Many workers have noted the apparent succession in ecological states along a river associated with changes in morphology. Thus a river was early classified as young in its headwaters, where erosional processes are dominant, mature in its mid-section where erosion and deposition are balanced and old in its lower reaches where deposition is the major process. Rejuvenation zones may also occur in mature or old reaches where increased gradient may temporarily reverse the more normal succession. These descriptions are based on sedimentology but corresponding biological changes are also very marked and have been summarized variously by Illies and Botasaneanu (1963), Hynes (1970) and Hawkes (1975) who demonstrate the existence of different associations of flora and fauna along the river which usually take the name of the dominant faunistic element, often fish. The nature of the flora and fauna depend on the slope, current and the type of bed material in the river. As a river ideally follows an orderly parabolic profile, the succession of the various elements appears correspondingly orderly. Recently the river continuum concepts, introduced by Vannote et al. (1980) has attempted to assemble the various morphological and biological changes along a river into a coherent description of this progression.
This concept assumes that the geo-physical variables within a river system present a continuous gradient from source to mouth. Communities of living organisms succeed each other along the length of the river in such a way as to minimize energy loss. This requires striking a balance between the most efficient use of available energy through specialization and a contrasting tendency towards a uniform rate of energy processing throughout the year. The structure of such communities is summarized in Fig. 3.1. In the temperate rivers examined by the river continuum group, the following shifts in community structure can be identified. Riverine communities can be separated into three main groups, headwaters (orders 1–3), medium-sized streams (orders 4–6) and large rivers (orders >6). Headwater streams are heavily influenced by riparian vegetation, which is responsible for large-scale inputs of allochthonous nutrients while at the same time hindering autotrophic production by shading. In some types of river, particularly blackwater rivers of the equatorial rainforests allochthonous inputs in headwater streams or on floodplains may represent a major source of new nutrients to the system. As stream size increases allochthonous inputs become less important and the aquatic communities tend to concentrate more on autochthonous processing of nutrient transported from upstream. This transport, with the exception of new allochthonous material arising from floodplains and feeder tributaries, is the basis for all subsequent living processes. The first effect of this process is in the nature of the allochthonous material which degrades from coarse particulate organic matter (CPOM) in the low order streams to progressively finer particulate organic matter (FPOM), ultrafine particulate organic matter and eventually to molecular components, amino acids, sugars, etc. as one proceeds downstream. The composition of the living aquatic communities reflect these changes in the nature of the nutrient substrates and in the physical form of the river ecosystems. Plants progress from anchored submerged macrophytes in upstream reaches through periphyton to phytoplanktonic communities within the main channel. Infrasubstrate communities shift from shredder-dominated areas in the low-order headwater streams through grazer-dominated areas in the medium-sized streams, to collector-dominated communities in the higher order streams. Fish communities also tend to undergo a similar transition from invertivorous predator dominance in low-order streams, through grazer-dominated communities in medium-sized rivers to iliophagous dominance in the potamon.
Since its proposal many workers have used the concept as a framework for the analysis of small river systems. Hawkins and Sedell (1981) for example, confirmed the predictions of the concept in four Oregon streams, and Culp and Davis (1982) similarly successfully applied it to the Oldman and South Saskatchewan river system. The ecological changes envisaged by the river continuum concept are mostly accomplished in the progression through smaller order streams and little change is predicted in rivers from order 6 onwards. This means that it may be used as a descriptor for the first 200 km or so of a river course during the transition from rhithron to potamon but once the stable potamonic phase is reached little further change can be anticipated for several thousand kilometres. Furthermore, within the potamon, the floodplain is somewhat of a special case, for here the recycling of nutrients and organic matter through the growth and decay of plants parallels, to a certain extent, the situation nearer the headwaters, with the renewed input of CPOM. However, the nutrient base for this productivity may be regarded as autochthnous in that it arises from the nutrients deposited in river borne silt.
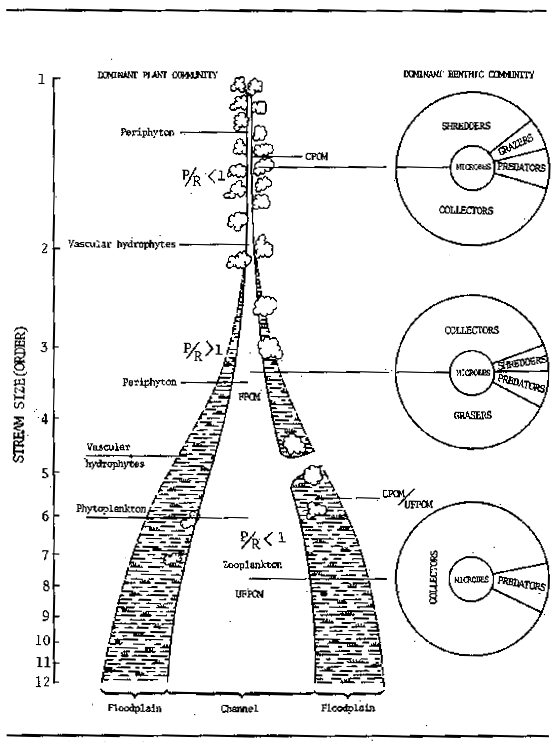
Figure 3.1 Conceptual relationship between stream size and the progressive shift in structural and functional attributes of lotic communities. (After Vannote et al., 1980)
Work by Bruns et al. (1984) indicates that, while this theoretical continuum may be applied to the spatial evolution of conditions within the main channel, it is strongly influenced by presence of tributaries. The ways in which these inflowing rivers affect the progression depends on the size of the tributary relative to the main channel. This explains the sometimes abrupt transitions that may occur at the confluence of two large water courses. Similarly the continuum may be interupted or even reversed by geomorphological irregularities in the normal catenary shape of the profile.
An important input into the aquatic system is the rain of small non-aquatic creatures and organic matter from terrestrial sources. As the floods advance, invertebrates, especially ants and termites, are caught by the rising flood and incorporated into the aquatic system. There is also a continuous input of insects, seeds, leaves, pollen and other material from flooded forests and grasslands which either enter the drift in flowing waters, or settles to the bottom where it is decayed by bacterial and fungal activity.
Workers on inundated forest regions all consider nutrients of allochthonous origin to be the single most important, if not the only, input into the system. Geisler, for example, has in preliminary experiments found that up to 56 individual pieces of organic material fell onto a quarter m² glue board in the course of one day (Geisler, et al., 1973). The extent of leaf fall is indicated by data from many parts of the world. For example, Blackburn and Petr (1979) summarizing data from low order temperate streams showed that averages of between 3.5 and 8 t of plant litter (leaf, bark and branches)/ha fall into the water every year. Comparable figures from the tropics indicate leaf falls of 6 t or more/ha/yr. Standing crops may be equally high, for instance in one small stream from the new territories Hong Kong, Dudgeon (1982) found the detrital standing crop to be one hundred times greater than that of the periphyton and more consistant at 80–120 gm/m² (= 1 t/ha). About 60% of this was organic matter and thus accessible to the food chain.
Studies on the flow of nutrient through the Volga system indicate that the nutrient cycle in the river and its reservoirs is greatly influenced by external inputs. The main trophic flows are thought to proceed from allochthonous organic matter via bacteria direct to invertebrates, primary production by phytoplankton and higher vegetation playing a very secondary role.
