Two groupings of small animal forms are found within the flowing waters of main river channels; the true zooplankton and the drift. It is often very difficult to distinguish between the two and in many cases the organisms comprising them are fairly similar. In torrential and low order streams the majority of the drift arises from the dislodging of aquatic benthic organisms and from terrestrial insects dropping from overhanging vegetation or swept into the channel with surface flow. In larger slow flowing potamonic rivers the role of these dislodged and entrained organisms diminishes, and the role of a true zooplankton increases accordingly. Kammeyn and Novotny, (1977) identified four components of the drift in the Missouri river: (i) particulate organic matter (POM); (ii) macroinvertebrates; (iii) larval fish, and (iv) zooplankton. Concentrations of POM and detritus ranged from 11 mg/m³ in anabranches through 783 mg/m³ at the main channel border to 2331 mg/m³ in the main channel in channelized reaches. Unchannelized reaches had less of the material with 18–135 mg/m³ in anabranches, 64–111 mg/m³ at the main channel border and 111–505 mg/m³ in the main channel. Although concentration of POM may increase in channelized reaches of the Missouri, Morris et al., 1968 recorded an opposing trend where mean values of drift organisms in unchannelized reaches were 1980 mg/m³ and only 230 mg/m³ in channelized reaches. These estimates indicate that about 526 kg of drift organism flow past a fixed point during the day in an unchannelized reach and 204 kg/day in a channelized reach. As with other workers Morris et al. found the species composition of the drift was very dissimilar from that of the benthos in large river channels and more closely resembled the composition of the aufwuchs, although about 20% of the drift by weight were terrestrial (allochthonous) forms. Numbers of drift organisms increased at night when densities were often double those during the day, and a marked diurnal periodicity in the drift has previously been described from many temperate rivers (see Hynes, 1970). In tropical waters too the number of organisms increases at night and particularly at nightfall (Elouard et Leveque, 1977). The abundance of larval and juvenile fish in the drift, which shows no such periodicity, may in part, be attributed to the downstream movement of yolk-sac and post yolk-sac larvae, and in part to the presence of individuals feeding on the drift organisms.
Information on zooplankton in rivers, particularly those of the tropics, is rather sparse but existing studies indicate that similar factors influence zooplankton densities as those influencing phytoplankton. The abundance of phytoplankton itself is possibly one of the major determining features and peaks in zooplankton abundance are apt to follow peaks in phytoplankton as exemplified for the Paranà river by Bonetto (1976) (Fig. 4.1).
Variations in zooplankton abundance have, however, been attributed primarily to differences in flow, with a number of other factors including turbidity, dissolved oxygen concentration and conductivity also playing a minor role. Under normal flow regimes only low densities of zooplankton are present in the main channel of rivers. For example only about 5 250 individuals/m³ of protozoa, crustacea and rotifers have been recorded from the Mekong river (Sidthimunkra, 1970). Rzoska (1974) also remarked on the small quantities of zooplankton found in the Sudd although Holden and Green (1960) had noted higher numbers of up to 16 000 individuals/m³ in the Sokoto river. In fact considerable differences are to be found between the planktonic fauna of the main channel and that of the lentic waters of side arms and floodplain and the zooplankton of both lotic and lentic waters fluctuates in abundance according to season. Thus whilst only 1 000 individuals/m³ of zooplankton were found in the Upper Paranà River at high waters 10 000 individuals/m³ were present at low water. Similarly in the Blue Nile at Kartoum planktonic Crustacea were present at about 20 000 individuals/m³ during periods of moderate flow but these rose to up to 100 000 individuals/m³ at low water (Tailing and Rzoska 1967). In the Nile Sudd densities were also higher in the dry season (mean of 4 460 individuals/m³ for four sites) than in the wet (mean of 2 070 individuals from the same four sites). The abundance of zooplankton in temperate rivers may be seasonally high relative to that in tropical waters, as in the Danube where Enaceanu (1964) recorded only 550 individuals/m³ in winter which rose to over 1 million individuals/m³ in the September peak. This superabundance of organisms may have been due to enrichment of the Danube waters by domestic and industrial contaminants but there is no doubt that, were flow conditions unfavourable, no such development would have occurred.
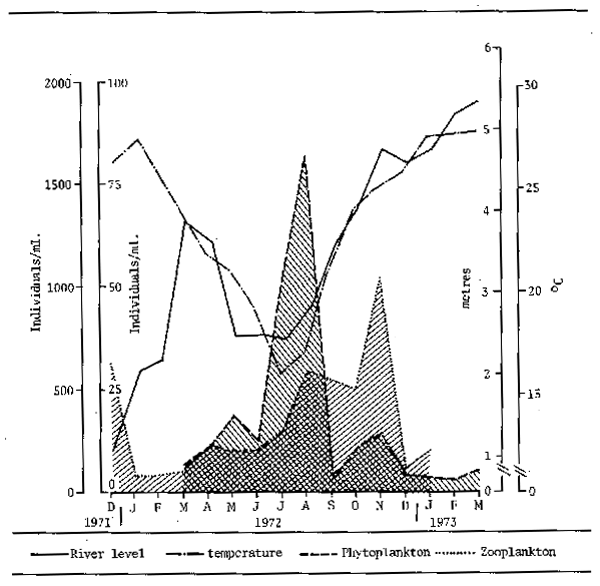
Figure 4.1 Variations in abundance of phyto- and zooplankton in the Parana river at the level of Parana relative to temperature and river level. (After Bonetto, 1976)
Zooplankton is much more common in the floodplain pools and backwaters where its abundance per unit volume is usually inversely correlated with the amount of water in the system. Vranovsky (1974), for instance, found the average biomass of zooplankton to be 14–15 times higher in the Baka side arm of the Danube than in the main stream (mean of 6.75 g/m³ for two years in the river). During periods of low or nil flow, greatly increased biomasses were present, especially in the isolated side arms where values of up to 30 times those of the main river were recorded. Similarly Sidthimunka (1970) was able to record 131 000 individuals/m³ in the Mong river, while only about 5 000 were present in the main stem of the Mekong. In the standing waters of tropical floodplains too, there is a widespread tendency for greater concentrations of organisms to be prsent in the dry season, as is the case with phytoplankton. Like phytoplankton, however, the decrease in relative density may only be a dilution effect and the number of organisms over the floodplain as a whole may in fact be higher during the wet season than during the dry. Bonetto (1975) recorded increases in numbers of zooplankters per unit volume even during the floods of the Parana, although elsewhere in the system the lowest values were found during the same period (Bonetto and de Ferrato, 1966). Increases in specific diversity often occur simultaneously with increases in absolute number. Rivers subject to marked annual thermal variations tend to have peaks of zooplankton abundance in the spring and summer months (Bonetto and de Ferrato, 1966; Bonetto, 1975), or in the spring and autumn (Ertl, 1966; Osmera, 1973) and these do not always coincide with the period of lowest water. The abundance of organisms varies and considerable numbers are occasionally found in permanent lakes. A summary of estimates of zooplankton abundance from floodplain standing waters is shown in Tables 4.1 and 4.2.
