456p.
DABROWSKI K and M. RUSIECK. 1983. Content of total and free amino acids in zooplanktonoic food of fish larvae Aquaculture 31: 31–-42.
DENDRINOS P.,S. DEWAN and J,P.THORPE. 1984. Improvement in the feeding efficiency of larval, post larval and juvenile Dover sole (Solea solea L) by the use of staining to improve the visibility of Artemia used as food. Aquaculture 38 : 137–144
GATESOUPE J.and P. LUQUET. 1981/1982. Weaning of the sole (Solea solea) before metamorphoisis. Aquaculture 26 : 359–368.
JOHNS D.M., W.J. BERRY and S. McLEAN 1981. International Study on Artemia. XXI. Investigations into why some strains of Artemia are better food than others, Further nutritional work with larvae of the mud crab, Rhithropanopeus harrisii J. Worle Maricul. soc 12 : 303–314.
JOHNS D.M., M.E. PETERS and A. D. BECK. 1980. International Study on Artemia. VI. Nutritional value of geographical and temporal strains of Artemia effects on survival and growth of two species of Brachyuran larvae . p.291–304, In : The brine shrimp Artemia. Vol, 3. Ecology, Culturing, Use in Aquaculture. Parsoone G., P. Sorgeloos, O. Roels and E, Jaspers (Eds). Universa Press, Wetteren, Belgium, 456p.
KANAZAWA A., S, TESHIMA and K. ONO. 1979. Relationship between essential fatty acid requirements of aquatic animals and the capacity for bioconversion of linolenic acid to highly unsaturated fatty acids. Comp Biochem Physiol 63B : 295–298.
KLEIN-MacPHEE G., W.H. HOWELL and A.D.BECK. 1980. International Study on Artemia. VII. Nutritional value of five geographical strains of Artemia to winter flounder Pseudopleuronectes americanus larvae. p. 305–312. In: The brine shrimp Artemia, Vol. 3. Ecology, Culturing, Use in Aquaculture, Persoone G., P. Sorgeloos, O. Roels and E. Jaspers (Eds), Universa Press, Wetteren, Belgium, 456p.
KLEIN-MacPHEE G., W.H. HOWELL and A.D.BECK, 1982, Comparison of a reference and four geographical strains of Artemia as food winter flounder (pseudopleuronectes amereicanus) larvae; Aquaculture 29: 279–288.
LAVENS P., P. LENGER and P.SORGELOOS. 1986. Manipulation of the fatty acid profile in Artemia offspring in a controlled production unit.
LAVENUS P. and P.SORGELOOS. 1984; Controlled cyst production of Artemia cysts under standard conditions in a recirculating culture system. Aquacultural Engineering 3: 221–235.
LEGER P.,D.A, BENFTSON, K.L. SIMPSON and P. SOREGELOOS. 1986. The use and nutritional value of Artemia as a food source, P, 000–000. In: Occanography and Marine Biology Annual Review, Vol. 24 Barnes M (Ed). Aberdeen University Press, Aberdeen, Scotland.
LERGER P., G. BIEBER and P. SORGELOOS. 1985 a International Study on Artemia, XXXIII. promising results in larval rearing of Penaeus stylirostris using a prepared diet as algal subtitute and of Artemia enrichment. J. world Maricul; Soc. 15;
LEGER P., P. LANDREVA, E. NAESSENS-FOUCQUAERT and P, SORGELOOS. 1985b.
International Study on Artemia XXXV. Techniques to manipulate the fatty-acid profile in Artemia
nauplii and the effect on its nutritional
Effectiveness for the marine crustacean Mysidopsis bahia (M). Book of Abstracts, second
International Symposium on the Brine Shrimp Artemia Antwerp (Belgium), Sept 1–5, 1985, P. 61.
LEGER P., P. SORGELOOS, O.M. MILLAMENA and K.L. SIMPSON. 1985 C. International Study on Artemia. XXI, Factors determining the nutritional effectiveness of Artemia; the relative impact of chlorianted hydrocarbons and essential fatty acids in san Francisco Bay and San Pablo Bay Artemia J, Exp. Mar Biol Ecol. 93: 71–82.
