Ramli Saad,
Fisheries Research Institute, Department of Fisheries,
Ministry of Agriculture, 11700 Glugor, Penang, Malaysia.
ABSTRACT
This study was undertaken to determine suitable species of Gracilaria for culture in Malyasia. Specimens were collected from five locations throughout the West Coast of Peninsular Malaysia. Physical parameters for each location were noted. The samples were brought back to the laboratory for preparation of the herbarium specimens and analysis of agar yields. Agar extraction was carried out using the hot water technique. The results showed that Gracilaria changii has the following desirable characteristics for cultivation: a high yield of good quality agar; abundant in many locations; euryhaline (22–32 ppt); a rapid growth rate; and easily cultured either using vegetative cuttings or spores. It was therefore recommended that Gracilaria changii be given the highest priority for culture in Malaysia.
1. INTRODUCTION
Seaweeds, either collected from the wild or cultured, are a very important resource for utilisation. They also play an important role in maintaining the well being of the aquatic environment, especially in buffering the impacts of organic pollution. In addition, seaweeds also contain a number of important compounds such as carrageenan, agar, alginic acid, manitol and iodine, which may be commercially extracted. Seaweeds can be consumed directly as food, or used as an additive in food products and other industrial products, as well as being utilised as fodder for animals.
Research in seaweed farming in Malaysia was initiated with limited success in 1983 (Faazaz, 1986). Since then, research efforts have been focused on the culture of Gracilaria in brackishwater ponds in Peninsular Malaysia. On the other hand, Eucheuma farming has been proven to be successful and is known to be expanding in East Malaysia.
At present there is no commercial production of Gracilaria in Malaysia, however, there is a small scale agar extraction factory (namely Algae Bio Tech) based in Selangor, which uses imported raw materials from Thailand. This factory processes 70 kg of dry Gracilaria spp. daily, producing 8 kg agar per day which is sold at RM 72.00/kg to wholesalers.1
1 1 US $ is approximately equal to 2.5 RM
In Sabah, Eucheuma spp. farms cover a total area of more man 1,000 hectares with a production of about 100 tonnes (dry weight) of Eucheuma spp. per month, which is mostly exported to Sweden. No processing is done in Malaysia.
Agar is imported into Malaysia in 4 main forms, namely: agar strips; bacteriological agar; agar desserts; and flavoured powder mixtures (Jahara and Phang, 1989). It was reported that as much as 90% of the agar imported for food is in the form of agar strips, while the remaining 10% is in the form of powdered or jelly desserts. Malaysia has a large domestic market for seaweed and agar products. In 1988, about RM 6.55 million worm of agar strips were imported into this country (Jahara and Phang 1989). The successful development of seaweed culture would therefore contribute towards foreign exchange earnings.
With an aim of promoting seaweed culture in Malaysia, several objectives have been identified, namely:
To obtain fanning technology which is economically viable on a commercial scale; and
To use cultured seaweed in the treatment of effluents from aquaculture activities, especially from shrimp farms.
2. ECOLOGY AND TAXONOMY OF GRACILARIA
2.1 Literature review
The first records of marine algal taxonomy from the Asian region were from the collections made during the Pruessische Expedition nach 0st-Asien from 1860–1862 by Eduard von Martens. Since then, many reports have been published on this subject and based on these reports, a preliminary list has been completed for Malaysia (Phang, 1986).
In 1991, a new checklist was published by Phang and Wee. A total of 50 collection sites were recorded and 212 taxa listed. This total included: 5 families, 13 genera and 12 species of Cyanophyta; 13 families, 24 genera and 74 species of Chlorophyta; 19 families, 39 genera and 59 species of Rhodophyta; and 8 families, 13 genera and 46 species of Phaeophyta. Out of these, 25 species are considered common, while information on the remaining 187 is insufficient. Subsequently, a few other publications have been released. These papers listed an additional 7 taxa of Chlorophyta, 18 taxa of Rhodophyta and 5 taxa of Phaeophyta (Phang, 1994). In addition, based on the collections of the Institute of Advanced Studies, University of Malaya, 19 new taxa have been reported.
2.2 Materials and methods
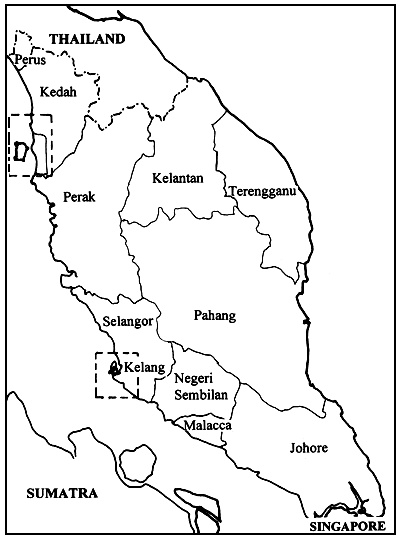
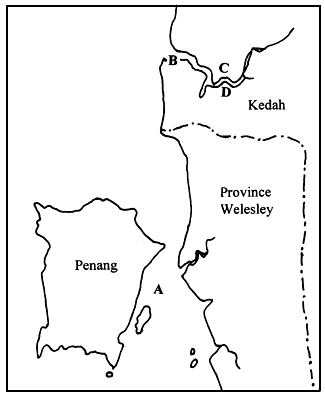
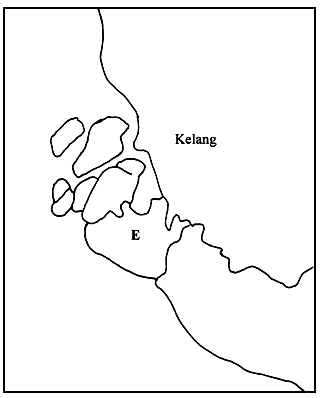
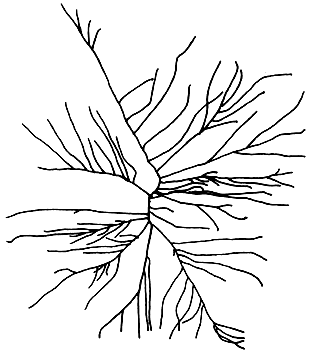
Gracilaria spp. specimens were collected from five locations, namely: at the Middle Bank in Penang (A); Tanjung Dawai (B), Ban Merbok (C) and Teluk Bayu (D) in Kedah and Pulau Carey (E) in Selangor (Figure 1). The samples were brought back to the laboratory in plastic bags for the preparation of the herbarium specimen and agar analysis. Physical factors, such as salinity, turbidity and temperature, were noted at each location.
Each location had its own unique characteristics and a brief description is given below:
Middle Bank. Penang. Located between Penang island and the mainland, the lagoon has a sandy loam bottom, is sheltered from strong wind and wave action and is exposed during low tides. Gracilaria spp. was found attached to the hairy roots of seagrass, plastic and other solid materials in the area.
Tanjung Dawai. Kedah. This area forms the estuary of the Merbok River. It is sheltered from wind and wave action and is submerged during low tide. Gracilaria spp. was found attached to the netting materials and other floating substances offish cages.
Ban Merbok. Kedah. A shrimp pond with a muddy bottom.
Teluk Bayu. Kedah. Upper region of the Merbok river with a brackishwater habitat, sheltered from strong wind and wave action and submerged during low tide. The area is affected by low salinities during the rainy season. Gracilaria spp. was found attached to net cages and floating substances of the fish cages.
Pulau Carey, Selangor. A mangrove forest, sheltered from strong wind and wave action and exposed during low tide with low light intensity. Gracilaria spp. was found attached to the aerial root of mangrove trees or dead branches which have fallen off onto the ground.
Figure 1: Map indicating collection sites A, B, C, D and E.
 |  |
2.3 Results
The results of the experiments are summarised in Table 1. The samples collected were identified as Gracilaria changii, Gracilaria fastigiata, Gracilaria sp. 1 and Hypnea sp. Gracilaria sp. 1, which looked very similar to G. changii, was identified as Gracilaria changii initially, but after growing this species in the pond, the morphological characteristics were found to be far different. The species was then termed Gracilaria sp. 1, but the exact taxonomy of this species needs further confirmation.
All of the species were collected from sheltered and nutrient rich areas with a salinity range of between 22 to 32 ppt. They were found to be dominant in these locations. The sample, MSW 005, which was identified as Hypnea sp. and was found growing together with cultured Gracilaria sp. in the ponds. It has a very fast growth rate (about 4 times as fast as Gracilaria sp.) and was found to grow well even on muddy bottoms.
3. PROCESSING (AGAR YIELD AND QUALITY ) OF GRACILARIA SPECIES
3.1 Literature review
Faazaz (1986) reported that Gracilaria cylindrica (now Gracilaria changii) produced good quality agar which is suitable for agar production. Gracilaria changii produced more than 45% agar (Ramli, 1989) without alkali treatment and between 33 to 37.5% with alkali treatment. Kasim and Ismail (1983) found that Gracilaria changii yielded 16.4% agar without alkali treatment and 24.5% with pre alkali treatment. Pre alkali treatment was found to increase the gel strength of agar from 547.7 gm/cm2 to 844.8 gm/cm2 .
3.2 Materials and methods
The collected Gracilaria spp. samples were cleaned with sea water and weighed. The samples were then dried in the sun and re-weighed. The dried samples were soaked in fresh water for one to two hours and then sun dried. The clean anhydrous weight (CAW) of the samples was then calculated. The samples were kept in plastic bag until it was time for extraction.
A 100 g sample of dried Gracilaria spp. was soaked in freshwater for 30 minutes before agar extraction. After dripping, the sample was boiled with 4 litres of freshwater for 1 hour in a stainless steel container. The mixture was screened through 2 layers of cotton and nylon cloth mesh with the aid of coconut presses. The agar solution was then collected in an aluminium tray and kept aside to cool and form a gel. The trays were then kept in the refrigerator for two to three hours to harden the gel, which was then cut into 2–3 cm thick pieces and kept in the deep freeze for another 24 to 48 hours. The gel was exposed to air for thawing and dried with sunlight thereafter. The dried agar was weighed and the percentage yield was calculated.
3.3 Results
The results of this study are summarised in Table 1. Agar yield of Gracilaria changii varied from 22.1% to 41.7%. Gracilaria fastigiata yielded 15.8% of agar while Hypnea sp. yielded 13.5% of carrageenan, using the same procedure for agar extraction (not in acid condition).