| River | Biomass g/m³ | Authority |
|---|---|---|
| Danube: | ||
| Dyje: | ||
| Locality 1 | 2.0–4.6 (471.6 max.) | Osmera, 1973 |
| Locality 2 | 7.75 |
|
| Locality 3 | 7.5 |
|
| Baka: | ||
| Side arms | 6.75 (mean of 2 years) | Vranovsky, 1974 |
| Amazon: | ||
| Lago Redondo | 1.61 |
Marlier, 1968 |
| Nile Sudd: | ||
| Aljab | 0.002–0.001 | Monakov, 1968 |
| Shambe | 0.002–0.40 | |
| Jor | 0.021–0.049 | |
| No 0.138–0.107 | ||
| Atar | 0.246 |
|
| Magdalena: | 0.92 (mean for 4 lagoons April) | |
| 1.95 (mean for 5 lagoons June) | Mikkola & Arias, 1976 | |
| 1.53 (mean for 6 lagoons August) |
a These figures are often based on only a few observations and do not therefore necessarilyreflect the full range of variations of the parameters measured
Flow is also slowed where waters are impounded behind dams and concentrations of zooplankton reach high levels in the reservoirs thus formed. This high concentration of organisms forms a locally increased concentration of zooplanktonic organisms at the point of discharge from the dam which diminishes slowly as the water mass moves downstream, Dzyuban in Mordukhai-Boltovskoi (1979) was thus able to state that flow regulation following the construction of the Volgograd reservoir has brought about great changes in the composition and abundance of the zooplankton in the lower Volga. These are now determined by zooplankton discharged from reservoirs together with the tendency of the river to restore a rheophilic type community. Thus zooplankton decreases in abundance downstream of the discharge point. Similar changes have occurred in the delta, mainly because of the decrease in the overall volume of water and the suppression of the spring flood. High concentrations of zooplankton were much more common in the floodplain pools of the upper delta where species with high capacities for parthenogenetic reproduction attained up to 3 g/m³. The life of these pools has been shortened and many have disappeared with a consequent reduction in overall zooplankton abundance. By contrast an increase in emergent vegetation has provided favourable habitats for phytophylic, periphytic and benthic forms. Extensive work on the zooplankton of the Nile showed similar changes below the various dams. For instance Brook and Rzoska's (1954) data for river plankton above and below Gebel Aulia showed a change in dominance from Copepoda upstream of the lake to Cladocera below the dam. With the addition of the Roseires and Aswan dams the Nile has undergone multiple regulation turning it into a cascade system in which the zooplankton community has changed from a riverine species dominated fauna to one more typical of lacustrine systems. In the lower Nile below the Aswan dam zooplankton density varied between 12 000 and 133 000 individuals/m³ with a more or less steady increase in density downstream. Since the closure of the dam, this lowest river reach has been converted to a semi-lenitic body of water.
| River | No. of individuals/m | Remarks | Authority |
|---|---|---|---|
| Danube: | |||
| Erec lagoon | 110 000 | Minimum: December | Ertl, 1966 |
| 4 006 000 | Maximum: April | ||
| Husie lagoon | 616 000 | Minimum: February | |
| 8 493 000 | Maximum: June | ||
| Dyje: locality 1 | 10 000 | Minimum: February | Osmera, 1973 |
| 500 000 | Maximum: May-July | ||
| locality 2 | 2 000 | Minimum: Spring flood | |
| 10 000 000 | Maximum: June | ||
| Missouri: | |||
| Backwaters | 6 670 | Mean: April-October | Kallemeyn & Novotny, 1977 |
| Dvina: | 247 999 | 400 000 | Zhadin and Gerd (1970) |
| Oka: | 950 000 | Spring | |
| 1 281 000 | Summer | ||
| Parana: | |||
| Don Felipe lagoon | 17 000 | Minimum: June | Bonetto & De Ferrate, 1966 |
| 1 200 000 | Maximum: October | ||
| Los Espejos lagoon | 26 000 | Minimum: July | |
| 277 000 | Maximum: December | ||
| Flores lagoon | 12 000 | Minimum: January | |
| 830 000 | Maximum: September | ||
| Laguna Totoras | 80 000 | Minimum: Falling water | |
November |
Bonetto et al., 1948 | ||
| 930 000 | Maximum: High water | ||
August |
|||
| Laguna Gonzalez | 6 330 000 | Minimum: Falling water | |
October |
Bonetto et al., 1948 | ||
| 24 000 000 | Maximum: Low water | ||
December |
|||
| Laguna La Brava | 536 000 | Minimum: High water | |
May |
Bonetto et al., 1978 | ||
| 761 000 | Maximum: Low water | ||
| Laguna Sirena | 12 000 | Minimum: Low water | CECOAL, 1977 |
| 536 000 | Maximum: High water | ||
| Magdalena: | |||
| Los Ponches | 5 380 000 | Maximum: Low water | Arias, 1977 |
| Machado | 4 360 000 | Maximum: Low water | |
| Pinalito | 25 300 | Maximum: Low water | |
| Sokoto: | 27 000 | Maximum: Dry season | Holden & Green, 1961 |
| Mekong: | |||
| Nong pla pak swamp | 38 500 | Random sample | Sidthimunka, 1970 |
| Ping and Nan Rivers | 136 234 | Outflow | Junk, 1976 |
| Bung Borapet | 122 279 | Inflow | |
| Amazon: | |||
| Lago Preto de Eva | 738 000 | Mean | Marlier, 1967 |
| Lago Calado | 107 087 | Junk, 1976 | |
| Nile: | |||
| Aljab lagoon | 180 | January | Monakov, 1968 |
| 3 000 | May | ||
| Shambe lagoon | 600 | February | |
| 3 000 | May | ||
| Jor lagoon | 2 700 | February | |
| 12 400 | May | ||
| No lagoon | 8 000 | February | |
| 53 000 | May | ||
| Atar lagoon | 59 000 | April |
a These figures are often based on only a few observations and do not therefore necessarilyreflect the full range of variations of the parameters measured
Kallemeyn and Novotney (1977) also found that numbers of zooplalkters declined downstream of the major impoundments in the Missouri river where the presence of zooplankton is strongly correlated with discharge from the Lewis and Clark lakes. A 51% decrease in abundance 16 km downstream of the point of discharge, 61% at 38 km and 70% at 145 km was shown by Hesse et al. (1982). High concentrations of zooplankton also arise from the injection of organisms into the main river channel from chutes and backwaters. In the Missouri this resulted in greater numbers in unchannelized section of the river where 6 670 individuals/m³ were present in backwaters, 12 110 individuals/m³ in chutes, 10 864 individuals/m³ in the main channel adjacent to the river bank and 12 245 individuals/m³ in the main channel itself. There was a strong peak of abundance in May at 370 mg/m³ when the rains flushed out the backwaters, deminishing to less than 20 mg/m³ in winter. Vranovsky (1974) noted the same phenomenon in the Danube where maximum values of zooplankton biomass (300–600 mg/m³) occurred in autumn and the average amount of zooplankton drifting past a fixed point reached as high as 260 g/s in 1967 due to the wash out of organisms from the side arms.
