Purita O. dela Pena
National Project Co-ordinator, UNDP/FAO/PHI/89/004
ABSTRACT
This paper (Part II) deals with the processing (analysis of agar content and quality) of Gracilaria spp in the Philippines.
Over two hundred samples were collected for this study from wild stocks, particularly from Sorsogon. The experiments consisted of pre-treatment with 5% alkali (NaOH) at 90°C for 3 hours, extraction of agar and determination of agar quality. Results showed that the average mean yield of agar ranged between 16.18%-24.67% with G. tenuistipitata having the least and G. changii the highest yield, respectively. Gel strength was determined using a 1.5% agar solution and was highest for the agar from G. heteroclada (892 g/cm2). Other species with high gel strength were G. tenuistipitata (726 g/cm2 ), G. firma (606 g/cm ) and G. changii (583 g/cm2). G. salicornia (287 g/cm2), and G. fastigiata (250 g/cm2 ) showed the lowest gel strength. Melting temperatures ranged from a high of 92 °C in the agar from G. firma to a low of 79 °C from G. fastigiata. Most of the species showed high quality agar which can be used for bacto-agar and agarose production.
1. INTRODUCTION
The objective of the present study was to evaluate the commercial potential of seaweeds resources, identify the species suitable for culture and to provide guidelines on Gracilaria spp. farming.
There are numerous species of seaweeds in Philippine waters and they have become an important aquatic resource in the country. The species which are traditionally used for food include: Caulerpa lentillifera; Gelidiella acerosa; Gracilaria sp; Eucheuma denticullatum; Kappaphycus alvarezii; and Sargassum sp. Gracilaria spp. is gathered from wild stocks or farmed, it is then dried for export to developed countries as raw materials. Processing plants for Eucheuma spp. and Gracilaria spp. were established in the country in the late 60's, so processing for food product agar and carrageenan, semi-refined and refined carrageenan from Euchuema spp. and Kappaphycus spp. is now in existence. An estimated 70% of carrageenan world-wide is being produced today from K. alvarezii and E. denticulatum.
The Philippine species of Gracilaria spp. occurs naturally over the entire country. The chief sources of agar are Gracilaria spp., Gelidiella spp. and other related seaweeds (Santos and Doty, 1978). However, in recent years the demand for dried Gracilaria spp. raw material has increased both to meet local supply and to fulfil export demand. The natural stocks of the agaropyhyte species started to decline and are still declining. Agarophytes are higher priced than other colloid bearing seaweeds. The demand for agar exists in developed and developing countries for food industry, Pharmaceuticals, microbiological research and genetic engineering. Agarose, a purified agar extract, is the most expensive and has been utilised for medical diagnosis, cell and tissue cultures.
2. PROCESSING (AGAR YIELD AND QUALITY) OF GRACILARIA
Studies have been made on agar from Philippine seaweeds. Gracilaria verrucosa (synonymous with G. confervoides) and Gelidiella spp. were studied as a source of agar in the early 1950's by the Philippines Fisheries Commission (now Bureau of Fisheries and Aquatic Resources) and the National Institute of Science and Technology. Other species of Gracilaria studied were G. eucheumoides, G. arcuata, and G. salicornia (Santos and Doty, 1978); G. coronopifolia, G. arcuata, G. edulis, G. eucheumoides, G. verrucosa, G. salicornia and Gracilaria sp. A. (Hurtado-Ponce and Umezaki, 1988); G.firma, G. fastigiata, G. cylindrica, G. salicornia and G. tenuistipitata, (Santos, 1993;. de la Pena and de Jesus, 1993). Similar studies were undertaken at UP-MSI, Diliman, SEAFDEC, in Iloilo and other universities. Most of the results of the studies showed that species of Gracilaria have potential quality of agar useful for food and other specialised agar products. The production of high quality agar from Gracilaria spp. for local processing and for the international market is the brightest prospect for the Philippine agar industry. Since Gracilaria spp. is the major raw material for agarophytes, a knowledge and understanding of their taxonomy and processing would be of importance for commercial culture and industry.
2.1 Materials and methods
The Gracilaria spp. samples used for this study were collected from Sorsogon and different coastal sites in the Philippines. Several species were identified. The collected samples were cleaned, sorted, coded and then weighed fresh. The seaweeds were then dried under the sun.
i. Moisture content
20 g of dry seaweed samples were weighed and dried further in an oven at 105 °C to a constant weight.
ii. Clean anhydrous weight (CAW)
20 g of dry seaweed samples were weighed and soaked in 600 ml of water 3 times for 10 minutes. The water was drained off and the seaweed dried to constant weight at 60°C. The % CAW obtained is used for calculation of agar yield.
iii. Alkali treatment
Two 100 g samples of dried Gracilaria spp. were placed in 2 litre capacity stainless steel pots containing 1.2 litres of 5% alkali solution and heated on a water bath for 3 hours at 90°C.
iv. Extraction of agar
Pre-treated samples were washed with water until free of alkali and put back stainless pots containing 600 ml of water. The pH of the water and sample was adjusted to 6.0 with 1% acetic acid.
The samples were heated for 2 hours at 85–90°C in a water bath and blended. 75 g of filter aid was added to the blended sample and stirred for half and hour. The extract was then filtered while hot under pressure at 60–85 psi. Filtrate was collected in a rectangular aluminium pan and gelled at room temperature. The gel was cut into cubes and frozen overnight. The next day, the gel was thawed and washed with water until free of colour. Isopropanol was added to facilitate drying at 60°C. Percentage agar yield was then calculated. The dried agar was powdered for quality analysis.
v. Moisture content - Oven method (AOAC, 1975)
Moisture content was determined in triplicate by weighing 1 g of agar into a tared porcelain crucible which was placed in an oven at 105°C for 5 hours. The porcelain crucibles were transferred to a desiccator, cooled and re-weighed.
vi. Gel strength - FMC gel tester
A 1.5% solution of agar sample was heated in a water bath until dissolved and allowed to set for 2 hours at 20°C before measurement of the gel strength.
vii. Melting temperature
A 1.5% solution of agar sample was heated and dissolved in a water bath. The agar solution was transferred to a test tube and gelled for 1 hour at 20°C. The tube was then placed in a water bath at 60°C and a lead shot dropped inside. The temperature was noted when the lead shot dropped to the bottom of the tube.
vii. Sulphate-turbidimetric method
The agar sample was dissolved in magnesium nitrate and hydrochloric acid. The sulphate content was determined by turbidimetric method by spectrophotometer with absorbance of 425 nm
3. RESULTS AND DISCUSSION
More than two hundred Gracilaria samples were collected at different sites in the coastal areas of the Philippines particularly in Sorsogon. Table 1 shows the environmental requirements of the different species of Gracilaria studied (Taw, 1994). However, only the common species of Gracilaria were analysed for their physico-chemical qualities.
Table 1: Environmental parameters suitable for Gracilaria spp. farming.
| Species | Environment | ||||||
| Salinity (ppt) | pH | Temp. (°C) | Transparency | Substrate | Tide (cm) | Area | |
| G. changii | 33–35 | 7–8 | 27–33 | clear | coraline | 30–20 | Open sea |
| G. firma | 25–35 | 6–8 | 25–35 | semi clear | sandy/mud | 30–250 | Bays, |
| G. fastigiata | mangrove | ||||||
| G. heteroclada | channels | ||||||
| G. heteroclada | 10–25 | 6–8 | 25–33 | turbid | muddy | 30 | Brackish |
| G. tenuistipitata | water ponds | ||||||
It was observed that Gracilaria species appeared in the intertidal zone at different times of the year depending on the location. Nevertheless, during the rainy season the thalli deteriorated and disintegrated, which may be related to changes in seawater temperatures. It was also found that during summer the thalli remains healthy even at above 35°C (Wang et al. 1984). This observation correlated with the properties of agar. The physico-chemical properties of agar were generally good during the summer months. Table 2 summarises the yield of agar from the ten species of Gracilaria studied with their corresponding yield, clean anhydrous weight (CAW), moisture content, gel strength, total sulphate, and melting temperature and pre-treatment at 5% NaOH. The CAW ranged between 35 to 56% and moisture content between 12–17% (Table 3).
Table 2: Mean values of physico-chemical analysis of agar extracted by 5% NaOH pre-treatment of Gracilaria species in the Philippines
| Species | % CAW | % Moisture content | % Yield | Gel Strength (g/cm2) | Total Sulphate | Melting Temp. (°C) |
| G. fastigiata | 44.4 | 16.89 | 19.24 | 250 | 3.28 | 79 |
| G. salicornia | 41.8 | 12.06 | 20.07 | 287 | 2.42 | 80 |
| G. changii | 56.7 | 14.48 | 24.67 | 583 | 0.61 | 86 |
| G. tenuistipitata | 52.8 | 15.51 | 16.18 | 726 | 0.90 | 88 |
| G. firma | 54 | 16.11 | 17.61 | 606 | 0.22 | 89 |
| G. heteroclada | 48.5 | 17.39 | 20.05 | 892 | 0.78 | 92 |
The physical properties varied from species to species and, in addition, the monthly sampling, post harvest handling, seasonal variation and drying may contribute to the quality of dried Gracilaria spp.. The average yield ranged from 16 to 24%, G. changii gave the highest yield of agar followed by G. salicornia, G. heteroclada, G. fastigiata, G. firma, and G. tenuistipitata. Other species analysed also gave high yields but this has to be confirmed by more sampling. The gel strength of agar ranged from 250–892 g/cm2 with the agar from G. fastigiata and G. salicornia providing the lowest gel strength and the highest gel strength provided by G. heteroclada, G. tenuistipitata, G. changii, and G. firma at 92°C.
G. fastigiata gave the highest total sulphate content and lowest gel strength as compared to the other species. From the data gathered, it appeared that the higher the total sulphate content, the lower the gel strength (Santos and Doty, 1978). G. firma and G. changii showed the lowest total sulphate content of 0.22% and 0.61%, respectively. Agar quality from all species in the present study showed similar results to previous reports (Santos, 1992 and de la Pena and de Jesus, 1993). Our results showed that among the species tested, G. heteroclada, G. firma, G. changii, and G. tenuistipitata showed high gel strength and melting temperature. The results of agar properties showed that Gracilaria spp. samples collected during summer seasons generally gave high yield and gel strength, although it varies with the species, season and location. The properties of the different species of Gracilaria spp. tested, showed that the agar of some species could be used for specialised agar products in microbiological culture media or agarose and the species with soft gel strength could be utilised for food industry.
