Khanjanapaj Lewmanomont,
Professor, Department of Fishery Biology,
Faculty of Fisheries, Kasetsart University, Bangkok, Thailand
ABSTRACT
From 9 countries in the region only Bangladesh reported the absence of Gracilaria spp. The reports from the other eight countries, namely: China; India; Indonesia; Malaysia; Myanmar; Philippines; Thailand; and Vietnam, included 25 identified species with 3 varieties and 4 unidentified species. Among these, 10 species appear to be correctly identified, the rest have to be confirmed. The most common species was G. edulis, followed by G. tenuistipitata, G. salicornia and G. changii. The species recommended for culture were G. edulis, G. changii and G. tenuistipitata.
The main problem in identifying the species of Gracilaria was the lack of sexual reproductive organs. Only a few species could be identified by their morphological characteristics such as G. eucheumoides and G. salicornia.
1. INTRODUCTION
Marine red algae, or red seaweeds, comprise the largest and most diverse assemblage of the marine plants. They are important to the marine environment and used as raw materials for the extraction of valuable products. Gracilaria is one of the red seaweeds used as an important source of agar extracts and related products. This seaweed grows naturally in many Asian countries, but the taxonomic status and ecological requirements for culture of the various species are not properly known.
2. STUDIES ON GRACILARIA
The genus Gracilaria was established by Greville in 1830 and consisted of four species: G. confervoides; G. compressa, G. purpurascens and G. erecta. In 1852, J. Agardh revised and redefined the generic circumscription for the genus, and designated G. confervoides as its species. Early concepts of the species of Gracilaria were mainly based on external structure. A detailed anatomical study was first reported by Sjostedt in 1926 with G. confervoides, G. compressa and G. robusta.
In 1949, Dawson regarded nutritive filaments as a diagnostic characteristic at the generic level and distinguished the genus Gracilariopsis from Gracilaria on the basis of the absence of nutritive filaments and the small size of the gonimoblast cells. In 1966, Papenfuss reported that the presence of nutritive filaments could not always be confirmed in British material of Gracilaria verrucosa. He concluded that the presence of nutritive filaments could not be used as a basis for separating Gracilaria and Gracilariopsis and, for that reason, he reduced Gracilariopsis to synonymy with the original genus Gracilaria.
In 1963, Chang and Xia described the genus Polycavernosa with P. fastigiata as the type species. This genus consists of rhizome-like creeping parts from which arise erect, free branches; a creeping portion fastened at frequent intervals by a disc-like attachment; spermatangial conceptacles in clusters and basal absorbing filaments with many long branches. Many authors supported the recognition of Polycavernosa and some species of Gracilaria were transferred into Polycavernosa and new species have been described. Wynne (1989), re-instated Hydropuntia Montagne (1842) which is shown to be the earliest validly published name with H. urvillei as the type species. He transferred 14 species of Polycavernosa and two species of Gracilaria to Hydropuntia.
In 1991, Abbott, Zhang and Xia described a new species, Gracilaria mixta, according to the mixture of two kinds of spermatangial conceptacles, Verrucosa type and Polycavernosa type, in the same branchlets. Re-examination of male plants of the western Pacific taxa placed in Polycavernosa (= Hydropuntia) also shows both types of configurations in the same thalli. Therefore, 16 species of Hydropuntia were transferred to Gracilaria.
The genus Gracilaria is cosmopolitan in its distribution. It has been reported from most parts of the world, the Arctic, temperate, tropical and even Antarctic region. According to Bird et al. (1982), the apparent centre of distribution lies in the tropics with the largest number of species, and a rapid decline in species numbers occurs with increasing latitude in both directions. More than 150 species have been reported. The identification of the species of Gracilaria is difficult owing to the great variability of the plants and poorly understood species limits. Anatomical characteristics seem to be more stable than morphological features.
2.1 Life cycle of Gracilaria (Figure 1)
Gracilaria shows an alternation of isomorphic generations between haploid gametophyte and diploid tetrasporophyte. The gametophyes are dioecious. The fertile male thallus produces spermatangia and the female thallus produces carpogonia. After fertilisation a structure called a “cystocarp” is formed. The cystocarps are prominent, protruding, globose or hemispherical, with or without rostrum, scattered on the surface of the female thallus. Each cystocarp consists of pericarp, gonimoblast filaments and carposporangia, with or without absorbing filaments. Carpospores are liberated through a small hole or ostiole at the top of the cystocarp and germinate into tetrasporic thalli or tetrasporophytes.
Figure 1: Life cycle of Gracilaria
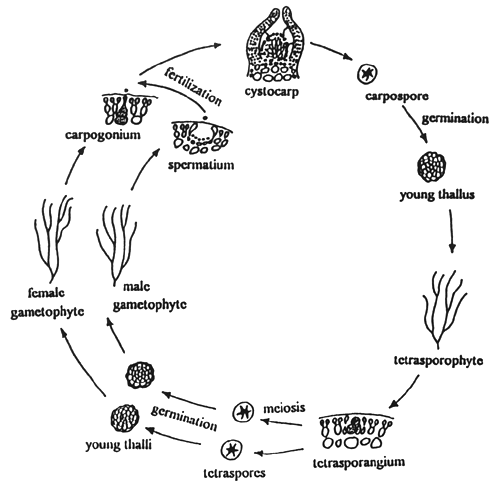
The mature tetrasporophyte produces tetrasporangia occurring generally in the cortex of the thallus. The tetrasporangium is cruciately divided and forms four spores or tetraspores which germinate into four gametophytic thalli or gametophytes, of which two are male and two are female thalli.
3. CHARACTERISTICS OF GRACILARIA
The genus Gracilaria is characterised by an erect, fleshy and succulent thallus which consists of a small discoid holdfast with lateral branches. The thalli of most species are cylindrical, some are compressed (G. eucheumoides) and some are foliose (G. textorii).
Important characteristics used for identifying species of Gracilaria are :
| A. | Morphological features (external features) | ||
| 1. | From of thalli | - | cylindrical or terete |
| - | compressed or flattened | ||
| - | foliose | ||
| 2. | Branching | - | alternate |
| - | di or trichotomous | ||
| - | secund | ||
| - | irregular | ||
| 3. | Constriction of branches - frequently Gracilaria shows a constriction at the point where a lateral branch joins a main axis. | ||
| 4. | Branch apex | - | attenuate |
| - | rostrate | ||
| - | tapering | ||
| - | apiculate | ||
| - | obtuse | ||
| - | blunt | ||
| B. | Anatomical characteristics (internal features) | ||
| 1. | Size and number of medullary cells | ||
| 2. | Number of cortical cells | ||
| 3. | Change of cells in medullary layers | ||
C. Characteristics of reproductive organs
Types of spermatangial conceptacles:
Polycavernosa type - compound Verrucosa type
Characteristics of cystocarps:
With or without rostrum
Anatomy of cystocarp:
iv. Absorbing filament
The most important characteristics used in identifying the species are the male and female reproductive organs. Without these, it is almost impossible to determine the specific names.
3.1 Studies on Gracilaria in Asian waters
Taxonomic studies of Gracilaria in Asian waters were reported in “Taxonomy of Economic Seaweeds”, publications of California Sea Grant College, Volume 1–4. A review of work carried out in seven Asian countries is presented below.
Studies on Gracilaria have been actively carried out in China by Zhang and Xia. They described many new species and revised the name of some similar species previously identified. In total, 25 species were reported from China.
Japan has produced many taxonomic works on Gracilaria especially those by Yamamoto. Seventeen species were reported with some replaced names for the previous identifications. Phang (1994), reported 5 species of Gracilaria from Malaysia and Singapore, two of which are new records. These five species were added to the three previously known in Malaysia.
Taxonomic studies of Gracilaria in the Philippines have been conducted since 1963. Twenty four species are listed, about half are mis-identified or have had their names changed recently. Abbott (1994), examined specimens that had been collected in the Philippines and reported 9 species. Three of the nine were added as new records and two new species were described by Yamamoto and Trono (1994).
In Taiwan, Chiang (1985) reported only 8 species with key, list, description and distribution of the species.
Studies of Gracilaria in Thailand have been conducted recently. Abbott (1988), described 4 species of Gracilaria and 4 species of Polycavernosa, two of these are new species. Lewmanomont (1994) described 13 species of Gracilaria from Thailand, four are new records and one new species.
