Gavino C. Trono Jr.,
Marine Science Institute, College of Science, University of the Philippines,
Quezon City, Philippines.
ABSTRACT
The current status of Gracilaria culture in the region, different culture methods, production of planting materials and the future development of Gracilaria culture are discussed in this paper.
1. INTRODUCTION
At present, a large proportion of the agarophytes used in the manufacture of agar still come from natural stocks. There are no accurate data available on the contributions of agarophytes produced through cultivation but, based on the genera imported by Japan in 1984 from different countries (Armisen and Galatas, 1987), about one half of the seaweed processed into agar is harvested from natural stocks. The main genera of seaweeds utilised in the manufacture of agar are: Gracilaria; Gelidium; Pterocladia; Gelidiella; Ahnfeltia; and Ceramium. In 1984, approximately 6,683 tonnes of agar were produced by 14 countries (Armisen and Galatas, 1987). About 48% of this amount was processed from Gelidium and Pterocladia and the other 52% from “other seaweed” which undoubtedly would include other genera such as Gracilaria and Gelidiella More recent estimates place world agar production at 7–10,000 tonnes (Globefish, 1988).
As far as is known today only the genus Gracilaria is presently produced in commercial quantities though cultivation. In the early 1980's, Chile, Brazil, Taiwan and the Philippines were the main suppliers of cultured Gracilaria to Japan. Recently, Vietnam, Indonesia , Thailand, Hawaii and others have applied culture techniques in Gracilaria production. Even now a large proportion of Gracilaria production still comes from natural stocks. Gelidium and Pterocladia are used mainly for the production of bacteriological agar and agarose and are still derived from the harvest of natural stocks. Their commercial production through farming is still a long way from realisation.
2. GRACILARIA CULTURE
2.1 Species of Gracilaria presently cultured
Seven species of Gracilaria have been reported to be cultured in the region. These are: Gracilaria changii (Xia et Abbott) Abbott, Zhang and Xia; G. flrma Chang et Xia; G. fisheri (Xia et Abbott) Zhang et Xia; G. heteroclada Zhang et Xia; G. gigas Harvey, G. lemanaeformis (Bony) Weber van Bosse; G. lichenoides (Linne) Harvey and G. tenuistipitata Chang et Xia.
2.2 Selection of culture areas
Site selection for farms is an important consideration which partly determines the success or failure of the farming venture. In general, sites which support natural stocks of the species to be cultured are considered good sites. However, if culture is to be carried out in areas where there are no natural stocks of the species, the area should have comparable ecological conditions to the sites where the natural stocks are found. Basic ecological parameters which should be evaluated are: salinity; water temperature; turbidity; water movement; depth at low tides; and nutrients.
Natural stocks of many species found in commercial quantities grow quite well in brackish and highly fertilised waters like protected bays and lagoons. Some species with potential for culture are also found on reefs, sandy flats to sandy-rocky wave exposed habitats. The culture technology to be applied should be adapted to the ecological conditions of the sites.
2.3 Selection of highly productive seedstocks
The utilisation of highly productive species/ strains with high quality agar is always a plus factor in the successful farming of Gracilaria. In species where it is easy to manipulate the individuals to improve hybridisation, using the gametes may be a normal and logical way of improving the seedstocks. However, in Gracilaria and other species where the technique in the manipulation of the sexual process to produce hybrids is not well known, other methods may be used in selecting the species.
One of these methods-is through species/strain selection. Different strains/species present in a natural populations are selected, e.g. different species, various coloured strains, morphologically different thalli. These different strains/species are then multiplied through cloning. Comparative eco-physiological studies are then conducted using these stocks. Physiological studies to determine the tolerance range of the various species/strains to the changes in ecological factors may be done employing manometric techniques using a respirometer. The photosynthetic/respiratory responses can be monitored under varying conditions of stress. Analyses of yields and agar qualities of the various species/strains are also an indispensable tool in determining the qualities of the various strains or species as a basis for the selection of seedstocks.
In situ studies on seasonality and production capacities of the various strains/species, relative to changes in ecological factors (such as salinity, temperature, nutrients and water movement), are important in determining the field conditions which influence the productivity of the various strains as well as the seasonal aspects of the reproductive states and regenerative capacities of the various strains/species.
2.4 Production of seedstocks
The term “seeds” refers both the vegetative propagules (cuttings) and spores which can be utilised as planting materials. The availability of local stocks as seed materials is important. Gracilaria exhibits triphasic alternation of generation consisting of the gametophyte, sporophyte and carposporophyte. The large somatic stages, the gametophyte and sporophyte are sources of cuttings and spores.
Vegetative propagules (cuttings)
Healthy thalli which are fleshy and elastic in texture, dark-reddish brown in colour, robust, well-branched with smooth and shiny surfaces should be selected as planting materials. These must be clean, free of dirt and epiphytes. Preparation of the “seeds” varies depending on the type of culture used, which are described later in this paper.
Spore and seeding materials
Two methods are presently used in the production of sporelings from spores: natural spore-recruitment and induced spore-shedding in hatcheries.
Natural spore recruitment
In the spore recruitment method, artificial substrates like wooden stakes, rocks and netting materials may be used. However, braided ropes and netting materials are generally preferred because of the ease in handling during the process of recruitment as well as during cultivation. The ropes are anchored or tied to wooden stakes below the low tide level among the dense populations of Gracilaria. These are left in the area for about two weeks to allow the naturally shed spores to settle on them. The sporelings developing from the spores become visible after 3 to 4 weeks. The seeded ropes with sporelings are then transferred to the culture sites for outgrowing.
Hatchery production of sporelings from spores
The production of sporelings from spores requires some skill to recognise fertile materials. The fertile female materials are quite easy to recognise because of the presence of cystocarps. These fertile structures appear as elevated mammillate “bumps” on the surface of the thalli with dark coloured contents. Cystocarps which have already shed spores (“empty”) are pale in colour. In contrast, the fertile sporophytic thalli is quite hard to recognise because the tetrasporangia are microscopic and do not form apparent recognisable structures. The tetrasporangia appear as dark purplish microscopic spots on the surface of the thalli when examined under a stereomicroscope. Their presence, however has to be counter checked by the examination of sections of the suspected thalli under a compound microscope. The tetrasporangia are of the cruciate type and are embedded in the cortex of the thallus.
Hatchery production of sporelings requires a land-based set-up consisting of a seeding tank with provision for control of water depth and an adjustable seeding material support structure. These structures consist of a wooden frame with monofilament netting material which can fit into the seeding tank and a mechanism to adjust the height from the bottom of the tank. Various types of substrate can be seeded, e.g., pieces of gravel, shells or lines. These materials are placed at the bottom of the seeding tank. The use of lines as setting substrate requires additional structures, i.e., a frame which also fits the tank when laid flat on the bottom. The line may be nylon braided ropes (ca. 3mm dia) or plastic tying raffia materials. The “raffia line” or rope is wound evenly to the seeding frame. The frame with “raffia line” is placed at the bottom of the tank.
Fertile materials of Gracilaria are collected from the natural stocks and brought to the seeding facility. The moist materials are placed in containers such as styrofoam boxes provided with aeration holes to avoid stress. The fertile Gracilaria are then placed on the seeding support structure and submerged in water in the seeding tank. The distance of the seeding materials from the seeding substrates (rocks, stones, or raffia in frames) should be adjusted to ensure the uniform distribution and density of the spores. The seeding materials are left in the tanks for 2 to 3 days to allow the shedding of spores.
The seeded materials may be left: in the tank for another 1 to 3 days to allow the spores to germinate and completely attach to the raffia or substrates. The seeded rope is transferred to a holding tank or to a nursery area while waiting for the spores to develop into sporelings.
3. CULTURE METHODS PRESENTLY USED
3.1 Pond culture
The determination of pond culture system for Gracilaria consists of the following stages:
Site selection
The success in pond culture of Gracilaria is highly dependent on the selection of appropriate sites. The following criteria are used in the selection of sites for pond culture:
the site should be located near seawater and freshwater sources;
the site should be protected from strong winds;
the pond bottom should be at or near the zero tide level; and
the pH of seawater should be slightly alkaline, e.g., 8.2 to 8.7.
Many species of Gracilaria are euryhaline and can grow in brackish water under a wide range of salinities. A salinity range of 15 to 24 ppt has been found to be optimal. Salinity increases during the sunny months due to evaporation, reaching values as high as 35 ppt or drops to as low as 8 ppt during the rainy season. These variations have been shown to be detrimental to the crop. The maintenance of optimal salinity in ponds requires available sources of both freshwater and seawater. The ponds should be located in areas protected from strong winds to prevent the Gracilaria accumulating on the leeward side of the pond. The formation of thick heaps of Gracilaria on one side of the pond has adverse effects on growth due to shading. Water movement is greatly influenced by tidal changes in relation to the elevation of the pond bottom. Ponds located in areas where the bottom is at, or a little above, the zero tide level can easily be managed as water exchange is easy.
Culture ponds
The average size of ponds for the culture of Gracilaria is about one hectare or smaller. Smaller ponds are easier to manage than larger ones. Pond management is also easier when Gracilaria is polycultured with shrimp and or crab. Provisions of entrance and exit gates facilitate proper water management.
The depth of ponds vary from 50 to 80 cm. The bottom generally, is of clayish loam, silly loams or sandy loam. It was observed that Gracilaria easily gets buried in ponds with soft sandy or muddy bottom. This problem can be prevented by increasing the depth of the water during windy periods. In large ponds, wind breakers consisting of bamboo slots are installed perpendicular to the direction of the wind to prevent the seaweed being transported to one side of the pond.
Preparation and stocking of ponds
The ponds are dried for several days after which water is men introduced. Healthy stocks (characterised by their elastic feel to touch, reddish brown colour, brittle texture and well branched thalli) are selected as planting materials. The seedstocks are transported from the source to the pond site early in the morning to prevent exposure to the sun. During long-distance transport, it is frequently sprinkled with seawater and perforated bamboo or plastic pipes are inserted into the bottom of the heap to provide aeration. The plants must immediately be placed in the pond upon arrival. The planting materials are cleansed, cut into pieces and broadcast uniformly on the bottom of the pond. The stocking rate is 5,000 to 6,000 kg of chopped Gracilaria per hectare.
Pond management
The water is maintained at a depth approximately 30 to 40 cm above the top of the algae. However, the depth is increased to 60 to 80 cm during the warm summer months to prevent a significant rise in water temperature. Water depth is also increased during the cold winter months to avoid temperatures dropping to below 8 oC, which is lethal to Gracilaria.
Frequent exchange of water is necessary to maintain optimum water temperatures in the ponds. About 50 to 75% of the pond water is drained and replaced with fresh seawater every two to three days.
Fertilisation with organic or inorganic fertiliser is necessary to enhance the growth of Gracilaria. Weekly application of three kilograms of urea per hectare was found to be sufficient Fermented pig manure may be applied at a rate of 160 to 180 kg per hectare two to three days after the exchange of the water. The manure is placed in sacks and the sacks are placed at strategic areas in the pond.
Harvest and post-harvest activities
Under optimum conditions, the crop may be harvested 2 to 3 month after seeding. Cropping may be done every 10 to 40 days manually by using scoop nets. The frequency of harvest is primarily dictated by the amount of biomass, the market price and the season. Approximately 30 to 40% of the biomass is harvested during each cropping. The crop is thoroughly washed in pond water to remove silt, sand, pieces of shell and other extraneous materials, such as snails and other algae. The clean Gracilaria is spread uniformly on bamboo screens or plastic sheets for drying. An average wet to dry ratio of 7:1 is generally attained.
3.2. Field culture
There are several methods used in the field culture of Gracilaria. The “seeds” used in these various methods are cuttings or sporelings.
Fixed off-bottom long line method
In this method, three-strand polyethylene ropes 3 to 4 mm in thickness and 5 meters long (or longer) are generally used. Other type of materials, such as coir or abaca ropes, may be used as a substitute. The seeding of long lines using cuttings consists of untwisting the rope and inserting bunches of cuttings between the strands of the rope, the seedlings passing two or three times through the strands. This method ensures that the cuttings are securely tucked in place. The seeding of the ropes is done in the shade and the seedlings are placed in basin of sea water to prevent the cuttings from dehydrating.
The support system for the long line consists of wooden stakes driven into the ground. These are usually arranged in rows, the distance between stakes in the row is about one meter while the distance between rows of stakes varies between 4 to 5 meters. One end of the seeded rope is tied to a stake, stretched tightly and the other end is tied to the opposite stake in the next row.
The same technique is applied for ropes seeded with spores. Unbraided plastic raffia seeded with hatchery produced sporelings are used as long lines. Longer ropes may be used but additional stakes are utilised as support to prevent the ropes from sagging to the ground. The distance of the seeded ropes from the ground vary depending on the depth and clarity of the water.
Raft method
The raft method is popularly used in deep areas and where bamboo is available. The raft generally measures 2 × 4 meters in size. Seeded long-lines are also used. One end of the seeded rope is tied to the shorter bamboo frame, stretched tightly and the other end fixed to the opposite side of the raft. The distance between ropes vary but about 12 to 14 lines or more may be attached to one raft. In India, the use of coir ropes fabricated into nets of 7 cm mesh size was used on a 2 × 2 m raft. The same method of seeding was used. Variations of this method have been developed in the People's Republic of China. Pieces of rope about 70 cm long seeded with Gracilaria cuttings are hung horizontally on the low fixed raft or vertically along a long line floating raft.
Improvement of substrates
This method can only be utilised in areas where natural beds are found. The introduction of artificial substrates such as rocks, shells, bamboo or wooden stakes to the site provides additional substrates for the spores to settle or fragments of thalli to attach. Most of the commercially important species of Gracilaria (beds) are found in shallow bays and coves where the substrate is generally particulate (sandy-muddy). The survival and success of spores growing in this habitat is greatly enhanced by the availability of solid substrates. Results of studies on the influence of solid substrate on biomass production has shown that production more than doubles in portions of the beds where solid substrates were introduced.
3.3 Polyculture with shrimp and/or crab
Gracilaria can be polycultured with shrimp (Penaeus monodon) and/or crab (Scylla serrata). Stocking material for a hectare of farm consists of 4,000 to 5,000 kg of Gracilaria, 5,000 to 10,000 crab and 10,000 to 20,000 shrimp. Crushed trash fish and snails are generally used as feed for the crabs. Crabs are harvested after three months, the shrimp after four to seven months. Survival rates as high as 80% for crabs and 80 to 90% for shrimp, have been documented making this polyculture one of the most profitable aquaculture methods in Taiwan. The net income from polyculture has been proven to be three times as much as from monoculture.
3.4 Polyculture with fish in cages
Gracilaria may be polycultured with carnivorous fish species in cages. Seeded nylon twines are attached to a support structure at the opposite sides of the cages. The distance of the: seeded lines below the surface of the water is determined by the light intensity requirements of the species for maximum photosynthesis.
4. FUTURE DIRECTION FOR DEVELOPMENT
4.1 Selection and development of fast growing seedstocks with high quality agar
Two of the factors which influence the income of farmers are the production capacity of the farms and the agar quality of the seedstock. Thus, comparative studies of local species of Gracilaria should be done to select the most productive, fast growing species or strain with high quality agar. The selected species should then be developed and multiplied and used as seedstocks for commercial culture.
4.2 Polyculture
Studies on polyculture with other species in ponds should be done to increase the productivity of the farms and make efficient use of the space and nutrients available.
4.3 Appropriate methods and culture site
The species respond differently to the culture methods being used, thus the method to be applied to the species should be one which will enhance the ecological conditions resulting in an increase in the productivity of the species. Culture sites to be selected should conform to the ecological requirements of the species to be cultured.