4. RECOMMENDATIONS
The results obtained at time of observation in 1992–1993 indicated that several samples of G. fastigiata, G. changii, G. firma, G. tenuistipitata, and G. heteroclada have an average yield of 16.18–24.67% and the gel strength as high as 892 g/cm2. G. firma, G. changii, G. tenuistipitata, G. heteroclada, and G. fastigiata were found to have good quality and the environmental parameters for optimum growth were observed. G. changii grows in the open sea with coraline substrate, clear, transparency and salinity of 33–35 ppt and water temperatures between 27–33°C. G. firma, G. fastigiata, G. heteroclada and G. tenuistipitata were both found to adapt and grow in brackishwater ponds with muddy substrate and turbid transparency. They also tolerate low salinity. The species mentioned above are recommended for farming having the required quality and environmental tolerance suitable for the agar industry.
Table 3: Collection sites, dates and quality of agar extracted from different Gracilaria species from the Philippines
| Sample code | Place of Collection | Date of Collection | Wet weight (g) | Dry Weight (g) | CAW (%) | Moisture (%) | Agar Yield (%) | Gel Strength (g/cm2) | Total Sulphate (%) | Melting Temp. (°C) |
| G. fastigiata | ||||||||||
| SW 0001 | Bagacay, Gubat, Sorsogon | 5/18/92 | 500 | 600 | 42 | 18.0 | 21.43 | 150 | 3.93 | 76 |
| SW 0012 | Layog, Barcelona, Sorsogon | 6/18/92 | 20,050 | 2,467 | 44.5 | 15.81 | 20.9 | 106 | 5.2 | 75 |
| SW 0055 | Bagacay, Gubat, Sorsogon | 9/17/92 | 12,100 | 1,720 | 45 | 16.61 | 15.77 | 63 | 0.24 | 67 |
| SW 0094 | Bagacay, Gubat, Sorsogon | 12/12/92 | 8,000 | 766 | 41 | 14.65 | 21.70 | 335 | 4.07 | 75 |
| SW 0115 | Bagacay, Gubat, Sorsogon | 2/3/93 | 8,500 | 922 | 48 | 16.22 | 12.50 | 670 | 1.66 | 90 |
| SW 0119 | Bagacay, Gubat, Sorsogon | 3/16/93 | 600 | 100 | 45 | 17.06 | 14.00 | 167 | 2.97 | 82 |
| SW 0125 | Bagacay, Gubat, Sorsogon | 4/14/93 | 6,500 | 800 | 43.5 | 17.33 | 26.89 | 136 | 4.38 | 85 |
| SW 0134 | Bagacay, Gubat, Sorsogon | 5/19/93 | 2,200 | 250 | 46 | 19.44 | 23.00 | 370 | 3.75 | 80 |
| G. salicornia | ||||||||||
| SW 0070 | Casiguran, Sorsogon | 10/29/92 | 4,766 | 592 | 47 | 9.22 | 18.5 | 329 | 1.86 | 79 |
| SW 0181 | Bongansaran, Gubat, Bor. | 9/2/93 | 5,000 | 621 | 36.5 | 14.89 | 21.64 | 245 | 2.98 | 81 |
| Gracilaria sp. | ||||||||||
| SW 0148 | Malaweste, uguey, Cagayan | 6/17/93 | 16,000 | 2,000 | 35 | 17.87 | 29.71 | 1033 | 0.51 | 90 |
| SW 0155 | Mabuhay, Bulusan | 7/13/93 | 6,000 | 1,030 | 65.5 | 18.79 | 25 | 912 | 0.95 | 89 |
| SW 0223 | Bongansaran, Gubat, Sor. | 11/16/93 | 4,750 | 327 | 46.5 | 13.69 | 26.24 | 408 | 0.98 | 89 |
| G. changii | ||||||||||
| SW 0020 | Layog, Barcelona, Sorsogon | 6/19/92 | 16,500 | 1,973 | 56 | 19.83 | 15.71 | 216 | 0.51 | 81 |
| SW 0050 | Mapapac, Barcelona, Sor. | 9/9/92 | 7,899 | 945 | 59 | 11.13 | 26.0 | 277 | 0.41 | 78 |
| SW 0065 | Dancalan, Bulusan, Sor. | 10/28/92 | 3,834 | 458 | 50 | 11.67 | 21.0 | 456 | 0.41 | 85 |
| SW 0136 | Layog, Barcelona, Sorsogon | 5/19/93 | 4,000 | 750 | 66 | 10.6 | 14.1 | 857 | 0.36 | 93 |
| SW 0154 | Layog, Barcelona, Sorsogon | 7/13/93 | 35,000 | 680 | 57 | 15.75 | 27.0 | 534 | 1.01 | 87 |
| SW 0161 | Layog, Barcelona, Sorsogon | 8/3/93 | 2,200 | 320 | 60.5 | 18.27 | 33.0 | 839 | 0.64 | 88 |
| SW 0183 | Layog, Barcelona, Sorsogon | 9/3/93 | 6,500 | 1,035 | 57 | 14.25 | 26.14 | 527 | 0.69 | 86 |
| SW 0217 | Layog, Barcelona, Sorsogon | 10/10/93 | 2,500 | 373 | 49.5 | 16 | 29 | 908 | 0.64 | 87 |
| SW 0229 | Layog, Barcelona, Sorsogon | 12/12/93 | 10,500 | 1,285 | 56 | 12.78 | 30.1 | 630 | 0.82 | 91 |
| G. tenuistipitata | ||||||||||
| SW 0003 | Tughan, Juban, Sorsogon | 3/16/93 | 2,306.4 | 166.9 | 52.5 | 12.50 | 23.24 | 68 | 4.06 | 73 |
| SW 0058 | Bagacay, Gubat, Sorsogon | 4/25/93 | 21,000 | 1,828.4 | 55 | 19.35 | 20.4 | 1051 | 0.20 | 88 |
| SW 0112 | Buhong, Juban, Sorsogon | 5/18/93 | 15,620 | 1,945.3 | 18.55 | 14.91 | 982 | 0.58 | 93 | |
| SW 0122 | Catanagan, Juban, Sorsogon | 6/14/93 | 6,750 | 780 | 17.45 | 8.43 | 780 | 0.52 | 91 | |
| SW 0133 | Tughan, Juban, Sorsogon | 6/21/93 | 11,000 | 1,100 | 51 | 9.72 | 13.92 | 750 | 0.17 | 96 |
| G. firma | ||||||||||
| SW 0002 | Bagacay, Gubat, Sorsogon | 5/8/92 | 5,000 | 500 | 38 | 20.5 | 14.46 | 1002 | 0.29 | 92 |
| SW0010 | Bagacay, Gubat, Sorsogon | 6/18/92 | 42,320 | 4,122 | 54 | 15.86 | 15.25 | 583 | 0.31 | 88 |
| SW 0025 | Bagacay, Gubat, Sorsogon | 7/14/92 | 12,000 | 1,445 | 47.5 | 16 | 23 | 820 | 0.17 | 93 |
| SW 0054 | Bagacay, Gubat, Sorsogon | 9/17/93 | 27,500 | 3,825 | 61 | 15.35 | 18.36 | 324 | 0.32 | 87 |
| SW 0179 | Bagacay, Gubat, Sorsogon | 9/2/93 | 3,000 | 420 | 64.5 | 16.04 | 28 | 878 | 0.16 | 89 |
| SW 0193 | Baruy-baruy, Samar | 9/12/93 | 7,000 | 480 | 80 | 15.60 | 7.62 | 219 | 0.26 | 85 |
| SW 0207 | Calampong, Samar | 10/26/93 | 1,000 | 48 | 16.72 | 18.29 | 239 | 0.18 | 86 | |
| SW 0228 | Bongansaran, Gubat, Sor. | 12/12/92 | 3,710 | 356 | 39 | 12.75 | 15.90 | 778 | 0.29 | 91 |
| G. heteroclada | ||||||||||
| SW 0021 | Tughan, Juban, Sorsogon | 6/19/92 | 5,000 | 120 | 40 | 15.74 | 15.85 | 450 | 3.63 | 89 |
| SW 0140 | Binakayan, Cavite | 5/25/93 | 31,100 | 2,522 | 46 | 17.36 | 15.21 | 808 | 0.76 | 94 |
| SW 0156 | Tughan, Juban, Sorsogon | 7/13/93 | 4,000 | 479 | 51 | 17.43 | 22.6 | 1487 | 0.48 | 92 |
| SW 0163 | Caditaan, Magallanes, Sor. | 8/8/93 | 9,500 | 916 | 37.5 | 19.13 | 24 | 1016 | 0.66 | 96 |
| SW 0216 | Tughan, Juban, Sorsogon | 10/27/93 | 3,500 | 295 | 68 | 17.29 | 22.6 | 700 | 0.92 | 91 |
REFERENCES
AOAC, 1975. Official methods of analysis. 12th edition. Association of Official Analytical Chemists, Washington D.C.
dela Pena, P.O. and De Jesus, F.I. 1993. Quality of agar extracted from Gracilaria spp. in Eastern Sorsogon and Sorsogon Bay. Field document No. 11 Seaweed Production Development Project PHI/89/004, DA-BFAR/UNDP/FAO, Manila, Philippines.
Hurtado-Ponce, and Umesaki. 1988. Physical properties of agar gel from Gracilaria (Rhodophyta) of the Philippines. Britannica Marina, Vol. 31 pp 171–174.
Santos, G. 1993, Processing of Gracilaria. Training Report Processing Consultant, Quezon City Philippines.
Santos, G. and Doty, M. 1978. Gracilaria for the manufacture of agar. Fisheries Journal of the Philippines. Vol. III (2): p29–34.
Taw, N, 1994. Guide on the farming of seaweed Gracilaria species. Field Document No. 17. Seaweed Production Development Project PHI/89/004, DA-BFARUNDP/FAO Manila, Philippines.
Wang, Y. C, Pan, G. Y. and Chen, L. C. M. 1984. Studies on agarophytes: II Field Observations and Growth of Gracilaria verrucosa (Phodophyta) in Shantou District, Gusangdong, PRC. Botanica Marina Vol XXVII: p265–268.
Vithya Srimanobhas,
Faculty of Environment and Resources Studies,
Mahidol University, Nakhon Pathom, Thailand.
and
Attaya Kungsuwan,
Food Technologist, Fishery Technological Development Division, Bangkok, Thailand.
ABSTRACT
Seven species of Gracilaria were studied for their taxonomy and ecology: G. changii, G. edulis, G. firma, G. fisheri, G. irregularis, G. salicornia and G. tenuistipitata. In terms of agar quality, G. fisheri and G. tenuistipitata had the highest gel strength of 768 and 758 g/cm , respectively. This finding is in accord with the results reported by Dr Suwalee Chandrkrachang of BRU that G. edulis and G. tenuistipitata from Pattani have the highest potential as a source of agar in Thailand. It was recommended that further studies should be conducted on these two species with the aim of developing them as a main source of agar for the processing industry.