LEGER P., P. VANIIAECKE and P. SOREGELOOS. 1983. International Study on Artemia. XXIV. Cold storage of live Artemia nauplii from various geographical sources - potentials in aquaculture Aquacultural Engineering 2: 69–78.
McLEANS., C.E.OLNEY, G. KLEIN-MacPHEE and K,L, SIMPSON, 1985. Effect of chlordane and dieldrin on the Artemia to winter flounder food-chain. Book of Abstracts, Seconds International Symposium on the Brine Shrimp Artemia, Antwerp (Belgium), Sept. 1–5. 1985, p. 72.
MILLAMENA O. M., R.F. BOMBEO, N.A. JUMALON and K.L. SIMPSON. 1985. The effects of various diets on the nutritional value of Artemia as feed for Penaeus monodon larvae; Book of Abstracts, World Mariculture Society, 16th Annual Meeting, Orlando, Florida, USA, 13–17 January, 1985.
OLNEY C, E., P.S SCHAUER, S. McLEAN, YLU, and K.L. SIMPSON, 1980. International study on Artemia. VII. Comparison of the chlorinated hydrocarbons and heavy metals in five different strains of newly hatched Artemia and a laboratory reared marine fish p. 342–352. In: The brine shrimp Artemia. Vol. 3. Ecology, Culturing, Use in Aquaculture, Persoone G., P. Sorgeloos, O. Roels and E.Jaspers (Eds). Universa Press, Wetteren, Belgium. 455p.
ROBIN J., C. LEMILLINAIRE, and G. STEPHAN. 1985. Production to Artemia using mixed diets. Control of fatty acid content for marine fish larvae culture. Book of Abstracts, Second International Symposium on the Brine Shrimp Artemia, Antwerp (Belgium), Sept. 1–5, 1985, p. 98.
ROLLEFSEN G 1939. Artificial rearing of fry of seawater fish - preliminary communication. Rapp. p - V. Reun. Cons perm. Explor Mer 109; 133.
SAMAIN J.F., J. MOAL. J.Y. DANIEL., J.R., LeCOZ and M. JEZEQUEL. 1980. The digestive enzyms amylase and trypsin during the development of Artemia: effect of conditions. p. 239– 255. In: The brine shrimp Artemia. Vol. 2. Physiology, Biochemistry, Molecular Biology. Persoone G., P. Sorgeloos, O. Roels and LE. Jaspers (Eds.). Universa Press, Wetteren, Belgium. 664 p.
SCHAUER P.S., D.M. JOHNS, C.E. OLNEY, and K.L. SIMPSON. 1980. International study on Artemia IX. Lipid level, energy content and fatty acid composition of the cysts and newly hatched nauplii from five geographical strains of Artemia. p. 365–373. In: The brine shrimp Artemia. Vol. 3. Ecology, Culturing, Use in Aquaculture. Persoone G., P. Sorgeloos, O. Roels and E. JAspers (Eds.). Universa Press, Wetteren, Belgium. 456 p.
SCHAUER P.S. and K.L. SIMPSONl. 1985. Bioaccumulation and bioconversion of dietary labeled fatty acids in Artemia and winter flounder (pseudopleuronects americanus). Can. J. Fish. Aquat. Sci. 42: 1430–1438.
SEALE A. 1933. Brine shrimp ((Artemia) as a satisfactory live food for fishes. Trans. Am. Fish. Soc. 63: 129–130.
SEIDEL C.R., D.M. JOHNS, P.S. SCHAUER, and C.E. OLNEY. 1982. International study on Artemia. XXVI. The value of the nauplii from Reference Artemia Cysts and four geographical collections of Artemia as a food source for mud crab, Rhithropanopeus harrisii, larvae. Mar. Ecol. Prog. Ser. 8 : 309–312.