4.4 Environmental aspects of polyculture
Although the polyculture of Gracilaria with other species is definitely known to improve the quality of pond effluents, the absorption and accumulation of pesticides and other toxic substances by the seaweeds should be a primary concern of the farmers which should be resolved. Studies on the pesticides which are not harmful to humans should be carried out.
4.5 Improvement in the post harvest methods
The quality of the crop is by large influenced by the post harvest methods used. The removal of dirt and associated materials by thorough washing and sorting and the use of fast sun-drying methods are some of the easy but important post harvest activities which will enhance the quality of the crop.
REFERENCES
Armisen, R. and F. Galatas. 1987. Production, properties and uses of agar. In: D. J. Mc Hugh (ed.) Production and utilisation of products from commercial seaweeds. FAO Fish Tech. Pap. (288): 1–57.
Bird, K. T. 1988. Agar production and quality from Gracilaria sp. strain G-16: Effects of environmental factors. Botanica Marina 31:33–39.
Bird, K. T., Hanisak, M. D. and Ryther, J. 1981. Chemical quality and production of agar from Gracilaria tikvahiae grown in different enrichment conditions. Botanica Marina 24:441–444.
Chiang, Y. M. 1981. Cultivation of Gracilaria (Rhodophyta, Gigartinales) in Taiwan. Proc. 10th Intl. Seaweed Symp.: 569–574.
Daugherty, B. and Bird, K. T. 1988. Salinity and temperature effects on agar production for Gracilaria verrucosa Strain G-16. Aquaculture 74: 1–9.
FAO, 1983. World seaweed industry and trade. Joint ADB/FAO (SCS-INFOFISH) Market Studies. Vol. 6.30 pp. SCS/DEV/83/26. FAO, Manila.
Globefish, 1988. Seaweed- the least known of marine based industries - Special Feature. Globefish Highlights No. 3/88. FAO Publication.
Naylor, J. 1976. Production, trade and utilization of seaweeds and seaweed products. Fisheries Technical Paper No. 159. FAO, Rome.
Neisch, A. C. 1978. Developments in the culture of algae and seaweeds. In: T. V. R. Pillay and W. A. Dill (eds). Advances in Aquaculture. pp. 395–402. Fishing News Books Ltd., Farhein, Surrey, England.
Raju, P. V. and Thomas, P. C. 1971. Experimental field cultivation of Gracilaria edulis (Gmel.) Silva. Botanica Marina, 14:71–75.
Rueness, J., Mathisen, H. J. and Tanager, T. 1987. Culture and field observations on Gracilaria verrucosa from Norway. Botanica Marina 30:267–276.
Tachnavarong, S. 1988. Gracilaria culture in Thailand. In: Report on Training Course on Seaweed Farming. ASEAN/SF/88/GEN/6: pp. 136–141. FAO Publication.
Trono, G. C. Jr. 1981. Field culture of Gracilaria and other species. In: Trono, G. C. Jr. and Ganzon-Fortes, E. T. (eds.). Report on the training course on Gracilaria algae. SCS/GEN/81/29:45–46.
Trono, G. C. Jr. 1986. Seaweed culture in the Asia-Pacific region RAPA Publication 1987/8. Regional Office for Asia and the Pacific. FAO of the United Nations, Bangkok Thailand. 41 pp.
Trono, G. C. Jr. 1988. Production of economically important seaweeds through culture and harvesting of natural stocks. In: Report on the Training Course on Seaweed Farming. ASEAN/SF/88/GEN/6: 59–70. FAO Publication.
Chen Jiaxin,
Director, Yellow Sea Fisheries Research Institute,
19 Laiyang Road, Qingdao, China 266003.
and
Xia Bang Mei,
Institute of Oceanology, Academic Sinica,
7 Nan Hai Road, Qingdao, China 266071.
ABSTRACT
This review report combines the country papers presented by China, India, Myanmar and Vietnam and the literature dealing with genus Gracilaria. Detailed biological characteristics of Gracilaria spp., including basic features of taxonomy, life cycle and ecological conditions for cultivation are described. Sixty-six species of Gracilaria and their distribution in the region are listed and 25 species are introduced in detail. Culture methods, such as pond culture and floating-raft culture, are briefly discussed.
1. INTRODUCTION
Red seaweeds of the genus Gracilaria have been the main source of agarophytes in the world since the 1960s, due to their euryhaline and eurythermal characteristics, as well as their higher growth ratios and agar contents. For example, 450 tonnes of agar products were produced in China in 1993, of which 260 tonnes were from Gracilaria spp. The taxonomic status of many of the Gracilaria species is not properly known in many countries of the Asia-Pacific region because Gracilaria species vary a great deal, even within the same habitat and population. This has often created confusion when comparing the information and data provided by different countries on red seaweeds with respect to production, culture methods, ecological parameters and phycocolloid properties. In addition, several other algae occurring in the region form an important resource of the phycocolloid industry, but little is known about their correct taxonomic status and ecological conditions for cultivation. In the absence of information on the productivity, biological and bio-chemical characteristics of the different strains and species, it is difficult to develop a viable culture technology for the lesser known species that would be economically efficient and sustainable. This paper is a review report that is based on the country reports presented by China, India, Myanmar and Vietnam, as well as a number of references, including, “Taxonomy of Economic Seaweeds” edited by Isabella A. Abbott.
2. A BRIEF HISTORY OF THE TAXONOMIC STUDY OF GENUS GRACILARIA
The genus Gracilaria was established by Greville in 1830 and consisted of four species: G. confervoides, G. compressa, G. purpurascens and G. erecta. In 1852, J. Agardh revised and redefined the generic circumscription for the genus and designated G. confervoides as its species. Early concepts of the species of Gracilaria were mainly based on external structure. A detailed anatomical study was first reported by Sjostedt in 1926 with G. confervoides, G. compressa and G. robusta.
In 1949, Dawson regarded nutritive filaments as a diagnostic characteristic at the generic level and distinguished the genus Gracilariopsis from Gracilaria on the basis of the absence of nutritive filaments and the small size of the gonimoblast cells. In 1966, Papenfuss reported that the presence of nutritive filaments could not always be confirmed in British samples of Gracilaria verrucosa. He concluded that the presence of nutritive filaments could not be used as a basis for separating Gracilaria and Gracilariopsis and for that reason he reduced Gracilariopsis to a synonymy with the original genus Gracilaria.
In 1963, Chang and Xia described the genus Polycavernosa with P. fastigiata as the species type. This genus consists of rhizome-like creeping parts from which arise erect, free branches. The creeping portion is fastened at frequent intervals by a disc-like attachment; spermatangial conceptacles are in clusters and basal absorbing filaments have many long branches. Many authors supported the recognition of Polycavernosa, some species of Gracilaria were transferred into Polycavernosa and new species were described. Wynne (1989), re-instated Hydropuntia Montagne (1842) which was shown to be the earliest validly published name with H. urvillei as the species type. He transferred 14 species of Polycavernosa and two species of Gracilaria to Hydropuntia.
In 1991, Abbott, Zhang and Xia described a new species, Gracilaria mixta according to the mixture of two kinds of spermatangial conceptacles, Verrucosa type and Polycavernosa type, in the same branches. Re-examination of male plants of the western Pacific taxa placed in Polycavernosa (= Hydropuntia) also shows both types of configurations in the same thalli. Therefore, 16 species of Hydropuntia were transferred to Gracilaria.
The genus Gracilaria is cosmopolitan in distribution. It has been reported in most parts of the world, the Arctic, temperate, tropical and even Antarctic regions. According to Bird et al (1982), the apparent centre of distribution lies in the tropics with the largest number of species and a rapid decline in species numbers occurs with increasing latitude in both directions. More than 150 species have been reported. The species of Gracilaria in the region were listed, of which 27 species were from China, 32 species were from India, 7 species were from Myanmar, 9 species were from Peninsular Malaysia and Singapore, 13 species were from Thailand, 25 species were from the Philippines and 13 species were from Vietnam. The identification of all the species of Gracilaria listed here still remains a vexing problem owing to the great variability of the plants and poorly understood species limitations. Nevertheless, the information provided by country reports gives us a opportunity to exchange information with each other.
3. FEATURES OF GRACILARIA AND THEIR TERMINOLOGY
3.1 Morphological features
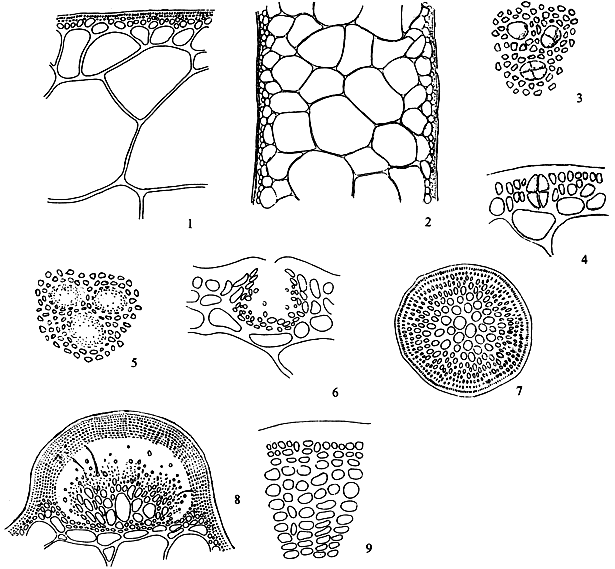
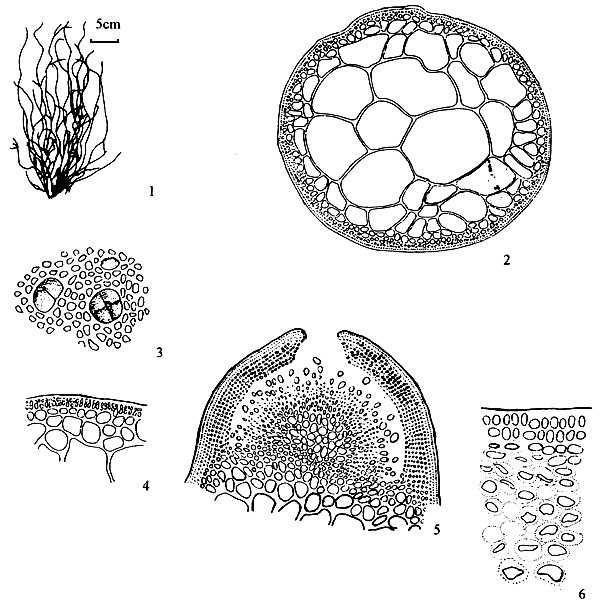
Thallus construction
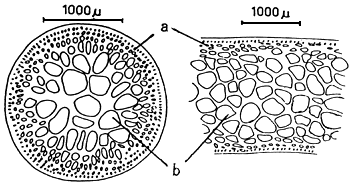
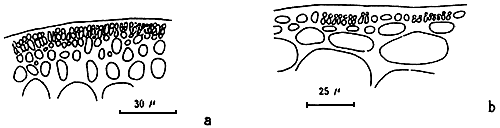
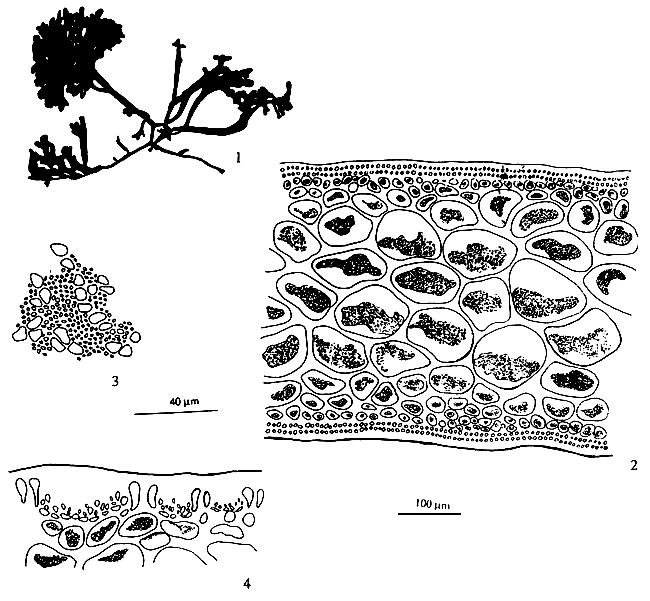
A group of apical meristematic cells divided into multitaxial form thallus. The main structure of thallus is composed of pseudo-parenchymatous or parenchymatous cells. Throughout the thallus, commonly isodiametric cells of outer cortex layers and inner medulla layers are observed (Figure 1.1).
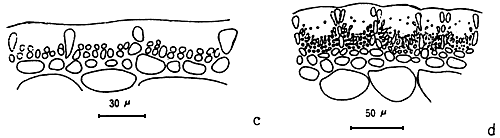
Apical cells
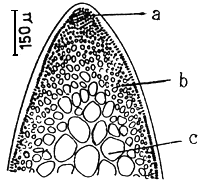
A group of apical cells are situated at the meristematic region of the thallus. It is difficult to distinguish them from the neighbouring cells of the broad apical region, except when a little protrusion occurs at the sharp apex (Figure 1.2).
Cortex
The cortex is the outer tissue of the thallus consisting of 1 to 4 layers of cortical cells. The cells are small, ovoid, strongly pigmented, densely protoplasmic and arranged in anticlinal plane. The outermost cortical cells (surface cells) are more or less elongated and are smaller than the inner cells. Sometimes more cortical layers are observed in the basal part due to the secondary growth of the outermost cells (Figures 1.1–1.2).
Figure 1: Morphological features of Gracilaria spp.
| 1.1: | Transversal and longitudinal section of a sterile plant. | |
| a. | Cortex layers | |
| b. | Medulla layers | |
 |  | |||
| 1.2 | Transversal and longitudinal section of a sterile plant. | 1.3 | Transversal section of a sterile frond, showing several florideam starch grains. | |
| a. | Apical cells | |||
| b. | Cortex cells | |||
| c. | Medulla layers | |||
1.4 A holdfast and its longitudinal section.
Medulla
The term medulla has been used for the pseudo-parenchymatous tissue of the thallus beneath the cortex and consists of non-pigmented, irregularly polygonal, large cells with thin to thick walls. The medulla may be separated into two parts, the central medulla (inner medullar) usually with empty cells and peripheral layer (outer medulla) which is generally filled with the floridean starch grains. The transition from outer medulla to cortex is either abrupt or gradual (Figures 1.1–1.2 and Figures 1.3).
Hairs
Elongated, deciduous, colourless, unicellular hairs up to 40 long, are found abundantly on the cortical layer of the thallus in most species, but are scarcely present in G. verrucosa. Hairs are attached by means of a special foot-cell or hair base-cell. Hair base-cells are distinctly large, darkly staining and different in shape from other cortical cells.
Branches
Branches are erect or decumbent, cylindrical, compressed or flattened. The cylindrical branches may be conspicuously articulated into oblong, obovate to ovoid segments in some species (e.g. G. crassa). Branching may be highly variable irregularly or regularly dichotomously, sometimes trichotomously branched in one plant (e.g. G. foliifera). The shape and breadth of the branches may be uniform throughout the thallus (e.g. G. foliifera) or slightly broad or narrow at the apex and narrow at the base (e.g. G. textorii). The apices of the branches may be acute, obtuse or rounded. The base of the branches are slightly or distinctly constricted.
Branchlets
The branchlets are short and are probably potentially indeterminate branches arising on all sides of the branches. These occur generally at the upper parts of the branches (e.g. G. edulis).
Margins
The margins may be entire, sometimes undulated or spiny with minute processes or outgrowths.
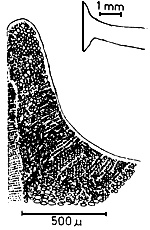
Holdfast (Figure 1.4)
The plants are attached on the substratum by means of a discoid holdfast. One to several branches may arise from a single holdfast.
Haptera
The decumbent branches may be produce multi-cellular haptera or secondary attachment organs on reaching a substratum (e.g., G. crassa).