Table 1: Data on the taxonomy, ecology and processing of collected seaweeds.
| Sample code | Species | Locality | Date | Salinity (%) | Turbidity (cm) | Temp. (°C) | Wet/dry | % Agar | Note: Tidal exposure, epiphytes, grazing. |
| MS W001 | Gracilaria changii | Middle Bank Penang | 15.5.92 | 30 | 20 | 28 | 11.1 | 41.7 | Sheltered, Exposed, Ulva sp. Siganus spp. |
| MSW 002 | G. changii | Tg. Dawai, Kedah | 28.5.92 | 32 | 90 | 30 | 15.6 | 39 | Sheltered, Not Exposed Siganus spp. |
| MSW 003 | G. changii | Tg. Dawai, Kedah | 9.79.92 | 32 | 85 | 29 | 14.7 | 35.8 | Sheltered, Not Exposed Enteromorpha. |
| MSW 004 | G. changii | Merbok Kedah | 9.9.92 | 28 | 115 | 29 | 16.1 | 30.5 | Sheltered, Not Exposed Enteromorpha. |
| MSW 005 | Hypnea sp. | Merbok Kedah | 9.9.92 | 28 | 115 | 29 | 39.1 | 13.5 | Sheltered, Not Exposed Enteromorpha. |
| MSW 006 | G. changii | Middle Bank Penang | 24.4.93 | 28 | 20 | 28 | 12.4 | 20 | Sheltered, Not Exposed Ulva sp, Siganus spp |
| MSW 007 | G. fastigiata | Middle Bank Penang | 24.4.93 | 28 | 20 | 28 | 12.4 | 15.8 | Sheltered, Exposed Ulva sp, Siganus spp |
| MSW 008 | G. changii | Teluk Bayu, Kedah | 14.5.93 | 25 | 75 | 30 | 10.6 | 25.4 | Sheltered, Not Exposed Enteromorpha, Siganus spp |
| MSW 009 | Gracilaria .sp. 1 | Pulau Carey Selangor | 14.4.94 | 31 | - | 30 | 16.7 | 20.4 | Sheltered, Exposed Enteromorpha.. |
| MSW 010 | Gracilaria .sp. 1 | Pulau Carey Selangor | 27.7.94 | 30 | - | 29 | 16.4 | 24.4 | Sheltered, Exposed Enteromorpha. |
| MSW 011 | G. changii | Merbok Kedah | 17.9.94 | 22 | 130 | 28 | 16 | 25.2 | Sheltered, Not Exposed Enteromorpha. |
| MSW 012 | G. changii | Merbok Kedah | 8.10.94 | 25 | 125 | 29 | 15.4 | 22.1 | Sheltered, Not Exposed Enteromorpha. |
4. DISCUSSION AND CONCLUSIONS
The percentage yield of agar in Gracilaria changii varied with the locality and time of collection, which may be due to differences in environmental factors, such as salinity, turbidity, nutrients, temperature and seasons. Even though the percentage yields of agar varied, the values were still relatively high and economic for seaweed farming.
Agar quality was not analysed due to a lack of the necessary equipment but, based on a study by Kasim and Ismail (1993), this species produced high quality agar. Gel strength in this study was 547.7 g/cm2, which is Grade 1 classification under the Japanese standard for processed agar quality (as quoted by Chandrkrachang and Chinadit, 1988 in Table 2). These authors also reported that its gel strength can be increased to 844.8 g/cm2 if given pre alkali treatment. This would then be classified as special grade agar, based on the same criteria.
Table 2: Processed agar quality standard set by the Japanese.
| Agar Grade | Gel strength (g/cm2) |
| Special Grade Agar | 600 or more |
| Grade 1 | 350 or more |
| Grade 2 | 250 or more |
| Grade 3 | 150 or more |
Gracilaria changii is found in many locations throughout the country, especially in nutrient-rich areas such as fish cage culture sites. This species is also easily grown in shrimp ponds either by using spore or vegetative cuttings as seedstock and has a fast growth rate.
Our preliminary research has shown that Gracilaria changii can produce more than 14 tonnes (dry weight)/hectare/year using vegetative cuttings as a seedstock. On the oilier hand, using spores as seed material, production can be increased to more than 19 tonnes (dry weight)/hectare/year. These studies were performed in brackishwater shrimp ponds and the production figures show that they are economically viable. Based on the above study, Gracilaria changii is undoubtedly the seaweed species that should be given the highest priority for culture in Malaysia.
This species has the following desirable characteristics for culture purposes:
It has high agar yield and quality.
It is abundant in many locations.
It is euryhaline (22–32 ppt).
It has a rapid growth rate.
It is easily cultured either using vegetative cuttings or spores.
As for the other two alternative species, Gracilaria fastigiata and Hypnea sp., further studies should be conducted to confirm their culture requirements. During the study, we found several other species, such as Gracilaria verrucosa, but the quantity was insufficient for analysis. These species need to be propagated and cultured to increase their biomass for analysis.
5. RECOMMENDATIONS
Further research should be geared towards producing Gracilaria spp. on a commercially viable scale.
Problems of epiphytes such as Enteromorpha, often found in Gracilaria spp. culture ponds, must be overcome.
Methods to overcome or minimise predatory problems (Siganus sp.) when Gracilaria spp. is cultured in open waters should be devised.
Small commercial extraction plants should be developed (capacity of 50–200 tonnes dried seaweed per day) for agar production.
Financial and technical support should be available for purchasing up-to-date equipment for seaweed research.
There should be support from international experts.
Funding of support staff would accelerate research projects.
REFERENCES
Chandrkrachang, S. and Chinadit, U. 1988. Seaweeds production and processing in Thailand. Proceedings of the Seminar and Workshop on “From Seaweeds to Biotechnology”, Bangkok, Thailand.
Faazaz A. L., 1986. Preliminary results on the experimental culture of the red seaweed Gracilaria spp. in Malaysia. Bulletin Perikanan Bil. No. 40,9pp, Department of Fisheries, Ministry of Agriculture, Malaysia.
Jahaya, J. and Phang, S. M. 1989. Seaweed marketing and agar utilising industries in Malaysia, 65 pp. Presented in Seminar on Gracilaria production and utilisation in the Bay of Bengal, 23–27 October 1989, Songkhla, Thailand (Mimeo).
Kasim, A. and Ismail, A. 1993. Penentuan Penghasilan dan Kekuatan Gel Agar Daripada Gracilaria changii dan G. edulis (Rhodophyta: Gracilariaceae): Journal of Bioscience 49 (1): 85–92. (Abstract in English).
Phang, S. M. 1986. Malaysia's Seaweed Flora. Proc. Ninth Ann. Seminar Malaysia Society of Marine Science: 17–45.
Phang, S. M. and Wee, Y. C. 1991. Benthic Marine algae: In Kiew, R. (Ed.), The State of Nature Conservation in Malaysia, Malayan Nature Society, Kuala Lumpur: 51–61.
Phang, S. M. 1994. New record of Malaysian Marine Algae. In A. Sasekumar, N. Marshall & D.J. Macintosh (eds.), Ecology and Conservation of Southeast Asian Marine and Freshwater Environment including wetlands. Hydrobiologia 285: 125–129.
Ramli. S., 1989. Simple process for agar extraction from Polycanernosa changii, 5 pp. Presented in Seminar on Gracilaria production and utilisation in the Bay of Bengal, 23–27 October 1989, Songkhla, Thailand (Mimeo).
U. Kwi Shwe,
Principal Scientist, Applied Chemical Research Department,
and
U. Aung Khine,
Pharmaceutical Department,
Ministry of No. 2 Industry, Myanmar Scientific and Technological Research Department.
ABSTRACT
The taxonomic studies identified seven species, namely: G. verrucosa (Hudson); G. edulis (Gmelin) Silva; G. crassa (Harvey) J. Agardh; G. foliifera (Forsskal) Boergesen; G. millardetii (Montagne) J. Agardh; G. textorii Suringer and G. eucheumoides Harvey. The first six of those listed were studied for agar yield and quality. Four species, G. crassa, G. foliifera, G. verrucosa and G. edulis were cultured in a small-scale experimental system. The results of the culture experiment showed G. edulis to be the most promising. The study recommends its culture in inland ponds and coastal waters. Identification of the species reported as “verrucosa” is not confirmed.
1. INTRODUCTION
The abundant seaweeds in Myanmar are considered an important natural resource. It has been reported that at least 35 species of alginophyte, 19 species of carageenophyte, 7 species of agarophyte and altogether 162 species of different kinds of seaweeds occur throughout the coastal areas of Myanmar. Amongst those species, some economic species have been utilised by people for many years. Red seaweeds, such as Hypnea spp., Catenella spp., Solieria spp. and Gracilaria spp. are well known in the market as a raw material for the production of phycocolloid. A great deal of research and experimental work has been carried out by Myanmar researchers to exploit and develop seaweed utilisation. In 1969 and the 1970s, coastal surveys, cultivation practices and extraction work on seaweeds was carried out.
According to taxonomic studies on the genus Gracilaria, seven species of Gracilaria have been identified in Myanmar. Since 1982, four out of the seven species have been tested for agar-extraction. In the present study, a total of six species of Gracilaria were studied for agar processing potential, emphasising the agar yield and quality of the different species of Gracilaria in Myanmar.
Eight species of useful seaweeds were experimentally cultured including four species of Gracilaria: G. crassa; G. foliifera; G. verrucosa; and G. edulis. The Myanmar government is paying special attention to cultivating Gracilaria at the laboratory scale, with the intention of developing it to commercial scale. Important results and valuable experience have been obtained, some of which are reported in this paper. Previous research has produced useful results for the culture of Gracilaria, but all of these projects are still at the small scale research level.
2. TAXONOMIC STUDIES AND SOME ECOLOGICAL NOTES ON SEVEN SPECIES OF GRACILARIA IN MYANMAR
2.1 Background and current status
Common and widespread species of Gracilaria along the coastal waters of Myanmar (Kyi Win, 1972), include:
Gracilaria confervoides Greville
G. verrucosa (Hudson) Papenfuss
G. minor (Sonder) Comb. nov.
G. corticata J. Agardh
G. foliifera (Forsskal) Boergesen
G. textorii Suringer
G. edulis (Gmelin) Silva
G. ornata Areschoug
G. cylindrica Boergesen
G. curtissae J. Agardh
According to more detailed taxonomic studies on the genus Gracilaria (Hla Hla Cho, 1975; Kyi Shwe and Aung Myint, 1991), seven species have been identified:
Gracilaria verrucosa (Hudson) Papenfuss
G. edulis (Gmelin) Silva
G. crassa (Harvey) J. Agardh
G. foliifera (Forsskal) Boergesen
G. millardetii (Montagne) J. Agardh
G. textorii Suringar
G. eucheumoides Harvey
In this study, the above seven species of Gracilaria are referred to as a part of post-workshop activities under NACA-FAO/ Government Programme. The taxonomic studies and some ecological notes are also presented.