In Vietnam, Nguyen (1992) re-examined the specimens of Gracilaria and Gracilariopsis and reported 15 species.
In conclusion, 53 species of Gracilaria are listed in this region, almost half are mis-identified or have had their names changed recently. In some cases, the old names are repeated, but the new one has also been added.
3.2 Species determination of Gracilaria from Asian countries
Examination of herbarium specimens from member countries in the region found that most of them lacked male reproductive organs and there were some without both male and female ones. This made it difficult to determine the species. Only a few species could be identified by their morphological characteristics, such as G. eucheumoides by its compressed, prostrate, thick and succulent thallus with dentate margins, and G. salicornia by its prostrate to semi-erect thallus with constricted segments.
From the reports of the countries, 25 species with 3 varieties were identified and 4 species were unidentified (Table 1). Among these, some names are not currently used. They are :
G. crassa - current name G. salicornia. Xia (1986), examined specimens from various countries and found all Gracilaria with constricted segments either at the main axes or lateral branches, namely G. cacalia, G. crassa, G. minor, G. canaliculata and G. salicornia were the same species. Since G. salicornia is the oldest available name for this group, the other names were placed in synonymy.
G. fastigiata - this name is supposed to be Polycavernosa fastigiata which was transferred to its former name G. edulis (Abbott et al. 1991).
G. verrucosa - this is the most confused species and many countries reported its occurrence. It is not a regular inhabitant of Asian waters, it belongs to European algal flora.
According to Abbott et al. (1985) “G. verrucosa”, was recorded from “everywhere” with the widest kinds of ecological conditions. Some of the descriptions or specimens in general match those from the English coast (type locality), but critical comparisons show differences in details of the gonimoblast, pericarp, or spermatangia. The Japanese and Chinese specimens referred to G. “verrucosa” were found to be a similar taxon, but taxonomically not the same as the English G. verrucosa. Further, it is different from the Taiwan specimens under that name (Yang and Chiang, 1982) especially in the gonimoblast structure. Also different were specimens from California called “G. verrucosa” (Abbott and Hollenberg, 1976). Clearly, it is necessary to understand the features and their variation by which the British topotype material of G. verrucosa is to be recognised.
The Taiwanese G. verrucosa reported by Chiang (1985, p.81) was re-examined and identified as G. tenuistipitata var. liui by Zhang and Xia (1988). The Chinese G. verrucosa reported by Zhang and Xia (1985) was named as G. asiatica.
3.3 Problems with identification of Gracilaria
The taxonomy of this economically important agar-producing genus is confused by the high degree of morphological and anatomical variation exhibited within species. Although Gracilaria is a very polymorphic genus vegetatively, it has remarkably constant reproductive structures.
The main problems in identification of Gracilaria are the lack of sexual reproductive organs in most of the specimens collected and also lack sufficient numbers of specimens for an evaluation of the boundaries of species. The comparison of the type specimens is not easy because the type specimens are widely distributed and many specimens are difficult to find.
Table 1: Distribution of Gracilaria in some Asian countries (From: Taxonomy of Economic Seaweeds. Vol. 1–4).
| SPECIES | CPR | JAP | MAL | PHI | TAI | THA | VIE | Male | Remarks | |
| 1. | G. arcuata | X | X | X | X | X | V | |||
| 2. | G. articulata | X | X | V | ||||||
| 3. | G. asiatica | X | X | V | Replaced name for G. verrucosa | |||||
| 4. | G. bangmeiana | X | X | X | P | Replaced name for Polycavernosa ramulosa | ||||
| 5. | G. blodgettii | X | X | X | T | |||||
| 6. | G. bursa-pastoris | X | X | T | Replaced name for G. compressa | |||||
| 7. | G. cacalia | X | V | Current name is G. salicornia | ||||||
| 8. | G. canaliculata | X | V | Current name is G. salicornia | ||||||
| 9. | G. changii | X | X | X | V | |||||
| 10 | G. chorda | X | X | C | ||||||
| 11 | G. chouae | X | T | |||||||
| 12 | G. compressa | X | T | Synonym for G. bursa-pastoris | ||||||
| 13 | G. confervoides | X | V | Synonym for G. verrucosa | ||||||
| 14 | G. coronopifolia | X | X | X | X | X | V | |||
| 15 | G. crassa | X | X | X | X | V | Current name is G. salicornia | |||
| 16 | G. cuneifolia | X | T | |||||||
| 17 | G. dactyloides | X | ||||||||
| 18 | G. denticulata | X | X | X | T | Current name is G. vieillardii | ||||
| 19 | G. disticha | X | ||||||||
| 20 | G. edulis | X | X | X | X | X | X | P | Replaced name for P. fastigiata | |
| 21 | G. eucheumoides | X | X | X | X | X | X | |||
| 22 | G. firma | X | X | X | X | X | V | |||
| 23 | G. fisheri | V | ||||||||
| 24 | G. gigas | X | X | X | X | T | ||||
| 25 | G. glomerata | X | T | |||||||
| 26 | G. hainanensis | X | X | V | ||||||
| 27 | G. heteroclada | X | X | C | ||||||
| 28 | G. incurvata | X | ||||||||
| 29 | G. irregularis | X | C | |||||||
| 30 | G. lacinulata | X | ||||||||
| 31 | G. lemaneiformis | X | X | X | C | |||||
| 32 | G. lichenoides | X | Synonym of G. edulis | |||||||
| 33 | G. manilaensis | X | V | Replaced name for G. verrucosa | ||||||
| 34 | G. megaspora | X | ||||||||
| 35 | G. minor | X | V | Current name is G. salicornia | ||||||
| 36 | G. minuta | X | V | |||||||
| 37. | G. percurrens | X | V | |||||||
| 38. | G. punctata | X | X | |||||||
| 39. | G. purpurascens | X | ||||||||
| 40. | G. rubra | X | V | |||||||
| 41. | G. salicornia | X | X | X | X | X | X | V | ||
| 42. | G. spinulosa | X | X | |||||||
| 43. | G. sublittoralis | X | ||||||||
| 44. | G. subtilis | X | V | |||||||
| 45. | G. sullivanii | X | uk | |||||||
| 46. | G. tenuistipitata | X | X | X | X | T | ||||
| 47. | G. textorii | X | X | X | X | X | X | T | ||
| 48. | G. turgida | X | ||||||||
| 49. | G. urvillei | X | ||||||||
| 50. | G. verrucosa | X | X | X | X | V | ||||
| 51. | G. vermiculophylla | X | V | Replaced name for G. verrucosa | ||||||
| 52. | G. vieillardii | X | T | Replaced name for G. denticulata | ||||||
| 53. | G. yamamotoi | |||||||||
| TOTAL | 30 | 17 | 8 | 24 | 8 | 13 | 15 |
Key: V = Verrucosa type
T = Textorii type
C = Chorda type
P = Polycavernosa type
| CPR | China |
| JAP | Japan |
| MAL | Malaysia |
| PHI | Philippines |
| TAI | Taiwan |
| THA | Thailand |
| VIE | Vietnam |
Table 2: List of Gracilaria reported by the eight participating countries.
| SPECIES | CPR | IND | INS | MAL | MYA | PHI | THA | VIE | ||
| 1. | G. arcuata | X | X | |||||||
| 2. | G. asiatica | X | ||||||||
| 3. | G. blodgettii | X | ||||||||
| 4. | G. bursa-pastoris | X | ||||||||
| 5. | G. changii | X | X | X | ||||||
| 6. | G. chorda | X | ||||||||
| 7. | G. corticata var. corticata | X | ||||||||
| var. cylindrica | X | |||||||||
| 8. | G. crassa1 | X | X | |||||||
| 9. | G. edulis | X | X | X | X | |||||
| 10. | G. eucheumoides | X | X | X | ||||||
| 11. | G. fastigiata2 | X | X | |||||||
| 12. | G. flrma | X | X | |||||||
| 13. | G. fisheri | X | ||||||||
| 14. | G. foliifera | X | ||||||||
| 15. | G. gigas | X | X | |||||||
| 16. | G. hainanensis | X | ||||||||
| 17. | G. heteroclada | X | ||||||||
| 18. | G. irregularis | X | ||||||||
| 19. | G. lemaneiformis | X | X | X | ||||||
| 20. | G. manilaensis | X | ||||||||
| 21. | G. millardetii | X | ||||||||
| 22. | G. salicornia | X | X | X | ||||||
| 23. | G. tenuistipitata | X | ||||||||
| var. liui | X | X | X | |||||||
| 24. | G. textorii | X | ||||||||
| 25. | G. vemicosa3 | X | X* | |||||||
| 26. | Gracilaria sp. 1 | X | ||||||||
| 27. | Gracilaria sp. 2 | X | ||||||||
| 28. | Gracilaria sp. 3 | X | ||||||||
| 29. | Gracilaria sp. 4 | X | ||||||||
* Name changed to G. asiatica
1. Current name is G. salicornia
2. Current name is G. edulis
3. Does not exist in Asian waters
REFERENCES
Abbott, I. A. 1985. Gracilaria from the Philippines: List and distribution of the species. Taxonomy of Economic Seaweeds with reference to some Pacific and Caribbean species. California Sea Grant College, La Jolla. p. 89–90.