SEIDEL C.R., J. KRYZNOWEK, and K.L. SIMPSON. 1980. International Study on Artemia. XI. Amino acid composition and electrophoretic protein patterns of Artemia from five geographical locations. P. 375–382. In: The brine shrimp Artemia. Val. 3. Ecology, Culturing, Use in Aquaculture. Persoone G., P. Sorgeloos, O. Roels and E. Jaspers (Eds.). Universa Press, Wetteren, Belgium. 456 p.
SHELBOURNE J.E. 1968. The culture of marine fish larvae with special reference to the plaice Pleuronectes platessa and the sole (Solea solea). Thesis, London University, 143 p.
SIMPSON K.L., G. KLEIN-MCPHEE, and A.D. BECK. 1983. Zooplanton as a food source. p. 180– 201. In: proceedings of the Second International conference on Aquaculture Nutrition: Biochemical and Physiological Approaches to Shellfish Nutrition. Pruder G.D., C. Langdon and D. Conklin (Eds.). Division of Continuing Education, Louisiana State University, Baton Rouge, Louisiana, USA.
SOEJIMA T., T. KATAYAMA, and K. L. SIMPSON. 1980. International Study on Artemia. XII. The carotenoid composition of eight geographical strains of Artemia and the effect of diet on the carotenoid composition of Artemia. P. 613–622. In: The brine shrimp Artemia. Vol. 2. Physiology, Biochemistry, Molecular Biology. Persoone, G., P. Sorgeloos, O. Roels and E. Jaspers (Eds.). Universa Press, Wetteren, Belgium. 664 p.
SORGELOOS P. 1980. The use of the brine shrimp Artemia in aquaculture. p. 25–46. In: The brine shrimp Artemia. Vol. 3. Ecology, Culturing, Use in Aquaculture. Persoone G., P. Sorgeloos, O. Roels and E. Jaspers (Eds.). Universa Press, Wetteren, Belgium. 456 p.
SORGELOOS P., E. BOSSUYT, E. LAVINA, M. BAEZA-MESA, and G. PERLSOONE. 1977. Decapsulation of artemia cysts: a simple technique for the improvement of the use of brine shrimp in aquaculture. Aquaculture 12: 311316. SORGELOOS P., E. BOSSUYT. P. LAVENS. P. LEGER, P. VANHAECKE and D. VERSICHELE. 1983. The use of brine shrimp Artemia in crustacean hatcheries and nurseries. p. 71–96. In: CRC Handbook of Mariculture. Vol. 1. Crustacean Aquaculture. McVey J.P. (Ed.) CRC Press.
TESHIMA S. 1978. Requirements of essential fatty acids and sterols in crustaceans. p. 60–77. In: Dietary Lipids in Aquaculture. Japanese Society of Scientific Fisheries (Ed.). Suisangaku Ser. No. 22, Koseisha Koseikaku, Tokyo.
VAN BALLAER E., F. AMAT, F. HONTORIA, P. LEGER, and P. SORGELOOS. 1985. Preliminary results on the nutritional evaluation of W3-HUFA-enriched Artemia nauplii for larvae of the sea bass, Dicentrarchus labrax. Aquaculture 49: 223–229.
VANHAECKE P., P. LAVES, and P. SORGELOOS. 1983. International Study on Artemia. XVII. Energy consumption in cysts and early larval stages of various geographical strains of Artemia. Ann. Soc. R. Zool.Belg. 113: 155–164.
VANHAECKE P. and P. SORGELOOS. 1980. International Study on Artemia. IV. The biometrics of Artemia strains from different geographical origin. p. 393–405. In: The brine shrimp Artemia. Vol. 3. Ecology, Culturing, Use in Aquaculture. Persoone G., P. Sorgeloos, O. Roels and E. Jaspers (Eds.). Universa Press, Wetteren, Belgium. 456 p.