Sudden seasonal pulses in total zooplankton numbers seem to arise mainly by increases in rotifers, although the other major components of the zooplankton, the copepods and cladocera, also have characteristic peaks. Nauplii are common and are often washed out of the standing waters into the main channel during rising floods. Rhizopods have also been noted as an important element of the plankton at this time (Holden and Green, 1960; Green, 1963).
Some correlation between conductivity and zooplankton numbers has been found in vegetation flanking the main channel of the Zambezi river. Here maxima of both conductivity and zooplankton numbers (110,000 Cladocera and Copepoda m³) occurred during the dry season. Both parameters were minimal during the peak flood when zooplankton were nearly absent.
The degree of vegetation also appears critical and the greatest number of zooplanktonic organisms are commonly found in waters with at least sparse vegetation (Bonetto, 1975). Their density also increases locally at or near the open water/vegetation interface, under mats of floating vegetation and associated with submersed plants. This implies that there is a succession of planktonic organisms within the standing waters of the floodplain, which corresponds to the general evolution of such bodies of water, from the open water to the heavily vegetated state, as discussed by Botnariuc (1967). Comparative studies that demonstrate this are few, although Green (1972 and 1972a) has demonstrated such a succession in the floodplain lakes of the River Suià Missú in Brazil. As the vegetated areas are generally more productive than openwater areas, there is presumably a comparable evolution in productivity per unit area. This would, however, be compensated for by the diminution in size of the individual water body as it progresses through silting from the open water to the marshy state. Work on lagoons of the Danube floodplain by Ertl (1966) and the Elbe floodplain by Novotna and Korinek (1966), indicates that the density of the fish population influences the composition and abundance of the zooplankton. Detailed work on ecological inter-reactions of this nature is unfortunately lacking in tropical lagoons, although it is to be suspected that similar relationships may also be found there.
Zooplankton densities are usually considered to be low in the main mass of water on the floodplain, but the shallow littoral of the flood zone may support considerable quantities f planktonic forms. The higher conductivity and temperature, and local availability of oxygen can support blooms of zooplankton that can be readily observed at the waters edge. Unfortunately little quantitative information supports this as such areas have been relatively little investigated, but the high values for the zooplankton from the Baka and Dyje backwaters of the Danube in Tables 4.1 and 4.2 may be attributed to the fact that these water bodies are mainly littoral in nature (less than 1.5 m deep) with considerable submersed vegetation. In a comparison between littoral and pelagic zones in backwaters, Osmera (1973) showed that mean biomass from the littoral zone was 7.75 mg/1 as compared with 1.64 mg/1 in the pelagic zone of the same lagoon.
Survival of the adverse conditions of the flood poses certain problems for planktonic organisms which are readily washed away by the increased flows. Nevertheless, zooplanktonic organisms reappear rapidly when more favourable low flow patterns are reestablished. This has been attributed to reservoirs of organisms in upstream habitats, but Moghraby (1977) considered the 50–100 organisms/m present in riverside pools of the Blue Nile as inadequate to serve as sources for the regeneration of the population. Instead he produced evidence that the adults or the eggs of many species enter a diapause as temperatures are lowered and silt concentrations increased during the earlier part of the flood. He found abundant pockets of diapausing individuals in various types of bottom deposit and showed experimentally that they were only released when silt concentrations dropped and temperatures rose to the dry season norms.
Floating vegetation supports rich and varied animal communities either as a free living mesofauna in the open water among the plant stems or as an epifauna in the root masses of floating plants (i.e., pleuston). These have been studied in most detail in the root masses of the floating meadow grasses Paspalum repens and Echinochloa polystachya of the Amazon system from where many species of crustacea, insect nymphs, oligochaetes and molluscs were identified. Junk (1973a) distinguished three major biotopes according to fauna and flora. The first of these, the flowing whitewater biotope-consists of the stands of vegetation bordering the main river channel. Here faunal densities on the exposed outer fringe were low, possibly due to current, which sweeps organisms away, and to large amounts of inorganic sediment, which hinder feeding. Faunal abundance increased from the fringe towards the centre of the stand or mat and total abundance at the centre could be as-high as 100 000 individuals/m² although the norm was around 50 000 individuals/m². Biomass increased from 0.3 g/m² (dry weight) or 1.5 g/m² (wet weight) at the fringes to 4.2 g/m² (dry weight) or 20 g/m² (wet weight) in sheltered places within the stand. A second biotope, well-oxygenated sedimented whitewater lagoons, undergoes fairly wide annual fluctuations in level which periodically destroy the aquatic vegetation. During the optimal growing period large numbers of individuals occurred, abundance usually ranged between 100 000 to 300 000/m², although densities of up to 700 000/m² were recorded. Faunal density was evenly distributed and biomass varied between 2.5 and 11.6 g/m² (dry weight) or 12–62 g/m² (wet weight). Marlier (1967) found similar values ranging from 3.9 to 13.9 g/m² (dry weight) or 18.9–39.7 g/m² (wet weight) from three samples of floating vegetation in another lake of this type. The third biotope - sedimented whitewater lakes - had thick stands of vegetation supporting little or no dissolved oxygen. Water regimes are more stable than those of the former type of lagoon. In the peripheral zone of the vegetation mats, faunal abundance was about the same as in the well-oxygenated lake, but various groups of organisms rapidly disappeared due to oxygen stress and abundance was very low (0.16–0.29 g/m² - dry weight) even only a few metres from the outer edge.