1. INTRODUCTION
Thailand has a coastline of 2,614 kilometers bordering the Gulf of Thailand in the east and the Andaman Sea in the west. Along the coastal shelf are shallow mud flats and rocky or sandy beaches, some parts are mangrove forests, seagrass beds or coral reefs. Coastal inhabitants have utilised several species of red seaweeds, such as Gracilaria spp., as supplementary foods for many years. Recently, the agar requirement of the food industries and microbiological laboratories has increased. Data from foreign trade statistics reveal that Thailand increasingly imports agar annually while small amounts are exported (Table 1). Studies on agar containing seaweeds has been made since 1986 by various institutes including the Faculty of Fisheries, Kasetsart University, concerning seaweed production, and Srinakarinvirot University, concerning agar processing. Abbott (1988) and Lewmanomont (1994) have provided excellent works on the taxonomy of Gracilaria spp. in Thailand.
Table 1: Agar imported and exported to Thailand from 1984–1993.
| Years | Imported | Exported | ||
| Quantity (tonnes) | Value (million Baht) | Quantity (tonnes) | Value (million Baht) | |
| 1984 | 259.92 | 87.98 | 1.22 | 0.08 |
| 1985 | 234.13 | 95.33 | 11.80 | 2.06 |
| 1986 | 251.87 | 108.24 | 11.66 | 2.13 |
| 1987 | 276.77 | 112.93 | 10.83 | 2.67 |
| 1988 | 262.45 | 111.10 | 5.06 | 0.97 |
| 1989 | 274.78 | 129.40 | 2.97 | 0.82 |
| 1990 | 346.56 | 199.32 | 0.76 | 0.17 |
| 1991 | 325.99 | 190.16 | 1.69 | 1.11 |
| 1992 | 435.44 | 233.17 | 5.00 | 1.67 |
| 1993 | 472.53 | 226.48 | 7.78 | 3.50 |
1.1 Material and methods
Seaweed samples were collected from the eastern and southern provinces of Thailand (Figure 1). For taxonomic study, material was placed in 5% formalin. After return to the laboratory some of the seaweeds were dried. Sections were cut by freezing microtome or free hand, stained in 1% aqueous aniline blue and 50% glucose syrup was applied as a mounting medium. For agar yield and quality studies, all materials were collected fresh and transported to the Fishery Technological Department Institute for further study.
At the Institute, the seaweeds were cleaned with water which included removing sand, stones and shells, and sun dried. Dried seaweed thus obtained was stored until extraction and analysis.
i. Extraction method.
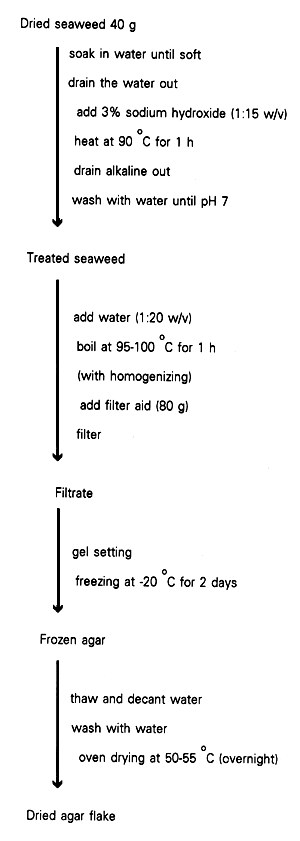
For extraction, a 100 g sample of dried seaweed was cleaned in water 3 times and dried again to determine the final dry weight. The results obtained were calculated to find the CAW (Clean Anhydrous Weight). The dried seaweed obtained was then weighed out in samples of 40 g for agar extraction. The flow diagram of extraction method is shown in Figure 2. To extract the agar, the dried seaweed was submerged in water until soft and then the water was drained out. The wet seaweed sample was boiled with 3% sodium hydroxide (1:15 w/v) at 90 ± 4° C for one hour and washed with water until the pH was neutral. Further boiling was performed in water (1:20 w/v) at 100 °C for one hour with homogenising and addition of a filtration aid. Filtration was performed on a vacuum press and the filtrate was set and kept in a freezer at -20 °C for 48 hours. After thawing the frozen agar, the agar cake was dried in an oven overnight at 50–55 °C and further analysis of agar quality was performed.
ii. Ash content
Analysis of ash content was carried out at 550°C, until the sample turned white and had a stable weight according to a method laid out by AOAC (1980).
iii. Moisture content
Moisture content analysis was carried out at 100°C for 6 hours AOAC (1980).
iv. Gel strength.
A 1.5% aqueous solution of agar was prepared and poured into a beaker which gave an agar thickness of 3 cm and a diameter of 6 cm. The agar in these beakers was set in a water bath at room temperature for 12 hours and the gel strength of the agar cakes obtained was measured using a Nikanui gel tester.
v. Sulphate content
0.1 g of the sample was weighed out, 2 ml of saturated Mg (NO3)2 in HNO3 was added and the mixture evaporated to dryness. After heating in a muffle furnace at 400 °C for 4–5 hours, the sample was cooled to 80 °C and 10 ml of 1M HCI was added. The mixture was then filtered and the volume made up to 50 ml. All of the solution was transferred to a 150 ml beaker and 5 ml of NaCI-HCI (67g of NaCI with 200 ml of water and 8 ml of HCI) was added together with 10 ml of glycerol-ether (1:2) and 0.2g of BaCl2. The mixture was stirred for one minute and left to stand for 4 minutes, before being stirred again for 5 seconds.
Figure 1: Map of Thailand, showing the collecting places.
Figure 2: Flow diagram of agar extraction from seaweed.
The absorbance of the final solution was measured at 425 nm. The sulphate content was then calculated from the standard curve previously prepared (ASTM, 1985; ACE, 1961).
vi. Melting temperature.
A 1.5% agar solution was prepared and poured into a test tube (2.5 × 20 cm) to a height of 10 cm and a thermometer was dipped halfway into the agar solution. After the agar had congealed completely, the test tube was heated in a water bath of 100°C. A bead was placed on the surface of the gel and the temperature was observed as the bead dropped through the solution.
2. RESULTS
2.1 Taxonomy and ecology
1. Gracilaria changii (Xia et Abbott) Abbott, Zhang et Xia (Figure 3).
| Reference: | Abbott, Zhang and Xia, Pac. Sci. 45:23,1991. |
| Basionym: | Polycavernosa changii Xia et Abbott, Phycologia 26:407, Figure 3, 1987. |
| Description: | Plants bushy, 5–7 cm high, branches cylindrical, main branches 1.5–2.5 mm in diameter; branching alternate or irregular to four orders, most of the branches are abruptly constricted at the base and taper towards apices. |
| Collections: | Ao Len, Changwat Trat, 12° 04' N, 102° 331 E, NACA 002. 28 April 1993; NACA 007 8 June 1993. |
| Ecology: | G. changii was found growing on rocks, gravel and shells in sand-mud areas which were exposed to air at low tide. Water highly turbid, salinity 32–33 ppt. |
2. Gracilaria edulis (Gmelin) Silva (Figure 4).
| Reference: | Univ. Calif. Publ. Bot. 25:293, 1952. |
| Synonym: | Polycavernosa fastigiata Chang et Xia, Stud. Mar. Sinica, 3:125,1963. |
| Description: | Plants form a loose clump 15–25 cm high, branches cylindrical, main branches 0.7–1.0 mm in diameter, branching dichotomous or trichotomous to six orders, branches apices frequently hook-like in appearance. |
| Collections: | Ao Len, Changwat Trat, 12° 04' N, 102° 33' E, NACA 004. 28 April 1993. |
| Ecology: | G. edulis was found growing on rocks in sandy-muddy areas which are exposed to air during extreme low tide. Water was highly turbid and salinity was around 33 ppt. |
3. Gracilaria firma Chang et Xia (Figure 5).
| Reference: | Stud. Mar. Sinica. I 1: 143–145, fig. 38, 1–8; fig. 39,1–4, pl 2, fig.4. 1976. |
| Description: | Plants succulent, erect 8–12 cm high; branches cylindrical, main branches 1.5–2.0 mm in diameter, branching alternate or irregular to five orders, lower branches frequently constricted at the base. |
| Collections: | Ban Learn Tian, Changwat Trat, 12O 051 N, 102O 35' E, NACA 001.28 April 1993; NACA 008.4 April 1994. |
| Ecology: | Gracilaria firma was found growing on gravel and rock fragments in sandy muddy areas. Not exposed to air during low tide, water turbid, salinity 31–33 ppt. |
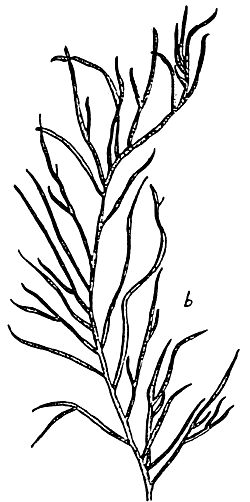
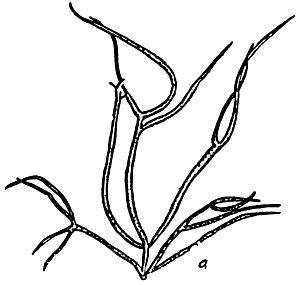
Figure 3: Habit of Gracilaria changii.
Figure 4: Habit of Gracilaria edulis.