Abbott, I. A. 1988. Some species of Gracilaria and Polycavernosa from Thailand. Taxonomy of Economic Seaweeds. Vol. 2, p. 137–150.
Abbott, I. A., Y. M. Chiang, S. Frederick, J. N. Norris, R. T. Stud, B. M. Xia and H. Yamamoto. 1985. The red alga Gracilaria Greville (Gracilariaceae, Gigartinales) : Introduction. In : Abbott, I. A. and J. N. Norris, (eds.) Taxonomy of Economic Seaweeds; with references to some Pacific and Caribbean species. California Sea Grant College Program, La Jolla, Calif, pp.67–68.
Abbott, I. A., Zhang J. Fu and Xia B. M 1991. Gracilaria mixta, sp. nov. and other western Pacific species of the genus (Rhodophyta: Gracilariaceae) Pac. Sci. 45(1) : 12–27.
Agardh, J. G. 1852. Species genera et ordines algarum. Vol.2(2) : 337–720 pp. Lund.
Bird, C. J., J. P. van der Meer and J. Malachlan. 1982. A comment on Gracilaria verrucosa (Huds.) Papenf. (Rhodophyta: Gigartinales) J. Mar. Biol. Ass. U.K. 62: 453–459.
Chang, C.F. and Xia B. M. 1963. Polycavernosa, a new genus in the Gracilariaceae. Stud. Mar. Sinica 3 : 119–126.
Chiang, Y.M. 1985. Gracilaria from Taiwan; Key, list and distribution of the species. Taxonomy of Economic Seaweeds. 1: 137–150
Dawson, E. Y. 1949. Studies of north-east Pacific Gracilariaceae. Occasional Papers Hancock Found. 7 : 1–54, 25 pls.
Greville, R. K. 1830. Algae Britannicae. XXXVIII + 215 pp., 19 pls. Maclachlan and Stewart, Edinburgh.
Lewmanomont, K. 1994. The species of Gracilaria from Thailand. In: Abbott, I. A. (ed). Taxonomy of Economic Seaweeds. 4: 145–148
Nguyen H. D., 1992. Vietnamese species of Gracilaria and Gracilariopsis. Taxonomy of Economic Seaweeds. 3:207–210.
Papenfuss, G. F. 1966. Notes on algal nomenclature V. Various Chlorophyceae and Rhodophyceae. Phycos 5 : 95–105.
Phang, S. M. 1994. Some species of Gracilaria from Peninsular Malaysia and Singapore. Taxonomy of Economic Seaweeds. 4: 125–133.
Sjostedt, L. G. 1926. Floridean Studies. Lunds Univ. Arssk. N.F. Avd. 2, Bd. 22 : 1–94.
Wynne, M. J. 1989. The reinstatement of Hydropuntia Montagne (Gracilariace, Rhodophyta). Taxon 38 : 476–479.
Xia B. M. 1986. On Gracilaria salicornia (C. Agardh) Dawson. Chinese Journal of Oceanology and Limnology 4(1) : 100–105, pl.1.
Yamamoto, H. and G. C. Trono, Jr. 1995. Two new species of Gracilaria from the Philippines. Taxonomy of Economic Seaweeds: Vol 4, p. 95–101.
Zhang J. F. and Xia B. M. 1985. On Gracilaria asiatica Zhang et Xia and G. verrucosa (Huds.) Papenf. Oceanol. Limnol. Sin. 16 : 175–180.
Zhang J. F. and Xia B. M. 1988. On two new Gracilaria (Gigartinales, Rhodophyta) from South China. In.: Abbott, I. A., ed. Taxonomy of Economic Seaweeds; with references to some Pacific and Caribbean species. California Sea Grant College Program, La Jolla, Calif. Vol.2, pp. 131–136.
by
R. Perez and O. Barbaroux,
IFREMER Centre de Nantes, Rue de I'lle d'Yue,
Nantes, France.
ABSTRACT
There are four main uses for cultured Gracilaria: fodder for fish and mollusc aquaculture; human consumption; fertiliser; and high quality agar. Fish farmers in China and the Philippines were probably the first to cultivate this algae by cutting it into little pieces and throwing it into ponds where herbivorous fish (milkfish) are grown. In this symbiotic system, algal growth is stimulated by nitrogen excretion by the fish, which also feed on the seaweeds. Gracilaria spp., was not considered an agarophyte before 1949 when there was a shortage of Japanese agar and alkali treatment techniques were developed to improve agar quality. Production has increased steadily since that time.
Despite the simplicity of culture, two major problems remain to achieve production of high quality agar: (i) taxonomic problems due to difficulties in reliably determining species; and (ii) increasing culture yields lowers agar quality and vice versa.
1. INTRODUCTION
1.1 Geographical distribution
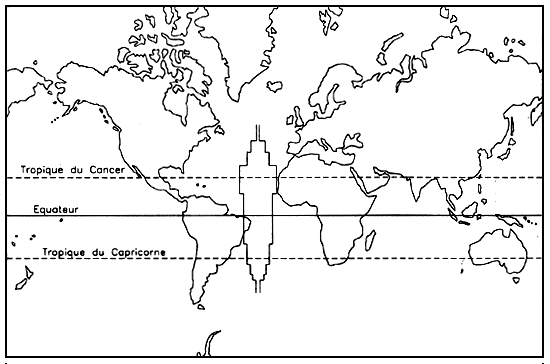
Gracilaria spp. has a world-wide distribution (Figure 1) because of its ability to withstand great ecological variations. It is found in all the seas of the world, from 60° north to 40° south latitude (Kling, 1978) and from cool temperate to tropical waters. Chapman (1977), supposed that it could exist up to 47° north latitude, and Mayer (1986) down to 45° south latitude. Development is more limited in cool temperate areas, but the quality of the agar is similar to that of bacteriological agar. The grey distribution zone is valid for all oceans and not just for the Atlantic.
Figure 1: Worldwide distribution of Gracilaria spp. between 47°N and 45°S.
The biology of Gracilaria spp. was recently reviewed by Yamamoto (1978) in Japan; Chang and Xia (1976) in continental China; Shang (1976) in Taiwan; Umaheswara Rao (1972) in India; Kim (1970), Cable (1974) and Santelices (1989) in Chile; Kraft (1977) in New Zealand; Bodard (1966), Kling (1978), Destombe (1987), Lefèbvre (1986) and Christiaen (1986) in Europe; and MacLachlan and Edelstein (1977) in North America.
1.2 Bathymetric distribution
Gracilaria spp. is found on tidal mudflats, in sandy or muddy basins with scattered rocks where it is rarely out of the water (Lefèbvre, 1986). In the sea, it occurs to depths of up to 25 m, although 98% of the population is found between 0.5 m and 10 m, with an optimum depth of 3 to 4 m (Kim, 1970). It cannot remain out of the water for more than an hour. By means of its basal disk, it attaches to all sorts of substrates (sand, shell debris, rocks), even to mussel byssus. It can continue to develop even when partly buried in the sand.
2. BIOLOGY
2.1 Regeneration
Like most Gigartinales, Gracilaria possesses the remarkable ability of regeneration at the level of breaks or damaged areas in order to reconstitute missing tissues and ensure growth. This property concerns attached plants as well as detached fragments and the basal disk. The thallus, once cut, can thus be expected to grow again, and a piece can be used as a cutting to produce an entire frond. The cutting buried 10 cm in the sand, which prevents any photosynthesis, can subsist on its own reserves for more than 90 days without any increase in size or weight (Santelices et al, 1984). Re-growth only occurs once movements of the sea bottom displace the sand and uncover the fragment. These fragments as well as basal disks are as important as sexual reproduction in maintaining and expanding populations.