This community is also very important in the middle Paraná river where the abundance and density of Eichhornia, Pistia, Salvinia and Azolla supplies an sample substrate (Bonetto, Dioni and Pignalberi, 1969). As examples Bonetto et al. (1978) found 17 000 individuals/m² of molluscs, oligochaetes, leeches, mites, crustacea and insects in Salvinia herzogii during the summer (corresponding to high water) and 9 200 individuals/m² during winter in laguna La Brava of the Upper Parana; Varela et al. (1978) describe in detail the floral and faunal associations in the floating islands or “embalsados” of that lagoon; CECOAL (1977) found a maximum value of 20 000 individuals/m² in summer falling to 4 000 individuals/m² in winter for Azolla and Pistia. Also in the Parana system over 100 taxa at specific or generic level were found in the submerged stoloniferous masses of Eichhornia (Poi de Neiff and Neiff, 1980) where seasonal changes in the dominance of organisms and differences between the fauna associated with plants in river and lagoon were also observed. Here maxima reached 262 000 individuals/m² within the Paspalum repens/Salvinia hertzogii complex (Poi de Neiff, 1981). Also in Latin America, Zarate and Cubides (1977) found a range of biomass from 6.56 and 130.7 gm/m² wet weight (Mean 35.14 g/m²) for 17 stations on lagoons of the Magdalena system. The authors identified 67 taxa of invertebrates in the root masses of Eichhornia. Similar distributions of organisms have been found in African papyrus swamps where the zone close to the interface with the open water was considered the richest habitat in the system by Rzoská (1974). Many species of oligochaetes, Bryozoa, Protozoa, Crustacea, insects and molluscs have been found among the papyrus, reeds, Echinochloa and Eichhornia of the Nile Sudd, Monakov (1969). Few quantitative samples have been taken of this habitat, but 5 minute pond net sweeps captured as many as 547 individual Crustacea (Rzoska, 1974). Monakov (loc. cit.) who also attempted to quantify the biomass of the Entomostraca, found that the fringe was 10–100 times richer than the open water of the Sudd.
Petr (1968) found between 5 000 and 16 000 individuals/m² in the roots of Pistia from Volta Lake. In the Sudd, Rzoská (1974) found up to 300 individual animals per pistia plant, although densities increased in quiet stretches of the river, notably in the swamps and reached a peak in river lagoons. Fewer individuals were noted in floating vegetation in Bangula lagoon by Shepherd (1976). Here a mean of 388 individuals of insect, oligochaete, crustacea and molluscs were present per m² of Nymphaea and a similar assemblage at 210 individuals/m² was found in Pistia.
The weight of animals associated with the roots of Eichhornia was also high in the cienagas of the Magdalena river, where Kapetsky et al. (1977) reported a mean of 35.1 g/m² from 17 sample sites in 10 different water bodies. Values ranged from 6.9 to 130 g/m².
Investigations of submersed vegetation in two lakes of the maritime delta of the Danube show the considerable differences that can arise in faunal densities depending on relative distribution of support plants. In Porcu lake Ceratophyllum demersum covers about 64% of the bottom and Nitellopsis obtusa about 4% whereas in Rosu lake C. demersum occupied 14% and N. obtusa 53% of the bottom. Both plants form a similar type of substrate and host similar species of Oligochaeta, Trichoptera and (Chironomida) yet total quantities in Porcu lake, at 1.8 kcal/m² (0.36 g/m²) in May and 93.4 kcal/m² (18.7 g/m²) in August exceeded the 2.2 kcal/m² (0.44 g/m²) in May and a maximum of 18.7 kcal/m² (3.74 g/m²) in June. The differences in biomass were attributed to the greater density of organisms supported by Ceratophyllum (Izvoranu, 1982).
A particular community has been identified in the umbels of Cyperus papyrus (Thornton, 1957). This consists principally of terrestrial forms which pass their lives in this specialized habitat, but which contribute to the allochthonous food supply of the swamp waters.
The literature on the benthos of running waters has been reviewed by Hynes (1970) who concluded that similar elements of the fauna of hard substracts are common to streams and rivers all over the world. The benthic fauna of hard, stony runs and riffles is richer than that of the silty reaches and pools of the rhithron in both number of species and in total biomass. The communities of Soviet rivers have been classified on the basis of both substrate and current velocity (Zhadin and Gerd, 1970) as follows:
- Lithorheophilic communities inhabit solid substrates in flowing waters and consist mainly of large insects.
- Psammorheophilic communities occur over sand bottoms in flowing waters and consist of small arthropods and protozoons living in the interstices of the sand.
- Argillorheophilic communities inhabit clay substrates and are mostly sessile or burrowing forms.
- Pelorheophilic communities live in silt in flowing waters.
- Pelophylic communities are found in silt in still waters.
- Phytophylic forms live in backwaters rich in plants.
Benthic fauna is, as a rule, better developed on stable bottoms. On mobile bottoms sessile insect larvae with their sensitive ducts or tissue structures and also moving molluscs are readily displaced. Other more mobile forms such as Asellus or Gammarus may equally be at risk through the risks of mechanical damage.
Biomass was thus found to be closely related to the type of community. For instance, in the Dnieper Lithorheophils had a standing crop of about 1 kg/m², Pelorheophils about 10 g/m² and psalmophils about 1 g/m² although isolated pelophils in cut-off lakes attained over 100 g/m².