4. Gracilaria fisheri (Xia et Abbott) Abbott, Zhang et Xia (Figure 6).
| Reference: | Abbott, Zhang et Xia, Pac. Sci. 45:23,1991. |
| Basionym: | Polycavernosa fisheri Xia et Abbot, Phycologia 26:411–413, figs 5,13,1987. |
| Description: | Plants bushy, 16–44 cm high, branches cylindrical, main branches 0.7–1.5 mm in diameter; branching alternate to four orders, constricted at the base and tapering towards the apices. |
| Collections: | Ko Yo, Changwat Songkhla, 07° 10'N, 100° 33'E, NACA 009.21 August 1994. |
| Ecology: | G.fisheri was found growing on nets of fish cages in muddy, sandy areas. Not exposed to air during low tide, water turbid, salinity 11 ppt. |
5. Gracilaria irregularis Abbott (Figure 7).
| Reference: | Abbott. Tax. Econ. Seaweeds, vol. 2, p 141, figs. 1,5–6. |
| Description: | Plant erect, 4–8 cm high; branches cylindrical, main branches 2.5–3.0 mm in diameter; branching mostly secund. |
| Collections: | Ao Len, Changwat Trat, 12° 04' N, 102° 33' E, NACA 003. 28 April 1993. |
| Ecology: | G. irregularis was found growing on rock fragments, gravel and shells in sandy mud areas exposed to air during low tide, water highly turbid, salinity about 33 ppt. |
6. Gracilaria salicornia (C. Agardh) Dawson (Figure 8).
| Reference: | Dawson. Bull. S. Calif. Acad. Sci. 53:4, fig 3,1954. |
| Basionym: | Sphaerococcus salicornia C. Agardh Sp. algarum, vol I, p 302, 1822. |
| Description: | Plants clump, thalli rigid, prostrate to semi-erect; branches cylindrical, frequently constricted into segments, 2.5–4 mm in diameter, branching irregular. |
| Collections: | Leam Tian, Trat Province 12° 05' N, 102° 35'E, NACA 005.28 April 1993; NACA 006, 8 June 1993. |
| Ecology: | G. salicornia was found growing on rocks or gravel in muddy/ sandy mud areas not exposed to air during low tide. Water turbid, salinity about 31 ppt. |
7. Gracilaria tenuistipitata var. liui Zhang et Xia (Figure 9).
| Reference: | Zhang et Xia. Tax. Econ. Seaweeds, Vol. 2, pp. 131 – 132, figs. 1, 3–9, 1988. |
| Description: | Plants bushy, 22–32 cm high; branches slender, cylindrical, main axes 0.5 mm in diameter, branching alternate to irregular to three orders, numerous delicate branchlets. |
| Collections: | Ban Da To, Ao Pattani, Changwat Pattani, 06° 55f N, 101° 20' E. NACA 010. 20 August 1994. |
| Ecology: | G. tenuistipitata var. liui was found growing on gravels and shell in sandy mud areas, exposed to air during extreme low tide, water turbid, salinity around 31 ppt. |
Figure 5: Habit of Gracilaria firma.
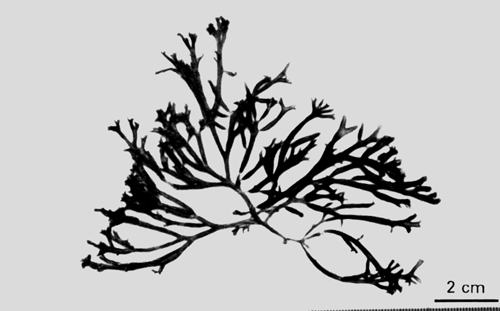
Figure 6: Habit of Gracilaria fisheri
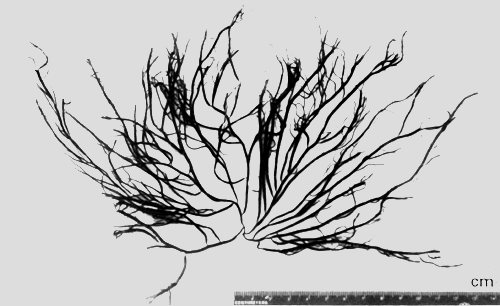
Figure 7: Habit of Gracilaria irregularis.
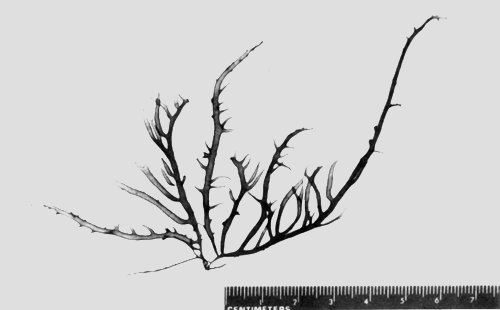
Figure 8: Habit of Gracilaria salicornia.

Figure 9: Habit of Gracilaria tenuistipitata var. liui.
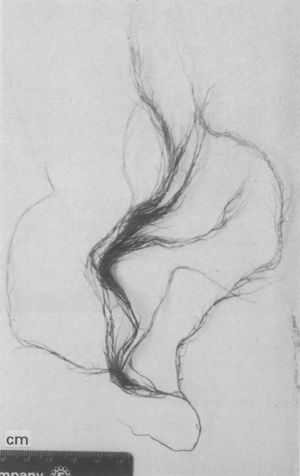
2.2 Agar yield and quality
The results of agar quality are summarised in Table 2.
Table 2: Results of agar quality analysis of all samples.
| Species | Yield (%) | Ash (%) | Moisture Content (%) | Melting Temp. (°C) | Sulphate content (%) | Gel strength (g/cm2) |
| G. salicornia | 4.75 | 5.60 | 16.80 | 88 | - | 180 |
| G. changii | 9.50 | 5.90 | 15.00 | 90 | - | 190 |
| G. firma | 19.96 | 2.46 | 6.90 | 93 | 2.04 | 692 |
| G. fisheri | 12.84 | 2.13 | 6.67 | 94 | 2.01 | 758 |
| G. tenuistipitata var. liui. | 12.70 | 1.85 | 6.24 | 98 | 1.81 | 768 |
It was found that sample number NACA 009 (Gracilaria fisheri) and NACA 010 (G. tenuistipitata) had the highest gel strengths, with values of 768 and 758 g/cm , respectively. It was apparent that samples number NACA 006 and NACA 007, which had a very high ash content also had a very low gel strength. These results are in agreement with those reported by Chandrkrachang and Chinadit 1988, where G. fisheri from Songkhla and G. tenuistipitata from Pattani were identified as having greatest potential as raw materials for agar processing in Thailand. Further study on production of these two species should be made to eliminate the problem of raw material shortages for agar processing in Thailand.
REFERENCES
Abbott, I. A. 1988. Some species of Gracilaria and Polycavernosa from Thailand. In: Abbott, I. A., (ed.) Taxonomy of economic seaweeds: With reference to some Pacific and Caribbean species. Volume 11. Calif. Sea Grant College Programme, La Jolla, Calif, pp 137–150.
ACE, 1961. Treatise on Analytical Chemistry. Part III. Analytical Chemistry of Elements. John Wiley & Sons Inc,Vol 7, 103–105(1961).
AOAC (1980). Official methods of analysis of the Association of Official Analytical Chemists. 13th Edition, 1980 507 pp.
ASTM, 1985. Standard test methods for the sulphate ion in water. ASTM, 1985: 685–688. Chandrkrachang, S. and Chinadit, U. 1988. Infoflsh No 4/88,22–25.
Lewmanomont, K. 1994. The species of Gracilaria from Thailand. In: Abbott, I.A., (ed.) Taxonomy of economic seaweeds: With reference to some Pacific species. Volume IV. Calif. Sea Grant College Programme, La Jolla, Calif, pp 135–148.
Do Van Khuong,
and
Nguyen Van Thuc
Research Institute of Marine Products, Ministry of Fisheries,
Hai Phong, Socialist Republic of Vietnam.
ABSTRACT
Samples of Gracilaria spp. were collected from northern Vietnam and 8 of the 13 species reported by Nguyen Huu Dinh and Ngugen Van Tein (1993) were identified, namely: G. asiatica Zhang et Xia (syn. G. verrucosa); G. tenuistipitata Zhang et Xia; G. blodgettii Harvey; G. arcuata Zan.; G. hainemensis Zhang et Zia (similar to G. edulis); G. chorda Holm (syn. G. lemaneiformis), G. gigas Harv. and G. bursa-pastoris Silva.
The taxonomic identification of Gracilaria species usually meets with the following difficulties: variability among species is not clearly defined; morphological features of the species are influenced by environmental conditions which tend to change at different locations; and some of the specimens collected lack reproductive organs. The taxonomy of Gracilaria spp. is quite complicated and synonyms are commonly used. To correctly identify the taxonomic status, it is necessary to collect Gracilaria spp. specimens continuously when it is mature and has reproductive organs (tetrasporangium, spermatangial conceptacle and cystocarp).
Among the 8 species identified during this study, 4 were found to be of high economic value. Of these 4 species, 3 were studied and cultivated: G. asiatica; G. blodgettii and G. tenuistipitata. G. chorda still has to be studied in trial cultivation.
Analysis of results from 6 Gracilaria species collected from Hai Phong and Quang Ninh showed that the dried/fresh ratio, agar yield and gel strength were highest in G. asiatica. Its also has the lowest ash content, is widely distributed in Vietnam and could be the main species cultured in brackish water swamps in northern Vietnam. Other species of high economic value for culture and agar processing in Vietnam are G. blodgettii and G. tenuistipitata.
1. INTRODUCTION
Along the coast of Vietnam, economic red algae are widely distributed. Since ancient times, they have been naturally harvested and utilised as food and medicines in the form of raw or processed products. Only in recent decades has attention been paid to the development of seaweed culture to meet the demands of domestic consumption and the export market. In Vietnam, the cultivation and processing of economic seaweeds is quite new and has only really developed since 1987, when seaweed markets began expanding in other countries in the region.
One of the main genus of seaweeds which has been studied and cultivated is Gracilaria. At present, the cultivation of three species of Gracilaria has developed in Vietnam, namely: Gracilaria asiatica (syn. G. verrucosa ); G. blodgettii; and G. tenuistipitata. In 1987, Vietnam produced about 1,500 tonnes of dried Gracilaria spp., but by 1993 that quantity had reached to 2,800–3,000 tonnes. Most of the dried Gracilaria spp. is used for agar processing for domestic consumption and the remainder is exported to China. In addition, trial cultivation of Eucheuma gelatinae has begun in Vietnam.
The agar extraction industry from Gracilaria spp. has clearly expanded. In 1987, Vietnam produced only 20 tonnes of agar, but by 1993 this had increased to 250 tonnes. In addition to industrial scale agar processing factories, many small scale and family scale ones have appeared with high economic efficiency. However, this industry only meets local demands and the agar is used for the candy and foodstuff industry. The export of agar from Vietnam is facing difficulties due to its poor quality, especially its low gel-strength and high melting point.
In Vietnam, as with other countries in the region, the identification of species of economic seaweeds belonging to the genera Gracilaria, Eucheuma and Porphyra, is difficult. Some species may be an important source of raw material for the agar extraction industry, but knowledge of the correct taxonomy and the ecological requirements for cultivation is still limited.
2. ECOLOGY AND TAXONOMY OF GRACILARIA
Morphological features traditionally used to distinguish species of Gracilaria are the hold fast, form of thalli, main branches and branching patterns. In recent years, attention has been given to the structure of reproductive organs which varied little in one species. Zhang, and Xia (1976) suggested using the structure of the pericarp, Yamamoto (1978) suggested using the arrangement of spematangia as an important feature to identify the species. For Gracilaria, taxonomists combine comparative methods of morphology, function structure, reproductive organs structure and cell rearing for taxonomic identification.