Lefèbvre (1986), noted that low temperatures inhibit the emergence of fronds but favour the development of the disk, which becomes a means of resistance and survival. Thus, during severe winters in which fronds are destroyed, the base survives and produces re-growth the following year.
2.2 Growth
Growth is ensured by the apex of the main stem and those of ramifications. Growth rate and development differ depending on whether the plants live in warm areas without great seasonal variations, in marshes in which the water is replenished or in brackish waters. In Israel, Friedlander and Lipkin (1982) found a maximal growth of 6.6 g.m-2.day-1 (wet weight). Doty (1977) noted 5.6 g.m-2.day-1 and Edelstein (1977) 8.9 g.m-2.day-'. Optimal theoretical production can attain 125 tons.hectare-1.year-1 (dry weight), i.e., one of the highest productions in the plant world.
2.3 Variables affecting production
The fact that Gracilaria spp. is a cosmopolite is indicative of its capacity to withstand considerable environmental variations.
Salinity: Gracilaria spp. is euryhaline, adapting to salinities of 15 to 50 ppt. The best growth is obtained between 20 and 35 ppt.
Temperature: Optimal development occurs between 20 and 28°C (Causey et al. 1946).
Lighting: Light intensity does not appear to be a basic factor since the species can survive with very reduced lighting.
Epiphytism: Beginning in June, certain Gracilaria spp. plants bear epiphytes which become increasingly abundant, ultimately causing cessation of growth and degeneration of thalli.
Turbidity: As Gracilaria spp. lives preferentially in sandy areas, it needs to withstand great variations in turbidity which limit photosynthesis by reducing light intensity. Some fields implanted near the estuaries of streams carrying considerable silt are more fragile and paler than those growing in clearer waters.
Immersion: When the climate is quite humid, Gracilaria spp. can survive an entire day out of the water, but in sunlight it resists no more than an hour.
Nutrition: As Gracilaria. spp. develops in quite varied sites, it has been impossible by natural observation to demonstrate the influence of nutrient levels on the metabolism of the species. Jones (1959) reported that, paradoxically, the greatest growth takes place when the nitrogen level is lowest.
3. NATURAL POPULATIONS AND COLLECTION METHODS
The largest resources in Gracilaria spp. are found in the following locations:
in Asia, along the Pacific coasts of Japan and the Philippines; along the shores of Java, Sri Lanka and south India; in the Bay of Bengal; around Taiwan and Hong Kong; in Thailand, Vietnam, Cambodia and Malaysia;
in Africa, along the coasts of Mozambique and South Africa;
in South Europe (southern and Adriatic Italy);
in South America, along most of the coast of Peru and Chile; along the southern shores of Argentina and Brazil.
Exploitation is particularly active in Taiwan, the Philippines and Chile. In Taiwan and the Philippines, harvesting is done manually in the marshes and along the coast where plant populations show high densities (4.2 kg. m-2) and growth rates of 4.70 g. m-2 day-1. Peak growth rates are reached between November and February when the sea is calm, salinity high and rains infrequent. The minimal period for harvesting corresponds to July and August when the south-west monsoon brings big waves, violent rains and a drop in salinity.
In Chile, the dominant Gracilaria have been identified as G. lemaneiformis (Bory) Weber Van Bosse by Santelices et al. (1979), G. chilensis by Bird et al. (1987), Gracilaria verrucosa (Hudson) Papenfuss by Tawaga, Kojima and Kono (1963) and G. confervoïdes by the Department of Hunting and Fishing (Cable, 1974). Harvesting is performed manually at mid-thigh level in the intertidal zone and by boat using a tool in the subtidal zone. Three types of tools are used (Figure 2): a long-handled rake; the “trebol” a hook attached perpendicularly to a pole; and the “araña” or “spider”, which looks like an umbrella turned inside out. The two traditional systems used (trebol and araña) are in fact being abandoned in favour of the rake and diving which provide higher yields and cause less plant deterioration.
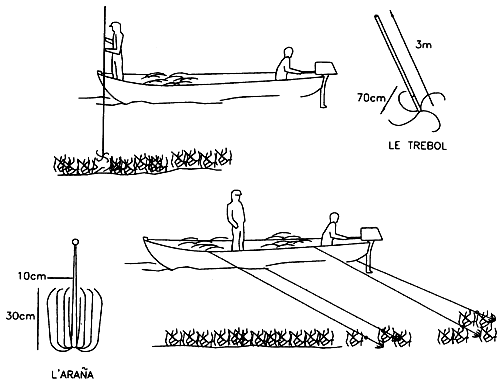
Figure 2: Gathering wild populations in Chile.
4. CULTIVATION OF GRACILARIA
The first efforts at cultivating Gracilaria spp. were undertaken in Taiwan about 25 years ago. They were initially empirical, but the technique was improved as scientific knowledge increased. Chile began this type of activity around 1983 and now produces 1,200 tonnes of cultivated Gracilaria spp. The government plans to increase production to 4,000 tonnes, having granted 420 concessions for a total of 2,700 hectares. Other countries will probably follow this example since many experiments are now in progress.
4.1 Sowing from cuttings
Cultivation in Taiwan, Vietnam and China
Cultivation is performed by scattering fragments of Gracilaria spp. in marshes communicating with the sea by a system of sluices or in basins dug directly in the intertidal zone.
Type of marshes: Each marsh covers a mean area of one hectare and is connected to the sea as well as to a fresh water source, allowing salinity to be maintained at around the optimal value (25 ppt). Water depth is 50 to 150 cm, and the bottom should be about 10 cm below sea level at low tide. The substrate is theoretically sandy. Gracilaria spp. prefers fine sand (3.9 mm in diameter) to very fine sand (less than 2 mm in diameter) and mud which are more easily resuspended by turbulence. If the marsh area is greater than a hectare, it is necessary to erect a bamboo fence around it to keep out the wind which would otherwise cause currents and waves and displace the alga, accumulating it in piles. A net with 15-cm mesh is placed downstream from the marsh in order to capture alga which would otherwise be carried away. The pH must be kept slightly alkaline (8 to 9) by replenishing the water.
Sowing: The farmer selects a stock of Gracilaria spp. whose good condition is attested by its elasticity, dark colour and ramification. Cuttings (20 to 30 cm) are scattered uniformly at a rate of 4,000 to 5,000 kg per hectare (rarely more), corresponding to a density of 0.4 to 0.5 kg. m-2.
Growth management: Replenishment of the water allows the marsh to be maintained at near-optimal conditions for algal growth. In general, water replenishment 2 to 3 times per day represents 50 to 70% of the total volume. The water layer above the algae must not be less than 30 cm between March and June, nor less than 80 cm from July to October.
Epiphytes and commensal algae: It is not unusual for commensal algae and epiphytes to invade the marshes and cuttings, reducing light and rapidly absorbing nutrient salts. The invaders include Ulva spp., Enteromorpha spp., Chaetomorpha spp., Cladophora spp., Ectocarpus spp., Elachista spp., Polysiphonia spp., Ceramium spp. and diatoms. The fight against these “weeds” is particularly difficult. The farmer must necessarily remove them with a rake or by hand while they are still floating. The second means of reducing epiphytes consists in working with a high density of thalli per m2 (2 to 3 kg). Thirdly, browsing animals can be used, which prefer the tender fronds of the epiphytes to the tougher ones of Gracilaria sp.. Among these animals, the fishes Chanos chanos, Fundulus heteroclitus and Tilapia sp. are most often employed to destroy Chlorophyceae and Cyanobacteria. These fish must of course be removed once the epiphytes are eliminated since they are then likely to attack the young ramifications of Gracilaria spp.
Harvesting: The first harvest can be made 2 to 3 months after sowing, with subsequent ones every 20 to 40 days from June to November depending on algal growth. As the tide allows only 1 or 2 daily replenishments of water, the yield is between 11 and 27 tonnes.hectare-1year-1(dry weight). Total annual production for Taiwan has been around 13,000 tonnes (dry weight) since 1981 (Trono, 1988).