2.2 Materials and methods
The material used for detailed study was collected from intertidal regions (including drift) of the Rakhine and Taninthari coast including the Mergui Archipelago in Myanmar. After field collection, the materials were first preserved in 10% formalin in seawater, then either kept in formalin and seawater or 70% alcohol with glycerine and mounted on the herbarium sheets. The specimens were kept in the Phycological Herbarium of the Marine Science Department, Mawlamyaing University, Mawlamyaing and Applied Chemistry Research Department, Myanmar Scientific and Technological Research Department (MSTRD) Yangon, Myanmar. Distribution areas are given only for those species whose full herbarium records have been obtained.
Dried herbarium materials were found to be more suitable for cutting sections to prepare microscope slides. A fragment of the material was sectioned (25–50 um thick) under a dissecting microscope using a double edged razor blade. A fragment of the material was put on a piece of white paper and pressed lightly with a microscope slide as a guide for the razor blade. The object parallel to the end of the guide slide which was pressed gently with the left hand was cut transversely in continuous series. The flattened materials were more suitable for cutting longitudinal sections. The sections were stained with 1% aqueous aniline blue, acidified by adding one to two drops of 0.1 N hydrochloric acid and washed with water. The excess water from the materials was drawn off with a piece of blotting paper from one side and 20%–50% Karo (corn syrup) with phenol was applied in series from the opposite side of the cover glass.
2.3 Summary
Seven species of Gracilaria, namely: G. verrucosa, G. edulis, G. crassa, G. foliifera, G. millardetii, G. textorii and G. eucheumoides are reported in the present study (Figures 1–9).
The form of the thalli varied being either cylindrical, compressed or flattened. G verrucosa and G. edulis have cylindrical branches, G. foliifera is compressed or flattened and G. millardetii, G. textorii and G. euchomoides are flattened throughout. A wide range of morphological variations were observed within the different species or even in a single species. Although the form of the plants differ greatly from one another, the anatomy of the thallus, shape of spermatangial cavities and the structure of the cystocarps are considered determining factors to differentiate one species from another.
The sexual plants are dioecious. Three forms of spermatangial cavities were observed. The depressions occur in G. textorii, cup-like cavities in G. verrucosa and deep conceptacles in G. edulis, G. crassa, G. foliifera and G. millardetii. Sex organs were not found in G. eucheumoides.
Two-celled carpogonial branch projects singly and outwardly. The supporting cell produces two to four vegetative filaments each with 1–3 cells. The fertilised carpogonium fuses with the adjacent cells forming a fusion cell. The fusion cell cuts off several gonimoblast initial cells outwardly and further developed into gonimoblast parenchyma. These produce the short-celled gonimoblast filaments. The peripheral layer of these cells transform into chains of carposporangia each with a conspicuous central body.
The gonimoblast parenchyma cells are small or large having dense or less protoplasmic contents and may or may not produce nutritive filaments. Cystocarps are globose or dome-shaped, rostrate or non-rostrate, ostiolate or non-ostiolate and scattered or aggregated on all sides of the cylindrical species or on surfaces of the flattened species. The cruciate tetrasporangia are scattered and sunken in the cortex surrounded by unmodified or modified cortex.
In some parts of the Myanmar coastline, economically important agarophytes occur abundantly, especially on the Rakhine coastal region. The plants studied so far do not necessarily represent all the species of the genus Gracilaria of the coastal regions of Myanmar. It is possible that more species may be discovered with further exploration in different localities at different times. The present study may not be sufficient to give a comprehensive account on the ecological notes on each species. Therefore further study on the species of Gracilaria in Myanmar will be required.
Figure 1: Gracilaria verrucosa (Hudson) Papenfuss.
Cystocarpic plant showing very irregular and luxuriant branching.
Figure 2: Gracilaria edulis (Gmelin) Silva.
Tetrasporic plants showing numerous curved, short branchlets spreading through the branches.
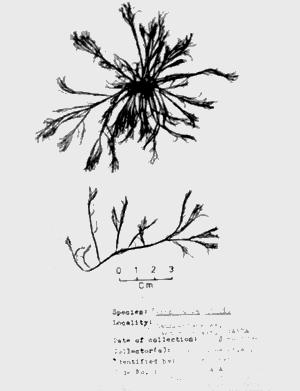
Figure 3: Gracilaria edulis (Gmelin) Silva.
Cystocarpic plant showing numerous short branchlets spreading through the branches.
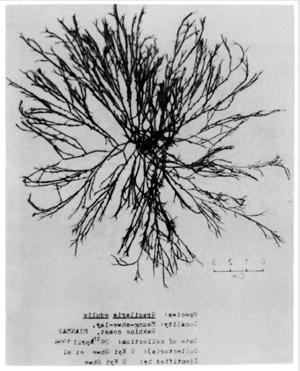
Figure 4: Gracilaria crassa (Harvey) J. Agardh.
A Male plant showing the irregular constrictions of some branches.
B Tetrasporic plant showing the swollen part of the branches.
C Cystocarpic plant showing the unconstricted thallus.
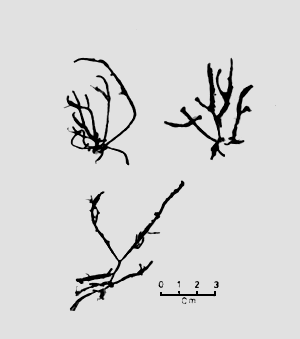
Figure 5: Gracilaria foliifera (Forsskal) Boergessen. Tetrasporic thalli showing the fastigiate branches and a few marginal proliferations at the upper parts of the branches.
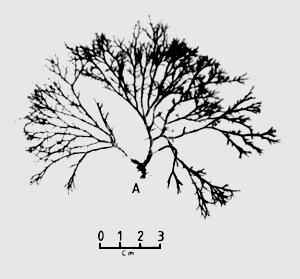
Figure 6: Gracilaria foliifera (Forsskal) Boergesen.
Cystocarpic plant showing subterete branches throughout the thallus except close to the base of the plant.
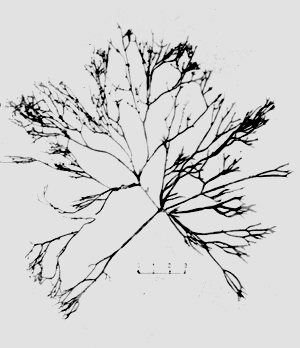
Figure 7: Gracilaria millardetii (Montagne) J. Agardh.
A. Habit of sterile plants.
B. Cystocarpic plant showing cylindrical stripe near the base of the plant.

Figures 8: Gracilaria textorii (Suringar) J. Agardh.
A. Cystocarpic plants of broad thalli showing proliferations.
B. Tetrasporic plants of narrow thalli with a few proliferations.
C. Broad thalli showing entire margins.
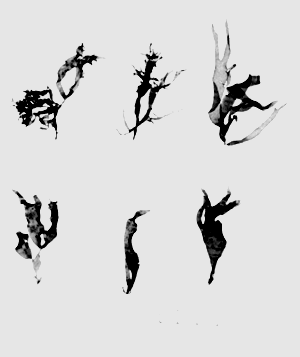
Figure 9: Gracilaria eucheumoides Harvey. Habit of sterile plants.
3. PROCESSING (AGAR YIELD AND QUALITY) OF GRACILARIA IN MYANMAR
3.1 Background and current status
Studies on the availability of agarophytes in Myanmar, methods of extraction, yield and the quality (gel strength, gelling and melting temperatures) of agar obtained have been carried out by the Department of Marine Science, Mawlamyaing University and the Central Research Organisation. Recently collected seaweeds were processed and the agar yield and quality were studied so that the results could be compared with those of previous studies. Altogether six species of Gracilaria were collected and their respective agar yield and quality was determined. A few connections between yield, quality and ecological notes were established. The results will partially fulfil the requirements of NACA's post-workshop activities and provide some data to the Myanmar Scientific and Technological Research Department for their objective of progressing to developmental research for agar production at an economic scale.
3.2 Materials and methods
i. Extraction of agar
100 g of sun-bleached Gracilaria spp. seaweed was washed with water and put into a 2.5 1 stainless steel beaker adding 1 litre of 2–6% NaOH solution. After heating at 90°C for 1.5 hrs the contents were filtered and washed with water and several ml of 0.1 mol/L HC1 was added to neutralise the excess of alkali. After filtering and washing, the seaweeds were then extracted with water at 100°C for 2 hours, which was finally filtered. The filtrate was transferred into a tray and allowed to congeal at room temperature. The gel was cut to short sticks and put into a refrigerator to freeze at about -10 °C for one day. The frozen gels were thawed with tap water and then sun-dried.
ii. Determination of agar yield
1–2 g. of dry seaweed and agar product were weighed separately and dried in an oven at 105°C for 2 hrs. After cooling in a desiccator the agar yield was calculated.
iii.. Determination of moisture content
2.5 g of seaweed powder or agar was weighed in a weighed bottle and dried in an oven at 105°C for 2 hrs. After cooling it was weighed and the moisture content was calculated.
iv. Determination of ash content
A crucible was treated with 3 mol/L HC1 solution and washed with water and then dried in a furnace at 500°C for 1/2ahr. The crucible was weighed after cooling in a desiccator. 2–5 g of seaweeds or agar were weighed into the weighed crucible and calcined in the furnace of 500 °C for 4 hrs. If it remained dark, 1 ml. of concentrated HNO3 was added after cooling and further calcined until combustion was complete. The ash content was then calculated.
v. Determination of gel strength of agar
a) Preparation of 1.5 % solution of agar:
1.5 g of agar was weighed and put into a 250 ml beaker and 100 ml of water was added. The agar was soaked for two hours at room temperature and then heated (while covering the beaker) until it dissolved completely.