WATANABE T. 1985. Use of Artemia in fish and crustacean farming in Japan - a review. Book of Abstracts, Second International Symposium on the Brine Shrimp Artemia, Antwerp (Belgium), Sept. 1–5, 1985, p. 142.
WATANABE T., T. ARAKAWA, C. KITAJIMA, and S. FUJITA. 1978a. Nutritional evaluation of proteins of living feeds used in seed production of fish. Bull. Jap. Soc. Sci. Fish. 44: 985–988.
WATANABE T., F. OOWA, C. KITAJIMA, and S. FUJITA. 1978b. Nutritional quality of brine shrimp, Artemia salina as a living feed from the viewpoint of essential fatty acids of fish. Bull. Jap. Soc. Sci. Fish. 44: 1115–1121.
WATANABE T.M. OHTA, C.KITAJIMA, and S. FUJITA, 1982. Improvement of the dietary value of brine shrimp Artemia salina for fish larvae by feeding them on w3 highly unsaturated fatty acids. Bull. Jap. Soc. Sci. Fish. 48: 1775–1782.
WICKINS J.F. 1972. The food value of brine shrimp, Artemia salina L., to larvae of the prawn, Palaemon serratus Pennant. J. esp. mar. Biol. Ecol. 10: 151–170.
YONE Y. 1978. Essential fatty acids and lipid requirements of marine fish. p. 43–59. In: Dietary Lipids in Aquaculture. Japanese Society of Scientific Fisheries (Ed.) Suisangaku Ser. No. 22, Koseisha Koseigaku, Tokyo.
Table I: Average proximate composition of Artemia nauplii and adults as calculated from data presented in 26 and 15 references, respectively.
| Nauplii | Adults | |
| Protein | 52.2 ± 8.8% | 56.4 ± 5.6% |
| Lipid | 18.9 ± 4.511.8 ± 5.0 | |
| Carbohydrate | 14.8 ± 4.8 12.1 ± 4.4 | |
| Ash | 9.7 ± 4.617.4 ± 6.3 |
TABLE II: Summary of nutritional bioassay results for several categories of aquatic organisms fed 10 geographical strains of Artemia
| Artemia geografical strain | ||||||||||
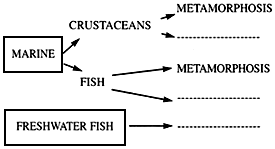 | AUSTRALIA | BRAZIL | CANADA | CHINA | FRANCE | ITALY | UTAH | SAN PABLO BAY | SAN FRANCISCO BAY | R.A.C. |
| + | + | ± | + | + | + | - | - | + | + | |
| + | + | ± | + | + | + | + | - | + | + | |
| + | + | + | + | + | + | - | - | + | ||
| ± | + | ± | ± | ± | - | ± | - | + | + | |
| + | + | + | + | + | + | + | + | + | ||
Table III: Intra-strain variability in levels of the essential fatty acid 20: 5ω3. Data are given as percentage of total fatty acid methyl esters and represent analyses of samples taken over several seasons or years.
| Artemia Georgraphical strain | 20: 5ω3 content (area %) | ||
| San Francisco Bay | 0.3 – 13.3 | ||
| Brazil | 3.5 – 10.6 | ||
| China | 1.3 – 15.4 | ||
| Canada | 5.2 – 9.5 | ||
| Utah - Southern Arm | 2.7 – 3.6 | ||
| - Northem Arm | 0.3 – 0.4 | ||
Fig. 1 Summary of the requirements a food must meet to be a practical diet for larval organisms.
Fig. 2 The size ranges of various Artemia life stages.
Fig. 3 Change in energy content and dry weight of different forms of Artemia. Instar I nauplii (newly hatched) are considered to have 100% values for those variables. The percent decrease or increase from 100% is shown for, successively, instar II-III nauplii, cold-stored instar I nauplii, and decapsulated cysts.
Fig. 4 Percent survival of winter flounder larvae fed on five geographical strains of Artemia (after Klein-MCPhee et al., 1980).