Annual litterfall on floodplain wetlands is very high, although the precise classification of material generated on the floodplain as allochthonous or authochthonous is arguable. Records from the United States range from 350 g (dry wt.)/m²/yr for an Illinois swamp to over 700 g (dry wt.)/m²/yr in North Carolina (Mulholland, 1981). In Amazonian rain forests litter fall may be as high as 1 000 g (dry wt.)/m²/yr although Adis et al. (1979) calculated that in the poorer Rio Negro floodplain forest value are closer to 580–790 g (dry wt.)/m²/yr. Even in savanna floodplains the presence of gallery forest and floodable scrub vegetation provides a substrate from which materials fall into the water.
Much of the initial breakdown of CPOM in headwater streams is carried out by fungi and bacteria which are abundant in the leaf letter of these zones. Sesile bacteria also form an important part of the biomass of the riffle zones where they form epilithic slimes. Bacteria may became detached during period of high flow and between 0.4 an 9.2 × 106 cells have been found suspended in flowing waters depending on discharge (Marxsen, 1980).
Little information is available on the abundance of microorganisms in unpolluted rivers and floodplain lakes, although they are obviously of immense importance both in the breakdown of vegetation debris, dung and other organic remains, as well as in the diet of many species of detritivore fish. The distribution of such microorganisms as fungi, actinomycetes, or starch, pectine and hemi-cellulose decomposers, which obtain their energy from organic substrates, as well as aerobic and anaerobic nitrogen fixing bacteria, were substantially higher in the swamps now submerged by the Kainii reservoir, than in the river (Imevbore and bakare, 1974). For example, a total of 6.3 × 107 organisms/ml were estimated to be present in the surface water of the swamps and 5.1 × 1011/ml were estimated in swamp mud. This contrasts with the 3.5 × 104 and 1.3 × 1011/ml found in river water and mud respectively. Fairly constant amounts of bacteria, between 2.3 × 105 organisms/ml for the Rio Negro and 5 × 105/ml for the Solimoes were found in the rivers of the Amazon basin (Schmidt, 1970). Greater numbers were found in the Lago do Castanho, a representative várzea lake. Here total estimates of up to 7.3 × 106/ml were made although there is considerable seasonal and spatial variation in density (Schmidt, 1969). In the lake, bacterial activity closely follows that of algae and seasonal maxima of algae are always associated with maxima in bacterial number. Similarly there is a distinct vertical stratification in both algal and bacterial numbers, with differences of between 0.5–4 × 106 organisms/ml. Maximum densities occurred at about 1 m depth and at the bottom. Rai (1979) also found a strong correlation between bacterial counts and water level in four Amazonian lakes. The numbers of saprobic bacteria in a blackwater lake, ranged from 4 × 10³ - 2.2 × 105/ml during high water to 1.1 × 105 - 9 × 105/ml during low water, and total bacterial counts in varzea lakes ranged from 2.1 to 11.6 × 108/ml during high water and 4.2 to 15.6 × 109/ml during low water. Rai further confirmed Schmidt's findings that bacterial maxima were strongly correlated with algal maxima.
These examples from tropical systems indicate the richness of the bacterial flora in the more sheltered tropical waters. The values do not in fact differ greatly from those quoted for the Danube by Mucha (1967), where 1.5–2.6 × 106 organisms/ml were present in the bacterial plankton in Czechoslovakia and Yugoslavia. In the Soviet portion of the delta 3.2–23.6 × 106 organisms/ml have been noted from the river and 3.6–12 × 106 organisms/ml from the standing water Kilia arm. The Danube, however, is highly polluted, particularly with organic matter. Other Ponto-Caspian rivers show similar ranges of bacterial plankton numbers: 9-23.7 × 106 organisms/ml from the Volga and 1–3 × 106 organisms/ml from the Dniester (Gavrishova et al., 1982).
The contribution made by phytoplankton to primary production within rivers is generally regarded to be low when compared to other types of aquatic systems. However, phytoplankton is present in rivers and contributes to the nutrient balance and to the trophic requirements of some of the fish species.
The major factors determining the presence and abundance of phytoplankton are temperature, velocity of the current, availability of nutrients and availability of light.
TemperatureIn high latitudes temperature is one of the principal regulators of planktonic abundance with a well defined seasonal cycle based on the alternation of winter and summer. Thus in temperate rivers there is a minimum in phytoplankton production and biomass during the winter. Even in the lower Parana, where large seasonal temperature differences are normal, the summer high water figures for phytoplankton numbers are frquently higher than the low water figures suggesting that temperature is the major factor influencing phyto-plankton abundance in this portion of the river (CECOAL, 1977). In tropical rivers temperature plays a much diminished role and the greatest densities of phytoplankton coincide with low water.
CurrentPhytoplanktonic organisms are sensitive to velocity and turbulence of flow in rivers as the rapid currents and mechanical stresses of rapids and waterfalls inhibit the development of new plankton and rapidly suppress any existing organisms discharged from associated lentic waters. Thus the agitated waters of the rhithron generally support little plankton, although some does develop in the occasional quiet backwater and pool. In addition a drift is present in the main channel made up of primarily bottom living algal forms which are dislodged by the rapidity of the flow.
In the potamon, studies confirm the strong influence of flow and, consistent with this, show that phytoplankton is more common in the lentic components of the system than in the lotic.
Prowse and Talling (1958) demonstrated the strong correlation between phytoplankton growth and current velocity in the Nile at the Gebel Aulia dam. The dam slowed the Nile current and produced a rapid increase in planktonic concentration. When the dam was open the flow was faster and plankton concentration dropped. The build up of phytoplankton from this source has been thought to account for the majority of potamonic plankton; a conclusion justified by the progressive disappearance of such phytoplankton downstream from the point of discharge. Storage and discharge of water from mainstream or major tributary reservoirs can also alter the indigenous patterns of phytoplankton abundance. This has occured in the Volga river where the whole algal fauna has been considerably modified following the conversion of the river into a cascade of reservoirs (Kuzmin in Mordukhai - Boltovskoi, 1979). Before impoundment the spring flood peak of phytoplankton biomass was less than the summer low water peak. After reservoir construction species composition changed, with a reduction in the number of taxa present in the unmodified lower floodplain and delta. Here the seasonal cycle of abundance has remained relatively unmodified although now the spring peak in biomass (6.3 g/m³), when diatoms predominate, is greater than the prolonged period of high biomass through the summer low water (2.4 – 3.8 g/m³) when green and blue algae are most common. The phytoplankton of the Dniester river likewise underwent qualitative and quantitative changes after the closure of the Dubassery reservoir with increases in Cyanophyta downstream of the dam. The phytoplankton biomass varied between 0.66–0.85 g/m³ before the dam was built but rose to about 4 g/m³ in the river after its construction.
Discharge is assigned the major role in regulating phytoplankton abundance in the Mississippi river (Baker and Kromerbaker, 1979), although in the river seasonal variations of temperature are high and influence the succession of the various components of the phytoplankton. Phytoplankton abundance, as represented by chlorophyll ‘A’ concentrations, was less in the main channels of the river than in the backwaters or in a river lake where the current was slowed. In the Missouri river, Berner (1951) associated the low plantonic densities (0.067 cells/ml) with high current and turbidity and a lack of subsidiary floodplain waterbodies to feed into the mainstream. The Illinois river, a tributary of the Missouri/Mississippi system had much higher phytoplankton densities (about 400 cells/ml) when it was still connected to its floodplain lake and backwaters (Kofoid, 1908). The literature of the Mississippi system between St. Louis and Cairo has been summarized by Schrammn et al., 1974 who confirm these fundings and commented on the difference in species composition and density between main channel, riverine backwaters and floodplain lakes. Diversity is far higher in the lentic environments where chloro-phycea and Cyanophycea are dominant than in the river where Chrysophycea are the major element. Similar findings are recorded by Bryan et al. (1975 and 1976) who commented that phytoplankton has a very characeristic distribution within the Atchafalaya system. When the flood is in progress habitats are inundated and flushed out producing an homogeneity of community structure. After the floods receed communities differentiate and representative forms characteristic of the different types of water body again exert themselves. Clearly if much of the main stream phytoplankton originates from flushing and discharge from the lentic components of the system, build up in numbers must occur where the flow of a river is slowed or halted in backwaters or in the standing waters of the floodplain. Rzoska and Talling (1966) found phytoplankton to be much more abundant in backwaters of the Nile Sudd than in the main channels and thus Rzoska (1974) quoted values of between 40 to 140 cells/ml for the river, whereas densities in lagoons reached from 1 720–2 330 cells/ml at river post 12. Differences in the specific composition of the phytoplankton were also common. Blue-green algae such as Anabeana and Lyngbya dominated in the standing waters, whereas in the river the sparse flora consisted mainly of diatoms especially Melosira. Samples from a small West Africa river, the Oshun, showed similar trends to occur there. The main river is inhabited mainly by desmids and diatoms, and colonial cholorophycea were the first to colonize the backwaters.