Product conditioning: The harvest is carefully washed in marsh water to remove mud, sand, fish or mollusc larvae and shellfish. Epiphytes are removed by hand. The plants are then spread out on the ground or on a layer of bamboo or on a horizontal trellis until water content is reduced to 18 to 20%.
Cultivation methods in other parts of the world
Figure 3 shows common methods of Gracilaria culture in Chile: cuttings are pushed down into the sand with a pitchfork (top) and a spade is used to partially bury the cuttings (middle). The bottom of Figure 3, shows the system used in deep areas: cuttings are attached to a sausage-shaped plastic bag filled with sifted sand; many fields are created in this way. The advantage of the method concerns the rapidity with which the cuttings can be planted. The disadvantage is that the plastic bags are not biodegradable and thus a source of pollution. The mean yield from such plantings is 20 tons.hectare-1 year-1 (dry weight).
The stretched rope method is used in Chile and the Caribbean: (Figure 4) This method is similar to the “mono-line” approach used in the Philippines for Eucheuma. It is used when the bottom is too muddy or, on the contrary, too stony. The algae benefit from more growing space, and water circulates both above and below the plants. Although better growth is obtained and losses are relatively low with this system, it is rather time-consuming to set up. Yields of 50 to 60 tonnes hectare-1 year-1 can be obtained in winter (the warmest season in Chile).
Other methods of cultivation include raft culture (Figure 5) where the rafts either float (cuttings being placed every 10 cm along ropes hanging in the water) or are anchored so as to be near the surface at low tide and under 5 to 6 m of water at high tide (in which case the ropes with the cuttings ar horizontal).
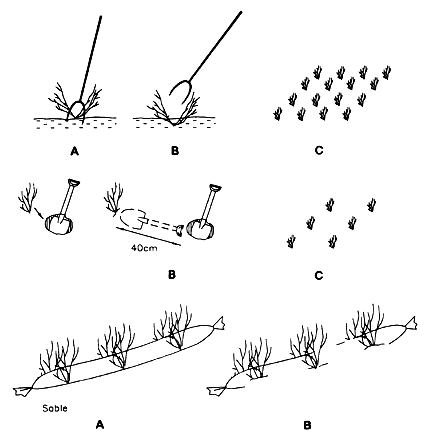
Figure 3: Cultivation procedures in Chile.
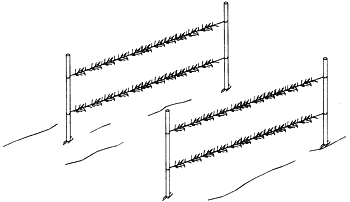
Figure 4: Stretched lime method tested in the Caribbean.
Culture trials in raceways (ponds with water current) have also been undertaken. Cultivation by spraying: Gracilaria thalli are placed on a plastic-coated grid and continually sprayed with enriched seawater at a rate of 50 l.m-2 min-1 . The water recovered under the grid is returned to the sprayer by a pump.
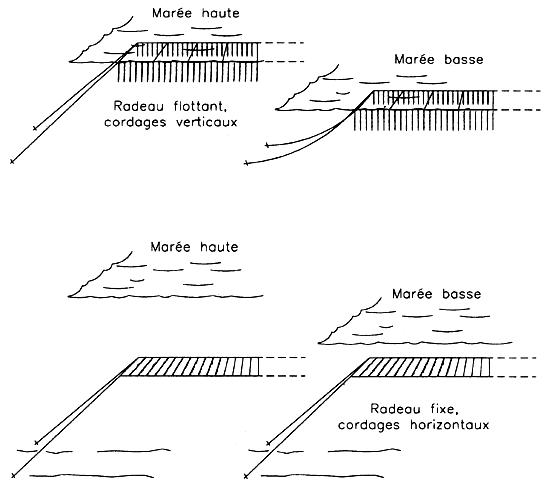
Figure 5: Cultivation on rafts in the Caribbean and the Republic of China.
Different systems for cultivating Gracilaria in the Caribbean are shown in Figure 6. Cuttings are placed between strands on rafts (A) submerged 2 to 3 m below the surface. The crop is cut, and the part remaining under the strand generates a new tuft. Figure 7, shows cultivation management during development of the cuttings, those with low growth (A) are replaced with fragments obtained from plants with high growth (B). The farmer thus “homogenizes” the development of the plants.
According to Lopehandia (1986), 10% of the production in Chile is obtained by the profitable method using sausage-shaped plastic bags of sand. This is why 420 new concessions were requested and granted in 1987. Nearly 2,700 hectares are currently devoted to the cultivation of Gracilaria, which may soon make Chile is the world's leading producer. The crop, once carefully dried, provides food agar of good quality, with a gel strength of 900 g.cm2 after alkaline extraction. In some cases, the production can be used as bacteriologic agar.

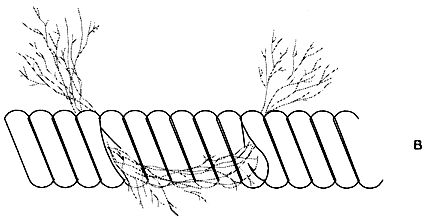
Figure 6: Different systems for cultivating Gracilaria in the Caribbean.
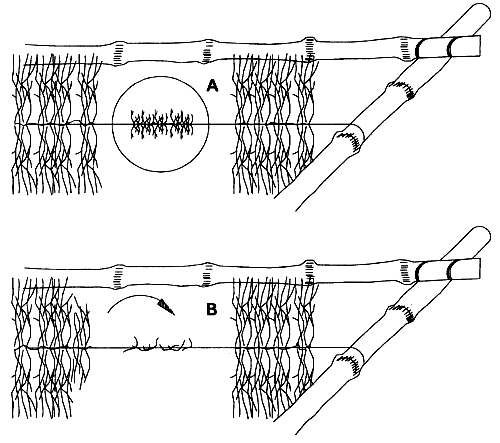
Figure 7: Cultivation management during development of the cuttings.
In all cases, harvesting takes place when the tufts are between 80 and 100 cm in length. It is done on foot in the intertidal zone and by diving in deep waters. The alga is cut with a knife or sickle, leaving in place a piece 15 to 20 cm long which will generate a new plant. If sowing is performed in January, February or March, the first crop can be obtained in May and subsequent ones in September–October, December-January, April–May, etc.
4.2 Sowing from spores or carpospores
The use of reproductive elements is more difficult than that of fragments, in as much as the factors causing sudden, massive emission must be controlled. Besides, the interval between germination of the reproductive element and the first crop is much longer than that between attachment of the cutting and the first harvest. As Gracilaria is a small alga, the creation of populations by means of cuttings requires considerable work and manpower, implying higher costs. The use of spores would allow rapid sowing on a large number of supports, rocks, nets or ropes. It would also be possible to produce hybridizations to improve either growth capacities or gel quality.
At Santa Lucia in the Caribbean, ropes were seeded using spores placed within a dense population of Gracilaria.. However, an element of chance enters into such operations since the ropes must be placed in the water precisely at the peak of the emission (which is difficult to predict) in order to prevent too many commensal algae and animals from colonising them, which in fact is what generally happens.
Good seeding by means of spores or carpospores can only be obtained in the laboratory where a massive release can be produced at the desired time by acting on certain factors. The most efficient process would appear to be that used in Thailand.
5. USES OF GRACILARIA
Gracilaria spp. is used for agriculture, human food, animal feed and agar extraction. In agriculture, it provides a means of enrichment either in the form of fermented algae mixed with seeds sown in the spring or of liquid or powder spread over roots or leaves. People in the Philippines, Indonesia, Korea and China consume Gracilaria spp. either fresh (known as “gonori”), in salads, dried or in a jelly made from powdered fronds.
5.1 Agar production
When extracted according to the process applied to Gelidiales, agar from Gracilaria spp. has no market value since its gel strength (upon gelation) is very low. Thus, it is preferable first to apply an alkaline treatment with soda for 50 to 70 min at 95°C, in the proportion of 5 tonnes of algae (dry weight) to 100,000 litres of 3 or 5% soda. This operation eliminates sulphuric esters and converts a-L-galactopyranose units into 3,6-anhydro-a-L-galactopyranose, thereby increasing gel strength (Wang and Yang, 1980) but not to the level of that of the polymer extracted from Gelidiales. These are generally food and not pharmaceutical agars.