Fig. 5 Percent survival of mud crab larvae fed on five geographical strains of Artemia (after Johns et al., 1980).
Fig. 6 Linear relationship between the essential fatty acid (20:5ω3) (20:5ω3) content of several Artemia collections from San Francisco Bay and the biomass of mysids to which the Artemia were fed (data from Leger et al., 1985c).
Fig. 7. Linear relationship between the chlorinated hydrocarbon content of several Artemia collections from San Francisco Bay and the biomass of mysids to which the Artemia were fed (data from Leger et al., 1985c).
Fig.1: Food requirements for larval organisms
For the culturist-
- consistent availability
- simple production procedures
- euryplasticity and versatility
- salinity / temperature tolerance
- handling
- disinfection
- different sizes and forms
- use as a carrier
For the predator-
- physical requirements
- clean
- no alien materials
- no diseases
- acceptable
- perceptible
- cacenable
- palatable
- ingestable
- nutritional requirements
- digestible
- nutrient requirements
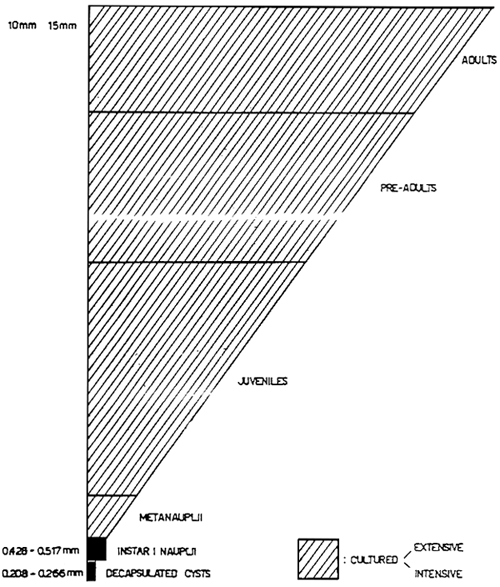
Figure 2
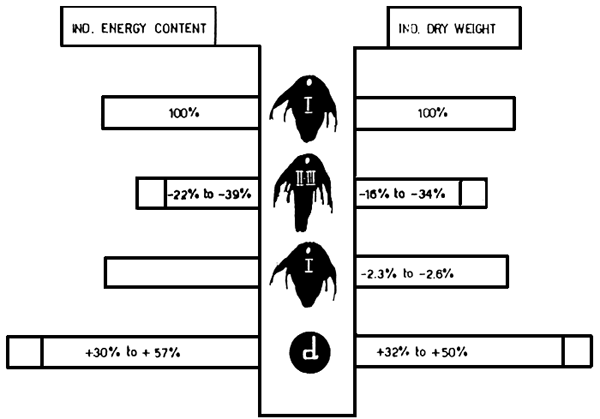
Figure 3
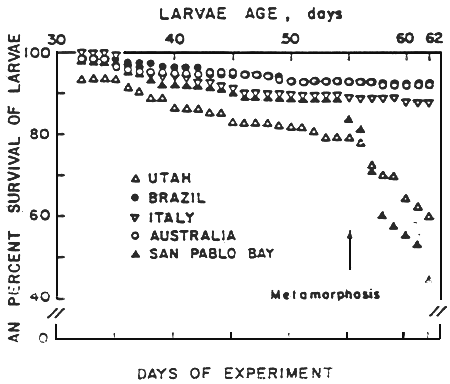
Figure 4
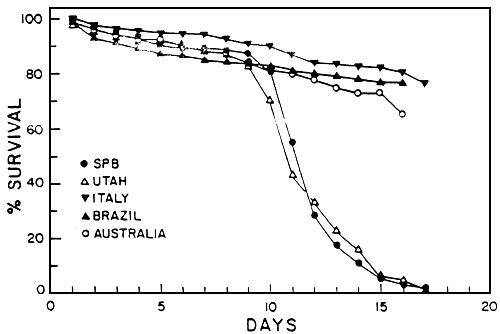
Figure 5