Phytoplankton abundance is also associated with seasonal differences in flow. Densities generally reach a peak in the dry season and diminish in the floods in both types of water unless otherwise influenced by temperature. Thus Egborge (1974) found a good negative correlation in the Oshun between phytoplankton abundance and both water level and current velocity, with maximum abundance at times of low water, although even then the total numbers of organisms were very low. Iltis (1982) also found that algal pop ulations were maximal during low waters and that the floods were characterized by very poorphyto-plankton in the main channels of six rivers of the Ivory Coast. Carey (1971) had earlier found phytoplankton densities to be less during the floods in the Kafue river with dense blooms occurring in the river at Nampongwe and in Namatenga lagoon between August and November when the floods had receded. Phytoplankton was generally scarce in the Sokoto river at most times of year, but was maximal in the dry season between March and June, especially in a floodplain lagoon. Holden and Green (1960) suggested that, although the relative abundance of organisms in terms of numbers per unit volume is lower during the floods, the absolute abundance may well remain the same due to the dilution of the number of organisms by the enormously increased volume of water in the system.
A similar argument was proposed by Bonetto, Dioni and Pignalberi (1969) who remarked at the same time on the generally low contribution made by phytoplankton to the primary production of the Parana river. This is particularly small during the floods or in water bodies with dense vegetation due to high turbidity and shading effects, but may rise in some lagoons during the dry season. For instance, CECOAL (1977) showed cycles of phytoplankton abundance in the upper Parana which flunctuated between about 25 cells/ml in the floods to over 250 cells/ml during low water. In the Middle Parana values of 2 331 cells/ml (low water) and 44 cells/ml (flood) represent values midway between those of the two confluent systems (Bonetto, 1982). The algal population of the main channel of the Amazon was also found to be higher at low water (15 000 cells/ml) than at high water (3 000 cells/ml) (Schmidt, 1970), and the same held true in a varzea lake with 500 000 algal cells/ml at the period of minimum water level. Much of the rise in the number of phytoplankton in the river was attributed to discharge of algal rich waters from lagoons. This cyclic pattern of activity is by no means universal in the Amazon system as the Rio Negro showed a remarkably constant regime of about 10 000 algal cells/ml, and in lake Redondo the peak of algal production was reached during the rising waters when the nutrient-rich whitewaters were invading the lagoon (Marlier, 1967). Because of the interaction between the various water types in the Amazon system the nutrient regimes are likely to differ considerably from the more normal regimes of rivers with only one dominant water type, and in certain circumstances generally nutrient concentrations may be greater in the floods when lagoons are invaded by nutrient-rich waters than during the dry season when nutrients have been diluted by rainfall and inflow of poorer groundwaters.
Availability of nutrientsThere are several pointers to the important role nutrient availability plays in determining the abundance of the phytoplankton and in particular in limiting it development beyond a certain level. In the Gebel Aulia Dam for example, Prowse and Talling (1958) attributed the failure of phytoplankton to develop beyond a certain level at slack water to nutrient depletion, particularly of nitrates. Talling (1957) had earlier traced the high negative correlation of phytoplankton abundance and nitrate concentration in the Nile. On the other end of the scale, extraordinary high concentration of nutrient associated3 with eutrophication may result in blooms in excess of the usual.
The normal cycle of abundance associated with water velocity is shown in the Danube, where in Romania 0.8 cells/ml were found during the June floods and 4.0 cells/ml were found during the October low water (Szemes, 1967). Similarly in the slow reaches of the Russian part of the delta, cell counts ranged from 192 cells/ml in the floods to 2 621 cells/ml at low water. This pattern was disturbed by heavy pollution in the upper reaches of the river, where in the fast flowing Austrian stretch 300 cells/ml were already present. Densities of 10–15 000 cells/ml were attained in the highly polluted Czechoslovakian and Hungarian reaches, where water blooms are common during the autumn giving a foul taste to drinking water. Juris (1975) even records numbers as high as 20 000 cells/ml in periods of maximum development, which may rise in high as 50 000 cells/ml in years with especially low water. Further downstream, in Yugoslavia, cell counts dropped as low as 320–1 060 cells/ml and continued to fall to the figures shown for the delta. The Danube pattern indicates that when abundant nutrients are available, flow becomes a secondary consideration in limiting phytoplankton number.
Elevated standing stocks of phytoplankton in the Laguna Gonzales (54 642 cells/ml - 252 906 cells/ml) as compared to other lagoons of the Riachuelo (Parana river) - Laguna Totoras 129–1 330 cells/ml Laguna La Brava 335–9 235 cells/ml, Laguna Sirena 194–434 cells/ml and Laguna Meritta 72–654 i/ml are traceable to the highly eutrophicated state of Laguna Gonzalez (Bonetto et al., 1978).
The actual families of algae comprising the plankton vary much with water quality and in the Danube Blue-green algae predominated under eutrophicated conditions. In the tropics too the major forms present differ, for instance desmids tended to dominate in the flora of black-water streams both in the Kapuas R., Borneo (Vaas, 1953) and in the Amazon.
In clear white waters of neutral pH diatoms and green algae are more abundant and in eutrophicated waters or those with high pH, blue-green algae are the more common and indeed are often the only element of the flora.
Availability of lightThe amount of suspended matter in the water affects the penetration of light into the water, for example Bonetto (1980) found a relationship S = a e bh for the Parana river, where S is the light penetration as measured by Secchi disk, h is the velocity of the current as measured by the height of the river and a and b are constants. It would thus appear that in many cases the limitation of plankton development in the main channel of swift flowing rivers stems not from the current per se but from the low penetration of light in such waters. In the main channel of the Parana just below the confluence with the Paraguay river, for instance, the primary production varied between 0–285 mg C/m²/day and the number of organisms varied between 80–2 000 cells/ml. The maxima and minima were very strongly correlated with flow (Bonetto et al., 1979), abundance and production during the flood being reduced not only by the strong current but by the poor light penetration. In many Latin American floodplain waters, and probably in African areas too the productive zone is limited to a relatively thin layer near the surface. This rarely exceeds 3 m in the Amazonian Lago de Castanho (Figure 3.2) or in the floodlakes of the Riachuelo river (Bonetto et al., 1978a and b), or 2 m in the Cienagas of the Magdalena (Mikkola and Arias, 1976). Limitation of photosynthetic activity during the rainy season occurs when rising waters bring silt into the lagoon. It may be restricted in a similar manner during the period of low waters when wind induced turbulence resuspends bottom mud.