3.2 Reproductive features
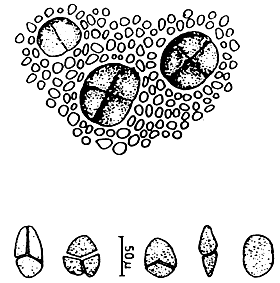
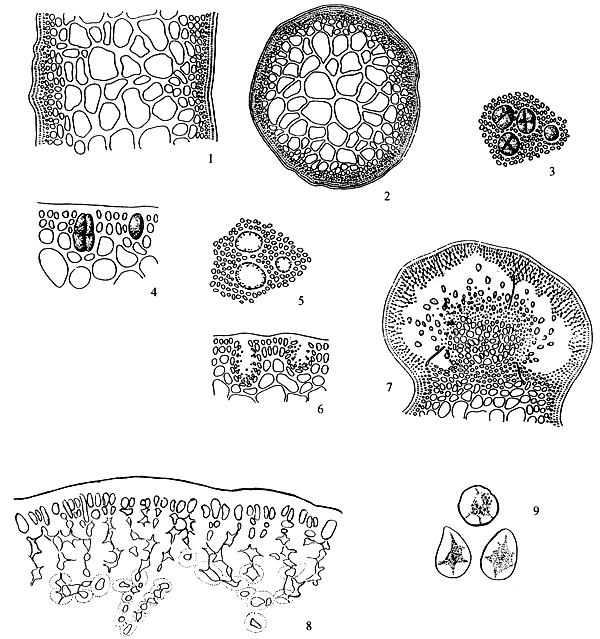
Spermatangia (Figure 2.1)
Spermatangia are found on the surface of the thallus in variously shaped cavities. The spermatangia are ovoid to pyriform, 2.5–6.0 μm in diameter, more weakly staining than the spermatangial mother cells which are arranged compactly or loosely in anticlinal position on the surface cells of spermatangial cavities.
Figure 2: Reproductive features of Gracilaria spp.
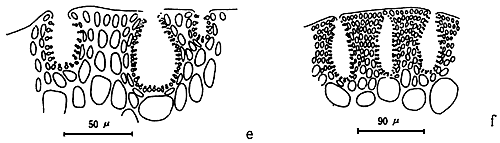
2.1: Different types of spermatangia
a-b. Chorda-type; c-d. Textorii-type; e-f. Verrucosa-type
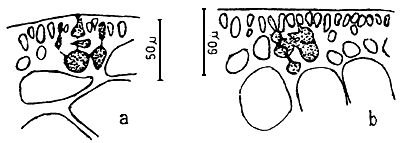
2.2 Carpongonial branches
a. Unfertilised carpongonial branch to show clearly trychogyne.
b. Early post-fertilisation stage of the carpogonial apparatus.
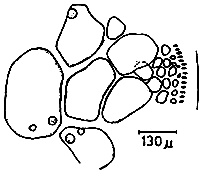
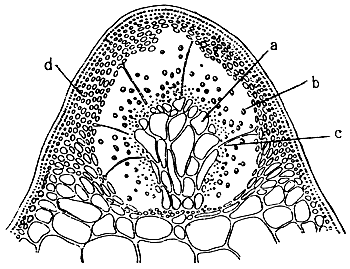 | 2.3 | A mature cystocarp. |
| a. | Gonimoblast | |
| b. | Carposporangia | |
| c. | Nutritive filaments | |
| d. | Pericarp |
 |  | ||
| 2.4 | Carposporangia. | 2.5 | Tetrasporangia showing various styles of division. |
Spermatangial conceptacles (Figure 2.2)
The shape of spermatangial conceptacles varies with different species. Three types of spermatangial cavities: (a) chorda type with superficial or wide opening, (b) textorii type with shallow cup-like cavities and (c) verrucosa type with deep and ovoid to oblong conceptacles with narrow openings. Dawson (Searles and Hommersand, 1971) proposed a system for classifying genera and species of Gracilariaceae based on spermatangial cavities.
Carpogonial branches (Figure 2.2)
The carpogonical branch is two to four cells and occurs singly (unicarpogonical) and outwardly on a supporting cell which is an inner cortical cell of the thallus. The carpogonium is conical and the basal cell is usually ovoid or angular and generally large, sometimes similar or smaller in size to the carpogonium. The trichogyne is long, not constricted at the base, projecting directly outward or slightly twisted at the tip. The supporting cell is large, ovoid, darkly stained and also produces two to four deeply stained celled vegetative filaments which are similar in size and shape to carpogonial branch.
Post-fertilisation (Figure 2.2)
After fertilisation, the fertilised carpogonium probably fuses directly with an adjacent cell from one of the vegetative filaments.
Auxiliary cell
Richly protoplasmic cell from one of the vegetative filaments borne on the supporting cell of the carpogonial branch probably functions as the auxiliary cell.
Fusion cell
The results of the extended connection of the fertilised carpogonium with neighbouring cells is a large ramified fusion cell consisting or carpogonium and adjacent cells of the vegetative filaments bearing from the supporting cell.
Gonimoblast (Figures 2.3, a)
After the formation of the fusion cell, gonimoblast initial cells arise from several points of the fusion cell outwardly. These further develop into gonimoblast tissue in the same direction. Earlier formed gonimoblast cells are large, polygonal in shape, densely protoplasmic and form a parenchymatous tissue around the upper part of the fusion cell. Gonimoblast parenchyma produces short-celled filaments which are irregularly shaped, rounded toward the tips and transformed into carposporangia.
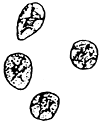
Carposporangia (Figures 2.3,b and 2.4)
The carposporangia are ovoid to pyriform with a conspicuous central body and form the periphery of the short-celled gonimoblast filaments in chains.
Nutritive filaments (Figures 2.3 ,c)
Nutritive filaments connecting the gonimoblast parenchyma with the pericarp may or may not be present in different species or in the same species. These filaments are filiform, possess a base of greater or lesser width and haustorium-like ramification at the tips which pierce the pericarp cells.
Carposporophyte
Carposporophytes occur on the female gametophyte. This generation consists of a large irregular ramified fusion cell, bearing small or large gonimoblast parenchyma, with or without nutritive filaments and short-celled filaments which transformed into carposporangia in regular or irregular radial chains.
Pericarp (Figure 2.3,d)
The sterile cortical cells surrounding the developing carposporophyte, divide simultaneously and form the cortical layer of considerable thickness which becomes the cystocarpic wall (pericarp) enveloping the protruding carposporophyte.
Cystocarp (Figure 2.3)
The term cystocarp refers to the reproductive structure, found on the female plant, which forms as a result of the fertilisation of the carpogonium. The mature cystocarp consists of the carposporophyte and pericarp. They are either globose or dome-shaped, scattered or aggregated and protrude from the surface of the thallus.
Ostiole
The ostiole is an opening formed by successive divisions of cortical cells surrounding the developing carposporophyte. Carposporangia are liberated through the ostiole which are present in most species but absent in some species.
Tetrasporangia (Figure 2.5)
Tetrasporangia are cruciately divided, scattered on the surface of the thallus and surrounded by modified or unmodified cortical cells. Tetrasporangia initially may be cut off from any of the surface cortical cells.
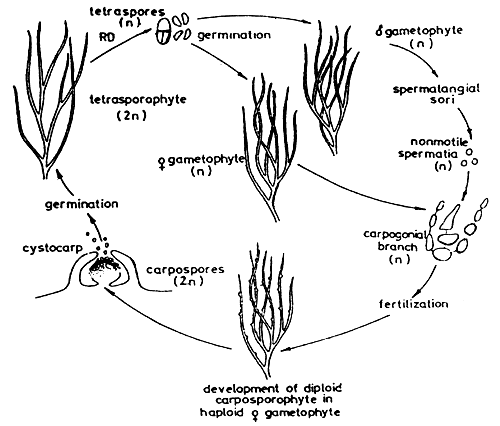
4. LIFE CYCLE OF GRACILAR1A (Figure 3)
Gracilaria shows an alternation of isomorphic generations between haploid gametophyte and diploid tetrasporophyte. The gametophyes are dioecious. The fertile male thallus produces spermatangia and the female thallus produces carpogonia. After fertilisation, a structure called a “cystocarp” is formed. The cystocarps are prominent, protruding, globose or hemispherical, with or without rostrum, scattered on the surface of the female thallus. Each cystocarp consists of pericarp, gonimoblast filaments and carposporangia, with or without absorbing filaments. Carpospores are liberated through a small hole or ostiole at the top of the cystocarp and germinate into tetrasporic thalli or tetrasporophytes. The mature tetrasporophyte produces tetrasporangia occurring generally in the cortex of the thallus. The tetrasporangium is cruciately divided and forms four spores or tetraspores which germinate into four gametophytic thalli or gametophytes, of which two are male and two are female thalli (Figure 3).
Figure 3: The life cycle of Gracilaria.
5. LIST OF GRACILARIA SPECIES FROM SOME ASIAN COUNTRIES
| BGD | CPR | IND | INS | JAP | ROK | MYA | MAL | PHI | THA | VIE | ||
| 1. | G. arcuata | + | + | + | + | + | ||||||
| 2. | G. armata | + | ||||||||||
| 3. | G. articulata | + | + | |||||||||
| 4. | G. asiatica (G. verrucosa) | + | + | + | ||||||||
| 5. | G. bailinae | + | + | |||||||||
| 6. | G. bangmeiana (P. ramulosa) | + | + | |||||||||
| 7. | G. blodgettii | + | + | + | + | |||||||
| 8. | G. bursa-pastoris (G. compressa) | + | + | + | ||||||||
| 9. | G. cacalia | + | ||||||||||
| 10. | G. canaliculata | + | + | |||||||||
| 11. | G.changii (P. changii) | + | + | + | + | |||||||
| 12. | G. chorda | + | + | |||||||||
| 13. | G. chouae | + | ||||||||||
| 14. | G. coronopifolia | + | + | + | ||||||||
| 15. | G. corticata | + | ||||||||||
| 16. | G. crassa | + | + | + | ||||||||
| 17. | G. cuneifolia | + | ||||||||||
| 18. | G. cylindrica | + | + | + | ||||||||
| 19. | G. dactyloides | + | ||||||||||
| 20. | G. debilis | + | + | |||||||||
| 21. | G. denticulata | + | ||||||||||
| 22. | G. disticha | + | + | |||||||||
| 23. | G. dura | + | ||||||||||
| 24. | G. edulis (P. fastigiata) | + | + | + | + | + | + | |||||
| 25. | G. eucheumoides | + | + | + | + | + | + | + | ||||
| 26. | G. fergusonii | + | ||||||||||
| 27. | G. firma | + | + | + | + | + | ||||||
| 28. | G. fisheri (P.fisheri) | + | ||||||||||
| 29. | G. foliifera | + | + | |||||||||
| 30. | G. gigas | + | + | + | + | |||||||
| 31. | G. glomerata | + | ||||||||||
| 32. | G. hainanensis | + | + | + | ||||||||
| 33. | G. incurvata | + | ||||||||||
| 34. | G. indica | + | ||||||||||
| 35. | G. irregularis | + | ||||||||||
| 36. | G. kanyakumariensis | + | ||||||||||
| 37. | G. kilakaraiensis | + | ||||||||||
| 38. | G. lacinulata | + | ||||||||||
| 39. | G. lemaneiformis | + | + | + | + | |||||||
| 40. | G. lichenoides | + | ||||||||||
| 41. | G. manilaensis | + | ||||||||||
| 42. | G. megaspora | + | + | |||||||||
| 43. | G. millardetii | + | + | |||||||||
| 44. | G. mixta | + | ||||||||||
| 45. | G. obtusa | + | ||||||||||
| 46. | G. opuntia | + | ||||||||||
| 47. | G. percurrens (P. percurrens) | + | ||||||||||
| 48. | G. pudumadamensis | + | ||||||||||
| 49. | G. punctata | + | + | + | ||||||||
| 50. | G. purpurascens | + | ||||||||||
| 51. | G. pygmae | + | ||||||||||
| 52. | G. salicornia | + | + | + | + | + | ||||||
| 53. | G. spinulosa | + | ||||||||||
| 54. | G. stellata (P. divergent) | + | ||||||||||
| 55. | G. sublittoralis | + | ||||||||||
| 56. | G. subtilis (P. subtilis) | + | ||||||||||
| 57. | G. tenuistipitata | + | + | + | + | + | ||||||
| 58. | G. textorii | + | + | + | + | + | + | + | + | |||
| 59. | G. turgida | + | ||||||||||
| 60. | G. tuticorinensis | + | ||||||||||
| 61. | G. urvillei | |||||||||||
| 62. | G. vanbosseae | + | ||||||||||
| 63. | G. vermiculophylla | + | ||||||||||
| 64. | G. yamamotoi |
6. DESCRIPTION OF THE MAIN SPECIES
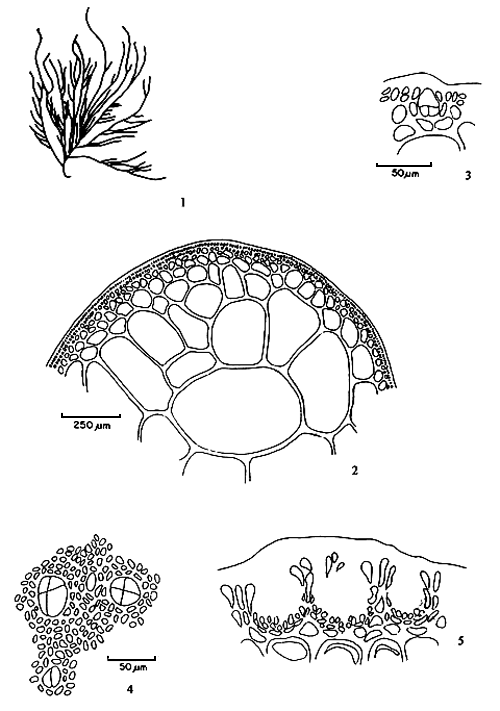
6.1 G. articulata C. F. Chang et B. M. Xia (Figure 4)
Thallus erect, solitary or caespitose, arising from small discs, cylindrical throughout, usually 10–18 cm in length, alternately, secundly or irregularly dichotomously branched; common mango in colour, succulent, brittle, easily broken, adhering rather well to paper on drying; branches and branchlets conspicuously articulate-constricted to a sharp spine; articulations elongated club-shaped, arcuated, upwards, 10–20 times as long as broad, ending in blunt, almost equal in breadth, abruptly constricted at the base. Thallus in transverse section consisting of a large-celled medulla bordered by one to two layers of much smaller cortical cells; medulla composed of 4–6 layers of large roundish cells ca. 1030 μm in the greatest diameter, possessing a wall 3–7 μm thick; cortical cells roundish or elongated, 7–10 × 3–7 μm in dimensions, with cuticle 10 μm thick; transition from medulla to cortex strikingly abrupt.
Tetrasporangia scattered on the surface of thallus, circular or ovoid in shape as seen from surface, ca 33–36 μm in diameter, ovoid in transverse section, ca. 33 × 24 μm in dimensions, surrounded by somewhat modified cortical cells, cruciately divided. Antheridia borne in conceptacular pockets mostly with a broad opening, ca. 41 μm deep and 45 μm wide, well separated by modified cortex.
Figure 4: Gracilaria articulata Chang et Xia.
Cystocarps prominently protruding, semiglobose, 530–950 μm in diameter, non-rostrate, not constricted at base, the gonimoblast developing from a large fusion-cell and connected to the pericarp by numerous nutritive filaments; carpospores round or ovoid in shape, 25–30 μm in diameter, containing a conspicuous stellate central body; pericarp thin, 60–85 μm in thickness, consisting of 9–12 layers of cells, of which the outer cells of 1–2 layers are cuboid or oblong in shape while the inner layers of cells are horizontally elliptical with distinct cell wall, cells in distinct longitudinal rows, under low power.