Content: Agar content differs appreciably depending on the variety of Gracilaria spp. considered and, for the same variety, according to the collection site, season, physiological state, development stage, conditions of cultivation, degree of cleaning, extraction mode, etc.
Quality; Quality is directly related to the composition of the molecule which, even more than the content, is subject to variations.
6. THE MARKET FOR GRACILARIA
The annual average global production of Gracilaria amounts to about 370,000 tonnes (wet weight). At present, cultivation accounts for a little more than a third of this output (130,000 tonnes). Taiwan remains the leading cultivator, followed by Chile. Contrary to what happens for carrageenans and alginates, agar is extracted by many small units located near the producing areas.
Seaweed production is not exclusively used for agar extraction. Its used for human food in the Far East (notably Southeast Asia) during Islamic holidays, and for feeding farmed fish and molluscs (mainly abalone). These uses absorb a good part of the total production, to the extent that fluctuations in the agarophyte market have only a limited impact on prices (except in South America where Gracilaria is used only for its agar content). The prices charged depend more on local negotiations than on international rules, except when it is a question of exportation, in which case the price of a tonne of dried, clean algae ranges between $500 and $700. The production cost per year and per hectare has been determined (Table 1).
The operation, whether gathering, extensive farming or intensive farming, takes the form of a family business involving a hectare (rarely two), with the use of additional labour only during sowing and harvesting. It generally depends on mixed farming since Gracilaria, shrimp, crabs and fish are developed harmoniously in the same marshes, thereby increasing income appreciably.
Table 1: Production cost and income for a hectare of Gracilaria cultivated at Taiwan in 1989.
| Costs/Income | Cost (US $) | |
| Variable costs | ||
| Labour | $1,800 | |
| Investment | $1,450 | |
| (cuttings, fertilisers, maintenance) | ||
| Taxes and losses | $ 350 | |
| TOTAL COSTS | $3,600 | |
| SALE PRICE | $7,350 | |
| PROFIT | $3,750 | |
The selection of efficient individuals or the creation of laboratory strains through genetic engineering might change this situation. In fact, the uncertain nomenclature for the genus does not facilitate studies but probably reflects the existence of various genomes whose combinations could be enriching. There is also a possibility for the future use of the protoplasts that Christiaen and Stadler (personal communication, 1986) have been able to obtain. The cultivation of Gracilaria is in fact in abeyance. If research currently in progress leads to cost-efficient cultivation of species of the Gelidium genus, which provides high quality agar, that of Gracilaria could be limited to the quantity intended for human and animal food. On the other hand, Gracilaria might well remain the agarophyte of recourse.
The degradation of the economic situation in Eastern European countries, which are high consumers of food agars but currently not very creditworthy, led to a decrease in 1991 in the world demand for agar and thus for agarophytes of the Gracilaria type. At the same time, production prices dropped. Even Chile, despite the production of good quality Gracilaria, is in difficulty. It is not easy to know how this situation will evolve. Moreover, “gelanes” (competing colloids of bacterial origin) have made their appearance, showing comparable properties to those of Floridean agar.
7. AGARS
Algae used for agar production belong to three orders:
- Gelidiales, including the genera Gelidium, Gelidiella and Pterocladia.
- Gigartinales, with the genus Gracilaria.
- Ceramiales, with the genus Ceramium.
Gelidium is harvested mainly in France, Spain, Portugal, Morocco, South Africa, Japan, Korea, China, the United States and Chile, involving notably G. corneum, G. cartilagineum, G. pacificum, G. pristoïdes, G. latifolium, G. sesquipedale and G. amansii. Mexico now has an annual production of 1,200 tonnes of G. robustum. Gelidiella comes mainly from Egypt, India and Madagascar. Pterocladia is abundant in the Azores (P. capillacea) and New Zealand (P. lucidd).
The genus Gracilaria is found world-wide, and its many species yield agars of quite variable quality. Noteworthy ones are Gracilaria sp., G. crassa, G. pichenoïdes, G. canaliculata, G. lemaeiformis, G. foliifera, G. corticata and G. multipartita. These species are harvested in Chile, Argentina, Peru, Brazil, the Caribbean, Japan, China, Taiwan, Indonesia, Vietnam, India, Sri Lanka and South Africa. Other algae, such as Anhfeltia plicata (Japan, Sakhalin), Acanthopeltis japonica and Ceramium sp., are blended with these species. The difference in quality among the agars is such that it is often necessary to indicate not only the genus and species but also the country of origin and the site at which the alga was harvested.
Table 2: World agar production in 1990.
| COUNTRY | Tonnes | % |
| Japan | 2,260 | 31.3 |
| Spain and Portugal | 1,910 | 26.4 |
| Korea | 1,440 | 19.9 |
| Chile | 900 | 12.5 |
| Morocco | 250 | 3.5 |
| USA and Mexico | 150 | 2.1 |
| Canada | 150 | 2.1 |
| France | 80 | 1.1 |
| India | 10 | 0.1 |
| Argentina | 10 | 0.1 |
| Others | 70 | - |
| TOTAL | 7,230 | - |
The total harvest of agarophytes amounted to 540,000 tonnes (wet weight), comprising 120,000 tonnes for Gelidium, 50,000 tonnes for Pterocladia and Gelidiella and 370,000 tonnes for Gracilaria. Agar contents relative to dry weight differed greatly (19 to 29%) from one alga to another and for a given place and time. The two great areas of cultivation are Taiwan and Chile (Figure 8).
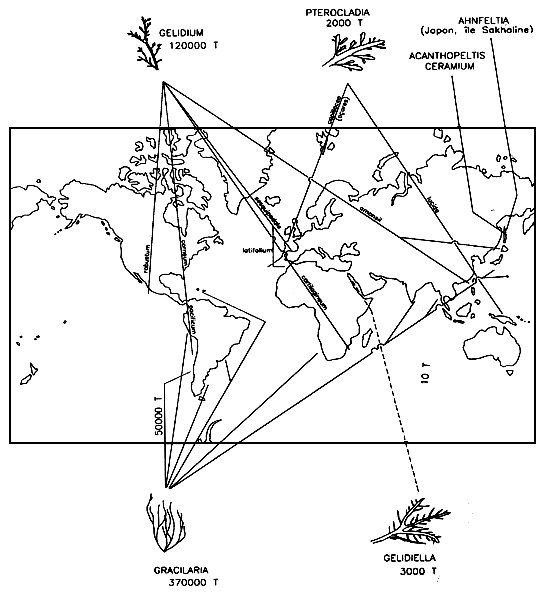
Figure 8: World agarophyte sources.
7.1 Extraction
The general process is based on the fact that agar is insoluble in cold water and soluble in warm water. The principle consists in obtaining a solution containing about 1% of agar at the end of extraction. Beyond this value, the separation between agar and water becomes practically impossible if a suitable product is to be obtained.
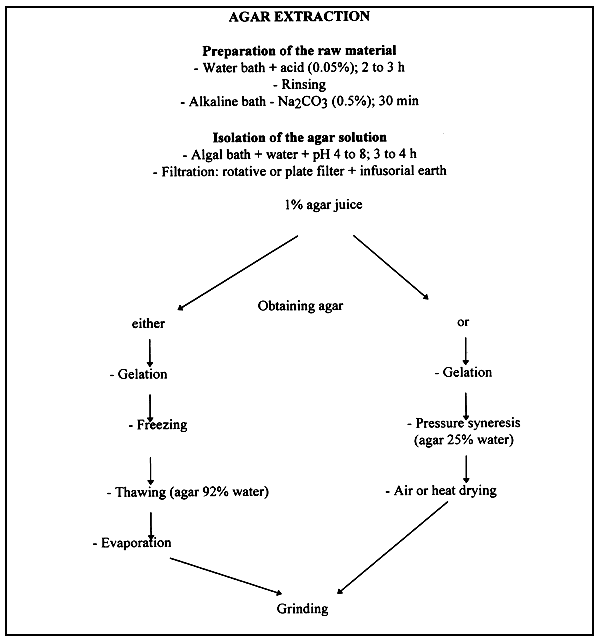
Except for these two points, it is impossible to describe an extraction method that is valid for all agarophytes. It is essential each time to adapt the technique to the physicochemical characteristics of the alga to be processed.