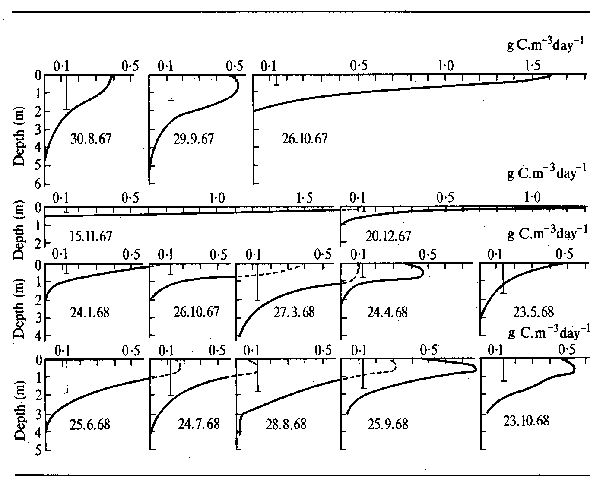
Figure 3.2 Vertical patterns of primary production by phytoplankton and secchi disc transparency (vertical bar) in Lago do Castanho from August 1967 to October 1968. (After Schmidt, 1973b)
Other factorsHigher vegetation may also influence plankton abundance. In Bangula lagoon in Malawi, the waters within patches of Nymphaea supported some 16 731 = 5 512 algal units (cells, filaments or colonies)/ml. Here shading effects possibly reduce plankton densities. Over submersed vegetation, higher but very variable figures were obtained of 38 107 – 52 188 units/ml (Shepherd, 1976).
centration of planktonic organisms were some 13 times greater in patches of open water within floating vegetation in the Laguna la Brava of the Riachuelo river than they were in the open water of the lagoons on the same date (Bonetto et al., 1978a). Reduced phytoplankton densities near emergent and floating vegetation and higher values over submersed vegetation have also been remarked upon from the Danube.
Bonetto (1982) has proposed a model linking transparency with solar radiation, temperature and the existing plankton density to predict the productivity of phytoplankton in the Parana river:
P = [10 + 0.28 (AmaxF)]e-0.019S | |
| where: | A max = -0.018 + 0.002Q + 0.021T |
and
P = Productivity per unit area (mgC m³/day)
T = Mean temperature (°C)
F = Density of phytoplankton
A = Photosynthetic activity as defined by (2)
Q = Daily radiant solar energy (Cal/m²)
S = Secchi disc reading (in cm)
Considerable work on primary productivity of floodplain lagoons has also been carried out by Schmidt (1973a) on the Lago de Castanho of the Amazonian várzea. His estimates of biological production ranged from 2.15 gC/m/day at the lowest water level to 0.32 gC/m³/day during the inflow of new river water. The net annual production was 297 gC m equivalent to a gross productivity of 358 gC/m²/year. The algal biomass was 1.9 gC/m² or 17 kg/ha with a gross productivity of 1.1 gC/m²/day. Production from the Rio Negro was found to be considerably lower, ranging from 0.030 – 0.434 gC/m³/day giving a net productivity of 0.063 gC/m³/day or a gross annual production of 23 gC/m²/year (Schmidt, 1976). Other workers have found values between these two extremes. Bonetto, Dioni and Pignalberi (1969) obtained values between 0.050 gC/m²/day and 1.0 gC/m²/day from two different lagoons in the Paraná floodplain and two other lagoons, L. Totoras and L. Gonzalez of the Riachuelo tributary to the Parana give somewhat higher readings of 0.2 – 2.5 gC/m²/day and 0.45 – 2.46 gC/m²/day respectively (Caro et al., 1979). Marlier (1967) found productions of between 0.14 and 0.7 gC/m²/day on the Lago Redondo. Values for various Magdalena cienagas range from 0.16 to 1.77 gC/m²/day (Mikkola and Arias, 1967) (Table 3.1), with mean values of 0.09 gC/m³/h from 18 lagoons of the Magdalena system (Arias, 1977). Values for gross primary productivity in the main stream of the Godavari river (India) ranged from 0.30 to 1.06 gC/m³/day (Rajalakshmi and Premswarup, 1975). Maximum values were recorded during the post flood period as transparency rises and flow rate falls. At this time there was a good phytoplankton bloom. A second bloom appeared after the summer rains. These figures are influenced by the discharge of organic polluting effluents at one of the sites where mean values of primary production were the highest and also by the anicut wiers which slow the flow and even out water level fluctuations.
These figures from tropical systems compare with temperate zone production rates of between 0 – 15 g 02/m²/day (equivalent to 0–4.7 gC/m²/day tabulated by FAO/UN (1973), and show the productivity of phytoplankton in rivers to be extremely low, although peak production at low water from isolated floodplain pools may temporarily reach the order of magnitude of production from lakes.
| System | Production gC/m²/day | Authority | |
|---|---|---|---|
| Amazon: | Lago do Castanho | 0.82 | Schmidt, 1973 |
| Lago Redondo | 0.29 | Marlier, 1967 | |
| Rio Negro | 0.063 | Schmidt, 1976 | |
| Rio Tapajos (0.44–2.41) | 1.366 | Schmidt, 1982 | |
| Paraná: | Lago Los Espejos | 0.05 | Bonetto, Dioni, |
| Pignalberi, 1969 | |||
| Lago El Aleman | 1.00 | Bonetto, Dioni, | |
| Pignalberi, 1969 | |||
| Laguna Totoras | 1.2 –1.6 | Bonetto et al., 1978 | |
| 0.2 –2.5 | Cara et al., 1979 | ||
| Laguna Gonzalez | 0.8 –2.5 | Bonetto et al., 1978 | |
| 0.45–2.45 | Cara et al., 1979 | ||
| Magdalena: | Cienaga Guajaro | 0.67 | Calculated from data |
| Cienaga Maria la Baja | 0.34 | Mikkola & Arias, 1975 | |
| Cienaga Carabali | 1.77 | ||
| Cienaga Palotal | 0.16 | ||
| Godavari R.: | 0.30–1.06 | Rajalakshmi-Premswarup, 1975 | |
| Paraguay. | Laguna Herradura | 0.14–4.50 | Zalocar et al., 1981 |
a N.B. These figures are often based on only a few observations and do not therefore necessarily reflect the full range of variations of the parameters measured
Production by algae, especially diatoms growing on rocks, submerged wood and floating or submersed vegetation may well be more important than the phytoplankton. This is certainly true in the rhithronic headwaters where phytoplankton is virtually absent but where the rocks of the riffles support. Mats of epiliths which in turn, provide the substrate for the complex of organisms comprising the “aufwuchs”. In the pools too, the floating leafed vegetation of the slacks are colonized by dense aggregations of epiphytes. The ecology of such communities in the running waters of the temperate zone has been surveyed by Hynes (1970).
Few quantitative data appear to be available on this community in the potamon, although several authors have remarked upon the abundance of such organisms. Rzoska, (1974) described the stems and surfaces of emergent, submerged and floating vegetation in the Nile Sudd as being covered with epiphytes including the red algae Compsogon. Indeed, Mefit Babtie (1982) record epiphytes as comprising 16.6% of the total dry weight of Naias pectinata where 0.17 g of epiphytes were found per gram of Naias. In the flood the proportion dropped to only 2.6%. Epiphytic algae were also recorded as being very abundant on the Kafue flats by Carey (1971). The littoral of the Lake Chilwa swamps, which closely resemble these of riverine floodplains, also support considerable populations of epiphytes on Typha stems and on floating dead plant material wherever there is sufficient light (Howard-Williams and Lenton, 1975). In Latin American waters Ducharme (1975), and Mikkola and Arias (1976) considered the production by periphyton to be considerably superior to that of the phytoplankton in the cienagas of the Magdalena river floodplain. The epiphytic community has also been considered very important in the Middle Parana because of the abundance and density of support in the form of floating and emergent vegetation, (Bonetto, Dioni and Pignalberi, 1969).