According to Nguyen Van Tien (1988), 13 species of Gracilaria were reported in Northern Vietnam, and Gracilaria asiatica Zhang et Xia with its high natural productivity, has been widely cultivated for many years. Seasonal changes in salinity in the northern coastal waters is an important factor influencing the appearance and biomass of Gracilaria spp. in these areas. In the littoral zone in the rainy season (end of June to early October) when the salinity reduces to 5–6 ppt, and sometimes reaches 3–4 ppt, Gracilaria spp. stops growing and begins to fade. In Cat Hai island, which is far from river mouths, salinities reach 14–28 ppt in the rainy season, Gracilaria spp. grows and develops well from April until December and fades only in January, February and March when salinity is over 30 ppt.
During 1992–1993, as part of this research programme, the following studies were carried out:
Selected Gracilaria spp. resource surveys in some locations (wild and cultivated plants) in Northern Vietnam.
Collection of specimens of Gracilaria spp. growing naturally and cultured in brackish water ponds.
Determination of the ecological and environmental characteristics of the collection site.
Taxonomic identification of specimens under the modern concepts and up-to-date documents on the taxonomy of Gracilaria spp.
2.1. Materials and methods
Materials were collected from various brackishwater swamps of Quang Ninh, Hai Phong, Nam Ha provinces of Northern Vietnam including all field-collected and cultivated Gracilaria species. Specimens of Gracilaria species collected were preserved in 5% buffered formalin/ sea-water. One part of the specimens was sliced in longitudinal and transverse sections of thallus by Microtome or razor and were stained with acetocarmin and aniline blue. Taxonomic identification of the specimens was done by Nguyen Van Tien from the Oceanographic Institute. Herbaria was comparatively studied with the Herbaria set of Gracilaria spp. of northern Vietnam housed at the Oceanographic Institute of Vietnam Science Institute. In addition to traditional documents, new ones were used to identify Gracilaria species, particularly the volumes of “Taxonomy of Economic Seaweeds” edited by Abbott and Norris (1985; 1988).
2.2. Results
1. Gracilaria asiatica Zhang et Xia (Figures 1 and 2).
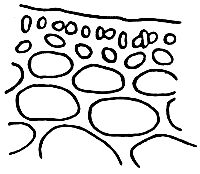
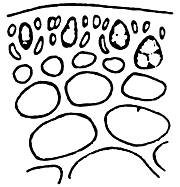
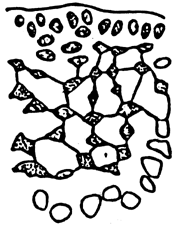
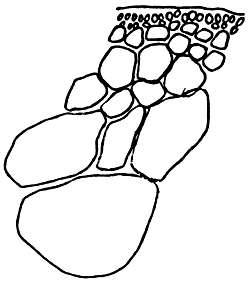
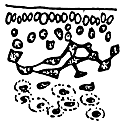
Alga solitary or large caespitose; brown or dark-brown in colour. Holdfast discoid or unattached. Thallus cylindrical or terete, about 1–1.5 mm in diameter, branching alternate, main branches elongate with shorter branchlets, acute apices. In transverse section, the central part consisted of large roundish cells, the diameter of central cells was 240–336 um. Cortex consisted of 1–3 layers of small cells.
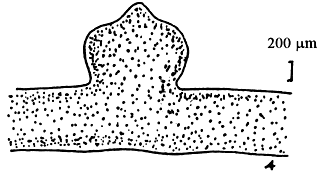
Tetrasporangium, divided cruciate or tetrapartite in cortex scattered over the whole thallus. Cystocarps hemispherical or conical, slightly constricted at the base, without rostrum, 580–700 um in diameter. Pericarp consisted of 8–11 layers of cells, with few absorbing filaments (Code No. 92001). This species is distributed at the medium littoral zone and in brackishwater swamps along the coast of northern Vietnam from October until the following July.
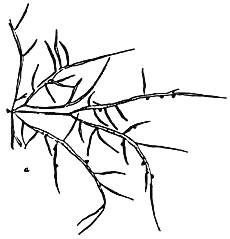
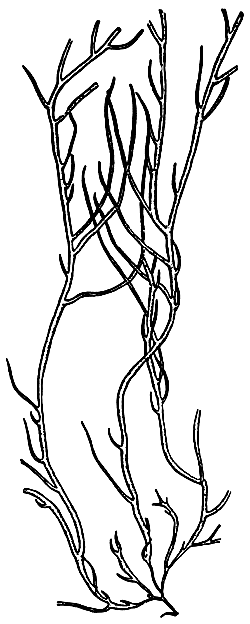
2. Gracilaria tennuistipitata Zhang et Xia (Figure 3).
Alga solitary or big caespitose. Thallus cylindrical, delicate as thin as thread at young period 18–30 cm long or more. Main axes with maximum diameter 1–2 mm, average 0.5–0. 6 mm. Dark-brown or green-brown.
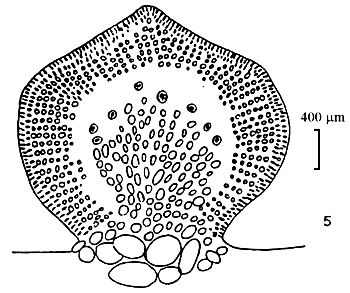
Simple branching, generally of one to three orders, branching alternate with one or two orders of branching. In mature alga it is difficult to separate main axes and main branches. In transverse section, the central cell is 260–275 um in diameter, next are 2–3 layers of pericentral cells, the layer of cells reduce their size from medulla to cortex. Outermost layer is 1–2 layers of small pigmented cells. Cystocarp hemispherical with constriction at the base, with rostrum, their diameter normally same as one of branch, In transverse section of cystocarp, pericarp consists of 8–11 layers. This species is distributed in most central and northern provinces of Vietnam and is mainly a species cultivated in the brackishwater swamps of the central provinces.
Figure 1: Gracilaria asiatica.
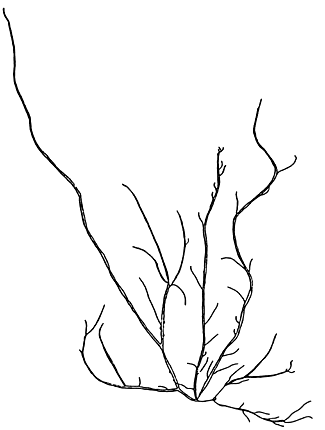
A. External features
 |  |
| B. Section of cylindrical thallus | C. Section of tetrasporic thallus |

D. Section of pericarp
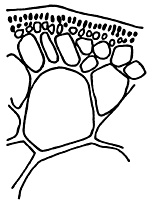
Figure 2: Gracilaria asiatica of Yen hung, Quang Ninh.
A. and B. Morphological cystocarp
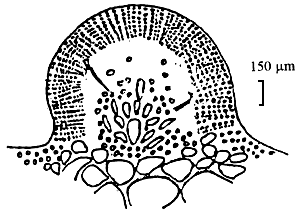 |  |
| C. Section of cystocarp | D. Section of cylindrical thallus |
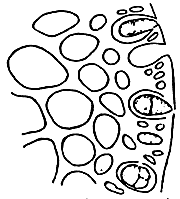
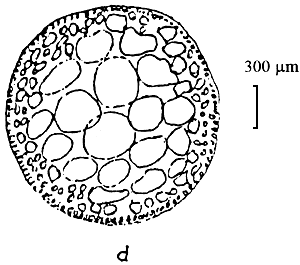
Figure 3: Gracilaria tenuistipitata of Lienvi, Quang Ninh.
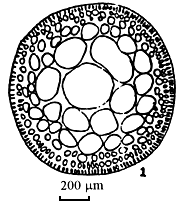 | 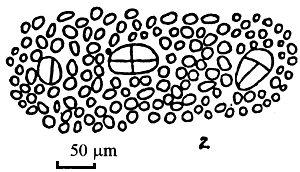 |
| A. Section of cylindrical thallus | B. Tetrasporic thallus |
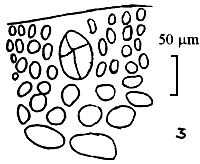 |  |
| C. Section of tetrasporic thallus | D. External morphology of cystocarp |
 | 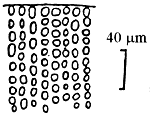 |
| E. Section of cystocarp | F. Section of pericarp |
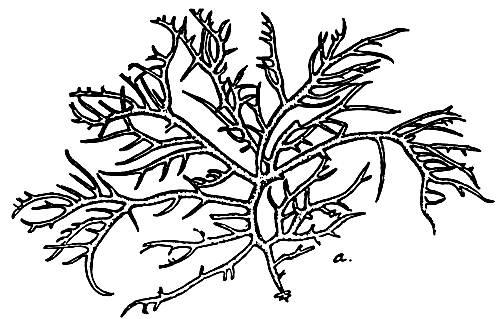
3. Gracilaria arcuata Zan (Figure 4).
Alga caespitose, red-brown, 10–15 cm tall. Holdfast discoid. Thallus cylindrical, 1–1.2 mm in diameter, branching alternate or dichotomous, slightly constricted at the base, some branch apex curved. In transverse section, the central part is a big medullary cell and layers of pericentral cells, cortex consisted of 3–4 layers of small roundish or oval, the size of cell becomes smaller from medulla to cortex. Tetrasporangium divided tetrapartite in the cortex layer. Cystocarp hemispherical, pericarp consisted of 2–15 layers of rectangular or squared, with clearly longitudinal rows. Plants attached to rocks, abundant in spring and summer (from March – July) at lower littoral zone of Hai Phong coastal area.
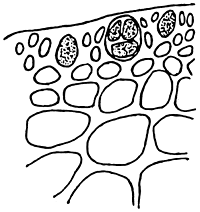
4. Gracilaria hainanensis (Figure 5).
Alga caespitose. Holdfast discoid. Thallus terete, dark purple or dark brown, 15–20 cm tall or more, 1–1.4 mm in diameter, constriction at the base, branching sparse, no main thalli, main branch dichotomous narrowly to the base, apex almost at the same height. In transverse section, medulla cell large, surrounded by big pericentral cells. Cortex consists of 1–2 layers of small pigmented cells. Tetrasporangium divided cruciate or tetrapartite, cystocarp hemispherical, pericarp consisted of 9–11 layers of ellipse cells. Central cells of pericarp are triangular or star-shape. Plant attached on rocks at lower littoral zone, appear in winter, spring and early summer with high biomass from March –June. It is necessary to collect more samples with reproductive organs.