b) Determination of gel strength with arm-balance type gel tester:
Twenty five (25 ml) of 1.5% agar solution was poured separately into four 50 ml beakers. After gelling at room temperature for at least two hours, the beaker with gel sample was placed on one hand of an arm-balance type gel tester, and a beaker with water was placed on the opposite balance arm. Adjustment was made by adding or subtracting water so as to have the gel and water beakers in an exactly balanced position. After zero setting the arm-balance, the stainless steel surface (1 cm × 1 cm) was placed on the surface of agar sample just to touch the uppermost layer of the gel and firmly clamped. Water was filled into the opposite beaker using a burette. The burette reading was equivalent to the weight in grams forced to the surface of the agar sample. The addition of water stopped as soon as the initial crack of agar was observed and the volume of water in the burette or weight in grams gives the gel strength in gm/cm2.
vi. Determination of gelling temperature of agar
50 ml of 1.5% solution of agar is poured into a test tube (2.5 × 20 cm) to 10 cm height. A thermometer was inserted through a rubber stopper into the solution and the temperature was set at room temperature to congeal the solution slowly. At around 40 °c, the test tube was inclined to a 45° angle and the temperature when the surface of the solution stops moving, was observed.
vii. Determination of melting temperature of agar
The test-tube used above was put in cold water allowing it to congeal completely, and then heated in a water bath. At the same time a stainless steel ball (Æ.3 mm) was dropped down through another hole in the stopper onto the gel surface. The temperature that the ball dropped down through the solution was observed.
viii. Determination of the sulphate content of agar
A weighed sample (2–3 g) and 25 ml of concentrated nitric acid were boiled gently in a 400 ml tall-form beaker covered with a watch glass. When the evolution of brown fumes has ceased, 1 g of potassium chlorate was added in portions. If the residue was not white, the oxidation was repeated. Concentrated HC1 (25 ml) was then added and the solution evaporated to dryness to remove the last traces of nitric acid.
The residue was dissolved in water and filtered through paper (No. 40, 11 cm, ashless). The filtrate was diluted to 200 ml. Two drops of concentrated HC1 were added and the solution was heated to boiling in a covered beaker. 10 ml of 10% barium chloride solution was then added dropwise with stirring. The boiling was continued for a few minutes and the solution was kept in hot water for 6 hours. The precipitate of barium sulphate was filtered on a weighed porcelain filter crucible. The latter was heated 1 hour at 800°C, cooled in a dessicator and weighed.
It was essential to remove all nitric acid in the above procedure as barium nitrate may be precipitated with barium sulphate giving a high value. Any tendency of barium chloride to co-precipitate can be avoided by its slow addition to the hot solution. Chloric acid is decomposed during the digestion with HC1.
ix. Infrared spectroscopic analysis of agar
4–6 mg agar was weighed into a test tube and 2 ml of water was added. It was then heated to dissolve the colloid (agar). An appropriate amount of solution was transferred to a plastic cover and dried at 40–50°C to allow a film to form, this was then subject to Infra red spectrometric identification.
3.3 Results
Six species of Gracilaria in Myanmar were collected, processed and analysed for agar yield and quality. For the analysis, the laboratory methods for red seaweeds and their polysaccharides, recommended and provided by NACA, were mostly applied. Due to the lack of proper equipment and apparatus, in some cases (e.g. determination of gel strength or sulphate contents) other standardised procedures had to be employed.
The data in Table 1 shows a wide range of variations in results. These variations depend predominantly on the age or maturity and the degree of development of the seaweed. The results, however, do not indicate what species of Gracilaria gives the best results for agar production, all of the species gave agar of fair quality for food grade.
Table 1: Yield and quality of agar extracted from some Gracilaria species in Myanmar.
| 1.5% solution of agar | Sulphate content (%) | Ash | ||||||
| No. | Gracilaria sp. | Agar Yield (%) | Gel Temp (°C) | Melt Temp (°C) | Gel Strength gm/cm | Pre treated | Non treated | Content (%) |
| 1 | G. verrucosa | 9 – 22 | 27 – 40 | 52 – 70 | 62 – 120 | 1.5 – 3.0 | 3.5 – 4.2 | 4.9 – 6.5 |
| 2 | G. edulis | 10 – 22 | 27 – 39 | 52 – 73 | 60 – 120 | 1.5 – 2.9 | 3.1 – 4.02 | 4.8 – 5.9 |
| 3 | G. crassa | 12 – 21 | 28 – 40 | 55 – 73 | 70 – 120 | 1.5 – 2.9 | 3.0 – 4.02 | 5.0 – 6.5 |
| 4 | G. foliifera | 11 – 19 | 28 – 39 | 50 – 82 | 60 – 98 | 1.5 – 3.1 | 4.1 – 5.0 | 4.8 – 6.5 |
| 5 | G. millardetii | 10 – 19 | 29 – 39 | 50 – 80 | 60 – 92 | 1.5 – 3.0 | 3.4 – 4.2 | 4.8 – 5.9 |
| 6 | G. textorii | 11 – 19 | 27 – 39 | 52 – 73 | 60 – 110 | 1.5 – 3.0 | 3.6 – 4.2 | 4.8 – 6.5 |
4. EXPERIENCE OF GRACILARIA CULTURE IN MYANMAR (Figures 10–12)
Eight species of useful seaweeds were experimentally cultured including four species of Gracilaria: G. edulis; G. verrucosa; G. crassa; and G. foliifera. Basically, constant depth or constant level methods were applied depending on the species to be farmed. Different artificial substrata were used except in broadcasting methods, which was done in ponds. Ropes and bamboo were mostly used to form artificial substrata, constructed in a way that takes into consideration the optimum requirements of the plants.
Some seaweeds were cultured experimentally in the intertidal area, tidal creeks, the open sea and in ponds. Seed stocks used were selected from natural stocks. The initial plants should be free from weeds and unhealthy signs. The luxuriant strain with fresh and healthy thallus were the most suitable initial seed stocks used. Various kinds of culture methods including different systems constructed to hold the artificial substrata, were devised considering the following factors:
Readily available materials were used in construction;
Easily manipulated structures were designed;
Environmental requirements of the cultured species were considered;
The seaweed should be easily planted, weeded and harvested;
High durability in seawater; and
Lowest investment cost.
Large amounts of spore producing plants of G. edulis and G. verrucosa were obtained while these species were growing. G. edulis had shown good potential for culture. There were obvious changes into sexual plants and spores were produced which settled and germinated on the culture bed. The germlings grew rapidly to harvestable size. Three main problems were found to be the principal constraints to successful farming: seed availability; grazing by fish; and weeds and pests.
The culture experiments were carried out according to the following timescale:
Construction and preparation of culture beds : November to January
Planting, fixing the initial plants to the nets : January to February
Weeding : February to March, (regular work)
Harvesting: (March to September)
Nursery: (March to September)
Drying: (throughout the harvesting period)
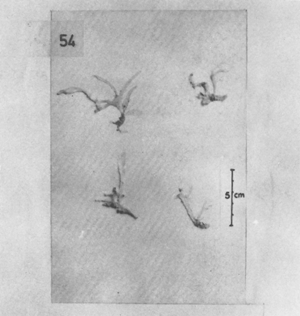
Figure 10: Myanmar coastline showing the distribution of different species of Gracilaria observed in the present study.
Figure 11: Hydrographic map showing the situation of the seaweed farm (G. edulis), Maung-shwe-lay, Rakhine Coast, Myanmar.
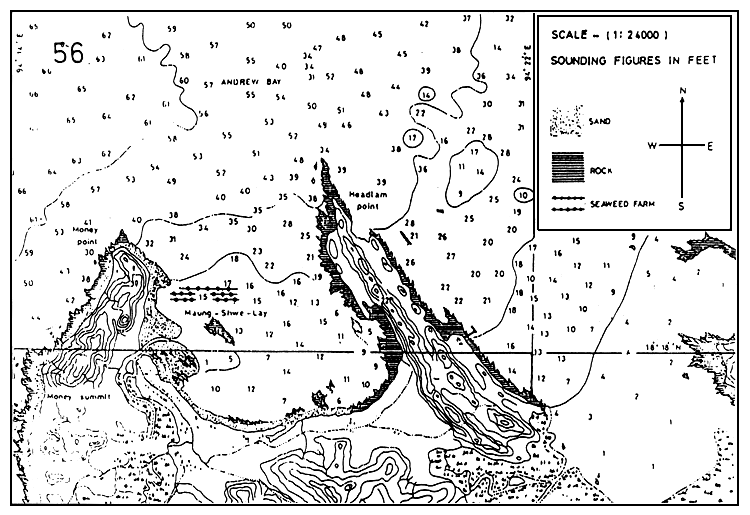
Farming season for G. edulis at the Maung-shwe-lay site was as follows:
| • | February: | Start of growth period. |
| • | March to September: | Growing period for production. |
| • | September to November: | Deteriorating period. |
| • | November to February: | Resting period. |
G. edulis grows through 8 months of the year on culture beds. The plants can be harvested once a month from April to September. The results show that the cultivation of G. edulis has commercial potential. Data collected from this experiment are shown in Table 2.
Table 2: Annual harvest of G. edulis from Maung-shwe-lay seaweed farming site.
| Sr. No. | Year | No of net line system | Wet wt (kg) | Dry wt (kg) | Remark |
| 1 | 1980 | 25 | 243 | 34 | |
| 2 | 1981 | 40 | 567 | 81 | |
| 3 | 1982 | 50 | 2,835 | 405 | |
| 4 | 1983 | 50 | 3,965 | 567 | |
| 5 | 1984 | 90 | 1,134 | 167 | Damage by storm |
| 6 | 1985 | 90 | 2,268 | 324 | |
| 7 | 1986 | 90 | 2,438 | 348 | |
| Total | 13,450 | 1,926 |
Figure 12: Some records of G. edulis culture experiments at Maung-shwe-lay, Rakhine Coast, Myanmar.