Although few figures are available it does appear that biological production by epiphytes is very high in most flood zones, especially at the periphery of vegetation masses where light is adequate for growth. In Bangula lagoon, Malawi, for example, the number of cells loosely attached to Ceratopyllum was estimated by Shepherd (1976) at 3.35 ± 2.10 ×106/g fresh wt. of Ceratophyllum. This gives an extrapolated number of 45.21 ± 17.70 ×109 cells/m² of lagoon surface, which was between 0.8 and 20 (mean 9) times the number of cells of the same organisms found free in the water. Investigations using artificial substrates in the Danube (Ertl et al., 1972) showed that periphyton abundance was limited by frequent fluctuations in water level and by scouring. These effects may have been magnified by the fixed nature of the artificial substrates used, which more closely simulated rocks or emergent vegetation, than floating vegetation which could move with the water level. Because of these effects, spring maxima in periphyton biomass were low and annual maxima of up to 650 g/m² were achieved in autumn, and often persisted until early winter when flows were minimal. Similar conclusions on the correlation between periphyton abundance and the flood cycle were reached by Iltis 1982, in some West African rivers. Here the abundance of periphyton per unit area followed the same fluctuations as those of the phytoplankton with the peak in relative abundance being reached at the end of the dry season. However, the greatly increased area of substrate available during the floods in the form of root masses of floating vegetation probably compensates for the low density and absolute abundance may well be maximal at this time.
Colonization with diatoms such is Melosira or Oscillatoria produce biomasses of 2–15 /m² in the muddy bottoms of the Volga and in the Dnieper biomasses exceed these of the zoobenthos. There are indications that the density of epiphytes decreases towards the shaded interior of stands and mats of vegetation along with the rest of the Aufuruchs community. For instance, in the Danube, Juris (1969, 1973) found 7.7 × 106 cells/cm² were present in shaded areas. Similarly standing crops of periphyton are reduced with depth (Fig. 3.3) both because the lower light penetration limits growth and because predatory consumer populations increase slightly with depth.
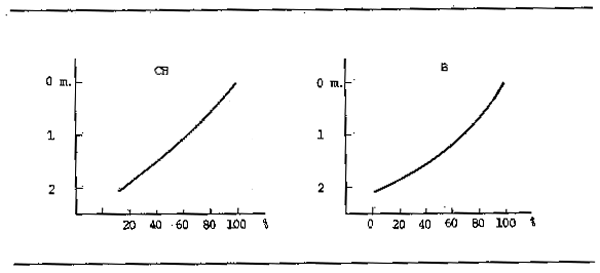
Figure 3.3 Biomass (B) and chlorophyll (CH) of periphyton at different depths in the littoral of the Danube River (Ertl et al., 1972)
Because of the concentrated nature of the periphyton it obviously is a major locus of production in the aquatic system. However, epiphytes may also perform subsidiary roles in the nutrient ecology of the system. Heeg and Breen (1982), for example, found that epiphyton on Potamogeton crispus had a considerable nitrogen fixing capacity of up to 23 mg N per 24 hours or 1.27 mg N/m² during the inundation of the Pongolo floodplain. It also forms the base of a particular community which is associated with the periphytic and perilithic habit, including the complex known as Periphytic Detrital Aggregate (P.D.A.). In samples from L. Valencia, Venezuela, Bowen (1979) has shown P.D.A. to contain 42.7% dry weight of organic matter. Algae, on the other hand, only contributed a small portion of the percentage (0.2 – 2.8%) by dry weight of the same sample even though they contribute a greater volume (17–279 mg/ml of sample).
Higher plants provide the major biotic structural elements in fluvial ecosystems. Not only does their distribution depend on the geology and morphology of the environment, but the presence of vegetation can itself modify the form of the system.
Although only rarely used directly for food by fish, vegetation has a range of ecological values for fish communities. It provides refuge, shade, a substrate for spawning and a support for many organisms which are of dietary importance for fish.
Longitudinal zonation of vegetation within rivers is based mainly upon the related factors of depth, flow, and mechanical stress. In torrential headwaters liverworts and mosses are the earliest forms to appear on rocks, both submersed and in the splash zone. This type of vegetation persists into the rocky riffles of the rhithron, but in these reaches the pools increasingly support rooted, floating leafed species in the slacks and emergent vegetation along the banks as slope decreases. As the river enters the more mature potamon, the ideal channel is fringed with emergent plants and floating grasses which give way to floating leafed plants and submersed species as the depth increases toward the centre of the river. Such a stable state is rarely attained in natural rivers, where one or other of these elements may be lacking. Similar successions can be seen in islands within the river channel where sand banks laid down by the current became colonised by plants which fix the bank and lead to further siltation. Such islands eventually form part of the seasonally floodable area taking the form of internalized floodplains. Gosse (1963) describes the distribution of vegetation on such islands in the Zaire R. (Fig. 3.4).
The river floodplain shows much more complex lateral successions based on the degree of flooding such as that proposed by Adams, 1964 which contains the following zones:
(a) permanently flooded waters with submersed vegetation only (open waters);
(b) permanently flooded areas with rooted or floating emergent vegetation;
(c) regularly seasonally flooded areas with rooted and floating emergent vegetation;
(d) areas that are occasionally flooded (between mean flood and highest flood levels;
(e) areas that are not flooded but whose water table is influenced by the flood regime.
In the Shire river Elephant marshes, Howard-Williams (in Hastings, 1972) distin- guished the following zones:
(a) aquatic plant zone: with floating sudd islands composed of Echinochloa pyramidalis, Ludwigia stolonifera and Ipomoea aquatica, together with true floating plants such as Azolla nilotica, Salvinia hastata and Pistia stratiotes;
(b) a swamp zone with water between 50 cm and 2 m in depth which consists of floating meadows of Echinochloa pyramidalis, Vossia cuspidata, Leersia hexandra, Cyperus papyrus, and Echinochloa stagnina;
(c) flooded grassland 1.5–6 m deep, mainly dominated by Oryza barthii;
(d) shallow flooded grasslands and levees with depths of flooding between 0.25 and 25 cm with tussocks of Setaria avettae, Vetivaria nigritana;
(e) floodplain margin regions with Hyparrhenia rufa, Panicum coloratum, Vetiveria nigritana and Setaria sphacelata.
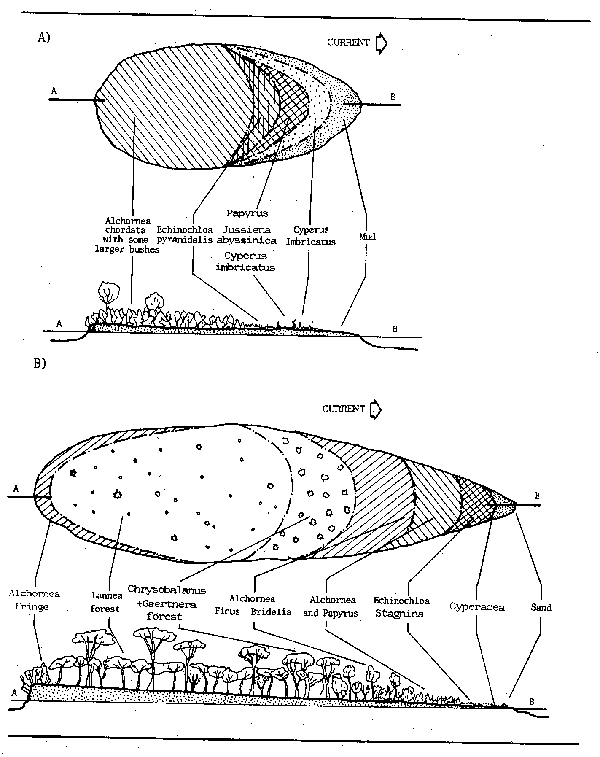
Figure 3.4 Successions of vegetation on islands in the Zaire River (A) Young Island; (B) Older Island (from J. Louis, 1947, quoted by Gosse, 1963)
Zonation of vegetation fringing the Parana river and its floodplain lakes is similar and also varies according to the type of terrain as shown in Fig. 3.5.