Freezing: Freezing is based on the fact that agar below 0°C becomes insoluble in water. The process consists in freezing the filtrate F containing 1% agar at a temperature between -2 and -10°C. Until I960, the prevailing method consisted in placing the filtrate F with 1% agar in 45 × 30 × 5 cm wooden boxes until gelation occurred. The gel was then cut into thin slices. The slices were treated differently according to season. In winter they were exposed to bad weather so that they could undergo a series of freezings and thawings. In summer they were soaked in a saline solution at -5°C. In both cases, the effect was the same. The water separated partly from the agar, taking pigments and other impurities with it.
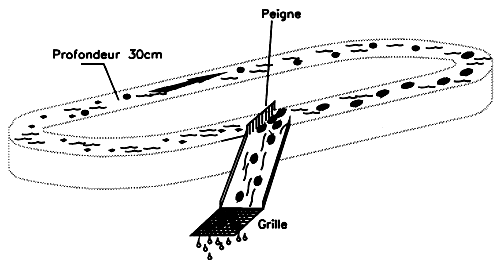
Figure 9: Cooling circuit allowing isolation of the agar.
Since 1967, the agar solution is cooled progressively (Figure 9), and the agar, having become insoluble, floats on the surface. A comb channels the flocculent masses toward a grid where they are drained. This type of rather costly separation is only used for high quality agars (Gelidium or Pterocladia agar).
Syneresis: This method is applied more particularly to the Gracilaria genus. Syneresis is the gel property which tends to compress and exude the water which it contains. This evacuation of water can be accelerated by applying pressure, in which case energy consumption is relatively low since dehydration requires only 8,360 to 9,196 kJ per kg of agar. However, implementation of the industrial technology is not easy.
7.2 Special case of Gracilaria and Porphyra in East Asia
Species of the Gracilaria and Porphyra genera were previously considered to provide soft gels or nongelling viscous solutions. Now they can be made to produce an acceptable gel by subjecting the alga to a highly alkaline heat treatment using an O.025 to 0.5 N soda solution, depending on the species and place of origin. This operation, known as “alkaline hydrolysis”, converts the a-L-galactopyranose-6-sulfate of the second monomer of neoagarobiose into 3,6-anhydro-a-L-galactopyranose.
7.3 Characteristics of the extraction factory
The operation of an agar-producing factory should take four basic elements into account: availability of water, the need for a control laboratory, maintenance of extreme cleanliness and the existence of well-designed storage areas.
7.4 Agar properties
Although agar possesses a certain number of characteristics which make it a quite special colloid, the basic property is its spontaneous ability to provide very resistant gels at a low concentration.
Gelation
When agar is mixed with water, the resulting solution has a gelling power. It is only necessary to heat the solution to 85–90°C and let it cool again. Other components need not be added, contrary to the situation for alginic acid (bivalent and trivalent cations and acids are required to produce gels), carrageenans (gelation occurs only in the presence of proteins or cations such as K+ or Ca++) and pectins (sugars or acids must be added to produce gels).
Measurement of gel strength
Measurement of gel strength constitutes the basic control element in the agar business. Industrialists and their customers generally use the Nikan Sui process which consists in determining the weight load capable of breaking a standard 1.5% gel in 20 seconds.
Gel strengths range from 150 to 1,200 g.cm-2. Top quality values are between 600 and 900 g.cm-2 medium quality between 400 and 600 g.cm-2 and poor quality less than 350 g.cm-2. Only Gelidia, because of their high agarose level, produce gels reaching and sometimes exceeding (Gelidium latifolium) a break force of 1,000 g.cm-2 , which corresponds to a gel strength 10 times as great as that of other colloids with the same concentration measured in the same conditions.
Gel properties
Agar produces a translucent gel which is tasteless and odourless and can therefore be used for the gelation of food products without altering colour or flavour. It enhances the taste and acts as a long-term aromatic fixative. The refractive index can be increased by the adjunction of sugar (glucose) or glycerine, giving an attractive appearance. Agar gel has excellent reversibility.
7.5 The special case of bacteriological agar
One of the greatest uses of agar is in the bacteriologic field. In fact, bacteriology is indebted to agar for the progress it has made since 1881 when Robert Koch used this product for the first time to solidify culture media. Bacteriologic agar is prepared mainly from Gelidium and sometimes from Pterocladia. For these genera, a 1% solution reaches gel state between 34 and 38°C, temperatures which allow bacterial culture. However, the extract obtained from Gracilaria and Gelidiella solidifies at 41 to 43 °C, temperatures too high for bacterial life.
Agarose
Agarose is the chemical component of agar which is the poorest in sulphuric esters and the richest in 3,6-anhydro-a-L-galactopyranose. It is very difficult to obtain agarose in pure state, i.e., free both of residues from the extraction process and of the chemical products used to isolate it from agar. Various techniques (roughly 15) have been developed to reduce purification costs or more particularly to ensure the elimination of impurities incompatible with certain applications.
Agarose is defined by
8. AGAR APPLICATIONS
Twenty years ago, agar was used mainly as a thickener of textile colorants. For this purpose, it has been totally replaced by alginates.
8.1 Use in the food industry
The very wide range of agar applications in the food industry is attributable to its particular gelation characteristics not found in any other vegetable or animal colloid. Accordingly, its price on the world market is higher than that of other colloids. As agar was the first phycocolloid used, some 300 years ago, it can be safely assumed that it has no toxicity for man. The countries with the most demanding regulations have approved its use. Under the code E 406, it is used mainly for gelation but also as a stabiliser for controlling viscosity. The customary doses are less than 1%. Studies have shown that human agar digestion is imperfect since less than 10% is assimilated. Given its limited calorific contribution, reduced assimilation and low percentage in foods, agar cannot be said to modify the calorific value of the foods to which it is added. Its numerous applications in the food industry include the following:
8.2 Uses of agar in agriculture
Seeds are preserved from bacteria and moulds by an agar gel of the same type as that used for the protection of food preparations. The fight against insects requires the raising of insect larvae, a great number of which are then sterilised before being released. Sterile males play the role of fertile males with regard to females, so that there is no reproduction. The harmful action of the fly Pectinopora glosipeis, which attacks cotton plantations, has been limited in this way. Agar enters into the preparation of food for the larvae of these insects.
In orchid nurseries, agar is the substrate which receives the culture medium for meristems or cell tissues for clone formation. The agar must be absolutely free of growth inhibitor. This application has increased considerably since meristem cell cultures have become the classic technique in agriculture for producing and reproducing certain varieties.
8.3 Use of agar for pharmaceutical purposes
Agar was first used as a bulk laxative (Molagar) and is now used for many other purposes. It is an expedient in pharmaceutical preparations and enters into the composition of gastric plasters (phosphalugel, gelogastrin, anacidase). Agar is used to thicken and stabilise many cholesterol solutions. It is also used as an emulsifier in creams, suppositories and surgical liquids and as a dispersive agent in tablets. It is mixed with potentially inflammable alcohol concentrations to avoid this risk. Added to dietetic substances, it serves as an appetite suppressant by expanding within the stomach.
8.4 Use of agar for moulding and casting
Agar gel allows precision moulding to be achieved in sculpture and archaeology as well as in dentistry where it is used most. One advantage of agar is that a series of equally fine secondary moulds can be made based on the first one.
8.5 Use of agar in bacteriology
Applications in bacteriology constitute one of the most remarkable uses of agar. A great number of culture media use agar as basic substrate. Agar has contributed to the development of most vaccines. To prevent syneresis, an 0.75%–5% agar-gelatine mixture is prepared. As agar is not altered by bacteria and does not affect them, their biology can be studied as a function of the substances introduced into the gel. A typical medium is composed of:
| - | agar | 15 to 30 g |
| - | sodium chloride | 5g |
| - | water | 1,000 g |
| - | pectin | 10g |
| - | protein | 50 g |
The medium is different for each bacterial species, but the main ones are the following:
glycerine-agar
blood-agar (from the horse or sheep)
serum-agar
egg-agar
8.6 Use of agar in biochemistry and biotechnology
Agarose is generally used for protein separation, mainly in analytic laboratories. Industrial applications have also appeared because of advances in genetic engineering productive of substances such as interferons, interleukins and insulin which are often separated and purified on an agarose support.
Electrophoresis
Electroendosmotic gels constitute the most suitable medium for the separation of polyelectrolytes
according to their charge or mass.