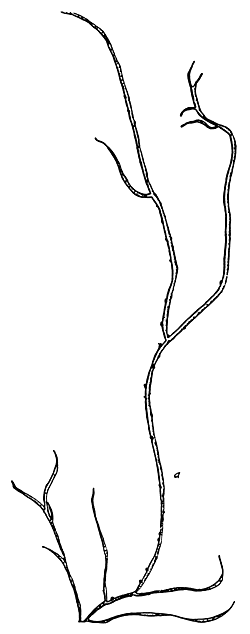
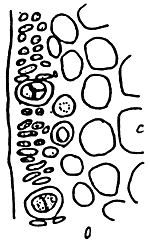
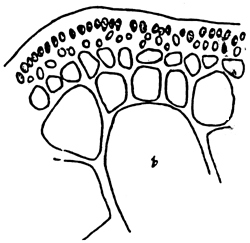
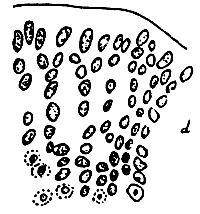
5. Gracilaria chorda (Figure 6).
Alga caespitose, reddish or yellow-red. Holdfast discoid. Thallus cylindrical usually higher than 30 cm, 1–1.3 cm in diameter, main axes, branching alternate, slightly constricted at the base. In transverse section, it consists of a large medulla surrounded by a layer of pericentral cells. The cortex consists of 3 layers of small cells.
Tetrasporagium, divided cruciate or tetrapartite. Cystocarp hemispherical, scattered on surface of thallus. Pericarp consisted of 8–11 layers of cells arranged vertically, 1–3 inner layers with roundish cells. Remainder are ellipse or oval. Plants attached to rocks at medium littoral and lower littoral zones. Abundant in Spring and Summer (from March to July ). It is necessary to collect more samples with reproductive organs.
6. Gracilaria gigas Harv. (Figure 7).
Alga caespitose, 30–40 cm tall, reddish or red-brown. Holdfast discoid. Thallus terete, 2–5 mm in diameter, tapering at the base, sparse branching, alternate, no or slight constriction, acute apices. In transverse section, medulla consisted of 2 layers of large multiangular cells, colourless. When the plant is old, these cells fade making hollows in the thallus. Cortex consisted of 3–4 layers of small roundish or ellipse cells.
Tetrasporangium, divided cruciate or tetrapartite, cystocarp hemispherical, seated in cortex of whole thallus, pericarp consisted of 7–9 layers of cells longitudinally arranged, inner cell star-shape or multiangular central cells oblong, outer roundish or oblong. Plants attached on rocks, shell, alluvial soil (sand) at medium littoral zone and lower littoral zone in bay, occurring abundantly in Spring and early Summer at Hai Phong, Quang Ninh. It is necessary to collect samples with reproductive organs.
Figure 4: Gracilaria arcuata.
A. External morphology
 |  |
| B. Section of tetrasporic thallus | C. Cross-section of pericarp |
Figure 5: Gracilaria hainanensis.
 | 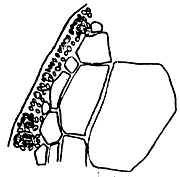 |
| A. External morphology | B. Cross-section of tetrasporic thallus |
Figure 6: Gracilaria chorda.
 |  |
| C. Section of tetrasporic thallus | |
 | |
| B. Section of cylindrical thallus | |
 | |
| D. Cross-section of pericarp | |
| A. External morphology |
Figure 7: Gracilaria gigas.
 |  |
A and B External morphology
 |  |  |
| C. Section of cylindrical D. thallus | Section of tetrasporic E. thallus. | Cross-section of pericarp |
7. Gracilaria bursa-pastoris Silva (Figure 8).
Alga large caespitose, 20–35 cm tall or more, reddish or red. Holdfast conical. Thallus cylindrical, brittle, coarse, 1–2 mm in diameter with main axis. Branching abundant, botryoid. On main branch, coarse branchlet large with acute apex. In transverse section, medulla consisted of 2–3 layers of quite big cells, multiangular, colourless. Cortex consisted of 1–2 layers of small roundish or oblong cells. Tetrasporangium, divided cruciate or tetrapartite, occurring sparsely among cortex cells of whole thallus. Cystocarp hemispherial or conical, tapering at the base. Pericarp consisted of 7–8 layers of small cells, irregularly. Inner cells horizon-oblong, outer vertical-oblong, medulla star-shape or multiangular with not clearly walls. Plants attached to rocks, shells at medium and lower littoral zones. Abundant from March to July. It is necessary to collect samples with reproductive organs.
8. Gracilaria blodgettii Harv. (Figure 9).
Alga caespitose, lightly red or dark-brown, 15–40 cm tall or more. Holdfast discoid. Thallus terete, 1. 5–2 mm cm in diameter. Branching abundant, alternate unilateral, botryoid or annular. Main branch with short-small branchlets. Branches prolonged, base hardly constricted. Apices of branches somewhat constricted.
In transverse section, medulla consisted of a central cell surrounded by a pericentral cell. Cortex consisted of 3–4 layers of roundish or elongate cells, smaller from medulla to cortex.
Tetrasporangium divided cruciate or tetrapartite. Spermatangial conceptacle oblong or oval. Cystocarp hemispherical or conical. Pericarp consisted of 9–13 layers of horizontal - oblong cells, longitudinal arranged rows.
Plants attached on rocks, shells and other plants at medium and lower littoral zones. Found abundantly from October to the next July. This species is commonly cultivated in brackish water swamps along the coastal area of Hai Phong and Cat Hai island.
2.3. Discussion
During this study, specimens of Gracilaria spp. were collected in northern Vietnam and 8 species of the 13 reported by Nguyen Huu Dinh and Nguyen Van Tien, (1992) were identified. The 8 species of Gracilaria spp. identified in this study were:
Gracilaria asiatica Zhang et Xia (Syn. G. verrucosa)
G. tenuistipitata Zhang et Xia
G. arcuata Zan
G. hainanensis Zhang et Xia (Similar to G. edulis)
G. chorda Holm (Syn. G. lemaneiformis)
G. gigas Harv.
G. bursa-pastoris Silva
G. blodgettii Harv.
Figure 8: Gracilaria bursa-pastoris.
 |  |
| B. Section of cylindrical thallus | |
 | |
| C. Cross-section of pericarp | |
| A. External morphology |
Figure 9: Gracilaria blodgettii.
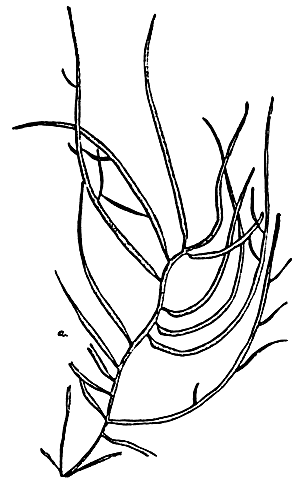
A. External morphology
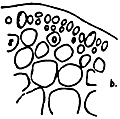 |  | 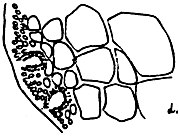 |
| B. Section of tetrasporic thallus | C. Cross-section of pericarp | D. Section of male thallus |
The determination of Gracilaria species usually meets with the following difficulties:
variability among species is not clearly defined.
morphological features of species are influenced by environmental conditions leading to changes at different locations. The specimens collected normally lack of reproductive organs, for this reason, Gracilaria verrucosa has previously been named as follows:
Fucus verrucosa Hudson 1762
F. confervoides Linnaeus 1786
Gracilaria confervoides Greville 1830
Sphaerocoscus confervoides Martens 1886
Gracilaria verrucosa Papenfuss 1950.
Gracilaria asiatica Zhang et Xia 1985
The Gracilaria variety has also been separated into many varieties such as Hydropuntia, Carollopisis, Gracilariopsis and Polycavernosa. Recently, some researchers agreed that Gracilariopsis rhodotricha and 16 species of the genus Polycavernosa belonged to the variety Gracilaria.
In 1978, a well-known Japanese taxonomist (Yamamoto) used spermatangial conceptacles position as a feature to determine Gracilaria species. In 1985, Zhang and Xia suggested using cystocarp form and pericarp structure as important characteristics for identifying Gracilaria species. This seems acceptable, as the structure of these reproductive organs change little.
In general, the taxonomy of Gracilaria species is quite complicated and synonyms are commonly used. In order to gain the correct taxonomic status it is necessary to collect specimens continuously when the seaweed is mature and has reproductive organs (tetrasporangium, spermatangial conceptacle and cystocarp).
3. PROCESSING OF GRACILARIA SPECIES
The technology of agar processing from Gracilaria spp. in Vietnam has been studied since 1963, however, although the procedure is simple, the yield and quality of agar is low.
From 1973–1980, Vietnam and Germany co-operated in researching industrial scale procedures and installing a pilot production line with a capacity of 50 tonnes agar/year at Ha Long canning factory in Hai Phong city. In 1981, this pilot plant was put on trial operation and only had a yield of 20 tonnes/year. With the improvement of equipment and some steps in the production line, the Ha Long canning factory currently produces around 120 tonnes agar/year.
From 1985 to 1990, the Research Institute of Marine Products (RIMP) concentrated on developing a stable procedure of agar processing on a small scale (10–15 tonnes/year ) and family scale (3–10 kg/day). Now the Research Institute of Marine Products continuously studies techniques to upgrade agar quality and technology transferring for other enterprises.
In implementing the research programme of NACA for agar extraction for different Gracilaria species, it is hoped that new species will be founded to supply more raw materials for agar processing industry in Vietnam.
3.1 Materials and methods
All specimens of Gracilaria species collected during 1992 and 1993 were used. However, for two of the species collected, Gracilaria hainanensis and G. gigas, there was not enough of the specimens for agar analysis. Agar extraction was carried out under the procedure set up by Research Institute of Marine Products which was modified from Chandrkrachang (1992). A diagram of the agar extraction procedure of RIMP is shown in Figure 10.
The moisture content of seaweed, agar yield, gel strength and ash content were determined according to NACA, 1992.
3.2. Results
A sample of Gracilaria spp. was extracted and its agar yield analysed in duplicate or triplicate to gain an average figure. Analytical results were obtained from 6 Gracilaria species collected from Hai Phong and Quang Ninh provinces (See Table 1 and Figure 2) and showed that the dried/fresh ratio, agar yield and gel strength were highest in G. asiatica, and its ash content was also the lowest. It is a widely distributed species of Gracilaria in Vietnam and may become the main culture species in brackish water swamps in northern Vietnam.