Detailed analyses of the vegetational zonation of floodplains carried out by Schmid (1961) for the Tonle Sap in Cambodia and Smith (1976) for the Okavango Delta, show relatively little difference in the basic type of spatial zonation for tropical systems.
In the Danube the zonation of vegetation is based on a hydrographic index where hg = 0.1 (mean high water level - mean low water level) and vegetation complexes are correlated with hg in the following manner:
Hg 6–7 highest river banks forested with willows, poplars, and ash
Hg 5–6 water meadow pastures
Hg 8–5 emergent water plants, cat tails and reeds
Hg 3 well developed reed beds
Hg 0.3 backwaters, lakes with floating vegetation and submersed plants
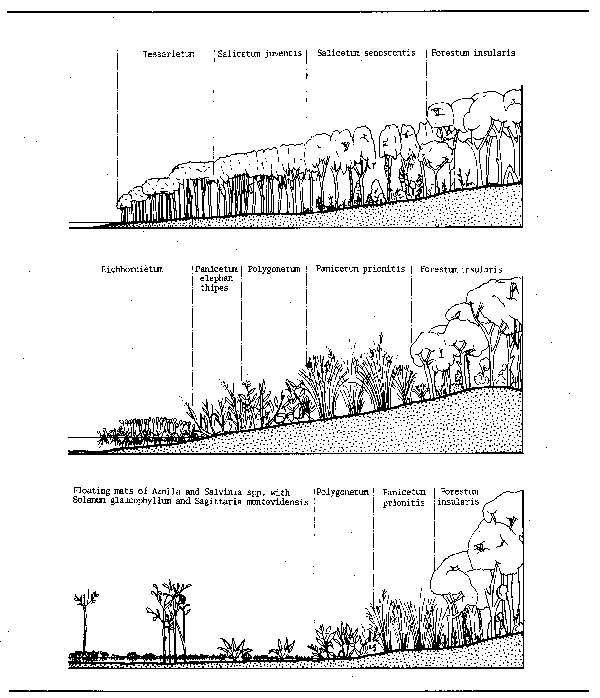
Figure 3.5 Zonation of vegetation fringing the Parana river: A. Sand banks; B. Bordering deep lagoons and channels; C. Shallow lagoons ad marshes. (After Franceschi and Lewis, 1979)
Because of the regular seasonal variations in water level in many river systems there is a temporal succession as well as spatial one. In the long term the colonization of river channels, backwaters and the various types of floodplain water body by floating and emergent vegetation accelerates siltation and tends to catalyze the transition of such waters to dry land. This type of succession has already been described for the Danube river in considering the morphology of the floodplain. More detailed profiles of the shorelines of two of the numerous lakes from the Riachuelo river of the Parana system (Bonetto et al., 1978a and 1978b) (Fig. 3.6) show the difference between Lago Totoras, a clear, moderately eutrophicated lake with prairie like marginal vegetation which is presumably young in temporal succession whereas L. la Brava, which is covered with floating emergent vegetation, possibly represents an older and mature lake of the same system.
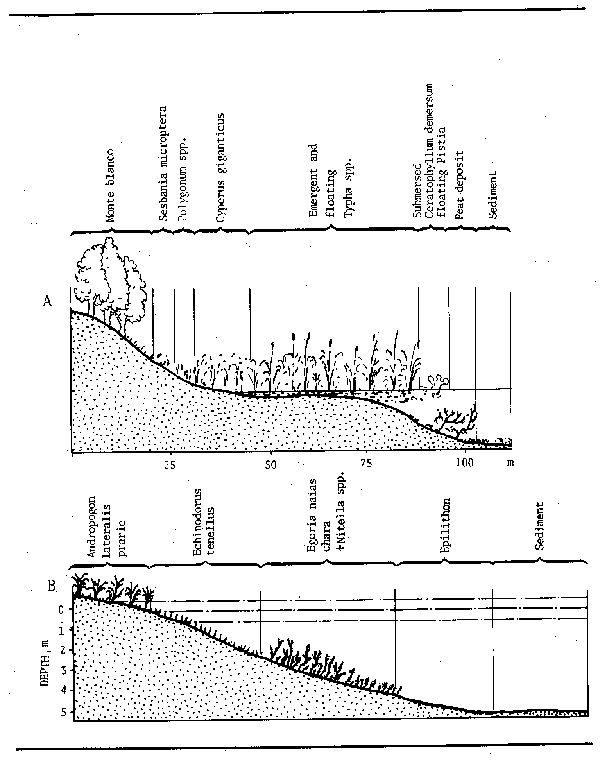
Figure 3.6 Zonation of littoral vegetation in two lagoons of the Riachuelo River A. Laguna La Brava; B. Laguna Totoras. (After Bonetto et al., 1978a and b)
In the short term there is an annual sequence of replacement of one species by another in response to the different degrees of flooding. In individual lagoons, vegetation increases in density and biomass throughout the dry season until it occupies most of the water surface. As the water rises much of the vegetation is washed out and biomass within the lagoon falls rapidly. Nieff (1975) has further defined the changes in the relative abundance of the species found in the lagoon showing the progressive occupation of the free waters by floating forms such as Azolla and Salvinia during rising waters. The subsequent growth of Polygonium punctatum, Ludwigia peploides and Mycrophyllum brasiliensis shades out the free floating forms and as water level falls, these plants are in turn replaced by Nymphoides indica.
Riparian forest is an important element in the vegetation complex of rivers in that, in the natural state, fallen wood structures the environment, leaf fall provides a major source of organic and nutrient inputs and the overhanging vegetation gives a mosaic of light and shade which conditions the distribution of many aquatic organisms. Originally many of the world's floodplains would appear to have been forested, at least by a strip of gallery along the channels. However, the influence of man and his domestic animals in colonizing the rich alluvial bottom lands has resulted in their clearance to produce the type of agricultural or savanna plains so familiar throughout the world. Two examples of this historical development are given by Yon and Tendron, 1981, for the alluvial forests of Europe (Fig. 3.7) and by Van Leynsele (1979) for the Ngiri floodplain. The Ngiri, which lies in the densely forested Zaire system is kept free of trees by regular burning of vegetation.