Habitat: Grows on shells in the sublittoral region of low salinity.
6.2 G. asiatica Chang et Xia (Figure 5)
Thallus erect, solitary or caespitose, cylindrical throughout, habit variable, 3–50 (-200) cm in length, 1–3 mm in diameter, arising from a small discoid holdfast with 1–4 order of branches; purplish brown to dark brown, sometimes to greenish or yellowish, subcartilaginous in substance, adhering imperfectly to paper on drying; branches irregularly alternately, secundly or subdichotomously, sometimes with shorter or longer ramuli, 0.5–2. 5 mm in diameter, gradually tapering towards the apex and slightly or occasionally abruptly constricted or nonconstricted at the base. Thallus in transverse section consisting of a medulla of large parenchymatous cells, 165–365 μm in diameter with thicker walls, 8–24 (-40) μm, surrounded by 3–5 or more layers of cells, 7–10 × 5–7 urn in dimensions, pigmented and with surface jelly 10 μm thick; transition from medulla to cortex gradual; empty in central portion when old.
Tetrasporangia scattered among the surface layer of frond, subspherical or ovoid in shape in surface view, 40–46 μm in diameter, ovoid or oblong in transverse section, 49–69 μm × 39–49 μm in dimensions, surrounded by unmodified cortical cells, cruciately or occasionally tetrahedrally divided. Carpogonial branches two-celled; cystocarps prominently protruding, subspherical to hemispherical in shape, 660 x 750 μm in diameter nonrostrate or slightly rostrate, unconstricted or slightly constricted at base. Gonimoblast developing from a fusion-cell, parenchymatous, connected with the pericarp by rare (or without) absorbing filaments; carpospores round or ovoid in shape, 16–23 μm in diameter; pericarp 115–250 urn in thickness, consisting of 7–13 layers of cells; of which one layer of outermost pigmented cells and about 6–8 layers of cells with obscure cell walls except for 2–5 innermost layers of cells with clearer cell walls, the distinct stellate cell protoplasm of inner layer cells connected anticlinally and radially with each other by means of more distinct pit-connections. Spermatangial conceptacles scattered over the surface of the blade in well-separated, spherical to oblong in shape in surface view, 33–50 μm or more ovoid to long elliptical conceptacle in sectional view, 80–180 μm deep and 33–100 μm wide, well-separated by modified or unmodified cortex cells.
Habitat: Grows on gravel, pebbles and rocks which are often covered with sand and mud in the intertidal to upper subtidal.
Remarks: For more than two hundred years Gracilaria verrucosa (Hunds.) Papenfuss, (known as G. confervoides (L.) Greville) originally described in Devon, England by Hudson in 1762, has been reported from various places in the world from the arctic to the tropics and is regarded as a cosmopolitan species. In China, since Martens first reported the occurrence of this species from Macao, China in 1866, many other phycologists (Gepp in 1904; Cotton, 1915; Ariga, 1919; Tseng and Li 1935; Howe 1924; 1934 Setchell, 1931; Chang and Xia 1976; Zhang and Xia, 1984) have reported the same species from various places in China.
Figure 5: Gracilaria asiatica J. F. Zhang et B. M. Xia.
Determination of this species of Gracilaria is difficult because of their great variability and the lack of reliable characteristics in differentiating related species. Several years ago, Zhang and Xia found the structure of the pericarp to be quite constant with the species and valuable in differentiating species (Zhang and Xia, 1976). Although they identified the common Chinese species as G. verrucosa (Huds.) Papenfuss, they suspected the cosmopolitan distribution of the English species and that the Chinese species was different from the English species, but the lack of specimens from England and other places for comparative studies prevented them from dealing with this problem any further. After getting the specimens collected from Wemoury, Devon, England, comparative studies revealed that the Chinese species does differ specifically from the English Gracilaria verrucosa (Huds.) Papenfuss.
6.3 G. changii (Xia et Abbott) Abbott, Zhang and Xia (Figure 6)
Thalli 5–7 cm tall, with many branches arising from a small discoid holdfast or from a percurrent axis; branching alternate or irregular of two to four orders; branches cylindrical or inflated, 1.0–2.5 mm in diameter abruptly constricted at bases and tapering towards apices. Fronds in transverse section consist of large medullary cells with thick walls, two to three layers of cortical cells; transition of cells abrupt. Tetrasporangia ovoid, 13–17 x 28–36 μm in diameter, cruciately divided. Spermatangial conceptacles verrucosa type; adjoining ones frequently coalescing, forming polycavernosa-type spermatangia. Cystocarps conical or semiglobose, 1.0–1.4 mm wide, 0.5–0.8 mm high, some slightly rostrate, not constricted at base. Gonimoblasts consist of numerous small cells; absorbing filaments few, lateral and upper, carpospores round, 18–24 μm in diameter; pericarp thick, consisting of two kinds of cells, the outer five to six rows rounded, the inner six to nine rows compressed.
Habitat: Grows on gravel, shell, rock fragments, mangrove roots, or fish cages in sandy- muddy areas with high turbidity.
Remarks: This species has often been mis-identified as G. cylindrica (Doty et al. 1983, Santos and Doty, 1983, Doty and Fisher, 1987) and as G. blodgettii (Phang, 1986, Phang and Maheswary 1990) because of the constrictions at the branch bases. Gracilaria changii is quite widely distributed in Malaysian waters. The agar content and gel strength vary with local conditions

Figure 6: Gracilaria changii (Xia et Abbott) Abbott, Zhang and Xia.
Figure 7: Gracilaria chouae J. F. Zhang et B. M. Xia.
6.4 G. chouae Chang et Xia (Figure 7)
Thallus erect, solitary or caespitose, arising from a small disc, cylindrical throughout, usually 15–20, but up to 40 cm long, 2–3 mm in diameter, with two to four orders of branches. Texture succulent; easily broken, and brittle when dry; adheres well to paper on drying. Light red when fresh. Branches irregularly alternate, occasionally furcate, but strongly mixed with unilateral or secund branching in whole or in part; gradually tapers toward apices and not constricted where attached.
In transverse section, medullar consists of large pseudo-parenchymatous cells, 232–598 μm in diameter, with walls 6.6–8.3 μm in diameter. Medullar surrounded by one to two layers of cortical cells, which are roundish and 23–33 μm in diameter; outermost layer pigmented and consisting of cells 10–17 μm by 7–10 μm in diameter. Transition from medulla to cortex abrupt. Tetrasporangia scattered among cortical cells, ovoid or subspherical in surface view, 31–43 μm in diameter, ovoid or oblong in transverse section, 26–63 μn by 17–33 urn in diameter, surrounded by slightly modified cortical cells; cruciately divided. Spermatangia conceptacles of “textorii-type”, scattered over surface of frond in a small, shallow, well-defined depressions, 30– 53 μm deep and 30–43 μm in diameter; separated by modified, elongated cortical cells, characteristic of this type of conceptacle.
Cystocarps protrude prominently, conical or hemispherical, 664–913 μm by 650–880 μm , slightly rostrate, not constricted at base. Gonimoblast composed of some large pseudo-parenchymatous cells developing from a fusion cell, with many upper absorbing filaments connecting gonimoblast to pericarp. Carpospores round or ovoid, 20–36 μm by 10–20 μm in diameter. Pericarp 100–191 μm thick, consisting of 7–12 layers of cells; outermost layer pigmented cells ovoid-oblong; remaining pericarp cells 7–10 rows of rounded or horizontally elliptical cells, arranged in somewhat irregular vertical rows, with secondary pit connections inconspicuous; three to five innermost layers of small irregularly shaped cells.
Remarks: G. bursa-pastoris was characterised by Harvey (1849), and later by Ohmi (1958), as having succulent, brittle, alternate or subsecund branches, with broad insertions of the nonconstricted branches that had patent and rounded axils, numerous absorbing filaments in the cystocarp and shallow saucer-like spermatangial cavities. Chang and Xia (1976) thought that this characterisation matched the Chinese material rather well. However, the structure of the pericarp that Ohmi (1958) had depicted as having star-shaped cells with prominent secondary pit connections contrasted sharply with the Chinese material, which had pericarpic cells that were rounded to oval-obovate (Chang and Xia, 1976).
Recently, Abbott (1985), named a new species of Gracilaria for the Hawaiian material previously identified as G. bursa-pastoris, using as distinguishing features the small cells of the pericarp and their star-shaped contents, together with the large cells of the gonimoblast. This description of G. parvispora appears to include what has been called G. bursa-pastoris in Japan, and although Chang and Xia did not examine the Japanese material, they think that it is more like the Hawaiian than the Chinese material, particularly with respect to the structure of the pericarp.
In external appearance, particularly the unilateral branching pattern, G. chouae and G. parvispora look alike, although plants of G. parvispora tend to be more robust and shorter than the Chinese plants. The differences lie in some characteristics of the pericarp, such as thickness, shape, and arrangement of pericarp cells and contents, and in the sizes of the cystocarps. The cystocarp of G. chouae is conspicuously smaller than that of G. parvispora. G. parvispora has the largest cystocarp, G. bursa-pastoris has an intermediate sized one, and G. chouae has the smallest of the three. In Chang and Xia's earlier described species and varieties, G. asiatica, G. tenuistipitata var. liui, G. chouae and G. asiatica var. zhengii are all mainly based on pericarp features.
Because of a re-examination and critical study of Chinese specimens, the earlier reported 21 species of Gracilaria in China have been reduced to 18 species. This was accomplished by Xia (Xia, 1986), who placed G. cacalia, G. crassa, and G. minor into the synonymy of G. salicornia.
6.5. G. coronopifolia J. Agardh (Figure 8)
Plants are short, from 4–6 cm, irregular and sub-dichotomous, branching to form an entangled mass; purplish-red when fresh but red when dry. Tertiary branches (up to 1.5 m) are much thicker than branches (0.5 mm), probably because of anastomosing of the branches; ultimate branches have pointed apices. Cuticle is thick, one to two rows of small cortical cells before large medullary cells, and transition from cortex to medulla is abrupt. Only young cystocarps are found, rostrate at ostiole and constricted at the base. No distinct size variation is found in pericarp cells, gonimoblast cells are small and compressed, and upward growing absorbing filaments are present.
Remarks: This species resembles G. edulis in having subdichotomous branching, rostrate ostiole and constricted base in the cystocarp. However, unlike G. edulis, it has no “thick-walled brick-like cells” in the pericarp and no basal absorbing filaments (Xia and Abbott, 1987).
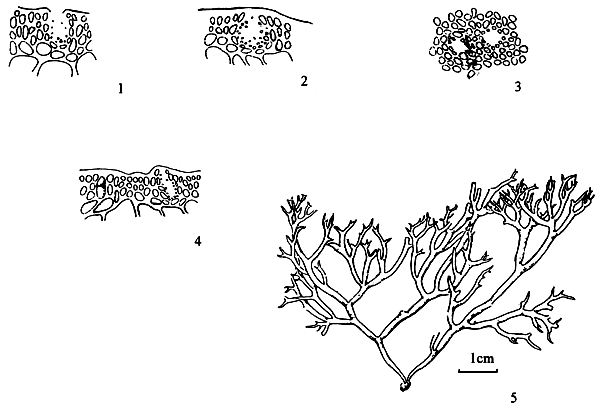
Figure 8: Gracilaria cornopifolia J. Ag.
6.6. G. cuneifolia (Okamura) Lee et Kurogi (Figure 9)
Thallus erect, solitary or caespitose, flattened, 4–7 cm tall, arising from a small disc by a short, slender subterete stipe 2–6 mm long; purplish red; thin, membranous adhering imperfectly to paper on drying; two to three times dichotomously branched, segments 1.5–9.0 mm wide, with broad, round, patent axils, ending in blunt or notched apices, with entire margins. Thallus in transverse section consisting of a medulla of two to three layers of large parenchymatous cells, 112–224 μm x 66–120 μm in diameter, with walls 6 urn thick, surrounded by one to two layers of small cells, 10–33 μm x 17–43 μm in diameter. The outermost layer 4–7 μm x 3.3–6.6 μm in diameter, pigmented and surface jelly 10 μm thick; sterile thallus in transverse section 297–322 μm thick. Tetrasporangia cruciately divided, scattered among the surface layers of frond, circular or ovoid in surface view, 26–33 μm x 23–30 μm in diameter, ovoid or oblong in transverse section, 26–36 μn x 17–23 μm, surrounded by modified cortical cells. Cystocarps on both surface of frond, prominently protruding, hemispherical, dome-like in appearance, slightly rostrate, up to 0.7 mm x 1.23 mm in diameter, nonconstricted at base; gonimoblast consisting of branched pseudo-parenchymatous filaments developing from a fusion cell; gonimoblast cells polygomal, 46–92 μm x 26– 46 μm, upper traversing filaments abundant, connecting gonimoblast to the pericarp; carposporangia round to ovoid, 13–23 μm x 10–13 μm. Pericarp of one kind of tissue, 191–198 μm thick, consisting of 13–16 layers of cells, the cells with obscure cell walls, the contents star-shaped. Spematangia textorii- type in cortical depressions, 23–30 urn x 20–33 μm deep.
Figure 9: Gracilaria cuneifolia (Okamura) Lee et Kurogi.
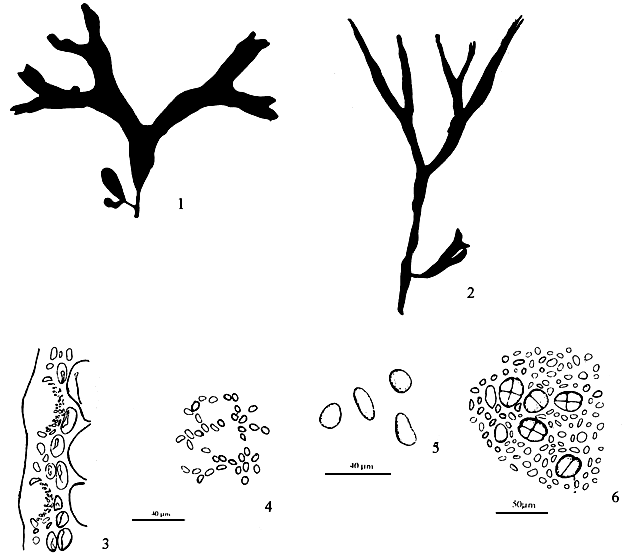
6.7 G. edulis (Gmelin) Silva (Figure 10)
Thallus growing from a disk-like holdfast, with prostrate rhizome forming a tuft or a cluster 6–27 cm tall; branching dichotomous or trichotomous, five to seven orders; branches 0.4–1.0 mm in diameter. Two groups of plants can be distinguished. The first grows in fastigiate tufts with five to seven (up to 10) orders of branches with narrow-angle furcations, branch intervals gradually in length, the last order ending in bifurcate or trifurcate apices. This group is found growing on fish cages or on rocks in rather clear water. The second group grows on rocks or mud surfaces in sandy mud areas, forming an entangled mass or loose clump with hooks or root-like discs on branch apices; branching of five to seven orders with wide-angle furcation, lower branch intervals much longer than the last two orders; branches cylindrical, lower branches about 1 mm thick and becoming thinner, to 0.3 mm, for terminal segments with attenuate apices.
Frond in transverse section consist of roundish thin-walled medulla to cortex abrupt. Tetrasporangia ovoid in transverse section 20–60 × 32–38 μm, surrounded by elongated cortical cells. Spermatangia in groups of six to ten deep sac-like cavities (polycavernosa type), slightly elevated from the surface of thallus. Cystocarps globose, 0.7–1.2 mm in diameter with rostrate tips and constricted at bases; gonimoblasts consisting of elongated cells; carpospore roundish, 15 × 22 μm in diameter; pericarps thick consisting of 9 to 14 rows, cells of the outrows oval, inner cells horizontally compressed; basal absorbing filaments robust with many branches, lateral absorbing filaments rare.