Table 1: Results of the analysis of Gracilaria spp.
| Species | Place of collection | Date of collection | Dry/wet weight | Moisture Content (%) | Agar Yield (%) | Gel Strength (g/cm2) | Ash of Agar (%) |
| G. verrucosa | Dinh Vu, Hai Phong | 20.06.92 | 11.25 | 10.2 | 29.5 | 516 | 3.2 |
| G. blodgettii | Phu Long, Hai Phong. | 27.05.93 | 10.64 | 10.0 | 26.5 | 278 | 3.6 |
| G. tenuistipitata | Lien Vi, Quang Ninh | 21.06.92 | 9.60 | 11.3 | 19.7 | 338 | 3.4 |
| G. chorda | Hai Ninh, Quang Ninh | 28.05.93 | 10.40 | 12.5 | 21.5 | 290 | 4.0 |
| G. arcuata | Do Son, Hai Phong | 07.05.93 | 8.06 | 16.0 | 17.6 | 258 | 4.2 |
| G. bursa-pastoris | Quan Land, Quang Ninh | 29.05.93 | 9.65 | 15.0 | 15.2 | 128 | 4.5 |
G. blodgettii has an agar yield higher man that of G. tenuistipitata, but its gel strength is lower. G. boldgettii, having a high productivity, has been cultured a lot in Hai Phong and Cat Hai island, whereas G. tenuistipitata is cultured in the central provinces of Vietnam.
G. chorda has a agar yield higher man that of G. tenuispitata and its gel strength is higher than that of G. blodgettii. The latter has not been studied for the purpose of cultivation but it is a species which should be paid more attention to in Vietnam.
G. arcuata and G. bursa-pastoris have little natural biomass and the yield and quality of agar are lower than those of other 4 species above-mentioned. These species are not principal objects for study in Vietnam.
Figure 10: Agar extraction procedure at RIMP.

The quality of Gracilaria spp. and its agar in one species differed in various areas depending on ecological conditions. For cultivated Gracilaria spp., the yield and quality of agar still depends on culturing methods (seeding density, water-changing ability, fertilising, harvesting). In this study, Gracilaria asiatica samples were collected from various areas of 4 the coastal provinces of Northern Vietnam and analysed for the quality of seaweed and its agar (See Table 2). The analytical results showed that Gracilaria asiatica growing in Dinh Vu, Hai Phong and Yen Hung, Quang Ninh had the best quality. These areas are determined as having rich nutritional waters and other ecological conditions which are suitable for the growth and development of Gracilaria spp.
Table 2: Results of the analysis of Gracilaria spp. from different locations.
| Place of collection | Date of collection | Dry/wet weight | Agar Yield (%) | Gel Strength (g/cm2) | Ash of Agar (%) |
| Dinh vu, Hai Phong | 20.06.92 | 11.25 | 29.5 | 516 | 3.2 |
| Bang La, Hai Phong. | 20.06.92 | 11.4 | 27.0 | 456 | 3.4 |
| Cat Hai, Hai Phong | 20.06.92 | 10.5 | 24.5 | 319 | 3.6 |
| Yen hung, Quang ninh | 20.06.92 | 10.86 | 31.0 | 474 | 3.25 |
| Thai thuy, Thia binh | 20.06.92 | 11.0 | 28.0 | 396 | 3.2 |
| Hai hau, Nam Ha | 20.06.92 | 10.5 | 26.9 | 387 | 3.4 |
3.3. Discussion
The identification of 3 Gracilaria species: G. asiatica, G. blodgettii and G. tenuispitata with the highest quality among 13 species existing in Northern Vietnam corresponds with previous research results by Do and Nguyen (1990) and Nguyen Van Tien (1992 ). The above 3 species have also been researched and cultured for raw material for agar extraction in Vietnam. In addition, to the above, Gracilaria species and G. chorda with high economic value are also being studied.
The cultivation of Gracilaria species for high quality agar depends on many factors including site selection, the structure of ponds (in case culturing in brackish water area), culture methods, suitable harvest time and semi-processing. Studies on the biological characteristics of Gracilaria spp. are necessary in order to produce high-grade, quality products. During previous years, 3 species of cultivated Gracilaria spp. have been thoroughly studied in Vietnam. The introduction of new culture species is needed as are relevant studies to gain products of high economic value.
4. CONCLUSIONS
During this study, only 8 of the 13 species in northern Vietnam, listed by Nguyen Huu Dinh and Nguyen Van Tien (1990), were found.
Among the 8 species found, 4 had a high economic value. Of these 4 species, 3 species were studied and cultivated, namely: Gracilaria asiatica, G. blodgettii and G. tenuistipitata. G. chorda needs further study and trial cultivation.
The quality of raw materials, quality and yield of agar depend on the ecological conditions of each region and harvesting time. Specimens should be collected at the peak of development during the mature period for correct analytical results.
The collection of specimens in season (the period that Gracilaria spp. has reproductive organs), will make identification of the species easier. It is necessary to supply enough funds for field-collecting continuously at the mature period in order to gain specimens with reproductive organs.
5. PROPOSALS
It is necessary to invest in the systematic study of the components and ecosystems of the Gracilaria spp. flora of Vietnam. On the basis of this an accurate estimate of the natural resources, preservation measures as well as a development plan can be made for cultivating and agar processing in whole country.
Further study is needed and the application of new-techniques to culture seaweeds with higher economic value in Vietnamese coastal areas. More attention should be paid to species that can be cultured in gulfs and bays.
New technologies in agar processing should be promptly applied to produce high- quality products which can meet the requirements of both domestic and international markets.
Vietnam must co-operate with other countries in the region for training and study of Gracilaria spp. and agar processing. Taxonomic methods for Gracilaria spp. should be standardised and manuals for culture methods and analysis of agar quality produced.
Regional Centres for research into Gracilaria spp. and agar should be established under NACA so that researchers can exchange information on ecology, taxonomy, biophysiology, biochemistry, technique of cultivation of Gracilaria spp. and agar processing technology.
REFERENCES
Abbott, I. A., 1985 New species of Gracilaria from California and Hawaii. Taxonomy of Economic Seaweeds, Vol. I, Univ. of California, La Jolla Calif.
Abbott., I. A., 1988. Some Species of Gracilaria and Polycavernosa from Thailand . Taxonomy of Economic Seaweeds, Vol. II, Univ. of California, La. Jolla Calif.
Abbott., I. A., 1992. Taxonomy of Economic Seaweeds With reference to some pacific and western Atlantic species, Vol. HI. A publication of the California Sea Grand College, p. 193 – 232.
Chandkrachang, S. 1992 . A step by step guide on the small scale processing of Agar - agar and a comment on aquasanitation. FAO Fisheries Circular No. 848 , FIIU/C848 .
Do. V. K., 1990. Status of production and utilisation of seaweeds in Vietnam .Report of the Regional Workshop on the culture and utilisation of seaweeds. Cebu City, Philippines, 27 – 31 August 1990.
NACA , 1992 . Action plan for the regional study on Taxonomy , Ecology and Analysis of the Commercially Important Red Seaweeds . Training Workshop on Taxonomy, Ecology and Analysis of Commercially Important Red Seaweeds, Bangkok Thailand, 1992 .
Nguyen, V. T., 1988. Quantitative distribution of Gracilaria in Hai Phong Quang Ninh coastal zone. Annual Report of Oceanographic Institute, Haiphong, 1988 .
Nguyen H. D. and Nguyen V. T. 1993. Marine Algae of North Vietnam Publishing House of Science and Technology, 1993 .
Yamamoto, H. (1978). Systematic and anatomical studies of the Genus Gracilaria in Japan. Mem. Fec. Fish. Hokkaido University. 25:97–152.
Zhang, C. F. and Xia B. M. 1976. Studies on Chinese Species of Gracilaria . Stud . Mar. Sin . 11 , p. 157 –163.
Zhang, C. F. and Xia, B. M. 1985. On Gracilaria asiatica sp. nov. and G. verrucosa. Oceanol. lim. sin. 16:175–180.
Zhang C. F. and Xia B. M. , 1988. On two new Gracilaria from South China . Taxonomy of Economic Seaweeds, Vol. II. Univ. of California. La Jolla Calif.
H. Shoghi*
Iranian Offshore Fisheries Research Centre
Port of Chabahar, Iran
A number of studies have been carried out to identify and map the distribution of Iranian coastal algae. The first attempt to identify algae in the Persian Gulf was made by Diesing and Eadlicher in 1845. This study was followed by Boergesan and Koie (1939) and Newton (1955). It is worth mentioning that all these studies were carried out in the Persian gulf coasts and this is the first time that an article has been presented describing Iranian algae in Oman sea.
During four study trips fielded in 1993, 23 species of brown algae, 16 species of green algae and 29 species of red algae were identified in coasts of Persian Gulf and Oman Sea.
It was observed that Sargassum spp., Ulva lactuca, and Chondrus crispus species were common in the Oman Sea and Padina spp., Enteromorpha intestinalis, Chodrus crispus species were quite well distributed in the Persian Gulf.
The Iranian Fishing Company (Shilat) has a programme to develop the culture of economic and commercial algal species in Iranian waters and to establish new processing industries to obtain agar, alginate and carrageenan from algae. Any co-operation with relevant companies and organisations would be accepted and appreciated. Iran looks forward to joining the regional co-operation efforts in seaweed development organised under NACA.
V. Pahalawattaarchchi*,
National Aquatic Resources Agency,
Crow Island, Colombo 15, Sri Lanka.
ABSTRACT
Of the 260 seaweed species found growing along the coast of Sri Lanka, Gracilaria species are the most commonly used for food. Two species, G. verrucosa and G. edulis, occur in commercially valuable quantities in Kalpitiya, Trincomalee and Mannar.
G. edulis was recorded in the 1950's in the Kalpitiya area. Large quantities of G. verrucosa (G. confervoides) have been recorded in Koddiyar Bay near Trincomalee. G. edulis has the highest yield of good quality agar when extracted at pH 5. The ideal heating time was 4 hours. Comparison of cultured and wild seaweeds showed that the latter can give a satisfactory amount of gel content and strength. Comparative studies of three of the most common species found in the northern part of Sri Lanka, namely Hypnea spp., Gracilaria lichenoides and Gelidium spp. have shown a seasonal variation in agar content in these species, the plants showing the highest agar content in January. During May to November, wild Gracilaria spp. grows well in the Puttalam lagoon. This coincides with the south-west monsoon period and also the season for prawn fishing in the lagoon.
Sri Lanka exported an average of 100 tonnes/year of dried raw Gracilaria spp. since early in the last century. Exports have, however, dwindled over the years for various reasons. There is an increasing domestic demand for agar especially for medical research purposes and the confectionery industry. During 1985, Sri Lanka imported a wide range of seaweed products processed from red and brown seaweeds.
The National Aquatic Resources Agency launched a project in 1988 at Kalpitiya (north-west coast) to find out optimum growth conditions and culture sites for G. edulis in Puttalam lagoon. Two methods were followed for cultivation, namely vegetative and spore setting techniques. The culture of Gracilaria on a commercial scale and improvement of processing methods are considered of immense importance to national development from seaweed products and cut down the import expenditure on refined agar. Polyculture of Gracilaria spp. with brackish water fish species is recommended for future development.
