C.B. COWEY
The method most commonly employed to determine protein requirements is that of dose-response curve.
This method consists in including in a basal diet all essential nutrients with the exception of the one under investigation. This nutrient is then added in stepwise amounts to other portions of the basal diet to enable a graded intake be given. The response measured is some index of growth (weight gain, length increase, etc…). generally a dose-response curve for a nutrient, which the animal cannot synthesize itself from ordinarily available materials, will slowly increase up to a plateau stage and then remain at that level or even decrease when the requirement level has been reached.
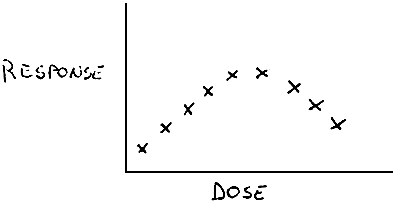
When this happens, at the intersection of the two lines, the apparent requirement for the nutrient will appear.
With protein the test diet must include all the nutrients with the exception of the one under investigation. If we increase the level of protein, we also increase the content of energy. But, when we are doing a dose-response curve,we have to ensure that the diet with low protein content has the same energy level as the diet with high protein content. One of the most common methods employed to solve this problem is to substitute carbohydrates for proteins. This assumes that the energy furnished by carbohydrates is the same that of protein. But normally a completely digested protein has an energy value of perhaps 4. 5 Kcal/gr. while for a well digested carbohydrate it has an energy value of 4 Kcal/gr. The problem arising is that if we increase the carbohydrate content of the diet we lower the extent to which it is utilized. Another important question is that protein requirement may change during the life of the fish. Evidence was presented to illustrate this. The protein requirements of some fish, which were determined essentially at the juveniles stage are shown below. In fact in a laboratory, people normally use fry or juveniles for experiments and requirements will decrease in size. All the values shown are high with the exception of that for channel catfish. A possible explanation is that if fish or any animals are given the choice of substrates for use as energy source (either proteins or carbohydrates), the animal will preferentially use the protein as energy source rather than carbohydrates. So that in doing a dose-response curve with a higher level of protein intake, you tend to increase the use of protein as a source of energy.
PROTEIN REQUIREMENTS OF DIFFERENT SPECIES OF FISH
| SPECIES | g/kg | Ref. | |
| Rainbow trout | 400–460 | SATIA, 1974; TIEWS et al., 1976 | |
| Common carp | 380 | OGINO and SAITO, 1970 | |
| Chinook salmon | 400 | DE LONG et al., 1958 | |
| Japanese eel | 445 | NOSE and ARAI, 1972 | |
| Gilthead sea-bream | 400 | SABAUT et LUQUET, 1973 | |
| GRASS carp | 410–430 | DABROWSKI, 1977 | |
| Channel catfish | 220–320 | GARLING and WILSON, 1976 | |
| Tilapia zilli | 350–400 | TESHIMA et., 1978 |
Fig.1 shows another experiment on channel catfish. There are four groups of fish of 2 different diets, one with 250 g. of protein/kg and the other with 350 g/kg. At the end of the experiment, there was a significant difference was noted for larger fish. This seems a reasonable demonstration that smaller fish need more protein than larger ones.
Another claim that was made was that the water temperature affected the requirement for protein. With chinook salmon, the dose-response curve has been interpreted as showing a differences in protein requirements between 8° and 15° C, but the curve can be interpreted in other ways.
Concerning salinity an experiment was carried out with rainbow trout at two different levels of salinity: 10 ppt and 20 ppt. 10 ppt corresponds to about 1/3 of the sea water salinity and it is probably isotonic with the tissue of fish. With 20 ppt fish have to osmoregulate to some extent to get rid of the excess salt. The protein requirement is around 40 % at 10 ppt while at 20 ppt it is 45 %. A possible conclusion is that salinity can affect the protein requirement of the fish capable of living at these salinities.
AMINO-ACID REQUIRTEMENT
The essential amino-acids (EAA) are those that fish cannot synthetize by themselves from ordinarily available materials. They number ten in all.
The main way of determining these nutrients is by the dose-response curve. Preparation of test diets presents some problems. For example, if we have to carry out a dose-response curve for tryptophan and the fish require 40 % protein, we can give this amount by putting 20 % protein in the diet, this will fix the minimum level of tryptophan to make up the protein content of the diet. Then we prepare diets containing gradually increasing levels of tryptophan. The use of this type of diet entails an important assumption: the free amino-acids are used equally as well as proteins. This means that a free amino-acid mixture which has the same composition as a protein gives the same rate of growth. This assumption as we shall see later on, is questionable.
Another important point is that deficiencies in some nutrients, like vitamins cause high morality rates, in fish. Often amino-acid deficiencies show very few pathological signs and mortality rates are low. Growth is very poor. One of the few amino-acids which does whow pathological symptoms in deficiency is tryptophan. In rainbow trout deficiency of tryptiphan causes scoliosis, lordosis and renal calcinosis.