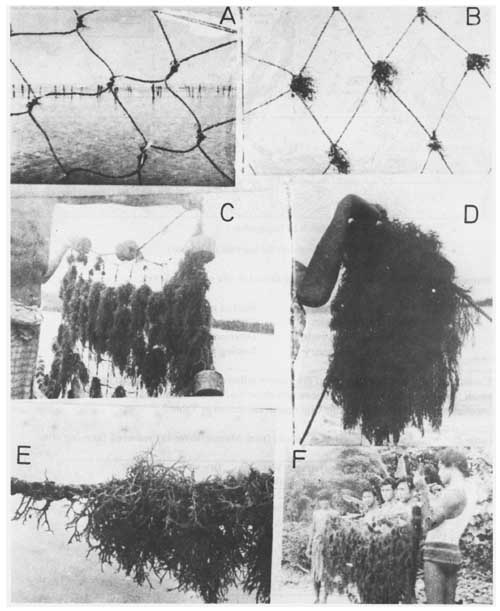
Our experiences in the experimental culture of the seaweeds had resulted in the following important considerations:
The simplest design or system is usually the most appropriate one;
Locally made large fibre-glass balls are available to use as floats on calm sea surface with wave action and currents;
Single floating lines are the most suitable system to cultivate G. edulis in the open sea area;
Sequential work activities have to be followed and carried out as scheduled;
Knowledge on the biology of species such as scientific names, growing nature (habit and habitat) and ecological relationship are essential to successful cultivation;
The local inhabitants must be convinced on the feasibility of farming. They should also be organised into a farmers co-operative;
Problems of harvesting and drying the farm produce (seaweed) may become one of the problems for operation of commercial farms; and
Seaweed farming projects for commercial scale culture is a long term activity.
5. DISCUSSION AND CONCLUSION
Detailed taxonomic studies on Gracilaria spp. in Myanmar and agar-extraction research work have shown some economic potential. Our research data show that all of the Gracilaria species gave a fair yield and quality as food grade agar.
Gracilaria species are one of the most common species of seaweeds in Myanmar. The economically important species, such as G. verrucosa and G. edulis are, however, restricted to estuarine areas. Best development of those two species occurs in the salinity range of 20 to 30 ppt and a temperature between 18 and 28°C. G. foliifera is characteristically different and thrives in a typically marine environment. Experience of G. edulis culture from 1978 to 1987 showed that maximum thallus development occurred during the month of February to September every year. The following conclusions may be formulated from our studies of G. edulis:
It has been noted that G. edulis is the most common species whose thallus development is best compared with other species. Because of this dominant characteristic, it can be concluded that Myanmar coastal waters are naturally suited for this species.
Growing habit is commonly restricted at the well-protruded tissues and it grows luxuriantly on small holdfast which attaches to any kind of hard substrate. It is important to consider the problem of site and selection of artificial substrata for use in farming.
The results of our experiments showed that G. edulis can grow successfully and its potential for large scale production is high. The results also showed that two different methods are applicable, inland pond culture and coastal open sea culture.
6. RECOMMENDATIONS
The objective of this study was to evaluate and pinpoint the suitability of specific species of Gracilaria for culture in Myanmar. Based on the study results, Myanmar will concentrate on studies relating to the species Gracilaria edulis as a post workshop activity.
The phycocolloid industry in Myanmar has been practised since 1974. Successful laboratory extraction in 1970 led to the implementation of commercial scale phycocolloid industries. This extraction technology was first exploited by the Government and later many private cottage industries began commercial operation. It partially fulfils the country's need for phycocolloid products.
Much experience has been gained by our previous research and farming experiments on G. edulis therefore problems and difficulties which will inevitably occur in culture may be anticipated and avoided. The following are a few points which should be considered when further production and expansion of seaweed culture are planned:
There should be more co-operation and collaboration between people concerned with seaweed cultivation and agar production to develop a processing industry;
Raw material procurement and processing of the seaweeds must be synchronised properly to gain maximum profit and reduce the cost of production. A continuous flow of well-grown harvested material to the processing plant site should be guaranteed and planned accordingly;
Priority should be given to research work for commercial production;
Budget allocation or investment should be sufficient to meet the goals of the project;
For open sea culture, a well-planned and functioning hatchery and nursery pond for spore collection and seed stock should be established;
For pond culture, our experience indicates that polyculture or mixed culture should be practised. Mixed culture with seaweed and prawn (Penaeus monodon) or brine shrimp (Artemia spp.), in coastal ponds have been getting a lot of attention in Myanmar recently;
Agar processing from seasonally harvested farmed seaweeds should be carried out to determine which species gives an agar product of good quality with high yield;
To correlate the most productive season and agar quality in order to obtain optimum conditions from an economic standpoint, seasonal crops should be systematically harvested and processed under constant parameters.
To upgrade agar quality, more research work on processing techniques and purification of products should be carried out. Furthermore, research work on advanced culture techniques and cross-breeding experiments leading to cultivating a high yield crop for economic size farming experiments should be planned. For that purpose, proper equipment, well-trained personnel as well as other supporting materials should be provided. This research work should be performed at a suitable coastal area by a group which must have support and co-operation from various sources within the country as well as abroad.
ACKNOWLEDGEMENTS
First and foremost the authors would like to express their gratitude to NACA-FAO Government Co-operative Programme Authorities who partially fulfil the development of Asia-Pacific region including Myanmar. Secondly to the Directorate General, Deputy Director General and superior departmental officials of Myanmar Scientific and Technological Research Department (MSTRD) who always encourage me to carry out this research work on the utilisation of seaweeds in Myanmar. Thirdly to U Aung Myint (Marine Biologist, IFE) and to U Soe Tun (Manager, DOF) for their intensive discussions and suggestions concerning seaweed culture experiences. Lastly to my commemorate researchers in Maung-shwe-lay farm and Applied Chemistry Research Department of MSTRD, particularly to Daw Mo Mo Than, Daw Mi Mi Soe and U Than Soe, for their intensive participation.
REFERENCES
Hla Hla, C. 1975. Studies on the genus Gracilaria (Rhodophyta, Gigartinales) of Burma. Thesis for the degree of Master of Science in Marine Biology, Rangoon University.
Kyi Shwe, U. and Aung Myint, U. 1991. List of identified seaweeds of Myanmar. (Read at a Research Seminar in Myanmar).
Kyi Win, U. 1972. A classification of the seaweeds of Burma. (Read at the Burma Research Congress in Myanmar, 1972).
Ma. Ethel G. Liana,
Bureau of Fisheries and Aquatic Resources,
P.O. Box 623, Manila, Philippines.
ABSTRACT
This paper (Part I) discusses the taxonomy and ecology of 11 Gracilaria species, namely: G. arcuata; G. changii; G. eucheumoides; G. fastigiata; G. firma; G. gigas; G. heteroclada; G. lemaneiformis; G. manilaensis; G. salicornia; and G. tenuistipitata, identified from the materials collected in Philippine waters between June 1992 and December 1994. Collecting sites include several areas in eight provinces of the Philippines, namely: Cagayan, La Union, Cavite and Sorsogon in Luzon Island; Aklan, Samar and Bohol in the Visayas region; and Sulu (Tawi-Tawi and Sitankai) in Southern Mindanao.
Of the 11 species reported, five (G. changii, G. fastigiata, G. firma, G. heteroclada and G. tenuistipitata) have been identified as potential species for culture or farming purposes, based on the quality of their phycocolloid as well as number of ecological considerations. Of these five, G. firma, G. heteroclada and G. tenuistipitata were considered priority culture species based primarily on their wide distribution in the country. This paper also includes a discussion of the present status of the country's seaweed industry and a brief review of previous related studies on Philippine Gracilaria spp.
1. INTRODUCTION
Seaweeds constitute the Philippines' third largest fishery export (after tuna and shrimp), contributing to national economy in terms of foreign exchange, as well as domestic revenue and employment. Seaweeds contribute 41% to overall aquaculture production, thereby topping the list of major cultured species; and 90% of the country's total mariculture production. The Philippines is the leading producer of raw (dried) Eucheuma spp. and semi-refined carrageenan, also called Philippine Natural Grade (PNG) carrageenan, contributing 70% of the world's supply. The country still ranks fourth among the largest seaweed-producing countries in the world and second (after Japan) in the production of red seaweeds.
One of the world's most important agarophytes is Gracilaria spp. which is also highly utilised (at least for the commercial species) in the Philippines. Although ranking only third in importance (after Eucheuma spp. and Caulerpa spp.) in terms of production, it has more potential than Caulerpa spp. in terms of utilisation or application. The utilisation of Gracilaria spp. is not limited to human food or animal feed. The seaweed can also be dried for export or domestic markets, or used as a raw material for local agar production. Agar is very popular in the Philippine market as processed agar bars, locally called “gulaman”.
1.1 Statistics of production and value of seaweeds
The trend in seaweed production in the Philippines is generally still increasing. Table 1 shows the annual production data on cultured seaweeds (mainly Kappaphycus sp. and Eucheuma sp.) which account for 99.5 % of the country's total seaweed production. The remaining 0.5 % comes from the gathering of wild or natural stocks.
Table 1: Five-year production data on cultured seaweeds in the Philippines, 1989–1993.
(Source: BAS, Fishery Statistics, 1993.)
| Year | Quantity (mt) | Value ('000 Peso2s) |
| 1989 | 268,701 | 604,578 |
| 1990 | 291,176 | 796,077 |
| 1991 | 283,783 | 1,039,771 |
| 1992 | 349,505 | 1,526,896 |
| 1993 | 380,010 | 1,544,288 |
2 1 US $ = 25 Pesos approximately
The volume of seaweeds produced in 1989 increased in 1990 by 8.36% and the value by 31.67%. However, in 1991 seaweed production declined in volume by 2.5%, but increased in value by 30.6%. In 1992, there was a remarkable increase in production, both in quantity (23.2%) and value (46.85%). In 1993, there was again an increase in the volume (8.72%) and value (1.14%) of seaweed production.
The volume of Gracilaria spp. gathered from the wild stocks has fluctuated annually (Table 2), but the value of production has maintained an increasing trend.
Table 2: Production data on Gracilaria spp., 1983–1991. (Sources: BFAR, Fishery Statistics of the Philippines, 1983–1987; BAS, Fishery Statistics Bulletin, 1990–1991.)
| Year | Quantity (tonnes) | Value ('000 Pesos) |
| 1983 | 371 | - |
| 1984 | 415 | - |
| 1985 | 655 | - |
| 1986 | 728 | - |
| 1987 | 434 | - |
| 1988 | - | - |
| 1989 | 398 | 1,218 |
| 1990 | 359 | 1,865 |
| 1991 | 452 | 2,671 |
Production volume dropped by 9.8% in 1990 from the previous year's level. However, the value of production increased by 53.1%. In 1991, there was a remarkable increase of 25.9% in the quantity of Gracilaria spp. produced as well as the value, which increased by 43.2%. The largest volume of gathered Gracilaria spp. seaweeds came from the Southern Tagalog and the Central Visayas regions.