1. INTRODUCTION
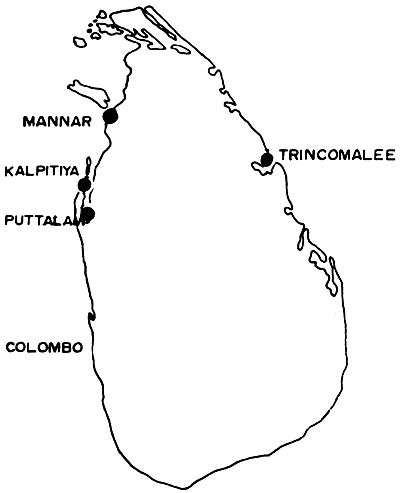
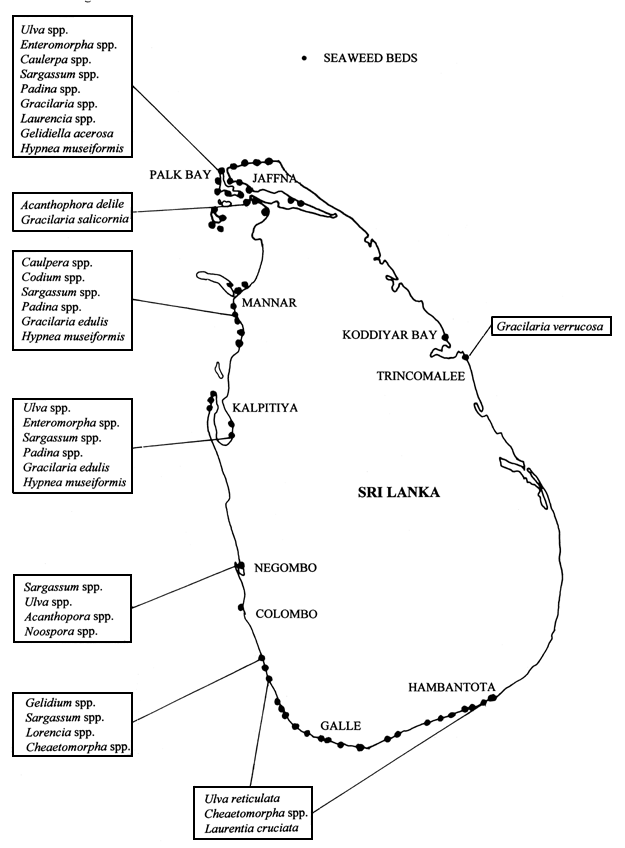
There are nearly 260 species of seaweeds growing along the Sri Lankan coastline (which is approximately 1700 km long), of which only 2 species are of commercial interest (Durairtnam, 1961). Gracilaria varieties, known as “Ceylon moss” are the varieties most commonly used as food. G. verrucosa and G. edulis are the two main species of commercial importance at present and occur in commercially valuable quantities in three main areas of Sri Lanka (Figure 1), namely Kalpitiya, Trincomalee and Mannar (Anon., 1952).
Figure 1: Locations where seaweed is collected for commercial purposes in Sri Lanka.
Gracilaria edulis was reported in the 1950's in the Kalpitiya area. Large quantities of Gracilaria verrucosa (Gracilaria confervoides) have been recorded in Koddiyar Bay near Trincomalee (Duariratnam 1965). According to the author G. verrucosa gives a 20–25 % yield of agar. Recent studies regarding the agar yield of G. edulis revealed that it has the highest constant (48.7%) of best quality agar when extract at pH 5. The ideal heating time was 4 hours, on the basis of yield and quality.(Jayasinghe & Jayasinghe 1994). According to the comparison of cultured and wild seaweeds, the latter has given satisfactorily amounts of gel content, working strength, moisture content, ash content and insoluble ash contents (Jayasinghe & Jayasinghe ,1994, unpublished data). Studies in the northern part of Sri Lanka, have shown a seasonal variation in agar content in G. lichenoides, the plant showing the highest agar content in January.(Table 1).
Table 1: Agar content (dry weight) of different red algae from northern Sri Lanka (Mandativu).
| 1977 | 1978 | ||||||
| Species | October | December | January | March | May | July | August |
| Gracilaria | .37% | - | 50% | 35% | 28% | 33% | 27% |
Source: Arumugam et al. (1981)
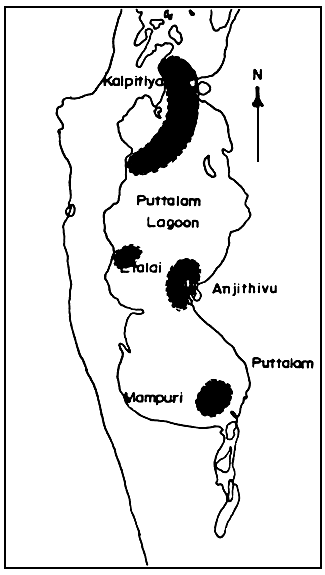
During May to November wild Gracilaria spp. grows well in the Puttalam lagoon (Subasinghe & Jayasuriya 1989a). This coincides with the south west monsoon period and also the season for prawn fishing in the lagoon. Figure 2 shows the areas of Puttalam Lagoon where natural beds of seaweeds are found. People who collect seaweeds are mainly engaged in prawn fishing and only when the local buying agents request seaweeds do they collect them (Subasinghe, & Jayasooriya, 1989b).
Figure 2: Locations in the Puttalam Lagoon where natural beds of seaweeds are found.
2. PRESENT STATUS OF THE GRACILARIA INDUSTRY
2.1 Wild stocks of Gracilaria
Sri Lanka exports about 100 tonnes/yr of dried raw Gracilaria spp., which has been actively collected, dried and exported since early in the last century, though there has been a recent decline in wild stocks (Subasinghe & Jayasuriya, 1989a). Figure 3 shows the distribution of seaweed beds of commercial importance. The quantity exported in 1972 was around SO tonnes with an export value of Rs.76,000 (FOB). In 1986, these exports increased to 150 tonnes. Of this, 70 tonnes were produced from the Kalpitiya area, the balance came from the Trincomalee area (Subasinghe & Jayasooriya 1989a,b).
Over the last 7 years, no harvesting of Gracilaria spp. has been done in Trincomalee due to the unsettled conditions prevailing in the northern part of the country. According to export data, only 10 tonnes of dried Gracilaria spp. were exported in 1987. In 1988, it was further reduced to 5 tonnes. At present, according to the information provided by the export companies there is a very high renewed demand for this resource. Hence, the export companies find it difficult to cope with the present demand due to limited natural stocks.
2.2 Status of the seaweed processing industry
Most countries exporting seaweeds have realised the benefits of exporting processed seaweed products rather than the dried seaweeds. Understanding the distinct advantages of exporting unprocessed seaweed products, the government launched several pilot projects to examine the feasibility of extracting alginic acid and agar from local seaweeds, during the period of 1960–1970. Even at that time those products were found to be of international standard.
The government decided to expand this industry by setting up a network of collecting centres and processing plants under the management of District development councils. To accelerate the programme, the government introduced an import quota system for manufactures. Nevertheless, a change in government policy in the mid 1970's cancelled the import quota system and the local market once again started to import seaweed products. Since then Sri Lanka has remained as an exporter of dried seaweed.
2.3 Demand for the seaweed products in Sri Lanka
In Sri Lanka, with the development of new industries, there is a demand for agar especially for medical research purposes and the confectionery industry. During 1985, Sri Lanka imported a wide range of seaweed products processed from red and brown seaweeds at a value of Rs 14 million. (Customs Department Report).
2.4 Present status of culture practices of Gracilaria spp.
An alternative way to prevent the exploitation of natural seaweed stocks is cultivation. Taking into account the importance of establishing a steady source of Gracilaria spp. for that purpose, the National Aquatic Resources Agency launched a project in 1988 at Kalpitiya (north west coast) to establish optimum growth conditions and culture sites for G. edulis in Puttalam lagoon.(Jayasooriya 1993).
Two culture methods were used, namely vegetative and spore setting techniques. From the vegetative propagation technique the plant growth was 0.42 cm/day and from the spore setting technique it was 0.25 cm/day. Gracilaria edulis showed high relative growth rates in Puttalam lagoon during the south west and the north east monsoon.
Figure 3: Distribution of seaweed of commercial importance in Sri Lanka.
The most effective substratum found for the vegetative propagation was plastic nets in an optimum depth of 20 cm with optimum density of 100 fragments/m2 (Jayasooriya 1993). The author also recommended the spore settlement technique for the development of small scale culture practices oilier than commercial culture due to the lack of spore bearing plants in the lagoon during parts of the year. The author also revealed that fluctuations in environmental factors such as salinity and turbidity of water adversely affected the growth of Gracilaria spp.. Hence, more work is needed on the aspect of turbidity in relation to light attenuation for finding suitable sites in the lagoon for the culture of Gracilaria edulis.
The culture of Gracilaria spp. on a commercial scale and improvement of processing methods are considered to be of immense importance to increase the GNP from sea weed products and cut down the import expenditure on refined agar.
Polyculture of Gracilaria spp. with edible brackish water fish species can be recommended for future development of seaweed culture.
REFERENCES
Arumugam et al (1981). Preliminary studies on the Alginic acid and Agar contents of some marine algae.
Anon, 1952. Report of Ceylon Fisheries Institute.
Durairatnam, M. 1961 Contribution to the study of marine algae of Ceylon, Bulletin of the Fisheries Resource Station. Ceylon, No. 10: 5–117.
Duariratnam, M. 1965. The ecology of Gracilaria verrucosa (Hudson) Papenfuss in Koddiyar Bay, Trincomalee. Ceylon Bulletin of the Fisheries Research Station, Ceylon, 19:11–12.
Jayasinghe, P.& Jayasinghe, C. 1994. Extraction of agar from seaweeds. Paper presented in FAO-IPFC Working Party Meet SIFR-World Bank Meet 7–12 March. CIFT, Cochin, India.
Jayasooriya, P. M. A. 1993. Biomass estimation of economically important seaweed species along the west coast of Sri Lanka and culture trials of Gracilaria edulis in the Puttalam lagoon of Sri Lanka.(Phd thesis).
Subasinghe, S. & Jayasooriya, P. M. A. 1989a A study of the present status of the seaweed industry in Sri Lanka.(unpublished).
Subasinghe, S & Jayasooriya, P. M. A. (1989b) Utilisation and marketing of seaweed in Sri Lanka. Paper presented in seminar on Gracilaria spp. production and utilisation in the Bay of Bengal region. Songkhla, Thailand, 23–27 October 1989.