Habitat: Growing in loose clumps on rocks or mud surface in muddy areas of intertidal zone, in dense tufts when growing on fish cages, and in loose fastigiate tufts on rocks in clear water.
Remarks: This species is often found with G. changii, sharing the same intertidal muddy substrate. The two can be easily distinguished because the plants of G. edulis are bushier than those of G. changii and are red when dry rather than black.
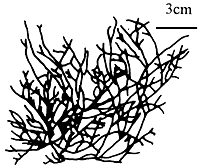
Figure 10: Gracilaria edulis (Gmelin) Silva.
6.8 G. eucheumoides Harvey (Figure 11)
Thallus compressed, prostrate, thick and succulent; branching pinnate to dichotomous; branches 1–3 cm long, 0.5–1.0 cm wide, 0.2–0.9 (up to 1.1) cm thick, with dentate margins, attached by discoid holdfasts. Fronds in transverse section consisting of many layers of cells with stellate plastids, transition of cells from medulla to cortex gradual, medullary cells 100–200 μm in diameter. Only a few specimens of tetrasporic plants found; tetrasporangia roundish on surface view and elongated in transverse section. Gametophytic plants not found.
Habitat: Grows on dead coral fragments.
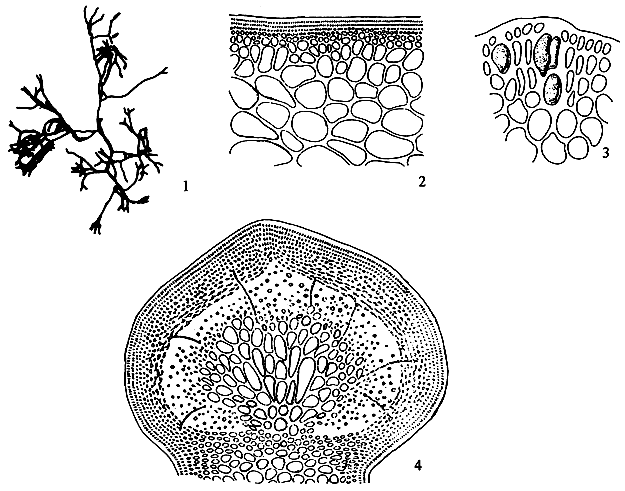
Figure 11: Gracilaria eucheumoides Harvey.
6.9 G. firma Chang et Xia (Figure 12)
Thallus erect, caespitose or solitary, arising from a small disk-like holdfast, cylindrical throughout, usually 10–20 cm in length, whitish brown in colour, with slight green when fresh, firm in texture, adhering imperfectly to paper on drying; branches alternately or secundly, 1–2 mm, up to 3 mm in diameter, gradually tapering toward apex and abruptly constricted at the base. The structure of the fronds often having a somewhat more gradual transition from medulla to cortex; medulla composed of large parenchymatous cells which are 230–360 μm, up to 450 μm in diameter, with wall 10–20 μrn thick, surrounded by intracortical layers of 3–5 layers roundish cells filled with globular floridean starch grains; outer small cortical cells are pigmented, 6.6–13.2 μm x 3.3– 6.6 μm in dimensions and with surface jelly ca. 7 μm thick.
Figure 12: Gracilaria firma Chang et .
Tetrasporangia densely scattered over the greater part of the frond, cruciate, round in surface view, 33–37 μm in diameter, ovate in shape as seen in transverse section of the frond, 59–66 μm × 26–43 μm in dimensions, bordered by more or less modified cortical cells; tetraspore containing a conspicuous pigmented stellate central body. Cystocarps prominently protruding, conical or hemispherical in shape, 580– 630 μm in diameter, with a large gonimoblast parenchyma of small, densely massed, thicker wall, richly protoplasmic cells, 23–46 urn × 16.5–23 μm in dimensions, producing dense, radiating chains of ovoid or elliptical carpospores filling the cystocarpic cavity; carpospore 23–40 μm x 16–20 μm in dimensions; without nutritive filaments. Pericarp thin, 83–95 μm in thickness, consisting of 8–10 layers of cells, of which the outermost cells of 1–2 layers are pigmented and roundish square to oblong in shape, while the inner layers of cells are horizontally oblong with distinct cell wall, cells in distinct longitudinal rows, the innermost 2–3 layers of cells are ellipical to roundish in shape. Antheridia occur densely on the frond which are round in shape or sometimes irregularly as seen in surface view, elliptical cavities, 66–116 urn x 33–66 μm in dimensions in transverse view, bordered by modified cortical cells.
Habitat: Grows on shells, gravel and rock fragments in muddy areas of turbid water.
Remarks: The species resembles G. changii, and G. edulis. It can be separated from G. edulis on the basis of the abundance of basal absorbing filaments (few in G.firma) and structure of pericarp cells. It has a less branched plant than G. changii, with gradual transition from cortex to medulla, cystocarp constricted at base and no horizontally oriented inner pericarp cells.
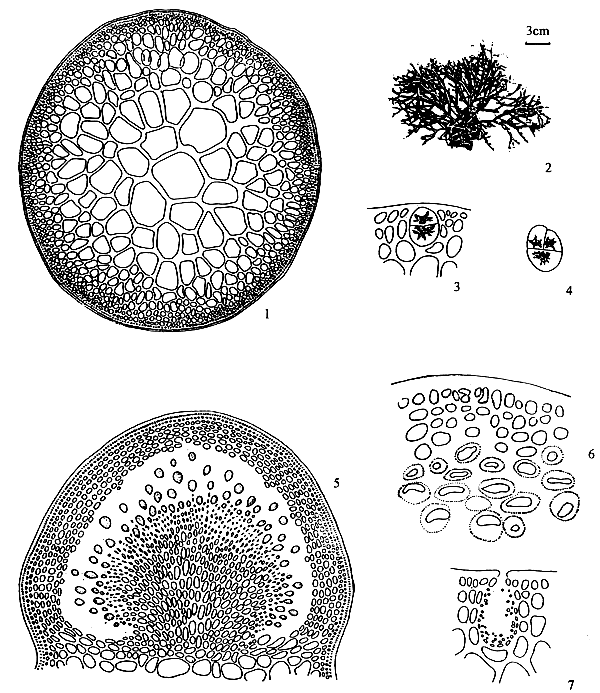
6.10 G. fisheri (Xia et Abbott) Abbott, Zhang and Xia (Figure 13)
Thallus bushy, 13–30 (up to 45) cm tall, with many branches coming from a short stipe or from percurrent axis; branching alternate, three to four orders; branches cylindrical, 0.6–2.3 mm in diameter, constricted at bases and tapering toward apices. Frond in transverse section consisting of medulla 220–620 μm in diameter, with thick layers of cortex; transition form medulla to cortex gradual. Tetrasporangia ovoid, tetraspores 20–25 urn in diameter. Spermatangia ovoid, single or in groups of two to three cavities. Cystocarps conical, rostrate, unconstricted at bases, 0.3–0.7 mm high, and 1.0– 1.3 mm wide; gonimoblasts consisting of many small cells; absorbing filaments lateral and upper; pericarp thick with inconspicuous cell walls and star- shaped contents; carpospores rounded, 18–24 μm in diameter, or ovoid, 8–14 × 14–20 μm.
Habitat: Commonly found growing on living and empty shells (Cerithium sp.) and on broken rocks, gravel, polyethylene bags, and fish cages in sandy-muddy areas of turbid water.
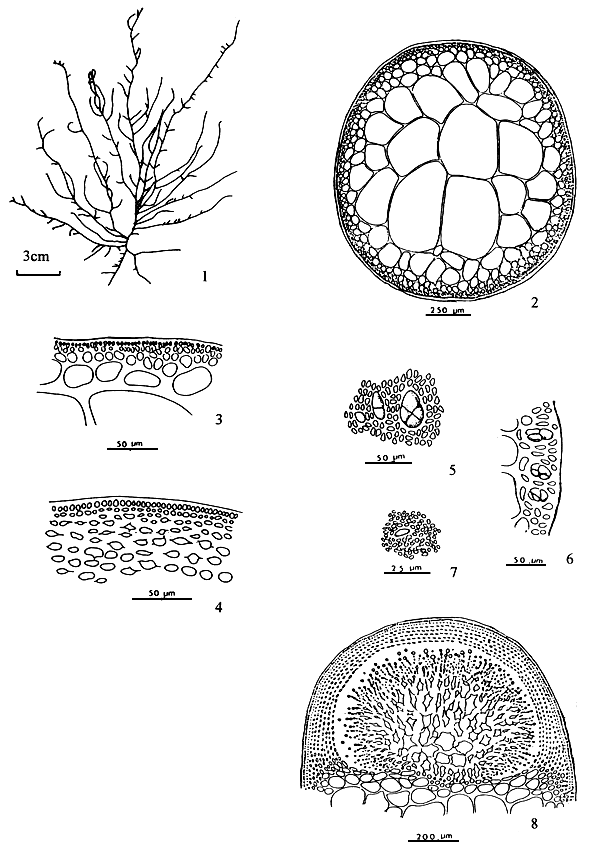
6.11. G. glomerata Zhang and Xia (Figure 14)
Plants 4.0–4.5 cm tall, complanate, dark purplish brown, attached below by a discoid holdfast, with a short and slender subterete stipe 1–2 mm long; frond 1–2 mm wide, irregularly dichotomously branched, to six to seven orders, usually branching one to three times subdichotomously below. Otherwise naked for 1.0–2.5 cm in basal and median parts, bearing toward their apices dense branches, often somewhat glomerate; texture cartilaginous, with entire margins and blunt apices. Frond in transverse section consisting of a medulla of large, thin-walled cells 79–145 urn × 73–112 μm in diameter, and one to two layers of small pigmented cortical cells 3.0–6.6 μm x 5–6 μm; transection 448– 481 μm thick. Spermatangia textorii-type in cortical depressions, 23–30 μm deep. Tetrasporangial and female plants unknown.
Figure 13: Gracilaria fisheri (Xia et Abbott) Abbott, Zhang and Xia.
Figure 14: Gracilaria glomerata J. F. Zhang et B. M. Xia.
Remarks: The dense branches in the upper parts together with rather thick segments are so distinctive as to separate this species readily from all others now reported in this genus. None of the other complanate species is reported as having glomerate branches. Although tetrasporophytes and female material have not been seen, the habitat of these male plants is different from that of other species and the plants represent an independent species.
6.12. G. hainanensis Chang et Xia (Figure 15)
Thallus solitary or caespitose, arising from a small disc, cylindrical throughout, usually 15–35 cm, up to 45 cm in length, one to two orders of branches; chestnut purple or kelp green in colour, succulent in substance, becoming soft and adhering imperfectly to paper upon drying; branches generally supple, elongated, up to 30–40 cm, 2–3 mm in diameter, attenuated to a fine apex, flagelliform, irregularly alternately or secundly and sparingly branched, abruptly constricted at the base; thallus consisting of three kinds of tissues; medulla composed of large parenchymatous isodiametric or polygonal cells which are 0.5–0.6 mm, up to 0.95 mm in diameter, with walls 7–10 μm thick, merging rather abruptly into the subcortex of one to two layers of irregularly ovoidal cells, 23–33 μm in diameter, and one to three layers of small, pigmented, more or less anticlinally arranged cells, which are roundish, cuboid or ovate in shape, 6.5–9 μm × 5–6.6 μm in dimensions and with surface jelly ca. 7 μm up to 13 μm thick.
Tetrasporangia densely scattered over almost the entire surface of frond, round or ovate in shape in surface view, ca. 33 μm in diameter, ovoid or oblong in transverse section, ca. 37–39 μm × 21–26 urn in dimensions, surrounded by somewhat elongated ovoid cortical cells, cruciately divided. Antheridia borne in well-separated, deep, open, conceptacular pockets 37 × 39 μm in dimensions in the cortex. Cystocarps remote, scattered over the thallus surface above and below, rather small, mostly less than 450 μm diameter, conical, non-rostrate, not constricted at base, gonimoblast consisting of parenchymatous cells, connected to the pericarp by several nutritive filaments; mature carpospores round or ovoid in shape, 26–30 μm. in diameter, containing a conspicuous stellate central body; pericarp thin, ca. 90–120 μm in thickness, consisting of 9–11 layers of cells, of which the outermost layer cells are pigmented and roundish square to oblong in shape, whereas inner layer cells are horizontally elliptical to horizontally roundish oblong with distinct cell wall, cells in distinct longitudinal rows, under low power.
Habitat: Growing on shells in the sublittoral region of low salinity.
6.13. G. bailinae (Zhang et Xia) Zhang et Xia (Figure 16)
Thallus erect, solitary or caespitose, arising from a small disc, cylindrical throughout, usually 10–50 (up to 70) cm long; main axis percurrent or not, 1–2 (up to 3) mm in diameter, with two to four orders of branches; succulent, brittle and easily broken; fleshy, light red to kelp (dark) green, dark brown and hardened when dry, adhering to paper on drying; branches irregularly alternate, secund or furcate, with second order long, third order short, sometimes spinose, 0.2– 0.5 mm in diameter, gradually tapering towards the apex and nonconstricted at the base; branches and branchlets are distinguished clearly. Thallus in transverse section consists of a medulla of large parenchymatous cells, 200– 531 μm in diameter, with walls 8–10 μm in diameter, surrounded by two to three layers of small roundish cells, 13–33 μm in diameter, the outermost layer 7–10 by 4–7 urn in diameter, pigmented and with surface jelly 3 urn thick; transition from medulla to cortex abrupt.
Tetrasporangia scattered among the surface layers of frond, ovoid or oblong in surface view, 33–36 by 16– 26 μm in diameter, ovoid to cylindrical in transverse section 20–30 μm by 13–19 μm in diameter, surrounded by slightly modified cortical cells, cruciately or occasionally irregularly tetrahedrally divided. Cystocarps prominently protruding or subcornical, 500–780 μm by 830–1000 μm in diameter, nonrostrate or slightly rostrate, nonconstricted at base; gonimoblast consisting of parenchymatous cells, the cell walls obscure and cell contents irregularly connected to each other, about 23– 82 by 13–20 μm in diameter; pericarp thin, 76– 100 μm thick, consisting of seven to eight layers of cells, of which the outermost layer is pigmented and cells roundish-cuboidal to oval, whereas the inner layers have cells that are horizontally oval-elliptical with rounded corners and obscure cell walls. Spermatangia superficial and continuous over the thallus surface.
Figure 15: Gracilaria hainanensis C. F. Chang et B. M. Xia.
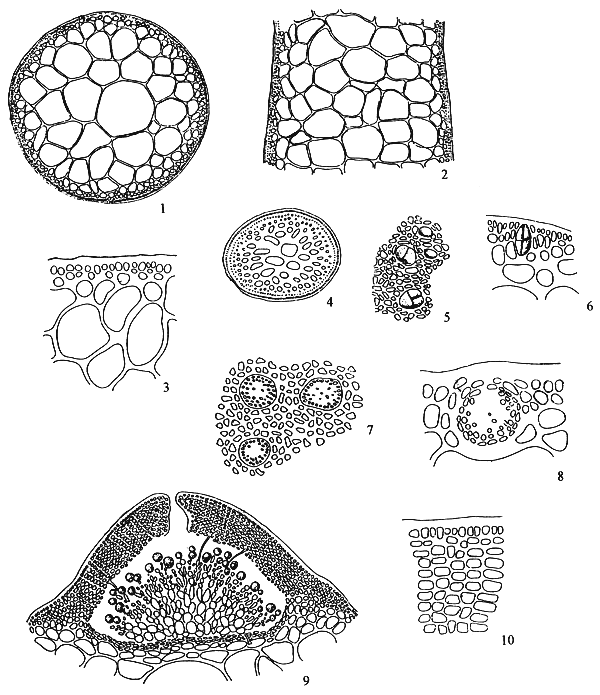
Figure 16: Gracilaria bailinae (Zhang et Xia) Zhang et Xia.