There are numerous applications for this property, including analysis of biological fluids, separation of DNA combinations and development of the genetic map, recovery of isolated substances, preservation of electrophoretic plates, isoelectric focusing and two-dimensional gel electrophoresis.
Immunology
There are many applications of agarose in immunology for the detection and study of antigenic material, particularly that responsible for diseases.
Culturing of micro-organisms
Agar is a suitable substrate for culturing micro-organisms and cells. However, even those agars considered typically bacteriologic can contain infinitesimal proportions of unknown substances capable of disturbing the development of micro-organisms and animal or vegetable cells. For this reason, scientists prefer to use agarose which is known to have a high degree of purity and consistency.
Gel-filtration and affinity chromatography
Immobilisation of biological systems. Agarose in capsule or bead form is used to enclose active coal and ion-exchange resins during blood infusions to detoxify patients after an overdose.
Other uses. Agar is also used for many special purposes, including lubrication of certain parts involved in the processing of tungsten and tantalum; graphite preparation; protection of aluminium upon contact with caustic environments; stabilisation of nitroglycerin; manufacture of ultra-sensitive photographic films and of paint, batteries and storage batteries; and preparation of primers or glues for wallpapering.
9. WORLD MARKET
The quality standards used are generally based on those established by the Japanese merchants who have dominated the agar market for about SO years. The four qualities differentiated by them are summarised in Table 3.
Table 3: Characteristics of the different commercial agars.
| QUALITY | SPECIAL | 1 | 2 | 3 |
| Water content (%) | 22 | 22 | 22 | 22 |
| Gel resistance (g.cm2) | + than 600 | + than 350 | + than 250 | 150 |
| Protein content (%) | 1.5 | 1.5 | 2 | 3 |
| Warm insoluble matter (%) | 0.5 | 2 | 3 | 4 |
| Maximum ash (%) at 550°C for 4 h | 3 | 4 | 5 | 5.5 |
The sale price ranges from $36 per kg (special) to $10 per kg (quality 3). Food agar represents the greatest part used in the world (88 to 93%), mainly in Asia. Contrary to that of carrageenans, agar production is performed in relatively small factories, of which there are 220 in the world (170 in Japan). The two largest factories, in Spain and Chile, have an annual capacity of 400 tonnes.
The market for bacteriologic agar represents only 4 to 5% of the 7,530 tonnes produced world-wide, and the kg sells for $36 to $100. The price of agarose ranges from $190 to $1,170 per kg and stands for 2% of the bacteriologic agar. Table 4 shows the quantities of agar consumed in each country in 1990:
Table 4: Quantities of agar consumed in the world in 1990.
| COUNTRY | Consumption in tonnes |
| Japan | 3,300 |
| United States | 830 |
| United Kingdom | 700 |
| Germany | 600 |
| Korea | 215 |
| Denmark and Spain | 250 |
| France | 100 |
| Italy | 150 |
| Argentina | 125 |
| Brazil | 100 |
| Chile | 90 |
| Africa | 100 |
| South Sea Islands | 170 |
| Eastern Europe | 400 |
| Others | 400 |
| TOTAL | 7,530 |
References
Bird, C. J., Helleur, R. J., Hayes, E. R. and Mac Lacklan, J. 1987. Analytical pyrolysis as a taxonomic tool in Gracilaria (Rhodophyta: Gigartinales). Hydrobiologia, 151/152, 207–211.
Bodard, M., 1966. Les Gracilaria et Graciliopsis du Senegal. Ann. Fac. Sc. Univ. Dakar. 19, 27–55.
Cable, W. D. 1974. A description of the activities of the Maullin (Chile) Fishing Co-operative in the extraction of the marine alga Gracilaria sp. Bot. Mar., 17, 60–62.
Causey, N. B., Prytherch, J. P., Mac Caskill, J., Humms, H. J. and Wolf, F. A. 1946. Influence of environmental factors upon the growth of Gracilaria confervoides. Bull. Duke. Univ. Mar. Sta, 3, 19–24.
Chang, C.F. and Xia,B.M., 1976. Studies on Chinese species of Gracilaria Studio. Mar. Sinica 11, 91–163.
Chapman, A. R. O., Edelstein, T. and Power, P. J. 1977. Studies on Gracilaria I. Morphological and anatonomical variation in samples from the lower Gulf of St Lawrence and New England. Bot. Mar. 20, 149–153.
Christiaen, D. 1986. Structure et fonction des polyosides matricielles de la paroi de Gracilaria verrucosa (Huds.) Papenfuss. These Doc,, es Sc. Nat, Univ. Lille 673,173 p.
Destombe, C, Godin, J. and Bodard, M., 1987. Decay in the life history of Gracilaria verrucosa: the consequences in cultivation. In: Advances in biotechnology, Lille, 22, 17–25.
Doty, M. S. 1977. Seaweed resources and their culture in the South China Sea Region. FAO Publication SCS/7/WP60 (Workshop in Manila), 22p.
Edelstein, T. 1977. Studies on Gracilaria sp. Experiments on inocula incubated under greenhouse conditions. J. Exp. Mar. Biol. Ecol, 30, 249–259.
Friedlander, M. and Lipkin, Y. 1982. Rearing of Agarophytes and Carrageenophytes under field conditions in the Eastern Mediterranean. Bot. Mar., 25,101–105.
Jones, W. E., 1959. Experiments on some effects of certain environmental factors on Gracilaria verrucosa (Hudson) Papenfuss. J. Mar. Biol. Ass. U.K., 38, 153–157.
Kim, D. H., 1970. Economically important seaweeds in Chile. 1. Gracilaria Bot. Mar., 13, 140–162.
Kling, R, 1978. Researches sur les conditions optimales de croissance de Gracilaria verrucosa (Hudson) Papenfuss (Gigartinales, Gracilariacees). These Doc. Univ. Lille 1,157p.
Kraft, G. T., 1977. Transfer of New Zealand red alga Tylotus proliferus (Gracilariacia, Gigartinales), to the genus Gracilaria New Zealand Journal Bot, 15, 492–502.
Lefebvre, C. 1986. Comportement, en cultures experimentales des deux generations diploides du Gracilaria verrucosa (Hudson) Papenfuss: le carposporophyte a carpospores et le tetrasporophyte immature. Thesis Doc., Univ. Lille, 132p.
Lopehandia, J. 1986. Problemas y perspectiasen la utilisation de la algas chilenas. Monographia biologicas. In: Santelices, B. Usos y funciones ecologicas de la algas marinas bentonicas. 4, 29–43.
Mac Lachlan, J. and Edelstein, T. 1977. Life-history and culture of Gracilaria foliifera (Rhodophyta) from South Devon. J. Mar. Biol. Ass. UK, 57, 577–586.
Mayer, A. M.S. and Krotz, L. 1986. Biological activity in Macrocystis from Argentina. Anti-tumor cytotoxicity humoral immune response, genotoxicity, antiviral activity. Hydrobiologia, 151/152, 483–496.
Santelices, B. 1989. Algas marinas de Chile: Distribucion, ecologia, utilization, diversidad. Publ. Fac. Cien. Biol. Pont. Univ. Catol. de Chile, inscription 70 554 ISBN., 156–231.
Santelices, B., Vasquez, J., Ohme, U. and Fonck, E. 1984. Managing wild crpos of Gracilaria in central Chile. Hydrobiologia, 116/117, 77–81.
Santelices, B. and Fonck, E. 1979. Ecologia y cultivo de Gracilaria lemanaeformis. Acta. 1st Symp. Algas Mar. Chilenas, 1, 165–200.
Shang, Y. C. 1976. Economic aspects of Gracilaria culture in Taiwan. Aquaculture, 8, 1–7.
Tagawa, S., Kojima, Y. and Kono, M. 1963. Gracilaria verrucosa from Japan, J. Shimonoseki Univ. Fish, 13, 1, 12–27.
Umaheswara, Rao, H., 1972. On Gracilariaceae of the seas around India. J. Mar. Biol. Ass. India, 14, 671–696.
Wang, C. Y. and Yang, S. S. 1980. Seasonal variation in the quality of Gracilaria cultivated in Taiwan. Proc. Nat. Sc. Council, 4, 78–86.
Yamamoto, H. 1987. Systematic and anatomical study of the genus Gracilaria in Japan. Mem. Fac. Fish. Hokkaido Univ. 25, 97–152.