In the Danube pelorheophilic (with 16.5–99.2 g/m²), psammopelorheophilic (with 22.7–69.2 g/m²) and Argillorheophilic (with 71.6 g/m²) communities all and considerably higher studing crops than the psamorheopilhic community.
Russev (1967 and 1981) has also described the distribution of biomass according to location and bottom type in the Danube with mean values of 72.5 g/m² over leaf litter, 47.4 g/m² over mud, 26.4 g/m² over clay and 0.24 g/m² over sand which is far less stable a substrate. Furthermore water speeds in excess of 2–3 m/s¹ inhibit the development of benthos in the centre of the channel forcing it to concentrate mainly in protected shingle areas was of the littoral (Russev, 1982). The mean biomass on the Bulgaria reaches of the river was 35.22 g/m² of which 34.21 g/m² were molluscs. Similar mean values of 38.9 g/m² (total) and 34.21 g/m² (molluscs) were obtained in the autumn. Biomass per unit area varied considerably with water level. The mean biomass (without molluscs) was only 0.76 g/m² at high water (April-August), whereas at mid-water it was 1.94 g/m² and at low water 6.34 g/m². The much higher values obtained as water level decreased were attributed to the dislodging and transport downstream of benthos at times of high flood, and the dispersion of benthic organisms over a wider area during the flood period.
In tropical rivers Bishop (1973) commented on the similarity of rheophilic rainforest communities in Africa, Sri Lanka, tropical S. America and the Gomback river Malaysia. The lowland reaches of rivers share many features, but relatively little information is available3 on the benthic fauna of the slow-flowing silt-laden rivers associated with floodplains.3 Such that does exist indicates a generally poor fauna consisting of a relatively small number of species consistent with Zhadin and Gerds conclusions of the low biomass of pelorheophils in Russian rivers. Bonetto and Ezcura (1964) reached similar conclusions based on observations on the Parana river which showed the benthic fauna to diminish in diversity and, abundance as the current slows. Maximum values may reach over 71 000 individuals/m² in the region of Yacireta as compared with 17 000 individuals/m² at Posadas, 23 000 at Ituzaingo and 13 000 at Ita bate (CECOAL 1979). Data from the Amazon (Junk 1971) also indicate a poor fauna in the main river, although experiments with artificial substrates where 12 900 individuals/m² settled in four days showed populations in blackwater rivers to be higher than expected. Monakov (1969) also concluded that the bottom fauna is monotonous in the main channels of the main channels of the Sudd where only small groups of Chironomids and Oligochaetes inhabit the sand and mud bottoms. In general biomass ranged between 0 and 0.2 g/m² although Rzoska questioned this conclusion on the basis of the small number of samples taken. Further downstream, between the Aswan dam and the sea, the macrobenthos is dominated by Molluscs although some Polychaetes and Oligochaetes were also present. Quantitatively biomass varied between 1–9 g/m³ rising to the unusual value of 53.61 g/m³.
Differences in species composition have also been recorded from some rivers, for instance the mollusc fauna of the Niger consists mainly of abundant Aetheria elliptica, Aspatharia, Mutela dubia and Viviparus unicolor in muddy reaches, constrasted with rare individuals of Corbula fluminialis, Mutela rostrata, Caelatura aegyptica and Cleopatra bulminoides in sandy reaches (Blanc et al., 1955). Large aggregations bivalve molluscs also occur in the Volga, Dneister, Dneiper and Don. In the Volga Dreissena contribute local standing crops of several kg/m². Otherwise the benthos of the river is poor in the main channel. A high benthic biomass of 400 g/m² has also been recorded from the many tributaries of the Mekong (Sidthmunka, 1970) which again was almost entirely due to a concentration of molluscs.
In the Missouri river there are no data to show the benthic populations before impoundment, but studies by Morris et al. (1968) indicated that there is relatively little difference between the benthic biomass per unit area in channelized and unchannelized reaches. In the first case mean biomass was calculated as 0.08 g/m² and in the second at 0.07 g/m². These means, however, conceal considerable variations with locality as within the river biomass varied between 0.019 (0.048) 0.99 g/m² in unchannelized reaches of the main stream whereas in channelized reaches it varied between 0.002 (0.007) 0.022 g/m². On mud banks the biomass was higher in channelized reaches at O.058 (0.803) 2.678 g/m² than in unchannelized reaches where it was 0.111 (0.267) 0.349 g/m². The general scarcity of benthos was attributed to the general nature of the Missouri river in that it has shifting substrates, high silt load, fluctuating water levels and absence of aquatic vegetation, all of which militate against high production of benthic organisms. However an overall loss of benthos occurred in channelized reaches where the area available for colonization was reduced by 67% over the unchannelized reaches. Earlier Berner (1951) had found similar values ranging between 0.024 g m-2 in the middle of the channel to 0.258 g/m² nearer the edge, and had constrasted the low values from the Missouri with the clearer, slower flowing Illinois where a mean of 29.23 g/m² was found by Richardson (1921) at a time when the river still had its floodplain lakes. Schramm et al. (1974) summarized the data for benthos from the Middle Mississippi which show similar differences between channelized and unchannelized reaches. On the basis for this they emphasize the importance of the channel area for benthic production.
Information obtained from backwaters and standing waters of the floodplain indicate somewhat higher standing crops than in the main river. In the Romanian portions of the Danube floodplain a mean biomass of 20.06 ± 9.35 g/m² was calcualted from 12 lakes (Academia Republicii Socialiste Romania, 1967) and in lakes of the Volga delta values of between 7–48 g/m² have been found. Monakov (1969) recorded biomasses of zoobenthos consisting mainly of Oligochaetes, chironomids and molluscs ranging from 0.58–4.7 g/m² from five of the floodplain lakes of the Sudd. The mean values of 2.95 g/m² for the period of high but falling water and 2.07 g/m² for low water compared with the 0–0.2 g/m² found for the main channel. Benthic biomass quoted for the Nong Pla Pak swamps of the Mekong floodplain (Sidthimunka, 1970) were of the same order at 1.75 g/m² at a time when, benthic biomass in the main river was only 0.12 g/m². Similarly a mean of 1.51 g/m² (range 0–6.51 g/m²) of benthic organisms was found in cienagas of the Magdalena system by Mikkola and Arias (1976). Here a mean of 1.9 g/m² was found in the open waters, of the Cienagas whereas the more sheltered bay habitats had a mean density of 3.1 g/m². Arias (1977) found values of 1.76±1.81 g/m² for the open waters of 18 cienagas in this system when the bay areas had values of 2.96 ± 1.51 g/m². These variations in biomass were attributed to the often deoxygenatedconditions in the deeper waters of the cienagas.