The species shows a close morphological similarity to G. bursa-pastoris (Gmelin) Silva from China, but the latter has spermatangia in shallow depressions as opposed to being borne superficially and shows large gonimoblast cells and numerous absorbing filaments in the cystocarp. According to the spermatangial configuration, G. bailinae resembles G. chorda Holmes and G. lemaneiformis (Bory) Weber-van Bosse, but differs from G. chorda on the smaller medullary cells, by thicker cell walls and the structure of the pericarp; from G. lemaneiformis by the thin cuticle, thicker cell wall, small cystocarps, smaller size of carpospores, and by the large cells of the gonimoblast filaments that have numerous secondary pit connections. Gonimoblast cells of G. lemaneiformis from the type locality (Patia, Peru) are notably small, and the gonimoblast is stalked. Some elongated plants were found in a shallow ditch where there was more water motion than in other places.
6.14. G. irregularis Abbott (Figure 17)
Thalli erect, succulent, 2.5–13.8 cm tall, percurrent axes 1–5 mm in diameter; branching mostly secund; branches always smaller than main axes, the last order of branches sometimes clustered. Fronds in transverse section consisting of medulla of large cells, 300–775 μm in diameter, cortex one to two cells thick, sometimes three to five cells; transition of cells from medulla to cortex abrupt. Tetrasporangia cruciate, 23–36 μm in diameter, tetrasporic plants with many large “gland” cells. Spermatangia in superficial layer (chorda type); male plants pale. Cystocarps conspicuous, dome-shaped, 0.6–0.8 mm high and 0.9–1.6 mm wide, not constricted at bases; gonimoblast consisting of many small cells with obscure cell walls and star-shaped contents, giving a reticulate appearance; pericarps roundish or slightly compressed, 9 to 12 rows; absorbing filament lacking; carpospores oval to rounded, 21–25 μm in diameter.
Habitat: Growing on gravel, shells, and rock fragments in sandy-muddy areas together with G. changii and G.flrma.
Remarks: Abbott (1988), described the male plants as having spermatangial conceptacles of the verrucosa type, oval to obovate, numerous, adjoining ones frequently coalescing. Lewnanomont has been unable, so far, to find any verrucosa type male plants, only the chorda type. In order to confirm the male type, gametophytic plants from tetraspores were cultured.

Figure 17: Gracilaria irregularis Abbott.
They showed the chorda type spermatangial arrangement. Gracilaria irregularis can be distinguished from other Gracilaria species in Thailand by its short and succulent axis and secund branching.
6.15 G. lemaneiformis (Bory) Weber-van Bosse (Figure 18)
Thallus solitary or caespitose, up to 46 cm tall, with few to several long branches 0.5–1.4 mm in diameter; branching irregular mostly from lower portion, branches simple, two to five branches frequently occurring from a single branch apex. Frond in transverse section consisting of medulla of large thin-wall cells, 130–260 μm in diameter, two layers of cells in cortex; transition from medulla to cortex abrupt. Tetrasporangia ovoid 15–28 × 30–50 μm. Spermatangia superficial (chorda type). Cystocarps spherical, 0.7– 0. 9 μm in diameter, slightly rostrate or nonrostrate and constricted at bases; gonimoblasts consisting of filaments of many small cells with obscure cell walls and reticulate contents; carpospores roundish to ovoid, 18–25 μm in diameter; pericarp 10–14 layers; without absorbing filaments.
Figure 18: Gracilaria lemaneiformis (Bory) Weber-Van Bosse.
Habitat: Grows on fish cages.
Remarks: Gracilaria lemaneiformis resembles G. tenuistipitata var. liui and can be separated with certainty only on the basis of spermatangial configuration. Spermatangia are superficial in G. lemaneiformis (chorda type) and in shallow saucer-like depressions in G. tenuistipitata var. liui. The latter species is grown under mariculture conditions in Taiwan and South China.
6.16. G. manilaensis Yamamoto et Trono (Figure 19)
Fronds caespitose, up to 60 cm or more tall; main axes up to 1.5 mm thick, cylindrical throughout, usually percurrent; branching alternate, sometimes secund or irregular; branches abundant, long, similar to main axis, with lateral branchlets; bases of branches and branchlets sharply constricted at their point of attachment; purplish red or sometimes greenish; fleshy to somewhat cartilaginous. Cortical layer consisting of one to two rows of densely protoplasmic cells; outermost cells 7.2–12.8 urn high, 8.8–13.6 μm wide, with primary pit connections only; hair basal cells about 16 μm high, about 15 μm wide, scattered. Medulla consisting of polygonal cells, increasing in size toward centre, up to 570 μm. Outer layer of medulla more or less compressed parallel to frond surface; transition in cell size from cortex to medulla abrupt. Cystocarps globose, up to 1000 μm in diameter, gonimoblast cells elongated, up to 23 x 71 μm; absorbing filaments present, penetrating into pericarp. Spermatangia formed in conceptacles (verrucosa type); conceptacles cup-shaped, roundish, or oval, up to 71 μm deep, up to 50 μm wide, crowded but separated by vegetative tissue. Tetrasporangia 35–40 μm high, 24–27 μm wide, regularly cruciate, surrounded by several tiers of elongated vegetative cells. Life history is typical Polysiphonia type (Yamamoto 1991). Chromosome Number: n=24.
Habitat: This species grows on muddy bottom in shallow water .
6.17. G. percurrens (Abbott) Abbott (Figure 20)
Thallus erect, with percurrent axis, 12–21 cm tall; branching alternate to irregular, branches constricted at base and broadened distally, 0.5–2.2 mm in diameter, with blunt apices. Frond in transverse section consisting of large thin walled medullary cells, 123–370 μrn in diameter, two layers of cells in cortex; transition of cells from medulla to cortex, abrupt. Tetrasporangia 15–18 × 25– 29 μm. Spermatangial conceptacles sac-like, single (verrucosa type) and in groups of two to four cavities (polycavernosa type). Cystocarps conical, rostrate, not constricted at bases, 0.5–0.8 mm high, 0.7–1. 0 mm wide; gonimoblast filaments consisting of small cells; basal absorbing filaments few, lateral and upper absorbing filaments few to many; carpospores roundish to ovoid, 13–20 μm in diameter; pericarps thick, 10–14 rows of cells.
Habitat: Growing on rock fragments in intertidal zone of turbid water.
Figure 19: Gracilaria manilaensis Yamamoto et Trono.
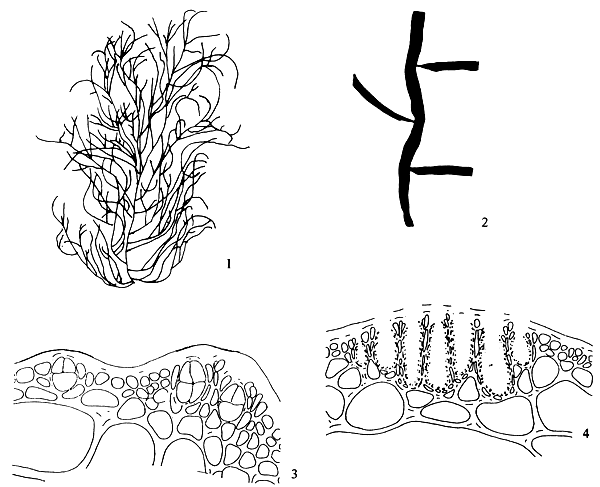
Figure 20: Gracilaria percurrens (Abbott) Abbott
6.18. G. rubra Chang et Xia (Figure 21)
Thallus erect, cylindrical, slender, 16–40 cm high, 0.5–1 mm thick, arising from a small disk-like holdfast, with 1–3 orders of remote, alternately or secundly, long attenuate branches, up to 15 cm or more in length, abruptly constricted at the base; thallus in transverse section consisting of a medulla of large parenchymatous cells, ca. 250–350 urn in diameter, with walls 3–7 μm thick, surrounded by a narrow, infracortical layer of one to two layers of roundish cells, 16.5–30 μm in diameter, and one to two layers of smaller cortical cells, pigmented, ovoid or cuboid, 7–10 μm × 6–10 μm in dimensions. Transition from medulla to cortex extremely abrupt; light purplish red in colour, membranous-cartilaginous in substance, adhering imperfectly to paper on drying.
Tetrasporangia scattered over surface of thallus, spherical of subspherical in shape as seen from surface, 26–33 μm in diameter; ovoid in transverse section, 26–30 × 20–23 μm in dimensions, surrounded by unmodified cortical cells, cruciately divided. Cystocarps scattered, prominent, protruding, globose or semiglobose, non-rostrate, unconstricted, 450–700 μm 700– 863 μm in dimensions; gonimoblast arising from a large fusion cell. Parenchymatous connected to the pericarp by nutritive filaments; carpospores about 20–33 μm diameter, with a conspicuous stellate central body; pericarp ca. 82–170 μm in thickness, consisting of 8–14 layers of cells, of which the outermost layer cells are pigmented and roundish square to oblong in shape, whereas inner layer cells are roundish, and becoming horizontally oblong in mature with obscure cell wall, cells in distinct longitudinal rows, under low power. Antheridia borne in well-separated, deep, open, conceptacular pockets, 33– 43 μm deep, 23–36 urn diameter.
Habitat: Dredged at 10 m, growing on shells.
6.19. G. salicornia (J. Agardh) Dawson (Figure 22)
Thalli prostrate to semierect, branching of prostrate from dichotomous to irregular, branches cylindrical, 3–5 mm in diameter, partly constricted; forming a rough entangled mass of various sizes with root-like discs on branch apices. In semi-erect form, segments constricted throughout, with two to four branches at each node, 10–15 cm tall; yellow to bright orange. Fronds in transverse section consisting of many layers of thin -walled cells, 150–400 μm in diameter, cortical layer of two to four cells with abundant “gland” cells; transition of cells from medulla to cortex gradual. Tetrasporangia cruciately divided, 25–30 × 37–45 μm in diameter, scattered over the surface of thallus. Spermatangial conceptacles oval (verrucosa type), single or in groups of two to three cavities. Cystocarps globose, nonrostrate, slightly constricted at bases, 0.8–2.0 μm in diameter, gonimoblasts consisting of many small cells, pericarp thick, consisting of two kinds of cells, six to eight elongate cells in outer layer, five to eight rounded cells in inner layer, absorbing filaments lateral and upper; carpospores spherical, 16–24 μm in diameter.
Habitat: Growing on various kinds of substrates, rocks, gravel, shells and mangrove roots in clear to turbid water. Specimens growing in clear water are always orange, those growing in muddy areas are dark brown.
Figure 21: Gracilaria rubra C. F. Chang et B. M. Xia.
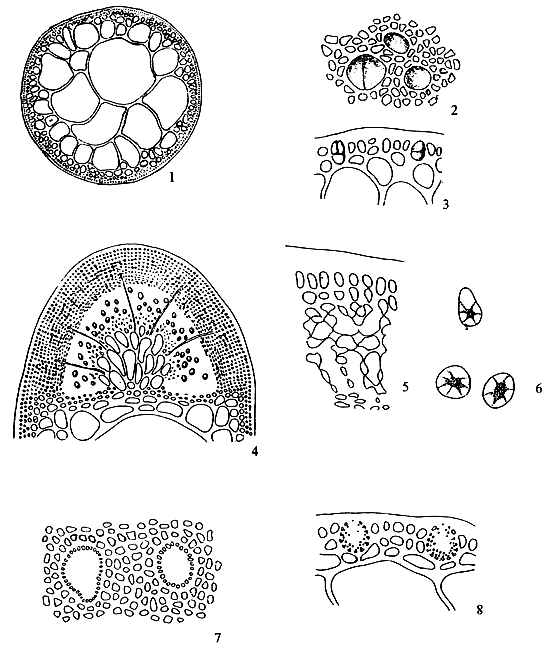
Figure 22: Gracilaria salicornia (Ag.) Dawson.
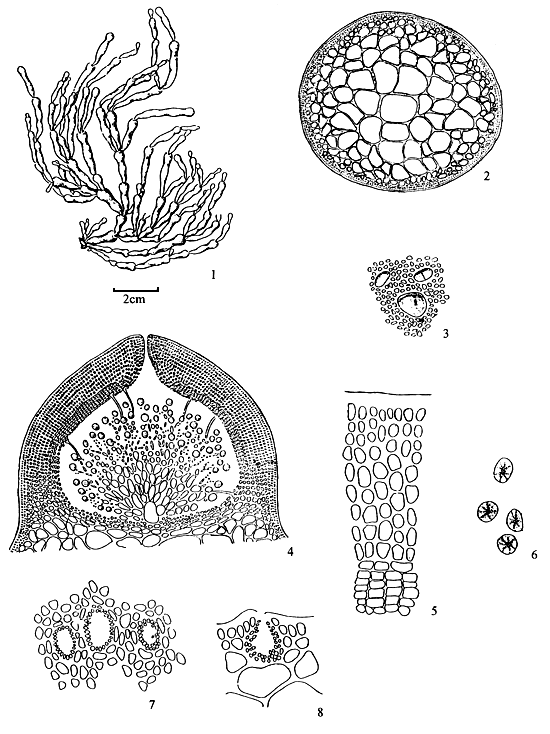
6.20. G. sullivanii Yamamoto et Trono (Figure 23)
Fronds prostrate, attached to substrate by hapters originating from ventral side of the fronds, up to 10 cm long. Main axes compressed, up to 5 mm wide and 3 mm thick; branching dichotomously in opposite manner upper portion of branches almost cylindrical, branch tips blunt; reddish brown; cartilaginous. Cortical layer consisting of one to two rows of densely protoplasmic cells; outermost cells 5.6–16 urn high , 4–8 μm wide, with primary pit connections only; hair basal cells about 20 urn high, about 18 μm wide, in groups of 20– 40 cells. Medulla consisting of polygonal cells, increasing in size towards the centre, reaching up to 410 μm. Outer layer of medulla more or less compressed parallel to frond surface; transition in cell size from cortex to medulla abrupt. Tetrasporangia formed in upper half portion of nemathecium-like elevation on dorsal side of the frond, regularly cruciate, 44.5–50.5 μm high, 20–26.3 μm wide, surrounded by rows of elongated vegetative cells; nemathecium-like structure consisting of layers of up to eight cells, up to 140 μm thick. Cystocarps and spematangia unknown.
Habitat: This species grows prostrate on rock at a depth of 2–3 m at the edge of coral reef.
Remarks: This species is characterised by regular dichotomous branching and prostrate habit. The several layers of elongated vegetative cells that surround the tetrasporangia form prominent and distinctive nemathecium-like elevated structures not present in other Gracilaria species. These features appear to be distinctive enough for separating this species from other taxa, notwithstanding the absence of cystocarps and male reproductive organs.
6.21 G. tenuistipitata var. tenuistipitata Chang et Xia (Figure 24)
Thallus from a small disk-like holdfast arising to a height of 20–40 cm, rarely up to 1 m, simple or moderately alternately branched near the base, the branches becoming like the main axis, terete throughout, very slender near the base of the plant, above expanding to 0.5–1.5 mm diameter, 1–2 times branched; structurally showing a broad medulla of large thick walled cells 225–390 urn, with walls, 13–16 μm thick, in the centre, toward the surface considerably smaller, the cortex of 1–2 layers of rounded cells 10–20 μm diameter, with cuticle 10–13 urn thick; fleshy red in colour, cartilaginous in substance, adhering rather well to paper on drying.