Dried Gracilaria spp. costs from three to five pesos per kilo, depending on the species and quality of the product. The retail value of processed Gracilaria bars or “gulaman” is Pesos 6.50–7.50 per bar (approximately weighing five grams) or Pesos 1,300–1,500 per kilogram (Guanio, 1993).
1.3 Local consumption, export and import
About 99% of total seaweed production is absorbed by local processors and export traders while only 1% is consumed locally as food. It is estimated that 75 % to 97 % of seaweed production is exported as dried seaweeds. The remaining 3 % to 25 % is utilised by local processors. Seaweed exports have been declining since 1990, as shown in Table 3.
Table 3: Export data on dried seaweeds.
| Year | Quantity (tonnes) | Value ('000 Pesos) |
| 1990 | 35,346 | 1,192,331 |
| 1991 | 26,830 | 581,950 |
| 1992 | 22,756 | 480,398 |
Denmark has absorbed most of the country's seaweed exports, about 26% to 33% of the total volume. Many other countries like France, the United Kingdom, U.S.A. and Korea also absorbed larger shares (11–17 %) compared to the other importing countries. The country's imports of dried seaweeds are minimal and declining, as shown in Table 4.
Table 4: Import of dried seaweeds to the Philippines.
| Year | Quantity (tonnes) | Value ('000 Pesos) |
| 1989 | 723 | 1,297 |
| 1990 | 473 | 923 |
| 1991 | 143 | 377 |
The largest volume of imported dried seaweeds was supplied by Indonesia. In 1991, import from this country abruptly decreased as the Philippines started to import dried seaweeds from Taiwan.
2. GRACILARIA OF THE PHILIPPINES
2.1 Literature review
Among the first of the early reports on Philippine Gracilaria spp. (citing Abbott, 1994) were those of Montagne (1844) of G. confervoides, and Dickie (1876) of G. dactyloides and G. eucheumoides from the Challenger Expedition specimens. The report of Abbott (1985) which included 17 species and one variety, and that of Silva et al. (1987) with 23 species, one variety and one form, represent compilations of species from earlier literature on Philippine Gracilaria spp.
Previous studies which dealt specifically with Gracilaria spp. taxonomy and ecology were those by Manuel (1979), Trono and Azanza-Corrales (1980, 1981), Trono et al. (1983) and Yamamoto (1989). Recent studies include those by Trono (1992, 1993), Abbott (1994) and Yamamoto and Trono (1994). Among other previous works on benthic marine algae dealing with taxonomic descriptions and ecological aspects, including those of Gracilaria spp., were those by Galutira and Velasquez (1963), Velasquez et al. (1972), Trono-and Ganzon-Fortes (1980, 1988), Cordero (1981), Trono and De Lara (1981), Trono and Ang (1982) and Hurtado-Ponce et al. (1992). Other related literature include the more recent reports by Hurtado-Ponce (1990), Liana (1992, 1994) and Nyan Taw (1993a, 1993b).
The literature review revealed that there have been many studies already conducted on Philippine Gracilaria, however, the identification of Gracilaria species has not been established until now. New names have been added and old names have been renamed so confusion exists in the recognition of species. Moreover, according to Yamamoto and Trono (1994), several of the species reported probably have not been identified correctly. Abbott (1994), stressed mat nearly all the Gracilaria spp. reported for the Philippines require a critical re-examination.
2.2 Materials and methods
Materials were collected from the wild or natural stocks of Gracilaria spp. seaweeds from eight different areas in the Philippines, namely: Cagayan, La Union, Cavite and Sorsogon in Luzon Island; Aklan in Panay Island in the Western Visayas region; Samar in the Eastern Visayas; Bohol in the Central Visayas; and Tawi-Tawi, Sulu Province, in Southern Mindanao (Figure 1). Once only collection of materials was done in most of these areas, except in Sorsogon and Samar.
Collection of samples and ecological data gathering, between June 1992 and December 1993, was done in Sorsogon, the site of the FAO/UNDP Seaweed Production Development Project (PHI/89/004) being implemented by the Bureau of Fisheries and Aquatic Resources (BFAR). The following areas were covered: Bagacay, Gubat; Layog, Barcelona; and Dancalan, Bulusan, along the eastern coast of Sorsogon Province; and Tughan, Juban, along Sorsogon Bay, designated as Stations 1 to 4, respectively, including Casiguran, Castilla, Prieto Diaz and Caditaan in Magallanes (Figure 2) which were also visited during the monitoring period.
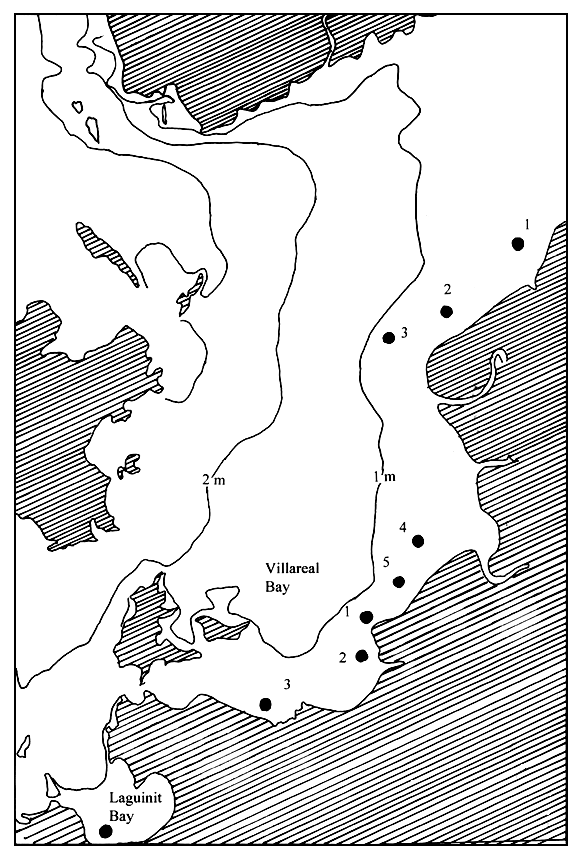
In Western Samar, the site of last year's seaweed resources assessment project of the BFAR, collection of Gracilaria samples and ecological data gathering was carried out monthly, starting February until December 1994, in three adjoining bays, namely: Maqueda Bay, Villareal Bay and Laguinit Bay. Five stations were occupied in Maqueda Bay (San Sebastian, Hitaasan, Barubaybay, Pinabacdao and San Isidro), three stations in Villareal Bay (Mayongpayong Pt, San Rafael and Mahayag) and only one station in Laguinit Bay (San Roque) (Figure 3).
Figure 1: Philippine map showing the areas (provinces) from where the Gracilaria spp. materials were collected.
Figure 2: Map of Sorsogon Province showing the stations occupied for sampling/monitoring activities.
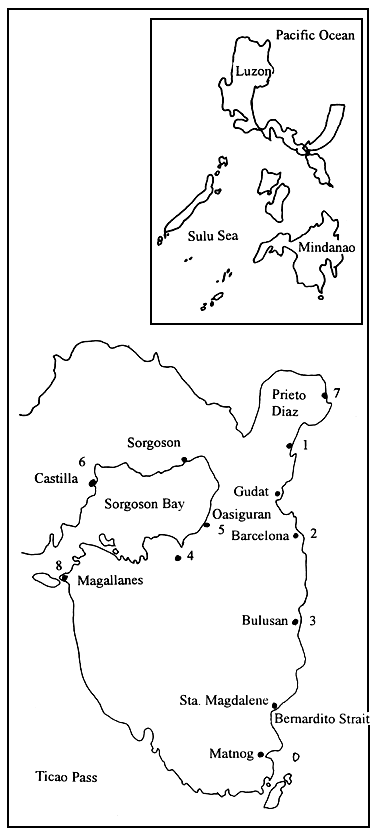
Figure 3: Map of Samar (Philippines) showing the location of the stations used for sampling activities in Maqueda Bay, Villareal Bay and Laguinit Bay.
Collected materials were sorted by species. A few of these were segregated as specimens for the herbarium collection and taxonomic studies; the rest were dried for processing and analysis. Sections were made to aid further taxonomic analysis.
2.3 Description of the species
Eleven species of Gracilaria identified from the materials collected are described here, with reference to previous studies particularly those of Trono (1993).
1. Gracilaria arcuata Zanardini (Figure 4).
Plants cartilaginous and greenish to purplish in colour. Thallus erect; branches terete or cylindrical throughout and slightly constricted at the base. Branching at the primary and secondary axes generally secund. Main branches arcuate, distal portion of branches cervicorn, ultimate branchlets coarse spinose.
Cortex consists of one to two layers of round to oval cells. Cell size transition from cortex to medulla very abrupt. Cystocarps hemispherical, not constricted at the base and scattered along the entire thallus. Pericarp consists of one layer of elongated cells at the surface; the inner layer of thin-walled cells arranged anticlinally. Base of gonimoblast made up of small irregularly shaped and flattened cells. Carpospores in chain.
2. Gracilaria changii Xia et Abbott (Figure 5).
Plants erect to spreading, attached to substratum by disc-like holdfast. Branching irregular. Main axis percurrent, branches cylindrical, lateral branches distinctly constricted and somewhat enlarged near the base, and tapering toward the apices. Cortex with two to three layers of cells; medulla with large, thin-walled parenchymatous cells; cell size transition from cortex to medulla very abrupt. Cystocarps hemispherical, some slightly rostrate, base not constricted. Gonimoblast with small cells; nutritive filaments few, pericarp thick, with three to six layers of oval outer cells and six to seven flattened inner cells. Spermatangial conceptacles verrucosa type to Polycavernosa type.
Figure 4: G. arcuata.