The tendency for littoral waters to support more benthos than the deeper waters has been confirmed by numerous other workers. Bonetto et al. (1978) furnished detailed information about the benthic fauna of two lagoons of the Riachuelo River, a tributary of the Parana system. In the laguna Totoras benthos was maximal during low water when total numbers exceeded 114,000 individuals/m². In summer, during high water, maximum abundance was much less at 46 500 individuals/m². In this lagoon too, there were considerable differences between the various zones of the lake with maximum abundance in the sub-littoral zone and the least in the centre. Similar differencies between the littoral and open water zones on one hand and between seasons on the other, were found in Laguna Gonzalez which, because of its great eutrophication, supported less organisms. In Laguna La Brava the meso and macro benthos contained between 17 500 and 95 300 individuals/m² at low water and 1 700–57 000 individuals/m² at high water depending on location in the lake. Particularly significant in this water body were thecamoebans which numbers sometimes exceeded 1.5 × 106 cells/m² at low wate but only 0.6 × 106 cells/m² at high water.
Three temporary lagoons in the savanna floodplain of the Rio Branco, N. Brazil, supported mean weights ranging from 0.16–0.88 g of benthos/m². There were some variations with depth and individual samples gave values as high as 2.52 g/m². The 0.25 g/m² (dry weight) obtained from the Lago de Rodondo appears consistant with the range of biomass found in the lagoons, despite Marlier (1967) opinion that these were very low. Nevertheless higher values have been reported for some Amazonian lakes, Eittkau et al. (1975) for example, found a mean annual weight of between 0.14 and 6.20 g/m² of benthos, and studies by Reiss (quoted in Sioli, 1975) also revealed biomasses up to 6.2 g/m² in the centre of Amazonian Varzea lakes. By contrast, samples from lake Tupe, a typical river lake, and some blackwater várzea lakes, led Reiss (1977) to conclude that the profundal zone of such bodies of water supports the poorest benthic fauna of any Amazonian lacustrine biotope. Values from the littoral zone are however much higher, reaching up to 104 g/m².
The range of densities from both African and Latin American floodplain lagoons suggests extreme values ranging from 0 to about 6 g/m² and mean values of about 2 g/m² for tropical rivers. Rzoska (1974), however, considered that values from such areas should be treated with caution as the cyclic energences of Ephemoptera, Chaoborus and chironomids can influence the standing crop and biomass profoundly. Very dense localized populations of other organisms can also arise where conditions are particularly favourable. Botnaruic (1967), for instance, recorded densities of molluscs of up to 2 657 g/m² in the deep water of Crapina lake of the Danube floodplain. Values are not normally this high, and Russev (1967) quoted Russian work on the 8 580 km² Kiliya arm in the Danube delta, where the mean biomass was 9.45 g/m². The same work indicated an annual production of 19 235 t (224.19 g/m²) from the whole arm. Work by Lellak (1966) on the benthos of Czechoslovakian backwaters showed this to be strongly affected by the presence or absence of fish. Where fish were absent, population densities of Chironomus and Chaoborus larvae were very high. Similarly, Enaceanu (1957) showed benthic biomass to be higher in enclosures from which fish were excluded (226.12 kg/hm² mean July-October) than in bottoms freely accessible to fish (70.98 kg/hm² for the same period) in the same lagoon. In floodplain pools where the composition and density of the fish populations isolated by receding flood waters are likely to vary, differences in benthos are liable to arise as a result of diversity in predation pressures. Although the authors quoted above have noted seasonal fluctuations in the benthos of the standing waters of the floodplain, Bonetto (1975) considered this the least affected by differences in water level of all communities inhabiting such habitats.
Submersed vegetation, where present, also acts as a centre of concentration for benthic invertebrates. Examination by Academia Republicii Socialiste Romania (1967) of 1 550 g of Potomogeton perfoliatus, collected from an area of 0.5 m², showed 10.489 individuals of insect larvae, Crustacea and molluscs to be present. The total weight of 87.3 g equivalent to 175 g/m² is reasonably high for the benthic fauna of this system, which normally varies around a mean of 20 ± 9 g/m². Values from the Bangula lagoon ranged between 0 005 g/m² under Pistia, 0.06 g/m² in open water, 0.378 g/m² in Ceratophyllum and 0.977 g/m² under Nymphaea. The numbers of individuals present in Ceratophyllum was still relatively low, 4 385 individuals/m² but was significantly greater than the densities found in Nymphaea and Pistia. Very large concentrations of invertebrate, including acarine mites, snails and phytophagous species of insect and mollusc were present in Egeria naias of the Totoras lagoon where between 10 000 individuals per 1 000 g of plant were present in winter and 320 000 individuals per 1 000 g of plant were present in summer.
On the floodplain itself, where seasonal desiccation occurs, flood season populations of macro-invertebrates are less well studied. Personal observations have shown enormous densities of pulmonate snails to be present on the bottom and at the water surface of inundated areas. Carey (1967 and 1971) reported Ephemoptera nymphs, Trichoptera larvae, chironomid larvae, Hemiptera and molluscs to be very abundant and widely distributed in the inundated zones of the Kafue River, especially in submerged banks of Najas and Ceratophyllum. The profusion of molluscs on the floodplain has also been noted on the Central Delta of the Niger by Blanc et al. (1955). In the Parana river, Bonetto, Dioni and Pignalberi (1969) commented on the restricted number of species which contributed to the great biomass. Particularly abundant were Unionacea, which made the most important contribution, despite the low calcium content and relatively low pH. The fact that molluscs can make a significant contribution to the benthos under these conditions is emphasized by their presence in Amazonian blackwaters. Certain of these are, however, so poor in minerals that only small Ferrissidae with conchiolin cases are present (Fittkau, 1967).