Tetrasporangia scattered among the surface layer of frond, round in shape in surface view, 30–33 μm in diameter, ovoid or oblong in transverse section, 30–46 μm × 18–30 μm in dimensions, surrounded by somewhat modified cortical cells, cruciately divided. Cystocarps prominently protruding, up to 830–950 μm high, globose, rostrate, constricted at base; gonimoblast composed of large parenchymatous cells, connected with the pericarp by very rare nutritive filaments; carpospores round or ovoid in shape, 33– 49 μm in diameter; pericarp ca. 72–102 μm in thickness, consisting of 8–11 layers of cells, of which the outer cells of 1–2 layers are elliptical to roundish in shape with obscured cell wall, while the innermost few layers of cells are roundish to horizontally oval with distinct cell wall, cells not in distinct longitudinal series under low power. Antheridia are scattered over the surface of the blade in small, shallow, well-defined depressions 10–23 μm diameter and separated by modified, elongated cortical cells.
Habitat: Grows on gravel and shells in the sublittoral region of lower salinity.
Figure 23: Gracilaria sullivanii Yamamoto et Trono.
Figure 24: Gracilaria tenuistipitata var. tenuistipitata Chang et Xia.
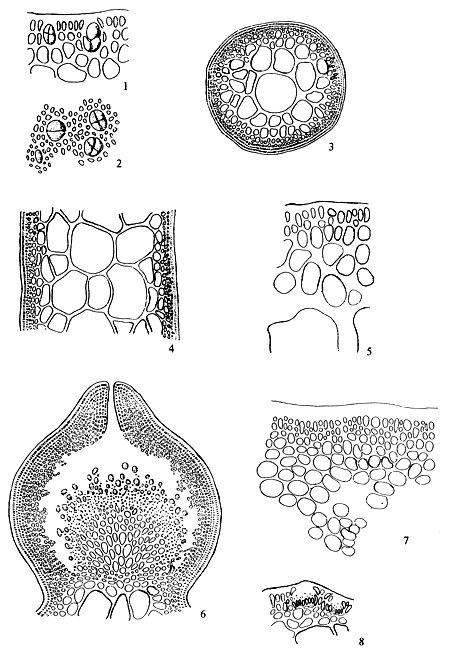
6.22. G. tenuistipitata var. liui Zhang et Xia (Figure 25)
Differing from the variety G. tenuistipitata by the slender thalli bearing numerous, delicate, short to long flagelliform lateral branchlets, frequently only 0.25 mm in diameter. Branching mostly from percurrent axes. Cystocarpic structure and shallow textorii-type spermatangia similar to variety G. tenuistipitata. This new variety frequently occurs naturally in fish ponds and shallow intertidal areas on muddy substrate. When cultivated, as in Hepu, Guangxi Province and in Taiwan (as G. verrucosa of Chiang 1981 and Shang 1976), the thalli are usually detached and without holdfasts. They may then tumble about in fairly large masses.
6.23 G. textorii (Suringar) De Toni
Thallus foliose, attached below by a small discoid holdfast, 4.2–13.2 cm tall and 6. 1–18. 8 cm wide; blade branched dichotomously or sub-dichotomously in one plant, segments 0.7–2.0 cm wide, membranous when dried, with entire margins or proliferous, apices blunt, bifurcate; dark red to greenish red. Frond in transverse section 147–185 μm thick, consisting of a few layers of medullary cells, transition of cells from medulla to cortex abrupt. Gametophytic plants, both male and female, smaller than tetrasporic plants; male plants very rare, tetrasporic plants abundant. Tetrasporangia ovate, scattered on both surfaces 16–25 × 23–27 μm, surrounded by elongate cortical cells. Male frond pale, spermatangia in shallow or saucer-like depressions (textorii type). Cystocarps large and prominent. Semiglobose to globose, 0.7–1.0 mm in diameter, slightly rostrate, not constricted at bases; gonimoblast cells small, numerous, almost occupying the cystocarpic cavity; absorbing filaments upper and lateral.
Habitat: Grows on rocks and fish cages.
6.24. G. verrucosa (Hudson) Papenfuss (Figure 26)
Cylindrical thalli, extremely variable in size, colour and habitat. Branches gradually attenuating to blunt apex, constrictions taking place at the base of the branches. Fronds 1–2.5 mm in diameter (-3 mm or more), usually 20–50 mm in length. In transversal section the medulla cells connected with each other by numerous pits and composed of 4–5 layers of large roundish cells, 133–240 μm in diameter, bordered by 2– 3 layers of much smaller and thinner cortical cells. The outermost cortical cells elongated more or less anticlinally, measuring 6–8 μm × 10–17 μm. Cystocarps hemispherical, globose, ostiolate scattered over the whole frond. Carpospores ovates or roundish, distributed radially, 13–47 μm thick, 23–67 high. tetrasporangia ovoid or oblong, 14–42 μm × 45–65 μm in dimensions, cruciate, densely scattered on the surface of frond, surrounded by slightly modified cortical cells, Antheridia, in the bottom of a rather shallow conceptacle, 15–51 μm diameter, 18–43 μm depth, usually well separated by unmodified cortical cells. They occur with different densities according to the surface of the frond. Small globular antherozoids, 3–5 μm diameter.
This species has been studied and reported in detail from many locations throughout the world. Originally, this species was believed to be cosmopolitan. As early as 1976, Zhang and Xia suspected the cosmopolitan distribution of the English species and found the structure of the pericarp to be quite constant with the species. As they expected, the Chinese species does differ specifically from the English Gracilaria verrucosa (Huds.) Papenfuss. Meanwhile they also found that the “Gracilaria verrucosa” of Japan, at least the specimen collected by H. Yamamoto from Hokkaido, belonged to the same species as the Chinese species, now described as Gracilaria asiatica Zhang and Xia. The asiatica species may be readily distinguished from the Gracilaria verrucosa from Wemburg, Devon, England by the deeper spermatangia conceptacles, by the larger tetraspores, by the smaller carpospores and by the structure of pericarp, as already comparatively studied by Zhang and Xia.
Figure 25: Gracilaria tenuistipitata var. liui Zhang et Xia.
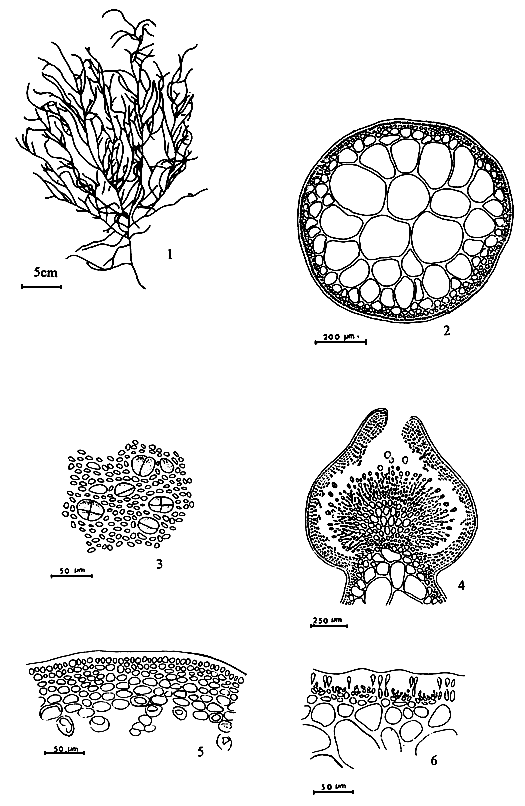
Figure 26: Gracilaria verrucosa (Hudson) Papenfuss.
Habit of a cystocarpic plant.
6.25 G. yamamotoi Zhang et Xia (Figure 27)
Plants 3–7 cm tall, foliose, dark purplish red, attached below by a discoid holdfast, with a short and slender subterete stipe 2–6 mm long; blade 1–3 mm wide, irregularly dichotomously branched in one plane, thick coriaceous to cartilaginous, with entire margins and attenuated apices; branches of one to two (up to three) orders; never adhering to paper on drying. Frond in transverse section consisting of a medulla of large, thin-walled cells, 99–106 μm in diameter, and one to two layers of small pigmented cortical cells, 7–10 μm × 3–7 μm; transection 310–320 μm thick.
Spermatangia (textorii-type) in individual to confluent saucer-like depressions, 30–33 μm deep, surrounded by modified cortical cells, distributed over thallus surface. Cystocarp nearly globose, 1.2–1.5 mm in diameter, prominently protruding, non-rostrate to only slightly rostrate, unconstricted at the base; gonimoblast consisting of many small cells, 20– 36 μm × 10–17 μm, with several cells toward the apex of each filament developing into carposporangia; carpospores roundish, ovoid or oblong, 40–53 μm in diameter, traversing filaments absent; pericarp 264–304 μm thick, consisting of two kinds of tissue, the outer of six to seven rows of smaller dense, oblong shaped contents, the inner 10–11 rows of large, loose, horizontally oblong cells.
Remarks: This species is similar to slender forms of G. textorii (Suringar) De Toni in external appearance. It is distinguishable, however, by the lack of traversing filaments in the cystocarp, by the small gonimoblast cells, and by two kinds of cell layers in the pericarp. In G. textorii, the traversing filaments are conspicuous, and the pericarps are constricted of only one kind of cells.
7. CULTIVATION
7.1 Selective criteria of species for culture
The species targeted for culture should have the following properties:
It must be a fast growing species which can be propagated utilising vegetative propagules or spores. Species with large and robust thalli are preferred because these can produce large amounts of biomass within relatively short cropping periods.
It must have a high, good quality, agar content.
The above considerations require that the available species be screened. Comparative studies on their productivity should be conducted to determine the species to be selected. Because the properties of agar differ among species it is terribly necessary that the correct name be applied. Thus, the taxonomy of the species should be clarified. The names are indices of the kind and quality of agar the Gracilaria contains and are used as basis for determining the price.
7.2 Selection of culture sites
The selection of sites is a very important factor to consider in pond and field culture because this could be the primary factor determining success or failure of the farming venture. In general, areas that support natural stocks of species to be cultured are good sites. If culture is to be done in an area where no stock of the species exists, then one should select sites which have comparable ecological conditions as the sites where the stocks are found. This will entail the gathering of data on various parameters such as salinity ranges, nutrient levels, turbidity, water temperature and type of substrate, among which temperature and salinity are most important.
Figure 27: Gracilaria yamamotoi Zhang et Xia.
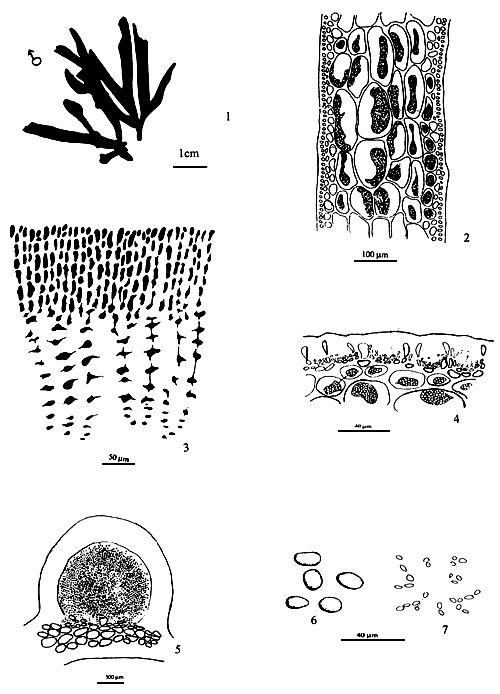
7.3 Production of seeds and sporelings
The term “seed” here refers to both the vegetative propagules and spores which can be utilised as planting materials. The sporelings are specially used as the young sporophytes and gametophytes, which are from carpospores or tetraspores, respectively.
The vegetative propagules are to select healthy thalli, which are fleshy and elastic in texture, dark reddish brown colour, robust well branched with smooth and shiny surfaces. In addition, these must be clear, free from dirt and epiphytes. The production of sporelings have two methods: natural spore-recruitment and induced spore-shedding in hatcheries.
Natural spore recruitment. The use of spores as seeding materials for the production of sporelings for culture necessitates the availability of natural stocks. In the natural spore recruitment methods, artificial substrates such as ropes, rocks, shells and netting materials are used. Ropes and netting materials are generally preferred. These are left in the area for about two weeks to allow the naturally shed spores to settle on them. The sporeling developing from the spores visible after 3 to 4 weeks. The seeded material are then transferred to the culture sites for outgrowing.
Hatchery production of sporeling from spores. The production of sporelings in hatcheries is quite similar to those in the seedling facilities for Porphyra and Laminaria, although less sophisticated. It consists of a seeding tank with provision for control of water depth with an adjustable seeding material support structure. This structure consists of a wooden frame with monofilament netting material which can fit into the seeding tank. It is provided with mechanisms, so that its height from the bottom of the tank can be adjusted.
Farmers collecting spores, either naturally or artificially, must possess knowledge of the reproductive habits and ecological properties of the culture species. A brief introduction to the biology of G. asiatica and G. tenuistipitata is given below.
7.4 Biology of Gracilaria
Gracilaria asiatica is the most common species in China. It grows rapidly and has a high agar content. A discussion on the influence of various environmental factors on the release and germination of spores growth of thallus of the spores is given below.
Influence of environmental factors on spore release
Both carpospores and tetraspores are released naturally into seawater after their maturation. Experiments have shown that the maximum quantity of spores is released at 8–10 am., gradually decreasing thereafter. The minimum quantity is released at 10 pm. to 6 am. the following day, after which the next maximum will take place. Besides the diurnal changes of releasing rhythmicity, the released quantity of spores also depends on ambient environmental factors.
Desiccation
In general, a mature Gracilaria is taken out of seawater and kept in a shady spot for 2 to 4 hours (air temperature 15–25 °C), the tetraspores or the carpospores will be released if the plant is dipped in seawater again. Table 1 gives the different quantities of tetraspores or carpospores released at different durations of desiccation, or removal from the seawater.
Table 1: The relationship between the quantity of tetraspores and carpospores released
| Time (hr.) | 0.5 | 1 | 2 | 3 | 4 | 5 | 6 |
| Tetraspores | 887 | 1011 | 3033 | 1921 | 1940 | 1904 | 782 |
| Carpospores | 201 | 226 | 686 | 683 | 458 | 477 | 421 |
Source: Zheng, 1987.
Seawater temperature
Experiments have shown that the highest number of spores are released at 20–25 °C (Table 2). According to field surveys, the reproductive season of Gracilaria is between June to August in northern coastal provinces or between March to May in southern China. During these seasons, the natural seawater temperature is 20–25 °C. The experimental results coincide with the natural reproductive season (Zheng, 1987).
Table 2: The relationship between released quantity and seawater temperature.
| Temperature (°C) | 8–10 | 12–15 | 20–22 | 25 |
| Tetraspore | 88 | 518 | 2295 | 1525 |
| Carpospore | 522 | 998 | 1343 | 824 |
Source: Zheng, 1987
Specific gravity of seawater
When mature plants were kept in seawater of different specific gravity, those in seawater of lower specific gravity release spores earlier than those kept in water of higher specific gravity. If the plants are put in seawater with an obviously low specific gravity (< 1.005), the released spores will swell and even break up due to osmosis caused by the low salinity, so that most of them would not complete normal germination. In order to obtain good results in the collection of spores, an optimum specific gravity range of 1.015 (19.0–20.2 ppt) to 1.020 (25. 5– 26. 9 ppt) should be observed (Zheng, 1987).
Influence of environmental factors on germination and growth of the spores
Gracilaria spores just released into seawater, measure around 30 μm in diameter and vary with species. Tetraspores are slightly bigger than carpospores. For example, the tetraspores of G. tenuistipitata have a diameter 24–56 μm, and carpospores 23–40 μm. Soon after release, the spores will attach to substrates and start first cleavage of cells. The germination and growth of spores are also influenced by ambient environmental conditions such as seawater temperature, light intensity, salinity, etc. (Zheng, 1987).