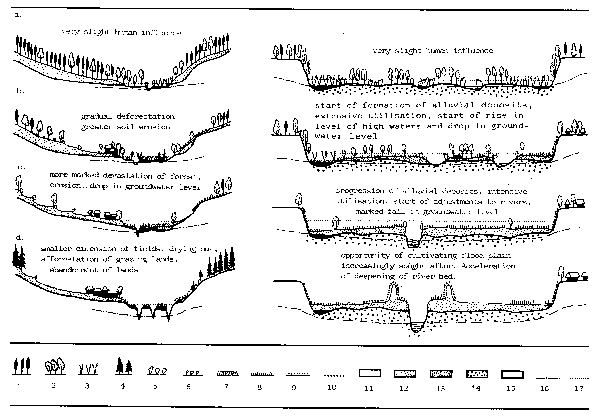
Figure 3.7 Schematic historical development of the landscape and vegetation of a river
valley in Europe. Middle reaches (right); Upper reaches (left).
a. At the beginning of the Christian era; b. Circa 1000; c. Circa 1800;
d. Circa 1900
1. beech gorves; 2. mixed forest and oak stands; 3. alder stands;
4. afforestation conifers; 5. bushy willows; 6. other bushes; 7. water
meadow; 8. fresh meadow; 9. dry meadow; 10. crops; 11. slope loam; 13. more;
14. gravel; 15. other soil; 16. average level of ground water; 17. average
level at high water. (After Yon and Tendron, 1981)
Deforestation is still proceeding throughout much of the tropical world, but considerable areas still remain in South America, Africa and Asia, where the floodplains are occupied by dense forests of flood-resistant trees. These may be of two main kinds which are best seen in the Amazon system where the ombrophilous lowland forests occupying the alluvial plains of the whitewater rivers are known as várzea forests, and the tropical evergreen peat forests occupying the floodable zones of the blackwater rivers are known as Igapá forest. River-side or gallery forests tend to occupy the levées on many wet savanna rivers and Bonetto (1975) described the Paraná as a corridor by which the Amazonian forest is able to penetrate far to the south of its normal distribution. Profiles of forested floodplains given by Sioli (1964) and Bonetto (1975) (Figs. 1.7a and b) describe the general distribution of the major vegetational zones of the Amazon and the Parandá Dry savanna rivers are frequently bordered by flood-resistent trees and scrub, mainly of the Acacia type, but also some palms especially on dry terrain ridges and leváes. In the Central Delta of the Niger, the boundary between unshaded and gallery forested floodplain channels was distinguished by Daget (1954) as following the 1 000 m isohyet; to the north drainage channels were unshaded and to the south were forested. The displacement southwards of the isohyet during the Sahelian drought has produced changes in the distribution of the woodlands bordering the river channels.
True submersed rooted aquatics form a high proportion of the macrophytes in temperate rivers and according to the data summarized by Westlake (1975) there are numerous studies indicating dry weight biomasses of around 5 000 kg/ha at fertile sites, such as backwaters and lagoons or within stabilized river channels. They are however, rarer in tropical systems. This appears to be mainly due to high turbidity or shading by floating meadows and other floating plants in the tropics, which prevent the development of species with no aerial parts. Aquatics with floating leaves are commoner in the slacks of pool systems, in quiet bays, openings and backwaters or just off the open waters fringe of the floating vegetation. Thus various species of Ceratophyllum, Trapa, Naja and Nymphaea are widely if sparsely distributed through quiet river channels and in most of the permanent waters of the world's floodplains. They also appear temporarily in the seasonally inundated area where they are concentrated in the major depressions and channels.
In the floodplain lakes of the Danube, Potomogeton perfoliatus, Valisneria spiralis, Ceratophyllum sp. as well as Trapa natans and Nymphaea alba contribute a significant portion of the biomass and total plant production (Academia Republicii Socialiste Romania, 1967). In Crapina lagoon, for instance, Nicolau (1952) calculated a biomass of P. perfoliatus of 1 749 kg/ha². Estimates in Academia Republicii Socialiste Romania (1967) indicated productions of between 2.52 and 4.55 gC/m². Production is high in June-July but had fallen by September. The lowest values were recorded in December. In the Pongolo river considerable standing crops of Potomogeton crispus of up to 1100 kg/ha² (dry wt) were recorded at the end of the growing season by Heeg and Breen (1982) indicating that submersed plants can also be important in semi-tropical systems.
One of the most conspicuous features of the tropical floodplain swamp communities are the vast areas occupied by floating vegetation; this may take the form of free floating types or of Sudd and meadow forming varieties.
Free floating forms. The same types of small free floating plant tend to recur throughout the world's swamps. Principal among these are Eichhornia crassipes, Pistia stratiotes, Azolla sp. and Salvinia sp., which form extensive mats which may choke water ways and induce deoxygenated conditions under themselves. They are influenced by the wind and current, and Bonetto (1975) has illustrated the manner in which this type of floating vegetation can accumulate at the outlet of depression lakes, clogging normal drainage until released into the main channel as “embalsados” (Fig. 3.8) Eichhornia crassipes, can double in number every 8–10 days in warm nutrient-rich waters (Wolverton and McDonald, 1976), but normal production is possibly less than this in the nutrient-poor swamps of floodplains. Dymond (in Westlake, 1963), for example, found a biomass corresponding to 1.4 kg dry weight/m² which is equivalent to an annual organic production of 11–33 t/ha.
Salvinia sp. can also form a major nuisance when introduced into waterways from which they were previously absent. In the Sepik River of Papua New Guinea, for instance, S. molesta occupied nearly all the free water in the system after its introduction and attained densities up to 6.8 kg of living material (fresh out) and 2.8 kg of dead and decomposing material/m² [equivalent to 96 t/ha of biomass], Mitchell et al. (1980). The mats effectively cut off all light from the underlying waters and also produce significant reductions in Dissolved Oxygen concentrations.
Although floating vegetation fringes temperate river channels, it is in the tropics that it finds its greatest expansion. In Africa four species dominate the more deeply inundated parts of the floodplains and regularly form vast floating mats fringing river channels and floodplain lagoons. During the floods portions of these mats are liable to break up to form floating islands or sudds. These are Cyperus papyrus, Echinochloa pyramidalis, E. stagnina and Vossia cuspidata. Of these, only C. papyrus is dependent on permanent water for its survival and reaches standing crops of between 10 and 34 t/ha dry (Thompson et al., 1979). In the Upemba basin production of C. papyrus ranged between 50 and 94 t/ha/yr.

Figure 3.8 Mechanism of release of floating vegetation masses “embalsados” during the flood cycle: (A) ponds with “embalsados” in flood condition; (B) drainage clogged by vegetation after flooding; (C) the same situation during the rains; (D) release of the “embalsados” into the river. (After Bonetto, 1975)
In the Amazon basin the varzea grasses desiccate during the dry season, although some may be semi-aquatic and have alternative dry season forms. During rising water there is an explosive growth phase which culminates after 4–6 months in flowering, followed by senescence and death. During the six month growing season Junk (1970) estimated Paspalam repens to attain 6–8 t (dry weight)/ha with a production surplus of 3–5 t/ha. Marlier (1967) estimated a mean standing crop of 96 t/ha fresh weight equivalent to 9.1 t/ha dry weight in Lago Redondo. The flora of Amazonian varzea lakes having high oscillations in water level (Fig. 3.9A) are composed mainly of P. repens and E. polystachya which die back completely at times of low water. In lakes with less extreme variations, more permanent islands of Leersia hexandra form and these become secondarily colonized with Cyperus sp. and eventually small trees and other non aquatic plants (Fig. 3.9B).