In the forest floodplains of the Amazon inundated with whitewaters, benthic organisms are common and have two production peaks, one at the beginning of the inundation and a second one after maximum high water in June/July. Reiss' (1977) work on the blackwater river lake Tupe, also indicated two peaks of abundance for littoral benthos. One peak occurred on the rising flood when 2 559 individuals/m² were recorded, and a second peak occurred at low water when 1 248 individuals/m² were present. Minimum densities during falling water were about 623 individuals/m². The faunal composition changed completely between the minimum and rising water level peaks. At low water chironomidae dominated the fauna with minor representation by oligochaetes, Acari and Corixidae. During rising water Chaoboridae and Ostracoda became steadily more important to the exclusion of other groups. Blackwater flooded Igapo forests, however, are generally very impoverished; Irmler (quoted by Sioli, 1975) found a benthic biomass of only 0.2 g/m² in such an area.
In areas of rapidly fluctuating water level which are subject to seasonal desiccation, it is evident that mechanisms must exist to survive the dry season. Fittkau et al. (1975) considered three possible mechanisms: migration, dormancy and recolonization. Hynes (1975) also considered that, in view of the high degree of adaptation required for resting eggs or burrowing and aestivating larvae, recolonication is by far the easiest strategy for drought survival. This implies very rapid growth of larvae, and in fact most larvae and nymphs in the Ghana river studied by him were fully grown after a month. Whyte (1971) has even noted some chironimid species which reached full size in three weeks. Studies on Rhodesian streams liable to seasonal desiccation have shown that the re-establishment of the fauna after the annual resumption of flow can be very rapid (Harrison, 1966). Oligochaetes, Crustacea and insect larvae appeared within ten days and there was a form of succession whereby the species composition typical of pools was re-established and stabilizing within a month. Harrison's conclusions also indicated that pulmonate snails and oligochaetes survived the drought by aestivating. Some smaller crustacea may have had dormant eggs. Most insects recolonized the area by the movement of flying adults from more permanent water bodies.
Decapod crustacea, which form part of the macro-benthic community, are a particularly important element of tropical river fauna. Ecologically they may be considered together with fish on the basis of their size, position in the food chain, behaviour and economic importance as food organisms. Species of Macrobrachium particularly form the basis for fisheries in many tropical rivers (see Kensley and Walker (1982) for the Amazon; Robertson (1983) for the Sepik river, FAO/UN (1971) for the Oueme River and Inyang (1984) for the Lower Niger river) and have also been widely adopted for fish culture. Similar activities are pursued with Astacus, Procamberus and Pacifastacus in the temperate waters of USA and Europe.
The neuston “community” is little discussed by workers on tropical floodplain ecology. Nevertheless, personal observations have shown the abundance of forms living at the air water interface. Mosquito larvae and various forms of pulmonate snails are extremely common, as are water striders (Hemiptera and Coleoptera), mites and spiders, particularly in the sheltered water among the stems of the floating vegetation. It is to be supposed that this community expands considerably during the flood season, although as yet there are no recorded observations to support this assumption.
Both larval and adult stages of various species of frogs and toads are plentiful on floodplains. Most fringing areas of the plain and all permanent and temporary swamps are colonized and in the most deoxygenated areas, tadpoles are often the only form of vertebrate life. Similarly amphibians rapidly appear in the most isolated of temporary pools. Little work appears to have been done on the ecology and dynamics of amphibian populations of rivers although they undoubtedly form an important component of the fauna of the floodplain, and probably contribute to the rapid recycling of detritus and mud by converting it into flesh which is useable by predatory species of fish, reptiles and birds. Frogs are exploited commercially for their legs particularly in China and Romania. However the resources have been either over-exploited, or the stocks have been reduced by pollution in some areas, for instance France, where they are mow protected.
Several families of reptiles have remained associated closely with water, and of these, four groups (Crocodiles, monitors, iguanas and turtles) are regularly found in and around rivers and the permanent and temporary standing waters of their floodplains. Certain species of snake too have adopted aquatic or semi-aquatic habits. Various forms of crocodile are distributed around the tropical world, and have been widely associated with flood rivers in the past. They have, however, been subject to widespread slaughter and are now virtually absent from many parts of their former range. The economic importance of the crocodile as a consumer of fish has been discussed, principally by Cott (1961). Although crocodiles eat a large quantity of fish, they also prey on other organisms which themselves are predators of fish. Their status as major competitors of man for fish is therefore somewhat obscure. A second function of crocodilia in the ecology of some types of tropical water has been suggested by Fittkau (1970 and 1973). In the nutrient-poor rain forest the larger elements of the community serve as nutrient sinks, which slowly accumulate the few minerals available (Fittkau and Klinge, 1973). Such a system depends much on the abundance of its species which are able to maximize the storage and recycling of nutrients of allochthonous origin. A similar process is thought to occur on the equally nutrient-poor blackwaters of the rainforest zones. In such aquatic systems the low level of nutrients does not permit much primary production and the food chain originates mostly in the rain of allochthonous material. Fish moving into the mouth lakes are allochthonous to that particular ecosystem and are fed upon by a variety of large predators, of which caimans are perhaps the most significant. A medium-sized caiman can eat between 0.6 and 0.8 percent of its body weight per day and excrete about 0.20 to 0.27 percent of its body weight of nitrogen, phosphorus, calcium, magnesium, sodium and potassium ions per day. Their contribution to the nutrient balance of such environments has been estimated by Fittkau to be locally superior and complementary to the nutrients derived from rain water, itself the major source of nutrients in the system as a whole. In places where crocodiles have been eliminated, declines in fish production have been noted, possibly because of a drop in the primary production based on the excreted nutrients.
Birds are a very conspicuous feature of the floodplain ecosystem. As with most wetlands various kinds of waterfowl are extremely common, but it is doubtful whether most of these react directly with fish populations. Piscivorous predators are also abundant, however, and represent possibly the greatest source of pressure from outside the aquatic system. Reizer (1974) for instance listed 37 species of ichthyopredator in the Senegal river and 19 species are listed by Shepherd (1976) from the Shire river. Birds are capable of taking a wide range of species of all sizes and the piscivorous bird community appears to be specialized toward the taking of particular sizes and types of prey.
In general, the life cycle of water birds is closely linked to the floods. Avian breeding seasons coincide with those of the fish and the fledgelings are being reared at just that time when small fish suitable for their feeding are most abundant. There is a particular heavy predation during the period of receding water when many fish are stranded in temporary pools (Lowe-McConnell, 1964; Bonetto, 1975).