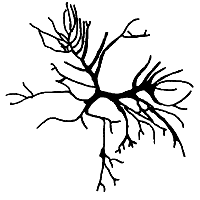
Figure 5: G. changii
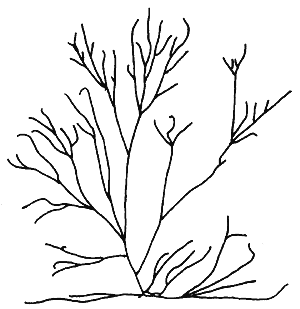
3. Gracilaria eucheumoides Harvey (Figure 6).
Plant prostrate with creeping thick branches attached to hard substrate by hapters. Greenish-brown to purplish in colour. Hapters strongly developed at basal ventral portions of branches. Branching is irregular. Branches distinctly compressed, with the margins serrate or with coarse, short dentations. Cortex thick, from two to four layers of cells, medulla consists of thick-walled cells, cell size transition from medulla to cortex, gradual.
4. Gracilaria fastigiata (Chang et Xia) Chang et Xia (Figure 7).
Plants erect, usually green but sometimes dark green in colour; exposed plants lighter, dry ones brownish to purplish in colour. Thallus terete or cylindrical. Branching irregular to alternate. Main axes more robust than branches. Branches not constricted, but sometimes slightly tapered at the base, generally bifurcating and usually becoming finer and fastigiate toward distal portions. Anastomosing or curving branches sometimes present, spinose branchlets commonly present. Cortex consists of one to three layers of cells; medullary cells thin-walled; cell size transition from cortex to medulla abrupt.
Cystocarps semi-globose to globose, slightly constricted at the base, rostrate, irregularly distributed along the thallus. Pericarp consists of 9 to 11 layers of cells; to three outermost layers anticlinally arranged, elongated to irregularly oval, becoming larger in the mid-layers with granular cell contents; two to three innermost layers flattened, small cells present in the bottom medial portion of pericarp.
Figure 6: G. eucheumoides.

Figure 7: G.fastigata.
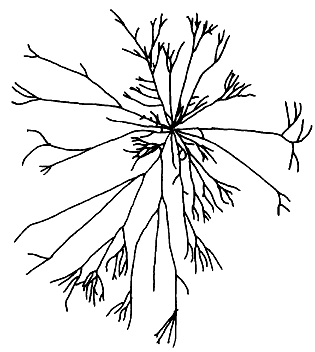
Nutritive filaments prominent, branching, found near the base or below gonimoblast. Centre of gonimoblast composed of large cells with granular cell contents.
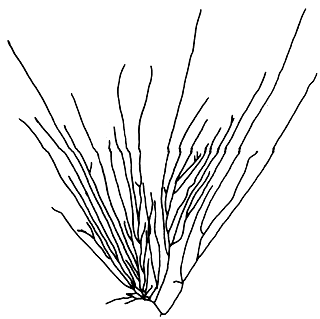
5. Gracilaria firma Zhang et Xia (Figure 8).
Plants erect, texture firm to leathery, yellowish brown or green in colour. Branches terete or cylindrical, distinctly constricted at the base. Branching irregular or alternate, sometimes unilateral. Axes and branches robust.
Cortical cells one to two layers, irregularly round to oval; medullary cells thin-walled; cell size transition from medulla to cortex gradual.
Cystocarps conical or semi-globose, not constricted at the base, somewhat embedded in thallus and rostrate. Pericarp consists of seven to 13 layers of cells thinning somewhat near the base; outermost layer of cells round to ovoid; middle layers flattened, ovoid or showing pit connections; innermost layer flattened or disintegrating. Nutritive filaments absent. Carpospores in chains; round, ovoid or tear-drop shaped. Centre of gonimoblast made up of densely packed small cells. Spermatangial conceptacles deep and oval in shape (verrucosa type).
6. Gracilaria gigas Harvey (Figure 9).
Plants erect, texture cartilaginous. Thallus axes coarse, thick and succulent. Branches few and cylindrical, with a short stipe showing slight constriction at the base, branch apices acute, sometimes blunt.
Cortex thick, with two to four layers of rounded cells. Cell size transition from medulla to cortex gradual.
Cystocarps prominent, semi-globose, slightly rostrate and constricted at the base. Pericarp consists of 13–15 layers of cells; outermost layer marked by elongated cells, followed by layers of anticlinally arranged cuboidal to elongated cells becoming smaller and loosely arranged towards the gonimoblast. Carpospores not forming chains, oval to obovate, with stellate central body. Nutritive filaments abundant between pericarp and gonimoblast.
7. Gracilaria heteroclada Zhang et Xia (Figure 10).
Plants erect, up to more than 60 cm tall, dark brown to purple, sometimes olive-green in colour, brittle when fresh. Thallus terete or cylindrical throughout. Branching irregularly alternate. Main branches percurrent with many spinose determinate branchlets; branches distinctly larger than the branchlets.
Cortex thin, consisting of one to two layers of irregularly spherical to oval cells. Medulla consists of large parenchymatous cells, surrounded by two to three layers of small roundish cells. Transition of cell size from medulla to cortex abrupt.
Cystocarps prominently protruding, hemispherical, nonrostrate to slightly rostrate, not constricted at the base. Centre of gonimoblast composed of small ovoid cells. Spermatangia superficial and continuous over surface of thallus (chorda type).
Figure 8: G. firma.
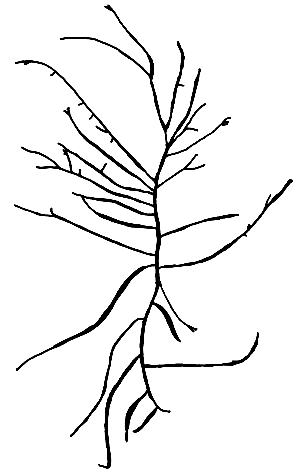
Figure 9: G. gigas.
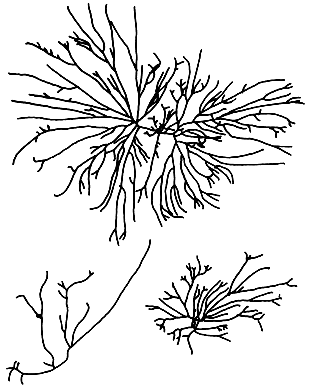
Figure 10: G. heteroclada.
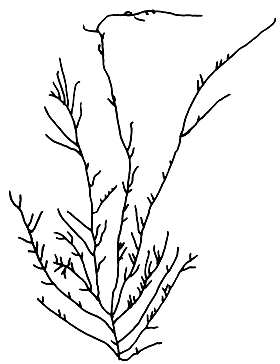
Figure 11: G. lemaneiformis.
8. Gracilaria lemaneiformis (Bory) Weber-van Bosse (Figure 11).
Plants solitary or caespitose, with few to several long branches. Branching irregular, mostly from lower portion. Cortex consists of two layers of cells, outermost layer elongate; medullary cells large and thin-walled; transition in cell size from medulla to cortex abrupt.
Cystocarps spherical, slightly rostrate or nonrostrate, constricted at the base; pericarp 10–14 layers; gonimoblast cells consist of filaments of many small cells with obscure cell walls; carpospores rounded to ovoid; absorbing filaments absent. Spermatangial conceptacles superficial (chorda type).
9. Gracilaria manilaensis Yamamoto et Trono (Figure 12).
Plants fleshy to somewhat cartilaginous, purplish red or sometimes greenish in colour. Fronds caespitose; main axis cylindrical throughout, usually percurrent. Branching alternate, sometimes secund or irregular. Branches abundant, long, similar to main axis, with lateral branchlets; both branches and branchlets sharply constricted at the base.
Cortex consists of one to two layers of dense protoplasmic cells; outermost cells with primary pit connections; hair basal cells scattered. Medullary cells polygonal, increasing in size toward the centre; outer layer more or less compressed parallel to frond surface. Cell size transition from cortex to medulla abrupt.
Cystocarps globose; gonimoblast cells elongated; absorbing filaments present, penetrating into pericarp. Spermatangial conceptacles cup-shaped, roundish or oval (verrucosa type).
Figure 12: G. manilaensis.
10. Gracilaria salicornia (C. Agardh) Dawson (Figure 13).
Plants semi-erect to erect, bright yellow to dark green in colour. Branching dichotomous to trichotomous, divaricately arranged. Branches distinctly divided into terete or cylindrical, subclavate to clavate segments which are swollen at the distal end and constricted at the base.
Cortical cells, two to three layers; gland cells numerous; cell size transition from medulla to cortex gradual.
Cystocarps prominent, globose, slightly rostrate, slightly constricted or not at the base; pericarp thick, 10–11 layers of cells; nutritive filaments present. Spermatangial conceptacles oval (verrucosa type).
11. Gracilaria tenuistipitata var. liui Zhang et Xia (Figure 14).
Plants generally small, yellowish brown to black in colour. Thallus slender, somewhat tough and firm. Branching irregular, mostly from percurrent axes. Branches not constricted at the base, tapering to acute apices occasionally bifurcating.
Cortex consists of two to three layers of round to oval cells; transition in cell size from medulla to cortex gradual.
Female fertile plants usually small and less branching. Cystocarps prominent, often broader than the branch, and scattered irregularly along main branches, semi-globose to globose, rostrate, constricted at the base. Pericarp thin, outer two layers cuboidal, inner layers flattened to oval, showing pit connections. Absorbing filaments rare. Centre of gonimoblast with small cells with strong pit connections. Carpospores in chains. Spermatangial conceptacles consist of shallow cavities (textorii type).
In addition to these species, there are at least three more species which have not been identified but have been recognised from the materials collected as being separate species from those reported in this paper.
2.4 Ecology of the species
Gracilaria spp. is widely distributed throughout the Philippines. Different species have been reported to occur in various habitats in many areas of the country, however, very few species occur in abundance in one locality, and not too many localities or areas have an abundance of the Gracilaria spp. resource throughout the year.
Gracilaria spp. seaweeds are generally found in shallow water, from the lower intertidal zone to the upper subtidal areas. They thrive in almost all types of habitats - from mangrove areas and brackish waters of soft and mud substrates or firm sandy type of bottom to open sea areas of rocky-coralline substrates. A species may be found in several areas of these habitats but may also be restricted to a particular substratum. The type of bottom generally has a profound effect on their distribution. A soft substratum, for example, characteristically supports a rich growth of G. heteroclada and G. tenuistipitata. However other species, like G. firma or G. fastigiata prefer a firm type of bottom to which they can be attached by means of holdfasts.
Figure 13: G. salicornia.
Figure 14: G. tenuistipitata var. liui.

Among the other species that prefer a firm substrate are G. arcuata, G. changii, G. eucheumoides, G. fastigiata, G. gigas and G. salicornia. G. manilaensis and G. lemaneiformis prefer soft-bottom areas.