Up to present, we have concentrated on weight gain as the response that we can measure and obviously we will be more confident in our results if we have more than one way of determining the requirements. Another method is to inject fish with 14C tryptophan, put the fish into a chamber and measure radioactivity in the expired carbon dioxide. What we can see is that at low level of tryptophan intake a small amount of the radioactivity in the tryptophan is expired in CO2. In other words, at low level, tryptophan intake, all the tryptophan is used by the fish for maintenance and also for growth and thus, above these levels, we will have excess tryptophan. One other method employed to find out how stepwise increases in dietary tryptophan will affect the animal is to exanine the level of free tryptophan in tissues (such as liver) and in the plasma.
Fig. 3 shows the amino-acid requirements of fish measured by various authors. The requirements are expressed in g/kg dry diet. What this figure suggests, is that for certain amino-acids, we get enormous differences between different species. But, if we express these values as mg/kg body weight, these differences becomes almost non-existent (See fig. 4). The same applies if we express the results as g, required per kJ of metabolisable energy in the diet.
Fig. 5 shows the relative proportions of essential amino-acids in fish muscle and the relative proportions required by 4 species of fish. The maintenance requirements for fish are probably realtively low in comparison with those of warm-blooded animals: The latter must maintain their body temperature, particularly at colder temperatures, they have special maintenance requirements for this purpose (production feathers or wool or hair). Fish do not need large amounts of energy to form non-toxic excretory products. Amounts can be excreted into surrounding water.
There is a similarity between the proportion of the amino-acid in the muscle and the proportion required by the fish. It seems advisable when preparing diets for species of fish which have only recently been cultivated (e. g. marine fish) to examine the relative proportions of the amino-acids in the muscle and to try and match these proportions in the food.
Fig. 6 shows the results of an experiment on the growth of fish which were given diets containing high levels of protein or a mixture of free amino-acids and protein; this mixture of free amino-acids was identical in amino-acid composition with that of the whole protein. The growth of the fish fed on a complete protein is considerably better than that obtained with fish fed on a mixture of free amino-acids alone or with protein.
This probably means that when we define the amino-acid requirement in a laboratory, we are probably not obtaining maximal growth rates. The poorer utilisation of free amino-acids is also of importance in practice, especially when we use a cheap protein of inferior quality in a diet and need to improve its biological value by supplementation with free amino-acids.
Fig. 7 shows an interesting experiment concerning the utilization of arginine, free or protein bound, by catfish. The authors gave a mixture of casein and gelatin to channel catfish. on the first line all the arginine was furnished by casein (1,2) and the weight gain was 11.6 %. In adding some arginine (free arginine) to the diet, no improvement in growth occurred. But when the authors added arginine as a component of protein (gelatin) they obtained an increase in weight gain. These authors concluded that free amino-acids can not be used to supplement a deficient protein. Protein bound amino-acids were effective in this respect. But (see fig. 8), in a similar experiment with lysine, other authors reached the opposite conclusion: a supplementation with lysine of a protein deficient in lysine was effective in promoting growth.
From a practical point of view, the problem may be resolved by the high price of free amino - acids, the less expensive amino - acids are lysine and methionine which are required by the animal feed industry, especially the poultry industry. Consequently, when using a protein which is deficient in some amino-acids (e.g. soyabean meal is deficient in methionine) a more practical solution is the addition of another protein of high quality (e.g. a good fish meal) to supplement this protein.
Some experiments demonstrated that when a fish is fed with a mixture of amino-acids, the amino-acid peak in the plasma is reached much quickly than when they are fed with protein (12 h. for amino-acids, 24 h. for protein). Free amino-acid concentration in plasma is higher when free amino-acids are fed leading to more rapid catabolism and less use being made of amino-acids for protein synthesis than when protein is fed.
It has been claimed by some people that differences in temperature may affect digestibility. Experiments carried out by CHO and SLINGER at 2 different temperatures (9° C and 18° C) showed that the digestibility varied slightly. At low temperatures, all metabolic processes are reduced in unison so that reduced environmental temperature has little effect on the measure value of protein digestibility.
Processing of the raw material can damage the proteins. This will affect the availability of some of the essential amino - acids. The problem arising is that although amino-acids are chemically measurable in the food protein they are not biologically available.