The impact of birds on the fish population is potentially very large, and studies from Africa indicate that the amount of fish taken by them can surpass the amount taken by the fishery. In the Senegal river, for instance, Reizer (1974) presented figures by Morel of between 100–200 000 herons and cormorants and 2 000 pelicans in the delta alone. Fish consumption was estimated at between 500–1 000 gm per day for the darter Anhinga rufa and for a heron, 1 000–2 000 gm per day for a pelican and 250 gm for the kingfisher, Ceryle rudis. On the basis of this birds take about 70 000 t/yr of fish as compared to a fish catch of about 50 000 t/yr.
Bowmaker (1963) gave an estimate for the daily ration of the cormorant Phalacrocorax africanus of 78 gm/day leading to a consumption of 286 t/yr from Lake Bangweulu. This estimate may be low by a factor of 10 in view of Reizer data and that of McIntosh (1978) who found that the European cormorant (Phalacrocorax carbo L.) eats some 650 to 700 gms of fish/day. Bowmaker concluded that the African cormorant is not harmful to the fishery, but could in fact be beneficial by its use of non-commercial species, and by its excreta which fertilize the water. Other figures from cold water streams indicate that kingfishers eat about 14 kg of fish/km, of stream terns consume about 63 kg of fish/km of river and Mergansers take some 25 kg/km. These are equivalent to 2.3%, 14.1% and 4.2% of total mortality respectively (Alexander, 1979).
Several aquatic or semi-aquatic mammals figure in the ecology of rivers although their role is by no means always clear. Beavers, which are confined to the north temperate zone, have had an enormous influence on the aquatic system. Their habit of building dams resulted in the more or less permanent inundation of large areas of floodplain,creating widespread swampy conditions in the valley bottoms. The dams themselves affected flow characteristics of streams, and the organic debris increase habitat diversity and cover for fish. Beaver populations have been drastically reduced in parts of Europe and North America and their dams have been cleared from rivers in an attempt to improve drainage. This process, together with the clearance of dead wood, stumps and other naturally occurring obstructions from the stream bed, has produced much dryer conditions in the valley bottoms and has effectively removed considerable areas of floodplain from lower order temperate streams. Otters are widespread throughout temperate and tropical systems. They apparently prey heavily on fish, although population densities are far from high so the impact of such predation is, in most cases, probably relatively low. They, do, however, have a bad reputation with fishermen for their habit of robbing and damaging setgear such as traps or gill nets. One record by Alexander (1979) shows otters to take about 13 kg of trout, km of cold water stream against 25 kg/km taken by mink. These figures were equivalent to 3.0% and 5.6% of total mortality respectively.
In African rivers hippopotami contribute a large amount of fertilizer to the aquatic system by cropping terrestrial vegetation and excreting it into the water in a form readily accesible to phytoplankton. Densities of hipopotamus were at one time very high in most African inland waters and they must have made a considerable contribution to nutrient inputs and recycling. They have, however, been eliminated through much of the continent and their numbers have been considerably reduced even within the confines of protected zones.
In Latin America and elsewhere, members of the family Sirenidae appear to play a similar role in nutrient balances particularly in the black water rivers and mouthlakes of the Amazon. The dugong (Dugong dugon) is distributed throughout the Indo-Pacific region (Husar, 1975). The african manatee Trichechus senegalensis is distributed throughout the rivers of West Africa and is present in considerable number in Cameroon, Gabon and in the Niger River System, (Nishiwaki et al., 1982). Trichechus manatus is distributed from Florida to North Eastern Brazil (Maranhao) and Trichechus inguinis is found throughout the Amazon river system. Studies on the feeding of Sirenidae (Best, 1981) show manatees to consume about 8% of their total body weight in aquatic plants daily whereas the dungong eats about 14%. Their selected foods are higher plants not normally eaten by other organisms and they are thus not in active trophic competition with other elements of the community.
Many large river systems have species of dolphin of the family Platanistidae. Platanista gangetica is found in the Ganges, Brahmaputra and other rivers of the Ganges basin. Platanista minor inhabits the Indus river and enters the Zambezi, Lipotes vexellifer occurs in the Yangtse, Inia geoffrensis is distributed throughout the Amazon system together with Sotalia fluviatilis, the only freshwater Delpinid, which is also found in the Orinoco river. Pontoporia blainvillei inhabits the lower reaches of the Parana and Uruguay rivers, the estuary of the Rio de la Plata and the adjacent coasted waters. Orcaella breviostris, which primarily inhabits shallow coastal waters of the S.E. Asian region, enters many including the Irrawaddy, Ganges, Brahmaputra and Mekong as well as minor water bodies of the Indonensian archipelago (Watson, 1981). All these dolphins feed on fish and Crustacea using their long beaks to root in the bottom. In the Amazon, the two coexisting species are separated by very different ecological characteristics (Ferreira, 1983) Sotalia is a social dolphin inhabiting the open waters of the river channel and feeding on small sized schooling fish eaten whole. It consumes about 6.6% of its body weight daily. Inia is a more solitary animal that depends on large solitary bottom dwelling fish and which are ripped apart before being consumed. It consumes 3.1% of its body weight daily. There is some overlap of diet at the edges of the channel. Inia feeds mainly during rising river levels as fish spread out onto the floodplain and has been recorded many kilometers from the river in the Igapo. Sotalia feeds principally during low waters as pelagic schooling fish are concentrated into the river. Extrapolting from what is known of the ecological role of other freshwater aquatic mammals dolphins may play two major roles within the ecosystem. Firstly, they may contribute to the maintenance of the nutrient balance especially in nutrient poor rivers in much the same way as that described for the Amazonian caimans. Secondly, they may consume significant amounts of fish thus affecting the dynamic of the populations within the river. Little information is available at present upon which to base any conclusions as to the role of these species, although indications from Ferreira (1983) are that large quantities of fish are consumed seasonally. This may be especially significant for fisheries in the case of Inia, which, by taking large and commercially preferred species, is in direct competition with fisheries. The smaller schooling fishes preyed upon by Sotalia have are not yet formed the subject of a fishery and are thus of little or no direct economic important. Despite this competition Inia, in common with most dolphins in freshwaters, is strongly protected by tradition, and is neither harmed nor eaten.
The floodplain is also used during the dry phase by a number of wild and domestic animals, but these will be considered separately in a later chapter of this review.