With respect to the salinity and water temperature requirements of Gracilaria spp., the data obtained from Sorsogon showed that variations in salinity were not very great at Station 1 compared to the other stations. Salinity values obtained, on average, ranged from about 25 ppt to 37 ppt. At Station 2, salinity dropped to as low as 2.0 ppt in December from a high salinity value of 35 ppt obtained in September. At Station 3, the salinity ranged from 5 ppt obtained in May to 36 ppt; while at Station 4, there was a tremendous drop in salinity from as high as 36 ppt to as low as 2.0 ppt.
Monthly fluctuations in water temperature were evident but the time of day that sampling was conducted could have made the difference. Nevertheless, a comparison of data obtained from different areas would indicate a uniform fluctuation pattern in all the stations. At Station 1, water temperature values ranged from 23.5 °C in February to 35 °C in June; Station 2, 25–36 °C; Station 3, 29–34 °C; and Station 4,24–35 °C.
3. DISCUSSION AND CONCLUSIONS
Although the species identification of the Gracilaria materials collected is still subject to verification, the species which are suitable for culture or farming purposes have been tentatively identified based on the quality of their agar content and ecological considerations, such as bottom-type preference and salinity.
The following species have been considered with potential for culture or farming: G. firma, G. changii, G. fastigiata, G. heteroclada and G. tenuistipitata. The first three species can be farmed in the open sea or in bays and coves, while the last two species are more suitable for farming in brackishwater ponds. Of these five species, however, G. firma, G. heteroclada and G. tenuistipitata can be considered priority culture species, considering not only their ecological characteristics but also their wide availability in many areas of the country.
G. firma, with quite a high percentage of agar yield and gel strength as analysed can be cultured in areas with a wide range of salinity (from 27 ppt to 37 ppt), in semi-turbid to clear water, and can withstand moderate to strong water movement. G. heteroclada and G. tenuistipitata, which have also good quality agar content and high yield, have the advantage of tolerating a wide range of salinity (as low as 2 ppt to as high as 36 ppt) and turbid waters, and may be cultured in ponds or sheltered bays where there is less wave action.
Among the areas where collection of Gracilaria spp. was undertaken, only Sorsogon and Samar (Maqueda Bay) are quite good sources of the raw material throughout the year. Cagayan (Buguey Bay), Bohol (Inabanga) and Tawi-Tawi (Buan Island) are also good sources; however, Gracilaria spp. is reportedly seasonal in these areas.
4. RECOMMENDATIONS
Considering the problem of Gracilaria spp. identification, it is recommended that follow-up studies on taxonomy and ecology should still be conducted to be able to gather sufficient samples, especially of the male materials.
Production development or culture studies should be continued and possibly be expanded to include other potential seaweed farming areas, for the three priority species: G. firma in open sea; G. heteroclada and G. tenuistipitata in sheltered bays and ponds.
REFERENCES
Abbott, I. A. 1985. Gracilaria from the Philippines: list and distribution of the species. In: Abbott, I. A. and J. N. Norris, (eds). Taxonomy of Economic Species with Reference to Some Pacific and Caribbean Species, pp. 89–90. California Sea Grant College Program, Univ. Calif., La Jolla, Calif. 167p.
Abbott, I. A. 1994. New records and a reassessment of Gracilaria (Rhodophyta) from the Philippines. In: Abbott, I. A., (e)d. Taxonomy of Economic Seaweeds with Reference to Some Pacific Species, Vol. IV, pp. 111–118. California Sea Grant College, Univ. Calif., La Jolla, Calif. 200p.
Bureau of Agricultural Statistics. 1991. Seaweeds situation report (1990–1991). Fishery Statistics Bulletin. Bureau Agricultural Statistics. 17p.
Bureau of Agricultural Statistics. 1993. Fishery Statistics 1984–1993. BAS, Dept. Agriculture, Quezon City. 49p.
Bureau of Fisheries and Aquatic Resources. 1983–87. Fisheries Statistics of the Philippines. BFAR, Dept Agric, Quezon City.
Cordero, P. 1981. Studies on Philippine marine red algae. National Museum of the Philippines., Manila 258p. 28 pls.
Dickie, G. 1876. Contributions to the botany of the expedition of HMS “Challenger”: Algae, chiefly Polynesian. J. Linn. Soc. London Bot 15:235–236.
Galutira, E. C. and G. T. Velasquez. 1963. Taxonomy, distribution and seasonal occurrence of edible marine algae in Ilocos Norte, Philippines. Philippine Journal of Science. 92:483–521.
Guanio, L. V. 1993. Preliminary assessment of the existing regional market of Gracilaria in the Sorsogon-Bicol area, Philippines. Field Document 07, Seaweed Production Development Project (PHI/89/004). Dept. Agriculture, Bu. Fish. & Aquatic Resources, UN FAO., Manila, Philippines. 8p.
Hurtado-Ponce, A. Q. 1990. Vertical rope cultivation of Gracilaria (Rhodophyta) using vegetative fragments. Botanica Marina 33:477–481.
Hurtado-Ponce, A. Q., Luhan, M. R. J. and Guanzon, N. G. Jr. 1992. Seaweeds of Panay. Aquaculture Dept, Southeast Asian Fish. Dev. Center, Tigbauan, Iloilo, Philippines. 114p.
Llana, M. E. G. 1992. Preliminary assessment of seaweeds and associated invertebrates in Eastern Sorsogon, Philippines. Field Document 01, Seaweed Production Development Project (PHI/89/004). Dept. Agriculture, Bu. Fish. & Aquatic Resources, UN FAO., Manila, Philippines. 11p.
Liana, M. E. G. 1994. The ecology of Gracilaria sites in Sorsogon Province, Philippines. Ms. 15p. (Submitted for publication in the Proceedings of the 3rd National Symposium on Marine Science).
Manuel, D. 1979. Studies on the taxonomy and distribution of Gracilaria species in the Philippines. (M.S. Thesis). University of the Philippines., College of Arts & Sciences, Diliman, Quezon City. 60p.
Montagne, C. 1844. Plantae cellulares quas in insulis philippinensibus a cl. Cuming collectae recensuit, observationibus non nullis descriptionibusque illustravit C. Montagne, D. M. London J. Bot 3: 658–662.
Nyan Taw. 1993a. Seaweed (Gracilaria) fanning trials in Sorsogon, the Philippines. Field Document 09, Seaweed Production Development Project (PHI/89/004). Department of Agriculture, Bureau of Fisheries & Aquatic Resources, UN FAO, Manila, Philipp. 19p. 2 pls.
Nyan Taw. 1993b. Manual on seaweed Gracilaria farming. Field Document 03, Seaweed Production Development Project (PHI/89/004). Department of Agriculture, Bureau Fisheries & Aquatic Resources, UN FAO, Manila, Philipp. 15p.
Philippine Council for Aquatic and Marine Research and Development. 1994. Seaweed R and D program evaluation. 27th Joint Governing Coun. & Tech. Advisory Comm. Meet, 28 Jan. 1994, PCAMRD HQ, Los Banos, Laguna. 28p.
Silva, P. C, Menez E. G. and Moe, R. L. 1987. Catalog of the benthic marine algae of the Philippines. Smithsonian Contributions to the Marine Sciences (27): 179p.
Trono, G. C. Jr. 1992. Notes on the taxonomy of Gracilaria and Sargassum. In: Calumpong, H. P. and E. G. Menez, (eds). Proceedings of the 2nd RP-USA Phycology Symposium/Workshop, pp. 45–51. Philippine Council for Aquatic and Marine Res. & Dev., Los Banos, Laguna. 376p.
Trono, G. C. Jr. 1993. The taxonomy of the genus Gracilaria in Sorsogon, Philippines. Field Document 13, Seaweed Production Development Project (PHI/89/004). Dept. Agriculture, Bu. Fish. & Aquatic Resources, UN FAO, Manila, Philipp. l1p.
Trono, G. C. Jr. and Ang P. Jr. 1982. Marine benthic algae from Bugsuk Island and vicinity, Palawan, Philippines. Kalikasan, Philipp. J. Biol. 11: 1–26.
Trono, G. C. Jr. and Azanza-Corrales R. 1980. The taxonomy and reproductive morphology of the Gracilaria species in Manila Bay, Philippines. Nat Sci. Dev. Bd., Univ. Philipp. Res. Highlights 2(4): 7–8.
Trono, G. C. Jr. and Azanza-Corrales R. 1981. The seasonal variation in the biomass and reproductive states of Gracilaria in Manila Bay. In: Levring, T., (ed.) Tenth Int'l. Seaweed Symposium, Part III: Special Sessions, pp. 743–748. Walter de Gruyter& Co., Berlin, Germany.
Trono, G. C. Jr., Azanza-Corrales, R. and Manuel, D.. 1983. The genus Gracilaria (Gigartinales, Rhodophyta) in the Philippines. Kalikasan, Philipp. J. Biol. 12(1–2): 15–41.
Trono, G. C. Jr. and de Lara A. V. 1981. Some marine benthic algae from Cabra and Lubang Islands, Occidental Mindoro, Philippines. Natural Applied Science Bulletin. 33: 1–49.
Trono, G. C. Jr. and Ganzon-Fortes E. T. 1980. An Illustrated Seaweed Flora of Calatagan, Batangas. Filipinas Foundation, Inc., Makati, Metro Manila. 114p.
Trono, G. C. Jr. and Ganzon-Fortes E. T. 1988. Philippine Seaweeds. National Book Store, Inc., Metro Manila. 330p.
Velasquez, G. T., Trono G. C, Jr. and Doty M. S. 1972. Algal species reported from the Philippines. Philipp. J. Sci. 101: 115–179.
Yamamoto, H. 1989. Gracilaria and Polycavernosa species of the Philippines. In: Umezaki, I. (ed). Scientific Survey of Marine Algae and their Resources in the Philippine Islands, pp. 35–40. Mombusho Int'l. Scient Res. Program, Kyoto Univ. Press, Kyoto, Japan.
Yamamoto, H. and G. C. Trono, Jr. 1994. Two new species of Gracilaria from the Philippines. In: Abbott, I. A., (ed). Taxonomy of Economic Seaweeds with Reference to Some Pacific Species, Vol. IV, pp. 95–101. California Sea Grant College, Univ. Calif., La Jolla, Calif. 200p.