Figure 1 - PROTEIN REQUIREMENTS OF CHANNEL CATFISH OF DIFFERENT SIZE
| DIETARY PROTEIN | DIGESTIBLE ENERGY | INITIAL WEIGHT | FINAL WEIGHT | INITIAL WEIGHT | FINAL WEIGHT |
| g/Kg | MJ/Kg | g | g | g | g |
| 250 | 9.7 | 14 | 97 | 114 | 526 |
| 350 | 10.7 | 14 | 126 | 114 | 497 |
Page & Andrews (1973) J, Nutr. 103 1339
Figure 2 - EFFECT OF DIETARY PROTEIN LEVEL ON GROWTH OF RAINBOW TROUT AT DIFFERENT SALINITIES
| % protein in diet | % increases initial weight | ||
| 10ppt | 20ppt | ||
| 30 | 127 | 111.5 | |
| 35 | 165.3 | 136.1 | |
| 40 | 206.7 | 187.8 | |
| 45 | 187.1 | 221.9 | |
| 50 | 204.0 | 213.8 | |
| 55 | 185.4 | 220.8 | |
date of ZIEIOUN et a1. (1973)
Figure 3 - AMINOACID REQUIREMENTS OF FISH (Various authors) g/Kg dry diet
| Arg. | Lysine | Histidine | Trypt. | |
| Chinook salmon | 24 | 20 | 7 | 2 |
| Eel | 17 | 20 | 8 | 4 |
| Carp | 16 | 22 | 8 | 3 |
| Rainbow trout | 18–27 | 28.7 | - | 2.5 |
| Channel catfish | 10.3–17 | 15 | 3.7 | 1.2 |
| Threonine | Leucine | Isoleucine | Valine | |
| Chinook salmon | 9 | 16 | 9 | 13 |
| Eel | 15 | 20 | 15 | 15 |
| Carp | 15 | 13 | 9 | 14 |
| Rainbow trout | - | - | - | - |
| Channel catfish | 5.3 | 8.4 | 6.2 | 7.1 |
| Methionine | Met + Cyst | Phenilal. | Phe + Tyr | |
| Chinook salmon | 16 | 6 +10 | 20 | 17 + 4 |
| Eel | 12 | 6 + 10 | 22 | 12 + 20 |
| Carp | 12 | 8 + 20 | 25 | 13 + 10 |
| Rainbow trout | 10 | 5 + 20 | - | - |
| Channel catfish | 5.8 | - | 4.7 | - |
Figure 4 - REQUIREMENTS OF TROUT AND CATFISH FOR CERTAIN AMINOACIDS
| Percent diet | mg/100g B.W./day | ||||
| Trout | Catfish | Trout | Catfish | ||
| Tryptophan | 0.24 | 0.12 | 4.7 | 3.6 | |
| Methionine | 1.00 | 0.56 | 20 | 20 | |
| Lysine | 2.18 | 1.50 | 43 | 45 | |
| Arginine | 1.80 | 1.03 | 28 | 31 | |
| Percent diet | g/Kg: metab.energy | ||||
| Trout | Catfish (1) | Trout | Catfish (1) | ||
| Tryptophan | 0.24 | 0.12 | 0.14 | 0.11 | |
| Methionine | 1.00 | 0.56 | 0.50 | 0.57 | |
| Lysine | 2.18 | 1.50 | 1.3 | 1.1 | |
Figure 5 - RELATIVE PROPORTIONS (%) OF ESSENTIAL AMINOACIDS IN FISH MUSCLE AND RELATIVE PROPORTIONS REQUIRED BY 4 SPECIES OF FISH
| Muscle | C. salmon | C. catfish | J. eel | C. carp | |
| Lys. | 16.9 | 14.6 | 20.1 | 13.5 | 16.1 |
| Hist. | 5.8 | 5.1 | 4.9 | 5.4 | 5.8 |
| Arg. | 11.2 | 17.5 | 19.8 | 11.5 | 11.7 |
| Tryp. | 2.3 | 1.5 | 0.9 | 2.7 | 2.2 |
| Thre. | 8.9 | 6.6 | 7.1 | 10.1 | 10.9 |
| Val. | 9.3 | 9.5 | 9.5 | 10.1 | 10.2 |
| Mer. | 5.3 | 11.7 | 7.5 | 8.1 | 8.8 |
| cys/2 | 2.3 |
Figure 6 - GROWTH OF FISH GIVEN DIETS CONTAINING EITHER PROTEIN OR A MIXTURE OF FREE AMINOACIDS (F.A.A.) AND PROTEIN
| % Increase in initial weight | ||
| Channel catfish | Trout | |
| 24 % whole EGG Protein | 319 | - |
| 5.7 % Protein + 22.1 % F.A. | 189 | - |
| 50 % Casein | 312 | |
| 25 % Casein + 25 % F.A.A. | 204 | |
Figure 7 - UTILIZATION OF ARGININE, FREE OR PROTEIN BOUND BY CATFISH
| Casein (%) | Gelatin (%) | ARG HCl (%) | Tot Arg. (%) |
| 35 | 0 | 0 | 1.2 |
| 35 | 0 | 0.58 | 1.7 |
| 25 | 10 | 0 | 1.7 |
| 35 | 6.5 | 0 | 1.7 |
(ANDREWS et al., 1977)
Figure 8 - RESPONSE OF CATFISH TO DIETS SUPPLEMENTED WITH LYSINE
| Diet | Available lysine | Initial weight | Weight gain |
| % | g. | % | |
| Basal | 0.88 | 201 | 81.1 |
| Basal + 0.33 Lys. | 1.22 | 201 | 170.7 |
| Basal + 0.66 Lys. | 1.54 | 196 | 73.6 |
| Basal + 0.68 % Lys | 0.88 | 196 | 73.6 |
(ROBINSON et al, 1980)