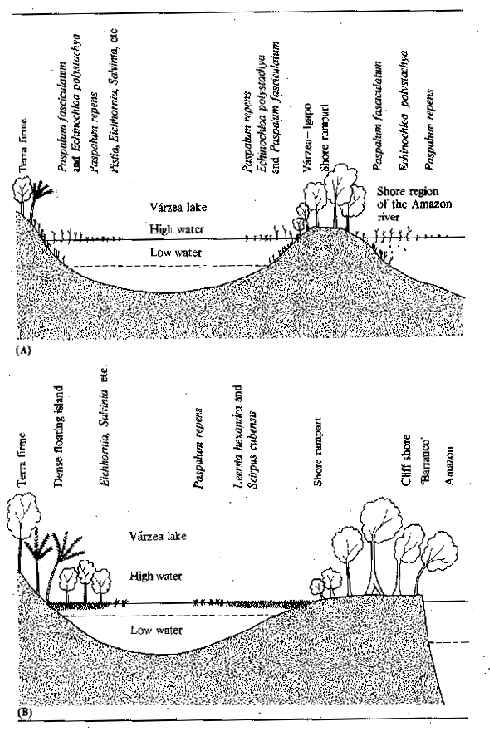
Figure 3.9 Schematic distribution of vegetation of two types of Amazonian varzea lake: (A) with big oscillations in water level; (B) with small oscillations in water level. (After Junk, 1970)
After the floods stranded floating vegetation decomposes extremely rapidly with 40–50% of dry matter being eliminated in the first 14 days and 60–70% within 50 days of desiccation. The nutrients concentrated within it are thus rapidly made available to other elements of the community, particularly to detritus feeders of various sorts. According to Howard-Williams and Junk (1976) the nutrient levels and nature of the decomposition litter varies very little with plant type, which means that, irrespective of origin, the detritus becomes chemically more and more uniform as decomposition proceeds.
The majority of savanna floodplains are covered with various types of grassland which follow a fairly typical annual course in all but the most highly cultivated plains. Junk illustrates such a temporal succession for grasses from the Amazonian floodplain (Fig. 3.10) whereby the dominant plants on the plain change annually in conjunction with the flood cycle. Most floodplain grasses are rhyzomatous and after the floods subside the enormous elaboration of wet season growth is burnt off either naturally or by man set fires. New growth of the dry season type is grazed by cattle or by wild game and burning may occur at intervals throughout the dry season. Very high productins have been recorded during this phase, of which the 23 kg/ha/day recorded by Heeg and Breen (1982) for Cynodon dactylon on the Pongolo plain may be considered normal. Although much of this is grazed some 825 kg/ha remain at the end of the dry season which means that in a single year some 34 tons of C. dactylon is submerged representing about 25 tons of wet organic matter as input to the aquatic system. In the floods some of these grasses may take on a wet season form with floating nodes which themselves develop roots, as in the case of Vossia and Echinochloa, or may remain rooted in the bottom but increase their stem length. Oryza barthii for instance, stands about 50 cm above the water surface irrespective of depth even when submerged under 2–3 m of water. Growth is very fast, as much as 1 m in two weeks, and productions of up to 2.5 t/ha can be achieved in five weeks. Total annual production can be reasonably high, and while dry grassland will not produce more than 2–3 t/ha/yr, productions of 10–20 t/ha/yr are not regarded as unreasonable for seasonally flooded grasslands by Thompson (1976).
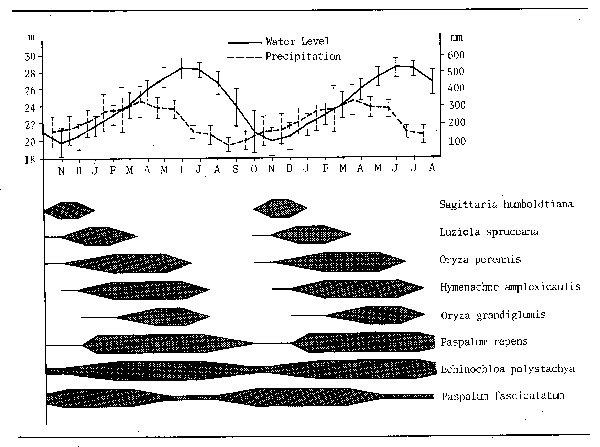
Figure 3.10 Temporal succession of grasses on Amazonian floodplains correlated with changes in water level throughout the hydrological cycle. (After Junk, 1983)
The floodplain grasslands are highly modified both by the natural flood regime, which selects for flood-resistent forms, but also by burning and grazing which prevent the recolonization of the plain by flood-resistent scrub bushes (Greenway and Vasey-FitzGerald, 1969).
Emergent vegetation: Emergent aquatic vegetation, such as Typha, Scirpus and Phragmites are widespread but localized in shallow muddy areas, and also tend to colonize sheltered alluvial banks of rivers in both temporate and tropical areas. Certain alkaline soils seem to favour these forms; the plain of reeds of the Mekong system where Eleocharis equisetima appears in great abundance (Le-Van-Dang, 1970) is a good example for this.
Stands of emergent vegetation, one established, are a major modifier of the ecosystem. By changing flow patterns they increase siltation accelerating the filling of floodplains depressions and contributing to the accretion of silt on islands on the main channel. They buffer the effects of scour and with their root and rhizome masses stabilize the river channel and its islands. On the temperate floodplain of the Danube, Bothnariuc (1967) recorded 1.2 kg/m² (dry weight) of plants from a marshy pond, and characterized the succession from ghiol to japse as a progression from a primary production based on phytoplankton in the open water “ghiol” to one based on higher emergent vegetation in the marshy “japse”.
Westlake (1963) placed tropical reed swamps as one of the most productive communities of plants with organic productions of up to 75 t/ha/yr. Analysis of Cyperus papyrus has shown it to have about 20 kg total biomass/m² in dense stands, the aerial portions forming 60–70 percent of the biomass (Thompson in Westlake, 1957), although 3–5 kg/m² were considered more likely over larger areas. Typha domingensis reaches a similar total biomass of 4.4 kg/m² with 52 percent underground. However, freshwater macrophytes in Malaysia are reported as having a standing stock of 370–520 g/m² (Wassink, 1975), a tenth of the values for African swamps.
There seems no doubt that the major part of the primary production of the floodplain is concentrated in the higher vegetation and principally in the perennial grasses. These die through desiccation, and by decay, burning or digestion by grazing animals are returned to the dry soil as nutrient, ash or dung ready for solution and utilization during the next flood phase. Floating weeds and sudd islands, which are often present in huge amounts, are also a mechanism for the translocation of nutrients within the system, representing a very real loss as they are swept downstream.
The remarks of Howard-Williams and Lenton (1975) summarizing the role attributed to higher vegetation in the littoral flood zone of lakes, can equally be applied to the floodplains and swamp vegetation:
(i) the vegetation provides a diverse habitat for animals and plants;
(ii) it acts as a filter and trap for allochthonous and autochthonous materials which in turn serve as nutrients for the plant communities themselves or for the associated aufwuchs and fish communities;
(iii) the nutrient pump effect of the emergent vegetation reputedly increases the concentration of elements in littoral areas of lakes and almost certainly does so in newly flooded waters of the floodplain;
(iv) it contributes to the autotrophic production in that as it decays it forms a rich detritus which is utilized as food by may organisms;
(v) furthermore, Howard-Williams and Junk (1977), suggested that the aquatic plants of the Amazon, and presumably of other nutrient poor systems, act as nutrient reservoirs and play a major role in the biogeochemical cycling of nutrients within the aquatic (floodable ecosystem) of the varzea. The vegetation which proliferates during the flood is deposited on the floodplain, on the exposed banks of the channels or in the lagoons where it decays. This material is then subjected to leaching and elution by tropical rainstorms and is stored in the soil until the next rainy season. This results in the conservation of salts in the floodplain rather than their being swept downstream disolved in the main water mass.
In forested rivers and streams, riparian or floodplain vegetation contributes considerable amounts of nutrients to the system. This has been best studied in low order temperate rivers where the autumn-shed leaves form an important source of allochthonous organic matter as well as food for invertebrates. Kaushik and Hynes (1971) have documented the decomposition of such leaves to show the build up of protein, nitrogen and phosphorus in water where the decomposing leaves serve not only to nourish invertebrates but to provide food for detritivore fish. Indeed in nutrient poor rivers the leaf fall may be the biggest single source of nutrients to the aquatic system.