Seawater temperature
To examine the germination and the growth of the spores those attached to slides for 12 hrs were moved to containers with sterilised seawater enriched with nutrients and incubated with a light intensity of 400 lux, photoperiod of 10:14 (D: L) for different periods of time. The results are shown in Tables 3 and 4.
Table 3: The effect of temperature on carpospore disc size (μm) after germination.
| Time | Temperature (°C) | ||||
| (days) | 7 | 10 | 15 | 20 | 28 |
| 10 | 39.6×39.6 | 42.9×42.9 | 46.2×46.2 | 46.3×46.3 | 42.9×42.9 |
| 20 | 39.6×39.6 | 45.9×45.9 | 52.8×52.8 | 59.4×59.4 | 49.5×42.5 |
| 30 | 46.2×46.2 | 59.4×59.4 | 59.4×59.4 | 56.1×56.1 | 56.1×56.1 |
| 40 | 56.9×49.5 | 62.7×59.0 | 69.3×62.7 | 75.3×75.9 | 72.6×59.4 |
| 50 | 56.9×49.5 | 66.0×59.4 | 66.0×66.0 | 95.7×82.5 | 99.0×82.5 |
Table 4: The effect of temperature on tetraspores disc size ((μm) after germination.
| Time | Temperature (°C) | ||||
| (days) | 7 | 10 | 15 | 20 | 28 |
| 10 | 39.6×39.6 | 36.3×36.3 | 39.6×39.6 | 36.0×36.0 | 39.1×39.1 |
| 20 | 39.6×39.6 | 42.9×42.9 | 49.5×49.5 | 42.9×42.9 | 42.9×42.9 |
| 30 | 46.2×46.7 | 62.7×62.7 | 62.8×62.8 | 52.8×52.8 | 52.8×52.8 |
| 40 | 52.8×49.8 | 59.3×62.7 | 75.9×61.9 | 75.9×66.0 | 75.9×66.0 |
| 50 | 49.5×42.9 | 66.0×59.0 | 95.7×82.5 | 95.9×85.0 | 99.0×82.5 |
The results illustrate that both tetraspore discs and carposore discs need a seawater temperature higher than 15 °C for their growth. If the temperature is below 15 °C, even if all other environmental conditions are suitable for their growth, they would survive but grow very slowly.
Light intensity
Light intensity is one of the most important factors influencing the germination and growth of spores. When the light intensity is stronger, the rate of germination and growth of spores are higher within 3000 Lux. If the spores attached to slides are kept in a dark place they will die in 20 days. The experimental results are shown in Table 5.
Table 5: The effect of light intensity on the spore disc growth (μm) after germination.
| Light intensity | Time (days) | |||
| (Lux) | 10 | 15 | 20 | 25 |
| 3,000 | 69 | 78 | 93 | 112 |
| 1500 | 50 | 63 | 85 | 104 |
| 200 | 40 | 48 | 60 | 76 |
| Dark | poor | poor | died | -- |
Specific gravity of seawater
Although Gracilaria plants prefer to inhabit estuarine areas, their spores are unable to withstand seawater with too low salinity. If the spores attached to substrate are in seawater with a specific gravity below 1.010 (12.5–13.7 ppt) their cells swell up due to the absorption of water into the cells from ambient environment. The colour of the pigments in the cells would change from red to pale, and the spores will die eventually. Experiments proved that the optimum specific gravity of seawater for germination and growth of spores ranges from 1.018 (23.0–24.2 ppt) to 1.025 (25.5–26.9 ppt).
The effect of environmental factors on growth of Gracilaria thalli
The effects of environmental factors on Gracilaria thalli are similar to that on their spores, but not entirely the same. For instance, as mentioned above, spores kept in seawater with a specific gravity below 1.010 will break up and die, but the thalli can grow very well in the same conditions. In view of this, ambient environmental factors required by Gracilaria thalli must be studied.
Specific gravity of seawater
Gracilaria is a group of euryhaline seaweeds. Under natural conditions, the specific gravity in which the plant can grow out ranges from 1.005 to 1.026 (5.2–38.1 ppt). Experiments and field surveys have shown that the optimum specific gravity is 1.010–1.020 (11.3–30.1 ppt) where fresh water regularly flows in.
Seawater temperature
Gracilaria is also an eurythermal plant which can grow at 5 to 30 °C. Optimum temperature varies with species. For example, the optimum temperature of G. asiatica is 15–25 °C. It can be found during May to August in northern China, but in south China only in winter and spring seasons. In summer, the growth of Gracilaria is almost completely stopped in the south until late autumn when the water temperature drops below 25 °C and the seaweeds will resuscitate again.
Field surveys have shown that the optimum seawater temperature for G. tenuistipitata, a species mainly distributed in Guangdong and Hainan Island, is identical with G. asiatica. When the water temperature is 30 °C, its diurnal growth rate measures 0.1–0.2 cm/day but as the temperature falls to 28 °C, the growth rate increases to 0.4–0. 5 cm/day. When the temperature is from 15 to 25 oC, growth rates can be higher than 1 cm / day.
7.5 Farming
The farming of Gracilaria seaweeds is not as complicated as that of Porphyra, Laminaria and Undaria. Generally, three methods have been adopted in the world: intertidal culture, floating culture, and pond culture.
Intertidal culture
The main feature of this culture method is that the spore collection and farming are usually the same. In southern China , in late autumn or early winter, young Gracilaria can grow up to 5–6 cm as they enter a period of fast growth rates. At this stage, the substrate to which young Gracilaria are attached are kept in lines on the bottom at an interval of 30–40 cm which will serve as a walk way. The routine management practices involves removal of miscellaneous seaweeds, collection of herbivorous gastropods and so on. Women and children often do it. In a normal season, 1500 kg (dry weight) per ha of Gracilaria plants can be harvested.
Floating culture
The method has been adopted from kelp farming. In a suitable season, such as January in southern China or May in the northern part, young seaweeds, either collected from fields or reared in hatchery, are pulled up from substrate and inserted into ropes called “sporeling rope” which are made of palm thread or artificial fibres. In each rope of 20 m length, 200 pieces of seaweeds 10 cm apart can be inserted. The sporeling ropes are fixed on floating raft. Three months later, the seaweeds will reach a length of over 1 m and can be harvested. Yields can reach about 3,000 kg (dry) per ha. This culture method is suited to the culture of G. asiatica and G. lemaneiformis.
Pond culture
Pond culture of seaweeds has been adopted by Chinese phycologists and farmers since 1960s. The stock seaweeeds are cut into pieces and spread in the fish pond at a density of 5–6 tonnes (fresh thalli) per ha. Chemical fertiliser or pig manure is applied regularly. Every 30–45 days, most biomass of the seaweeds can be harvested. The rest is continually left in the ponds as stock. The harvesting period lasts six months from June to November in northern China and in southern parts, like Hainan Island, almost all year. It is used mainly to culture G. tenuistipitata, especially for the variety G. tenuistipitata var. liui, as it is hard to find their reproductive thalli, both tetrasporphytes and carposporophytes. In the lagoon area of Vietnam (Hue province), the method has been used successfully and it is believed that the method could easily be extended throughout the region, particularly in the south-east and south Asia.
REFERENCES
Abbott, I. A. and Norris J. N. 1984. Taxonomy of economic seaweeds with reference to some Pacific and Caribbean species. Vol. I p. 67–114. California Sea Grant College, La Jolla.
Abbott, I. A. 1986. Taxonomy of economic seaweeds with reference to some Pacific and Caribbean species Vol. II, p. 127–156. California Sea Grant College, La Jolla.
Abbott, I. A, 1989. Taxonomy of economic seaweeds with reference to some Pacific and Caribbean species. Vol. III, p 193–231. California Sea Grant College, La Jolla.
Abbott, I. A, 1991. Taxonomy of economic seaweeds with reference to some Pacific and Caribbean species Vol. IV, p. 81–148. California Sea Grant College, La Jolla.
Abbott, I. A. 1991. New records and a re-assessment of Gracilaria (Rhodophyta) from the Philippines. Taxonomy of economic seaweeds. Vol. IV. p. 111–118. California Sea Grant College, La Jolla.
Abbott, I. A., Zhang J and Xia. B., 1991. Gracilaria mixta Nov. and other western Pacific species of the Genus. Pac. Sci.45(l):12–27.
Agardh, J. G., 1852. Species genera er Ordines Algaram. Volume 2. pp 337–720. Gleerup, Lund.
Bird, C. J., van der Meer, J. P. and McLachlan, J. 1982. A comment on Gracilaria verrucosa (Huds.) Papenf. ( Rhodophyta: Gigartinales).Journal of the Marine Biological Association of the U.K 62: 453–459.
Chang, C. F. and Xia, B. M., 1963. Polycavernosa, a new genus in the Gracilariaceae. Stud. Mar. Sinica. 3: 119–126.
Chang, C. F. and Xia, B. M. 1976. Studies of Chinese species of Gracilaria, Studia Marina Sinica, Vol. 11, P. 91–163
Chen, J. X. 1990. Gracilaria culture in China. Report on the Regional Workshop on the Culture and Utilization of Seaweeds. Vol. II, Network of Aquaculture Centres in Asia-Pacific, Bangkok, Thailand.
Chen, M. Q. and Ren, G. Z.,1985. The development process of sporelings of Gracilaria verrucosa (Hudson) Papenfuss. Oeanologia et Limnologia Sinica, Vol. 16, No. 3 p. 181–187.
Chiang, Y. M., 1981. Cultivation of Gracilaria (Rhodophycophyta, Gigartinales) in Taiwan, Proc. Int. Seaweed Symp., 10:569–574.
Chirapart, A., Ohno, M., and Yamamoto, H. 1991. Occurrence of a different Gracilaria in Japan. In: Norris, 1991 (ed). Taxonomy of economic seaweeds. Vol. IV. P. 119–124. California Sea Grant College, La Jolla.
Cotton, A. D., 1915. Some Chinese marine algae. Bull. Misc. Inform., Royal Botanical Gardens, Kew. 3 (15): 1–195, pls. 1–6.
Dawson, E. Y., 1949. Studies on the north-east Pacific gracilariaceae. Allan Hancock Foundation. Publs. Occ. Pap. 7:1–105, pls. 1–25.
Dinh, N. H. 1989. Vietnamese species of Gracilaria and Gracilariopsis. Taxonomy of economic seaweeds. Vol. III. P.207–210. California Sea Grant College, La Jolla.
Dong, H. K. 1970. Economically important seaweeds in Chile- Gracilaria, Botanica Marina, Vol. XIII, p. 140–162.
Doty, M. S. and Santos, G. A. 1983. Agar from Gracilaria cylindrica. Aquatic Botany 15:299–306.
Gepp, E. S. 1904. Chinese marine algae. Jour. Bot. 42: 161–165, pls. 460.
Greville, R. K. 1830. Algae Bruttabucae. 218 pp.19 pls. Published by the author, Edinburgh.
Harvey, W. H., 1849. Phycologia Britannica. London. Vol II, pls.121–240.
Howe, M. A. 1924. Chinese marine algae. Bull. Torr. Bot. Club. 38:489–514, pls. 27–34.
Lewmanomont, K. 1991. The Species of Gracilaria from Thailand. In: Norris, 1991 (ed). Taxonomy of Economic Seaweeds. Vol. IV. P. 135–148. California Sea Grant College, La Jolla.
Liu, S. J. 1987. Distribution, present production status and future of Gracilaria in Guangdong Province. Sci. and Tech. of Fisheries, 1:14–15.
Martens, G. V. 1866. Die Tange. Die Preussische Expedition nach Ost-Asien. Bot. Theil. pp 1–152. Pls. I–VIII.
Ohmi, H., 1958. The species of Gracilaria and Gracilariopsis from Japan and adjacent waters. Mem. Fac. Fish., Hokkaido Univ. 6: 1–66.
Papenfuss, G. F. 1966. Notes on algal nomenclature. V. Various Chlorophyceae and Rhodophyceae. Phykos 5, 95–105.
Phang, S. M. 1991. Some Species of Gracilaria from Peninsular Malaysia and Singapore. In: Norris, 1991 (ed). Taxonomy of Economic Seaweeds. Vol. IV. P. 125–134. California Sea Grant College, La Jolla.
Phang, S. M. and Maheswary, V. 1990. Phycocolloid content of some Malaysian seaweeds. In: Proceedings of the Twelfth Annual Seminar, Malaysian Society of Marine Sciences, pp 65–78.
Phang, S. M. and Wee, Y. C. 1991. Benthic marine algae. In: Kiew, R. (ed.) The state of nature conservation in Malaysia, pp 51–61.
Schneider, C. W. & R, B, Searles, 1973. North Carolina marine algae. II. New records and observations of the benthic offshore flora. Phycologia 12: 201–211.
Shang, S. J. 1976. Economic aspects of Gracilaria culture in Taiwan. Aquaculture 8:1–7.
Sjostedt, L. G. 1926. Floridean studies. Lunds University, Arsskr, N. F., Avd.2,22 (4): 1–94.
Taylor, W. R., 1960. Marine algae of the eastern tropical and subtropical coasts of the Americas. University of Michigan Press, Ann Arbor, 870 pp.
Trono, G. C. Jr. 1990. A review report of the production technologies of tropical species of economic seaweeds. Report on the Regional Workshop on the Culture and Utilization of Seaweeds Vol. II. Network of Aquaculture Centres in Asia-Pacific, Bangkok, Thailand.
Tseng, C. K. and Li, L. C. 1935. Some marine algae form Tsingtao and Chefoo, Shantung. Bull. Fan. Mem. Inst. Biol. (Bot) 6 (4): 183–235.
Xia, B. M. and Abbott, I. A. 1987. New species of Polycarvernosa Chang and Xia (Gracilariaceae, rhodophyta) from the western Pacific, Phycologia. Vol. 26 (4), 405–418.
Xia, B. M. 1986. On Gracilaria salicornia (C. Agardh) Dawson. Chin. J. Ocenaol. Limnol. 4: 100–106, 1pl.
Yamamoto, H., 1978. Systematic and anatomical study of the genus Gracilaria in Japan. Mem. Fac. Fish. Hokkaido Univ. 25: 97–152.
Yamamoto H. and Trono, G. C. Jr. 1991. Two new species of Gracilaria from the Philippines. In: Norris, 1991 (ed). Taxonomy of Economic Seaweeds. Vol. IV. P.95–102. California Sea Grant College, La Jolla.
Yang, S. S., Eang, C. Y. and Wang, H. H. 1981. Seasonal variation of agar produced in Taiwan area. Proc. Int. Seaweed Symp., 10:737–742.
Zhang, J. F. and Xia B. M. 1984. Some problems in the Chinese species of Gracilaria (Rhodophyta), Hydrobiologia 116/117, P.59–62.
Zhang, J. F. and Xia, B. M. 1985. On Gracilaria asiatica and Gracilaria verrucosa (Huds.) Papenfuss, Oceanologia et Limnologia Sinica, Vol. 16 (3) P. 175–180.
Zhang, J. F. and Xia, B. M. 1986. On two new Gracilaria (Gigartinales, Rhodophyta) from South China. In: Norris, 1986 (ed). Taxonomy of Economic Seaweeds. Vol. II. p. 131–136. California Sea Grant College, La Jolla.
Zhang, J. F. and Xia, B. M, 1989. Studies on two new Gracilaria from south China and summary of Gracilaria species in China, In: Norris, 1989 (ed). Taxonomy of economic seaweeds. Vol. III. P. 195–206. California Sea Grant College, La Jolla.
Zhang, J. F. and Xia, B. M., 1991, Three foliose species of Gracilaria from China. In: Norris, 1991 (ed.) Taxonomy of economic seaweeds, Vol. IV. P. 103–110. California Sea Grant College, La Jolla.
Zheng, C. K., Wang, S. J. and Liu, S. J., 1987. Phycoculture. Shanghai House of Science and Technology Publication. p. 225–254.